Abstract
BACKGROUND & AIMS:
Incidence and mortality associated with early-age onset colorectal cancer (EAO-CRC) is increasing, prompting professional society recommendations to lower the screening age in average-risk individuals. The yield of screening individuals younger than 50 years is not known.
METHODS:
A systematic review of 3 databases from inception through July 2020 was performed in all languages that reported colonoscopy findings in average-risk individuals younger than 50 years. The primary outcomes were EAO colorectal neoplasia (CRN) and advanced colorectal neoplasia (aCRN) prevalence. Subgroup analyses were performed based on sex, geographic location, time period, and age, including comparison with those aged 50–59 years. Generalized linear mixed model with random intercept logistic regression and fixed subgroup effects were performed.
RESULTS:
Of 10,123 unique articles, 17 studies published between 2002 and 2020, including 51,811 average-risk individuals from 4 continents, were included. The pooled rate of EAO-CRN was 13.7% (95% confidence interval [CI], 0.112%–0.168%) and EAO-aCRN was 2.2% (95% CI, 0.016%–0.031%). Prevalence of CRC was 0.05% (95% CI, 0.00029%–0.0008%). Rates of EAO-CRN were higher in men compared with women (relative risk, 1.71%; 95% CI, 1.49%–1.98%), and highest in the United States (15.6%; 95% CI, 12.2%–19.7%) compared with Europe (14.9%; 95% CI, 6.9%–29.3%), East Asia (13.4%; 95% CI, 10.3%–17.2%), and the Middle East (9.8%; 95% CI, 7.8%–12.2%) (P = .04) The rate of EAO-CRN in age groups 45–49 years and 50–59 years was 17.8% (95% CI, 14.5%–21.6%) and 24.8% (95% CI, 19.5%–30.8%), respectively (P = .04). The rate of EAO-aCRN in age group 45–49 years was 3.6% (95% CI, 1.9%–6.7%) and 4.2% (95% CI, 3.2%–5.7%), respectively (P = .69).
CONCLUSIONS:
The rate of aCRN in individuals aged 45–49 years was similar to the rate observed in individual aged 50–59 years, suggesting that expanding screening to this population could yield a similar impact on colorectal cancer risk reduction.
Keywords: Colon Cancer Screening, Colorectal Cancer, Colon Polyp
There has been a steady decrease in overall incidence and mortality associated with colorectal cancer (CRC) in the United States,1,2 due largely to screening in individuals 50 years and older,3 as supported by multiple professional society guidelines.4–6 Although the screening modalities have evolved over time, this starting age has remained the same because the majority of CRC cases have historically occurred in individuals older than 50 years,7 modeling studies show a favorable balance between the risks and life-years gained from screening,4 and population-based screening starting at age 50 years is cost-effective.8
In contrast, the incidence and mortality associated with CRC in individuals younger than 50 years, or early-age onset (EAO) CRC, is increasing.9,10 Updating microsimulation models with this epidemiologic data shows that starting screening in average-risk individuals (no established colorectal neoplasia [CRN] risk factors and no symptoms) at age 45 years has a favorable balance of benefit to potential harm. Accordingly, in 201811 the American Cancer Society issued a qualified recommendation to start CRC screening for average-risk individuals at age 45 years and the US Preventative Services Task Force currently has a similar draft grade B recommendation under review.12 Ladabaum et al13 demonstrated the cost-effectiveness of starting colonoscopy screening at age 45 years, with a cost of $33,900 per quality-adjusted life-year.
There are limited clinical data regarding the yield of screening younger individuals.14 Studies to date demonstrate the yield of screening in high-risk populations younger than 50 years, such as those with a family history of CRC15 or gastrointestinal symptoms,16 but there are few studies that report on the neoplastic findings in screening examinations among truly average-risk individuals younger than 50 years.17 Furthermore, the limited sample sizes preclude precise estimates of neoplasia prevalence among important sub-groups by sex and age ranges. Quantifying the expected yield of colonoscopy in average-risk individuals younger than 50 years and subsets of individuals at highest risk can inform new screening paradigms.
To address this gap, we performed a systematic review to describe the prevalence of colorectal neoplasia, advanced colorectal neoplasia, and CRC in average-risk, unselected individuals younger than 50 years.
Methods
Data Sources and Searches
A comprehensive literature search was performed by a medical librarian in July 2020. Publications were identified by searching a combination of keywords and database-specific indexing terms for the concepts of colon polyps, incidence and prevalence, and participant age younger than 50 years in Ovid Medline, Embase, and Web of Science (Supplementary Table 1). Results were limited to original research studies with human participants, with no limitations on language or publication date. All results were exported and de-duplicated in EndNote (Clarivate Analytics). A Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart is provided in Figure 1.18
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart for systematic review process.
Study Selection
Two independent reviewers (J.M.K., S.G.P.) evaluated all titles and abstracts and independently reviewed all designated articles for eligibility. If consensus could not be reached, a third reviewer (C.R.B.) provided input. Studies including only average-risk subjects younger than 50 years who underwent screening colonoscopy were included. Average risk was defined as individuals without established risk factors for CRC (ie, hereditary cancer syndromes, inflammatory bowel disease, or personal or family history of CRC) and without any symptoms (eg, abdominal pain, changes in bowel patterns, rectal bleeding, weight loss, anemia, or iron deficiency). Studies reporting other CRC screening methods (eg, flexible sigmoidoscopy, computed tomography colonography) were excluded. The following study types were excluded: meeting abstracts, systematic reviews, studies with fewer than 50 participants, and autopsy studies.
Data Extraction and Quality Assessment
Two reviewers (J.M.K., S.G.P.) independently extracted data from all full-text articles that met inclusion criteria and any differences were reconciled by re-review. If differences remained, a third reviewer (C.R.B.) was asked to perform data extraction as a tiebreaker. By convention, CRN was defined as adenoma, sessile serrated polyp, traditional serrated adenoma, or carcinoma. Advanced CRN (aCRN) was defined as any adenoma ≥1 cm, an adenoma with villous histology or high-grade dysplasia or carcinoma.19,20 Corresponding authors of included studies were contacted up to 3 times by e-mail to request data needed to complete sub-group analyses (eg, neoplasia rates by age group, sex, or location within colon).
Early-age onset is defined as occurring in adults younger than 50 years. The primary outcomes were prevalence of EAO-CRN, EAO-aCRN, and CRC. Secondary outcomes included the anatomic location within the colon (proximal or distal to splenic flexure).
