Abstract
Background:
After severe trauma, the older host experiences more dysfunctional hematopoiesis of bone marrow (BM) hematopoietic stem and progenitor cells (HSPCs), and dysfunctional differentiation of circulating myeloid cells into effective innate immune cells. Our main objective was to compare BM HSPC miR responses of old and young mice in a clinically relevant model of severe trauma and shock.
Methods:
C57BL/6 adult male mice aged 8–12 weeks (young) and 18–24 months (old) underwent polytrauma and hemorrhagic shock (PT) that engenders the equivalent of major trauma (injury severity score >15). Pseudomonas pneumonia (PNA) was induced in some young and old adult mice 24 hours after PT. miR expression patterns were determined from lineage-negative enriched BM HSPCs isolated from PT and PT+PNA mice at 24- and 48-hours post-injury, respectively. Genome-wide expression and pathway analyses were also performed on bronchoalveolar lavage (BAL) leukocytes from both mouse cohorts.
Results:
miR expression significantly differed among all experimental conditions (p<0.05), except for old-naïve vs old-injured (PT or PT+PNA) mice, suggesting an inability of old mice to mount a robust early miR response to severe shock and injury. In addition, young adult mice had significantly more leukocytes obtained from their BAL, and there were greater numbers of polymorphonuclear cells compared with old mice (59.8% vs 2.2%, p=0.0069). Despite increased gene expression changes, BAL leukocytes from old mice demonstrated a more dysfunctional transcriptomic response to PT+PNA than young adult murine BAL leukocytes, as reflected in predicted upstream functional pathway analysis.
Conclusion:
The miR expression pattern in BM HSPCs after PT(+/−PNA) is dissimilar in old versus young adult mice. In the acute post-injury phase, old adult mice are unable to mount a robust miR HSPC response. HSPC miR expression in old PT mice reflects a diminished functional status as well as a blunted capacity for terminal differentiation of myeloid cells.
Level of Evidence:
Basic Science.
Study Type:
Experimental murine model.
Keywords: trauma, microRNA, old, murine, hematopoietic stem and progenitor cells
BACKGROUND
Despite advances in healthcare, trauma is still one of the leading causes of death in all age groups (1). It is also the greatest cause of loss of productivity in the United States (2, 3). Advanced age is associated with significantly increased morbidity and mortality after trauma (4–6). The pathophysiology of these age-related trauma outcomes is still largely unknown, especially in regards to the host immune transcriptome and epigenome.
In a large prospective, multi-institutional observational study of human inflammatory conditions, an analysis of elderly versus young cohorts revealed that older adult patients demonstrated evidence of physiologic derangement during the first 24 hours following severe injury and hemorrhagic shock (4). These older patients had a higher incidence of ventilator-associated pneumonia (VAP), ventilator dependence, multi-organ failure, intensive care unit (ICU) length of stay, and 28-day mortality compared with their younger counterparts, as well as being more likely to be discharged to a skilled nursing facility (4, 7). Importantly, age greater than 55 years was identified as an independent predictor of mortality in severely injured blunt trauma patients with VAP, after controlling for injury severity, transfusion requirements, shock severity, physiologic derangement and comorbidities (4).
Previously, our laboratory developed a murine model of polytrauma and hemorrhagic shock (PT) that more closely recapitulates human trauma than historical models (8). Using our PT model, we determined that old adult mice have increased mortality after PT plus pneumonia compared with young adult mice (9). Interestingly, after PT plus pneumonia (PNA), elderly mice were unable to adequately induce emergency myelopoiesis, and experienced a prolonged return to homeostasis after severe injury and inflammation (9, 10). Their increased mortality was not linked to a worsened acute exacerbation of the inflammatory/cytokine/chemokine response (compared with the younger cohort), but rather the inability of their bone marrow (BM) hematopoietic stem and progenitor cells (HSPCs), as well as circulating myeloid cells, to initiate and complete a well-functioning myelopoietic response (9).
MicroRNAs (miR) are small noncoding RNAs that regulate gene expression and are considered part of the epigenome (11, 12). miRs can function in several ways, including RNA silencing and post-transcriptional regulation of gene expression (13), and they play key roles in immunomodulation, metabolism and cell differentiation, proliferation, and apoptosis (12). Growing evidence suggests miR control HSPC self-renewal and differentiation (14–16). However, the importance of miR in the pathology of HSPC dysfunction after severe injury or inflammation remains unclear (17). Using a murine model of multi-compartmental polytrauma and hemorrhagic shock with subsequent Pseudomonas pneumonia, our goal was to identify the unique miR expression profile produced by severe injury and inflammation in young and old adult mice. To assess potential downstream effects of the HSPC epigenetic patterns, we also analyzed the genomic pattern of bronchoalveolar leukocytes in young and old cohorts in our trauma model.
METHODS
Animals.
Adult C57BL/6J (B6) male mice were purchased at ages 6–8 weeks and 18–20 months from Jackson Laboratory (Bar Harbor, ME). Prior to initiation of the experiment, mice were acclimated to the humidity-controlled housing room programmed for a 12-hour light-dark cycle for a minimum of 2 weeks. Mice were cared for by the University of Florida Animal Care Services (Gainesville, FL). Mice of the same age cohort and treatment were housed in transparent cages (3–4 animals per cage) within specific pathogen-free facilities at ambient room temperatures (23°C). The animals were provided standard rodent chow and water ad libitum for the duration of the study. Due to the coprophagic nature of mice, caging them together for 2 weeks allowed the mice to obtain a similar microbiota composition and structure (18, 19), although our previous worked revealed that microbiome similarity occurred only among mice of the same age (20). The animals were cared for and used according to the Guide for the Care and Use of Laboratory Animals, and the experiments were approved by the University of Florida Institutional Animal Care and Use Committee.
Polytrauma and hemorrhagic shock model.
