Abstract
NLCs have provoked the incessant impulsion for the development of safe and valuable drug delivery systems owing to their exceptional physicochemical and then biocompatible characteristics. Throughout the earlier period, a lot of studies recounting NLCs based formulations have been noticeably increased. They are binary system which contains both solid and liquid lipids aiming to produce less ordered lipidic core. Their constituents particularly influence the physicochemical properties and effectiveness of the final product. NLCs can be fabricated by different techniques which are classified according to consumed energy. More utilization NLCs is essential due to overcome barriers surrounded by the technological procedure of lipid-based nanocarriers’ formulation and increased information of the core mechanisms of their transport via various routes of administration. They can be used in different applications and by different routes such as oral, cutaneous, ocular and pulmonary. This review article seeks to present an overview on the existing situation of the art of NLCs for future clinics through exposition of their applications which shall foster their lucid use. The reported records evidently demonstrate the promise of NLCs for innovate therapeutic applications in the future.
Keyword: NLCs, Drug delivery system, Lipid nanocarriers, Application of NLCs
1. Introduction
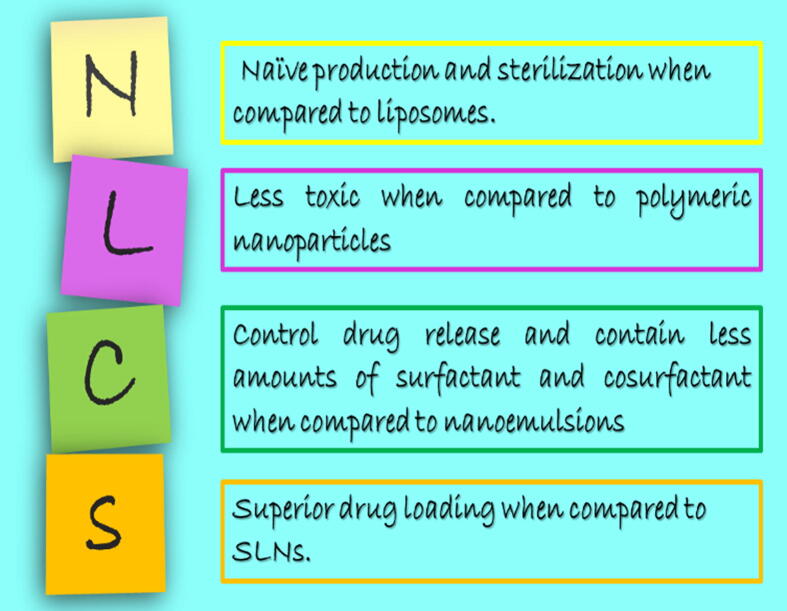
Nanoparticulate systems present potential platform for drug therapy that can improve its performance and rise above its limitations. Among the different investigated nano-systems, lipid nanoparticles keep immense promise in the area of drug delivery. Historically, Müller and Gasco around 1990s were firstly developed and nominated solid lipid nanoparticles (SLN) for the sake of avoiding organic solvents involved in preparation of polymeric nanoparticles (Muller et al., 2011). SLN became one of the most emerging systems as it provided better stability than liposomes (previously developed), solidified at body temperature which controlled drug release and deprived from toxic effects associated with organic solvent involvement (Teeranachaideekul et al., 2007a). As its lipid content represented only by solid lipid, low drug payload is the major challenge for applicability owing to internal rearrangement of crystal lattice and then drug expulsion. In order to increase drug loading, the second generation lipid nanoparticles; nanostructured lipid carriers (NLCs) was developed. NLCs are binary system which contains both solid and liquid lipids which in turn produced less ordered lipidic core (Muller et al., 2004, Radtke et al., 2005). This imperfection of internal arrangement aids more drug accommodation. So, NLCs overweigh SLN (Fig. 1) as the former can encapsulate higher drug amounts, contains lower water content and improves drug entrapment with minimized leakage during storage(Mehnert and Mäder 2012). They also since then, researchers paid attention to NLCs and discovered different applications.
Fig. 1.
Advantages of NLCs over SLN.
In this review paper, we have tried to present an inclusive elucidation of the following features: a) a concise overview of NLCs' components and their integration to technological aspects of the formulations; b) the current art in development of NLCs together with their different categories; c) major applications and current challenges for the development of NLCs as delivery systems by different routes of administration.
2. Components and formulation attributes
Basically and like emulsions, NLCs are composed lipid phase, aqueous phase and surfactant(s). However, selection of the components and their ratios can particularly influence the final behavior of the developed formulation. Regarding lipid phase, it consists of an imperfect solid lipid matrix prepared by mixing solid and liquid lipids. There are various types of lipids that had been used in formulations of NLCs (Table 1) such as triglycerides, partial glycerides, fatty acids, steroids and waxes. Oils (liquid lipids) and fats contain mixtures of mono-, di- and triglycerides of fatty acids of different chain length and degree of unsaturation (Hauss, 2007, Souto and Müller). In general, the selection of lipid relies on physiological tolerance, physiochemical structure, drug solubility and solid lipid/liquid lipid miscibility. Firstly, the lipids should be categorized as Generally Recognized As Safe (GRAS) which could not produce significant toxic effects in the concentration used. Secondly, physicochemical structure will determine the state of lipid at room temperature. Thirdly, before fabrication of NLCs, solubility of active drug in lipid should be essentially determined. If the drug is not preferentially solubilized in lipid core, it will attach to particles' surface or incorporate into micelles in the aqueous phase and hence the drug entrapment and loading will be very low. Fourthly, the compatibility between solid lipid and liquid is not admitted which in turn requires miscibility study by checking the macroscopic lipid phase homogeneity/separation below melting point of fat. Molten lipid phase should consist single phase (Doktorovová et al. 2010). It was reported that Miglyol was incompatible with Suppocire A, Geleol, cacao butter and Witepsol E75 (Elmowafy et al. 2016). It is preferred to blend solid lipids and liquid lipids in a ratio of 70:30 up to a ratio of 99.9:0.1 (Beloqui et al., 2016b). However and depending on the formulation attributes, the ratio may vary. For example, liquid lipid percentage increases in case of multiple emulsion NLC (Khosa, Reddi, and Saha 2018). Also higher encapsulation efficiencies with higher oil percentages have been recently reported by Elmowafy and coworkers in case of the drug is very soluble in oil as higher oil percentages created sufficient spaces for accommodation of lipophilic drugs in lipid matrix (Elmowafy et al. 2019). In addition, faster drug release was observed with higher liquid lipid content by Fathi and coworkers (Fathi et al. 2018). Long chain fatty acids and long chain triglycerides were reported to produce larger particle size when compared to medium chain fatty acids and medium chain triglycerides (Pokharkar, Patil-Gadhe, and Kaur 2018). The authors attributed the effect of chain length on particle size to increased mobility of internal lipid and fluidity of surfactant layer. Feasibility to combine different lipids made the NLC a facile system to attain the desired characteristics. On the other hand, long chain lipid based NLCs were reported to be absorbed via intestinal lymphatic system with enhanced bioavailability and prolonged half-life (Shete et al. 2013). Incorporation of combined fatty materials in lipid matrix also influenced the system pattern. Controlled release behavior of progesterone was observed by incorporation of fatty alcohol in lipid matrix of NLCs (Elmowafy et al. 2018a). Specifically, chain length of saturated fatty alcohols particularly influenced econazole nitrate flux and permeability through porcine skin. The higher the chain length, the higher flux values (Sanna, Caria, and Mariani 2010). The authors attributed that behavior to stronger interaction between solid lipid (precirol) and longer chain fatty alcohol which led to greater disorder of the densely packed lipids in the extracellular spaces of stratum corneum when compared to shorter chains fatty alcohols. On contrary to this study, shorter chain fatty alcohols (C10 and C12) were reported to be more effective as skin penetration enhancers of theophilline in non-nanoparticles' system (Sloan et al. 1998). In addition, bees wax was reported to improve in vitro release of carbamazepine due to higher degradation rate (Elmowafy et al., 2018b). Shifting towards natural lipids was also exploited as safe alternative to synthetic lipids. Ribeiro and coworkers developed lidocaine loaded NLCs with pure natural lipids (copaiba oil, sesame oil and sweet almond oil as liquid lipids and beeswax, shea butter and cacao butter as solid lipids). Authors demonstrated excellent biocompatibility in term of cellular viability and improved in vivo performance in terms of heart rate of zebra fish larvae and blockage of sciatic nerve in mice (Ribeiro et al. 2017).
Table 1.
