Abstract
Throughout their life cycle, parasitic organisms experience a variety of environmental conditions. To ensure persistence and transmission, some protozoan parasites are capable of adjusting their replication or converting to distinct life cycle stages. Trypanosoma cruzi is a 'generalisť parasite that is competent to infect various insect (triatomine) vectors and mammalian hosts. Within the mammalian host, T. cruzi replicates intracellularly as amastigotes and can persist for the lifetime of the host. The persistence of the parasites in tissues can lead to the development of Chagas disease. Recent work has identified growth plasticity and metabolic flexibility as aspects of amastigote biology that are important determinants of persistence in varied growth conditions and under drug pressure. A better understanding of the link between amastigote and host/tissue metabolism will aid in the development of new drugs or therapies that can limit disease pathology.
Keywords: metabolic flexibility, nutrient acquisition, T. cruzi, drug susceptibility, host-parasite
Introduction
Parasitic protozoans experience diversity in their environmental niches both within and between hosts. The variety of these environments includes variations in temperature, pH, oxygen saturation, nutrient availability, and immune activity. This heterogeneity can profoundly impact survival, metabolism [1], and the propensity to convert to distinct developmental stages to maximize transmission potential [2].
The kinetoplastid protozoan parasite Trypanosoma cruzi alternates between triatomine and mammalian hosts. T. cruzi can colonize a variety of mammalian species, earning its reputation as a 'generalist.' In humans, an infection can result in Chagas disease, which most commonly manifests as cardiomyopathy. In the mammalian host, T. cruzi exists in two forms; the extracellular trypomastigote is motile and invades nucleated host cells followed by an escape from a transient parasitophorous vacuole and conversion to the intracellular amastigote form (Figure 1A). Amastigotes replicate by binary fission and can do so in most tissues [3]. Tissue damage and clinical disease are primarily dependent on the presence of amastigotes [4], and therefore therapies aim to eliminate these parasites. Following several rounds of replication, amastigotes convert to trypomastigotes and lyse the host cell to continue the cycle of infection. Parasitemia and tissue burden are high in the acute phase of the disease, but most immunocompetent individuals progress to a chronic stage characterized by low parasite levels without parasite eradication.
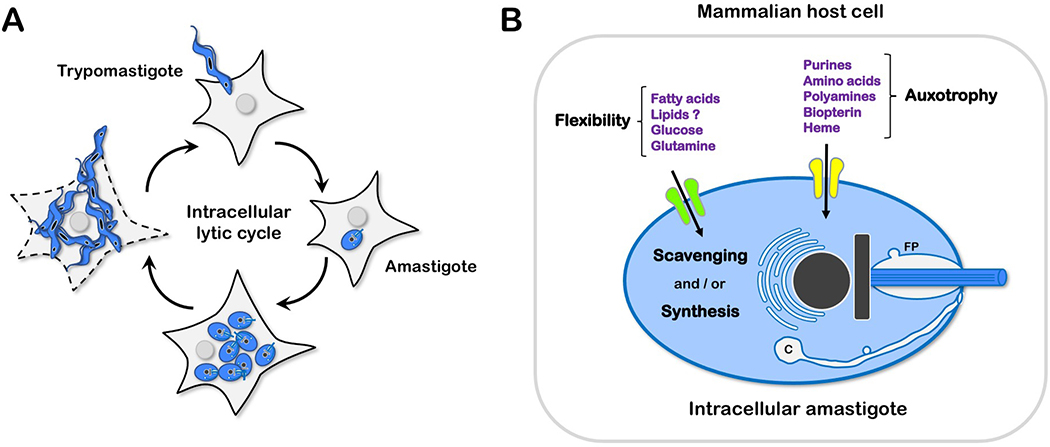
Figure 1.