Young (8–12 weeks) and old (18–24 months) adult mice underwent polytrauma, hemorrhagic shock and resuscitation as previously described in detail by Mira et al (8). Briefly, mice were anesthetized with inhalational isoflurane and restrained in the supine position. After cannulation of the femoral artery, mice were awakened from anesthesia and underwent 90 minutes of hemorrhagic shock (MAP 30–40 mmHg). After the hypotensive period, mice were resuscitated with four times shed-blood volume with Ringer’s lactate solution at a rate of 10 mL/hour. Mice then underwent repeat isoflurane anesthesia followed by femur fracture and cecectomy. The combined level of injury produces an equivalent injury severity score in humans of 18. After injury, mice were housed in treatment groups, and all mice were administered buprenorphine (0.2 mg/kg body weight) prior to arousal from anesthesia and every 12 hours afterward until euthanization on post-injury day 1 for PT mice and post-injury day 2 for PT+PNA mice. This model is generally nonlethal in both young and old mice, and the animals were able to maintain the ability to ambulate, groom, feed and drink.
Polytrauma and hemorrhagic shock followed by Pseudomonal pneumonia (PNA).
PNA was induced using Pseudomonas aeruginosa as previously described (PAK strain) one day after PT (21). Briefly, PAK was grown overnight, transferred to fresh medium and grown to mid-log phase. The bacterial density was measured at OD 600λ (DU 640 Spectrophotometer, Beckman Coulter, Inc., CA) and washed with PBS. The mice were delivered the equivalent of 1×107 bacteria in 50 μl intranasally.
Hematopoietic stem and progenitor cell isolation.
Bone marrow cells from young and old adult mice were aseptically collected from the non-injured lower extremity (femur bone) 1 day after polytrauma (old PT n=8, young PT n=8) or 1 day after pneumonia (old PT+PNA n=7, young PT+PNA n=7), as well as from naïve young and old adult control mice. Single cell suspensions were created by passing the cells through 70 μm pore sized cell strainers (BD Falcon, Durham, NC). Erythrocytes were lysed using ammonium chloride lysis buffer and washed with phosphate-buffered saline. HSPCs were isolated via immunomagnetic negative selection using EasySep™ Mouse Hematopoietic Progenitor Cell Isolation kit (StemCell Technologies, Vancouver, BC) according to the manufacturer’s protocol (9).
Murine HSPC miR expression.
Total RNA was isolated from HSPCs using RNeasy™ Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. miR profiling was performed using GeneChip™ miR 4.0 Array (ThermoFisher Scientific), with 3,163 transcripts passing filtering criteria. All miR arrays were normalized together using RMA as implemented in Partek Genomics Suite 6.6. miR expression patterns were calculated with a log2-transformed expression matrix with significant expression differences (fold expression changes over age-matched control) identified using BRBArrayTools®.
Bronchoalveolar lavage (BAL).
The trachea was cannulated and lavaged four times with 800 μl cold PBS containing 2 mM EDTA. The BAL cells were counted using a hematocytometer (Hausser Scientific, Horsham, PA). BAL single cell suspensions were created by passing the cells through 70 μm pore sized cell strainers (BD Falcon, Durham, NC). Cells were stained with the following antibodies for flow cytometric studies: PE Cy7 anti-CD11b, APC anti-siglec, and Pacific Blue anti-Ly6G, FITC anti CD11c, and PE anti-F4/80 (BD Pharmingen, Billerica, MA). Sytox Blue (Invitrogen, Carlsbad, CA) was used for cell viability analysis and samples were acquired and analyzed using an LSRII flow cytometer (BD Biosciences) and FACSDiva™.
BAL mRNA expression analysis.
BAL cells were collected 1 day after PT or PT+PNA mice and from naïve mice. Total RNA was isolated using RNeasy™ kit (Qiagen, Valencia, CA) and the quality and quantity was assessed using Agilent Bioanalyzer 2000. Nucleic acids were labeled using the 3′ IVT Express Kit and 15 μg of labeled cRNA was hybridized to Mouse Genome 430 2.0 Arrays (Affymetrix, Santa Clara, CA). Following hybridization, arrays were stained and washed using an FS450 Affymetrix fluidics station and Affymetrix FlexFS 450–0004 protocol. Arrays were then scanned in an Affymetrix GeneChip™ scanner 7G Plus. Genome-wide expression was performed on total BAL leukocytes. Expression patterns were compared between naïve, PT young/old and PT+PNA young/old mice at p<0.001 (F test). The significant, differentially expressed genes were further analyzed using Ingenuity Pathway Analysis™ (IPA) software. IPA Diseases and Functions software was employed to make downstream functional predictions from these groups of genes with a Z-score greater than two indicating significance.
Statistical analysis.
Results for continuous variables are reported as mean ± standard deviation (SD) for normally distributed variables or median and interquartile range for non-normally distributed variables. Normality was checked via the Shapiro-Wilk test. Student’s t-test or nonparametric Mann-Whitney test was used to compare normal or non-normal variables respectively between different groups or time points. Tukey’s multiple comparison procedure was used to adjust p-values for multiple comparisons. Data were analyzed using Prism 7 (GraphPad Software, CA) and SAS 9.4 (SAS Institute Inc., Cary NC).
RESULTS
HSPC miR expression patterns are dissimilar in old adult mice after polytrauma.
We sought to characterize miR expression of bone marrow HSPCs, the key cells involved in successful emergency myelopoiesis and hematopoiesis in general. Of our 32 samples, 3 samples failed RNA sample integrity validation leaving 29 total arrays composed of young PT (n=6), old PT (n=8), naïve young (n=7), and naïve old (n=8). Table 1 summarizes the miR expression analyses of each comparison completed in this study.
Table 1.
Summary of HSPC microRNA (miR) expression analyses (p < 0.05).
