Abstract
Chagas disease is a neglected tropical disease caused by Trypanosoma cruzi parasites. During mammalian infection, T. cruzi alternates between an intracellular stage and extracellular stage. T. cruzi adapts its metabolism to this lifestyle, while also reshaping host metabolic pathways. Such host metabolic adaptations compensate for parasite-induced stress, but may promote parasite survival and proliferation. Recent work has demonstrated that metabolism controls parasite tropism and location of Chagas disease symptoms, and regulates whether infection is mild or severe. Such findings have important translational applications with regards to treatment and diagnostic test development, though further research is needed with regards to in vivo parasite metabolic gene expression, relationship between magnitude of local metabolic perturbation, parasite strain and disease location, and host-parasite-microbiota co-metabolism.
Introduction
Chagas disease (CD) is caused by the protozoan parasite Trypanosoma cruzi, with a global prevalence of 6 million. Transmission occurs through fecal matter from infected triatomine insects, congenital transmission, blood transfusion, organ transplantation, contaminated food, and laboratory exposure [1]. Acute disease is initiated when metacyclic trypomastigotes penetrate a new host. Trypomastigotes invade host cells and differentiate into proliferative amastigotes. Intracellular replication is followed by differentiation back to the trypomastigote stage and trypomastigote release. Additional infection sites are then established [2]. Left untreated, acute infection usually transitions into an asymptomatic indeterminate chronic stage. Over decades of infection, approximately one-third of T. cruzi infections progress to symptomatic, chronic CD with life-threatening cardiovascular manifestations (cardiomegaly, apical aneurysms, arrhythmias, heart failure) and/or megacolon and megaoesophagus. Thus, acute T. cruzi infection shows broad tissue tropism, while chronic CD is associated with disease tropism specifically to the heart, colon and oesophagus [1].
This review focuses on metabolic adaptations to mammalian infection from T. cruzi and host perspectives, and then on recent findings demonstrating that these adaptations regulate CD tolerance and parasite and CD tropism. Lastly, we discuss knowledge gaps and future research directions (Figure 1).
Figure 1.
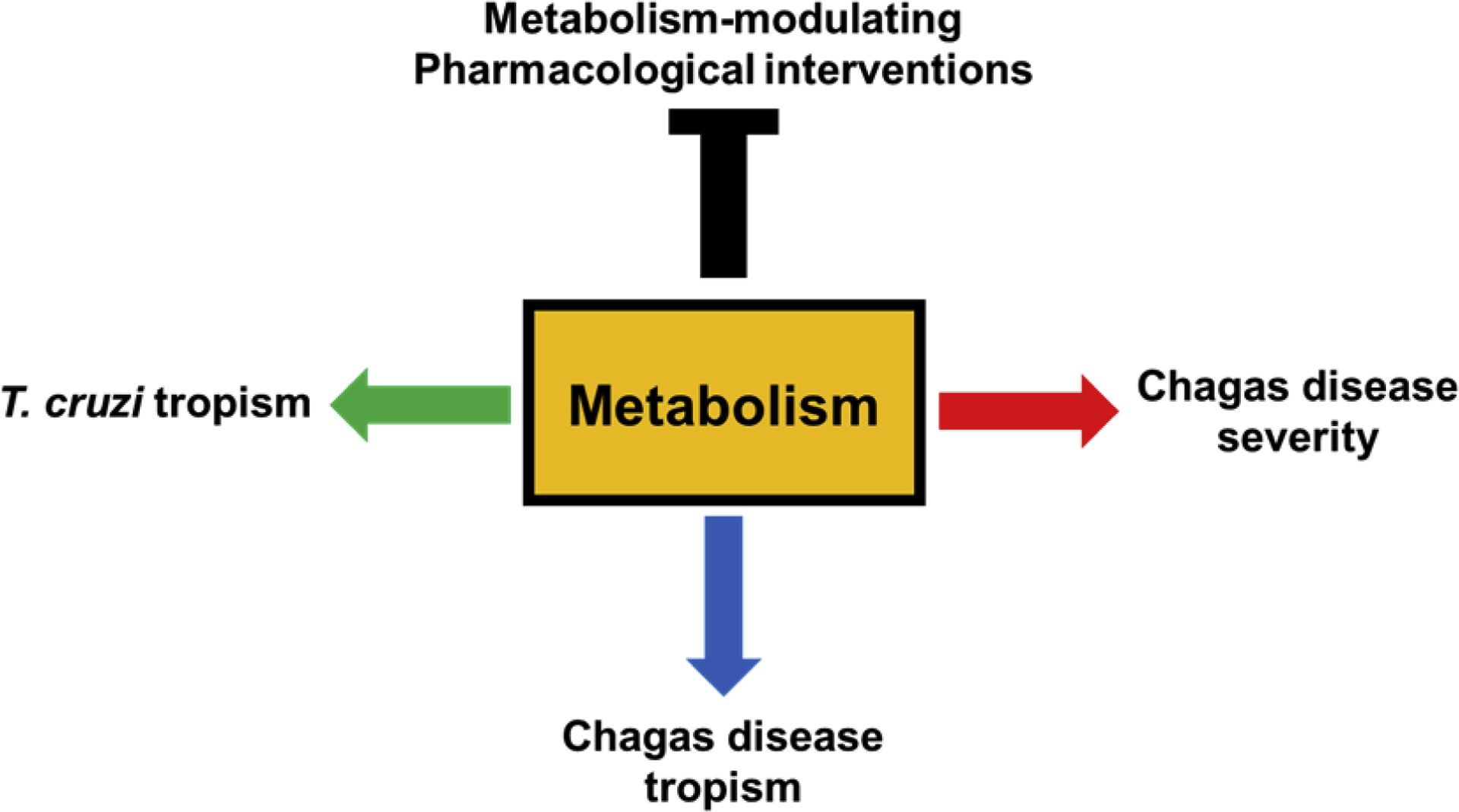
Intersection of metabolism, pathogen and disease tropism, and disease severity.
Metabolic adaptations to infection