(A) Trypanosoma cruzi infection cycle in mammalian host cells. Motile T. cruzi trypomastigotes actively invade nucleated mammalian cells to establish intracellular infection. Host cell entry triggers a developmental program [15], resulting in the formation of intracellular amastigotes that coincides with the localization of the parasite in the host cell cytoplasm. The timing of this process varies with parasite strain and host cell type. Replication competent amastigotes are typically formed by 16–20 hours post-infection (hpi) and begin to proliferate at ~24 hpi [27]. After several rounds of replication and cell division, intracellular amastigotes cease division and differentiate to trypomastigotes that eventually egress from the host cell where they can invade other cells. (B) Interaction of intracellular T. cruzi amastigotes with host nutrient sources. Generalized model depicting the transport of nutrients for which amastigotes have strict dependence on the host cell (auxotrophy) and those that can be acquired and synthesized by the parasite (flexibility). Transporters are drawn on the plasma membrane for simplicity but can be distributed within the flagellar pocket (FP) and cytostome/cytopharynx (C), and other endocytic organelles.
The mechanisms behind this remarkable persistence in diverse host environments, both between and within organisms, are still being explored, and their implications on drug efficacy are pertinent.
Metabolic flexibility and auxotrophy.
Parasites, by definition, are reliant on a host organism for growth and survival. The clearest examples of this dependence are instances where the parasite cannot synthesize a particular compound necessary for growth and instead have an absolute reliance on scavenging (i.e., auxotrophies). T. cruzi is auxotrophic for aromatic (phenylalanine, tryptophan, and tyrosine) and branched-chain acids (valine, leucine, and isoleucine) in addition to arginine, lysine, and histidine [5]. Similarly, T. cruzi is unable to synthesize heme [6], vitamins [7] and purines [8], making them reliant on salvage/transport pathways (Figure 1B). The free-living insect stage (epimastigote) has been commonly used to characterize these transport pathways, while less is known about the mechanisms of nutrient acquisition by the intracellular amastigote [5].
Conditions under which T. cruzi amastigotes retain the ability to synthesize and scavenge specific metabolites are less clear (Figure 1B). The evolutionary conservation of these anabolic pathways in the parasite may allow for metabolic flexibility that results in the ability of parasites to persist in a variety of environments. In this scenario, variability in amount, localization, or composition of scavenged metabolites is potentially balanced by de novo synthesis to maintain parasite viability, similar to other intracellular parasites [9–11]. Thus, the relative importance of specific host metabolic processes in T. cruzi amastigote replication is likely influenced by local environmental factors. These may include inherent metabolic differences in tissues colonized by the parasite [12] and arise with host immune responses that can control but not eliminate T. cruzi infection from the host.
Below, instances, where metabolites are synthesized and scavenged by T. cruzi are highlighted. We discuss the resulting implications for metabolic flexibility with a focus on the intracellular amastigote and its host.
Fatty Acids
Trypanosomes are capable of both scavenging fatty acids (FA) and de novo synthesis through a modular elongase (ELO) pathway to satisfy bulk fatty acid requirements, including glycosylphosphatidylinositol production [11,13] in addition to maintaining components of a conventional type II FA synthase that is localized to the mitochondrion [14]. In T. cruzi, conversion to the intracellular, replicative amastigote is accompanied by a global transcriptomic shift in the parasite that includes upregulation of the ELO pathway transcripts [15]. Yet, the reduction of host FA metabolism or triacylglycerol (TAG) synthesis slows intracellular amastigote replication, suggesting that parasite de novo FA synthesis cannot wholly compensate for changes to the extracellular lipid environment [16]. These data indicate that the FA requirements of intracellular T. cruzi amastigotes are fulfilled through a combination of scavenging and synthesis, similar to that described for other protozoan parasites. Maintaining the ability for both synthesis and scavenging may allow for a degree of flexibility in changing host environmental conditions.
Sterols
Unlike Plasmodium and Toxoplasma, which are sterol auxotrophs [17,18], T. cruzi has maintained a pathway for de novo sterol synthesis in addition to scavenging cholesterol [19]. The products of these sterol synthesis pathways are ergostane-type sterols, distinct from host-derived cholesterol. Disruption of the first committed step of endogenous sterol synthesis (i.e., squalene synthase) leads to profound growth and morphological effects in both T. cruzi and T. brucei [20,21], suggesting the essentiality of these endogenous sterol products. Nevertheless, inhibition of sterol synthesis at points within the de novo synthesis pathway is tolerated by Leishmania major, a related kinetoplastid parasite, but is accompanied by a reduction in tolerance to various stressors [22,23]. T. cruzi enzymes within the de novo sterol synthesis pathway have been explored as drug targets (i.e., TcCYP51) due to their assumed essentiality [24]. However, the concept that endogenously derived sterols are important for parasite resilience raises the possibility that their essentiality is at least partially dependent on external factors or internal metabolic states [22].
