Abstract
BACKGROUND
Adolescent and young adult (AYA) hematopoietic cell transplantation (HCT) survivors are at increased risk of metabolic syndrome and lean body mass (LBM) deficits. Resistance training (RT) is a potential intervention to improve LBM, metabolic fitness and reduce risk of cardiovascular disease.
PROCEDURE
Eligible participants ages 13–39 years, 80–120 days post-HCT, transfusion independent, and prednisone dose ≤1 mg/kg/day were approached. Baseline assessments of body composition (DXA), anthropometrics and strength testing were completed and participants were taught a 12-week, home-based RT intervention with weekly remote coaching. Follow-up assessments were at day +200 (FU1) and +365 post-HCT (FU2). Feasibility targets were 1) 60% enrollment of approached patients, 2) 80% completion of weekly phone calls and 3) 80% completion of the RT intervention and FU1 assessments. Acceptability was based on positive responses in qualitative interviews.
RESULTS
Twenty of 31 (65%) eligible AYAs enrolled. Three participants failed to complete baseline measurements (2=scheduling barriers, 1=passive refusal) and 4 participants who completed baseline assessments did not receive the intervention (1=medical reasons, 2=no longer interested). Of those who completed baseline assessments, 13 received the intervention, completed 88.5% of coaching calls, and 11 (65%) completed FU1. LBM (kg) increased or remained unchanged in 9/9 participants with complete body composition data at FU1 (mean 1.1 kg; 95%CI: 0.4,1.9). All participants who completed FU1 reported they would recommend the intervention to an AYA HCT survivor.
CONCLUSIONS
A home-based RT intervention in AYA HCT survivors early post HCT is both feasible and acceptable and may maintain or increase LBM.
Keywords: Adolescent and Young Adult, Resistance Training
INTRODUCTION:
There were more than 678,000 adolescent and young adult (AYA) cancer survivors in the US in 2019, and this number is projected to increase by more than 30% in the next 10 years.1 Unfortunately, one-third of childhood cancer survivors have severe or life-threatening medical complications 30 years after diagnosis and early mortality from cardiovascular disease (CVD) is one of the leading causes of non-relapse mortality.2–5 The growing number of survivors raises the importance of developing interventions to reduce these adverse late effects of cancer treatment, including after hematopoietic cell transplantation (HCT).1,6 HCT survivors are at increased risk of metabolic syndrome (central obesity, insulin resistance, glucose intolerance, dyslipidemia, and hypertension),7–10 lean muscle mass deficits and sarcopenic obesity compared to sibling controls.11,12 In a recent study, an exercise intervention including resistance training (RT) in adult breast cancer survivors was shown to attenuate metabolic syndrome, sarcopenic obesity, and circulating biomarkers (LDL cholesterol, total cholesterol, glycosylated hemoglobin, high-sensitivity C-reactive protein, insulin, leptin, and adiponectin).13 Lower muscle mass and higher central adiposity are highly predictive of insulin resistance and are potential targets for interventions designed to enhance metabolic fitness and reduce risk of CVD in adolescent cancer survivors.
Resistance training (RT) is a form of physical activity designed to improve muscular fitness by exercising a muscle or a muscle group against external resistance including traditional free weights and dumbbells, weight machines, body weight, elastic tubing, medicine balls, or even common household products like milk jugs filled with sand or soup cans.14 RT has been established as effective in increasing lean body mass, preventing abdominal and total fat gain, and reducing markers of inflammation (IL-6, TNF-alpha) and other cardio-metabolic risk factors in healthy adults.15–18 Studies in adults have demonstrated resistance training regimens are feasible and effective at improving abdominal adiposity, lipid metabolism and fat mass.15,19 Muscular strength has also been identified as an independent and powerful predictor of better insulin sensitivity in children.20
A 2019 systematic review of physical activity in AYA cancer patients highlighted the lack of high-quality studies aimed at improving physical functioning in this population,21 and even fewer studies evaluating RT interventions in these survivors.22,23 Furthermore, there are few studies evaluating RT interventions in pediatric and AYA HCT survivors and none of a home based intervention, which is likely to be more feasible, acceptable and generalizable than in-hospital supervised programs.22,23 Here in a small pilot study, we evaluate the feasibility and acceptability of a RT intervention uniquely suited to the needs of AYA cancer survivors, consisting of a brief in-person training at study entry followed by a home-based regimen for the duration of the study with remote coaching.
METHODS
Study Design
We conducted a single arm pilot study of an exercise intervention evaluating the feasibility and acceptability of home-based resistance training intervention in AYA HCT survivors. We recruited participants from the Seattle Children’s Hospital, Seattle Cancer Care Alliance and Fred Hutchinson Cancer Research Center (FHCRC) in Seattle, WA between November 2018 and February 2020. We targeted a sample size of 20–25 participants. The intervention period was 12 weeks. The primary endpoint was feasibility which was assessed through enrollment of approached and eligible patients, completion of weekly follow-up phone calls and completion of the full intervention among enrolled patients. As secondary endpoints, acceptability, change in body composition, and muscular strength were described.
Eligibility
Patients undergoing HCT for malignant diseases who were at least 80 days but less than 120 days out from an allogeneic or autologous HCT were recruited for participation in the study. Eligibility criteria included 13–39 years old, outpatient, ambulatory (able to walk) and without medical contraindication to increasing physical activity, transfusion independent, and ≤1 mg/kg/day of prednisone and on a tapering schedule. Given that study approach and enrollment were performed in person, scheduled follow-up at either Seattle Children’s Hospital or the Seattle Cancer Care Alliance was required for potential study participation. Additionally, because the study was performed at a tertiary referral center for HCT, patients who lived large distances from the cancer and reported inability to return for follow-up study visits were considered ineligible. After providing written informed consent (and assent if <18 years of age), patients were enrolled on the study. The study was approved by the IRB at Fred Hutchinson Cancer Research Center (no. 10046) and conducted in accordance with the ethical standards of the Declaration of Helsinki. It is registered at ClinicalTrials.gov (NCT03672981).
