Abstract
Background:
Prevalence rates of opioid use disorder (OUD) have increased dramatically, accompanied by a surge of overdose deaths. While opioid dependence has been extensively studied in preclinical models, an understanding of the biological alterations that occur in the brains of people who chronically use opioids and who are diagnosed with OUD remains limited. To address this limitation, RNA-sequencing (RNA-seq) was conducted on the dorsolateral prefrontal cortex (DLPFC) and nucleus accumbens (NAc), regions heavily implicated in OUD, from postmortem brains in subjects with OUD.
Methods:
We performed RNA-seq on the DLPFC and NAc from unaffected comparison subjects (n=20) and subjects diagnosed with OUD (n=20). Our transcriptomic analyses identified differentially expressed (DE) transcripts and investigated the transcriptional coherence between brain regions using rank-rank hypergeometric ordering (RRHO). Weighted gene co-expression analyses (WGCNA) also identified OUD-specific modules and gene networks. Integrative analyses between DE transcripts and GWAS datasets using linkage disequilibrium score (LDSC) assessed the genetic liability psychiatric-related phenotypes.
Results:
RRHO analyses revealed extensive overlap in transcripts between DLPFC and NAc in OUD, primarily relating to synaptic remodeling and neuroinflammation. Identified transcripts were enriched for factors that control pro-inflammatory cytokine-mediated, chondroitin sulfate, and extracellular matrix signaling. Cell-type deconvolution implicated a role for microglia as a potential driver for opioid-induced neuroplasticity. LDSC analysis suggested genetic liabilities for risky behavior, attention deficit hyperactivity disorder, and depression in subjects with OUD.
Conclusions:
Overall, our findings suggest connections between the brain’s immune system and opioid dependence in the human brain.
Introduction
Prevalence of opioid use disorder (OUD) and deaths (1) has spurred efforts to develop new treatments for OUD. This requires better understanding of brain alterations in those who develop dependence.
Impulsivity and deficits in cognition are hallmarks of OUD (2) and have been attributed to functional alterations in corticostriatal circuits in dorsolateral prefrontal cortex (DLPFC) and nucleus accumbens (NAc) (2, 3). Moreover, history of substance use is associated with corticostriatal circuit dysfunction contributing to cognitive impairment and risky behavior (4). However, we still have limited understanding of molecular alterations due to chronic opioid use and OUD that occur in these circuits in human brain.
Although few studies examined postmortem brains in subjects with OUD, the approach may uncover therapeutically viable pathways in opioid dependence. Previous work reported changes in opioid receptor expression in DLPFC (5-7) and altered expression of regulatory machinery for glutamate release in NAc (8, 9), potentially related to addiction severity in heroin users. Preclinical evidence corroborated these findings by demonstrating unique interactions between opioid and glutamate receptor signaling in opioid withdrawal and dependence (10-12). Nevertheless, deeper knowledge into the molecular alterations by chronic opioid use in the human DLPFC and NAc is limited.
We aimed to establish a more comprehensive understanding of the molecular changes across DLPFC and NAc in brains from subjects who were chronic opioid users also diagnosed with OUD. We used multiple levels of analysis by integrating transcriptomics across brain regions with traits related to OUD vulnerability using GWAS. Between DLPFC and NAc, we found remarkable overlap in both upregulated and downregulated transcripts. Further investigation into these overlapping transcripts revealed pathways enriched for factors controlling formation and degradation of extracellular matrix (ECM) and pro-inflammatory cytokine-mediated signaling. These pathways implicate neuroinflammation as a potential driver of ECM remodeling and synaptic reorganization, processes critical for opioid-induced neuroplasticity (13). Our analyses suggest microglia may play a role in OUD effects in both the DLPFC and NAc. Finally, we found links between neuroinflammation and OUD, and associations with a genetic liability to risky behavior using GWAS. Our findings suggest novel genetic and molecular changes in subjects with OUD that may contribute to opioid dependence.
Materials and Methods
Detailed procedures are provided in Supplementary Methods.
Human Subjects
Brains were obtained during routine autopsies conducted by the Office of the Allegheny County of the Medical Examiner (Pittsburgh, PA) after consent was obtained from next-of-kin. Procedures were approved by the University of Pittsburgh’s Committee for Oversight of Research and Clinical Training Involving Decedents and Institutional Review Board for Biomedical Research. Each subject meeting diagnostic criteria for OUD at time of death (n=20) was matched with one unaffected comparison subject (n=20) for sex and as closely as possible for age (Table 1; Table S1). Subjects with OUD had a diagnosis duration for at least five years (5-18 years since diagnosis, Table S1). DLPFC (area 9) and NAc were identified on fresh-frozen right hemisphere coronal tissue blocks using anatomical landmarks (14), and tissue (~50mg) was collected via cryostat, using an approach that minimizes contamination from white matter and other striatal subregions and ensures RNA preservation (15, 16).
Table 1.
Subject summary demographic and tissue characteristics
| Characteristic | Unaffected comparison (n=20) |
Opioid Use Disorder (n=20) |
|---|---|---|
| Age | 47.3 ± 9.5 | 46.9 ± 7.3 |
| Sex | 10M 10F | 10M 10F |
| Race | 13W 7B | 19W 1B |
| PMI (hours) | 15.7 ± 6.1 | 16.0 ± 5.3 |
| Brain pH | 6.6 ± 0.3 | 6.4 ± 0.2 |
| RIN | 8.0 ± 0.7 | 7.8 ± 0.7 |
| TOD (Time of death) | 6.16 ± 6.28 | 7.72 ± 7.42 |
| Tissue Storage Time (months) | 100.3 ± 86.1 | 103.0 ± 59.7 |
Values are mean ± SD. M, male; F, female; B, black; W, white
RNA-sequencing analyses
Differential expression (DE) was assessed using limma with covariate selection (17). Transcripts with corrected p<0.01 and log2FC>±0.26 (i.e., FC±1.2 or 20% expression change) were considered DE (18-23). The top 250 DE transcripts were ordered by log2FC for unsupervised subject clustering. MetaseqR (v3.11) determined transcript biotypes (24) and brain region enrichment using Log odds ratios and chi-square tests. Pathway overrepresentation was assessed using Metascape (GO, KEGG, Hallmark, Canonical Pathways, Reactome, BioCarta, CORUM; http://www.metascape.org) with expressed transcripts as background and with Gene Set Enrichment Analysis (GSEA; GO, KEGG) with genes ranked by −log10(pvalue) multiplied by the sign of the fold change. Networks were visualized with Cytoscape. INGENUITY® Pathway Analysis (Qiagen) and HOMER (v4.11) (25) was used to predict upstream regulators of DE transcripts. Rank-rank hypergeometric overlap (RRHO) (26, 27) was used to assess overlap of DE transcripts (p<0.01 in both regions). No batch effects for RNA-seq runs were found (p>0.05; Fig.S1). Data is available in GEO (GSE174409).
Identification of OUD-specific co-expression networks
We used weighted gene co-expression network analysis (WGCNA) to identify gene modules across samples (28, 29). Module differential connectivity (MDC) was used to quantify differences in co-expression within modules between OUD and unaffected comparison subjects. Fisher’s exact test determined whether DE transcripts were enriched within WGCNA modules. ARACNe was used to identify hub and OUD-specific hub genes for network analysis (30) and Cytoscape was used to visualize networks. Pathway overrepresentation categories for each module was assessed using Metascape, with the 5000 WGNCA-analyzed genes as background.
Cell type-specific DE analysis
To estimate cell-type fractions from bulk RNA-seq, Digital Sorting Algorithm (31) deconvolved astrocytes, endothelial cells, microglia, neurons, and oligodendrocytes from BRETIGEA (32). Unaffected comparison and OUD subjects were compared for cell-type enrichment using hypergeometric t-tests and cell type-specific DE analysis using CellDMC (33) adjusted for brain region (FDR<0.05). AUCell was used as an independent approach to compare DE transcripts in OUD to single-nuclei RNA-seq datasets in human postmortem brain (34, 35).
Integration of DE transcripts with GWAS
Region-specific differentially upregulated and downregulated transcripts (corrected p<0.01) constructed foregrounds for GWAS enrichment. The partitioned heritability linkage disequilibrium (LD) score regression pipeline calculated GWAS enrichment for brain region-specific noncoding regions containing and surrounding OUD transcript sets (36, 37). LD co-efficients were adjusted for FDR<0.1 on all GWAS.
Results
Enrichment of DE transcripts involved in neuroinflammation and extracellular matrix remodeling in DLPFC and NAc in OUD
We determined transcriptional differences by brain region in unaffected comparison subjects, finding unique transcriptional profiles in DLPFC and NAc (Fig.S2A-C; Data file S1). Established transcripts for cortex (e.g., BDNF (p<0.005); SLC17A7 (p<0.005)) and striatum (e.g., DRD1 (p<10−10), PDYN (p<10−9), PENK (p<10−6), PPP1R1B (p<10−7)) were enriched (Fig.S2D). Transcripts enriched in DLPFC were related to axon guidance and chemotaxis (Fig.S2E), versus NAc transcripts related to signal release, ion transmembrane transport, and cellular drug response (Fig.S2F). Threshold-free GSEA analyses yielded mostly overlapping pathways, with the DLPFC being enriched in pathways related to synapses and ion channels (Fig.S3) and the NAc being enriched in pathways related to translation (Fig.S4).