Oxidative metabolism
T. cruzi amastigote replication occurs after escape from a parasitophorous vacuole, and consequently, during replication, amastigotes have direct access to a variety of potential nutrient sources. It has generally been assumed that in the host cytosol, amino acids and FAs provide the primary sources for energy production [25]. However, amastigotes are competent to utilize exogenous glucose, in addition to glutamine to support respiration [15,26]. Additionally, the maintenance of spare respiratory capacity by amastigotes predicts the ability of the parasite to flexibly meet changing energetic demands [15] like their host cells of residence. Overall, the ability of intracellular T. cruzi amastigotes to utilize multiple fuel sources likely re-enforces their ability to persist in a variety of dynamic environments. Inhibition of amastigote mitochondrial respiration slows but does not prohibit replication [27], even though the capacity for β-oxidation, as inferred from enoyl-CoA hydratase knockout parasites, is important for amastigote replication [28]. These studies demonstrate that amastigote metabolic flexibility includes the ability to rely on non-respiratory carbon sources.
Identifying the relative importance of scavenged metabolites to intracellular T. cruzi amastigote metabolism is particularly challenging because the host cell remains metabolically active as parasites divide. As such, the host cell can readily metabolize metabolic tracers before reaching amastigotes, complicating the interpretation of metabolic data, particularly for the analysis of metabolites that are rapidly turned over. While the ultimate goal is to understand amastigote metabolism inside host cells, several approaches directly study amastigotes. Axenically derived amastigotes can be generated from trypomastigotes but have distinct growth, morphological and biochemical characteristics that differentiate them from intracellular amastigotes [29–31]. Alternatively, amastigotes directly isolated from infected cultures are metabolically active but have limited replicative capacity. The development of protocols to isolate and profile the metabolites directly from intracellular amastigotes should aim to minimize host contamination while being rapid to perform to effectively quench parasite metabolism to limit isolation-related metabolic shifts [32]. Direct measurement of metabolites from isolated amastigotes will be essential to validate the metabolic networks that are predicted from genomic and transcriptional data [15,33].
Sensing and responding to external cues.
Metabolites
Parasites can sense, either directly or indirectly, and adapt to changes in their environment to ensure persistence or growth within a host while optimizing the potential for transmission to a new host. Failure to regulate nutrient uptake in nutrient-rich environments [34] or adjust proliferation rates in nutrient limiting conditions [35] can result in parasite elimination. T. cruzi amastigotes are able to dynamically regulate their cell cycle by extending the G1 phase in response to shifting metabolic environments [27]. T. cruzi can also regulate the import of molecules such as heme that are necessary for replication but can be toxic when in excess [6]. While the sensing mechanisms of these processes are unknown, other parasites are capable of monitoring their intracellular metabolic states [35] and use external sensors (e.g., flagella) to probe their immediate environment [36,37]. Intriguingly, in addition to the predicted presence of intracellular sensors such as AMP-activated protein kinases [38], the short flagellum of intracellular T. cruzi amastigotes establishes close contact with host mitochondria and may provide a mechanism by which the parasite can gauge the immediate intracellular environment of the host cell [39].
Immune Responses
Infection with T. cruzi is followed by a brief incubation period and subsequent progression to the acute phase of the disease characterized by microscopically detectable parasitemia and nonspecific symptoms. Most infected individuals survive the acute phase and progress to a chronic stage where parasitemia is intermittently detectable by PCR. The majority of our understanding regarding the dynamics and localization of amastigotes in the chronic phase comes from mouse models of infection. In a murine host, T. cruzi can persist at low levels [40] in the gut, accompanied by scarce focal growth in other tissues [3,41]. During chronic infection, amastigotes remain replicative but have dramatically shifted cell cycle [42]. The specific determinants behind this process have not been characterized, but the induction of self-limiting replication to ensure protection from the immune response is not without precedence. For instance, Leishmania parasites induce a "stringent response" in tissue lesions, limiting parasite growth presumably to limit macrophage activation [43,44]. In addition, Leishmania parasites in chronic lesions do not natively utilize all nutrient sources available. Instead, they are primed to catabolize nutrients that have limited ROS production and therefore allow for persistence in vivo [45], highlighting the link between metabolic state and immune evasion. While little is known about switches in T. cruzi amastigote metabolism during chronic infection, localized alterations to the host metabolome and microbiome have potential implications for pathogenesis [12].