Commonly used liquid lipids and solid lipids for preparation of NLCs.
| Component | Name | Purpose and reference(s) |
|---|---|---|
| Liquid lipids | Oleic acid |
|
| Caprylic/Capric triglycerides (Miglyol 812)® |
|
|
| α-tocopherol/ Vitamin E |
|
|
| Soy bean oil |
|
|
| Black cumin oil |
|
|
| Caraway essential oil |
|
|
| Olive oil |
|
|
| Sweet almond oil |
|
|
| Squalene |
|
|
| Capmul MCM C8 |
|
|
| Solid lipids | Compritol 888 ATO |
|
| Precirol ATO 5 |
|
|
| Stearic acid |
|
|
| Glyceryl monostearate |
|
|
| Cetyl palmitate |
|
|
| Gelucire® |
|
Regarding surfactant, NLCs may be stabilized by single surfactant or combination of more than one surfactant with content ranging from 1.5% to 5% (w/v). However, surfactant type and concentration play important role in designing NLCs. NLCs are stabilized by different types of surfactant which are efficiently adsorbed onto particles' surfaces reducing the interfacial tension. Mostly, a mixture of (hydrophilic and lipophilic) surfactants is used for the preparation instead of sole surfactant, as the blend improved physical stability and functional properties of the developed system (Hasenhuettl 2008). In some cases, a combination of surfactants and biopolymers had been used (Zheng et al. 2013). Recently, Kanwar and coworkers have formulated biosurfactant (sophorolipids; microbial glycolipid) stabilized NLCs aiming to develop biocompatible drug delivery system. The concentration of the surfactant particularly influences the particle size of NLCs. Generally, the higher the surfactant concentration, the smaller the particle sizes. The most widely used surfactants in the literature are Poloxamer 188 (Liu et al., 2012, Han et al., 2008), Tween 80 (Sharma et al., 2016, Pradhan et al., 2015) and lecithin (Khan et al., 2016, Zhang et al., 2011).
2.1. Methods of preparation and classes of NLCs
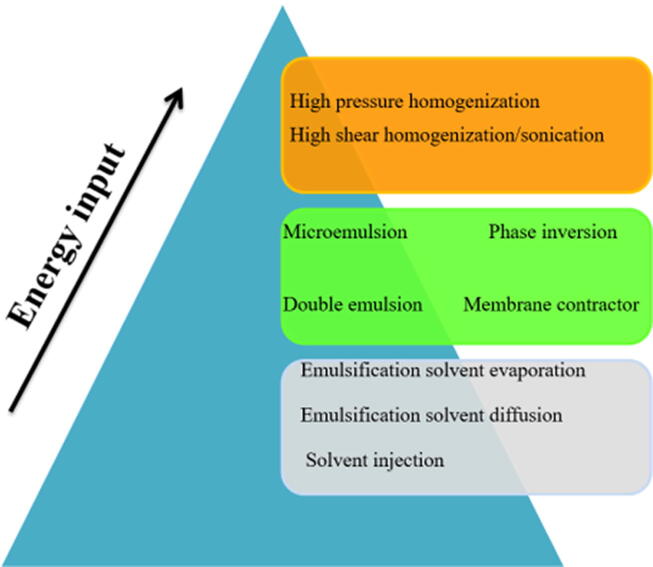
There are methods used for production of NLCs. Based on the energy required, methods can be categorized into three types (Fig. 2).
Fig. 2.
Methods of fabrication NLCs according to energy input.
2.2. High energy required methods
There are many techniques used for production of NLCs and required high energy input such as high pressure homogenization (HPH), high shear homogenization/sonication, supercritical fluids and microwave based. In this review, I will focus on HPH and high shear homogenization/sonication as they are the most widely used techniques.
2.2.1. High pressure homogenization
This method is considered as one of the most preferred methods because no solvents are added during the preparation. It has been considered as a consistent and powerful technique for the large-scale production of NLCs (Das and Chaudhury 2011) as it produces highly stable particles and require no organic solvent addition (Fang et al. 2008).
Hot homogenization: During this method, the drug is added to the molten lipid mixture which dispersed in heated aqueous solution of surfactant using high speed stirring. Finally, the pre-emulsion is further homogenized by high pressure homogenizer. NLCs are formed once the obtained nanoemulsions recrystallize at room temperature. The drawbacks of this method include heat degradation of thermolabile actives, reduction of emulsification power of some surfactants at higher temperatures and low drug encapsulation efficiencies as it may be partitioned in both lipid and aqueous surfactant solution at high temperature which promotes drug escaping into aqueous phase (Üner 2006).
Cold homogenization: In this method, the molten lipid mixture with the drug is solidified by rapid cooling under the effect of liquid nitrogen or dry ice. Subsequently, it is micronized and dispersed in a cold aqueous surfactant solution. The obtained dispersion is finally processed by high pressure homogenizer is applied. This technique can relatively overcome the drawbacks of hot methods such as avoiding heating of drugs and surfactants. Additionally, desired crystal structure can be obtained by controlling the crystallization process. On the other hand, the produced particles may exhibit higher particle sizes and heterogeneity when compared with hot homogenization method (del Pozo-Rodríguez et al. 2009).
2.2.2. High shear homogenization/sonication
In this method, lipophilic drug is dissolved or dispersed in molten solid lipid/liquid lipid mixture. The temperature used should be 10 °C above the melting point of solid lipid to make difficult to recrystallize. The aqueous surfactant solution of the same temperature is poured to lipid phase and pre-microemulsion is formed under the effect of high speed stirrer. The pre-emulsion is further homogenized using high shear homogenizers followed by probe sonicator treatment.
2.3. Low energy required methods:
2.3.1. Microemulsion
Microemulsion is prepared by similar procedure of high shear homogenization/sonication technique. Then the hot microemulsion is added to cold water to form nanoemulsion, which then recrystallizes to form NLC.
2.3.2. Double emulsion
In this method the prepared microemulsion is added to cold water (2–10 °C) which facilliate precipitation of uniformly distributed NLCs particles.
2.3.3. Phase inversion
In this method the whole components' mixture are exposed to three heating and cooling cycles. After that, the hot mixture is shocked by dilution with cold water and NLCs are formed by phase inversion.
2.3.4. Membrane contractor
Small lipid droplets are obtained by pressing the molten lipid against porous membrane. Concurrently, they are circulated inside the membrane module and sweeps away from the pore. NLCs are formed after cooling at room temperature.
2.4. Very low or no energy required methods:
2.4.1. Emulsification solvent evaporation
In this technique, active substance as well as lipids is dissolved in water immiscible solvent. The resultant solution is then emulsified with aqueous surfactant solution. Subsequently, the solvent is evaporated under continuous stirring resulting in NLCs formation. As there is no heat involved, this method is suitable for heat sensitive actives. The main disadvantages of this technique is solvent residue associated toxicity and diluted particles of NLCs due to inadequate solubility of the lipids in the solvents used (Shahgaldian et al. 2003).
2.4.2. Emulsification solvent diffusion
In this method, active substance and lipids are dissolved in organic solvent which is saturated with water for thermodynamic equilibrium. The resultant momentary o/w emulsion is distributed into water under stirring until solidification of the dispersed phase.
2.4.3. Solvent injection
In this method, active substance and lipids are dissolved in organic solvent and injected in with aqueous surfactant solution.
2.5. Types of the developed NLCs
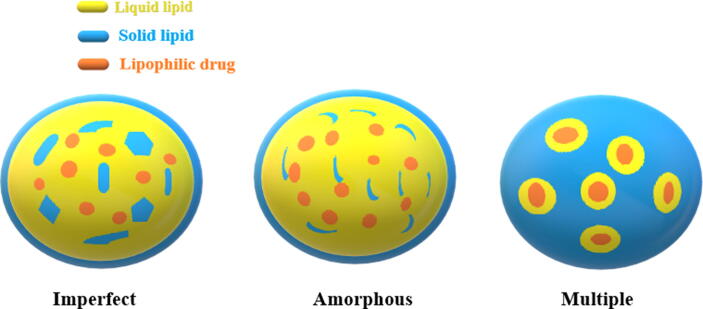
According to the lipidic structure of prepared NLCs, there are three categories of NLCs based on the composition of lipid mixture and the method used for their preparations (Fig. 3).
Fig. 3.
Types of NLCs.
2.5.1. Imperfect type
In this type, imperfection of the lipid matrix is obtained by using lipids which are different in their chemical characters such as carbon chain length and saturation degree. Mixing of such lipids creates crystal lattice disorder and crystallization is altered. Consequently, the lipid matrix can accommodate more drug amounts (Rainer H Müller, Radtke, and Wissing 2002) and it will be less likely to be expelled during storage than in case of using single lipid.
2.5.2. Amorphous type
In this type, obtaining structureless solid amorphous matrix leads to high drug payload as the lipid matrix will crystallize in less ordered amorphous state. Using medium chain triglycerides, hydroxyoctacosanylhydroxystearate, or isopropylmyristate with solid lipid can produce such pattern. Nuclear magnetic resonance (NMR) and differential scanning calorimetry confirm lipid solid state and transition temperature respectively.
2.5.3. Multiple type
In this type, the solid lipid matrix includes several nanosized liquid oil in which the drug is highly dissolved. As a result, the drug encapsulation is increased. In addition the drug is released in controlled release behavior and the drug leakage is less pronounced (Battaglia and Gallarate 2012) (stability factor) as the tiny oil droplets are bounded by solid lipid matrix.
3. Applications of NLCs
3.1. Oral application
As oral route of administration is the most favored route owing to its painlessness, accurate dosing, ease of administration and patient compliance, I will begin with application of NLCs in oral route.