Intervention
The RT exercise prescription was uniquely formulated for AYA patients according to RT principals outlined by the American College of Sports Medicine strength training guidelines.24 Participants were offered up to three initial in-person exercise teaching sessions with an exercise physiologist at the FHCRC Exercise Research Center where they were taught the home-based exercise program. The RT intervention was tailored to the participant’s baseline strength assessments by the exercise physiologist. Each participant was prescribed a progressive resistance program, with participants completing 1–2 sets of 8 to 10 exercises, 8 to 12 repetitions of each exercise, 2–3 days per week. Participants were given graded resistance bands to complement body weight exercises to target all major muscle groups with the primary goal of increasing muscle mass. An exercise physiologist then conducted weekly follow-up phone calls with each participant to assess adherence to the exercise program and to provide support including identifying and overcoming barriers to exercise, goal setting, assess for adverse events, and providing motivation to exercise.
Outcome Measures
Baseline assessments were performed within 2 weeks of study enrollment. The primary outcomes were assessed at Follow-up 1 (FU1) within 2 weeks after the 12-week intervention period. Outcomes were also assessed secondarily at Follow-up 2 (FU2) within 4 weeks of day +365 post HCT. Between FU1 and FU2, there were no further weekly follow-up phone calls, exercise prescriptions or assessments of weekly exercise. At all timepoints, anthropometrics, body composition, and strength testing were measured. For assessment of feasibility, we traced the number of eligible participants who enrolled in the study, the completion of weekly follow-up phone calls with participants and the number of participants who completed the 12-week intervention and FU1. To assess acceptability, one-on-one, semi-structured interviews were performed by a clinical research assistant via telephone following completion of the 12-week RT intervention. The interviews were conducted following a standardized script. Sessions were digitally recorded, transcribed verbatim and analyzed. Participants who completed FU1 were asked “Would you recommend this program to other adolescent/young adult hematopoietic cell transplant cancer survivors?”, “Would you recommend the home-based intervention to your friends?”, and “Did you enjoy the home-based resistance training intervention?”. Acceptability was measured by an affirmative response to these questions. Additionally, participants were asked to about positive and negative aspects of participating in the RT intervention for AYA HCT survivors.
Body Composition
Heigh and weight were obtained in the FHCRC Exercise Research Center or extracted from the medical record within 1 week of the evaluation time points. Dual x-ray absorptiometry (DXA) was used to evaluate body composition including lean body mass (LBM) and percent fat mass (PFM).
Strength Testing
Strength and endurance assessments were performed at the FHCRC Exercise Research Center under the direct supervision of an exercise physiologist. Assessments included hand grip strength25, 30-second chair stand assessment26, one repetition maximum chest press, one repetition maximum leg press27 and a 6-minute walk test28.
Primary Disease and Transplant Related Data
Primary disease and transplant related data were extracted from the electronic medical record. Data on donor source, conditioning regimen, radiation exposure, and steroid exposure for treatment of graft versus host disease (GVHD) were also collected.
Statistical analysis
Participants’ age, gender, and socio-demographic characteristics were summarized using descriptive statistics, as were participants’ medical characteristics, including underlying diagnosis, HCT conditioning regimen and donor source. Feasibility was defined as: (1) at least 60% enrollment of approached and eligible patients; (2) at least 80% completion of the full intervention and FU1 assessments among enrolled patients; and (3) at least 80% completion of weekly follow-up phone calls among those who received the intervention. Absolute and percentage change for muscle strength and body composition outcomes were calculated for the periods baseline to FU1 and baseline to FU2. Changes were summarized using means and 95% confidence intervals together with the number of participants experiencing no change, benefit or decline. Statistical analyses were performed using Stata version 16 (StataCorp., College Station, TX).
RESULTS
Feasibility and Acceptability
Between November 2018 and February 2020, 91 HCT survivors were screened for eligibility, 31 were eligible, 30 were approached, and 20 survivors (64.6%) were enrolled in the study. Study enrollment was stopped in February 2020 due to the SARS-CoV-2 pandemic limiting enrollment of up to an additional 5 participants. Participants ranged in age from 16–39 years, 6 (30%) were female, and 10 (50%) had a 4-year college degree or higher. (Table 1) Ten participants (50%) had a primary diagnosis of acute lymphoblastic leukemia, 5 (25%) acute myeloid leukemia, 4 (20%) Hodgkin lymphoma, and 1 participant had a germ cell tumor. Most participants (n=15, 75%) received total body irradiation (TBI) and had an allogeneic donor source (n=16, 80%). (Table 1) Seventeen participants (85%) completed baseline measurements. Of the 3 who did not complete baseline measurements, 2 participants cited lack of evening/weekend FHCRC Exercise Research Center hours and 1 passively refused participation after enrollment. Of the participants who completed baseline assessments, 13 (76%) completed the intervention and 11 (65%) completed FU1. Of the 6 participants who did not complete FU1 measurements, 2 had disease relapse, 1 suffered a non-intervention related medical condition preventing exercise, 2 declined further follow-up, and 1 declined to follow-up due to the SARS-CoV-2 pandemic. Six (55%) of the participants who completed FU1 required steroids for GVHD during the intervention period. There were no significant adverse events during the study. Of the 11 participants who completed assessments at FU1, 10 completed assessments at FU2. The patient who did not complete FU2 had disease relapse. (Fig. 1)
TABLE 1.
Participant demographics and transplant information.