Implications of metabolic heterogeneity and flexibility for drug development.
Benznidazole remains the standard for the treatment of T. cruzi infection. Treatment during the acute phase appears more efficacious than in the chronic phase, where baseline cardiac function and age may influence clinical benefit [46]. However, following treatment, a proportion of patients continue to harbor parasites, suggesting that parasites can withstand treatment or that benznidazole does not reach a proportion of tissue-resident parasites [47,48]. In response to non-lethal amounts of benznidazole in vitro, the amastigote cell cycle shifts towards a higher proportion of G1 parasites in a dose-dependent manner. This response is consistent with a DNA damage-based mechanism [49] of action followed by re-entry into the cell cycle at a rate inversely proportional to drug concentration [27]. By a similar mechanism, amastigotes tolerate inhibition of cytochrome b through a dramatically slowed cell cycle and have a rapid resumption of growth following the release of inhibition [27]. Combined, these observations suggest that the inherent ability of intracellular T. cruzi amastigotes to sense and respond through cell cycle plasticity should be taken into consideration when evaluating the effects of drug treatment.
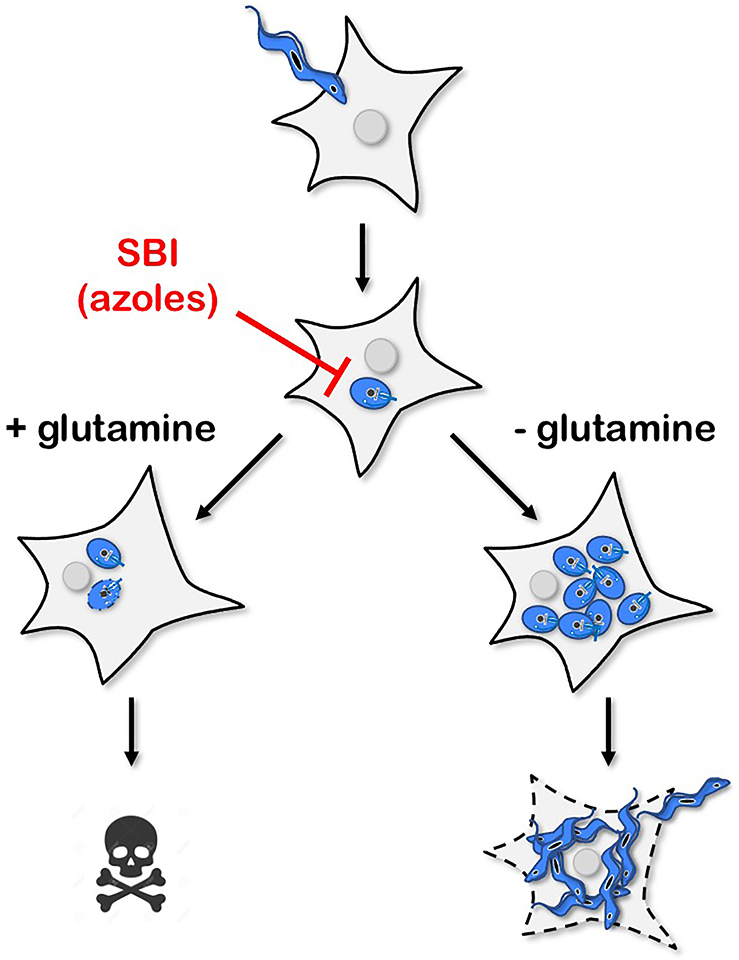
Drug screening traditionally employs nutritionally rich grow media and evaluates compound efficacies based on a failure of pathogen growth. While the metabolic state and growth rate of microorganisms is challenging to uncouple, in bacteria, the metabolic state has been shown to impact antibiotic efficacy [50]. Similarly, clinically relevant mutations in E. coli central metabolic genes, as opposed to mutations in the targeted pathway, can alter basal respiration and modulate drug activity [51]. The diversity of in vivo environmental niches coupled with metabolic and growth flexibility of parasitic organisms can complicate the utility of pharmacotherapies. For instance, Inhibitors of endogenous ergostane-type sterol biosynthesis (i.e., azoles) showed promising preclinical activity, but in contrast to benznidazole, a rapid rebound of parasitemia followed the cessation of therapy [47]. In vitro, the metabolic environment, particularly the amount of available glutamine (Figure 2), determines azole efficacy against T. cruzi amastigotes [52]. Interestingly, in the in vivo mouse model of chronic infection and under azole treatment, amastigotes persist in the large intestine, a site of reduced glutamine availability [53]. These data suggest that amastigote metabolic flexibility and the diversity of host environments can potentially result in infections that are recalcitrant to specific treatments due to variations in metabolic state.