The shift from an extracellular insect-stage lifestyle to a predominantly intracellular lifestyle in the mammalian host is associated with significant metabolic adaptations by T. cruzi [3][4]. Many of the insect stage (epimastigote)-elevated transcripts are associated with metabolism or nutrient uptake [4]. T. cruzi is able to sense nutrient availability and adapt its proliferation accordingly [5]. In vitro, at the mRNA level, amastigotes upregulate amino acid catabolism, fatty acid oxidation, oxidative phosphorylation, lipid and sterol biosynthesis, pyrimidine biosynthetic pathways, guanine salvage enzymes, and trypanothione antioxidant production compared to trypomastigotes or to timepoints early after host cell invasion [6][7][4]. Amastigotes supplement their intrinsic metabolic activities by scavenging host metabolites. A prominent example is parasite reliance on host purines [4], but amastigotes also scavenge host long chain fatty acids [8] and glucose [9]. However, these studies were performed during in vitro infection [6][7][4], with no data on how T. cruzi adapts on a metabolic level to infect different organs in vivo.
Host metabolic adaptations may represent health-promoting or pathological compensation for parasite nutrient usage, immune response metabolic control, or adaptations to tissue damage. Via metabolic control of immune pathways, glucose and the pentose phosphate pathway support antiparasitic reactive oxygen and nitrogen species production by infected macrophages stimulated with IFNɣ [10]. Genes related to mitochondria function were downregulated in in vitro-infected cardiomyocytes [11], and electron transport chain function was impaired [12]. Decreases in oxidative phosphorylation gene expression and electron transport chain function were also observed in mouse hearts [13][14][15][16] and in one human patient study [17], though this contrasts with reports of increased mitochondrial respiration in vitro in fibroblasts [9] and macrophages [18], and in other CD patient-derived samples [19]. These conflicting observations may reflect differences between in vitro and in vivo settings, between mRNA-based vs functional studies, and between timepoints and parasite strains. The increased oxidative metabolism in infected macrophages may help promote the production of antiparasitic nitric oxide [18]. Fatty acid synthesis was also decreased in acutely-infected mouse hearts [20][21]. Eliminating adipocytes worsens acute and chronic experimental CD, supporting a causative role of fatty acid storage in CD progression [22]. In addition, these metabolic changes may be impacting antiparasitic immune responses: fatty acid synthesis is required for Th17 differentiation [23], so that reduced fatty acid synthesis may impair parasite clearance and promote disease progression [24,25].
