Abstract
Objectives
To assess feasibility and effect of multimodal prehabilitation in patients with severe life-limiting intermittent claudication and complex infrainguinal disease.
Design
Case series of patients who underwent a 12-week prehabilitation program.
Setting
Outpatient clinic of a public tertiary hospital
Participants
Patients with a diagnosis of severe life-limiting intermittent claudication (Fontaine stage IIb and III) with complex infrainguinal disease or previous failed bypass attempts (N=5) who were referred to the prehabilitation clinic by a vascular surgeon.
Interventions
Patients underwent a baseline assessment that included quality of life questionnaires and functional capacity tests. After baseline assessment, they received a 12-week prehabilitation program that consisted of (1) a supervised exercise session 1 time per week; (2) home-based exercise prescription; (3) nutritional counseling; (4) smoking cessation; and (5) psychosocial intervention. Adherence to all components was recorded as well as the occurrence of any adverse event. After completion of the 12-week program, patients were reassessed.
Main Outcome Measure
Feasibility of prehabilitation measured by adherence to the different components of the program and occurrence of adverse events.
Results
All 5 patients completed the program. No serious adverse events occurred during the length of prehabiliation. Median adherence to each prehabilitation component was 91.7% (interquartile range [IQR], 33.5%) for supervised training, 91.7% (IQR, 40%) for home-based exercise, and 75% (IQR, 50%) for nutrition. Three of the 5 patients underwent psychosocial intervention and all who were active smokers enrolled in the smoking cessation program. Functional capacity measured with the 6-minute walk distance improved by 70 m (IQR, 99 m), and disease-specific quality of life measured with the Vascular Quality of Life Questionnaire improved by 25%.
Conclusion
Multimodal prehabilitation appears to be a feasible tool that could be used to increase functional capacity and quality of life for patients with complex infrainguinal disease and expected poor revascularization outcome or previous failed bypass attempts.
KEYWORDS: Intermittent claudication, Peripheral arterial disease, Preoperative exercise, Quality of life, Rehabilitation
Keywords: List of abbreviations: HADS, Hospital Anxiety and Depression Scale; IQR, interquartile range; PAD, peripheral arterial disease; VascuQol, Vascular Quality of Life Questionnaire
Peripheral arterial disease (PAD) affects up to 10% of the worldwide population and the prevalence increases to 20% among patients 70 years of age and older.1 Individuals with PAD are often sedentary and experience decreased functional capacity, severe disability, poor quality of life, and increased risk of premature mortality.2,3 Lifestyle modifications, especially smoking cessation, exercise, and nutrition are critical for patients with PAD and should be encouraged before contemplating surgery.4 Despite their importance in preventing disease progression, there is a lack of implementation of all these elements in the clinical setting.5,6
Multimodal prehabilitation, understood as a multidisciplinary optimization strategy that includes structured exercise, nutrition, smoking cessation, and psychological support, has been shown to improve surgical outcomes in different types of surgeries, and it is postulated as one of the most promising approaches to optimize a patient's functional capacity before and after surgery.7 There is currently no data on the effect of prehabilitation in PAD population.8 This multidisciplinary holistic approach could potentially improve patient-centered outcomes in patients with severe life-limiting intermittent claudication who have expected complex revascularization surgery.
Methods
Patient selection and diagnosis of PAD
This study was approved by the Hospital Research Ethics Board. Five consecutive patients with a diagnosis of severe life-limiting PAD and complex infrainguinal disease described as Trans-Atlantic Inter-Society Consensus II D lesions or previous failed bypass attempts9 were referred to the prehabilitation clinic by vascular surgeons at a tertiary hospital between the years 2018 and 2019. Patients agreed to participate in the program and signed the informed consent. Moderate to severe PAD diagnosis was made clinically by functional limitations in walking activity or pain at night, anatomically by imaging demonstrating arterial stenosis below the inguinal, and hemodynamically by an ankle brachial index ≤0.90. All patients were medically optimized and had stable PAD condition.