3.1.1. Enhancement of oral bioavailability
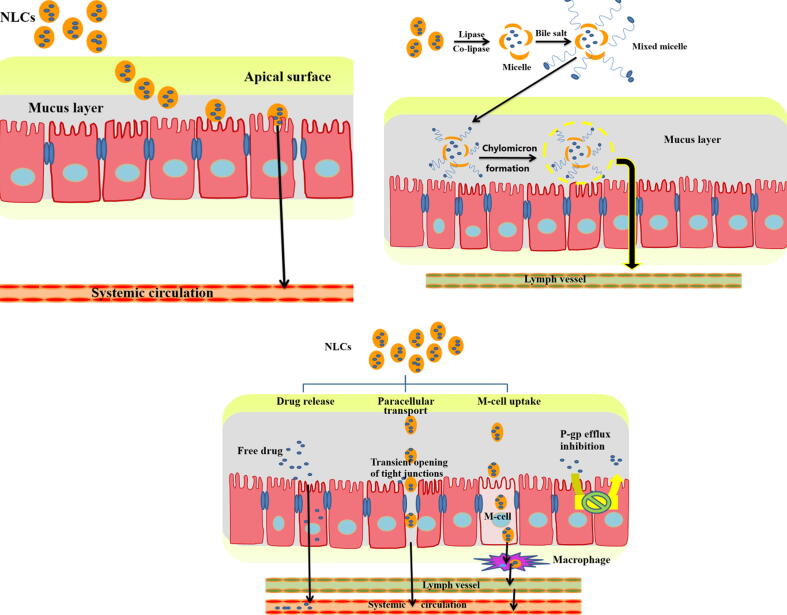
According to the US Food and Drug Administration (FDA), bioavailability can be defined as the rate (how fast the active ingredient goes into the general circulation) and extent (how much of the supposed strength get to the general circulation) to which the active ingredient or active moiety is absorbed from a drug product and becomes available at the site of action (Food 2014). Poor bioavailability is remaining an issue of orally administered drugs. However, it is mainly ascribed to either physiologically associated issues such as extensive first pass effect, enterocytes' efflux transportation, instability of the dug moiety in gastric fluids, fast gastric emptying and restriction by the intestinal barrier or physicochemically/formulatory associated issues such as poor solubility, improper drug partition coefficient and high molecular size of the drug. Interestingly, smart structure of NLCs can overcome most of mentioned poor bioavailability causing factors. However, there are many mechanisms in the literatures were reported for enhancing oral bioavailability (Fig. 4) which will be summarized as follows:
-
•
As low drug solubility represents one of the hurdles that roots low bioavailability, improving drug solubility is considered as one mechanism for enhancing bioavailability especially for class II (such as raloxifene (Shah et al. 2016), resveratrol (Neves et al. 2013), lovastatin (Chen et al. 2010; J. Zhou and Zhou 2015), spironolactone (Beloqui et al., 2014b) and vinpocetine (Zhuang et al. 2010) and class IV (such as saquinavir (Beloqui et al., 2013b) and etoposide (Zhang et al. 2011)) drugs.
-
•
Owing to the fatty nature of NLCs, they are digested by lipase and co-lipase into micelles (consisting of drug and lipis monoglycerides) which stimulate bile flow to form mixed micelles. Mixed micelles are absorbed by chylomicron formation into lymphatic vessels avoiding first pass effect and hence increase the carrier transport across the unstirred layer, presented between the bulk fluid and brush border membrane of enterocytes, enhancing drug absorption (X. Zhou et al. 2015). As the drug is incorporated of in the chylomicron, it will be absorbed alongside by fat absorption process (Trojan horse effect (Müller, Radtke, and Wissing 2002)).
-
•
Nanoparticulate systems including NLCs were reported to improve oral drug bioavailability by intracellular uptake by M−cells of Peyer’s patches (Harde, Das, and Jain 2011).
-
•
Inhibition of efflux transporter (P-gp) through the action of some surfactants used in the preparation such as Tween 80.
-
•
Slow degradation of the particles due to steric hindrance effect of Pluronics used as hydrophilic surfactant during preparation.
-
•
As the drug release from nanoparticles is efficient (large surface area), drug diffusion through gastrointestinal barrier will be maximized as the passive diffusion will continue as long as the concentration gradient is maintained.
-
•
Transient opening of tight junctions (gaps between two adjacent intestinal epithelial cells) due to the effect of highly lipophilic surfactants (Pathak and Raghuvanshi 2015) improving paracellular absorption.
-
•
Adhesion of nanoparticles to the intestinal underlying epithelium leading to improved retention and uptake (Beloqui et al., 2013).
-
•
Increases the residence time in stomach and upper small intestine owing to lipidic nature leading to enhanced absorption.
Fig. 4.
Mechanisms in the literatures by which NLCs can improve oral bioavailability of encapsulated drug.
Table 2 depicts list of some low bioavailable drugs which their bioavailability enhanced after incorporation into NLCs.
Table 2.
Recent studies aiming to improve oral bioavailability of poorly bioavailable drugs.
| Drug | Composition | research outcome |
|---|---|---|
| Olanzapine | The NLCs were composed of glyceryl tripalmitate (solid lipid), castor oil (liquid lipid), Pluronic F-68 and soy lecithin (surfactants). | In rats, NLC formulation was more than 5½-fold increase in oral bioavailability when compared to olanzapine suspension (Jawahar et al. 2018). |
| Raloxifene hydrochloride | The NLCs were composed of glyceryl monostearate (solid lipid) and Capmul MCM C8 (liquid lipid) and polyvinyl alcohol (surfactant). | In dogs, NLC optimized formulation was 3.75 fold enhancement in oral bioavailability when compared to raloxifene hydrochloride suspension (Shah et al. 2016). |
| Rosuvastatin | Lauric acid or stearic acid (solid lipid) and Capryol-90 or oleic acid (liquid lipid) and PEG-25-stearate (surfactant). | In rats, NLC formulations were six to nine fold improvement in the bioavailability of rosuvastatin when compared to Aqueous dispersion. Mixture of long chain fatty acid and medium chain fatty acids exhibited 1.5-fold increase in bioavailability when compared to only medium chain fatty acid (Pokharkar, Patil-Gadhe, and Kaur 2018). |
| Telmisartan | Glyceryl monostearate (solid lipid), oleic acid (liquid lipid) and Tween 20 (surfactant). | in rats, bioavailability of the telmisartan loaded NLC was increased by 2.17 and 3.46 fold compared to that of the marketed formulation and pure drug suspension, respectively (Thapa et al. 2018). |
| Olmesartan medoxomil | Gelucire 44/14 (solid lipid, Capmul MCM EP (liquid lipid) and TPGS (surfactant). | in rats, bioavailability of olmesartan medoxomil loaded NLC was increased by about 5 fold compared to that of the pure drug suspension (Kaithwas et al. 2017). |
| Fenofibrate | Precirol ATO 5 (solid lipid), Captex100 (liquid lipid) and tween-80 (surfactant). | in beagle dogs, bioavailability of Fenofibrate loaded NLCs suspension and solidified NLCs pellets revealed 3.6- and 3.5-fold respectively increase in bioavailability when compared to commercial product (Lipanthyl™ capsule) (Tian et al. 2013). |
| Atorvastatin | Gelucire 43/01 (solid lipid), Capryol PGMC (propylene glycol monocaprylate type I; liquid lipid) and Pluronic F68 (surfactant). | In rats, bioavailability of atorvastatin loade NLCs revealed 3.6- and 2.1-fold increase in bioavailability when compared to atorvastatin suspension and commercial product (Lipitor ™) (Elmowafy et al. 2017), |
| Ezetimibe | Monosteol™ (solid lipid), Capryol™ 90 (liquid lipid), Kolliphor® EL (surfactant) and Transcutol® HP (Cosurfactant). | In rats, bioavailability of ezetimibe loaded NLCs revealed 2.5- and 1.6-fold increase in bioavailability when compared to atorvastatin suspension and commercial product (Lipitor ™) (Shevalkar and Vavia 2019). |
| Amisulpride | Gelucire®43/01(solid lipid), Capryol™90 (liquid lipid) and Tween-80 (surfactant) | The relative bioavailability of NLCs capsules was found to be 252.78% when compared to Amipride® tablets (Assasy et al., 2019). |
| Simvastatin | Stearic acid (solid lipid), oleic acid (liquid lipid) and Pluronic F-68 (surfactant). | After oral administration of a single dose of simvastatin loaded NLC, 4-fold increase in bioavailability was observed, as compared to the simvastatin suspension (Fathi et al. 2018). |
3.1.2. Treatment of GIT local diseases
Local diseases in GIT such as inflammatory bowel diseases (as Crohn’s disease and ulcerative colitis) are characterized by highly secreted mucous, crypt distortions, ulcers and immune cell infiltration. Inflammatory bowel diseases are also considered as targets of orally administered NLCs due to their enhanced adhesion to and retention in gut wall epihthelium (Beloqui et al., 2014b). In addition, and regarding to such disease, the physiological characteristics of intestinal barrier are changed specially for intestinal lipids which are deficient in such cases (Beloqui et al., 2014a). Furthermore, NLCs have the ability to modulate immune response (Landesman-Milo and Peer 2012). Sinhmar and coworkers developed budesonide loaded Eudragit S100 coated NLCs in order to achieve colonic targeting (Sinhmar et al., 2018a). Though the authors performed only the in vitro evaluation of enteric-coated pellets, they concluded that Eudragit S100 coated NLCs could be used as a promising tool for the treatment of inflammatory bowel disease. Chanburee and Tiyaboonchai examined the influence of polymer types (polyethylene glycol, polyvinyl alcohol and chitosan) on the mucoadhesive properties of polymer-coated NLCs in order to deliver curcumin to intestinal mucosa (Chanburee and Tiyaboonchai 2017). Polyethylene glycol and polyvinyl alcohol coated NLCs exhibited higher adhesion with porcine intestinal mucosa than chitosan coated formulation. Authors attributed that to larger particle size and un-protonated chitosan at neutral pH leading to reduced interaction with mucin. Sinhmar and coworkers fabricated mannosylated NLCs for active targeting of budesonide to the inflamed bowel tissues (Sinhmar et al., 2018b). As the surface of macrophages present at the site of inflammation overexpress C-type lectin receptors which can easily recognize mannose, decoration of mannose group to the NLC surfaces could actively deliver the encapsulated drug to inflamed tissues. And aiming to maximize drug release at the desired location and protect the system from drastic gastric conditions, the system was further coated with Eudragit S100. Cytotoxicity results proved that the developed NLCs were non-toxic whereas in vivo evaluation revealed marked decrease in clinical activity scoring, macroscopic and microscopic indexing, colonic myeloperoxidase activity and inflammatory cytokines. Beloqui and coworkers successfully develop curcumin (Beloqui et al., 2016a) and budesonide (Beloqui et al., 2013c) loaded NLCs and deliver them to inflammatory bowel diseases. Authors suggested that using of anti-inflammatory drugs in lipid based nanocarrier could be a powerful strategy to target inflammatory bowel diseases. Regarding curcumin study, the authors compared NLCs with self nanoemulsifying drug delivery systems (SNEDDS) and lipid core–shell protamine nanocapsules (NC). In vitro results showed that NC exhibited the highest permeability across Caco-2 cell monolayers whereas only NLCs and SNEDDS could significantly reduce tumor necrosis factor alpha (TNF-α) secretion by lipopolysachharide-activated macrophages. Interestingly via in vivo evaluation, only NLCs could reduce submucosal edema and altered mucosa structures during histopathological examination and neutrophil infiltration and the expression of the pro-inflammatory cytokine TNF-α. Regarding budesonide, blank and drug loaded NLCs decreased reduced in vitro TNF-α secretion by about 100% and in vivo IL-1β and TNF-α in the colon with marked histological improvement of altered tissue characteristics.