We performed additional subgroup analyses to examine the stability of association and to identify sources of heterogeneity. Colonoscopy yield was examined according to the following subgroups: age groups (age younger than 40 years, 40–44 years, and 45–49 years), sex, geographic location where study was performed (North America, East Asia, Europe, Middle East), and study time period (pre-2005 and post-2005). The rationale for evaluating neoplasia according to time period is that studies from 1995–2004 predate any quality metrics for colonoscopy (ie, adenoma detection rate [ADR]) and availability of high-definition colonoscopy imaging. Any studies that took place during an overlapping time period were counted in the earlier time period.
Given recent interest by professional guideline groups to expand screening in individuals aged 45–49 years, we did further analysis of findings in this age group by sex and location to present expected results of screening this group. To compare yield of screening in those aged 45–49 years with those who currently qualify for screening, we extracted rates of CRN and aCRN for those aged 50–59 years in the studies included in our meta-analysis and performed a formal meta-analysis to report pooled rates of neoplasia in this older group among the same study populations.
The risk of bias was assessed independently by 2 reviewers (J.M.K., S.G.P.) and reconciled by a third reviewer (C.R.B.) using the Joanna Briggs Institute Critical Appraisal tool for prevalence studies as high (<50%), moderate (50–69%), or low (>70%), based on the percentage of items present.21,22
Data Synthesis and Analysis
Estimations of neoplasia outcomes were carried out with generalized linear mixed model using a random intercept logistic regression model.23 Pooled yield, along with their exact Clopper-Pearson 95% confidence intervals (CIs), were calculated for overall and subgroups of interest. The meta-analysis for the sex effect was conducted using Mantel-Haenszel method24,25 and Hartung-Knapp adjustment26 for the random study effects. The effect of sex in individual studies was estimated as relative risks in men and women. Subgroup analyses were conducted using the generalized linear mixed model with random study effects and fixed subgroup effects for age categories, geographic locations, and time period of the study start year. A formal global hypothesis test was conducted first to test whether there was any age group effect. If significant, post-hoc pair-wise comparison was conducted with Benjamini and Hochberg multiple comparison adjustment.27
Heterogeneity among studies was assessed with a χ2 test on Cochran’s Q statistic and quantified with the I2 statistic28 and the τ2 statistic, as described by DerSimonian and Laird.29 Heterogeneity was classified according to I2 as low (<30%), moderate (31%–60%), considerable 61%–75%), and substantial (>75%). All meta-analyses were adjusted by the random study effects so that the heterogeneity among the studies was taken into account in the pooled estimation of prevalence, 95% CIs, and hypothesis tests.
Sensitivity analyses were conducted for overall EAO-CRN and EAO-aCRN by removing the identified outliers with a 95% CI outside the 95% CI of the pooled effect. Publication bias was evaluated with a funnel plot, the asymmetry of which was assessed with Egger’s test.30 All computations were conducted with R, version 3.6.331 using “meta”32 and “dmetar”33 packages.
Results
Search Strategy and Study Characteristics
There were 10,123 unique articles identified from the 3 databases for title/abstract review and 332 full-length articles were assessed for eligibility. The most common reason for excluding full-text articles was that higher-than-average-risk individuals were included in the study, such as those with a family history of CRC or symptoms prompting the colonoscopy examination. There were 17 total articles included with 51,811 individuals younger than 50 years34–49 (Figure 1, Supplementary Table 2). Seven studies were from East Asia (4 from South Korea, 2 from Taiwan, and 1 from China), 5 from the United States, 3 from Europe (Romania, Germany, and Greece), and 2 from the Middle East (Israel and Iran). Of the 5 US studies, 239,47 included Black populations, in which American College of Gastroenterology guidelines50,51 support starting average-risk screening in black individuals at the age of 45 years. In the other US studies, colonoscopy was offered as part of an employee- or insurance-sponsored screening program.34,36 The majority of the articles (n = 13) took place during a contemporary study period (2005–2014), and 4 took place in an earlier period from 1995 to 2004. All 17 study authors were contacted for additional data; 8 authors responded and 6 provided data.
Neoplasia Prevalence on Colonoscopy
The pooled yield of EAO-CRN from 16 articles (41,111 individuals) was 13.7% (95% CI, 0.112%–0.166%) (Figure 2A). The Lieberman et al52 study was excluded because they reported advanced neoplasia only. Meta-analysis of 15 articles (51,324 individuals) demonstrated the rate of EAO-aCRN as 2.2% (95% CI, 0.016%–0.031%) (Figure 2B). Seventeen carcinomas were diagnosed among the 51,811 individuals included in the meta-analysis of single-episode screening colonoscopy, for a prevalence of 0.05%% (95% CI, 0.00029%–0.0008%).
Figure 2.