Assessments and intervention
Baseline assessment included sociodemographic and anthropometric data, disease-specific quality of life questionnaires (Vascular Quality of Life Questionnaire [VascuQol]-25 and Walking Impairment Questionnaire), presence of anxiety or depression (Hospital Anxiety and Depression Scale [HADS]10), and functional capacity (6-minute walk test,11 Gardner-Skinner's test12). Onset of claudication during the walk test was also recorded. After baseline assessment, patients started a 12-week multimodal prehabilitation program, summarized in table 1 and fully detailed in the supplemental appendix S1 (available online only at http://www.archives-pmr.org/). Patients were provided with a home-based exercise prescription notebook which they were expected to fill out every time the exercises were performed. Weekly compliance to home-based exercise as well as adherence to nutritional and psychosocial recommendations were recorded in a compliance sheet (see supplemental appendix S1, available online only at http://www.archives-pmr.org/) by the exercise specialist during the weekly supervised visit. During that visit, the exercise specialist also revised any limitations the patient might have had and progressed the exercises if necessary. Any exercise-related adverse events occurring during the supervised session were recorded. A reassessment was performed after completion of the 12-week prehabilitation program.
Table 1.
Description of the 12-week multimodal prehabilitation program for patients with PAD
Exercise
|
Supervised 1 time/wk |
|
|
Home-based 3 times/wk |
|
|
Nutrition
|
Nutritional education | |
|
|
|
| Nutritional intervention | ||
|
|
|
Psychosocial
|
|
|
| Smoking cessation |
|
|
Abbreviations: HADS-A, Hospital Anxiety and Depression Scale–Anxiety; HADS-D, Hospital Anxiety and Depression Scale–Depression.
Analysis
Statistical analyses were performed using SPSS Statistics.a Baseline and 12-week data were compared using the related-samples Wilcoxon signed rank test.
Results
A total of 5 patients with a median age of 76 years (range, 57-80y) underwent the 12-week prehabilitation program. Four patients had severe intermittent claudication (Fontaine stage IIb); 1 patient had rest pain (Fontaine III) and was using a wheelchair to move, because pain severely impaired his walking and he was not able to walk >50 m without needing to stop. All 5 patients had cardiovascular risk factors such as hypertension, dyslipidemia, and diabetes. There were 2 active smokers and 3 former smokers. Baseline functional capacity, disease-specific quality of life, and other parameters collected are shown in table 2.
Table 2.
Baseline and change in functional capacity, quality of life, and anxiety and depression after a 12-week multimodal prehabilitation program
| Measure | Baseline | 12-Week | Change | P Value |
|---|---|---|---|---|
| Functional capacity | ||||
| 6-MWT distance, m | 285 (69) | 362 (145) | +70 (99) | <.05 |
| Onset of pain, s | 98 (49) | 145 (161) | +31 (136) | <.05 |
| Gardner-Skinner, s | 97 (193) | 182 (363) | +90 (172) | <.05 |
| Quality of life | ||||
| VascuQol, total | 3.47 (1.93) | 4.84 (1.10) | +0.87 (1.24) | <.05 |
| Pain domain | 2.75 (2.13) | 4 (2.0) | +1.0 (1.5) | .104 |
| Social domain | 4.5 (2.25) | 5.5 (1.5) | +1.0 (1.75) | .257 |
| Activity domain | 2.75 (1.68) | 4.25 (1.65) | +1.45 (1.45) | <.05 |
| Symptoms domain | 3.5 (2.78) | 6 (1.38) | +1.03 (2.13) | .068 |
| Emotional domain | 3,29 (1.58) | 4.63 (1.59) | +0.86 (1.12) | <.05 |
| WIQ, total | 18.0 (10) | 45.2 (26.2) | +27.2 (24) | <.05 |
| WIQ distance | 5.9 (21.8) | 37.1 (36.7) | +18.6 (28.8) | <.05 |
| WIQ speed | 14.1 (10.7) | 39.1 (26.5) | +28.3 (18.4) | <.05 |
| WIQ stairs | 29.2 (20.8) | 54.2 (54.1) | +20.9 (35.4) | .104 |
| Anxiety and depression | ||||
| HADS-A | 9 (6.5) | 4 (4) | –3 (3.5) | <.05 |
| HADS-D | 7 (10.5) | 3 (4.5) | –4 (6) | .144 |
NOTE. Data are expressed as median (IQR). Change from baseline to 12 weeks is expressed as median of differences (IQR).
Abbreviations: 6-MWT, 6-minute walk test; HADS-A, Hospital Anxiety and Depression Scale–Anxiety; HADS-D, Hospital Anxiety and Depression Scale–Depression; WIQ, Walking Impairment Questionnaire.