3.1.3. Mitigation of drug associated toxic effects
In case of highly metabolized drugs in liver, overproduction of highly reactive metabolite is considered as the main toxicity predisposing factor. So, bypassing liver and absorption via lymphatic pathway could minimize the production of such metabolite and then alleviate hazardous effects. Elmowafy and coworkers formulated carbamazepine in bees wax containing NLCs in order to enhance drug dissolution, diminish plasmatic fluctuation and lessen carbamazepine induced toxicity (Elmowafy et al., 2018b). The authors studied the hepatic and testicular toxicity after 2 months of administration. NLCs exhibited the safest formulation when compared with carbamazepine suspension and market product (Tegretol™) in terms of biochemical, histological and immunohistochemical changes. As the toxicity of carbamazepine was directly correlated to the reactive metabolite; carbamazepine-10,11-epoxide (Kalapos, 2002, Martins et al., 2015), the authors suggested that bypassing liver by NLCs will help in lessen toxicity though they did not determine serum carbamazepine-10,11-epoxide concentrations.
3.2. Cutaneous application
Shifting of the administration to be through the skin is essential if the local cutaneous effect (dermal) is desired as the skin is then the target site of action (in case of local diseases such as acne, dematitis and dermal fungal infection) or systemic effect (transdermal) is desired as the active is subjected to acid degradation or first pass effect followed oral administration. For both effects, the outermost layer; stratum corneum (SC) is considered as the main barrier hurdles the absorption. SC is a horny layer formed by corneocytes due to apoptosis of keratinocytes consisting of ceramides (45–50%), cholesterol (25%), long chain free fatty acids (22 and 24 carbon atoms chain length; 15%) and other lipids (5%). These lipids are ordered in multilamellar bilayers. The role of SC is mainly protective against water loss and invading toxic molecules and microorganisms. The cutaneous drug diffusion may take place by transepidermal route (across intact epidermis and represents the predominant route) and/or transappendageal route (through skin appendages such as hair follicles) (Trommer and Neubert 2006). The later is often targeting sebaceous glands aiming to control sebaceous glands associated diseases such as seborrheic dermatitis, alopecia and acne.
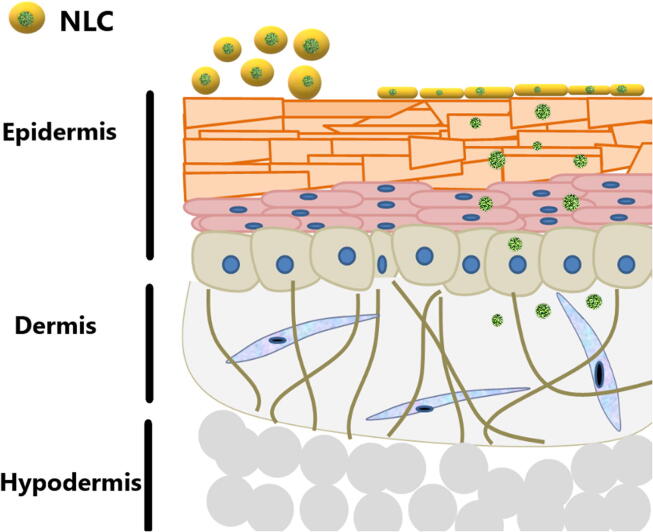
Conventional skin preparations such as ointments, creams gels and hydrogels particularly varied in their skin delivery pattern. For example fatty based ointment prolongs hydrophobic drug release whereas hydrogels and O/W creams offer a rapid release. In addition, the viscosity of the system remains more time at the site of application which in turn prolongs drug delivery. Undoubtly, NLCs overweigh conventional formulations in both dermal and transdermal routes (Fig. 5). NLCs can guard incorporated drugs against chemical degradation, allow adapting drug release and increase drug absorption, which cannot be achieved in conventional formulations (Souto, Müller, and Alemieda 2007). However, NLCs can overcome SC barrier and penetrate through the skin (Fig. 6) for therapeutic and cosmetic purposes via different mechanisms:
-
•
Formation of film above the skin surface. This film could consequently improve skin hydration by diminishing water loss (Muller et al., 2007) and improving drug penetration throughout the SC (Junyaprasert et al. 2009).
-
•
Creating controlled occlusive effect due to small particle size. This occlusion improves hydrates the SC, which subsequently increases the diffusion of the actives into the deeper skin layers (Gomes et al. 2014).
-
•
Rearrangement of SC lipids, which aids penetration of actives.
-
•
Miscibility/mixing of NLCs lipid constituents with stratum corneum lipids also helps penetration.
-
•
Inclusion of surfactant disrupts of skin structure and improves absorption.
-
•
Creation of distinct alteration in intercellular packing, decreased corneocyte packing and wider inter-corneocytes gaps (Zhai and Zhai 2014).
Fig. 5.
Advantages of NLCs over conventional formulations for cutaneous delivery.
Fig. 6.
Penetration of NLCs through SC for dermal and transdermal effects.
3.2.1. Dermal effect
Treatment of local cutaneous disorders can be achieved by NLCs in order to maximize therapeutic efficacy as NLCs are preferentially accumulated in the skin. Chen-yu fabricated quercetin-loaded NLCs in order to utilize drug activity against reactive oxygen species-mediated damage in the skin (Chen-yu et al. 2012). Authors found that the amount of quercetin deposited into epidermis and dermis from NLCs after 12 h of in vivo skin permeation study was significantly higher than (1.52 and 3.03 times respectively) that of quercetin solution. Histological examination of skin specimens showed weakened and swollen SC after NLCs application. Consequently, NLCs produced higher efficacy in inhibiting xylene induced ear edema than normal saline suspension injected intraperitoneally. Specifically, Elmowafy and coworkers studied skin delivery of dapsone via differently charged NLCs in order to achieve maximum anti-acne and anti-rosaceae activities (Elmowafy et al. 2019). The authors depended upon hydrophilic surfactant for creating surface charge in which; Tween 80 induced negatively charged particles, cetyltrimethylammonium bromide induced positively charged particles and Transcutol P induced neutral particles. Dapsone from positively charged particles deposited in higher amounts in full thickness skin than negatively charged and neutral particles. The authors attributed the results to electrostatic attraction between positively charged particles and negatively charged lipids in SC. As a result, complete healing of the skin layers in croton oil induced rosaceae model. On the contrary, Noor and co-workers developed dutasteride loaded NLCs coated with stearic acid-chitosan oligomer to improve delivery to the hair follicles (Noor et al. 2017). Recently, Chaiyana and coworkers have utilized NLCs for enhancing the Ocimum sanctum Linn. extract (anti-aging) skin delivery. The authors used rosmarinic acid as a marker for extract quantification and found that NLCs can efficiently deposited in the skin with no recorded skin permeation (Chaiyana et al. 2020). Arsenie and coworkers prepared NLCs loaded with azelaic acid, white willow bark extract and panthenol to augment overall skin care in terms of hydration and antioxidant efficacy (Arsenie et al. 2020). Müller and coworkers studied skin delivery of silver (Ag)-NLC complex for controlling different degrees of atopic dermatitis (Muller et al., 2007). As atopic dermatitis is manifested by an unorganized barrier of the skin and abundance of bacterial infection, adhesive NLC was relevant remedy as it is able to build up and repair the distorted barrier by adhesion and film formation. Coating of NLCs shifted the surface charge into positive value and had negative effect on skin deposition in coating concentration dependent manner. Authors attributed that to the different surface charge of the polysaccharide from chitosan and the epithelium. Puglia and co-workers formulated ketoprofen and naproxen loaded NLCs to achieve maximum dermal effect with controlled release behaviour (Puglia et al. 2008). The authors found that ketoprofen and naproxen formulated in NLCs showed enhanced accumulations in horny layer of the skin and decreased permeation through the skin when compared to corresponding free drugs. They also exhibited extended anti-inflammatory activity with prolonged release in the epidermis.