| Cohorts compared | # of significant genes |
|---|---|
| Young Naïve vs Old Naïve | 178 |
| Young PT vs Old PT vs Young naïve vs Old naive | 198 |
| Young PT vs Old PT | 182 |
| Young PT vs Young Naïve | 166 |
| Old PT vs Old Naïve | 0 |
| Young PT+PNA vs Old PT+PNA vs Young Naïve vs Old Naive | 638 |
| Young PT+PNA vs Old PT+PNA | 467 |
| Young PT+PNA vs Young Naïve | 530 |
| Old PT+PNA vs Old Naïve | 0 |
| All Cohorts - Young PT vs Old PT vs Young PT+PNA vs Old PT+PNA vs Young Naïve vs Old Naive | 313 |
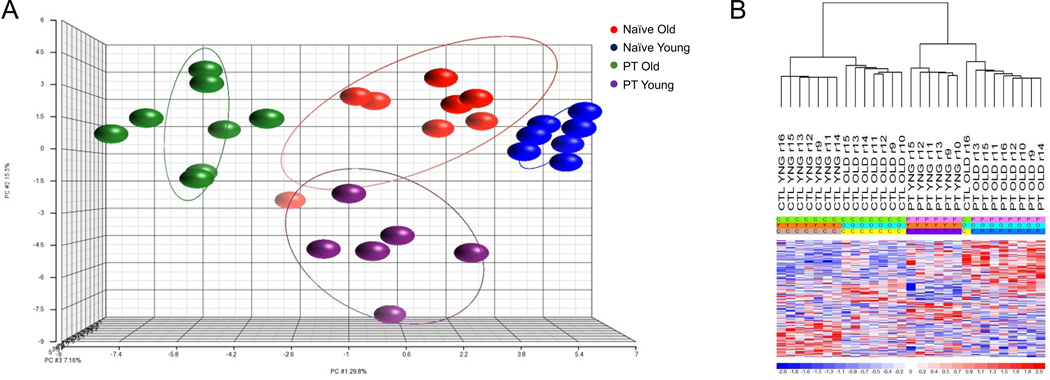
Supervised analysis identified 198 miRs (p<0.05) that defined each of our groups (old PT, naïve old, young PT, naïve young; Figure 1). Further analysis revealed that the miR expression pattern was significantly different among all conditions at 24 hours (p<0.05) except for old adult naïve vs old adult PT mice. The lack of significant differences in the miR expression between naïve and severely injured old adult mice suggests an inability of aged mice to mount a robust early miR response to injury.
Figure 1. Microarray Transcriptomic Analysis of Hematopoietic Progenitor and Stem Cells (HSPC) of Young Polytrauma (PT) Mice versus Old PT Mice versus Naïve Mice.
The transcriptomic response of isolated HSPCs in naïve and polytrauma mice. (A) Conditional principal component analysis of young PT mice, old PT mice and naïve miR expression patterns. (B) Heat map (log2) of the hierarchical clustering of HSPC miR expression patterns between old and young PT and naïve mice. Supervised analysis identified 198 miRs (p<0.05) that defined our groups (PT old, naïve old, PT young, naïve young). C, CTL = Control; Y, YNG = young; O = old; P, PT = polytrauma
Among cohorts, there was significantly different expression of 178 miRs between naïve young versus naïve old mice, 166 miRs between young PT versus naïve young mice, and 182 miRs between young PT vs old PT mice. Among the miRs with the highest differential expression between the trauma cohorts were miR-494–3p, miR-132–3p, miR-145a-5p and miR-125b-5p all of which are implicated in regulation of early progenitor cells (Supplemental Digital Content (SDC) 1) (22, 23).
Interestingly, in a comparison of relative changes in miR expression between old PT (versus naïve old) and young PT mice (versus naïve young), miR-132–3p and miR 145a-5p were amongst the 13 miRs with the highest fold-change difference between expressions of BM HSPC miR (SDC 2). Of note, miR-132–3p and miR-145a-5p have both previously been identified as playing a role in tumor suppression. Both were upregulated in older trauma patients and downregulated in younger trauma patients when compared with their age-matched controls (SDC 2).
HSPC miR expression patterns are dissimilar in old mice after polytrauma and pneumonia.
Pneumonia was induced in a separate cohort mice 24 hours following polytrauma (PT+PNA). All PT+PNA samples passed RNA sample integrity validation, leaving 22 total arrays composed of young adult PT+PNA (n=7), old adult PT+PNA (n=7), young adult naïve (n=4), and old adult naïve (n=4) murine groups.
Supervised analysis identified 638 significant miRs that defined each of our groups (old PT+PNA, young PT+PNA, naïve old, naïve young). The miR expression pattern was significantly different between young adult naïve vs. young adult PT+PNA as well as young adult PT+PNA vs. old adult PT+PNA at 48 hours. There was no significant difference between old adult naïve compared with old adult PT+PNA mice (Table 1), again suggesting an inability of aged mice to mount a robust early response to severe shock, injury, and PNA, unlike young adult mice.
There was differential expression of 530 miRs when comparing young PT+PNA vs. naïve young cohorts and 467 miRs between young PT+PNA vs. old PT+PNA cohorts. Among the miR with the highest differential expression between the PT+PNA cohorts were miR-125a-5p, miR-19b-3p, miR-21a-5p, miR196b-5p, miR-223, and miR-17–5p. Each of these have previously been demonstrated to be important for renewal, expansion, or differentiation of myelopoietic stem cells (24–30).
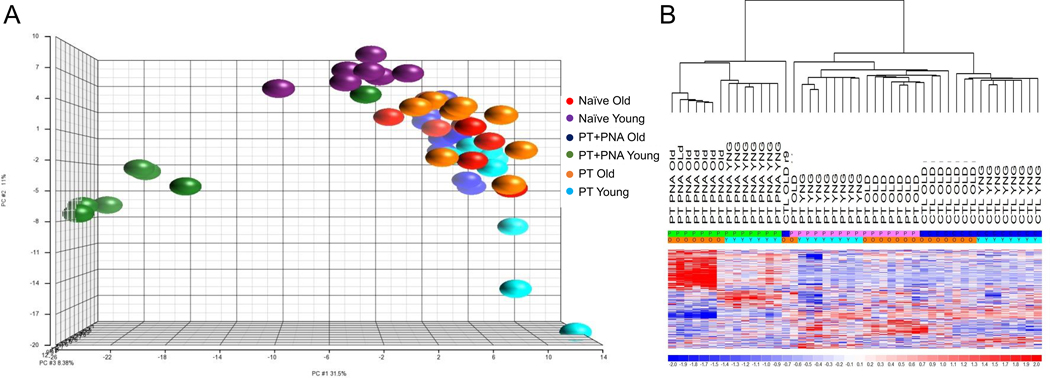
An additional supervised analysis among all groups (PT+PNA young and old, PT young and old, naïve young and old) identified 313 miRs whose differential pattern of expression defined each group (Figure 2). Among these differential miRs were the same miRs which had the highest differential expression in the individual analyses between PT and PT+PNA groups. These include miR-125a-5p, miR-19b-3p, miR-21a-5p, miR196b-5p, miR-223, miR-17–5p, miR-132–3p, miR 145a-5p, miR-494–3p, miR-132–3p, miR-145a-5p and miR-125b-5p, the functions of which as mentioned earlier, are crucial for HSPC function.