In contrast, T. cruzi benefits from host fatty acid beta-oxidation and amino acid catabolism [26], so that the observed reduction of fatty acid and amino acid catabolism at the mRNA level in acutely-infected [20] and chronically-infected [14] mouse hearts may slow parasite growth, though an increase in fatty acid catabolism gene expression was observed in one analysis of patient-derived samples [19]. On the other hand, T. cruzi-infected fibroblasts and myoblasts upregulate glucose uptake, but this favors T. cruzi proliferation [9]. Likewise, infected human foreskin fibroblasts upregulate tetrahydrobiopterin synthesis, lipid biosynthesis and mevalonate pathway gene expression. The latter has been postulated to be an adaptation to parasite sterol needs, or to mitigate reactive oxygen species produced in response to infection, while the former may help the parasite meet pterin needs [4]. Parasite reliance on host biopterin biosynthesis was confirmed via an RNAi screen, which also demonstrated reliance on host pyrimidine biosynthesis [26].
In vivo studies further revealed complex interactions between organs during infection, with variable metabolic adaptations depending on the organ system. For example, glucose uptake, sorbitol pathway and phospholipid synthesis were increased in heart tissue but decreased in plasma during acute T. cruzi Y strain infection [21]. Likewise, PC(20:4) was increased by acute infection with T. cruzi strain CL Brener (luciferase-expressing) in the oesophagus and large intestine, but decreased in the small intestine [27]. Acute infection was also associated with increased acylcarnitines in the oesophagus and small intestine, which persisted in the oesophagus during chronic infection, and elevated kynurenine in the large intestine at both disease stages [27]. Kynurenine is produced from tryptophan during inflammation and has immune-modulating properties. Kynurenine metabolites also directly inhibit T. cruzi growth and promote host survival in vivo during acute infection [28]. Cardiac glycerophosphocholines increased during mouse chronic infection with T. cruzi strains CL Brener and Sylvio X10/4, while infection depleted cardiac acylcarnitines [29]. This decrease in cardiac acylcarnitines may be an indicator of increased fatty acid beta oxidation flux, which may benefit T. cruzi [26]. The magnitude of cardiac phosphocholine perturbation was proportional to the levels of indicators of pro-fibrotic cytokines and to the magnitude of cardiac fibrosis and inflammation, in an independent chronic mouse infection model [30]. The representation of higher- vs lower-molecular-weight acylcarnitine and phosphatidylcholine family members also differed based on acute mouse T. cruzi infection severity [31]. Lastly, though T. cruzi is distal from sites of microbiota colonization [32], altered microbiota composition and metabolism has been observed during acute and chronic experimental infection [33][27].
Role of metabolism in T. cruzi tissue tropism and Chagas disease tropism
Acute-stage T. cruzi tropism is broad, while the gastrointestinal tract is the main site of chronic-stage T. cruzi persistence in mouse models, with increased parasite burden in the cecum during the transition from acute to chronic stage [34][32][27]. In the heart, parasite burden was highest at the heart base during acute mouse infection and chronic strain CL Brener infection [31]. Determinants of parasite tropism are still not fully understood, though immune mechanisms are certainly involved [31][34]. For example, lower parasite levels at the heart apex may be explained by higher antiparasitic IFNɣ at this site [31]. Metabolism may regulate parasite tropism directly due to parasite metabolic needs, or via regulation of immune responses. For example, eicosanoids are higher at the heart base and inhibit anti-T. cruzi immune responses [31]. Likewise, elevated colonic kynurenine may downregulate antiparasitic responses and contribute to parasite persistence in the large intestine during chronic infection [27]. Parasite tropism is also influenced by pleiotropic metabolic modulators such as diet, with high-fat diet altering parasitemia and cardiac vs adipose tissue parasite tropism, though contradictory effects have been reported [35][36].
CD also shows very specific disease tropism to the heart (especially heart apex), oesophagus and large intestine [1]. Cardiac specificity of damage may be related to higher mitochondrial dysfunction than in other organs [37]. Apical cardiac symptoms may also be due to the elevated apical IFNɣ, leading to immune-mediated tissue damage [31]. Sites of persistent metabolic alterations during chronic T. cruzi infection also concur with sites of CD symptoms. Chronic infection with strains CL Brener and Sylvio X10/4 was associated with significant metabolic alterations in lower and apical heart segments only, even though parasite burden in these regions is low [29]. Likewise, in the gastrointestinal tract during strain CL Brener infection, the magnitude of metabolic perturbation increased during the transition from acute to chronic stages in the oesophagus and the large intestine specifically, even as parasite load decreased [27]. Cardiac inflammation and fibrosis were greater in pericardial than endocardial heart segments, and the magnitude of infection-induced cardiac metabolic alterations was significantly correlated with inflammation in the pericardial segment only, in a mouse model [30]. These observations support a role of overall persistent metabolic alterations in determining the sites of chronic CD disease tropism, though studies with additional clinical isolates are needed.