| All | Completed FU1 | Didn’t Complete FU1 | |||||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||||
| n | 20 | 11 | 9 | ||||
| Age (years) | |||||||
| <18 | 2(10) | 1 (9) | 1 (11) | ||||
| 18–24 | 3 (15) | 3 (27) | 0 | ||||
| 25–29 | 1 (5) | 1 (9) | 0 | ||||
| 30–34 | 5 (25) | 3 (27) | 2 (22) | ||||
| 35–39 | 9 (45) | 3 (27) | 6 (67) | ||||
| Sex | |||||||
| Male | 14 (70) | 7 (63) | 7 (78) | ||||
| Race | |||||||
| Asian | 2 (10) | 1 (9) | 1 (11) | ||||
| Black or African American | 1 (5) | 0 | 1 (11) | ||||
| White | 17 (85) | 10 (91) | 7 (78) | ||||
| Ethnicity | |||||||
| Hispanic or Latino | 1 (5) | 1 (9) | 0 | ||||
| Non-Hispanic | 19 (95) | 10 (91) | 9 (100) | ||||
| Education | |||||||
| Less than high school | 2 (10) | 1 (9) | 1 (11) | ||||
| High school degree or GED | 2 (10) | 2 (18) | 0 | ||||
| Some vocational or college credit | 2 (10) | 1 (9) | 1 (11) | ||||
| 2-year college degree or trade degree | 4 (20) | 1 (9) | 3 (33) | ||||
| 4-year college degree | 7 (35) | 4 (36) | 3 (33) | ||||
| Post Graduate | 3 (15) | 2 (18) | 1 (11) | ||||
| Marital Status | |||||||
| Living with Partner | 1 (5) | 0 | 1 (11) | ||||
| Married | 10 (50) | 5 (45) | 5 (56) | ||||
| Separated | 1 (5) | 0 | 1 (11) | ||||
| Single | 8 (40) | 6 (55) | 2 (22) | ||||
| Total Household Income | |||||||
| $0-$20,000 | 3 (15) | 2 (18) | 1 (11) | ||||
| $21,000 – $40,000 | 3 (15) | 2 (18) | 1 (11) | ||||
| $41,000 – $60,000 | 2 (10) | 1 (9) | 1 (11) | ||||
| $61,000 – $80,000 | 2 (10) | 1 (9) | 1 (11) | ||||
| $81,000 – $100,000 | 1 (5) | 1 (9) | 0 | ||||
| $101,000 – $120,000 | 1 (5) | 0 | 1 (11) | ||||
| $121,000 or more | 5 (25) | 3 (27) | 2 (22) | ||||
| Choose not to answer | 3 (15) | 1 (9) | 2 (22) | ||||
| Household size | |||||||
| 1 | 1 (5) | 1 (9) | 0 | ||||
| 2 | 5 (25) | 2 (18) | 3 (33) | ||||
| 3 | 4 (20) | 3 (27) | 1 (11) | ||||
| 4 | 3 (15) | 2 (18) | 1 (11) | ||||
| 5 | 5 (25) | 2 (18) | 3 (33) | ||||
| 8 or More | 2 (10) | 1 (9) | 1 (11) | ||||
| Employment status | |||||||
| Disabled, not on disability | 2 (10) | 2 (18) | 0 | ||||
| Disabled, on disability | 4 (20) | 2 (18) | 2 (22) | ||||
| Homemaker | 1 (5) | 1 (9) | 0 | ||||
| In school, full-time | 2 (10) | 1 (9) | 1 (11) | ||||
| Not employed, looking for work | 1 (5) | 0 | 1 (11) | ||||
| Not employed, not looking for work | 2 (10) | 2 (18) | 0 | ||||
| Working, full-time | 2 (10) | 2 (18) | 0 | ||||
| Working, part-time | 1 (5) | 0 | 1 (11) | ||||
| Other | 4 (20) | 1 (9) | 3 (33) | ||||
| Choose Not To Answer | 1 (5) | 0 | 1 (11) | ||||
| Insurance provider | |||||||
| Medicaid | 3 (15) | 2 (18) | 1 (11) | ||||
| Medicare | 1 (5) | 0 | 1 (11) | ||||
| Employer | 6 (30) | 3 (27) | 3 (33) | ||||
| Through parent or guardian | 4 (20) | 3 (27) | 1 (11) | ||||
| Through previous employer | 2 (10) | 1 (9) | 1 (11) | ||||
| Through spouse’s or parent’s policy | 3 (15) | 2 (18) | 1 (11) | ||||
| Veteran’s Administration or Military (CHAMPUS) | 1 (5) | 0 | 1 (11) | ||||
| Primary diagnosis | |||||||
| ALL | 10 (50) | 4 (36) | 6 (67) | ||||
| AML | 5 (25) | 4 (36) | 1 (11) | ||||
| Hodgkin Lymphoma | 4 (20) | 2 (18) | 2 (22) | ||||
| Germ Cell Tumor | 1 (5) | 1 (9) | 0 | ||||
| Total body irradiation (TBI) | 15 (75) | 8 (73) | 7 (78) | ||||
| Cell type | |||||||
| Bone marrow | 1 (5) | 1 (9) | 0 | ||||
| Cord | 1 (5) | 1 (9) | 0 | ||||
| Peripheral blood stem cell | 18 (90) | 9 (82) | 9 (100) | ||||
| Donor type | |||||||
| Allogneic - related donor | 15 (75) | 8 (73) | 7 (78) | ||||
| Allogeneic - unrelated donor | 1 (5) | 1 (9) | 0 | ||||
| Autogeneic | 4 (20) | 2 (18) | 2 (22) | ||||
| Conditioning regimen | |||||||
| Chemotherapy only | 5 (25) | 3 (27) | 2 (22) | ||||
| High dose TBI (1200–1320 cGy) | 13 (65) | 7 (64) | 6 (67) | ||||
| Low dose TBI (300–300 cGy) | 2 (10) | 1 (9) | 1 (11) | ||||
FIGURE 1.