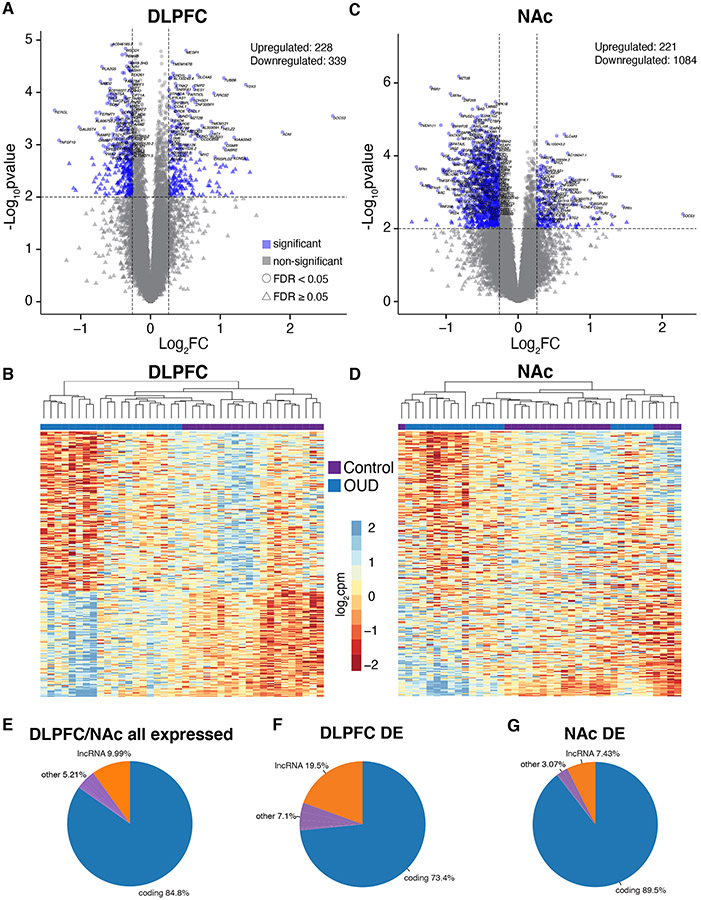
We investigated impact of OUD on region-specific transcriptional differences, finding profound effects on transcripts in DLPFC and NAc. High numbers of DE transcripts were present in both DLPFC (567) (Data file S2) and NAc (1306) (Data file S3; Table S2). The DLPFC DE volcano plot showed even distribution of downregulated (339) and upregulated (228) transcripts in OUD (Table S2, Fig.1A, Data file S2). Unaffected comparison subjects were differentiated from OUD subjects by unsupervised clustering based on the expression of the top 250 DE transcripts (log2FC) in DLPFC (Fig.1B). The NAc DE volcano plot for NAc showed many DE transcripts were downregulated (1085 versus 221 upregulated transcripts) in OUD (Fig.1C, Data file S3). Unsupervised clustering of subjects based on top 250 NAc DE transcripts identified subgroups in unaffected and OUD subjects (Fig.1D). Principal components analysis identified five subjects with OUD clustered together with four unaffected subjects (Fig.S5); each of these subjects was diagnosed with an inflammatory disease (e.g., asthma, arthritis; Table S1). However, another three subjects from the unaffected comparison cohort also had inflammatory disease history but did not cluster with the other subjects in inflammatory diagnoses (Fig.S5). This suggests inflammatory disorders do not drive NAc expression differences in OUD.
Fig. 1. Transcriptomic changes in DLPFC and NAc from OUD subjects.
A. Log2FC plotted relative to −log10p-value by volcano plot for differentially expressed (DE) transcripts in DLFPC. Horizontal dashed lines represent p-value significance cutoff of corrected p<0.01, while vertical dashed lines represent log2FC cutoffs of ≤−0.26 or ≥0.26 (FC≥1.2). Blue triangles represent DE transcripts that reach significance, log2FC, and FDR<0.05 cutoffs. B. Heatmap of the top 250 DE transcripts (corrected p<0.01 and log2FC of ≤−0.26 or ≥0.26) clustered by transcript and subject. Each column represents a subject (unaffected comparison, purple; OUD, blue). Subjects within groups cluster together. C. Volcano plot for DE transcripts in NAc. Note the large numbers of transcripts that are significantly reduced in expression compared to transcripts that are increased. D. Heatmap of the top 250 DE transcripts clustered by transcript and subject. Overall, subjects within groups cluster together with several groups of subjects forming separate clusters. E. Biotypes of all expressed transcripts in DLPFC and NAc in subjects with OUD. As expected, most of the transcripts encode protein coding genes. F. Biotypes of DE transcripts (corrected p<0.01 and log2FC of ≤−0.26 or ≥0.26) in DLPFC. Protein coding genes represent the majority of DE transcripts (blue; 73.4%) followed by lncRNAs (orange; 19.5%). G. Biotypes of DE transcripts in NAc. Protein coding genes represent the majority of DE transcripts (blue; 89.5%) followed by lncRNAs (orange; 7.43%).
Most transcripts expressed in DLPFC and NAc were protein-coding and long noncoding RNAs (lncRNAs) (84.8% protein-coding, 9.99% lncRNAs; Fig.1E). lncRNAs were overrepresented among DE transcripts (p<10−13) in DLPFC (19.5% lncRNAs, 73.4% protein-coding; Fig.1F; Table S3), but not NAc (7.43% lncRNAs, 89.5% protein-coding; Fig.1G; Table S4). Threshold and threshold-free analyses revealed pathways associated with inflammation in both DLPFC and NAc (Supplementary Results, Fig.S6-S11). Other pathways included chondroitin/dermatan sulfate metabolism and synapse organization in NAc, suggesting links between ECM remodeling, microglial cell migration, and synaptic plasticity, in line with recent work (38).
High transcriptional coherence between DLPFC and NAc converges on neuroinflammatory and extracellular matrix pathways in OUD
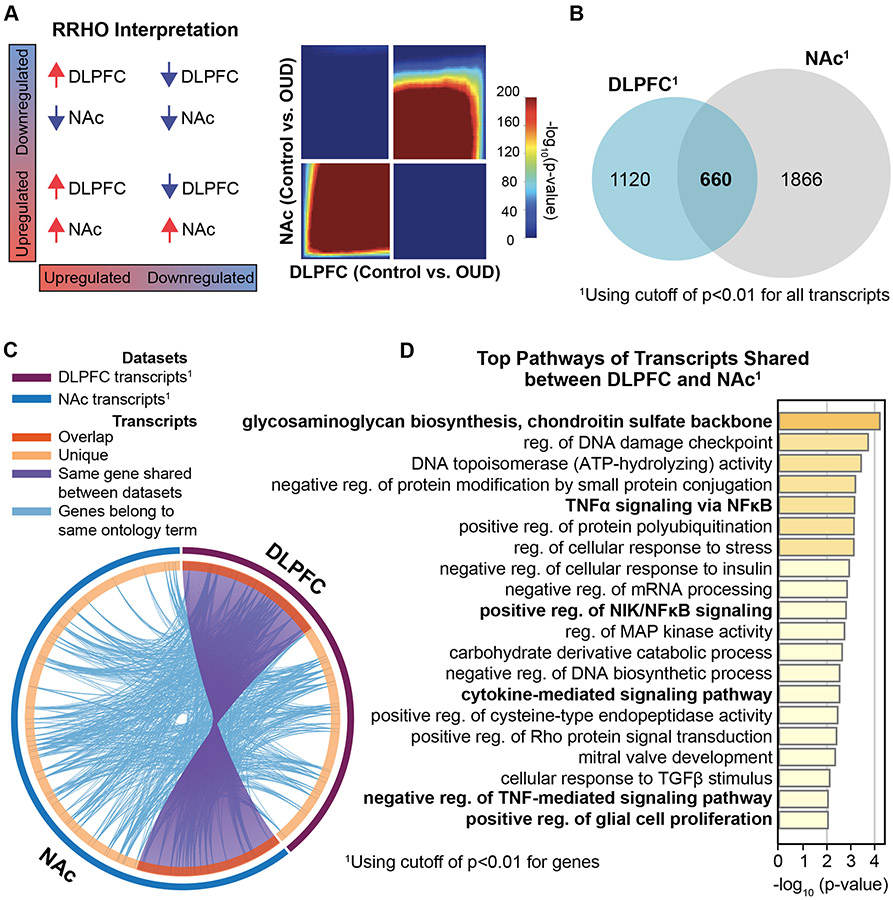
Since pathways were largely similar in OUD between brain regions, we explored the extent of transcriptional overlap between DLPFC and NAc using rank-rank hypergeometric ordering (39, 40), which orders transcripts by effect size direction and p-value. Substantial overlap in both upregulated and downregulated transcripts was found between DLPFC and NAc (Fig.2A-C, Fisher’s exact test, p<10−6; Fig.S12). Such analyses may provide insight into functional alterations across DLPFC-NAc circuits (2, 13, 41-44). Given the extent of overlap, pathway enrichment was conducted on DE transcripts shared between regions. Top shared pathways included those related to ECM (e.g., biosynthesis of glycosaminoglycans and chondroitin sulfate) and inflammation (e.g., cytokine-mediated immune signaling via tumor necrosis factor alpha (TNFα) and nuclear factor kappa B (NFκB) (Fig.2D). Other pathways were associated with cellular stress response (e.g., DNA damage repair), and epigenetic protein/histone modifications {e.g., ubiquitination and acetylation) (Fig.2D, Fig.S13).
Fig. 2. High transcriptional concordance reveals commonly altered molecular pathways between brain regions associated with opioid dependence.