Figure 2. Microarray Transcriptomic Analysis of Hematopoietic Stem and Progenitor Cells between all mouse groups.
The transcriptomic response of isolated HSPCs in naïve, polytrauma (PT), and polytrauma + pneumonia (PT+PNA) mice. (A) Conditional principal component analysis and (B) heat map (log2) of the hierarchical clustering of HSPC miR expression in PT+PNA old versus PT+PNA young versus PT old versus PT young versus naïve old versus naïve young mice (313 miRs significant at p<0.05). C, CTL = Control/Naïve; Y, YNG = young; O = old; P = treated; PT = polytrauma; PT+PNA = polytrauma + pneumonia
BAL messenger RNA (mRNA) expression patterns are dissimilar in old adult mice versus young adult mice after polytrauma +/− pneumonia.
Our previous work indicated that elderly patients are not only more likely to have pneumonia after trauma, but also among those who develop pneumonia, advanced age is associated with worse outcomes compared with younger cohorts (4, 9). Our laboratory used “reverse translation” to study this phenomenon in a clinically relevant murine trauma model in both young and old adult mice, illustrating that bone marrow progenitors and bronchoalveolar cells in the aged fail to effectively initiate as well as complete an emergency myelopoietic response, generating myeloid cells that are unable to appropriately resolve a secondary infection (9, 31). Thus, we sought to further examine the transcriptome of myeloid cells after PT+PNA in young and old adult mice to investigate the downstream effects of differential miR expression in old mice.
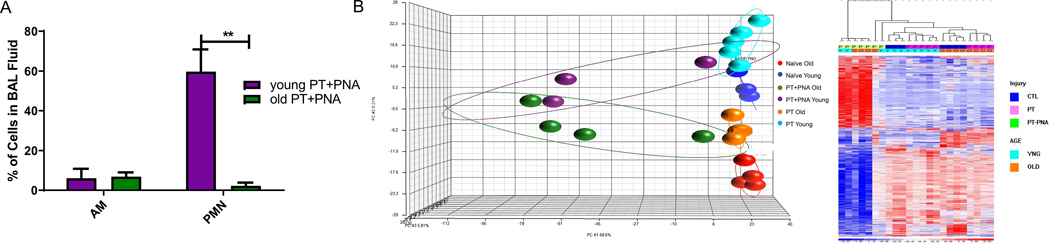
48 hours after polytrauma and 24 hours after induction of Pseudomonas pneumonia (PT+PNA), young adult PT+PNA mice had significantly more leukocytes obtained from their BALs, and were determined to have more PMNs compared with old mice (59.8% vs 2.2%, p=0.0069); however, there was no significant difference in the percentage of activated macrophages (6.0% vs 6.8%, p=0.89) (Figure 3A).
Figure 3. Analysis of Bronchoalveolar Lavage (BAL) Leukocytes.
(A) Cellular composition of leukocytes in bronchoalveolar lavage (BAL) after polytrauma + pneumonia (PT+PNA). Data represent the mean ± SD (n=3 in each group). **p < 0.01 (B) Microarray Transcriptomic Analysis of Bronchoalveolar Lavage Leukocytes. Heat map (log2) of the hierarchical clustering of BAL Leukocytes mRNA expression in (top) naïve old vs PT old vs PT+PNA old representing 979 Probe sets (829 Genes) at p<0.001 and (bottom) naïve young vs PT young vs PT+PNA young 262 Probe sets (95 Genes) at p<0.001. BAL leukocytes from old mice after PT+PNA had an increased number of changes in their transcriptome compared with young adult mice when compared with age matched controls. Data represent the mean ± SD (n=3 in each group). **p < 0.01Y, YNG = young; N = Naïve; O = old; P = treated; PT = polytrauma; PT+PAK = polytrauma + pneumonia
Next, we analyzed the transcriptomic differences of the BAL leukocytes in PT and PT+PNA cohorts. Surprisingly, the addition of a secondary infection (PT+PNA) led to a dramatically increased number of transcriptomic changes in the BAL-derived leukocytes of older adult relative to young adult mice when both were compared against age-matched controls (829 differential genes in a comparison of naïve old vs PT+PNA old vs PT old; 95 differential genes in a comparison of naïve young vs PT+PNA young vs PT young; Figure 3B). Old adult mice also demonstrated more extreme relative fold changes in both up- and downregulation of gene expression (SDC 3). However, even in this setting, IPA predicted more up- and downregulation of functional pathways necessary for the appropriate immune response in the PT+PNA young adult BAL leukocytes compared with the PT+PNA old adult mice. Specifically, there were 119 pathways altered in young adult BAL leukocytes vs. 96 pathways in old adult BAL leukocytes (2.0>z-score<2.0; SDC 3). There were only 3 pathways that were unique to PT+PNA old adult BAL leukocytes and each of these unique pathways highlight the potential for dysfunctional immune cells – predicted decrease in cell survival, predicted increase in necrosis of liver, predicted increase in apoptosis of neurons (SDC 3). Young adult BAL leukocytes had 26 unique pathways not seen in old adult BAL leukocytes. Many of these pathways, such as decreased fibroblast cell viability and increase in leukocyte accumulation and mobilization, are important for the appropriate response during acute inflammation.
DISCUSSION
Using a murine model of polytrauma and hemorrhagic shock that emulates severe, major human trauma, we determined that the miR expression pattern in bone marrow HSPCs after polytrauma and pneumonia is markedly dissimilar in old versus young adult mice. In the acute phase, old adult mice are unable to mount a robust miR response in HSPCs following PT±PNA. HSPC miR expression in old PT±PNA mice presages a diminished functional status as well as a blunted capacity for terminal differentiation of myeloid cells. The latter was illustrated demonstrated by the decreased percentage of PMNs in BAL leukocytes of old mice after PT±PNA and a greater dysregulation in their gene expression changes. This is important as miR expression is a modifiable epigenetic cellular component and miR modifications have been attempted in other disease states to improve outcomes (17).
miRs modify both pre- and post-transcriptional gene regulation (13) and studies have previously demonstrated that miRs can affect hematopoiesis and leukocyte differentiation (32, 33). We found that many of the differentially expressed HSPC miRs between old and young adult mice after PT are implicated in myeloid cell differentiation, and a summary of select HSPC miR functions is provided in Table 2. For example, miR-145 is known to regulate HSPC activity through suppression of canonical TGFβ signaling (34), while miR-494 has been associated with expansion of myeloid-derived stem cells through activation of NF-κB (35). Additionally, miR-125a expression is associated with an expansion of HSPCs, while overexpression of miR-125b in HSPCs promotes self-renewal, differentiation and expansion (22, 36). The upregulation miR-145, miR494 and miR-125b in old adult relative to young mice after PT suggests that there may be propensity for acute HSPC expansion in old mice in response to severe injury. All of the above miR may contribute to elderly mice entering ‘pathologic myeloid activation’ after severe injury and shock (37).