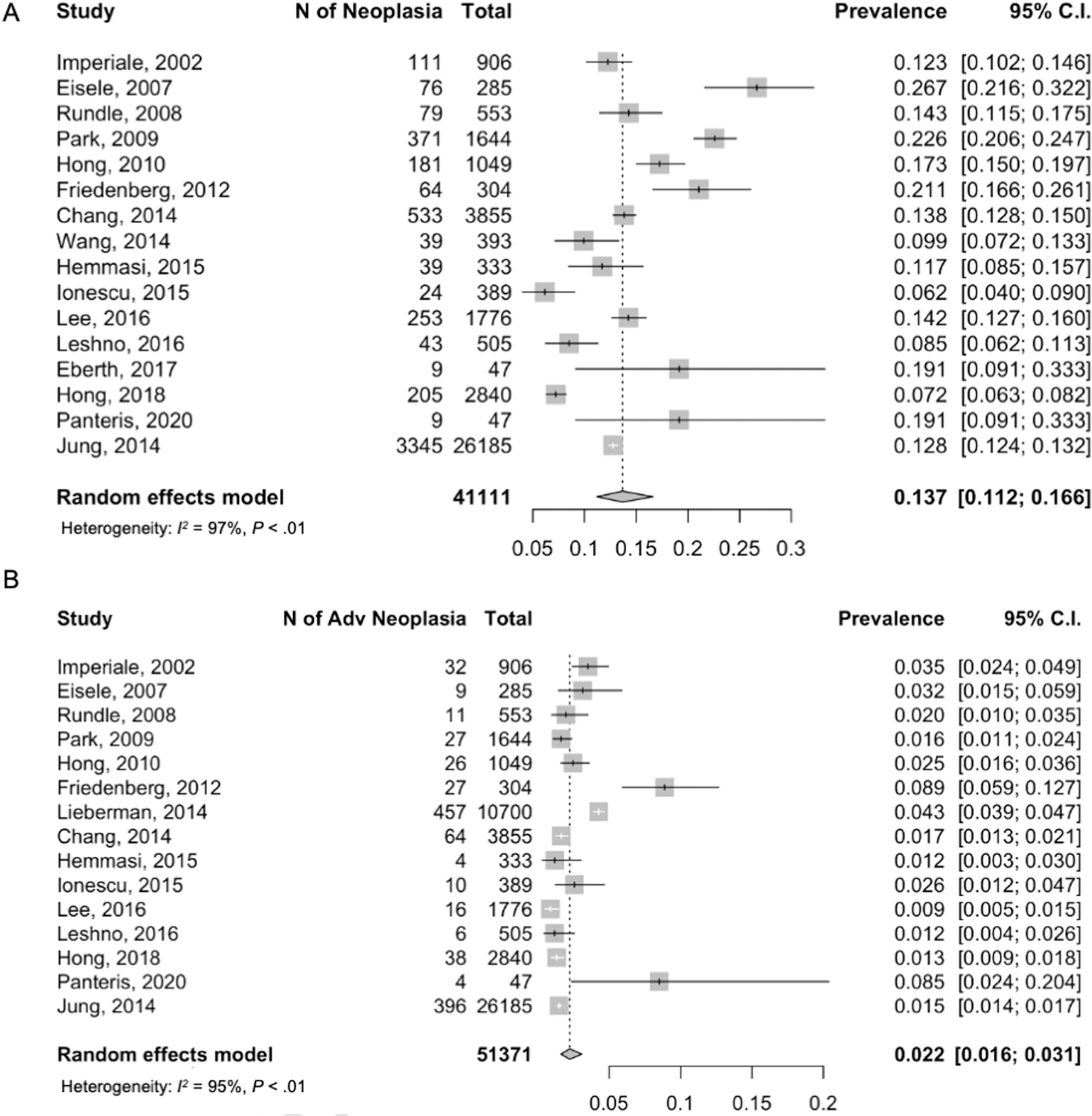
Colorectal neoplasia yield on screening colonoscopy for individuals younger than 50 years old. (A) Overall neoplasia (EAO-CRN). (B) Advanced neoplasia (EAO-aCRN).
Bias and Heterogeneity
Study quality assessments are presented in Supplementary Table 3. Because all of the studies were voluntary (not population-based observations), the sampling method was not a random probabilistic representation of the population younger than 50 years. Despite this, all studies had low risk of bias with acceptable design and reporting quality. Funnel plot analysis shows symmetry around the pooled outcome of EAO-CRN, indicating no significant publication bias (Egger’s test P = .65) (Supplementary Figure 1A). Funnel plot analyses of studies assessing findings of EAO-aCRN showed some asymmetry but no significant publication bias (Egger’s test P value .72) (Supplementary Figure 1B). The majority of included articles were large studies with low rates of aCRN, with the exceptions of Friedenberg et al39 and Panteris et al.49
Study heterogeneity was noted for EAO-CRN (I2 = 97%; τ2 = 0.19; χ214 = 321.92; P < .01) and EAO-aCRN (I2 = 95%; τ2 = 0.36; χ214 = 324.06; P < .01). Sensitivity analysis excluding studies reporting EAO-CRN and EAO-aCRN rates that were outside of the 95% CI of the pooled estimate did not change the results (Supplementary Figure 2A and B). To further explore sources of heterogeneity and stability of association across subgroups, we performed analyses according to subcategories of age, sex, geographic location, and study time period.
Yield of Colonoscopy by Age Groups
Meta-analysis was performed on additional age categories delineated as younger than 40 years,37,45,48 40–44 years,37,40,43,45,48 and 45–49 years.38–40,43,45,47–49 Neoplasia outcomes were significantly different by age groups (χ2 = 13.038; P = .001). Pooled rates of EAO-CRN increased from 8.1% (95% CI, 0.050%–0.127%) to 11.7% (95% CI, 0.092%–0.149%) to 17.8% (95% CI, 0.145%–0.216%) for ages younger than 40 years, 40–44 years, and 45–49 years, respectively (Figure 3A). Similarly, rates of EAO-aCRN varied by age group (χ2 = 18.267; P < .01) with rates of .07% (95% CI, 0.004%–0.011), 1.41% (95% CI, 0.011%–0.019%), and 3.6% (95% CI, 0.19%–0.067%) (Figure 3B).
Figure 3.