Taking physical state of applied NLCs into our consideration, they may be applied either as colloidal dispersion or incorporated into suitable semisolid base to offer proper formulation consistency for local application. Incorporation of NLCs into semisolid dosage form can be achieved by direct addition of NLCs to already prepared semisolid base (e.g. gels or creams) or adding viscosity enhancing agent (such as hydroxypropyl methylcellulose, pluronics, chitosan and Carbopol) to the aqueous phase or direct one-step production process of the final product (Pardeike, Hommoss, and Müller 2009). Agrawal and co-workers optimized acitretin loaded NLCs formulation with regard to mean particle size and % entrapment efficiency as responses (Agrawal, Petkar, and Sawant 2010). The optimized formulation was incorporated in Carbopol 934P (1% w/w). In vitro skin (human cadaver skin) accumulation study showed that significant higher amount of acitretin was deposited from NLC gel (81.38 ± 1.23%) when compared to acitretin plain gel (47.28 ± 1.02%). Clinically, anti-psoriatic activity was examined on 12 patients and NLC gel showed significant reduction in scaling and Psoriasis Area and Severity Index score from the initial level when compared to acitretin plain gel. Arora and co-workers studied the synergistic effect of Carbopol 934 hydrogels based on SLN and NLC-loaded cyclosporine and calcipotriol in order to treat psoriasis (Arora et al. 2017). Ex vivo results showed higher drug penetration and epidermis retention of NLCs, when compared to free drug. In vivo anti-psoriatic effect showed superiority of NLCs hydrogel over SLN and commercial gel. Gupta and Vyas formulated fluconazole loaded NLCs to improve skin accumulation and antifungal effect (Gupta and Vyas 2012). In vivo skin-retention studies exhibited higher fluconazole skin deposition (5-fold) in the case of NLC formulation. Gu and co-workers developed and evaluateed the triptolide loaded NLCs in order to increase dermal efficacy against edema and inflammation associated with rheumatoid arthritis (Gu et al. 2019). Pharmacokinetic study of NLCs revealed that triptolide skin concentration was higher than plasma level. However, NLCs decreased knee edema and the levels of inflammatory mediators accompanied with rheumatoid arthritis. Regarding market product, Cutanova Nanorepair Q10™ cream was the first commercial NLC product and introduced in October 2005 (Pardeike, Schwabe, and Müller 2010). FloraGlo™ is another example of NLCs commercial product which contains lutein (the active ingredient; antioxidant), safflower oil (liquid lipid) mixed with a solid lipid and surfactants to produce stable, controlled release and skin permeable NLC formulation (Carbone et al. 2013).
3.2.2. Transdermal effect
Transdermal drug delivery (TDD) offers an alternative route for reaching systemic circulation especially for these drugs which are suffering from low bioavailabilities and/or plasma fluctuations. TDD can avoid GIT conditions and first pass metabolism. In addition, it can control drug release and avoid overdosing of drugs suffering from fluctuation in plasma levels following oral and parenteral administrations. As transdermal delivery requires diffusion of drug molecule through skin layers and subsequent subcutaneous tissues to reach systemic circulation, TDD is governed by drug related factors such as lipid solubility, melting point and molecular weight. Lipid soluble drugs can diffuse across the skin mainly by the intracellular pathway, whereas water soluble drugs diffuse through the intercellular pathway (W. J. Lin and Duh 2016). The substance of molecular weight less than 0.6 kDa is well absorbed.
However, NLCs are considered as promising TDD system for encapsulated drugs. Mendes and co-workers designed donepezil loaded NLCs and incorporated them into Carbopol 940 gel base in order to increase transdermal permeability under the influence of different permeation enhancers (Mendes et al. 2019). In vitro skin permeation results showed that donepezil permeation from NLC gel was increased by the enhancing effect of their components and lipid nanocarriers themselves. The study demonstrated that only 0.56% of the amount of donepezil applied to the skin is retained. Chen and co-workers developed ropivacaine loaded NLCs in order to increase transdermal permeability and local anesthetic effect (Chen et al. 2015). In vitro skin permeation study showed that the steady-state flux (Js) of NLCs was about two fold when compared to propylene glycol drug solution for 24 h. pharmacodynamic activity showed about 89.1% inhibition rate of writhing response. Histologically, blank and drug loaded NLCs showed swollen structure within SC. Kapoor and co-workers designed, optimized and evaluated amlodipine loaded NLCs and incorporated them into Carbopol 940 gel base in order to increase transdermal permeability under the influence of different permeation enhancers (Kapoor et al. 2019). Confocal laser scanning microscopy study showed that the optimized NLCs were distributed deeply and to a greater extent throughout the subcutaneous, viable epidermis and dermis. Importantly, high AUC value was observed with optimized NLCs when compared to oral tablet (market product) indicating higher bioavailability (relative bioavailability % was about 1.23%). Acid sensitive lansoprazole was also protected through transdermal effect of NLCs (Lin and Duh 2016). The study showed that 3.75% isopropylmyristate containing NLC hydrogel greatly enhanced drug penetration. Pharmacokinetic study revealed that elimination rate constant of intravenous lansoprazole solution was higher than NLC hydrogel indicating prolonged plasma residence and hence extended inhibition of gastric acid secretion. Recently, Ocimum sanctum L. leaf extract (rich in ursolic acid) loaded NLCs were prepared and evaluated for anti-inflammatory effect after topical application (Ahmad et al. 2018). NLCs showed excellent anti-arthritic activity when compared to standard marketed diclofenac gel. In other study, Amorndoljai and coworkers developed ginger extract loaded NLCs for treatment of knee osteoarthritis (Amorndoljai et al. 2015).
3.3. Pulmonary application
Lung offers plentiful advantages as a delivery route for noninvasive actives for localized and generalized acting drugs. It offers large surface area (ca.100 m2), high perfusion (about 5 L/min) and slow drug metabolism. If local delivery is desired (as in case of asthma, lung cancer and cystic fibrosis), the particles should be adjusted to be more relevant to be accumulated in targeted pulmonary region. However, respiratory airway systems have biological barriers including mucus, ciliated cells and alveolar macrophages which hurdle pulmonary delivery. If the drug is targeting the upper respiratory tract, it is cleared by ciliated cells whereas those drugs localized in lower respiratory tract, they will be engulfed and digested by alveolar macrophages (Sung, Pulliam, and Edwards 2007). Lipid nanocarrires present one of the most suitable systems for pulmonary drug delivery as they can reach lower respiratory tract if their particle sizes are less than 0.5 μm resulting in high drug accumulation and diffusion (Jaques and Kim 2000). In addition, smaller particle sizes (less than 260 nm) were reported to avoid macrophagal clearance (Lauweryns and Baert 1977). Specifically, lipophilic constituents contributes enhanced bioadhesive characteristics and sustained release behavior of NLCs (Patlolla et al. 2010). Patlolla and coworkers examined celecoxib loaded NLCs for targeting lung through investigation of drug release, aerodynamic properties and cytotoxicity. Controlled release behavior and suitable aerodynamic diameter were obtained. The majority of nebulized NLCs were deposited in alveolar area of mice. Pharmacokinetics study revealed constant plasma levels for 6 h (Patlolla et al. 2010). Pardeike and coworkers developed isotonic, sterile and non-toxic itraconazole loaded NLCs in order to treat pulmonary aspergillosis in falcons (Pardeike et al. 2016). Nebulized NLCs particle size was in range of 100–200 nm confirming penetration and accumulation in deep respiratory tract. Gamma scintigraphic images showed that nebulized NLCs got in touch with two of the principal centers of infection indicating the efficacy of the system in treatment of aspergillosis. Mannosylated rifampicin loaded NLCs were also developed in order to target alveolar macrophages via recognition by via mannose receptor uptake for treatment of tuberculosis (Vieira et al. 2017). Nafee and coworkers developed sildenafil loaded NLCs in order to treat pulmonary hypertension (Nafee, Makled, and Boraie 2018). NLCs proved the superiority above free solution in terms of low incidence of intra-alveolar bleeding and normality of lung tissue parenchyma.