Median adherence to each prehabilitation component was 91.7% (interquartile range [IQR], 33.5%) for supervised training, 91.7% (IQR, 40%) for home-based, and 75% (IQR, 50%) for nutrition. Three of the 5 patients underwent psychosocial intervention and attended the follow-up visits. All participants who were active smokers (n=2) achieved smoking cessation by the end of the program with the smoking cessation program. No cardiovascular or other exercise-related events were observed during supervised exercise training, although 1 patient presented with a hypertensive peak (blood pressure, 210/90 mmHg) with symptomatology consisting of headache and dizziness upon arrival to one exercise session. He was immediately referred to the emergency department and did not perform the exercise session on that day. During the program, a reduction in the insulin requirements was possible in 2 patients owing to improvement in glycemic control.
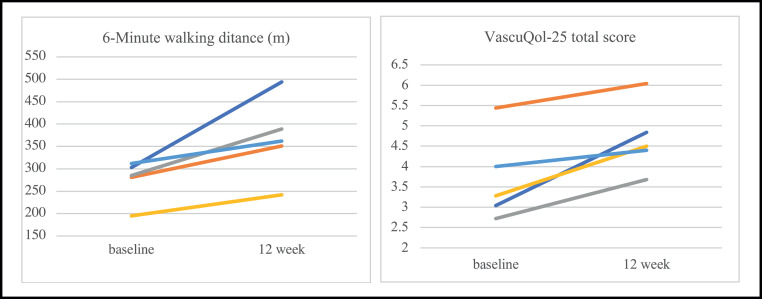
Baseline, 12-week, and changes in functional capacity, quality of life, and emotional status are summarized in table 1 and figure 1. The increase in functional capacity and onset of pain was clinically significant in all participants. Furthermore, the patient who used a wheelchair to ambulate owing to inability to walk for >50 m without needing to stop at baseline became fully functional and stopped using the wheelchair after completion of the 12-week program. Health-related quality of life and walking impairment measured by the VascuQol and Walking Impairment Questionnaire improved, with increases that almost doubled the baseline value in some cases.
Fig 1.
Individual changes for 6-minute walk test and VascuQol-25 at baseline and after 12 weeks of multimodal prehabilitation in 5 patients with PAD.
Discussion
This is the first study evaluating the effects of a multimodal prehabilitation intervention in patients experiencing intermittent claudication with complex infrainguinal disease. Our findings suggest that a 12-week program may improve functional capacity, quality of life, and anxiety. Despite that international vascular surgery societies have underlined the importance of exercise, nutrition, and smoking cessation for PAD prevention and control,9,13,14 there has been a lack of implementation of these interventions or they have been only partially implemented. Structured exercise for patients with PAD has been proven to be effective at increasing walking capacity and delaying onset of pain and has been shown to be as effective as medication and endovascular revascularization with respect to increasing pain-free walking distance.15 Nevertheless, very few studies have formally addressed quality of life in patients with PAD undergoing an exercise intervention and none have examined the effect of prehabilitation on quality of life or functional outcomes in PAD.8,16
The feasibility of multimodal prehabilitation has been demonstrated in these 5 cases of patients with severe life-limiting claudication and complex infrainguinal disease or with previous failed bypass attempts. The improvements in functional capacity, health-related quality of life, and emotional status shown in this case series are promising. This is especially relevant given the complexity of these patients, with higher rates of unsuccessful surgery and, in some cases, ending up with worse outcomes than before surgery. Therefore, nonsurgical interventions aimed at improving postoperative outcomes and delaying or avoiding the need for surgery in this population are crucial.
We believe that the reason for the good results attained rely on the synergy created when combining all the components (structured aerobic and resistance exercise, psychosocial support, nutritional counseling, smoking cessation). This holistic approach might have helped in breaking the enrooted poor health behavior and reluctance to change that is characteristic of this population. Furthermore, adherence to all components was higher compared with other trials with behavioral interventions in this population.17,18 As the literature shows, supervised exercise is better than unsupervised; however, in a clinical setting, asking a patient to come to a hospital facility 3 or 4 times per week might drop the adherence to the program owing to the economic and transportation burden for patients and caregivers. The mixed approach (supervised and home-based) of the prehabilitation program helped patients to remain adherent to the program by using the supervised sessions to track progress and make sure that patients felt accompanied during their exercise “journey,” whereas the home-based portion kept the program low-cost and made it more accessible for the patients. The high adherence to the program could also be attributed to the incorporation of the psychosocial support intervention and disease teaching, which are key components for patient empowerment and engagement.19
Study limitations
The main limitation of this case series is the small number of patients included, which does not allow us to reach strong and conclusive statements regarding the improvements in functional capacity and quality of life. Additionally, the fact that compliance of home-based exercise was logged subjectively by the patient and that there was no objective way of verifying that patients were truly doing the home-based portion of the program can be seen as a limitation.