Figure 2. Schematic illustrating the impact of environmental heterogeneity on the susceptibility of intracellular T. cruzi amastigotes to azole inhibitors of parasite sterol biosynthesis.
In cell culture models, a single change in the composition of the medium (the presence or absence of supplemental glutamine) alters the outcome of T. cruzi infection following exposure to lethal concentrations of azoles [52]. In the presence of supplemental glutamine, amastigotes succumb to azoles, whereas glutamine restriction is associated with parasite protection at the population level.
Conclusions:
Trypanosoma cruzi is a generalist parasite both in the host range and within-host distribution. While such flexibility is likely accomplished through the ability of these parasites to sense and respond to environmental perturbations via direct or indirect mechanisms, little is known about this capability. One crucial consequence of adaptability is that anti-parasitic drugs that induce metabolic changes in T. cruzi amastigotes can be contextually efficacious due to the parasite's capacity to exist in various metabolic states with the potential to confound new compound screening efforts. Future research will focus on understanding how amastigote metabolism allows for growth in diverse environments and unravel the processes by which amastigotes sense and respond to their environment. Understanding the link between amastigote metabolism and the resident host cell/tissue will aid the evaluation of candidate pharmacotherapies with the ultimate goal of disrupting processes integral to parasite persistence independent of their environment.
Highlights.
T. cruzi amastigotes can dynamically respond to changes in their environment
Growth and metabolic flexibility allow for T. cruzi growth in diverse environments
Mechanisms of T. cruzi growth plasticity have implications for drug development
Acknowledgments
Funding
This work was supported by the National Institutes of Health NIH-R21AI146815 and the American Heart Association AHA-19POST34380209.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Trindade S, Rijo-Ferreira F, Carvalho T, Pinto-Neves D, Guegan F, Aresta-Branco F, Bento F, Young SA, Pinto A, Van Den Abbeele J, et al. : Trypanosoma brucei Parasites Occupy and Functionally Adapt to the Adipose Tissue in Mice. Cell Host Microbe 2016, 19:837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Obaldia N, Meibalan E, Sa JM, Ma S, Clark MA, Mejia P, Moraes Barros RR, Otero W, Ferreira MU, Mitchell JR, et al. : Bone Marrow Is a Major Parasite Reservoir in Plasmodium vivax Infection. mBio 2018, 9:e00625–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lewis MD, Fortes Francisco A, Taylor MC, Burreil-Saward H, McLatchie AP, Miles MA, Kelly JM: Bioluminescence imaging of chronic Trypanosoma cruzi infections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cell Microbiol 2014, 16:1285–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tarleton RL, Zhang L, Downs MO: "Autoimmune rejection" of neonatal heart transplants in experimental Chagas disease is a parasite-specific response to infected host tissue. Proc Natl AcadSci U S A 1997, 94:3932–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marchese L, Nascimento J de F, Damasceno FS, Bringaud F, Michels PAM, Silber AM: The Uptake and Metabolism of Amino Acids, and Their Unique Role in the Biology of Pathogenic Trypanosomatids. Pathogens 2018, 7:E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Pagura L, Trevere E, Merli ML, Cricco JA: A new model for Trypanosoma cruzi heme homeostasis depends on modulation of TcHTE protein expression. J Biol Chem 2020, 295:13202–13212. * In this study, heme transport in T. cruzi is shown to be a dynamic process that allows for parasite replication under a variety of extracellular heme concentrations while avoiding toxicity from free heme.