3.4. Ocular application
As the eye has an exclusive anatomical structure, it is a highly sheltered organ and challenging for drug delivery systems. There are many obstacles that hamper ocular bioavailability such as tough blood-ocular barriers, muco-aqueous barrier, lymphatic tear turnover, non-pigmented layer of the ciliary epithelium, nasolacrimal drainage (more than 75% of solutions are drained through nasolacrimal duct) and reflex blinking (Diebold and Calonge 2010). The goals of ocular DDS are not only to improve ocular drug absorption but also to lessen the systemic absorption and hence generalized side effects. There are many drug delivery systems such as nanoparticles, microemulsions, spanlastics, liposomes and micelles were formulated to defeat the inadequacy of conventional ocular systems. Among them, lipid nanoparticles were reported to improve the corneal permeation of actives providing proficient ocular delivery (Sánchez-López et al. 2017). Generally, NLCs can overcome ocular barriers via different mechanisms:
-
•
Prolongation of drug release and hence residence time of the encapsulated drug.
-
•
Improvement of ocular bioavailability of the encapsulated drug via both transcellular and paracellular mechanisms.
-
•
Conquering blood ocular barriers.
-
•
Fortification of the encapsulated drugs against inactivation by lacrimal enzymes.
-
•
Raising the patient compliance by decreasing the dosing frequency.
In addition, NLCs are easily sterilized on large scale and reasonably harmless for ocular drug delivery due to their biocompatible lipids and surfactants and freedom from organic solvents. Lakhani and coworkers optimized amphotericin B loaded PEGylated NLCs (by using Box-Behnken design) and studied ocular biodistribution of optimized formulation after topical application (Lakhani et al. 2019). Results showed enhanced antifungal activity against wild type Candida albicans and amphotericin B resistant Candida albicans with superiority above commercial products (Fungizone™ and AmBisome™). In vitro transcorneal study showed that amphotericin B was detedcted at 7th hour in all ocular tissues after application of PEGylated NLCs and reached comparable levels with AmBisome™. Shen and coworkers developed cysteine-polyethylene glycol stearate and cysteine surface modified cyclosporine A loaded NLCs and examined mucoadhesion properties and corneal deposition (Shen et al. 2009). Results showed that the mucoadhesion of cysteine-polyethylene glycol stearate and cysteine surface modified NLCs were significantly enhanced when compared to the polyethylene glycol stearate and non-modified NLCs. Cysteine surface modified NLCs exhibited longer surface residence (up to 6 h) in the cul-desac when compared to non-modified NLCs. Chitosan coating was also point of interest of many researchers. Tian and coworkers developed flurbiprofen loaded NLCs partially coated with deacetylated chitosan. The coated formulation exhibited higher zeta potential, improved stability, high precorneal retention and enhanced transcorneal flurbiprofen permeation (B. Tian et al. 2012). Li and coworkers studied the transport mechanism of three chitosan derivatives surface modified NLCs to the anterior chamber through the cornea (Li et al., 2017a). The derivatives used for NLCs surface decoration were chitosan-N-acetyl-L-cysteine, chitosan oligosaccharides and carboxymethyl chitosan. Results showed that all coated formulations were of stronger resistant effect than solution and uncoated NLCs. However, Results showed that chitosan-N-acetyl-L-cysteine and chitosan oligosaccharides surface decorated NLCs higher penetration across the complete corneal epithelium barrier, lower conjunctival to corneal permeability ratio and higher bioavailability when compared to carboxymethyl chitosan decorated NLCs. Authors attributed weaker effect carboxymethyl chitosan to its time-limited hydrogel effect and the anion effect that carboxymethyl acts. Balguri and coworkers studied the effect of phospholipid chain length and molecular weight of polyethylene glycol on transcorneal permeation and posterior tissue penetration of ciprofloxacin loaded NLCs (Balguri et al. 2017). Results showed that phospholipid chain length did not influence transcorneal permeation whereas the most advantageous molecular weights of polyethylene glycol lied between 2 K and 5 K.
3.5. Brain application
To avoid invading by hazards, brain is highly shielded by diffusion restricting barrier called blood brain barrier (BBB). BBB can confines diffusion of the majority of macromolecules (100%) and small (98%) (Gabathuler 2010). Diffusion of the molecules into the brain by paracellular and transcellular pathways is restricted by the protective roles of tight junctions and efflux transporters (prominently Pgps and MRPs), respectively (Baratchi et al. 2009). So, the sole way to reach the brain in therapeutic concentration is via receptor-mediated endocytosis (Kreuter 2014). Wu and coworkers developed transferrin receptor monoclonal antibody OX26 surface decorated NLCs aiming to deliver salvianolic acid B and baicalin to the brain. Surface decorated NLCs enhanced brain delivery of both drugs when compared to NLCs and solution (Wu et al. 2019). Khan and coworkers investigated brain delivery by evaluation of anticonvulsant and anxiolytic effects of carbamazepine loaded NLCs (Khan et al. 2020). Zhao and coworkers designed lactoferrin decorated NLCs to deliver nimodipine (neuroprotective agent) to brain tissue efficiently (Zhao et al. 2018). In vivo biodistribution assay using DiR as the fluorescence probe showed that lactoferrin decorated NLCs the highest brain deposition.
4. Safety/toxicity aspects
Safety/toxicity of NLCs is considered as one of the main concerns. However, few researchers in the literature paid attention to safety profile. So, we have tried to gather a brief report on the safety/toxicity of the NLCs stated in the literature. Regarding oral application, NLCs are considered as relatively safe nanocarriers owing to the content of biodegradable and physiological lipids which are well tolerated in both in vitro cytotocity and in vivo studies. On the other hand, NLCs contain less quantities of surfactants and cosurfactants when compared to emulsions which improve their safety profile. Rahman et al. studied the toxicity of zerumbone-loaded NLCs on BALB/c mice model after oral administration (Rahman et al. 2014). Based on histopathological alterations, they reported that NLCs did not exhibit any signs of toxicity on lungs, liver and kidney and higher lethal dose (LD50) dose of NLCs was reported. In vitro Caco-2 cells cytotoxicity studies showed that NLCs system did not show significant cytotoxicity and cell viability was >90% (Zhou et al. 2012). In another study, it was demonstrated that the cytotoxicity (on lymphocytes) of NLCs was dependent on the number particles of NLCs in millilitre; 2.1 × 1011 particles/ml caused a decreased in the viability of lymphocytes (about 55%) (Mendes et al. 2015). Regarding cutaneous application, Bruge et al. studied cytotoxic effect of five commonly used solid lipids on human dermal fibroblast. They reported that Compritol ™ 888 ATO was the safest lipid owing to its neutral cytotoxic effect (Brugè et al. 2015). Fang et al. reported that enhancer could generally irritate the skin but not correlated to penetration power of the enhancer. They also reported that fatty acids usually presented the most irritating properties, followed by Azone, D-limonene, and L-alpha-lecithin. A complete portrait of the efficacy and safety of commonly used enhancers was therefore established in this study (Fang et al. 2003). Regarding ocular delivery, NLCs are considered relatively safer for ocular delivery owing to the their biocompatible lipids, non-ionic and biocompatible surfactants and organic solvent-free formulations (Salvi and Pawar 2019). However, the degree of clearance and the toxicity are mainly based on the site of administration (topical, intravitreal, intravenous, transscleral, suprochoroidal or subretinal) (Beloqui et al., 2016a). Liu et al. formulated mangiferin loaded NLCs for the treatment of cataract. They were investigated for its irritancy potential and ocular tolerability. They showed good safety profile (Liu et al. 2012). Gonzalez-Mira optimized flurbiprofen-loaded NLC by central composite factorial design based on concentration of drug, ratio of liquid and solid lipid and concentration of surfactant. The optimized formulation was safe and non-irritant to the eye (Gonzalez-Mira et al. 2010). Regarding pulmonary delivery, various studies showed minimal in vitro cytotoxicity of NLCs (Patil-Gadhe and Pokharkar, 2014, Li et al., 2017b, Patlolla et al., 2010, Liang et al., 2017). In addition, no inflammation or change in the integrity of alveoli (Jyoti et al. 2015), pulmonary edema(Patel et al. 2013) or pathological changes in lung and liver(Song et al. 2015) were observed after inhalation of NLCs.
5. Approaches to clinical trials
Although NLCs possess great potential as drug delivery carriers, preclinical and clinical studies are still insufficient. Therefore, there is a need to expand the spectrum of their applications to include clinical trial under appropriate ethical regulations. This might be attributed to the lack of critical analysis on the safety profile of NLCs as drug carriers. However, cutaneous and oral applications were the majors in that regard. For example, lovastatin, antihypercholesterolemic agent used for the treatment of patients with moderate hypercholesterolemia has been formulated in NLCs that showed increased stability and clinical efficacy (Chen et al. 2010). Acitretin, has been formulated in NLCs for topical treatment of psoriasis (Agrawal, Petkar, and Sawant 2010).
As a final point, the promising characteristics of NLCs can be further trailed with more studies on their absorption, distribution, metabolism, and excretion. Additionally, methods to upscale their production, and on their application in clinical trials in the near future should be also clinically investigated. The results are expected to offer an unconventional way for a safer and more competent delivery system.