A. Rank rank hypergeometric overlap (RRHO) plot indicating high degree of overlap, or transcriptional concordance, between DLPFC and NAc in OUD subjects. B. Venn diagram of differentially expressed (DE) transcripts between DLPFC and NAc. C. DE transcripts (purple lines) and their ontology (light blue lines) highly overlapped (orange) between DLPFC and NAc. D. Top 20 pathways of DE transcripts shared between DLPFC and NAc. Many of these pathways are related to extracellular matrix and neuroinflammatory signaling.
Given the association with epigenetic regulation, we investigated enrichment of chromatin states by comparing transcription start sites from DE transcripts in OUD to genome-wide maps of epigenetic modifications previously defined in postmortem brains from unaffected subjects (45). Transcription start sites of DE transcripts in DLPFC were enriched for genomic regions marked by weak polycomb repression (Fig.S14A, p<10−5). Weak polycomb repression diminishes histone marks (e.g., H3K27me3), which would otherwise inhibit transcription at the start site (45). Relative to unaffected subjects, enrichment of upregulated DLPFC DE transcripts in OUD suggests opioids induce activation of repressed genomic areas, promoting transcription (Fig.S14). In NAc, DE transcripts were enriched for genomic regions marked for quiescent states (Fig.S14A; p<10−6). Quiescent states are characterized by histone marks linked to transcriptional inactivity (45). Such findings suggest opioids repress genomic regions, resulting in downregulation of specific transcripts in NAc (Fig.S14B). Overall, these findings reflect a fundamental disturbance of chromatin states and transcriptional regulation by opioids relative to baseline epigenetic states.
Upregulation of microglia markers in OUD
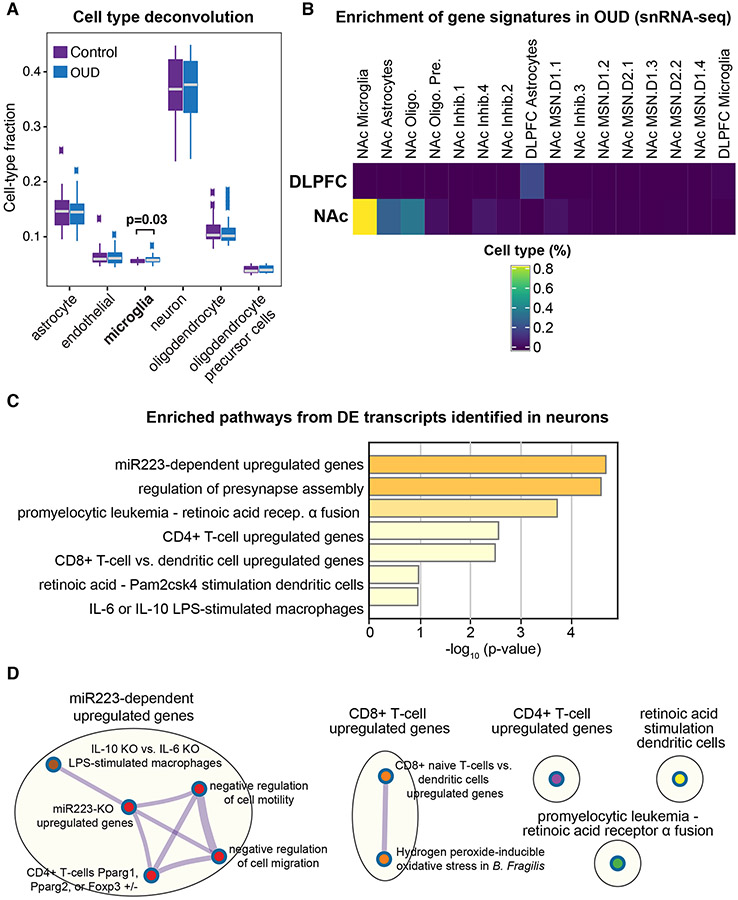
By leveraging the high transcriptional overlap between DLPFC and NAc, we identified specific DE transcripts across cell types using deconvolution of astrocytes, endothelial cells, microglia, neurons, and oligodendrocytes and oligodendrocyte precursor cells (33). Compared to unaffected subjects, markers for microglial fractions were significantly different in OUD (p=0.03; Fig.3A). We next conducted cell type-specific DE analysis in DLPFC and NAc by identifying DE transcripts in each cell type between unaffected and OUD subjects. Using brain region as a covariate, we detected a single DE transcript associated with microglia, HSD17B14 (Table S4). In microglia, reduced expression of HSD17B14 has been shown to augment pro-inflammatory glial activation (46). We independently validated microglial enrichment of transcriptional signatures using single-nuclei RNA-seq data from human postmortem brain (35) (Fig.3B). Our findings collectively support neuroinflammatory processes in DLPFC and NAc in OUD.
Fig. 3. Predicted microglial cell-type enrichment in both DLPFC and NAc.
A. Deconvolution analyses indicates modest difference of microglia fractions in DLPFC and NAc of OUD subjects (p<0.03; t-test controlling for brain region), consistent with enrichment of pathways in differentially expressed (DE) transcripts related to immune function. B. Using AUCell, gene signatures for DE transcripts in DLPFC and NAc in subjects with OUD was compared to signatures in various cell types from single nuclei RNA-sequencing datasets in human postmortem brains. Percent of cell type were significant in NAc microglia and DLPFC astrocytes, consistent with enrichment of transcripts related to immune function. C. Pathway enrichment analysis on the significantly altered DE transcripts in neurons identified pathways related to inflammation. D. Significantly enriched terms based on pathways included in multiple annotated sets (e.g., GO, KEGG, hallmark, etc.) using hypergeometric p-values and enrichment factors. The network is visualized with Cytoscape using Community cluster with categorical labels representing multiple clusters. Individual nodes are also labeled. Note nodes are related to presynaptic structure and function and miR233-dependent regulation of macrophage and T-cell activation. Oligo., oligodendrocytes; Oligo. Pre., oligodendrocyte precursor cells; Inhib., types of inhibitory neurons; MSN, types of medium spiny neurons, including D1 or D2, dopamine receptor subtype 1 or 2, respectively.
Focusing on transcript expression in neurons, we identified 25 DE transcripts significantly enriched for pathways related to neuroimmune signaling between neurons and local inflammatory cells (e.g., microglia) (Fig.3C, Table S5). We identified several upregulated transcripts controlled by a single microRNA (miR), miR223 (Fig.3C). miR223 is a crucial modulator of macrophage activation (47), further implicating microglia in the regulation of neuroimmune responses in OUD. A broader view indicated that immune-related pathways were largely distinct between microglia and other immune cell types including CD4+ and CD8+ T-cells (Fig.3D). This highlights the possibility of interactions between neurons and other immune cells in brains in OUD. Lastly, we also found strong enrichment of DE transcripts with synapse-related pathways (Fig.S15).
Increased connectivity of neuroinflammatory and extracellular matrix signaling gene modules in OUD
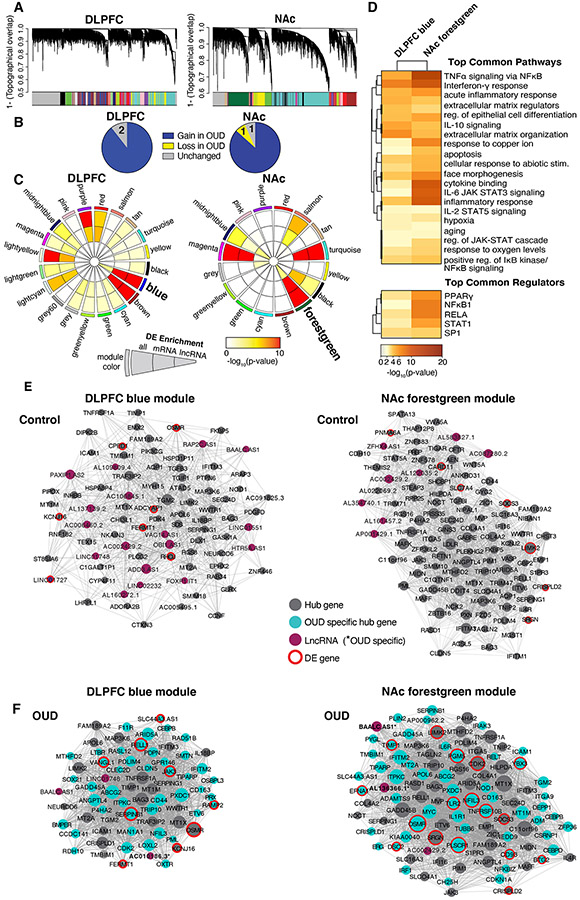
We used WGCNA to investigate correlations among transcripts in both unaffected comparison and OUD subjects. WGCNA identified 17 co-expression modules in DLPFC and 15 modules in NAc (Fig.4A). To identify OUD-specific modules, we compared network connectivity of each module between OUD and unaffected subjects. More coordinated expression of transcripts in unaffected subjects relative to OUD subjects indicates a ‘loss’ of connectivity in OUD. Conversely, a module that gains connectivity in OUD is more coordinated in OUD compared to unaffected subjects. In DLPFC, 15/17 modules gained connectivity and two remained unchanged in OUD subjects (Fig.4B). In NAc, 13/15 modules gained connectivity, while one module gained connectivity and one remained unchanged in OUD subjects (Fig.4B). These findings indicate that overall module structures were largely distinct between OUD and unaffected subjects.
Fig. 4. OUD associated gene networks in DLPFC and NAc.