- [7].Gazanion E, Vergnes B: Protozoan Parasite Auxotrophies and Metabolic Dependencies. Exp Suppl 2018, 109:351–375. [DOI] [PubMed] [Google Scholar]
- [8].Gutteridge WE, Gaborak M: A re-examination of purine and pyrimidine synthesis in the three main forms of Trypanosoma cruzi. Int J Biochem 1979, 10:415–422. [DOI] [PubMed] [Google Scholar]
- [9]. Krishnan A, Kloehn J, Lunghi M, Chiappino-Pepe A, Waldman BS, Nicolas D, Varesio E, Hehl A, Lourido S, Hatzimanikatis V, et al. : Functional and Computational Genomics Reveal Unprecedented Flexibility in Stage-Specific Toxoplasma Metabolism. Cell Host Microbe 2020, 27:290–306.e11. * In this work, computational and experimental methods are combined to identify important facets of T. gondii metabolic flexibility.
- [10].Liang X, Cui J, Yang X, Xia N, Li Y, Zhao J, Gupta N, Shen B: Acquisition of exogenous fatty acids renders apicoplast-based biosynthesis dispensable in tachyzoites of Toxoplasma. J Biol Chem 2020, 295:7743–7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Paul KS, Jiang D, Morita YS, Englund PT: Fatty acid synthesis in African trypanosomes: a solution to the myristate mystery. Trends Parasitol 2001, 17:381–387. [DOI] [PubMed] [Google Scholar]
- [12]. Hossain E, Khanam S, Dean DA, Wu C, Lostracco-Johnson S, Thomas D, Kane SS, Parab AR, Flores K, Katemauswa M, et al. : Mapping of host-parasite-microbiome interactions reveals metabolic determinants of tropism and tolerance in Chagas disease. Sci Adv 2020, 6:eaaz2015. ** In this study, small molecule focused LC/MS-MS and spatial metabolite mapping were exploited to identify metabolic disturbances in the gastrointestinal microenvironment in experimental T. cruzi infection with implications for alleviating damage and disease.
- [13].Lee SH, Stephens JL, Paul KS, Englund PT: Fatty acid synthesis by elongases in trypanosomes. Cell 2006, 126:691–699. [DOI] [PubMed] [Google Scholar]
- [14].Stephens JL, Lee SH, Paul KS, Englund PT: Mitochondrial fatty acid synthesis in Trypanosoma brucei. J Biol Chem 2007, 282:4427–4436. [DOI] [PubMed] [Google Scholar]
- [15].Li Y, Shah-Simpson S, Okrah K, Belew AT, Choi J, Caradonna KL, Padmanabhan P, Ndegwa DM, Temanni MR, Corrada Bravo H, et al. : Transcriptome Remodeling in Trypanosoma cruzi and Human Cells during Intracellular Infection. PLoS Pathog 2016, 12:e1005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gazos-Lopes F, Martin JL, Dumoulin PC, Burleigh BA: Host triacylglycerols shape the lipidome of intracellular trypanosomes and modulate their growth. PLoS Pathog 2017, 13:e1006800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Coppens I, Sinai AP, Joiner KA: Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J Cell Biol 2000, 149:167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Labaied M, Jayabalasingham B, Bano N, Cha S-J, Sandoval J, Guan G, Coppens I Plasmodium salvages cholesterol internalized by LDL and synthesized de novo in the liver. Cell Microbiol 2011, 13:569–586. [DOI] [PubMed] [Google Scholar]
- [19].Liendo A, Visbal G, Piras MM, Piras R, Urbina JA: Sterol composition and biosynthesis in Trypanosoma cruzi amastigotes. Mol Biochem Parasitol 1999, 104:81–91. [DOI] [PubMed] [Google Scholar]
- [20].Pérez-Moreno G, Sealey-Cardona M, Rodrigues-Poveda C, Gelb MH, Ruiz-Pérez LM, Castillo-Acosta V, Urbina JA, González-Pacanowska D: Endogenous sterol biosynthesis is important for mitochondrial function and cell morphology in procyclic forms of Trypanosoma brucei. IntJ Parasitol 2012, 42:975–989. [DOI] [PubMed] [Google Scholar]
- [21].Shang N, Li Q, Ko T-P, Chan H-C, Li J, Zheng Y, Huang C-H, Ren F, Chen C-C, Zhu Z, et al. : Squalene synthase as a target for Chagas disease therapeutics. PLoS Pathog 2014,10:e1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Mukherjee S, Xu W, Hsu F-F, Patel J, Huang J, Zhang K: Sterol methyltransferase is required for optimal mitochondrial function and virulence in Leishmania major. Mol Microbiol 2019, 111:65–81.. * This paper demonstrates that L. major does not require ergostane-type sterols for growth, but rather for proper mitochondrial function.