Role of metabolism in Chagas disease tolerance
Disease tolerance is the mechanism by which better health outcomes are attained, without changes in pathogen load [38]. Such mechanisms have been under-studied in CD, even though only 30–40% of patients develop symptomatic chronic-stage disease [1]. Metabolism regulates CD tolerance: acute-stage treatment with carnitine prevented acute-stage mortality, with no effects on parasite load or immune system. Instead, carnitine treatment restored infection-induced cardiac and circulatory metabolic alterations, leading to improved cardiac function [27]. Likewise, treatment with metformin, a non-specific activator of the central metabolic regulator AMPK, improved functional cardiac parameters in chronic experimental CD without reducing parasite burden [39].
Future directions
There is a strong need for new treatments for CD, particularly those that can improve disease parameters in addition to clearing parasites [1]. The multiple host metabolic adaptations described above (e.g. [27][21][29]) may represent pathways that can be targeted, with proven success in the case of carnitine metabolism during acute infection [27]. The role of the local metabolic environment in treatment efficacy has also been under-studied. Drug penetration or metabolism differed between endocardial and pericardial heart ventricle regions [30]. Recent in vitro work has also revealed that lower glutamine availability reduces T. cruzi sensitivity to azoles [40]. Strikingly, glutamine levels are lowest in the large intestine [27], one of the sites of T. cruzi persistence following incomplete in vivo azole treatment [41]. More studies of the intersection between local metabolism and CD treatment efficacy are thus required.
Metabolic alterations predicted infection outcome in acute-stage mouse models [31]. Given the intersections between metabolic adaptations and CD severity described above, metabolic phenotyping may represent a new way to predict patient progression and treatment efficacy. Machine learning algorithms may be particularly useful in this context [42]. Deconvoluting disease-specific metabolic alterations from behavioral confounders may be facilitated by the fact that several common metabolic alterations were observed in infected mice receiving a regular or a high-fat diet, compared to uninfected controls [43].
Studies of T. cruzi metabolic adaptations to infection have also been restricted to in vitro settings. To fully comprehend parasite metabolic flexibility, studies of parasite metabolism across different organs and organ regions at acute and chronic timepoints are required. These will be facilitated by approaches to selectively amplify parasite mRNAs, such as spliced leader RNA-seq [44], and new methods to identify small parasite foci in infected tissues [32], which can then be followed by mass spectrometry imaging, single-cell RNA-seq and single-cell metabolomics approaches (e.g. [45][46][47][48][49]). New fluorescence and bioluminescent technologies are overcoming longstanding challenges of locating and visualizing infected host cells during chronic infection [32,50], enabling more detailed studies of parasite tropism in vivo. These techniques should also help reveal the role of uninfected but infection-adjacent cells in CD pathogenesis. Improved in vitro culture systems such as organoids [51] or organs-on-a-chip systems [52] may help bridge the gap between in vitro and in vivo settings.
Likewise, the role of the microbiome and particularly microbiome metabolism has been under-studied in CD. Several of the microbiota metabolites affected by infection are immunomodulators [33][27] and thus may contribute to parasite tropism and disease pathogenesis, though further studies are necessary.
Lastly, recent work has revealed interesting connections between local magnitude of metabolic perturbation, metabolome-level resilience and sites of CD tropism using select T. cruzi strains [27][29]. Further studies should apply these approaches to a broader variety of T. cruzi strains with differential clinical disease manifestations.
Conclusions
Overall, both compensatory and disease-promoting host metabolic alterations occur in response to T. cruzi infection, while T. cruzi adapts its own metabolism to thrive in the mammalian environment. The magnitude of local host metabolic alterations may explain sites of CD tropism, and metabolism regulates tolerance to T. cruzi infection. Building on these findings could lead to the next generation of CD treatments and to new ways to monitor CD progression.