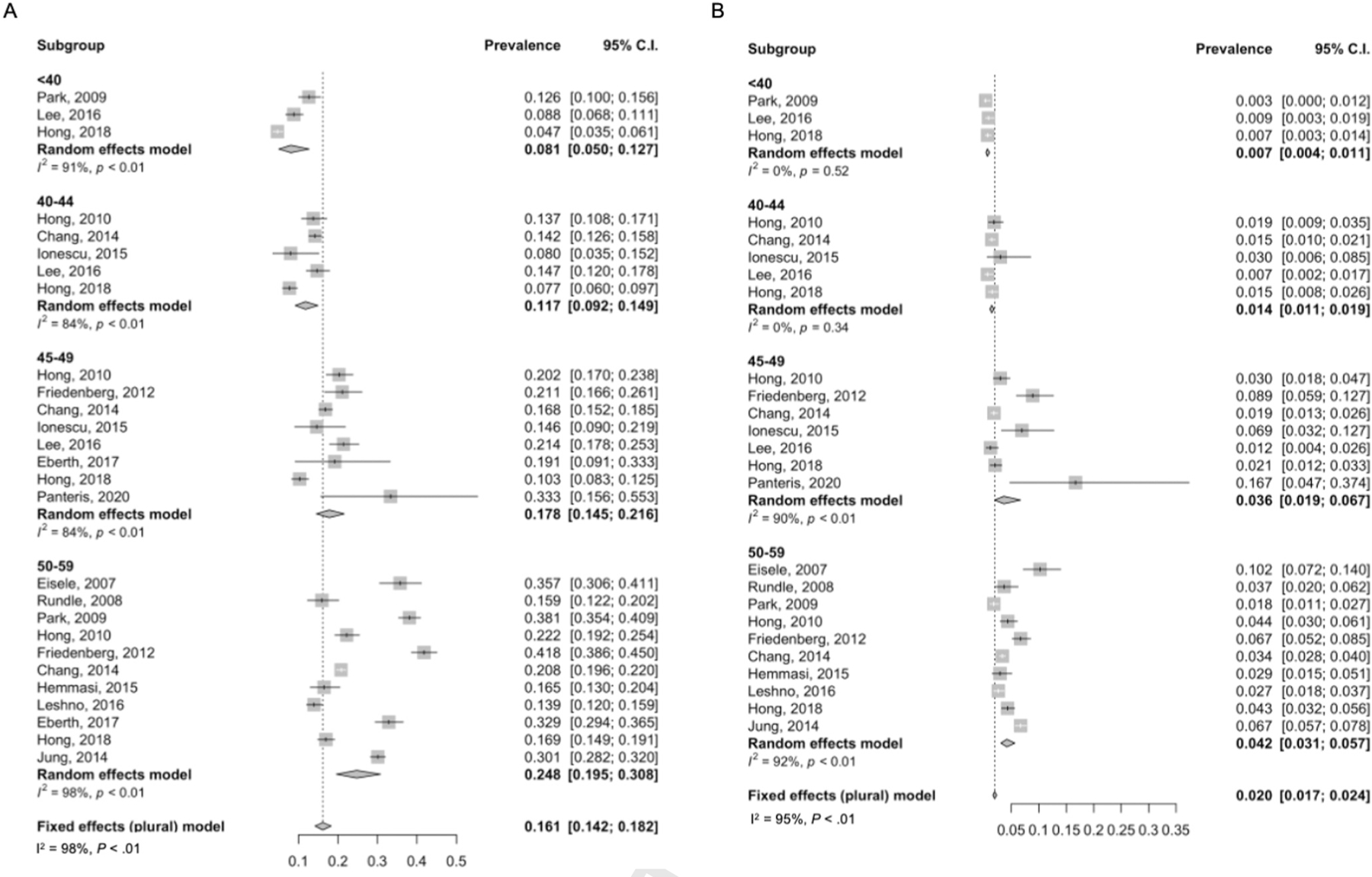
Colorectal neoplasia yield on screening colonoscopy by age groups (age younger than 40 years, 40–44 years, and 45–49 years). (A) Overall neoplasia (EAO-CRN). (B) Advanced neoplasia (EAO-aCRN).
Yield of Colonoscopy by Sex
The rate of EAO-CRN was 13.4% in men (95% CI, 0.107%–0.166%) and lower at 8.6% in women (95% CI, 0.070%–0.105%) (t = 8.95; P < .0001) (Figure 4A and B). The reported rate of EAO-CRN in men was higher compared with women in all 8 studies that provided data by sex (t = 8.95; P < .0001).34,36,38,40,42,43,46,48 Meta-analysis demonstrated a relative risk of men over women of 1.714 (95% CI, 1.486–1.976; I2 = 0.4%; τ2 = 0.0001; P = .43 (Figure 4C). The rate of EAO-aCRN was similar among men (1.9%; 95% CI, 0.010%–0.036%) and women (1.2%; 95% CI, 0.009%–0.016%) on meta-analysis of 7 studies (t = 1.87; P = .111) (Figure 4D–F).
Figure 4.
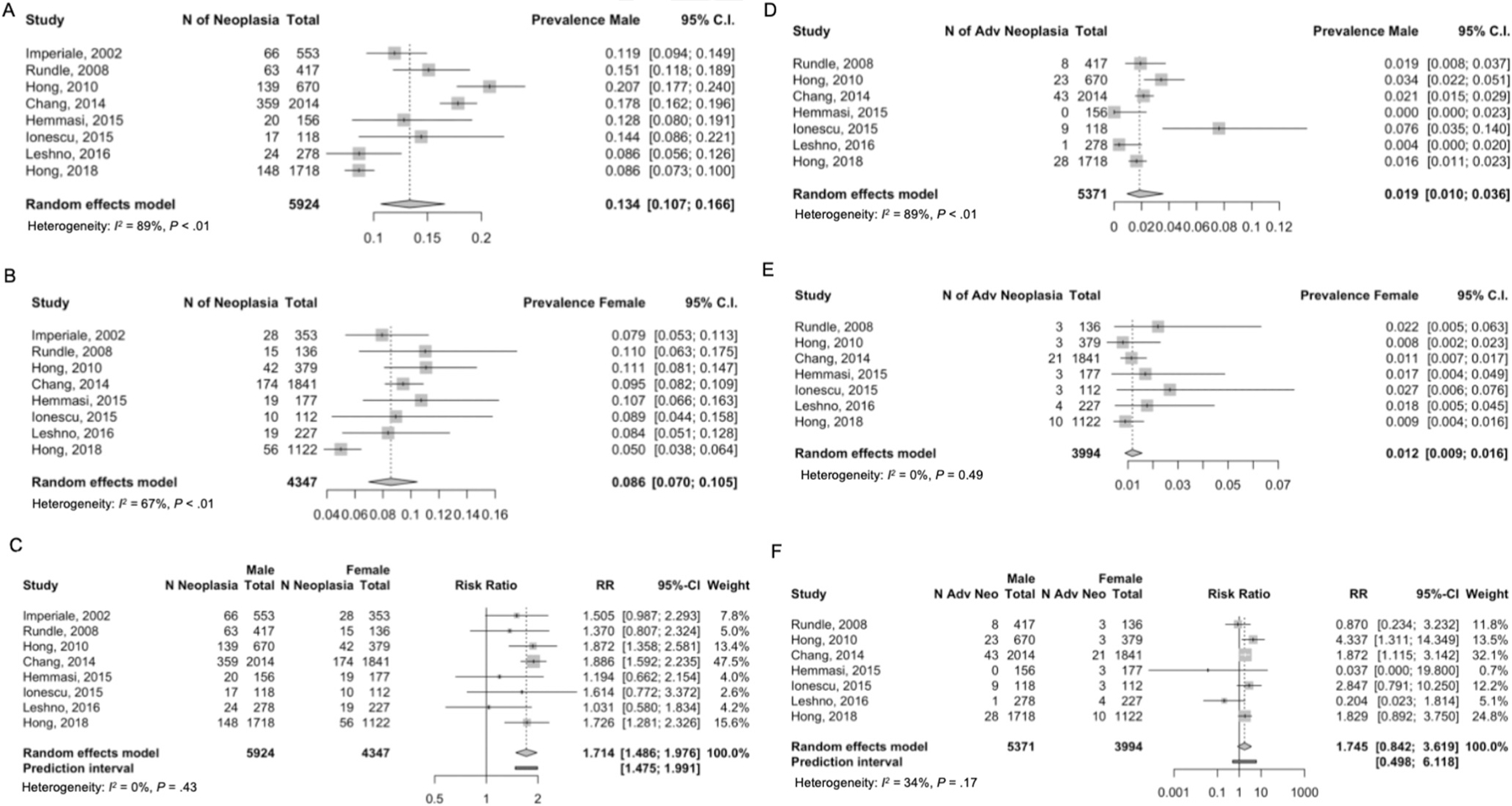
Colorectal neoplasia yield on screening colonoscopy by sex. (A) EAO-CRN, male. (B) EAO-CRN, female. (C) Relative risk EAO-CRN in male compared with female. (D) EAO-aCRN, male. (E) EAO-aCRN, female. (F) Relative risk of EAO-aCRN in male compared with female. Dotted red line represents the estimated risk ratio and the solid black line represent the risk ratio of 1. Each blue circle represents 1 of the 8 articles. And the size of the circle represents the sample size of the article (ie, larger circle indicated larger sample size of the individual study).