- [23].Xu W, Hsu F-F, Baykal E, Huang J, Zhang K: Sterol biosynthesis is required for heat resistance but not extracellular survival in leishmania. PLoS Pathog 2014, 10:e1004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lepesheva GI, Villalta F, Waterman MR: Targeting Trypanosoma cruzi sterol 14α-demethylase (CYP51). Adv Parasitol 2011, 75:65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Atwood JA, Weatherly DB, Minning TA, Bundy B, Cavola C, Opperdoes FR, Orlando R, Tarleton RL: The Trypanosoma cruzi proteome. Science 2005, 309:473–476. [DOI] [PubMed] [Google Scholar]
- [26].Shah-Simpson S, Lentini G, Dumoulin PC, Burleigh BA: Modulation of host central carbon metabolism and in situ glucose uptake by intracellular Trypanosoma cruzi amastigotes. PLoS Pathog 2017, 13:e1006747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dumoulin PC, Burleigh BA: Stress-Induced Proliferation and Cell Cycle Plasticity of Intracellular Trypanosoma cruzi Amastigotes. mBio 2018, 9:e00673–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Collins MH, Craft JM, Bustamante IM, Tarleton RL: Oral exposure to Trypanosoma cruzi elicits a systemic CD8+ T cell response and protection against heterotopic challenge. Infect Immun 2011, 79:3397–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Engel JC, Franke de Cazzulo BM, Stoppani AO, Cannata JJ, Cazzulo JJ: Aerobic glucose fermentation by Trypanosoma cruzi axenic culture amastigote-like forms during growth and differentiation to epimastigotes. Mol Biochem Parasitol 1987, 26:1–10. [DOI] [PubMed] [Google Scholar]
- [30].Navarro MC, De Lima AR, Askue J, Contreras VT: Morphological comparison of axenic amastigogenesis of trypomastigotes and metacyclic forms of Trypanosoma cruzi. Mem Inst Oswaldo Cruz 2003, 98:83–91. [DOI] [PubMed] [Google Scholar]
- [31].Takagi Y, Akutsu Y, Doi M, Furukawa K: Utilization of proliferable extracellular amastigotes for transient gene expression, drug sensitivity assay, and CRISPR/Cas9-mediated gene knockout in Trypanosoma cruzi. PLoS Negl Trop Dis 2019, 13:e0007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Carey MA, Covelli V, Brown A, Medlock GL, Haaren M, Cooper JG, Papin JA, Guler JL: Influential Parameters for the Analysis of Intracellular Parasite Metabolomics. mSphere 2018, 3:e00097–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shiratsubaki IS, Fang X, Souza ROO, Palsson BO, Silber AM, Siqueira-Neto JL: Genome-scale metabolic models highlight stage-specific differences in essential metabolic pathways in Trypanosoma cruzi. PLoS Negl Trop Dis 2020, 14:e0008728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nolan SJ, Romano JD, Kline JT, Coppens I: Novel Approaches To Kill Toxoplasma gondii by Exploiting the Uncontrolled Uptake of Unsaturated Fatty Acids and Vulnerability to Lipid Storage Inhibition of the Parasite. Antimicrob Agents Chemother 2018, 62:e00347–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Martin JL, Yates PA, Boitz JM, Koop DR, Fulwiler AL, Cassera MB, Ullman B, Carter NS: A role for adenine nucleotides in the sensing mechanism to purine starvation in Leishmania donovani. Mol Microbiol 2016, 101:299–313. [DOI] [PubMed] [Google Scholar]
- [36].Kelly FD, Yates PA, Landfear SM: Nutrient sensing in Leishmania: Flagellum and cytosol. Mol Microbiol 2021, 115:849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Oberholzer M, Saada EA, Hill KL: Cyclic AMP Regulates Social Behavior in African Trypanosomes. mBio 2015, 6:e01954–01914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Parsons M, Worthey EA, Ward PN, Mottram JC: Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics 2005, 6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lentini G, Dos Santos Pacheco N, Burleigh BA: Targeting host mitochondria: A role for the Trypanosoma cruzi amastigote flagellum. Cell Microbiol 2018, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lewis MD, Francisco AF, Taylor MC, Kelly JM: A new experimental model for assessing drug efficacy against Trypanosoma cruzi infection based on highly sensitive in vivo imaging. J Biomol Screen 2015, 20:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lewis MD, Kelly JM: Putting Infection Dynamics at the Heart of Chagas Disease. Trends Parasitol 2016, 32:899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Ward AI, Olmo F, Atherton RL, Taylor MC, Kelly JM: Trypanosoma cruzi amastigotes that persist in the colon during chronic stage murine infections have a reduced replication rate. Open Biol 2020, 10:200261. * This study shows that in experimental murine infection models, T. cruzi persistence in gastrointestinal smooth muscle is characterized by cycles of replication, host cell lysis and re-infection with no evidence of widespread dormancy.