Table 1.
Knowledge gaps.
| In situ T. cruzi metabolic adaptations to infecting different tissues |
| Role of microbiota metabolism in CD pathogenesis |
| Role of metabolic adaptations in uninfected but infection-adjacent cells in CD pathogenesis |
| Relationship between metabolic resilience and CD symptom localization, across clinical T. cruzi isolates |
| Cross-talk between immune system, localized metabolic perturbations, and CD tropism |
| Translatability of altered metabolic pathways into new CD treatments |
| Translatability of altered metabolic pathways into biomarkers of CD progression or treatment success |
| Role of the local metabolic environment in treatment failure |
Highlights.
Trypanosoma cruzi parasites and Chagas disease show specific tropism.
Metabolism is implicated in T. cruzi parasite tropism and Chagas disease tropism.
Several metabolic pathways have been implicated in Chagas disease pathogenesis.
Metabolic adaptations promote tolerance to T. cruzi infection.
Targeting these metabolic pathways may lead to new treatments for Chagas disease.
Acknowledgements
Work on Chagas disease metabolism in the McCall laboratory is supported by funding from the National Institutes of Health [grant number 1R21AI148886, R21AI156669]; and the PhRMA foundation [grant number 45188]. Laura-Isobel McCall, Ph.D. holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Nunes MCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverría LE, Dutra WO, Gascon J, Morillo CA, Oliveira-Filho J, et al. : Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement From the American Heart Association. Circulation 2018, 138:e169–e209. [DOI] [PubMed] [Google Scholar]
- [2].Lewis MD, Kelly JM: Putting Infection Dynamics at the Heart of Chagas Disease. Trends Parasitol 2016, 32:899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Minning TA, Weatherly DB, Atwood J 3rd, Orlando R, Tarleton RL: The steady-state transcriptome of the four major life-cycle stages of Trypanosoma cruzi. BMC Genomics 2009, 10:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li Y, Shah-Simpson S, Okrah K, Belew AT, Choi J, Caradonna KL, Padmanabhan P, Ndegwa DM, Temanni MR, Corrada Bravo H, et al. : Transcriptome Remodeling in Trypanosoma cruzi and Human Cells during Intracellular Infection. PLoS Pathog 2016, 12:e1005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dumoulin PC, Burleigh BA: Stress-Induced Proliferation and Cell Cycle Plasticity of Intracellular Amastigotes. MBio 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Houston-Ludlam GA, Trey Belew A, El-Sayed NM: Comparative Transcriptome Profiling of Human Foreskin Fibroblasts Infected with the Sylvio and Y Strains of Trypanosoma cruzi. PLOS ONE 2016, 11:e0159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Belew AT, Junqueira C, Rodrigues-Luiz GF, Valente BM, Oliveira AER, Polidoro RB, Zuccherato LW, Bartholomeu DC, Schenkman S, Gazzinelli RT, et al. : Comparative transcriptome profiling of virulent and non-virulent Trypanosoma cruzi underlines the role of surface proteins during infection. PLoS Pathog 2017, 13:e1006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gazos-Lopes F, Martin JL, Dumoulin PC, Burleigh BA: Host triacylglycerols shape the lipidome of intracellular trypanosomes and modulate their growth. PLoS Pathog 2017, 13:e1006800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shah-Simpson S, Lentini G, Dumoulin PC, Burleigh BA: Modulation of host central carbon metabolism and in situ glucose uptake by intracellular Trypanosoma cruzi amastigotes. PLoS Pathog 2017, 13:e1006747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Koo S-J, Szczesny B, Wan X, Putluri N, Garg NJ: Pentose Phosphate Shunt Modulates Reactive Oxygen Species and Nitric Oxide Production Controlling in Macrophages. Front Immunol 2018, 9:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Manque PA, Probst CM, Pereira MCS, Rampazzo RCP, Ozaki LS, Pavoni DP, Silva Neto DT, Ruth Carvalho M, Xu P, Serrano MG, et al. : Trypanosoma cruzi Infection Induces a Global Host Cell Response in Cardiomyocytes. Infection and Immunity 2011, 79:3471–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gupta S, Bhatia V, Wen J-J, Wu Y, Huang M-H, Garg NJ: Trypanosoma cruzi infection disturbs mitochondrial membrane potential and ROS production rate in cardiomyocytes. Free Radic Biol Med 2009, 47:1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Garg N, Popov VL, Papaconstantinou J: Profiling gene transcription reveals a deficiency of mitochondrial oxidative phosphorylation in Trypanosoma cruzi-infected murine hearts: implications in chagasic myocarditis development. Biochim Biophys Acta 2003, 1638:106–120. [DOI] [PubMed] [Google Scholar]