Yield of Colonoscopy in Proximal and Distal Colon
There were 7 articles included in the meta-analysis for neoplasia location within the colon.34,36,38,40,42,43,45 The rate of proximal EAO-CRN was 6.5% (95% CI, 0.055%–0.077%; I2 = 65%; τ2 = 0.03; P = .01 for study heterogeneity) and the rate in the distal colon was similar at 8.0% (95% CI, 0.071%–0.91%; I2 = 60%; τ2 = 0.019; P < .01 for study heterogeneity) (Supplementary Figure 3A and B). The rate of EAO-aCRN was 1.2% for proximal (95% CI, 0.010%–0.0016%; I2 = 0%, τ2 = 0; P = .147) and 1.6% for distal (95% CI, 0.006%–0.041%; I2 = 91%; τ2 = 0.61; P < .01) (Supplementary Figure 4A and B). The rates of CRN and aCRN were not different according to location within the colon.
Yield of Colonoscopy by Geographic Location
Neoplasia outcomes varied significantly across geographic region for 16 included studies for EAO-CRN (χ23 = 8.41; P = .04) and for EAO-aCRN (χ23 = 27.29; P < .01). The pooled rates of EAO-CRN were highest in the United States at 15.6% (95% CI, 0.122%–0.197%), then Europe at 14.9% (95% CI, 0.069%–0.293%), followed by 13.4% for East Asia (95% CI, 0.103%–0.172%), and 9.8% for the Middle East (95% CI, 0.078%–0.122%) (Supplementary Figure 5A). Rates of EAO-aCRN were highest in the United States (4%) followed by Europe (3.2%), East Asia (1.5%), and the Middle East (1.2%) (Supplementary Figure 5B).
Yield of Colonoscopy by Study Time Period
Yield of colonoscopy was analyzed according to study start time (before vs after 2005) and no significant difference was seen for EAO-CRN (16.2% vs 12.8%; P = .36) or EAO-aCRN (2.2% vs 2.3%; P = .89) (Supplementary Figure 6A and B).
Yield of Colonoscopy in Age Group 45–49 Years, by Sex
Meta-analysis by sex in age group 45–49 years40,43,48,49 showed higher prevalence of EAO-CRN in men (17.1%; 95% CI, 0.129%–0.224%) compared with women (10.8%; 95% CI, 0.074%–0.155%), for a relative risk of 1.531 (95% CI, 1.270–1.846) (Supplementary Figure 7). A similar pattern was seen for EAO-aCRN, with higher prevalence in men (4.4%; 95% CI, 0.019%–0.097%) compared with women (3.2%; 95% CI, 0.013%–0.077%), for a relative risk of 1.342 (95% CI, 1.039%–1.734%).
Yield of Colonoscopy in Age Group 45–49 Years, by Locations in the Colon
Among individuals aged 45–49 years, the rate of distal neoplasia was 11.5% (95% CI, 0.063%–0.201%) and proximal neoplasia was 9.4% (95% CI, 0.050%–0.171%).40,43,49 Higher rates of EAO-aCRN were seen in the distal colon (3.7%; 95% CI, 0.006%–0.201%) compared with the proximal colon (2.8%; 95% CI, 0.006%–0.117%) (Supplementary Figure 8).
Yield of Colonoscopy in Age Group 50–59 Years
There were 11 studies looking at neoplasia yield in individuals aged 50–59 years and the pooled rate of EAO-CRN was 24.8% (95% CI, 0.195%–0.308%) (Figure 3A). On pairwise comparison, the rate of EAO-CRN was higher in individuals aged 50–59 years vs age 45–49 years (P = .04). The rate of EAO-aCRN in individuals aged 50–59 years was 4.2% (95% CI, 0.031%–0.057%), which was similar to the rate in individuals aged 45–49 years (P = .7) (Figure 3B).
Discussion
In this systematic review and meta-analysis of average-risk individuals younger than 50 years undergoing screening colonoscopy, the rate of any colorectal neoplasia was 14% and advanced neoplasia was 2%. Higher rates were seen with increasing age, male sex, and in the distal colon. Based on our results, the number of individuals aged 45–49 years required to undergo colonoscopy to diagnose 1 individual with neoplasia (number needed to screen) is 6 for any CRN and 28 for aCRN, approaching the number of individuals needed to screen between ages 50 and 59 years to detect any CRN (n = 4) or aCRN (n = 24), respectively.