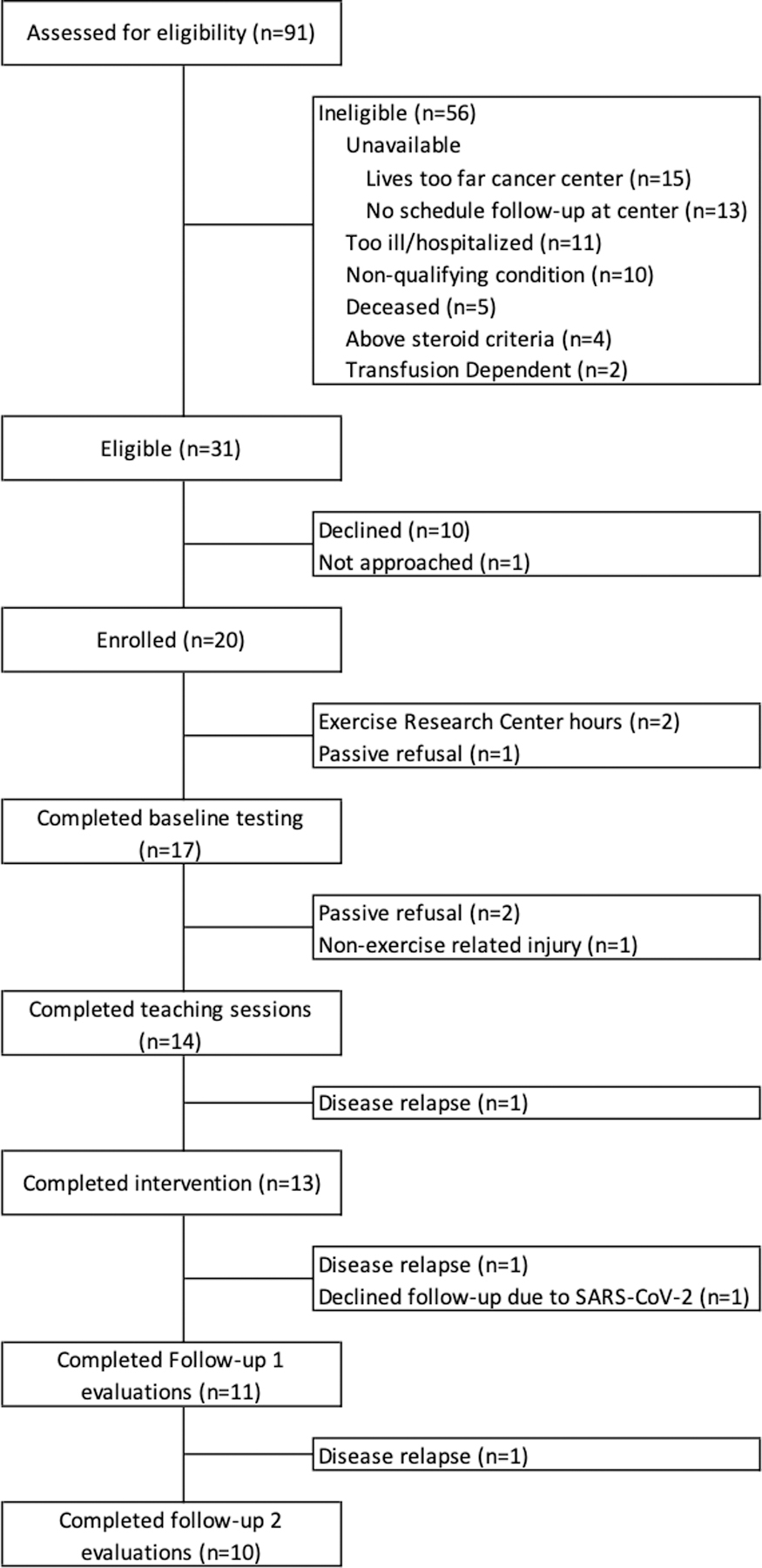
Flow of participants through the study.
Participants who completed the intervention, completed 89% of weekly coaching phone calls and reported a median of 2 exercise sessions per week. All participants who completed FU1 measurements reported during interviews they would recommend the intervention to a friend or HCT survivor. Additionally, all participants who completed FU1 stated they enjoyed the RT intervention.
During qualitative interviews, participants who completed FU1 listed the following as positive attributes of the RT intervention: could be performed at home and/or didn’t need a gym membership/expensive equipment (4); provided motivation to exercise (3); ability to observe measurable improvement from baseline testing (4); guidance for returning to exercise (2); improvement in mood or mental health (2); feeling as an active participant in improving their own health (2); improvement in strength and stamina (2); an exercise prescription to follow (2); support from exercise physiologists (1); accountability with weekly calls (1); improvement in confidence (1); all equipment provided (1); and a transition from inpatient physical therapy (1). Participants who completed FU1 did not report any negative aspects to the intervention. Of the participants who did not complete FU1 for non-medical reasons, 2 reported they were unable to come in for study visits at the Prevention Center during the hours of operation, 1 reported they “had too much on their plate” and withdrew prior to the baseline visit, 1 stated they lived 4 hours away and could not make it to the Prevention Center to complete the teaching sessions, 1 declined further participation and follow-up citing the SARS-CoV-2 pandemic and 1 decided not to participate after the in-person teaching but did not cite a reason. Additionally, participants who completed FU1 reported the following aspects of the intervention worked well: weekly phone calls (7); exercise prescription (2); access to an exercise physiologist for questions or modifications (4); initial in-person training sessions (2); and resistance bands and body weight exercises (2). The participants also made the following recommendations for improvement: more in-person sessions with the exercise physiologist at the beginning (1); in-person sessions with the exercise physiologist midway through the intervention (2); education on stretching and aerobic exercise (2); incorporation of mHealth activity monitoring such as Fitbit (1); educational materials on a healthy diet (1); option to video conference with the exercise physiologist (1); and a group social media page for motivation (1).
Body Composition
Paired baseline and FU1 and baseline and FU2 DXA body composition data were available on 9 participants. Baseline body composition data were unable to be extracted from DXA scans on 2 participants. Of the participants with evaluable data at FU1, all either maintained (n=3) or gained (n=6) LBM at FU1 compared with baseline (Table 3). Lean body mass increased for all 8 participants with paired baseline and FU2 body composition data. The mean increase in LBM at FU1 was 1.1 kg (95% CI: 0.4,1.9) or 2% (95% CI: 1, 4). At FU2 the mean increase in LBM was 2.1 kg (95% CI: 0.5, 3.8) or 5% (95% CI: 1, 8). Percent fat mass at FU1 decreased for 3 participants, remained the same for 1 participant and increased for 5 participants. The mean increase from baseline in PFM at FU1 was 1% (95% CI −2, 5) and 1% (95% CI −3, 5) at FU2. Of note, all participants who had an increase in PFM at FU1 were on oral prednisone for GVHD at some point during the intervention while all participants who decreased PFM or remained the same were not.
TABLE 3.