A. Weighted gene co-expression network analysis (WGCNA) was used to generate co-expression modules, with the network structure generated on each brain region separately. The identified modules that survived module preservation analysis were arbitrarily assigned colors and the dendrogram shows average linkage hierarchical clustering of genes. B. Pie charts summarize results from the module differential connectivity (MDC) analysis compared OUD to unaffected comparison subjects. The majority of modules gained in OUD subjects, revealing OUD-specific modules in both DLPFC and NAc. C. Circos plot identified by module names and colors. Enrichment for full list of differentially expressed (DE) transcripts, protein-coding (mRNA), and long non-coding RNAs (lncRNA), are indicated by semi-circle colors within each module, with increasing warm colors indicating increasing −log10 p-value. LncRNA enrichment was examined based on the high prevalence of these transcripts in the Biotype analysis (see Fig.1F,G). MDC analysis indicated a gain of connectivity in the DLPFC blue module and NAc forestgreen module in OUD. These modules were also enriched for DE mRNA and lncRNAs. D. Pathway enrichment analysis compared gene networks within the DLPFC blue module and NAc forestgreen module. Warmer colors indicate increasing −log10 p-value and highly shared pathways between the modules. Hub gene co-expression networks of the DLPFC blue module E. unaffected comparison subjects and F. OUD subjects, and networks of the forestgreen module in NAc E. unaffected comparison subjects and F. OUD subjects. Node size indicates the degree of connectivity for that gene. Turquoise nodes indicate OUD-specific hub genes, purple nodes indicate LncRNA gene, gray nodes indicate hub genes, and red halos indicate DE genes. Edges indicate significant co-expression between two particular genes.
To further investigate the biological significance of OUD-specific modules, we examined DE enrichment within each module focused on highly represented mRNAs and lncRNAs (Fig.1F-G). The DLPFC blue module and the NAc forestgreen module were of particular interest because of their increased connectivity in OUD compared to the other modules and significant enrichment of both DE mRNA and lncRNA transcripts in OUD (DLPFC blue full DE list q<10−26 mRNA q<10−23 lncRNA p=0.002; NAc forestgreen full DE list q<10−23, mRNA q<10−22, lncRNA p=0.004). The topography of DLPFC blue and NAc forestgreen networks were more correlated in OUD relative to unaffected subjects (Fig.4E-F), consistent with strengthening of module connectivity.
Given the substantial transcriptional overlap between brain regions, we tested the degree of overlap in transcript co-expression networks between DLPFC blue and NAc forestgreen modules. There was substantial overlap in transcripts between these modules (Fisher’s exact test, p<10−16) with pathway enrichment for neuroinflammation and ECM remodeling, consistent with our above findings (Fig.4D, Fig.S16). The top shared pathways include TNFα signaling via NFκB, interferon-γ response, and acute inflammatory response via IL-6 (48) and IL-2 (49) signaling (Fig.4D). Each of the top shared upstream regulators are modulators of inflammatory response: PPARγ (50), NFKB1 (51), RELA (51), STAT1 (52), and SP1 (53) (Fig.4D). We also found pathways related to ECM remodeling (Fig.4D). These data build upon our above analyses highlighting critical roles for neuroinflammation and ECM in OUD.
To identify potential drivers of co-expression networks, we detected highly connected “hub” genes within a module that were predicted to regulate the expression of other module genes. Many of the hub genes that were specific to OUD in DLPFC and NAc included inflammatory regulators, such as JAK3 (54), SERPINB1 (55), and RELL1 (56) in DLPFC (Fig.4F), and TLR2 (57), TNFRSF10B (58), and NFIL3 (59) in NAc (Fig.4F). We also found additional classes of highly connected transcriptional regulators within our co-expression networks including lncRNAs and RNA-binding proteins. Several lncRNAs were highly connected in OUD-specific networks in DLPFC and NAc (e.g., DLPFC: AC0101086.3 and NAc: BAALC.AS1) (Fig.4F, Table S6). AC0101086.3 is located proximal to CLEC2D in the genome, another OUD-specific hub gene. CLEC2D encodes ELT1, an activator of innate immunity (60, 61). BAALC.AS1 is highly expressed in brain and regulates astrocytes (62). In the NAc forestgreen module, we also identified YBX3, an RNA-binding protein. YBX3 regulates the expression of large neutral amino acid transporter 1 (LAT1) (63), which is necessary for the uptake of catecholamine precursor, L-DOPA in dopaminergic cells (64). Notably, changes in YBX3 expression in human midbrain has previously been implicated in opioid dependence (65). We identify putative mechanisms involved in neuroinflammation and dopamine neurotransmission in OUD.
Associations between DE transcripts in NAc and genetic liability for substance use-related traits in OUD
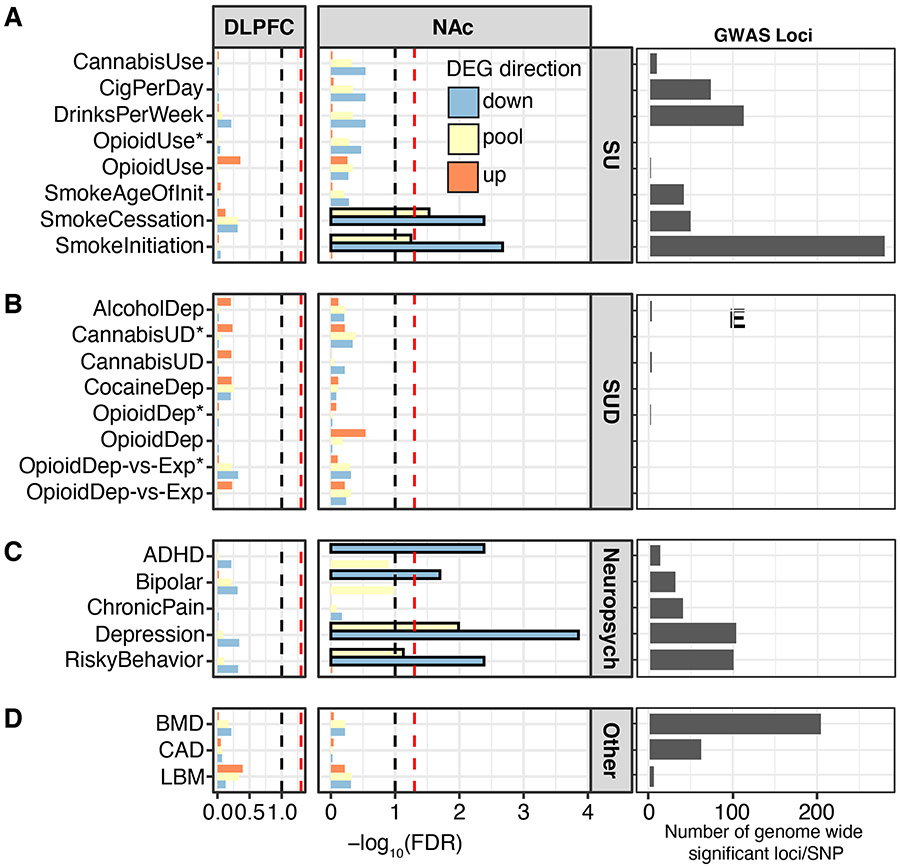
Opioid dependence is strongly linked to impulsivity and risk-taking. Given our results highlighting inflammation in OUD, we determined whether these pathways were linked to traits related to opioid use. To test this, we employed a GWAS-based approach that integrated risk loci of substance use-related traits (e.g., opioid dependence, smoking, and risky behavior) and psychiatric disorders (66-68) with DLPFC and NAc DE transcripts in OUD subjects. Loci identified by GWAS are known to overlap with intronic and distal intergenic noncoding regions within cis-acting regulators of gene expression (45). Using this information, we examined whether noncoding regions proximal to our DE transcripts were enriched for genetic risk variants associated with opioid dependence vulnerability. We discovered significant enrichment of downregulated DE transcripts in NAc of OUD subjects for genes associated with smoking initiation and cessation (Fig.5A), along with attention deficit hyperactivity disorder (ADHD), bipolar disorder, depression, and risky behavior GWAS (Fig.5C, FDR<0.05). Many of these transcripts were associated with neuroimmune signaling, along with the machinery involved in synaptic neurotransmission (Fig.2, Fig.4). We found a lack of DE transcript enrichment in chronic pain and opioid dependence GWAS (Fig.5A-B) likely due to small sample sizes, low numbers of significant loci, and variable enrichment co-efficients (Fig. 5E; Fig.S17). As expected, GWAS traits for bone mineral density, coronary artery disease, and lean body mass were unrelated to DE transcripts in OUD (Fig.5D; Fig.S17). Our results therefore bridge substance use related genetic risk factors to our transcriptomic findings in OUD.
Fig. 5. Differentially expressed transcripts in the DLPFC and NAc enrich for genetic liability of risky behavior.
Several well-powered genome-wide associated studies (GWAS) have identified risk loci associated with substance use (SU), substance use disorder (SUD)-, and neuropsychiatry (neuropsych)-related traits. Significant risk loci overlap with intronic and distal intergenic noncoding regions, presumably within cis-acting regulatory elements of gene expression. A. Proximal noncoding regions of differentially expressed (DE) transcripts in DLPFC and NAc from OUD subjects were investigated for enrichment of genetic risk variants of SU-related traits using partitioned heritability linkage-disequilibrium score regression analysis. No enrichment was found in the DLPFC, but significant enrichment was found in the NAc for upregulated and downregulated transcripts with smoking cessation and smoking initiation. B. No enrichment was found in the DLPFC or NAc for SUD-related GWAS. C. No enrichment was found for neuropsychiatry-related GWAS in the DLPFC, but there was enrichment in the NAc for attention deficit hyperactivity disorder (ADHD; down-regulated), bipolar disorder (downregulated), depression (up and downregulated), and risky behavior (up and downregulated) GWAS. D. No enrichment in DLPFC or NAc for unrelated GWAS traits, including bone mineral density (BMD), coronary artery disease (CAD), and lean body mass (LBM). Number of genome-wide significant loci for each GWAS dataset used to examine enrichment of DE transcripts in OUD. Current GWAS datasets for opioid used and dependence are comparatively small to other GWAS traits used in the analyses. *, indicates in African American ancestry; lack of asterisk indicates European American ancestry. SNP, single nucleotide polymorphism.