Our study provides a unique contribution to the existing literature by reporting the yield of screening colonoscopy in a strictly defined average-risk population, by reporting on the clinically significant outcome of advanced neoplasia and by providing yield stratified by age sub-groups. Although a recent meta-analysis examined the yield of colonoscopy in those younger than 50 years, it reported overall yield including those with symptoms,53 thus the reported rates of neoplasia may not be reflective of the asymptomatic, average-risk population. Furthermore, all results were pooled for any adenoma found in individuals aged 18–49 years, without yield separated by advanced findings or age sub-groups. Another recent meta-analysis of 70 studies described the global prevalence of colorectal adenomas, however, included studies in which up to 10% of the study populations had a family history of CRC.54 Our systematic review applied strict inclusion criteria to specifically address the yield of colorectal screening in asymptomatic, average-risk individuals, reported rates of aCRN (the intended target for screening), and presented results stratified by age to provide the expected yield if screening expanded to those aged 45–49 years.
Our results are consistent with a recent study by Butterly et al,17 which included 5656 patients younger than 50 years who had a colonoscopy for “low-risk symptoms,” such as abdominal pain and constipation. These symptoms had no association with advanced neoplasia (odds ratio, 1.00; 95% CI, 0.81–1.24), suggesting that patients with these symptoms likely represent an average-risk population. Like our results, they reported an overall EAO-CRN rate of 12.0% and EAO-aCRN rate of 2.4%. For patients in their cohort aged between 45 and 49 years, 17.5% (327 of 1869) had EAO-CRN and 3.3% (61 of 1869) had EAO-aCRN, which was comparable with our findings in which, for individuals aged 45–49 years, 17.8% had EAO-CRN and 3.6% had EAO-aCRN.
The rates of aCRN in ages 45–49 years approaches the rates in screen-eligible populations older than 50 years. In published screening cohorts, aCRN rates range from 3.6% to 6.8% in 50- to 54-year-olds.17,55 Strikingly, the rate of aCRN in individuals aged 45–49 years in our study (3.6%) was statistically similar to the rate observed in 50- to 59-year-olds (4.2%), suggesting that expanding screening to this population could yield a similar impact on CRC risk reduction. Although we cannot conclude from our results the proportion of aCRN lesions detected in individuals aged 45–49 years that would progress to cancer before age 50 years, earlier identification and surveillance of patients at increased risk of metachronous neoplasia can decrease lifetime cancer risk.56–59
The risk for EAO-CRN was significantly higher in men compared with women (relative risk, 1.714; 95% CI, 1.486–1.976), a similar pattern to sex differences in CRC among older adults.60–62 Although we did not find a statistically significant difference in aCRN between men and women, this may be due to the limited number of studies that provided aCRN rates by sex and resultant low event rates (28 of 1718 men vs 10 of 1122 women). The magnitude of difference between men and women needs to be assessed in large prospective screening cohorts, given the potential implications on age-specific ADR targets.
Up to 75% of EAO-CRC occurs in the left colon63 and our results showed a similar trend in EAO-CRN. This may be relevant when considering the most efficient and cost-effective screening strategy, given high sensitivity for left-sided neoplasia for fecal immunochemical testing and flexible sigmoidoscopy.64
We found significant differences in neoplasia prevalence based on geographic region of the world with highest rates in the United States, followed by Europe. EAO-CRC is a global phenomenon with increasing incidence in other countries, such as Australia, Canada, Germany, and the United Kingdom.65–67 Two of the US studies comprised entirely African American populations, which is known to be a high-risk group, thus there may be limited generalizability to the US population. Surprisingly, although there had been a steady rise in EAO-CRC incidence since the mid-1990s,10 we found no difference in prevalence by time periods, suggesting that improvements in colonoscopy quality and ADR over time did not impact rates of EAO-CRN.
This analysis has several strengths. We applied strict study selection criteria to capture only asymptomatic individuals without a family history of CRC to represent the average-risk individuals that professional societies have proposed screening beginning at age 45 years. Most other studies have looked at yield of screening in risk-enriched populations.53,54 Although there was a high level of heterogeneity in our pooled results for overall EAO-CRN and EAO-aCRN, our sub-group analyses explored the sources heterogeneity and, as expected, demonstrated significant differences by age sub-groups. This analysis provides stratified yield of screening by age groups younger than 50 years, which is particularly important in light of new recommendations to lower the screening age to 45 years, so understanding the yield of screening for individuals aged 45–49 years is most relevant. All 17 studies included in this meta-analysis had low risk of bias.
This study has several limitations. Similar to other studies that describe yield of colonoscopy screening as either part of clinical trials (in which participants self-select and provide informed consent to participate)68–70 or retrospective studies within opportunistic screening programs in which participants provide informed consent to undergo a colonoscopy,60–62 it is possible that our results are not generalizable to a truly average-risk, unselected population. It is possible that individuals with a family history of CRC were included if they were unaware of their family history or did not report it. However, this likely reflects clinical practice, given the large proportion of patients who do not know or report their family history.71,72 Our results are still generalizable to patients in clinical practice that are assumed to be average risk and consent to undergo colonoscopy. We were unable to account or adjust for colonoscopy quality, including equipment (high-definition imaging), bowel preparation quality, or endoscopist ADR. However, our time trend analyses suggest that newer equipment and awareness of quality benchmarks did not impact yield. We were unable to assess the outcome of serrated or advanced serrated neoplasia because only 2 of the 17 studies included in our analysis reported serrated neoplasia findings. Butterly et al17 found that 5.9% of individuals aged 45–49 years with low-risk indications for colonoscopy had a clinically significant serrated polyp. Our study included diverse international cohorts and identified differences in rates of neoplasia based on geographic location, thus generalizability to a specific population, such as average-risk individuals in the United States, may be limited. Although overall participant characteristics, such as race, ethnicity, smoking status, and obesity, were reported in the original studies, they were not stratified by age or neoplasia findings, thus we were unable to perform further sub-group analyses to assess these CRN risk factors. Although our results demonstrate high level of heterogeneity, it is unlikely a result of outliers because all of the articles were selected carefully, and the publication bias evaluation did not suggest significant selection bias. We used random study effects model to adjust for the heterogeneity among the studies. Sensitivity analysis for our primary end points obtained consistent results. Finally, due to the cross-sectional nature of the studies included, the rates of CRC were too low to perform sub-group analyses.
Our study provides robust pooled estimates of the yield of colonoscopy screening in average-risk individuals younger than 50 years, findings that can inform quality metrics for targeted ADRs in adults aged 45–49 years and serve as point estimates and ranges for simulation models. Large-scale prospective studies in well-defined average-risk young individuals need to be performed to further characterize sub-groups at higher risk for neoplasia who would benefit most from earlier screening. Additional work is needed to assess the impact on cost, risk, and colonoscopy access of entering young individuals with colorectal neoplasia into polyp surveillance programs.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
Given the rising incidence of colorectal cancer in young adults, multiple professional societies have recommended lowering the screening age from 50 years to 45 years in average-risk individuals.