Mean score and mean percentage change from baseline following the home-based resistance training intervention at follow-up 1 (FU1) and at 1 year post hematopoietic cell transplant (FU2) among participants who completed FU1 assessments.
| Mean Score (Range) | Baseline -> Follow-up 1 (n=11) | Baseline -> Follow-up 2 (n=10) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strength Test | Baseline | FU1 | FU2 | Mean % Change | 95% CI | P-value | Mean % Change | 95% CI | P-value | ||||||
| Chair stand test (stands) | 14.5 | (7.5–30) | 18.9 | (10–35) | 20.9 | (15.5–34) | 36 | (18–55) | <0.01 | 47 | (21–74) | <0.01 | |||
| 6-Minute walk (meters) * | 466 | (348–633) | 529 | (420–667) | 556 | (471–623) | 14 | (9–20) | <0.01 | 14 | (6–35) | <0.01 | |||
| Grip strength - dominant (kg) ** | 31 | (16–51) | 36 | (20–61) | 33 | (21–52) | 14 | (6–22) | <0.01 | 19 | (2–34) | 0.09 | |||
| Grip strength - non-dominant (lbs) | 29 | (13–43) | 34 | (20–59) | 33 | (20–49) | 19 | (6–32) | 0.01 | 30 | (2–34) | 0.02 | |||
| Chest press - one repetition maximum (lbs) | 79 | (30–163) | 98 | (45–180) | 100 | (40–188) | 30 | (16–45) | <0.01 | 35 | (16–54) | <0.01 | |||
| Leg press - one repetition maximum (lbs) | 178 | (70–280) | 216 | (100–320) | 221 | (130–320) | 26 | (13–39) | <0.01 | 33 | (14–52) | <0.01 | |||
Young adult 6-minute walk test norms are 593 ± 57 meters for women and 638 ± 44 m for men.
Young adult dominant hand grips strength norms are 30 ± 6.6 kg for women and 47 ± 9.5 kg for men.
Muscular Strength
Of the participants who completed FU1, all increased their 6-minute walk distance with a 14% (95% CI: 9, 20) mean increase from baseline (Table 3). 10 participants increased their 30-second chair stand score with the other participant obtaining the same score resulting in a mean 36% increase (95% CI: 16, 57) from baseline. Dominant grip strength increased in 9 participants and non-dominant grip strength increased in 8 participants corresponding to a 14% (95% CI: 5, 23) and 19% (95% CI 4, 33) mean increase respectively. One repetition maximum chest press increased in 10 participants and leg press one repetition max increased in 9 participants corresponding to a 30% (95% CI: 14, 47) and 26% (95% CI: 11, 41) mean increase respectively. (Fig. 2)
FIGURE 2.
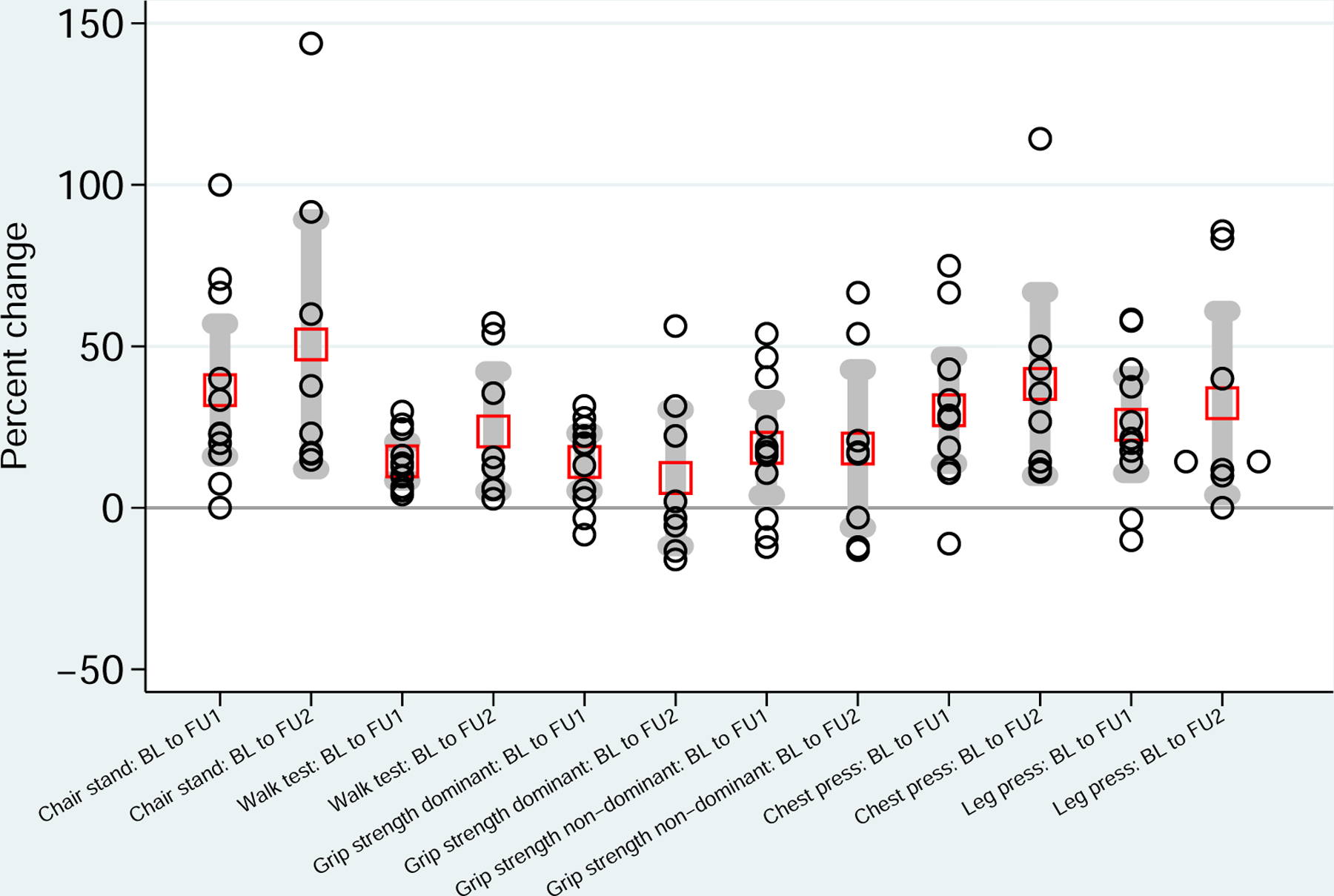
Percent change in muscular strength from baseline (BL) to follow-up 1 (FU1) and follow-up 2 (FU2). Red boxes indicate means and gray bars indicate 95% confidence intervals.