6. Perspectives and conclusion
The development of drug delivery system is an unending demanding scheme that combines multidisciplinary study attempts in different areas. NLCs are binary lipid-based nanocarriers containing blend of both solid lipid and liquid lipid which allow the entrapment of lipophilic actives, protecting them from degradation and improving their stability. They are composed of FDA approved surfactants and biocompatible lipids which makes them safe for use. Components of NLCs should be carefully selected as they will directly influence product stability and effectiveness. The easiness of successful fabrication shifted them into convenient large scale production especially by high pressure homogenization. However, they are extensively used in the last decade in the pharmaceutical and biomedical fields as they gather key points of smart formulation such as high drug payload, capability to target specific sites by surface modification and increased knowledge of the fundamental mechanisms of transport via various routes of administration. Consequently, they can be used for treatment and control of various conditions in different applications. Owing to reduced particle degradation and extended GIT residence times after oral administration, NLCs represent supreme contenders for enhancing drug bioavailability, treatment of inflammatory bowel diseases and alleviation of drug induced toxicity. With regard to the cutaneous applications, NLCs provide a convenient carrier for dermal and transdermal drug delivery as they can hydrate skin and mix with skin lipid eventually. For pulmonary application, NLCs present favorable aerosolization characteristics and convenient stability. In addition, they can overcome the resident barriers and accumulate in the lung. When applied to the eye, they offer extended residence time increasing ocular bioavailability of the actives with no/little toxic effects. Though the tenacious barrier shielding the brain, NLCs can attain the brain by surface decoration which in turn can pass BBB by receptor mediated transcytosis. Taking into account the growing number of patent in the last few years, NLCs should have a fair chance for clear clinical translation and pharmaceutical marketing in all applications.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this work through the project number “375213500”.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to extend their sincere appreciation to the central laboratory at Jouf University for support this study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agrawal Y., Petkar K.C., Sawant K.K. Development, evaluation and clinical studies of acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. Int. J. Pharm. 2010;401(1–2):93–102. doi: 10.1016/j.ijpharm.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Ahmad A., Abuzinadah M.F., Alkreathy H.d.M., Banaganapalli B., Mujeeb M. Ursolic acid rich ocimum sanctum L leaf extract loaded nanostructured lipid carriers ameliorate adjuvant induced arthritis in rats by inhibition of COX-1, COX-2, TNF-α and IL-1: pharmacological and docking studies. PLoS ONE. 2018;13(3) doi: 10.1371/journal.pone.0193451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorndoljai P., Taneepanichskul S., Niempoog S., Nimmannit U., Chasriniyom C. The efficacy and safety of ginger extract in nanostructure lipid carrier (NLC) for treatment of knee osteoarthritis (OA) Integr. Med. Res. 2015;4(1):92. doi: 10.1016/j.imr.2015.04.146. [DOI] [PubMed] [Google Scholar]
- Arora R., Katiyar S.S., Kushwah V., Jain S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: a comparative study. Exp. Opin. Drug Deliv. 2017;14(2):165–177. doi: 10.1080/17425247.2017.1264386. [DOI] [PubMed] [Google Scholar]
- Arsenie L.V., Lacatusu I., Oprea O., Bordei N., Bacalum M., Badea N. Azelaic acid-willow bark extract-panthenol – loaded lipid nanocarriers improve the hydration effect and antioxidant action of cosmetic formulations. Ind. Crops Prod. 2020;154(May) doi: 10.1016/j.indcrop.2020.112658. [DOI] [Google Scholar]
- Assasy A.-H., El I., Younes N.F., Makhlouf A.I.A. Enhanced oral absorption of amisulpride via a nanostructured lipid carrier-based capsules: development, optimization applying the desirability function approach and in vivo pharmacokinetic study. AAPS PharmSciTech. 2019;20(2):82. doi: 10.1208/s12249-018-1283-x. [DOI] [PubMed] [Google Scholar]
- Balguri S.P., Adelli G.R., Janga K.Y., Bhagav P., Majumdar S. Ocular disposition of ciprofloxacin from topical, PEGylated nanostructured lipid carriers: effect of molecular weight and density of poly (ethylene) glycol. Int. J. Pharm. 2017;529(1–2):32–43. doi: 10.1016/j.ijpharm.2017.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratchi S., Kanwar R.K., Khoshmanesh K., Vasu P., Ashok C., Hittu M., Parratt A., Krishnakumar S., Sun X., Sahoo S.K. Promises of nanotechnology for drug delivery to brain in neurodegenerative diseases. Curr. Nanosci. 2009;5(1):15–25. [Google Scholar]
- Bashiri S., Ghanbarzadeh B., Ayaseh A., Dehghannya J., Ehsani A., Ozyurt H. Essential oil-loaded nanostructured lipid carriers: the effects of liquid lipid type on the physicochemical properties in beverage models. Food Biosci. 2020;35(January) doi: 10.1016/j.fbio.2020.100526. [DOI] [Google Scholar]
- Battaglia L., Gallarate M. Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery. Exp. Opin. Drug Deliv. 2012;9(5):497–508. doi: 10.1517/17425247.2012.673278. [DOI] [PubMed] [Google Scholar]
- Beloqui A., Solinís M.A., Delgado A., Évora C., del Pozo-Rodríguez A., Rodríguez-Gascón A. Biodistribution of Nanostructured Lipid Carriers (NLCs) after intravenous administration to rats: influence of technological factors. Eur. J. Pharm. Biopharm. 2013;84(2):309–314. doi: 10.1016/j.ejpb.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Beloqui A., Coco R., Alhouayek M., Solinís M.Á., Rodríguez-Gascón A., Muccioli G.G., Préat V. Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. Int. J. Pharm. 2013;454(2):775–783. doi: 10.1016/j.ijpharm.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Beloqui A., Coco R., Memvanga P.B., Ucakar B., des Rieux A., Préat V. PH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. Int. J. Pharm. 2014;473(1-2):203–212. doi: 10.1016/j.ijpharm.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Beloqui A., Memvanga P.B., Coco R., Reimondez-Troitiño S., Alhouayek M., Muccioli G.G., Alonso M.J., Csaba N., de la Fuente M., Préat V. A comparative study of curcumin-loaded lipid-based nanocarriers in the treatment of inflammatory bowel disease. Colloids Surf., B. 2016;143:327–335. doi: 10.1016/j.colsurfb.2016.03.038. [DOI] [PubMed] [Google Scholar]
- Beloqui A., Solinís M.Á., Delgado A., Évora C., Isla A., Rodríguez-Gascón A. Fate of nanostructured lipid carriers (NLCs) following the oral route: design, pharmacokinetics and biodistribution. J. Microencapsul. 2014;31(1):1–8. doi: 10.3109/02652048.2013.788090. [DOI] [PubMed] [Google Scholar]
- Beloqui A., Solins M.N., Gascn A.R., del Pozo-Rodrguez A., des Rieux A., Prat V. Mechanism of transport of saquinavir-loaded nanostructured lipid carriers across the intestinal barrier. J. Control. Release. 2013;166(2):115–123. doi: 10.1016/j.jconrel.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Beloqui A., Solinís M.Á., Rodríguez-Gascón A., Almeida A.J., Préat V. Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016;12(1):143–161. doi: 10.1016/j.nano.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Brugè F., Damiani E., Marcheggiani F., Offerta A., Puglia C., Tiano L. A comparative study on the possible cytotoxic effects of different nanostructured lipid carrier (NLC) compositions in human dermal fibroblasts. Int. J. Pharm. 2015;495(2):879–885. doi: 10.1016/j.ijpharm.2015.09.033. [DOI] [PubMed] [Google Scholar]
- Carbone C., Cupri S., Leonardi A., Puglisi G., Pignatello R. Lipid-based nanocarriers for drug delivery and targeting: a patent survey of methods of production and characterization. Pharm. Pat. Anal. 2013;2(5):665–677. doi: 10.4155/ppa.13.43. [DOI] [PubMed] [Google Scholar]
- Chaiyana W., Anuchapreeda S., Somwongin S., Marsup P., Lee K.-H., Lin W.-C., Lue S.-C. Dermal delivery enhancement of natural anti-ageing compounds from ocimum sanctum linn. Extract by nanostructured lipid carriers. Pharmaceutics. 2020;12(4):309. doi: 10.3390/pharmaceutics12040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanburee S., Tiyaboonchai W. Mucoadhesive nanostructured lipid carriers (NLCs) as potential carriers for improving oral delivery of curcumin. Drug Dev. Ind. Pharm. 2017;43(3):432–440. doi: 10.1080/03639045.2016.1257020. [DOI] [PubMed] [Google Scholar]