Table 2.
Function of select significant HSPC microRNA (miR).
| miR | Function | Reference |
|---|---|---|
| miR-145 | differentially regulates hematopoietic stem and progenitor activity through suppression of canonical TGFβ signaling | (34) |
| miR-494 | associated with expansion of myeloid derived stem cells through activation of the NFkB | (35) |
| miR-125a | expression is associated with an expansion of HSPCs | (36) |
| miR-125b | overexpression in hematopoietic stem cells promotes self-renewal, differentiation and expansion | (22) |
| miR-132/124 | important for cycling, function, and survival | (55) |
| miR 196b | miR-21/miR-196b work inhibit myelopoiesis and completely block G-CSF–induced granulopoiesis | (56) |
| miR-21 | elevated levels in myeloid cells enhance proliferation and block myeloid differentiation; expression implicated in increased myeloid-derived suppressor cells and promote immunosuppression in late sepsis | (57) |
| miR-17–5p | highly expressed in HSPCs and markedly downregulated during monocytic differentiation and maturation | (33) |
| miR-223–5p | myeloid-specific miR-223 negatively regulates progenitor proliferation and granulocyte differentiation and activation. | (32) |
There was also differential expression of miR-223–5p, which has shown decreased expression in the young adults compared with old mice after PT+PNA. The decline of miR-223 is both an important event for erythroid differentiation that leads to the expansion of erythroblast cells as well as for both granulocyte development and mature homeostasis (30, 32). A key aspect of ‘pathologic myeloid activation’ is the overproduction of myeloid precursor cells and poorly functioning terminal myeloid cells at the expense of both erythropoiesis and lymphopoiesis (37, 38). Expression of miR-223 is detected at low levels in pluripotent HSPCs and common myeloid progenitor cells, and increases with differentiation of granulocyte-monocyte progenitor (immature bone marrow neutrophils) to mature peripheral blood granulocytes (32). Interestingly, we noted a relative increase of PMNs in BAL samples from young PT+PNA mice coupled with decreased bone marrow expression of miR-223 as compared with old PT+PNA mice. Old adult mice after PT+PNA demonstrated the opposite, suggesting a failure of granulocytic differentiation among aged mice that originates in the bone marrow.
The aging-related impaired miR response has been implicated in both the process of inflammaging and immuno-senescence, which can be exacerbated in many other disease processes (39, 40). Inflammaging, an age-related increase in systemic chronic inflammation, has been implicated in many disease processes as well as the differential responses to certain stresses in the elderly (39, 41). Our data is more consistent with immuno-senescence, the age-associated changes in the immune system resulting in a decreased ability to mount an effective immune response (42, 43). This study, as well as our other previously published analyses on old adult murine sepsis and trauma, suggests a failure to mount an appropriate response to acute injury contributes to the poor outcomes seen in the elderly (4, 9, 10, 44–49). Specifically, at time of injury and/or inflammation, aged bone marrow HSPCs are unable to mount an equivalent acute response to that of younger adults, including rapid myeloid demargination and repopulation of the BM with functional innate immune effector cells by HSPCs (50, 51).
This study also demonstrated that the failure of appropriate bone marrow emergency myelopoiesis in old mice is associated with a unique gene expression pattern in BAL leukocytes. BAL leukocytes in old mice after PT+PNA had increased gene expression changes compared with old PT and PT±PNA young mice. Of note, these leukocytes were isolated 48 hours after injury, and our previous murine work was conducted at 24 hours after injury. Importantly, in human trauma, the circulating leukocytes of the older adult patients (compared with young trauma patients) display a less aberrant transcriptomic response 12 to 24 hours after injury, which is followed by persistent and increased aberrant genomic expression that fails to return to baseline (4). This is consistent with and potentially contributes to the increase in gene expression changes seen in the BAL leukocytes from old mice with PT+PNA. In addition, increased genomic changes does not necessarily equate to better function; although there are increased genomic changes in the old mice after PT+PNA, further pathway analysis suggests reveals this response does not result in the appropriate transcriptome that allows proper leukocyte function as seen in young. IPA upstream functional analysis predicted fewer altered pathways in the old mice, suggesting a more dysfunctional transcriptomic response to PT+PNA. Although there was significant overlap in the predicted IPA pathways, the 26 unique pathways displayed in young adult mice BAL leukocytes suggest an early blunted response to the acute inflammation from PT+PNA in old adult mice. For example, young adult mice BAL leukocytes after PT+PNA are predicted to have an increase in accumulation of immune cells and cell movement of immune cells, which is not seen in old mice. This could contribute to the significantly decreased percentage of PMNs in the BAL of old adult mice acutely after PT+PNA. Overall, BM HSPC miR expression patterns in elderly mice after trauma reveal a diminished a functional status of their HSPCs and a blunted capacity for myeloid cell differentiation. This is associated with a circulating and tissue leukocyte gene expression pattern that suggests a dysfunctional immune cell response in old adult mice after trauma with subsequent pneumonia.