Of the participants who completed FU2, compared with baseline, all participants improved their 30-second chair stand score with a mean increase of 51% (95% CI: 12, 89) and 6-minute walk test with a mean increase of 24% (95% CI: 5, 42). Additionally, all participants increased their one repetition maximum chest press and leg press with a mean increase of 38% (95% CI: 10, 67) and 32% (95% CI: 4, 61) respectively. Dominant and non-dominant grip strength increased by a mean of 9% (95% CI: −12, 30) and 18% (95% CI: −6, 43).
DISCUSSION
We report the results of a pilot study, which demonstrates the feasibility and acceptability of a home-based RT intervention for AYA HCT survivors in the early post-HCT period. The feasibility goals of >60% enrollment of eligible HCT survivors and completion of >80% weekly phone coaching calls of those who started the intervention were achieved. Completion rates of the 12-week intervention and FU1 among enrolled participants were lower than the anticipated 80% rate due to the fact that 4 participants dropped out of the study due to medical issues/relapse or SARS-CoV-2 pandemic reasons and 2 due to lack of evening or weekend hours at the FHCRC Exercise Research Center for initial teaching sessions. Recruitment rates in other non-AYA exercise intervention studies of HCT survivors report enrollment rates ranging from 22–71% with completion of follow-up evaluations ranging from 58–79%.29–33 In a recent AYA oncology exercise study in patients who completed cancer treatment (not including HCT), a recruitment rates of 68% was reported, which is similar to 65% reported in our study.34
Promisingly, all participants increased or at least maintained LBM following the intervention (FU1) and at 1-year post-HCT (FU2). Previous studies have demonstrated that following allogeneic HCT, total lean body mass significantly decreases corresponding to increased incidence of sarcopenia.35–37 Additionally, an overwhelming majority of patients also demonstrated improvements in functional strength assessments. Respectively, for young adult women and men, 6-minute walk test norms are 593 ± 57 m and 638 ± 44 m and dominant hand grips strength norms are 30 ± 6.6 kg and 47 ± 9.5 kg.38,39 While mean scores for the 6-minute walk test and grip strength were all below population norms at baseline and FU1, both improved and approached the normal range by FU1. Previous studies of physical exercise programs in HCT survivors have either shown no statistically significant improvement in LBM or PFM.29 While we report the changes in body composition and strength testing, this study was not powered to detect differences in these metrics and only descriptive data are reported. However, with these early data, we feel the resistance training intervention we describe in this study serves as a promising intervention to increase LBM and prevent sarcopenia.
In addition to the improvements in LBM, we also saw corresponding improvements in strength assessments of major muscle groups from baseline at both follow-up timepoints. These results demonstrate not only improvement in body composition but also function. However, without a control group, it is difficult to attribute improvements to the RT intervention versus natural improvements that may occur with the passage of time and growth after HCT. Despite seeing improvements in LBM, these improvements were not universally seen for changes in PFM. All of the participants who had an increase in PFM at FU1 (n=5) were on steroids for GVHD at some point during the intervention period. Therefore, the increase in PFM following the intervention could largely be due to the use of steroids for treatment of graft versus host disease. Future interventions could combine both resistance and aerobic exercise to target both improvements in LBM and percent fat mass.
The intervention described above is unique in the fact that it utilizes both remote and in-person coaching, outpatient based, and is designed for the early post-HCT period. As the FHCRC standard of care, patients undergoing HCT have anthropometric measurements, body composition measured by DXA and other evaluations at day +80 and day +360 post-HCT. Given these standard of care timepoints, and the fact that most patients have achieved neutrophil engraftment, transfusion independence, and are nearing discharge from the outpatient HCT clinic by the day +80, day +80–120 was selected as a convenient timepoint for initiation of the RT intervention. It was observed that older patients had a trend towards not completing FU1. Potential contributing factors for this observation include increased rate relapse, comorbidities, conflicting priorities or increased distance from the cancer center. Despite limitations in completion of the intervention for some patients, a home-based RT intervention in the early post-HCT period is crucial because many AYA patients are at a teachable moment when they are still in close follow-up under the care of their primary transplant team. Furthermore, an early post-HCT intervention could prevent later health complications.
Previous literature to date has focused on older and young populations, as well as predominantly hospital-based interventions. A home-based resistance training intervention is particularly of value in this immunocompromised population which has been highlighted by the SARS-CoV-2 pandemic. Furthermore, as we also learned in our study, AYA survivors can find it hard to return to regional cancer centers for work, family, and educational reasons, making a home-based intervention desirable in this population. In future studies, combining baseline visits with teaching sessions could be beneficial for some AYA participants as to limit the number of returns to the cancer center. We have learned that having weekend and evening hours available for teaching sessions and follow-up assessments is particularly important in the AYA population and plan to implement this into future studies.
There are a number of limitations to our study, outside of the small number of participants, little racial and ethnic diversity, single transplant center, need to live near the transplant center or easily return for follow-up visits, heterogeneity of the primary diagnosis and donor type, and the lack of a control group. In future studies, we plan to explore the feasibility of remote testing to increase the opportunity for participation for those who live far away from the cancer center. Additionally, we plan to incorporate the recommendations from participants in this pilot study including incorporation of mHealth activity monitoring and providing information on stretching and aerobic exercise. Data regarding pre-HCT body composition were unavailable and not obtained as part of this study. Furthermore, we were unable to extract baseline body composition data from the DXA scans for 2 participants as only limited DXA scans for bone density were performed. Additionally, we did not collect data on nutrition and protein intake which can also have an impact on lean body mass gain.
This study provides preliminary evidence supporting a randomized controlled trial of a home-based resistance training exercise program in AYA survivors that is both feasible and acceptable. Future randomized trials are needed to confirm and validate these early findings. Given the prevalence of sarcopenia and metabolic syndrome observed in HCT survivors, exercise interventions that target skeletal muscle loss and cardiometabolic risk factors are needed to address the chronic metabolic complications in HCT survivors.
TABLE 2.