Discussion
Our work suggests that inflammatory processes are altered in the brains of subjects with OUD. Clinical findings linking inflammation to OUD are primarily from reports of elevated levels of circulating pro-inflammatory cytokines in opioid dependent individuals (69). Pro-inflammatory cytokines in periphery and brain may have functional consequences that contribute to OUD. In line with this, microglial inhibition mitigates subjective withdrawal (70), reduces motivation to consume opioids in dependent individuals (71), and attenuates opioid reward-seeking in rodents (72-75). However, the unanswered question was whether these findings were clinically relevant to OUD. By focusing on key brain regions involved in OUD, we reveal several key inflammatory pathways altered in DLPFC and NAc in the context of OUD.
One of the top pathways shared between DLPFC and NAc is TNFα signaling via NFκB. Receptors known to activate NFκB, TNF and TLR4 (76, 77), are among the top predicted upstream regulators of DE transcripts. Though still controversial, there is growing evidence opioids can also induce a neuroinflammatory response via direct activation of TLR4, a transmembrane receptor which activates NFκB signaling and inflammatory cascades (78). Upon activation, NFκB dimers translocate to the nucleus to drive transcription of cytokines, chemokines, and interleukins (76, 77). Additionally, opioids can activate NFκB via opioid receptors (79-83), and activation of NFκB signaling can, in turn, promote transcription of opioid receptors and peptides (84-89), involved in opioid reward (84, 90, 91). Thus, while opioids can influence immune function via NFκB, opioids may also activate NFkB, with downstream effects on addiction-related behaviors, independent of immune function.
An effective neuroinflammatory response involves the interplay between immune cells and local ECM remodeling. The ECM is an assembly of adhesion molecules, proteins, polysaccharides, and proteoglycans, critical for blood brain barrier integrity and synaptic function (92). Both the formation and degradation of ECM in the brain depend on the aggregation of a specific family of proteoglycans, chondroitin sulfate glycoaminoglycans (CS-GAGs) (93). Significantly, CS-GAGs constitute the top shared pathway enriched between DLPFC and NAc in OUD subjects. CS-GAGs aggregate in the perisynaptic space in response to inflammation. ECM remodeling is also implicated in synaptic plasticity (94), with roles in neurite outgrowth, dendritic spine formation and morphology (93, 95), and myelination (96, 97). Reduced myelination has been found in clinical neuroimaging and postmortem brain studies of chronic opioid users (98-102), as well as in rodent models of chronic opioid exposure (103, 104). Importantly, the cytokines we identified in DLPFC and NAc of OUD subjects, such as interferons and TNFs, modulate ECM remodeling (105, 106), suggesting links between neuroinflammation, ECM, and opioids.
We therefore posit that opioid-induced changes in CS-GAG signaling driven by neuroinflammation disrupt ECM structure and have profound consequences on dendritic, synaptic, and behavioral plasticity. For example, reorganization of ECM in NAc via matrix metalloproteinases, which facilitate matrix degradation and reassembly, leads to increased potentiation of glutamatergic synapses (107) and drives opioid relapse (108). Importantly, we identified TIMP1 and TLR2 as OUD-specific hub genes, both of which have crucial roles in ECM remodeling (109) and functional reorganization of excitatory synapses by directly inhibiting matrix metalloproteinases (107). We therefore speculate: 1) opioid use elicits release of pro-inflammatory cytokines in the brain that activate TIMP1 and TLR2, protein modulators of ECM organization (110); 2) these activated modulators modify matrix metalloproteinases activity to alter ECM organization; and 3) these disturbances to ECM remodeling alter synaptic plasticity136 and result in the behavioral changes related to OUD. Future experiments using animal models will directly test these possibilities.
Multiple lines of evidence point to the centrality of microglia, the primary resident immune cells in the brain, in our OUD subjects. First, microglial markers were significantly enriched across DLPFC and NAc in OUD, which we independently validated using single nuclei RNA-sequencing data from human postmortem brain. Moreover, our cell type-specific analysis revealed that HSD17B14 was the top DE transcript associated with microglia. HSD17B14 maintains microglial homeostasis during inflammation (46). Second, our enrichment analysis of cell type-specific DE transcripts revealed pathways involved in microglial activation and the activation of additional immune cell types, including CD4+ and CD8+ T-cells. Earlier work suggests synergistic relationships between microglia and resident or infiltrating peripheral T-cells, potentiating neuroinflammation in OUD (111-114). Third, the cytokines we identified, including IL-1β, IL-6, IL-2, and TNFα, are secreted by microglia (115), all of which are implicated in opioid reward and dependence (116-118). Fourth, pathways identified in OUD are related to transcriptional regulation in microglia during inflammation. Specifically, network analyses predicted the following top upstream regulators as PPARγ, NFκB1, RELA, STAT1, and SP1. While microglia rely on multiple families of transcription factors (119), our findings in OUD largely identify transcription factors that are important for guiding microglial response to inflammatory signals. For example, PPARγ is a nuclear transcription factor preferentially expressed in human microglia. PPARμ is activated in response to neuroinflammation and leads to anti-inflammatory responses that are neuroprotective (119). Aberrant activation of STAT1 in microglia upregulates several pro-inflammatory cytokines. Intriguingly, STAT1-dependent signaling regulates the expression of the human μ-opioid receptor via IL-6, another pro-inflammatory cytokine that we identified in our analyses (120). Finally, we found two pathways intimately linked to microglial function: ameboidal migration and integrin signaling. Integrins physically tether microglia and neurons to the ECM scaffold (121). Microglia rely on the ECM for ameboidal migration, further linking microglia, ECM, and neuroinflammation in OUD.
In addition to changes in protein-coding transcripts, subjects with OUD exhibited marked expression changes in lncRNAs. LncRNAs are key regulators of gene and protein expression (122). Several lncRNAs we identified in OUD, AC0101086.3, BAALC.AS1, and AL136366.1, are implicated in both neurotransmission and neuroinflammation. In DLPFC, we found AC0101086.3 to be an OUD-specific hub lncRNA. This lncRNA is located proximally to CLEC2D, which encodes ELT1, the functional ligand for the natural-killer cell receptor NKR-P1A. NKR-P1A controls immunosurveillance via natural-killer, dendritic, and B-cells (60, 61). In NAc, we found BAALC.AS1 (123) and AL136366.1 (124) as OUD-specific hub lncRNAs, both of which are involved in various brain functions (125, 126).
Functional alterations occur at the circuit-level across multiple brain regions in opioid dependence and treatment (127). One such change occurs at the transcriptional level, where different brain regions can increasingly synchronize transcriptional patterns. There is evidence that enhanced transcriptional synchrony across brain regions occurs in response to insults including stress and drugs of abuse (18, 128). Consistent with this, we found significant transcriptional synchrony between DLPFC and NAc in OUD. To date, the relevance of such synchrony remains unclear. We speculate that, because the ranking, effect size, and direction of change is highly similar between brain regions, such synchrony may represent a common pathophysiological response to neuroinflammation in OUD. Further work is required to explore these possibilities.
Integrating large-scale transcriptional profiles with relevant GWAS findings suggests novel gene-trait associations in OUD. We demonstrated that downregulated transcripts in NAc of OUD subjects were significantly enriched for risky behavior GWAS (66). Risky behavior forms a functional triad with mood and impulsivity (129), where impulsivity is a risk factor for substance use (130-132). We observed a lack of GWAS enrichment for opioid dependence, likely due to smaller sample sizes and limited genome-wide significant loci in both European and African ancestries (133). As newer, larger, more robust GWAS findings emerge, we expect DE transcripts in OUD will show higher concordance with risk loci associated with opioid use and dependence. Our findings support relationships between genetic risk, brain region-specific transcriptional changes, and OUD vulnerability.
Overall, our data suggest links between the immune system and opioid dependence in the human brain. These results provide a novel putative mechanism across transcriptional networks, biological pathways, and specific cell types for the detrimental neuroadaptations across corticostriatal circuitry that result from chronic opioids and OUD. These insights offer the opportunity for new therapeutic targets with improved efficacy to treat OUD.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Deposited Data; Public Database | Human postmortem brain; 20 control subjects and 20 OUD subjects | RRID:SCR_000139 | ||
| Deposited Data; Public Database | Single-nuclei RNA-seq human postmortem brains, unaffected subjects | https://github.com/LieberInstitute/10xPilot_snRNAseq-human | ||
| Software; Algorithm | R | The R Project for Statistical Computing | RRID:SCR_001905 | |
| Software; Algorithm | Metascape | http://metascape.org/gp/index.html#/main/step1 | RRID:SCR_016620 | |
| Software; Algorithm | Cytoscape 3.8.0 | http://cytoscape.org | RRID:SCR_003032 | |
| Software; Algorithm | Ingenuity Pathway Analysis | http://www.ingenuity.com/products/pathways_analysis.html | RRID:SCR_008653 | |
| Software; Algorithm | HOMER | http://homer.ucsd.edu/ | RRID:SCR_010881 | |
| Software; Algorithm | MetaseqR | http://www.bioconductor.org/packages/release/bioc/html/metaSeq.html | RRID:SCR_000056 | |
| Software; Algorithm | RRHO2 | PMID: 29942049 | ||
| Software; Algorithm | AUCELL | https://bioconductor.org/packages/release/bioc/html/AUCell.html | ||
| Software; Algorithm | ARACNE | http://wiki.c2b2.columbia.edu/califanolab/index.php/Software/ARACNE | RRID:SCR_002180 | |
| Software; Algorithm | GSEA | http://www.broadinstitute.org/gsea/ | RRID:SCR_003199 |
Acknowledgments:
We would like to thank the staff and technicians who work diligently as part of the Brain Tissue Program at the University of Pittsburgh. Human tissue was obtained from the NIH NeuroBioBank and the University of Pittsburgh Brain Tissue Donation Program. This study was funded by the Hamilton Family Prize for Basic Neuroscience Research in Psychiatry at the University of Pittsburgh School of Medicine to R.W.L., NHLBI R01HL150432 to R.W.L, and NIDA R01DA051390 to R.W.L and M.L.S. A version of the manuscript was posted on bioRxiv (doi.org/10.1101/2020.09.14.296707).