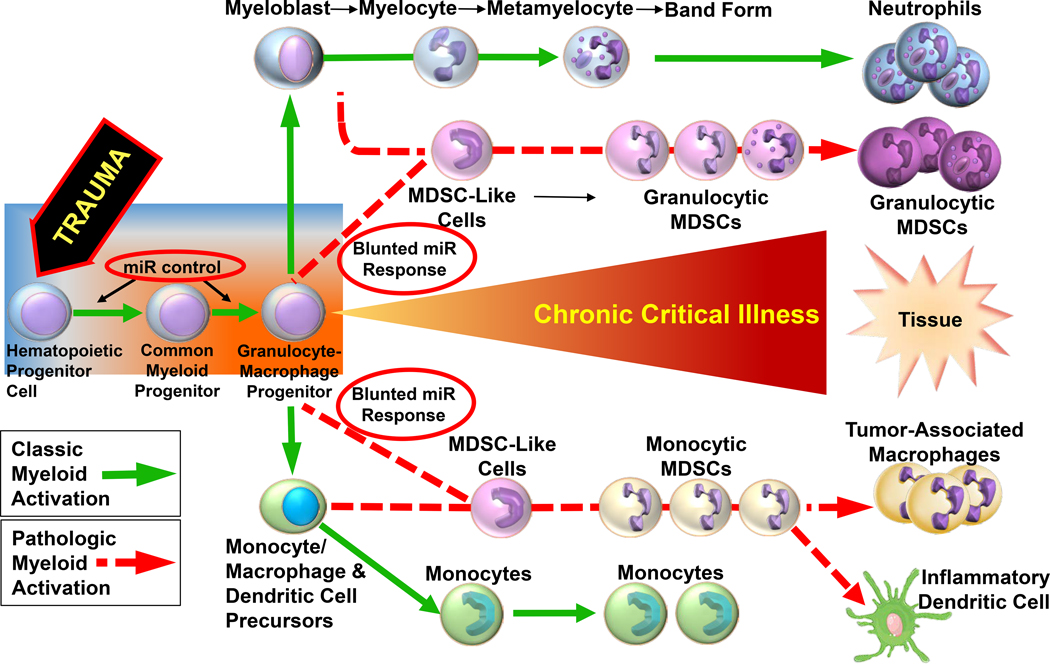
Our study has several limitations. First, this is a descriptive study. Further functional studies to elucidate the specific role of these miR in polytrauma is warranted. Specifically, functional studies to increase specific intracellular miRs, or blockade of miRs with small interfering RNAs (double stranded non-coding RNA that prevent translation of mRNA targets), or alter miR expression with epigenetic modifiers, both in vitro and in vivo, are required to discern if epigenetic modification of HSPCs in older adults after trauma can be modified to follow “classical activation” (17, 38, 52, 53) (Figure 4). For example, Apple et. al demonstrated that the administration of a beta blocker to rats after severe trauma mitigates persistent inflammation via upregulation of miR-25 and Rno-miR-27a (53). Further transcriptomic and epigenetic analysis of human BAL-derived leukocytes in young and old adult human trauma patients with subsequent pneumonia would also be of benefit. Additional analysis of other epigenetic regulatory mechanisms, like histone acetylation and DNA methylation, also warrant study. Additionally, the work should also be conducted in female mice, with specific investigation into differences between pre- and post-menopausal females. In general, it has been published that women have improved outcomes after hemorrhagic shock and severe injury (54). A better understanding of the differences in the female transcriptomic and epigenetic response at the progenitor cell level will help investigators offer a more personalized medical approach to improving outcomes in trauma patients.
Figure 4. Proposed pathway for classic and pathologic myeloid activation in chronic critical illness after trauma.
A blunted miR response (red circles) may be partly responsible for pathologic myeloid activation starting with HSPCs in the bone marrow of older adults. Modified from Kelly et al (37).
Conclusion
Our work highlights a dysfunctional and blunted response for the differentiation and functional capacity of peripheral myeloid cells that likely starts with the epigenetic response in the bone marrow of old mice after polytrauma. This study and our other previously published analyses on aged sepsis and trauma, suggest that it is a failure to mount an appropriate response to acute injury that contributes to the poor outcomes in the elderly.
Supplementary Material
SDC 1 (Table). HSPC miRs with fold-change (FC) > │2│ between old polytrauma (PT) mice vs young PT mice.
SDC 2 (Table). HSPC microRNA (miR) with the highest relative fold change (FC) differences between old and young PT adult mice compared with age-matched naïve.
SDC 3 (Data File). Gene datasets and IPA Diseases & Functions Pathways in PT+PNA young and old mice.
Funding:
This work was supported, in part, by National Institute of General Medical Sciences grants: R01 GM-113945 (PAE), R01 GM-104481 (LLM), R01 GM105893-01A1 (AMM), P50 GM-111152 (FAM, SCB, LLM, PAE, AMM) and K23 GM140268 (TJL). In addition, this work was supported, in part, by a postgraduate training grant T32 GM-008721 (DBD, JCM, JAS, BPF, LSK) in burns, trauma, and perioperative injury by NIGMS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST:
The authors declare that they have no relevant conflicts of interests.
No reprints will be requested.