Measured lean body mass and percent fat mass at baseline (BL), following the resistance training intervention at follow-up 1 (FU1) and at 1 year post HCT follow-up 2 (FU2) among participants who completed FU1 assessments.
| Lean Body Mass | Percent Fat Mass | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant | Measured (kg) | Change kg (%) | Measured (%) | Percent Change (%) | ||||||||||||
| BL | FU1 | FU2 | BL -> FU1 | BL -> FU2 | BL | FU1 | FU2 | BL -> FU1 | BL -> FU2 | |||||||
| 1 | 37.3 | 37.3 | 37.6 | 0.0 | (0) | 0.3 | (1) | 44.8 | 51.8 | 48.7 | 7.0 | 3.9 | ||||
| 2 | 64.0 | 66.7 | 68.7 | 2.7 | (4) | 4.7 | (7) | 36.0 | 41.8 | 35.5 | 5.8 | −0.5 | ||||
| 3 | 54.2 | 55.3 | 54.6 | 1.1 | (2) | 0.4 | (1) | 38.0 | 32.2 | 31.7 | −5.8 | −6.3 | ||||
| 6 | 36.1 | 37.3 | 39.1 | 1.2 | (3) | 3.0 | (8) | 40.4 | 36.8 | 38.0 | −3.6 | −2.4 | ||||
| 8 | UE | 56.1 | * | - | - | (0) | UE | 31.8 | * | - | - | |||||
| 11 | UE | 50.1 | 48.7 | - | - | (0) | UE | 35.4 | 38.4 | - | - | |||||
| 13 | 42.9 | 44.1 | 44.0 | 1.2 | (3) | 1.1 | (2) | 29.7 | 30.8 | 33.7 | 1.1 | 4.0 | ||||
| 14 | 38.9 | 41.1 | 43.0 | 2.2 | (6) | 4.1 | (10) | 50.1 | 51.6 | 51.7 | 1.5 | 1.6 | ||||
| 17 | 31.6 | 31.6 | 33.0 | 0.0 | (0) | 1.4 | (5) | 20.2 | 19.6 | 26.2 | −0.6 | 6.0 | ||||
| 18 | 50.4 | 50.4 | 51.9 | 0.0 | (0) | 1.5 | (3) | 34.0 | 41.9 | 40.2 | 8.0 | 6.2 | ||||
| 20 | 53.2 | 54.9 | 56.4 | 1.7 | (3) | 3.2 | (6) | 13.8 | 13.8 | 14.1 | 0.0 | 0.3 | ||||
UE = Body composition data was unable to be extracted from DXA.
Patient Relapse
ACKNOWLEDGMENTS:
This investigation was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32CA00935, Cancer Center Support Grant (P30 CA015704) the National Cancer Institute Transdisciplinary Research on Energetics and Cancer (TREC) Training Workshop (R25CA203650, PI: Melinda Irwin), Conquer Cancer Foundation and the Seattle Children’s Research Institute Clinical Research Scholars Program.
Abbreviations Table
- AYA
adolescent and young adult
- CVD
cardiovascular disease
- DXA
dual X-ray absorptiometry
- FHCRC
Fred Hutchinson Cancer Research Center
- FU1
follow-up 1
- FU2
follow-up 2
- GVHD
graft versus host disease
- HCT
hematopoietic cell transplantation
- LBM
lean body mass
- PFM
percent fat mass
- RT
resistance training
- TBI
total body irradiation
Footnotes
CONFLICT OF INTEREST STATEMENT:
The authors declare that there is no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
REFERENCES
- 1.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565 [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185 [DOI] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Buchanan GR, Eshelman DA, et al. Cardiovascular risk factors in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2001;23(7):424–430. [DOI] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Adams-Huet B, Victor RG, et al. Insulin Resistance and Risk Factors for Cardiovascular Disease in Young Adult Survivors of Childhood Acute Lymphoblastic Leukemia. J Clin Oncol. 2009;27(22):3698. doi: 10.1200/JCO.2008.19.7251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in Adult Survivors of Childhood Acute Lymphoblastic Leukemia: A Report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21(7):1359–1365. doi: 10.1200/JCO.2003.06.131 [DOI] [PubMed] [Google Scholar]
- 6.Majhail NS, Brazauskas R, Hassebroek A, et al. Outcomes of allogeneic hematopoietic cell transplantation for adolescent and young adults compared with children and older adults with acute myeloid leukemia. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2012;18(6):861–873. doi: 10.1016/j.bbmt.2011.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ketterl TG, Chow EJ, Leisenring WM, et al. Adipokines, Inflammation, and Adiposity in Hematopoietic Cell Transplantation Survivors. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2018;24(3):622–626. doi: 10.1016/j.bbmt.2017.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109(4):1765–1772. doi: 10.1182/blood-2006-05-022335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker KS, Chow E, Steinberger J. Metabolic syndrome and cardiovascular risk in survivors after hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47(5):619–625. doi: 10.1038/bmt.2011.118 [DOI] [PubMed] [Google Scholar]
- 10.Reusch JEB. Current concepts in insulin resistance, type 2 diabetes mellitus, and the metabolic syndrome. Am J Cardiol. 2002;90(5, Supplement 1):19–26. doi: 10.1016/S0002-9149(02)02555-9 [DOI] [PubMed] [Google Scholar]
- 11.Ketterl TG, Chow EJ, Leisenring WM, et al. Adipokine Concentrations and Adiposity in Hematopoietic Cell Transplant (HCT) Survivors. Biol Blood Marrow Transplant. 2017;23(3):S67–S68. doi: 10.1016/j.bbmt.2017.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei C, Thyagiarajan MS, Hunt LP, Shield JPH, Stevens MCG, Crowne EC. Reduced insulin sensitivity in childhood survivors of haematopoietic stem cell transplantation is associated with lipodystropic and sarcopenic phenotypes. Pediatr Blood Cancer. 2015;62(11):1992–1999. doi: 10.1002/pbc.25601 [DOI] [PubMed] [Google Scholar]
- 13.Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, et al. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(9):875–883. doi: 10.1200/JCO.2017.75.7526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ACSM’s resource manual for Guidelines for exercise testing and prescription | ACQUIRE Repository (3.4.3). Accessed August 22, 2017. http://acquire.cqu.edu.au:8080/vital/access/manager/Repository/cqu:4883