Conclusions
Multimodal prehabilitation appears to be feasible in patients with PAD experiencing severe life-limiting intermittent claudication with complex infrainguinal disease or previous failed bypass attempts. Multimodal prehabilitation could be an excellent tool to increase their quality of life and functional capacity, although further studies are needed to prove the safety and effect of this multimodal approach on quality of life, functional capacity, and postoperative outcomes, as well as its role in delaying or sparing the need for surgery in some cases.
Supplier
-
a.
SPSS Statistics for MacOS, version 23.0; IBM Corp.
Footnotes
Supported by the Alfonso Martin Escudero Foundation, Spain. The foundation was not involved in the study design, collection, analysis, interpretation of data, manuscript writing or in the decision to submit the manuscript for publication.
Disclosures: none.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.arrct.2021.100139.
Appendix. Supplementary materials
References
- 1.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 2.Regensteiner JG, Hiatt WR, Coll JR. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med. 2008;13:15–24. doi: 10.1177/1358863X07084911. [DOI] [PubMed] [Google Scholar]
- 3.Nowygrod R, Egorova N, Greco G. Trends, complications, and mortality in peripheral vascular surgery. J Vasc Surg. 2006;43:205–216. doi: 10.1016/j.jvs.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Mannava K, Money SR. Current management of peripheral arterial occlusive disease: a review of pharmacologic agents and other interventions. Am J Cardiovasc Drugs. 2007;7:59–66. doi: 10.2165/00129784-200707010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee D, Lingam P, Chetcuti S. Missed opportunities to treat atherosclerosis in patients undergoing peripheral vascular interventions: insights from the University of Michigan Peripheral Vascular Disease Quality Improvement Initiative (PVD-QI2) Circulation. 2002;106:1909–1912. doi: 10.1161/01.cir.0000035649.39669.ce. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch AT, Haskal ZJ, Hertzer NR. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. Circulation. 2006;113:1474–1547. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 7.Minnella EM, Coca-Martinez M, Carli F. Prehabilitation: the anesthesiologist's role and what is the evidence? Curr Opin Anaesthesiol. 2020;33:411–416. doi: 10.1097/ACO.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 8.Palmer J, Pymer S, Smith GE. Presurgery exercise-based conditioning interventions (prehabilitation) in adults undergoing lower limb surgery for peripheral arterial disease. Cochrane Database Syst Rev. 2020;9 doi: 10.1002/14651858.CD013407.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norgren L, Hiatt WR, Dorm Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45:S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Snaith RP. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ATS Committee on Profieciency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 12.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23:402–408. [PubMed] [Google Scholar]
- 13.Perk J, De Backer G, Gohlke H. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): the Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur J Prev Cardiol. 2012;19:585–667. doi: 10.1177/2047487312450228. [DOI] [PubMed] [Google Scholar]
- 14.O'Neill BJ, Rana SN, Bowman V. An integrated approach for vascular health: a call to action. Can J Cardiol. 2015;31:99–102. doi: 10.1016/j.cjca.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Murphy TP, Cutlip DE, Regensteiner JG. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: the CLEVER study. J Am Coll Cardiol. 2015;65:999–1009. doi: 10.1016/j.jacc.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coca-Martinez M, Kinio A, Hales L, Carli F, Gill HL. Combined exercise and nutrition optimization for peripheral arterial disease: a systematic review. Ann Vasc Surg. 2021;71:496–506. doi: 10.1016/j.avsg.2020.09.048. [DOI] [PubMed] [Google Scholar]
- 17.Harwood AE, Smith GE, Cayton T, Broadbent E, Chetter IC. A systematic review of the uptake and adherence rates to supervised exercise programs in patients with intermittent claudication. Ann Vasc Surg. 2016;34:280–289. doi: 10.1016/j.avsg.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Lin E, Nguyen CH, Thomas SG. Completion and adherence rates to exercise interventions in intermittent claudication: traditional exercise versus alternative exercise - a systematic review. Eur J Prev Cardiol. 2019;26:1625–1633. doi: 10.1177/2047487319846997. [DOI] [PubMed] [Google Scholar]
- 19.Abaraogu UO, Ezenwankwo EF, Dall PM, Seenan CA. Living a burdensome and demanding life: a qualitative systematic review of the patients experiences of peripheral arterial disease. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.