Footnotes
Competing Interests: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Jones CM, Einstein EB, and Compton WM (2018): Changes in Synthetic Opioid Involvement in Drug Overdose Deaths in the United States, 2010-2016. JAMA 319(17): 1819–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koob GF (2020): Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol Psychiatry 87(1): 44–53. [DOI] [PubMed] [Google Scholar]
- 3.Adinoff B, Rilling LM, Williams MJ, Schreffler E, Schepis TS, Rosvall T, et al. (2007): Impulsivity, neural deficits, and the addictions: the "oops" factor in relapse. J Addict Dis 26 Suppl 1: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldo BA (2016): Prefrontal Cortical Opioids and Dysregulated Motivation: A Network Hypothesis. Trends Neurosci 39(6): 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Lu Z, Xu M, Pan L, Deng Y, Xie X, et al. (2014): A heroin addiction severityassociated intronic single nucleotide polymorphism modulates alternative premRNA splicing of the mu opioid receptor gene OPRM1 via hnRNPH interactions. J Neurosci 34(33): 11048–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown TG, Xu J, Hurd YL, and Pan YX (2020): Dysregulated expression of the alternatively spliced variant mRNAs of the mu opioid receptor gene, OPRM1, in the medial prefrontal cortex of male human heroin abusers and heroin selfadministering male rats. J Neurosci Res: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sillivan SE, Whittard JD, Jacobs MM, Ren Y, Mazloom AR, Caputi FF, et al. (2013): ELK1 transcription factor linked to dysregulated striatal mu opioid receptor signaling network and OPRM1 polymorphism in human heroin abusers. Biol Psychiatry 74(7): 511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albertson DN, Schmidt CJ, Kapatos G, and Bannon MJ (2006): Distinctive profiles of gene expression in the human nucleus accumbens associated with cocaine and heroin abuse. Neuropsychopharmacology 31(10): 2304–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo JW, Mazei-Robison MS, LaPlant Q, Egervari G, Braunscheidel KM, Adank DN, et al. (2015): Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area. Nat. Neurosci 18(3): 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chartoff EH and Connery HS (2014): It's MORe exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front Pharmacol 5: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garzon J, Rodriguez-Munoz M, and Sanchez-Blazquez P (2012): Direct association of Mu-opioid and NMDA glutamate receptors supports their crossregulation: molecular implications for opioid tolerance. Curr Drug Abuse Rev 5(3): 199–226. [DOI] [PubMed] [Google Scholar]
- 12.Tokuyama S, Wakabayashi H, and Ho IK (1996): Direct evidence for a role of glutamate in the expression of the opioid withdrawal syndrome. Eur J Pharmacol 295(2-3): 123–9. [DOI] [PubMed] [Google Scholar]
- 13.Browne CJ, Godino A, Salery M, and Nestler EJ (2020): Epigenetic Mechanisms of Opioid Addiction. Biol Psychiatry 87(1): 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mai JK, Majtanik M, and Paxinos G (2016): Atlas of the human brain. [Google Scholar]
- 15.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, and Lewis DA (2008): Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. The American Journal of Psychiatry 165(4): 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, et al. (2012): Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry 169(10): 1082–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, et al. (2018): Opposite Molecular Signatures of Depression in Men and Women. Biol Psychiatry 84(1): 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker DM, Cates HM, Loh YE, Purushothaman I, Ramakrishnan A, Cahill KM, et al. (2018): Cocaine Self-administration Alters Transcriptome-wide Responses in the Brain's Reward Circuitry. Biol Psychiatry 84(12): 867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. (2017): Sex-specific transcriptional signatures in human depression. Nat. Med. 23(9): 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paden W, Barko K, Puralewski R, Cahill KM, Huo Z, Shelton MA, et al. (2020): Sex differences in adult mood and in stress-induced transcriptional coherence across mesocorticolimbic circuitry. Transl Psychiatry 10(1): 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pena CJ, Smith M, Ramakrishnan A, Cates HM, Bagot RC, Kronman HG, et al. (2019): Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat Commun 10(1): 5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logan RW, Ozburn AR, Arey RN, Ketchesin KD, Winquist A, Crain A, et al. (2020): Valproate reverses mania-like behaviors in mice via preferential targeting of HDAC2. Mol Psychiatry: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker DM, Zhou X, Cunningham AM, Lipschultz AP, Ramakrishnan A, Cates HM, et al. (2021): Sex-Specific Transcriptional Changes in Response to Adolescent Social Stress in the Brain's Reward Circuitry. Biol Psychiatry: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moulos P and Hatzis P (2015): Systematic integration of RNA-Seq statistical algorithms for accurate detection of differential gene expression patterns. Nucleic Acids Res 43(4): e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. (2010): Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38(4): 576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plaisier SB, Taschereau R, Wong JA, and Graeber TG (2010): Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res 38(17): e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cahill KM, Huo Z, Tseng GC, Logan RW, and Seney ML (2018): Improved identification of concordant and discordant gene expression signatures using an updated rank-rank hypergeometric overlap approach. Sci Rep 8(1): 9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B and Horvath S (2005): A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol 4: Article17. [DOI] [PubMed] [Google Scholar]
- 29.Langfelder P, Zhang B, and Horvath S (2008): Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics 24(5): 719–20. [DOI] [PubMed] [Google Scholar]
- 30.Margolin AA, Nemenman I, Basso K, Wiggins C, Stolovitzky G, Dalla Favera R, et al. (2006): ARACNE: an algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. BMC Bioinformatics 7 Suppl 1: S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong Y, Wan YW, Pang K, Chow LM, and Liu Z (2013): Digital sorting of complex tissues for cell type-specific gene expression profiles. BMC Bioinformatics 14: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKenzie AT, Wang M, Hauberg ME, Fullard JF, Kozlenkov A, Keenan A, et al. (2018): Brain Cell Type Specific Gene Expression and Co-expression Network Architectures. Sci Rep 8(1): 8868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng SC, Breeze CE, Beck S, and Teschendorff AE (2018): Identification of differentially methylated cell types in epigenome-wide association studies. Nat Methods 15(12): 1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aibar S, Gonzalez-Blas CB, Moerman T, Huynh-Thu VA, Imrichova H, Hulselmans G, et al. (2017): SCENIC: single-cell regulatory network inference and clustering. Nat Methods 14(11): 1083–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tran MN, Maynard KR, Spangler A, Collado-Torres L, Sadashivaiah V, Tippani M, et al. (2020): Single-nucleus transcriptome analysis reveals cell type-specific molecular signatures across reward circuitry in the human brain. bioRxiv: 2020.10.07.329839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finucane HK, Reshef YA, Anttila V, Slowikowski K, Gusev A, Byrnes A, et al. (2018): Heritability enrichment of specifically expressed genes identifies diseaserelevant tissues and cell types. Nat Genet 50(4): 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics C, et al. (2015): LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 47(3): 291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, et al. (2020): Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plaisier SB, Taschereau R, Wong JA, and Graeber TG (2010): Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic acids research 38(17): e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cahill KM, Huo Z, Tseng GC, Logan RW, and Seney ML (2018): Improved identification of concordant and discordant gene expression signatures using an updated rank-rank hypergeometric overlap approach. Scientific reports 8(1): 9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans CJ and Cahill CM (2016): Neurobiology of opioid dependence in creating addiction vulnerability. F1000Res 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Merrer J, Becker JA, Befort K, and Kieffer BL (2009): Reward processing by the opioid system in the brain. Physiol Rev 89(4): 1379–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nestler EJ (1997): Molecular mechanisms of opiate and cocaine addiction. Curr Opin Neurobiol 7(5): 713–9. [DOI] [PubMed] [Google Scholar]
- 44.Nestler EJ (2004): Historical review: Molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci 25(4): 210–8. [DOI] [PubMed] [Google Scholar]
- 45.Roadmap Epigenomics C, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, et al. (2015): Integrative analysis of 111 reference human epigenomes. Nature 518(7539): 317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saijo K, Collier JG, Li AC, Katzenellenbogen JA, and Glass CK (2011): An ADIOL-ERbeta-CtBP transrepression pathway negatively regulates microgliamediated inflammation. Cell 145(4): 584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, et al. (2012): A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation 125(23): 2892–903. [DOI] [PubMed] [Google Scholar]