- [43].Kloehn J, Saunders EC, O'Callaghan S, Dagley MJ, McConville MJ: Characterization of metabolically quiescent Leishmania parasites in murine lesions using heavy water labeling. PLoS Pathog 2015, 11:el004683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Saunders EC, Ng WW, Kloehn J, Chambers JM, Ng M, McConville MJ: Induction of a stringent metabolic response in intracellular stages of Leishmania mexicana leads to increased dependence on mitochondrial metabolism. PLoS Pathog 2014, 10:e1003888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Saunders EC, Naderer T, Chambers J, Landfear SM, McConville MJ: Leishmania mexicana can utilize amino acids as major carbon sources in macrophages but not in animal models. Mol Microbiol 2018, 108:143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, et al. : Randomized Trial of Benznidazole for Chronic Chagas' Cardiomyopathy. N Engl J Med 2015, 373:1295–1306. [DOI] [PubMed] [Google Scholar]
- [47].Molina I, Gómez i Prat J, Salvador F, Trevino B, Sulleiro E, Serre N, Pou D, Roure S, Cabezos J, Valerio, et al. : Randomized trial of posaconazole and benznidazole for chronic Chagas' disease. N Engl J Med 2014, 370:1899–1908. [DOI] [PubMed] [Google Scholar]
- [48].Murcia L, Carrilero B, Muñoz MJ, Iborra MA, Segovia M: Usefulness of PCR for monitoring benznidazole response in patients with chronic Chagas' disease: a prospective study in a nondisease-endemic country. J Antimicrob Chemother 2010, 65:1759–1764. [DOI] [PubMed] [Google Scholar]
- [49].Rajão MA, Furtado C, Alves CL, Passos-Silva DG, de Moura MB, Schamber-Reis BL, Kunrath-Lima M, Zuma AA, Vieira-da-Rocha JP, Garcia JBF, et al. : Unveiling benznidazole's mechanism of action through overexpression of DNA repair proteins in Trypanosoma cruzi. Environ Mol Mutagen 2014, 55:309–321. [DOI] [PubMed] [Google Scholar]
- [50].Lopatkin AJ, Stokes JM, Zheng EJ, Yang JH, Takahashi MK, You L, Collins JJ: Bacterial metabolic state more accurately predicts antibiotic lethality than growth rate. Nat Microbiol 2019, 4:2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Lopatkin AJ, Bening SC, Manson AL, Stokes JM, Kohanski MA, Badran AH, Earl AM, Cheney NJ, Yang JH, Collins JJ: Clinically relevant mutations in core metabolic genes confer antibiotic resistance. Science 2021, 371:eaba0862. ** This paper shows that changes to central metabolism of bacteria can alter antibiotic efficacy.
- [52].Dumoulin PC, Vollrath J, Tomko SS, Wang JX, Burleigh B: Glutamine metabolism modulates azole susceptibility in Trypanosoma cruzi amastigotes. Elife 2020, 9:e60226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Parab AR, McCall L-I: Tryp-ing Up Metabolism: Role of Metabolic Adaptations in Kinetoplastid Disease Pathogenesis. Infect Immun 2021, 89:e00644–20. [DOI] [PMC free article] [PubMed] [Google Scholar]