References
- 1.DiMaggio C, Ayoung-Chee P, Shinseki M, Wilson C, Marshall G, Lee DC, Wall S, Maulana S, Leon Pachter H, Frangos S. Traumatic injury in the United States: In-patient epidemiology 2000–2011. Injury. 2016;47(7):1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florence C, Haegerich T, Simon T, Zhou C, Luo F. Estimated Lifetime Medical and Work-Loss Costs of Emergency Department-Treated Nonfatal Injuries--United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(38):1078–82. [DOI] [PubMed] [Google Scholar]
- 3.Florence C, Simon T, Haegerich T, Luo F, Zhou C. Estimated Lifetime Medical and Work-Loss Costs of Fatal Injuries--United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(38):1074–7. [DOI] [PubMed] [Google Scholar]
- 4.Vanzant EL, Hilton RE, Lopez CM, Zhang J, Ungaro RF, Gentile LF, Szpila BE, Maier RV, Cuschieri J, Bihorac A, et al. Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care. 2015;19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor MD, Tracy JK, Meyer W, Pasquale M, Napolitano LM. Trauma in the elderly: intensive care unit resource use and outcome. J Trauma. 2002;53(3):407–14. [DOI] [PubMed] [Google Scholar]
- 6.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40(4):501–10; discussion 10–2. [DOI] [PubMed] [Google Scholar]
- 7.Cuschieri J, Johnson JL, Sperry J, West MA, Moore EE, Minei JP, Bankey PE, Nathens AB, Cuenca AG, Efron PA, et al. Benchmarking outcomes in the critically injured trauma patient and the effect of implementing standard operating procedures. Ann Surg. 2012;255(5):993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentile LF, Nacionales DC, Cuenca AG, Armbruster M, Ungaro RF, Abouhamze AS, Lopez C, Baker HV, Moore FA, Ang DN, et al. Identification and description of a novel murine model for polytrauma and shock. Crit Care Med. 2013;41(4):1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nacionales DC, Szpila B, Ungaro R, Lopez MC, Zhang J, Gentile LF, Cuenca AL, Vanzant E, Mathias B, Jyot J, et al. A Detailed Characterization of the Dysfunctional Immunity and Abnormal Myelopoiesis Induced by Severe Shock and Trauma in the Aged. J Immunol. 2015;195(5):2396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nacionales DC, Gentile LF, Vanzant E, Lopez MC, Cuenca A, Cuenca AG, Ungaro R, Li Y, Baslanti TO, Bihorac A, et al. Aged mice are unable to mount an effective myeloid response to sepsis. J Immunol. 2014;192(2):612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonson B, Das S. MicroRNA Therapeutics: the Next Magic Bullet? Mini Rev Med Chem. 2015;15(6):467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. 2014;13(8):622–38. [DOI] [PubMed] [Google Scholar]
- 13.Fleshner M, Crane CR. Exosomes, DAMPs and miRNA: Features of Stress Physiology and Immune Homeostasis. Trends Immunol. 2017;38(10):768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darden DB, Stortz JA, Hollen MK, Cox MC, Apple CG, Hawkins RB, Rincon JC, Lopez MC, Wang Z, Navarro E, et al. Identification of Unique mRNA and miRNA Expression Patterns in Bone Marrow Hematopoietic Stem and Progenitor Cells After Trauma in Older Adults. Front Immunol. 2020;11:1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bissels U, Bosio A, Wagner W. MicroRNAs are shaping the hematopoietic landscape. Haematologica. 2012;97(2):160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baue AE. Sepsis, systemic inflammatory response syndrome, multiple organ dysfunction syndrome, and multiple organ failure: are trauma surgeons lumpers or splitters? J Trauma. 2003;55(5):997–8. [DOI] [PubMed] [Google Scholar]
- 17.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–22. [DOI] [PubMed] [Google Scholar]
- 18.Franklin CL, Ericsson AC. Microbiota and reproducibility of rodent models. Lab Anim (NY). 2017;46(4):114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fay KT, Klingensmith NJ, Chen CW, Zhang W, Sun Y, Morrow KN, Liang Z, Burd EM, Ford ML, Coopersmith CM. The gut microbiome alters immunophenotype and survival from sepsis. FASEB J. 2019;33(10):11258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mankowski RT, Thomas RM, Darden DB, Gharaibeh RZ, Hawkins RB, Cox MC, Apple C, Nacionales DC, Ungaro RF, Dirain ML, et al. Septic Stability? Gut Microbiota in Young Adult Mice Maintains Overall Stability After Sepsis Compared to Old Adult Mice. Shock. 2021;55(4):519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, Cuenca AG, Warner E, Wallet SM, Wallet MA, et al. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 2011;186(1):195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaham L, Binder V, Gefen N, Borkhardt A, Izraeli S. MiR-125 in normal and malignant hematopoiesis. Leukemia. 2012;26(9):2011–8. [DOI] [PubMed] [Google Scholar]
- 23.Iio A, Nakagawa Y, Hirata I, Naoe T, Akao Y. Identification of non-coding RNAs embracing microRNA-143/145 cluster. Mol Cancer. 2010;9:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer SE, Muench DE, Rogers AM, Newkold TJ, Orr E, O’Brien E, Perentesis JP, Doench JG, Lal A, Morris PJ, et al. miR-196b target screen reveals mechanisms maintaining leukemia stemness with therapeutic potential. J Exp Med. 2018;215(8):2115–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen J, Huang Y, Li H, Zhang X, Cheng P, Deng D, Peng Z, Luo J, Zhao W, Lai Y, et al. Over-expression of miR-196b-5p is significantly associated with the progression of myelodysplastic syndrome. Int J Hematol. 2017;105(6):777–83. [DOI] [PubMed] [Google Scholar]
- 26.Guglielmelli P, Bisognin A, Saccoman C, Mannarelli C, Coppe A, Vannucchi AM, Bortoluzzi S. Small RNA Sequencing Uncovers New miRNAs and moRNAs Differentially Expressed in Normal and Primary Myelofibrosis CD34+ Cells. PLoS One. 2015;10(10):e0140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SW, Ramasamy K, Bouamar H, Lin AP, Jiang D, Aguiar RC. MicroRNAs miR-125a and miR-125b constitutively activate the NF-kappaB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20). Proc Natl Acad Sci U S A. 2012;109(20):7865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cammarata G, Augugliaro L, Salemi D, Agueli C, La Rosa M, Dagnino L, Civiletto G, Messana F, Marfia A, Bica MG, et al. Differential expression of specific microRNA and their targets in acute myeloid leukemia. Am J Hematol. 2010;85(5):331–9. [DOI] [PubMed] [Google Scholar]