- [14].Soares MBP, de Lima RS, Rocha LL, Vasconcelos JF, Rogatto SR, dos Santos RR, Iacobas S, Goldenberg RC, Iacobas DA, Tanowitz HB, et al. : Gene expression changes associated with myocarditis and fibrosis in hearts of mice with chronic chagasic cardiomyopathy. J Infect Dis 2010, 202:416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wen J-J, Garg NJ: Mitochondrial complex III defects contribute to inefficient respiration and ATP synthesis in the myocardium of Trypanosoma cruzi-infected mice. Antioxid Redox Signal 2010, 12:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vyatkina G, Bhatia V, Gerstner A, Papaconstantinou J, Garg N: Impaired mitochondrial respiratory chain and bioenergetics during chagasic cardiomyopathy development. Biochim Biophys Acta 2004, 1689:162–173. [DOI] [PubMed] [Google Scholar]
- [17].Wen J-J, Yachelini PC, Sembaj A, Manzur RE, Garg NJ: Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Radic Biol Med 2006, 41:270–276. [DOI] [PubMed] [Google Scholar]
- [18].Koo S-J, Chowdhury IH, Szczesny B, Wan X, Garg NJ: Macrophages Promote Oxidative Metabolism To Drive Nitric Oxide Generation in Response to Trypanosoma cruzi. Infect Immun 2016, 84:3527–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cunha-Neto E, Dzau VJ, Allen PD, Stamatiou D, Benvenutti L, Higuchi ML, Koyama NS, Silva JS, Kalil J, Liew C-C: Cardiac gene expression profiling provides evidence for cytokinopathy as a molecular mechanism in Chagas’ disease cardiomyopathy. Am J Pathol 2005, 167:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ferreira LRP, Ferreira FM, Laugier L, Cabantous S, Navarro IC, da Silva Cândido D, Rigaud VC, Real JM, Pereira GV, Pereira IR, et al. : Integration of miRNA and gene expression profiles suggest a role for miRNAs in the pathobiological processes of acute Trypanosoma cruzi infection. Sci Rep 2017, 7:17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gironès N, Carbajosa S, Guerrero NA, Poveda C, Chillón-Marinas C, Fresno M: Global metabolomic profiling of acute myocarditis caused by Trypanosoma cruzi infection. PLoS Negl Trop Dis 2014, 8:e3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22] .Lizardo K, Ayyappan JP, Oswal N, Weiss LM, Scherer PE, Nagajyothi JF: Fat Tissue Regulates the Pathogenesis and Severity of Cardiomyopathy in Murine Chagas Disease. PLoS Negl Trop Dis 2021, 15: e0008964. [DOI] [PMC free article] [PubMed] [Google Scholar]; •Demonstration of a causative role of lipid storage in Chagas disease progression using an innovative fat ablation technique.
- [23].Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, Sandouk A, Hesse C, Castro CN, Bähre H, et al. : De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nature Medicine 2014, 20:1327–1333. [DOI] [PubMed] [Google Scholar]
- [24].Magalhães LMD, Villani FNA, Nunes M do CP, Gollob KJ, Rocha MOC, Dutra WO: High interleukin 17 expression is correlated with better cardiac function in human Chagas disease. J Infect Dis 2013, 207:661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Guedes PM da M, da Matta Guedes PM, Gutierrez FRS, Maia FL, Milanezi CM, Silva GK, Pavanelli WR, Silva JS: IL-17 Produced during Trypanosoma cruzi Infection Plays a Central Role in Regulating Parasite-Induced Myocarditis. PLoS Neglected Tropical Diseases 2010, 4:e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Caradonna KL, Engel JC, Jacobi D, Lee C-H, Burleigh BA: Host metabolism regulates intracellular growth of Trypanosoma cruzi. Cell Host Microbe 2013, 13:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hossain E, Khanam S, Dean DA, Wu C, Lostracco-Johnson S, Thomas D, Kane SS, Parab AR, Flores K, Katemauswa M, et al. : Mapping of host-parasite-microbiome interactions reveals metabolic determinants of tropism and tolerance in Chagas disease. Sci Adv 2020, 6:eaaz2015. [DOI] [PMC free article] [PubMed] [Google Scholar]; ••Demonstrated correspondence between sites of persistent metabolic alteration and Chagas disease tropism, and identified a new role for acylcarnitine metabolism in Chagas disease tolerance.