- 48.Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Haegeman G, et al. (2011): Interleukin-6, a mental cytokine. Brain Res Rev 67(1-2): 157–83. [DOI] [PubMed] [Google Scholar]
- 49.Zelikoff JT, Parmalee NL, Corbett K, Gordon T, Klein CB, and Aschner M (2018): Microglia Activation and Gene Expression Alteration of Neurotrophins in the Hippocampus Following Early-Life Exposure to E-Cigarette Aerosols in a Murine Model. Toxicol Sci 162(1): 276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tufano M and Pinna G (2020): Is There a Future for PPARs in the Treatment of Neuropsychiatric Disorders? Molecules 25(5): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shih RH, Wang CY, and Yang CM (2015): NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front Mol Neurosci 8: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butturini E, Boriero D, Carcereri de Prati A, and Mariotto S (2019): STAT1 drives M1 microglia activation and neuroinflammation under hypoxia. Arch Biochem Biophys 669: 22–30. [DOI] [PubMed] [Google Scholar]
- 53.Mao XR, Moerman-Herzog AM, Chen Y, and Barger SW (2009): Unique aspects of transcriptional regulation in neurons--nuances in NFkappaB and Sp1-related factors. J Neuroinflammation 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H, Brown J, Gao S, Liang S, Jotwani R, Zhou H, et al. (2013): The role of JAK-3 in regulating TLR-mediated inflammatory cytokine production in innate immune cells. J Immunol 191(3): 1164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi YJ, Kim S, Choi Y, Nielsen TB, Yan J, Lu A, et al. (2019): SERPINB1-mediated checkpoint of inflammatory caspase activation. Nat Immunol 20(3): 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang HS, Huang XY, Yu HZ, Xue Y, and Zhu PL (2020): Circular RNA circ-RELL1 regulates inflammatory response by miR-6873-3p/MyD88/NF-kappaB axis in endothelial cells. Biochem Biophys Res Commun 525(2): 512–519. [DOI] [PubMed] [Google Scholar]
- 57.Luz A, Fainstein N, Einstein O, and Ben-Hur T (2015): The role of CNS TLR2 activation in mediating innate versus adaptive neuroinflammation. Exp Neurol 273: 234–42. [DOI] [PubMed] [Google Scholar]
- 58.Zelova H and Hosek J (2013): TNF-alpha signalling and inflammation: interactions between old acquaintances. Inflamm Res 62(7): 641–51. [DOI] [PubMed] [Google Scholar]
- 59.Kim HS, Sohn H, Jang SW, and Lee GR (2019): The transcription factor NFIL3 controls regulatory T-cell function and stability. Exp Mol Med 51(7): 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosen DB, Cao W, Avery DT, Tangye SG, Liu YJ, Houchins JP, et al. (2008): Functional consequences of interactions between human NKR-P1A and its ligand LLT1 expressed on activated dendritic cells and B cells. J Immunol 180(10): 6508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Germain C, Meier A, Jensen T, Knapnougel P, Poupon G, Lazzari A, et al. (2011): Induction of lectin-like transcript 1 (LLT1) protein cell surface expression by pathogens and interferon-gamma contributes to modulate immune responses. J Biol Chem 286(44): 37964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moodbidri MS and Shirsat NV (2006): Induction of BAALC and down regulation of RAMP3 in astrocytes treated with differentiation inducers. Cell Biol Int 30(3): 210–3. [DOI] [PubMed] [Google Scholar]
- 63.Cooke A, Schwarzl T, Huppertz I, Kramer G, Mantas P, Alleaume AM, et al. (2019): The RNA-Binding Protein YBX3 Controls Amino Acid Levels by Regulating SLC mRNA Abundance. Cell Rep 27(11): 3097–3106 e5. [DOI] [PubMed] [Google Scholar]
- 64.Singh N and Ecker GF (2018): Insights into the Structure, Function, and Ligand Discovery of the Large Neutral Amino Acid Transporter 1, LAT1. Int J Mol Sci 19(5): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saad MH, Rumschlag M, Guerra MH, Savonen CL, Jaster AM, Olson PD, et al. (2019): Differentially expressed gene networks, biomarkers, long noncoding RNAs, and shared responses with cocaine identified in the midbrains of human opioid abusers. Sci Rep 9(1): 1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karlsson Linner R, Biroli P, Kong E, Meddens SFW, Wedow R, Fontana MA, et al. (2019): Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat Genet 51(2): 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. (2019): Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 51(2): 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pasman JA, Verweij KJH, Gerring Z, Stringer S, Sanchez-Roige S, Treur JL, et al. (2019): Author Correction: GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal effect of schizophrenia liability. Nat Neurosci 22(7): 1196. [DOI] [PubMed] [Google Scholar]
- 69.Hofford RS, Russo SJ, and Kiraly DD (2019): Neuroimmune mechanisms of psychostimulant and opioid use disorders. Eur J Neurosci 50(3): 2562–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cooper ZD, Johnson KW, Pavlicova M, Glass A, Vosburg SK, Sullivan MA, et al. (2016): The effects of ibudilast, a glial activation inhibitor, on opioid withdrawal symptoms in opioid-dependent volunteers. Addict Biol 21(4): 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Metz VE, Jones JD, Manubay J, Sullivan MA, Mogali S, Segoshi A, et al. (2017): Effects of Ibudilast on the Subjective, Reinforcing, and Analgesic Effects of Oxycodone in Recently Detoxified Adults with Opioid Dependence. Neuropsychopharmacology 42(9): 1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bland ST, Hutchinson MR, Maier SF, Watkins LR, and Johnson KW (2009): The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain Behav Immun 23(4): 492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hutchinson MR, Northcutt AL, Chao LW, Kearney JJ, Zhang Y, Berkelhammer DL, et al. (2008): Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav Immun 22(8): 1248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Guglielmo G, Melis M, De Luca MA, Kallupi M, Li HW, Niswender K, et al. (2015): PPARgamma activation attenuates opioid consumption and modulates mesolimbic dopamine transmission. Neuropsychopharmacology 40(4): 927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM, et al. (2013): Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)- naltrexone on incubation of heroin craving. Biol Psychiatry 73(8): 729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oeckinghaus A and Ghosh S (2009): The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1(4): a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghosh S, May MJ, and Kopp EB (1998): NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16: 225–60. [DOI] [PubMed] [Google Scholar]
- 78.Eisenstein TK (2019): The Role of Opioid Receptors in Immune System Function. Front Immunol 10: 2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lieb K, Fiebich BL, Berger M, Bauer J, and Schulze-Osthoff K (1997): The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J Immunol 159(10): 4952–8. [PubMed] [Google Scholar]
- 80.Wang X, Douglas SD, Commons KG, Pleasure DE, Lai J, Ho C, et al. (2004): A non-peptide substance P antagonist (CP-96,345) inhibits morphine-induced NF-kappa B promoter activation in human NT2-N neurons. J Neurosci Res 75(4): 544–53. [DOI] [PubMed] [Google Scholar]
- 81.Sun J, Ramnath RD, Zhi L, Tamizhselvi R, and Bhatia M (2008): Substance P enhances NF-kappaB transactivation and chemokine response in murine macrophages via ERK1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol 294(6): C1586–96. [DOI] [PubMed] [Google Scholar]
- 82.Sawaya BE, Deshmane SL, Mukerjee R, Fan S, and Khalili K (2009): TNF alpha production in morphine-treated human neural cells is NF-kappaB-dependent. J Neuroimmune Pharmacol 4(1): 140–9. [DOI] [PubMed] [Google Scholar]
- 83.Hou YN, Vlaskovska M, Cebers G, Kasakov L, Liljequist S, and Terenius L (1996): A mu-receptor opioid agonist induces AP-1 and NF-kappa B transcription factor activity in primary cultures of rat cortical neurons. Neurosci Lett 212(3): 159–62. [DOI] [PubMed] [Google Scholar]
- 84.Rehni AK, Bhateja P, Singh TG, and Singh N (2008): Nuclear factor-kappa-B inhibitor modulates the development of opioid dependence in a mouse model of naloxone-induced opioid withdrawal syndrome. Behav Pharmacol 19(3): 265–9. [DOI] [PubMed] [Google Scholar]
- 85.Kraus J, Borner C, Giannini E, and Hollt V (2003): The role of nuclear factor kappaB in tumor necrosis factor-regulated transcription of the human mu-opioid receptor gene. Mol Pharmacol 64(4): 876–84. [DOI] [PubMed] [Google Scholar]
- 86.Karalis KP, Venihaki M, Zhao J, van Vlerken LE, and Chandras C (2004): NF-kappaB participates in the corticotropin-releasing, hormone-induced regulation of the pituitary proopiomelanocortin gene. J Biol Chem 279(12): 10837–40. [DOI] [PubMed] [Google Scholar]
- 87.Chen YL, Law PY, and Loh HH (2007): Action of NF-kappaB on the delta opioid receptor gene promoter. Biochem Biophys Res Commun 352(3): 818–22. [DOI] [PubMed] [Google Scholar]
- 88.Rattner A, Korner M, Rosen H, Baeuerle PA, and Citri Y (1991): Nuclear factor kappa B activates proenkephalin transcription in T lymphocytes. Mol Cell Biol 11(2): 1017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simeonidis S, Castagliuolo I, Pan A, Liu J, Wang CC, Mykoniatis A, et al. (2003): Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-kappa B site on its promoter. Proc Natl Acad Sci U S A 100(5): 2957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang X, Cui Y, Jing J, Cui Y, Xin W, and Liu X (2011): Involvement of p38/NF-kappaB signaling pathway in the nucleus accumbens in the rewarding effects of morphine in rats. Behav Brain Res 218(1): 184–9. [DOI] [PubMed] [Google Scholar]