- 15.Nunes PRP, Barcelos LC, Oliveira AA, et al. Effect of resistance training on muscular strength and indicators of abdominal adiposity, metabolic risk, and inflammation in postmenopausal women: controlled and randomized clinical trial of efficacy of training volume. Age Dordr Neth. 2016;38(2):40. doi: 10.1007/s11357-016-9901-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao C-D, Tsauo J-Y, Lin L-F, et al. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity: A CONSORT-compliant prospective randomized controlled trial. Medicine (Baltimore). 2017;96(23). doi: 10.1097/MD.0000000000007115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Artero EG, Lee D, Lavie CJ, et al. Effects of Muscular Strength on Cardiovascular Risk Factors and Prognosis. J Cardiopulm Rehabil Prev. 2012;32(6):351. doi: 10.1097/HCR.0b013e3182642688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vargas S, Petro JL, Romance R, et al. Comparison of changes in lean body mass with a strength- versus muscle endurance-based resistance training program. Eur J Appl Physiol. 2019;119(4):933–940. doi: 10.1007/s00421-019-04082-0 [DOI] [PubMed] [Google Scholar]
- 19.Huang SW, Ku JW, Lin LF, Liao CD, Chou LC, Liou TH. Body composition influenced by progressive elastic band resistance exercise of sarcopenic obesity elderly women: a pilot randomized controlled trial. Eur J Phys Rehabil Med. Published online January 12, 2017. doi: 10.23736/S1973-9087.17.04443-4 [DOI] [PubMed]
- 20.Benson AC, Torode ME, Singh MAF. Muscular strength and cardiorespiratory fitness is associated with higher insulin sensitivity in children and adolescents. Int J Pediatr Obes IJPO Off J Int Assoc Study Obes. 2006;1(4):222–231. [DOI] [PubMed] [Google Scholar]
- 21.Munsie C, Ebert J, Joske D, Ackland T. The Benefit of Physical Activity in Adolescent and Young Adult Cancer Patients During and After Treatment: A Systematic Review. J Adolesc Young Adult Oncol. 2019;8(5):512–524. doi: 10.1089/jayao.2019.0013 [DOI] [PubMed] [Google Scholar]
- 22.Chamorro-Viña C, Ruiz JR, Santana-Sosa E, et al. Exercise during hematopoietic stem cell transplant hospitalization in children. Med Sci Sports Exerc. 2010;42(6):1045–1053. doi: 10.1249/MSS.0b013e3181c4dac1 [DOI] [PubMed] [Google Scholar]
- 23.Järvelä LS, Kemppainen J, Niinikoski H, et al. Effects of a home-based exercise program on metabolic risk factors and fitness in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59(1):155–160. doi: 10.1002/pbc.24049 [DOI] [PubMed] [Google Scholar]
- 24.Irwin ML, American College of Sports Medicine, eds. ACSM’s Guide to Exercise and Cancer Survivorship. Human Kinetics; 2012. [Google Scholar]
- 25.Lee SH, Gong HS. Measurement and Interpretation of Handgrip Strength for Research on Sarcopenia and Osteoporosis. J Bone Metab. 2020;27(2):85–96. doi: 10.11005/jbm.2020.27.2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobson F, Hinman RS, Roos EM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(8):1042–1052. doi: 10.1016/j.joca.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 27.American College of Sports Medicine, Liguori G, Feito Y, Fountaine C, Roy B, eds. ACSM’s Guidelines for Exercise Testing and Prescription. Eleventh edition. Wolters Kluwer; 2021. [Google Scholar]
- 28.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 29.Knols RH, de Bruin ED, Uebelhart D, et al. Effects of an outpatient physical exercise program on hematopoietic stem-cell transplantation recipients: a randomized clinical trial. Bone Marrow Transplant. 2011;46(9):1245–1255. doi: 10.1038/bmt.2010.288 [DOI] [PubMed] [Google Scholar]
- 30.Hacker ED, Larson J, Kujath A, Peace D, Rondelli D, Gaston L. Strength training following hematopoietic stem cell transplantation. Cancer Nurs. 2011;34(3):238–249. doi: 10.1097/NCC.0b013e3181fb3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hacker ED, Collins E, Park C, Peters T, Patel P, Rondelli D. Strength Training to Enhance Early Recovery after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23(4):659–669. doi: 10.1016/j.bbmt.2016.12.637 [DOI] [PubMed] [Google Scholar]
- 32.Furzer BJ, Ackland TR, Wallman KE, et al. A randomised controlled trial comparing the effects of a 12-week supervised exercise versus usual care on outcomes in haematological cancer patients. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2016;24(4):1697–1707. doi: 10.1007/s00520-015-2955-7 [DOI] [PubMed] [Google Scholar]
- 33.Shelton ML, Lee JQ, Morris GS, et al. A randomized control trial of a supervised versus a self-directed exercise program for allogeneic stem cell transplant patients. Psychooncology. 2009;18(4):353–359. doi: 10.1002/pon.1505 [DOI] [PubMed] [Google Scholar]
- 34.Atkinson M, Murnane A, Goddard T, et al. A randomized controlled trial of a structured exercise intervention after the completion of acute cancer treatment in adolescents and young adults. Pediatr Blood Cancer. 2021;68(1):e28751. doi: 10.1002/pbc.28751 [DOI] [PubMed] [Google Scholar]
- 35.DeFilipp Z, Troschel FM, Qualls DA, et al. Evolution of Body Composition Following Autologous and Allogeneic Hematopoietic Cell Transplantation: Incidence of Sarcopenia and Association with Clinical Outcomes. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2018;24(8):1741–1747. doi: 10.1016/j.bbmt.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 36.Frisk P, Rössner SM, Norgren S, Arvidson J, Gustafsson J. Glucose metabolism and body composition in young adults treated with TBI during childhood. Bone Marrow Transplant. 2011;46(10):1303–1308. doi: 10.1038/bmt.2010.307 [DOI] [PubMed] [Google Scholar]
- 37.Slater ME, Steinberger J, Ross JA, et al. Physical Activity, Fitness, and Cardiometabolic Risk Factors in Adult Survivors of Childhood Cancer with a History of Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2015;21(7):1278–1283. doi: 10.1016/j.bbmt.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chetta A, Zanini A, Pisi G, et al. Reference values for the 6-min walk test in healthy subjects 20–50 years old. Respir Med. 2006;100(9):1573–1578. doi: 10.1016/j.rmed.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 39.Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL. Hand Grip Strength: age and gender stratified normative data in a population-based study. BMC Res Notes. 2011;4(1):127. doi: 10.1186/1756-0500-4-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.