NEW FINDINGS
The prevalence of overall and advanced colorectal neoplasia on screening colonoscopy for average-risk individuals younger than 50 years is 14% and 2%, respectively. In adults aged 45–49 years, 18% have any neoplasia and 4% have advanced neoplasia, similar to rates observed in adults aged 50–59 years.
LIMITATIONS
Included articles represent globally diverse, heterogenous populations and study designs.
IMPACT
Expanding colorectal screening to individuals aged 45–49 years could have a meaningful impact on colorectal cancer risk reduction.
Acknowledgments
This article is dedicated to our beloved mentor, colleague, and dear friend Dennis J. Ahnen, who was a champion for colorectal cancer prevention. His legacy lives on in the global research efforts he inspired in early-age onset colorectal cancer.
The authors thank Dr Wandong Hong (Wenzhou Medical University), Dr Mirela Ionescu (Elias Emergency University Hospital), and Dr Han-Mo Chiu (National Taiwan University), Dr Vasileios Panteris (Sismanogleio-Amalia Flemig General Hospital), Dr Jan M Eberth and Dr Franklin G. Berger (University of South Carolina), and Dr Frank Friedenberg (Temple University).
Funding
J. M. Kolb was supported in part by the National Institutes of Health Gastrointestinal Diseases Training Grant (T32-DK007038). J. Hu and Dexiang Gao were supported in part by the University of Colorado Cancer Center Support Grant (P30CA046934) and its population health shared resources. C. R. Boland funding was funded by R01 CA72851 (Familial and Early Onset Colorectal Cancer).
Abbreviations used in this paper:
- aCRN
advanced colorectal neoplasia
- ADR
adenoma detection rate
- CI
confidence interval
- CRC
colorectal cancer
- CRN
colorectal neoplasia
- EAO
early-age onset
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://doi.org/10.1053/j.gastro.2021.06.006.
Conflicts of interest
These authors disclose the following: C. R. Boland consults for Ambry Genetics. D. A. Lieberman is on the scientific advisory board of Cap-Check and Freenome. S. G. Patel is XXXX, Olympus America, Freenome, and ERBE USA. The remaining authors disclose no conflicts.
References
- 1.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020;70:145–164. [DOI] [PubMed] [Google Scholar]
- 2.Austin H, Henley SJ, King J, et al. Changes in colorectal cancer incidence rates in young and older adults in the United States: what does it tell us about screening. Cancer Causes Control 2014;25:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Preventative Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 2016;315:2564–2575. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Guidelines 2020 Colorectal Cancer Screening. Version 1 National Comprehensive Cancer Network, 2020. [Google Scholar]
- 6.Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;153:307–323. [DOI] [PubMed] [Google Scholar]
- 7.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev 2009; 18:1695–1698. [DOI] [PubMed] [Google Scholar]
- 8.Ladabaum U, Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology 2005;129:1151–1162. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Jemal A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970–2014. JAMA 2017;318:572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–281. [DOI] [PubMed] [Google Scholar]
- 12.US Preventative Services Task Force. Draft Recommendation Statement Colorectal Cancer: Screening US Preventative Services Task Force, 2020. [Google Scholar]
- 13.Ladabaum U, Mannalithara A, Meester RGS, et al. Cost-effectiveness and national effects of initiating colorectal cancer screening for average-risk persons at age 45 years instead of 50 years. Gastroenterology 2019; 157:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Lancet Gastroenterology and Hepatology. USPSTF recommends expansion of colorectal cancer screening. Lancet Gastroenterol Hepatol 2021;6(1):1. [DOI] [PubMed] [Google Scholar]
- 15.Lowery JT, Ahnen DJ, Schroy PC 3rd, et al. Understanding the contribution of family history to colorectal cancer risk and its clinical implications: a state-of-the-science review. Cancer 2016;122:2633–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ASGE Standards of Practice Committee, Pasha SF, Shergill A, et al. The role of endoscopy in the patient with lower GI bleeding. Gastrointest Endosc 2014;79:875–885. [DOI] [PubMed] [Google Scholar]
- 17.Butterly LF, Siegel RL, Fedewa S, et al. Colonoscopy outcomes in average-risk screening equivalent young adults: data from the New Hampshire Colonoscopy Registry. Am J Gastroenterol 2021;116:171–179. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolb JM, Molmenti CL, Patel SG, et al. Increased risk of colorectal cancer tied to advanced colorectal polyps: an untapped opportunity to screen first-degree relatives and decrease cancer burden. Am J Gastroenterol 2020; 115:9801–9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020;158:1131–1153.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. Int J Evid Based Healthc 2015;13:147–153. [DOI] [PubMed] [Google Scholar]
- 22.Mello FW, Miguel AFP, Dutra KL, et al. Prevalence of oral potentially malignant disorders: a systematic review and meta-analysis. J Oral Pathol Med 2018; 47:633–640. [DOI] [PubMed] [Google Scholar]
- 23.Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med 2010;29:3046–3067. [DOI] [PubMed] [Google Scholar]
- 24.Robins J, Breslow N, Greenland S. Estimators of the Mantel-Haenszel variance consistent in both sparse data and large-strata limiting models. Biometrics 1986; 42:311–323. [PubMed] [Google Scholar]
- 25.Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics 1985; 41:55–68. [PubMed] [Google Scholar]
- 26.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003; 22:2693–2710. [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995; 57:289–300. [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R: A language and environment for statistical computing R Foundation for Statistical Computing, 2020. [Google Scholar]
- 32.Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019;22:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrer M, Cuijpers P, Furukawa TD, et al. dmetar: companion R Package for the guide “Doing Meta-Analysis in R.” R package, version 0.0.9000, 2019.