- 91.Chen YL, Law PY, and Loh HH (2006): Nuclear factor kappaB signaling in opioid functions and receptor gene expression. J Neuroimmune Pharmacol 1(3): 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kangwantas K, Pinteaux E, and Penny J (2016): The extracellular matrix protein laminin-10 promotes blood-brain barrier repair after hypoxia and inflammation in vitro. J Neuroinflammation 13: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li HP, Komuta Y, Kimura-Kuroda J, van Kuppevelt TH, and Kawano H (2013): Roles of chondroitin sulfate and dermatan sulfate in the formation of a lesion scar and axonal regeneration after traumatic injury of the mouse brain. J Neurotrauma 30(5): 413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dityatev A, Schachner M, and Sonderegger P (2010): The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci 11(11): 735–46. [DOI] [PubMed] [Google Scholar]
- 95.Smith PD, Coulson-Thomas VJ, Foscarin S, Kwok JC, and Fawcett JW (2015): "GAG-ing with the neuron": The role of glycosaminoglycan patterning in the central nervous system. Exp Neurol 274(Pt B): 100–14. [DOI] [PubMed] [Google Scholar]
- 96.Harlow DE and Macklin WB (2014): Inhibitors of myelination: ECM changes, CSPGs and PTPs. Exp Neurol 251: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pendleton JC, Shamblott MJ, Gary DS, Belegu V, Hurtado A, Malone ML, et al. (2013): Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPsigma. Exp Neurol 247: 113–21. [DOI] [PubMed] [Google Scholar]
- 98.Bora E, Yucel M, Fornito A, Pantelis C, Harrison BJ, Cocchi L, et al. (2012): White matter microstructure in opiate addiction. Addict Biol 17(1): 141–8. [DOI] [PubMed] [Google Scholar]
- 99.Li W, Zhu J, Li Q, Ye J, Chen J, Liu J, et al. (2016): Brain white matter integrity in heroin addicts during methadone maintenance treatment is related to relapse propensity. Brain Behav 6(2): e00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu H, Li L, Hao Y, Cao D, Xu L, Rohrbaugh R, et al. (2008): Disrupted white matter integrity in heroin dependence: a controlled study utilizing diffusion tensor imaging. Am J Drug Alcohol Abuse 34(5): 562–75. [DOI] [PubMed] [Google Scholar]
- 101.Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, et al. (2010): Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain 133(Pt 7): 2098–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y, Li W, Li Q, Yang W, Zhu J, and Wang W (2011): White matter impairment in heroin addicts undergoing methadone maintenance treatment and prolonged abstinence: a preliminary DTI study. Neurosci Lett 494(1): 49–53. [DOI] [PubMed] [Google Scholar]
- 103.Avey D, Sankararaman S, Yim AKY, Barve R, Milbrandt J, and Mitra RD (2018): Single-Cell RNA-Seq Uncovers a Robust Transcriptional Response to Morphine by Glia. Cell Rep 24(13): 3619–3629 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fan R, Schrott LM, Arnold T, Snelling S, Rao M, Graham D, et al. (2018): Chronic oxycodone induces axonal degeneration in rat brain. BMC Neurosci 19(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Franitza S, Hershkoviz R, Kam N, Lichtenstein N, Vaday GG, Alon R, et al. (2000): TNF-alpha associated with extracellular matrix fibronectin provides a stop signal for chemotactically migrating T cells. J Immunol 165(5): 2738–47. [DOI] [PubMed] [Google Scholar]
- 106.Camejo EH, Rosengren B, Camejo G, Sartipy P, Fager G, and Bondjers G (1995): Interferon gamma binds to extracellular matrix chondroitin-sulfate proteoglycans, thus enhancing its cellular response. Arterioscler Thromb Vasc Biol 15(9): 1456–65. [DOI] [PubMed] [Google Scholar]
- 107.Beroun A, Mitra S, Michaluk P, Pijet B, Stefaniuk M, and Kaczmarek L (2019): MMPs in learning and memory and neuropsychiatric disorders. Cell Mol Life Sci 76(16): 3207–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kruyer A, Chioma VC, and Kalivas PW (2020): The Opioid-Addicted Tetrapartite Synapse. Biol Psychiatry 87(1): 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ries C (2014): Cytokine functions of TIMP-1. Cell Mol Life Sci 71(4): 659–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Akhter N, Nix M, Abdul Y, Singh S, and Husain S (2013): Delta-opioid receptors attenuate TNF-alpha-induced MMP-2 secretion from human ONH astrocytes. Invest Ophthalmol Vis Sci 54(10): 6605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schetters STT, Gomez-Nicola D, Garcia-Vallejo JJ, and Van Kooyk Y (2017): Neuroinflammation: Microglia and T Cells Get Ready to Tango. Front Immunol 8: 1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smolders J, Heutinck KM, Fransen NL, Remmerswaal EBM, Hombrink P, Ten Berge IJM, et al. (2018): Tissue-resident memory T cells populate the human brain. Nat Commun 9(1): 4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Daglas M, Draxler DF, Ho H, McCutcheon F, Galle A, Au AE, et al. (2019): Activated CD8(+) T Cells Cause Long-Term Neurological Impairment after Traumatic Brain Injury in Mice. Cell Rep 29(5): 1178–1191 e6. [DOI] [PubMed] [Google Scholar]
- 114.Steinbach K, Vincenti I, Egervari K, Kreutzfeldt M, van der Meer F, Page N, et al. (2019): Brain-resident memory T cells generated early in life predispose to autoimmune disease in mice. Sci Transl Med 11(498): [DOI] [PubMed] [Google Scholar]
- 115.Hanisch UK (2002): Microglia as a source and target of cytokines. Glia 40(2): 140–55. [DOI] [PubMed] [Google Scholar]
- 116.Eidson LN, Inoue K, Young LJ, Tansey MG, and Murphy AZ (2017): Toll-like Receptor 4 Mediates Morphine-Induced Neuroinflammation and Tolerance via Soluble Tumor Necrosis Factor Signaling. Neuropsychopharmacology 42(3): 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chan YY, Yang SN, Lin JC, Chang JL, Lin JG, and Lo WY (2015): Inflammatory response in heroin addicts undergoing methadone maintenance treatment. Psychiatry Res 226(1): 230–4. [DOI] [PubMed] [Google Scholar]
- 118.Lu RB, Wang TY, Lee SY, Chen SL, Chang YH, See Chen P, et al. (2019): Correlation between interleukin-6 levels and methadone maintenance therapy outcomes. Drug Alcohol Depend 204: 107516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Holtman IR, Skola D, and Glass CK (2017): Transcriptional control of microglia phenotypes in health and disease. J Clin Invest 127(9): 3220–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Borner C, Kraus J, Schroder H, Ammer H, and Hollt V (2004): Transcriptional regulation of the human mu-opioid receptor gene by interleukin-6. Mol Pharmacol 66(6): 1719–26. [DOI] [PubMed] [Google Scholar]
- 121.Dityatev A and Rusakov DA (2011): Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol 21(2): 353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yao RW, Wang Y, and Chen LL (2019): Cellular functions of long noncoding RNAs. Nat Cell Biol 21(5): 542–551. [DOI] [PubMed] [Google Scholar]
- 123.Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. (2018): Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet 50(8): 1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mez J, Chung J, Jun G, Kriegel J, Bourlas AP, Sherva R, et al. (2017): Two novel loci, COBL and SLC10A2, for Alzheimer's disease in African Americans. Alzheimers Dement 13(2): 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Canals I, Ginisty A, Quist E, Timmerman R, Fritze J, Miskinyte G, et al. (2018): Rapid and efficient induction of functional astrocytes from human pluripotent stem cells. Nat Methods 15(9): 693–696. [DOI] [PubMed] [Google Scholar]
- 126.Lozzi B, Huang TW, Sardar D, Huang AY, and Deneen B (2020): Regionally Distinct Astrocytes Display Unique Transcription Factor Profiles in the Adult Brain. Front Neurosci 14: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hanlon CA, Dowdle LT, and Henderson JS (2018): Modulating Neural Circuits with Transcranial Magnetic Stimulation: Implications for Addiction Treatment Development. Pharmacol Rev 70(3): 661–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bagot RC, Cates HM, Purushothaman I, Lorsch ZS, Walker DM, Wang J, et al. (2016): Circuit-wide Transcriptional Profiling Reveals Brain Region-Specific Gene Networks Regulating Depression Susceptibility. Neuron 90(5): 969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Torres A, Catena A, Megias A, Maldonado A, Candido A, Verdejo-Garcia A, et al. (2013): Emotional and non-emotional pathways to impulsive behavior and addiction. Front Hum Neurosci 7: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kreek MJ, Nielsen DA, Butelman ER, and LaForge KS (2005): Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci 8(11): 1450–7. [DOI] [PubMed] [Google Scholar]
- 131.Rodriguez-Cintas L, Daigre C, Grau-Lopez L, Barral C, Perez-Pazos J, Voltes N, et al. (2016): Impulsivity and addiction severity in cocaine and opioid dependent patients. Addict Behav 58: 104–9. [DOI] [PubMed] [Google Scholar]
- 132.Winstanley CA (2007): The orbitofrontal cortex, impulsivity, and addiction: probing orbitofrontal dysfunction at the neural, neurochemical, and molecular level. Ann N Y Acad Sci 1121: 639–55. [DOI] [PubMed] [Google Scholar]
- 133.Polimanti R, Walters RK, Johnson EC, McClintick JN, Adkins AE, Adkins DE, et al. (2020): Leveraging genome-wide data to investigate differences between opioid use vs. opioid dependence in 41,176 individuals from the Psychiatric Genomics Consortium. Mol Psychiatry 25(8): 1673–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.