- [28].Knubel CP, Martínez FF, Fretes RE, Díaz Lujan C, Theumer MG, Cervi L, Motrán CC: Indoleamine 2,3-dioxigenase (IDO) is critical for host resistance against Trypanosoma cruzi. FASEB J 2010, 24:2689–2701. [DOI] [PubMed] [Google Scholar]
- [29].Dean DA, Gautham, Siqueira-Neto JL, McKerrow JH, Dorrestein PC, McCall L-I: Spatial metabolomics identifies localized chemical changes in heart tissue during chronic cardiac Chagas disease. bioRxiv 2020, doi: 10.1101/2020.06.29.178038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hoffman K, Liu Z, Hossain E, Bottazzi ME, Hotez PJ, Jones KM, McCall L-I: Alterations to the cardiac metabolome induced by chronic T. cruzi infection relate to the degree of cardiac pathology. ACS Infect Dis 2021, 7:1638–49 [DOI] [PMC free article] [PubMed] [Google Scholar]; •Strengthened the link between metabolic changes and disease pathogenesis, by correlating magnitude of metabolic changes with indicators of disease severity. Findings of differential anesthetic drug levels between cardiac regions and between infected and uninfected animals support further analyses of detailed tissue drug distribution during T. cruzi infection, with implications for understanding the failure of existing treatments and to guide drug development.
- [31].McCall L-I, Morton JT, Bernatchez JA, de Siqueira-Neto JL, Knight R, Dorrestein PC, McKerrow JH: Mass Spectrometry-Based Chemical Cartography of a Cardiac Parasitic Infection. Anal Chem 2017, 89:10414–10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ward AI, Lewis MD, Khan AA, McCann CJ, Francisco AF, Jayawardhana S, Taylor MC, Kelly JM: Analysis of Trypanosoma cruzi Persistence Foci at Single-Cell Resolution. MBio 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]; •Fine mapping of infected cells, revealing parasite persistence specifically in the colon circular smooth muscle layer during chronic mouse infection. This technique is a key first step towards detailed understanding of local infection-induced changes.
- [33].McCall L-I, Tripathi A, Vargas F, Knight R, Dorrestein PC, Siqueira-Neto JL: Experimental Chagas disease-induced perturbations of the fecal microbiome and metabolome. PLoS Negl Trop Dis 2018, 12:e0006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lewis MD, Fortes Francisco A, Taylor MC, Burrell-Saward H, McLatchie AP, Miles MA, Kelly JM: Bioluminescence imaging of chronic Trypanosoma cruzi infections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cell Microbiol 2014, 16:1285–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nagajyothi F, Weiss LM, Zhao D, Koba W, Jelicks LA, Cui M-H, Factor SM, Scherer PE, Tanowitz HB: High fat diet modulates Trypanosoma cruzi infection associated myocarditis. PLoS Negl Trop Dis 2014, 8:e3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Figueiredo VP, Junior ESL, Lopes LR, Simões NF, Penitente AR, Bearzoti E, Vieira PM de A, Schulz R, Talvani A: High fat diet modulates inflammatory parameters in the heart and liver during acute Trypanosoma cruzi infection. Int Immunopharmacol 2018, 64:192–200. [DOI] [PubMed] [Google Scholar]
- [37].Wen J-J, Dhiman M, Whorton EB, Garg NJ: Tissue-specific oxidative imbalance and mitochondrial dysfunction during Trypanosoma cruzi infection in mice. Microbes Infect 2008, 10:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schneider DS, Ayres JS: Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol 2008, 8:889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vilar-Pereira G, Carneiro VC, Mata-Santos H, Vicentino ARR, Ramos IP, Giarola NLL, Feijó DF, Meyer-Fernandes JR, Paula-Neto HA, Medei E, et al. : Resveratrol Reverses Functional Chagas Heart Disease in Mice. PLoS Pathog 2016, 12:e1005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dumoulin PC, Vollrath J, Tomko SS, Wang JX, Burleigh B: Glutamine metabolism modulates azole susceptibility in amastigotes. Elife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]; ••An important demonstration of the role of the surrounding metabolic environment in determining T. cruzi drug susceptibility, with major implications with regards to causes of CD treatment failure.