- 29.Wong P, Iwasaki M, Somervaille TC, Ficara F, Carico C, Arnold C, Chen CZ, Cleary ML. The miR-17–92 microRNA polycistron regulates MLL leukemia stem cell potential by modulating p21 expression. Cancer Res. 2010;70(9):3833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felli N, Pedini F, Romania P, Biffoni M, Morsilli O, Castelli G, Santoro S, Chicarella S, Sorrentino A, Peschle C, et al. MicroRNA 223-dependent expression of LMO2 regulates normal erythropoiesis. Haematologica. 2009;94(4):479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efron PA, Mohr AM, Moore FA, Moldawer LL. The future of murine sepsis and trauma research models. J Leukoc Biol. 2015;98(6):945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451(7182):1125–9. [DOI] [PubMed] [Google Scholar]
- 33.Fontana L, Pelosi E, Greco P, Racanicchi S, Testa U, Liuzzi F, Croce CM, Brunetti E, Grignani F, Peschle C. MicroRNAs 17–5p-20a-106a control monocytopoiesis through AML1 targeting and M-CSF receptor upregulation. Nat Cell Biol. 2007;9(7):775–87. [DOI] [PubMed] [Google Scholar]
- 34.Lam J, van den Bosch M, Wegrzyn J, Parker J, Ibrahim R, Slowski K, Chang L, Martinez-Hoyer S, Condorelli G, Boldin M, et al. miR-143/145 differentially regulate hematopoietic stem and progenitor activity through suppression of canonical TGFbeta signaling. Nat Commun. 2018;9(1):2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Lai L, Chen Q, Song Y, Xu S, Ma F, Wang X, Wang J, Yu H, Cao X, et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J Immunol. 2012;188(11):5500–10. [DOI] [PubMed] [Google Scholar]
- 36.Guo S, Lu J, Schlanger R, Zhang H, Wang JY, Fox MC, Purton LE, Fleming HH, Cobb B, Merkenschlager M, et al. MicroRNA miR-125a controls hematopoietic stem cell number. Proc Natl Acad Sci U S A. 2010;107(32):14229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly LS, Darden DB, Fenner BP, Efron PA, Mohr AM. The Hematopoietic Stem/Progenitor Cell Response to Hemorrhage, Injury and Sepsis: A Review of Pathophysiology. Shock. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19(2):108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivieri F, Rippo MR, Prattichizzo F, Babini L, Graciotti L, Recchioni R, Procopio AD. Toll like receptor signaling in “inflammaging”: microRNA as new players. Immun Ageing. 2013;10(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aalaei-Andabili SH, Rezaei N. MicroRNAs (MiRs) Precisely Regulate Immune System Development and Function in Immunosenescence Process. Int Rev Immunol. 2016;35(1):57–66. [DOI] [PubMed] [Google Scholar]
- 41.Franceschi C Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. 2007;65(12 Pt 2):S173–6. [DOI] [PubMed] [Google Scholar]
- 42.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24(5):495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solana R, Pawelec G, Tarazona R. Aging and innate immunity. Immunity. 2006;24(5):491–4. [DOI] [PubMed] [Google Scholar]
- 44.Turnbull IR, Clark AT, Stromberg PE, Dixon DJ, Woolsey CA, Davis CG, Hotchkiss RS, Buchman TG, Coopersmith CM. Effects of aging on the immunopathologic response to sepsis. Crit Care Med. 2009;37(3):1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loftus TJ, Brakenridge SC, Murphy TW, Nguyen LL, Moore FA, Efron PA, Mohr AM. Anemia and blood transfusion in elderly trauma patients. J Surg Res. 2018;229:288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brakenridge SC, Efron PA, Stortz JA, Ozrazgat-Baslanti T, Ghita G, Wang Z, Bihorac A, Mohr AM, Brumback BA, Moldawer LL, et al. The impact of age on the innate immune response and outcomes after severe sepsis/septic shock in trauma and surgical intensive care unit patients. J Trauma Acute Care Surg. 2018;85(2):247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mira JC, Cuschieri J, Ozrazgat-Baslanti T, Wang Z, Ghita GL, Loftus TJ, Stortz JA, Raymond SL, Lanz JD, Hennessy LV, et al. The Epidemiology of Chronic Critical Illness After Severe Traumatic Injury at Two Level-One Trauma Centers. Crit Care Med. 2017;45(12):1989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loftus TJ, Thomas RM, Murphy TW, Nguyen LL, Moore FA, Brakenridge SC, Efron PA, Mohr AM. The effects of red cell transfusion donor age on nosocomial infection among trauma patients. Am J Surg. 2017;214(4):672–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell-Furtick M, Moore BJ, Overton TL, Laureano Phillips J, Simon KJ, Gandhi RR, Duane TM, Shafi S. Post-trauma mortality increase at age 60: a cutoff for defining elderly? Am J Surg. 2016;212(4):781–5. [DOI] [PubMed] [Google Scholar]
- 50.Luis TC, Tremblay CS, Manz MG, North TE, King KY, Challen GA. Inflammatory signals in HSPC development and homeostasis: Too much of a good thing? Exp Hematol. 2016;44(10):908–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14(5):302–14. [DOI] [PubMed] [Google Scholar]
- 52.Gore AV, Athans B, Iben JR, Johnson K, Russanova V, Castranova D, Pham VN, Butler MG, Williams-Simons L, Nichols JT, et al. Epigenetic regulation of hematopoiesis by DNA methylation. Elife. 2016;5:e11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Apple CG, Miller ES, Loftus TJ, Kannan KB, Thompson CW, Lopez MC, Baker HV, Moldawer LL, Efron PA, Mohr AM. Effect of Beta-Blockade on the Expression of Regulatory MicroRNA after Severe Trauma and Chronic Stress. J Am Coll Surg. 2020;230(1):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haider AH, Crompton JG, Oyetunji T, Stevens KA, Efron DT, Kieninger AN, Chang DC, Cornwell EE 3rd, Haut ER. Females have fewer complications and lower mortality following trauma than similarly injured males: a risk adjusted analysis of adults in the National Trauma Data Bank. Surgery. 2009;146(2):308–15. [DOI] [PubMed] [Google Scholar]
- 55.Mehta A, Zhao JL, Sinha N, Marinov GK, Mann M, Kowalczyk MS, Galimidi RP, Du X, Erikci E, Regev A, et al. The MicroRNA-132 and MicroRNA-212 Cluster Regulates Hematopoietic Stem Cell Maintenance and Survival with Age by Buffering FOXO3 Expression. Immunity. 2015;42(6):1021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Velu CS, Baktula AM, Grimes HL. Gfi1 regulates miR-21 and miR-196b to control myelopoiesis. Blood. 2009;113(19):4720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McClure C, Brudecki L, Ferguson DA, Yao ZQ, Moorman JP, McCall CE, El Gazzar M. MicroRNA 21 (miR-21) and miR-181b couple with NFI-A to generate myeloid-derived suppressor cells and promote immunosuppression in late sepsis. Infect Immun. 2014;82(9):3816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 1 (Table). HSPC miRs with fold-change (FC) > │2│ between old polytrauma (PT) mice vs young PT mice.
SDC 2 (Table). HSPC microRNA (miR) with the highest relative fold change (FC) differences between old and young PT adult mice compared with age-matched naïve.
SDC 3 (Data File). Gene datasets and IPA Diseases & Functions Pathways in PT+PNA young and old mice.