- 34.Imperiale TF, Wagner DR, Lin CY, et al. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med 2002;346:1781–1785. [DOI] [PubMed] [Google Scholar]
- 35.Eisele R, Vogelsang E, Kraft K, et al. Screening for colorectal lesions with high-resolution video colonoscopes in a German male average-risk population at 40 to 59 years of age. Z Gastroenterol 2007;45:952–957. [DOI] [PubMed] [Google Scholar]
- 36.Rundle AG, Lebwohl B, Vogel R, et al. Colonoscopic screening in average-risk individuals ages 40 to 49 vs 50 to 59 years. Gastroenterology 2008;134:1311–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park HW, Byeon JS, Yang SK, et al. Colorectal neoplasm in asymptomatic average-risk Koreans: the KASID prospective multicenter colonoscopy survey. Gut Liver 2009;3:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong SN, Kim JH, Choe WH, et al. Prevalence and risk of colorectal neoplasms in asymptomatic, average-risk screenees 40 to 49 years of age. Gastrointest Endos 2010;72:480–489. [DOI] [PubMed] [Google Scholar]
- 39.Friedenberg FK, Singh M, George NS, et al. Prevalence and distribution of adenomas in black Americans undergoing colorectal cancer screening. Dig Dis Sci 2012; 57:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang LC, Wu MS, Tu CH, et al. Metabolic syndrome and smoking may justify earlier colorectal cancer screening in men. Gastrointest Endosc 2014;79:961–969. [DOI] [PubMed] [Google Scholar]
- 41.Wang FW, Hsu PI, Chuang HY, et al. Prevalence and risk factors of asymptomatic colorectal polyps in taiwan. Gastroenterol Res Pract 2014;2014:985205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemmasi G, Sohrabi M, Zamani F, et al. Prevalence of colorectal adenoma in an average-risk population aged 40–50 versus 50–60 years. Eur J Cancer Prev 2015;24:386–390. [DOI] [PubMed] [Google Scholar]
- 43.Ionescu EM, Nicolaie T, Gologan SI, et al. Opportunistic colorectal cancer screening using colonoscopy. Comparative results between two historical cohorts in Bucharest, Romania. J Gastrointest Liver Dis 2015;24:171–176. [DOI] [PubMed] [Google Scholar]
- 44.Jung YS, Ryu S, Chang Y, et al. Risk factors for colorectal neoplasia in persons aged 30 to 39 years and 40 to 49 years. Gastrointest Endosc 2015;81:637–645.e7. [DOI] [PubMed] [Google Scholar]
- 45.Lee SE, Jo HB, Kwack WG, et al. Characteristics of and risk factors for colorectal neoplasms in young adults in a screening population. World J Gastroenterol 2016;22:2981–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leshno A, Moshkowitz M, David M, et al. Prevalence of colorectal neoplasms in young, average risk individuals: a turning tide between East and West. World J Gastroenterol 2016;22:7365–7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eberth JM, Thibault A, Caldwell R, et al. A statewide program providing colorectal cancer screening to the uninsured of South Carolina. Cancer 2018;124:1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong W, Dong L, Stock S, et al. Prevalence and characteristics of colonic adenoma in mainland China. Cancer Manage Res 2018;10:2743–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panteris V, Vasilakis N, Demonakou M, et al. Alarming endoscopic data in young and older asymptomatic people: results of an open access, unlimited age colonoscopic screening for colorectal cancer. Mol Clin Oncol 2020;12:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol 2005;100:515–523; discussion 514. [DOI] [PubMed] [Google Scholar]
- 51.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009;104:739–750. [DOI] [PubMed] [Google Scholar]
- 52.Lieberman DA, Williams JL, Holub JL, et al. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc 2014;80:133–143. [DOI] [PubMed] [Google Scholar]
- 53.Enwerem N, Cho MY, Demb J, et al. Systematic review of prevalence, risk factors, and risk for metachronous advanced neoplasia in patients with young-onset colorectal adenoma. Clin Gastroenterol Hepatol 2021;19:680–689.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong MCS, Huang J, Huang JLW, et al. Global prevalence of colorectal neoplasia: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020;18:553–561.e10. [DOI] [PubMed] [Google Scholar]
- 55.Brenner H, Zwink N, Ludwig L, et al. Should screening colonoscopy be offered from age 50? Dtsch Arztebl Int 2017;114:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cross AJ, Robbins EC, Pack K, et al. Long-term colorectal cancer incidence after adenoma removal and the effects of surveillance on incidence: a multicentre, retrospective, cohort study. Gut 2020;69:1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol 2017; 18:823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song M, Emilsson L, Bozorg SR, et al. Risk of colorectal cancer incidence and mortality after polypectomy: a Swedish record-linkage study. Lancet Gastroenterol Hepatol 2020;5:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loberg M, Kalager M, Holme O, et al. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med 2014;371:799–807. [DOI] [PubMed] [Google Scholar]
- 60.Brenner H, Altenhofen L, Stock C, et al. Incidence of colorectal adenomas: birth cohort analysis among 4.3 million participants of screening colonoscopy. Cancer Epidemiol Biomarkers Prev 2014;23:1920–1927. [DOI] [PubMed] [Google Scholar]
- 61.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010;362:1795–1803. [DOI] [PubMed] [Google Scholar]
- 63.Patel SG, Boland CR. Colorectal cancer in persons under age 50: seeking causes and solutions. Gastrointest Endosc Clin N Am 2020;30:441–455. [DOI] [PubMed] [Google Scholar]
- 64.Patel SG, Ahnen DJ. Current screening and surveillance guidelines. In: Shaukat A, Allen JI, eds. Colorectal Cancer Screening: Quality and Benchmarks Springer, 2015:13–43. [Google Scholar]
- 65.Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019;68:1820–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019;68:2179–2185. [DOI] [PubMed] [Google Scholar]
- 67.Araghi M, Soerjomataram I, Bardot A, et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol 2019;4:511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med 1993; 328:901–906. [DOI] [PubMed] [Google Scholar]
- 69.Dominitz JA, Robertson DJ, Ahnen DJ, et al. Colonoscopy vs. Fecal Immunochemical Test in Reducing Mortality From Colorectal Cancer (CONFIRM): rationale for study design. Am J Gastroenterol 2017;112:1736–1746. [DOI] [PubMed] [Google Scholar]
- 70.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med 2000;343:162–168. [DOI] [PubMed] [Google Scholar]
- 71.Patel SG, Ahnen DJ, Gumidyala A, et al. Poor knowledge of personal and familial colorectal cancer risk and screening recommendations associated with advanced colorectal polyps. Dig Dis Sci 2020;65:2542–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akhtar S, Sinha S, McKenzie S, et al. Awareness of risk factors amongst first degree relative patients with colorectal cancer. Colorectal Dis 2008;10:887–890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