- [41].Francisco AF, Lewis MD, Jayawardhana S, Taylor MC, Chatelain E, Kelly JM: Limited Ability of Posaconazole To Cure both Acute and Chronic Trypanosoma cruzi Infections Revealed by Highly Sensitive In Vivo Imaging. Antimicrob Agents Chemother 2015, 59:4653–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Poss AM, Maschek JA, Cox JE, Hauner BJ, Hopkins PN, Hunt SC, Holland WL, Summers SA, Playdon MC: Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J Clin Invest 2020, 130:1363–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lizardo K, Ayyappan JP, Ganapathi U, Dutra WO, Qiu Y, Weiss LM, Nagajyothi JF: Diet Alters Serum Metabolomic Profiling in the Mouse Model of Chronic Chagas Cardiomyopathy. Dis Markers 2019, 2019:4956016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cuypers B, Domagalska MA, Meysman P, Muylder G de, Vanaerschot M, Imamura H, Dumetz F, Verdonckt TW, Myler PJ, Ramasamy G, et al. : Multiplexed Spliced-Leader Sequencing: A high-throughput, selective method for RNA-seq in Trypanosomatids. Sci Rep 2017, 7:3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pan N, Rao W, Kothapalli NR, Liu R, Burgett AWG, Yang Z: The single-probe: a miniaturized multifunctional device for single cell mass spectrometry analysis. Anal Chem 2014, 86:9376–9380. [DOI] [PubMed] [Google Scholar]
- [46].Rappez L, Stadler M, Triana S, Gathungu RM, Ovchinnikova K, Phapale P, Heikenwalder M, Alexandrov T: SpaceM reveals metabolic states of single cells. Nature Methods 2021, 18: 799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]; •New analytical approach that could enable analyses of the local effects of infection on individual host cells and of bystander effects.
- [47].Passarelli MK, Pirkl A, Moellers R, Grinfeld D, Kollmer F, Havelund R, Newman CF, Marshall PS, Arlinghaus H, Alexander MR, et al. : The 3D OrbiSIMS—label-free metabolic imaging with subcellular lateral resolution and high mass-resolving power. Nature Methods 2017, 14:1175–1183. [DOI] [PubMed] [Google Scholar]
- [48].Steuerman Y, Cohen M, Peshes-Yaloz N, Valadarsky L, Cohn O, David E, Frishberg A, Mayo L, Bacharach E, Amit I, et al. : Dissection of Influenza Infection In Vivo by Single-Cell RNA Sequencing. Cell Syst 2018, 6:679–691.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Perry WJ, Spraggins JM, Sheldon JR, Grunenwald CM, Heinrichs DE, Cassat JE, Skaar EP, Caprioli RM: Staphylococcus aureus exhibits heterogeneous siderophore production within the vertebrate host. Proc Natl Acad Sci U S A 2019, 116:21980–21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Taylor MC, Ward AI, Olmo F, Francisco AF, Jayawardhana S, Costa FC, Lewis MD, Kelly JM: Bioluminescent:fluorescent Trypanosoma cruzi Reporter Strains as Tools for Exploring Chagas Disease Pathogenesis and Drug Activity. Curr Pharm Des 2020, doi: 10.2174/1381612826666201124113214. [DOI] [PubMed] [Google Scholar]
- [51].Garzoni LR, Adesse D, Soares MJ, Rossi MID, Borojevic R, de Nazareth Leal de Meirelles M: Fibrosis and Hypertrophy Induced by Trypanosoma cruzi in a Three-Dimensional Cardiomyocyte-Culture System. J Infect Dis 2008, 197:906–915. [DOI] [PubMed] [Google Scholar]
- [52].Steinway SN, Saleh J, Koo B-K, Delacour D, Kim D-H: Human Microphysiological Models of Intestinal Tissue and Gut Microbiome. Front Bioeng Biotechnol 2020, 8:725. [DOI] [PMC free article] [PubMed] [Google Scholar]