- Chen-yu G., Chun-fen Y., Qi-lu L.i., Qi T., Yan-wei X.i., Wei-na L., Guang-xi Z. Development of a quercetin-loaded nanostructured lipid carrier formulation for topical delivery. Int. J. Pharm. 2012;430(1-2):292–298. doi: 10.1016/j.ijpharm.2012.03.042. [DOI] [PubMed] [Google Scholar]
- Chen C.-C., Tsai T.-H., Huang Z.-R., Fang J.-Y. Effects of lipophilic emulsifiers on the oral administration of lovastatin from nanostructured lipid carriers: physicochemical characterization and pharmacokinetics. Eur. J. Pharm. Biopharm. 2010;74(3):474–482. doi: 10.1016/j.ejpb.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Chen H., Wang Y.i., Zhai Y., Zhai G., Wang Z., Liu J. Development of a ropivacaine-loaded nanostructured lipid carrier formulation for transdermal delivery. Colloids Surf., A. 2015;465:130–136. doi: 10.1016/j.colsurfa.2014.10.046. [DOI] [Google Scholar]
- Das S., Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. Aaps Pharmscitech. 2011;12(1):62–76. doi: 10.1208/s12249-010-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold Y., Calonge M. Applications of nanoparticles in ophthalmology. Prog. Retinal Eye Res. 2010;29(6):596–609. doi: 10.1016/j.preteyeres.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Doktorovová S., Araújo J., Garcia M.L., Rakovský E., Souto E.B. Formulating fluticasone propionate in novel PEG-containing nanostructured lipid carriers (PEG-NLC) Colloids Surf., B. 2010;75(2):538–542. doi: 10.1016/j.colsurfb.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Elmowafy M., Ibrahim H.M., Ahmed M.A., Shalaby K., Salama A., Hefesha H. Atorvastatin-loaded nanostructured lipid carriers (NLCs): strategy to overcome oral delivery drawbacks. Drug Deliv. 2017;24(1):932–941. doi: 10.1080/10717544.2017.1337823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmowafy M., Samy A., Raslan M.A., Salama A., Said R.A., Abdelaziz A.E., El-Eraky W., El Awdan S., Viitala T. Enhancement of bioavailability and pharmacodynamic effects of thymoquinone via nanostructured lipid carrier (NLC) formulation. AAPS PharmSciTech. 2016;17(3):663–672. doi: 10.1208/s12249-015-0391-0. [DOI] [PubMed] [Google Scholar]
- Elmowafy M., Shalaby K., Badran M.M., Ali H.M., Abdel-Bakky M.S., El-Bagory I. Fatty alcohol containing nanostructured lipid carrier (NLC) for progesterone oral delivery. in vitro and ex vivo studies. J. Drug Delivery Sci. Technol. 2018;45:230–239. doi: 10.1016/j.jddst.2018.03.007. [DOI] [Google Scholar]
- Elmowafy M., Shalaby K., Ali H.M., Alruwaili N.K., Salama A., Ibrahim M.F., Akl M.A., Ahmed T.A. Impact of nanostructured lipid carriers on dapsone delivery to the skin. In vitro and in vivo studies. Int. J. Pharm. 2019;572:118781. doi: 10.1016/j.ijpharm.2019.118781. [DOI] [PubMed] [Google Scholar]
- Elmowafy M., Shalaby K., Badran M.M., Ali H.M., Abdel-Bakky M.S., Ibrahim H.M. Multifunctional carbamazepine loaded nanostructured lipid carrier (NLC) formulation. Int. J. Pharm. 2018;550(1–2):359–371. doi: 10.1016/j.ijpharm.2018.08.062. [DOI] [PubMed] [Google Scholar]
- Fang J.-Y., Fang C.-L., Liu C.-H., Su Y.-H. Lipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC) Eur. J. Pharm. Biopharm. 2008;70(2):633–640. doi: 10.1016/j.ejpb.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Fang J.-Y., Hwang T.-L., Fang C.-L., Chiu H.-C. In vitro and in vivo evaluations of the efficacy and safety of skin permeation enhancers using flurbiprofen as a model drug. Int. J. Pharm. 2003;255(1-2):153–166. doi: 10.1016/s0378-5173(03)00086-3. [DOI] [PubMed] [Google Scholar]
- Fathi H.A., Allam A., Elsabahy M., Fetih G., El-Badry M. Nanostructured lipid carriers for improved oral delivery and prolonged antihyperlipidemic effect of simvastatin. Colloids Surf., B. 2018;162:236–245. doi: 10.1016/j.colsurfb.2017.11.064. [DOI] [PubMed] [Google Scholar]
- Food, U.S., 2014. Drug Administration Code of Federal Regulations Title 21. Department of Health and Human Services, Ed. 21CFR20157. US Food and Drug Administration, Washington.
- Gabathuler R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 2010;37(1):48–57. doi: 10.1016/j.nbd.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Gainza G., Bonafonte D.C., Moreno B., Aguirre J.J., Gutierrez F.B., Villullas S., Pedraz J.L., Igartua M., Hernandez R.M. The topical administration of RhEGF-loaded nanostructured lipid carriers (RhEGF-NLC) improves healing in a porcine full-thickness excisional wound model. J. Control. Release. 2015;197:41–47. doi: 10.1016/j.jconrel.2014.10.033. [DOI] [PubMed] [Google Scholar]
- Ghate V.M., Lewis S.A., Prabhu P., Dubey A., Patel N. Nanostructured lipid carriers for the topical delivery of tretinoin. Eur. J. Pharm. Biopharm. 2016;108:253–261. doi: 10.1016/j.ejpb.2016.07.026. [DOI] [PubMed] [Google Scholar]
- Gomes M.J., Martins S., Ferreira D., Segundo M.A., Reis S. Lipid nanoparticles for topical and transdermal application for alopecia treatment: development, physicochemical characterization, and in vitro release and penetration studies. Int. J. Nanomed. 2014;9:1231. doi: 10.2147/IJN.S45561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mira E., Egea M.A., Souto E.B., Calpena A.C., García M.L. Optimizing flurbiprofen-loaded NLC by central composite factorial design for ocular delivery. Nanotechnology. 2011;22(4):045101. doi: 10.1088/0957-4484/22/4/045101. [DOI] [PubMed] [Google Scholar]
- Gu Yongwei, Tang Xiaomeng, Yang Meng, Yang Dishun, Liu Jiyong. Transdermal drug delivery of triptolide-loaded nanostructured lipid carriers: preparation, pharmacokinetic, and evaluation for rheumatoid arthritis. Int. J. Pharm. 2019;554(September 2018):235–244. doi: 10.1016/j.ijpharm.2018.11.024. [DOI] [PubMed] [Google Scholar]
- Gupta M., Vyas S.P. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem. Phys. Lipids. 2012;165(4):454–461. doi: 10.1016/j.chemphyslip.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Muller H., Rainer R.S., Keck C.M. 20 Years of lipid nanoparticles (SLN & NLC): present state of development & industrial applications. Curr. Drug Discov. Technol. 2011;8(3):207–227. doi: 10.2174/157016311796799062. [DOI] [PubMed] [Google Scholar]
- Han F., Li S., Yin R., Liu H., Xu L.u. Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: nanostructured lipid carriers. Colloids Surf., A. 2008;315(1-3):210–216. [Google Scholar]
- Harde H., Das M., Jain S. Solid lipid nanoparticles: an oral bioavailability enhancer vehicle. Exp. Opin. Drug Deliv. 2011;8(11):1407–1424. doi: 10.1517/17425247.2011.604311. [DOI] [PubMed] [Google Scholar]
- Hasenhuettl, Gerard L., 2008. Overview of food emulsifiers. In: Food Emulsifiers and Their Applications. Springer, pp. 1–9.
- Hauss D.J. Oral lipid-based formulations. Adv. Drug Deliv. Rev. 2007;59(7):667–676. doi: 10.1016/j.addr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Huguet-Casquero A., Yining X.u., Gainza E., Pedraz J.L., Beloqui A. Oral delivery of oleuropein-loaded lipid nanocarriers alleviates inflammation and oxidative stress in acute colitis. Int. J. Pharm. 2020;586(June) doi: 10.1016/j.ijpharm.2020.119515. [DOI] [PubMed] [Google Scholar]
- Jaques P.A., Kim C.S. Measurement of total lung deposition of inhaled ultrafine particles in healthy men and women. Inhalation Toxicol. 2000;12(8):715–731. doi: 10.1080/08958370050085156. [DOI] [PubMed] [Google Scholar]
- Jawahar N., Hingarh P.K., Arun R., Selvaraj J., Anbarasan A., S S., G N. Enhanced oral bioavailability of an antipsychotic drug through nanostructured lipid carriers. Int. J. Biol. Macromol. 2018;110:269–275. doi: 10.1016/j.ijbiomac.2018.01.121. [DOI] [PubMed] [Google Scholar]
- Junyaprasert V.B., Teeranachaideekul V., Souto E.B., Boonme P., Müller R.H. Q10-Loaded NLC versus nanoemulsions: stability, rheology and in vitro skin permeation. Int. J. Pharm. 2009;377(1–2):207–214. doi: 10.1016/j.ijpharm.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Jyoti K., Kaur K., Pandey R.S., Jain U.K., Chandra R., Madan J. Inhalable nanostructured lipid particles of 9-bromo-noscapine, a tubulin-binding cytotoxic agent. In vitro and in vivo studies. J. Colloid Interface Sci. 2015;445:219–230. doi: 10.1016/j.jcis.2014.12.092. [DOI] [PubMed] [Google Scholar]
- Kaithwas V., Dora C.P., Kushwah V., Jain S. Nanostructured lipid carriers of olmesartan medoxomil with enhanced oral bioavailability. Colloids Surf., B. 2017;154:10–20. doi: 10.1016/j.colsurfb.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Kalapos Miklós Péter. Carbamazepine-provoked hepatotoxicity and possible aetiopathological role of glutathione in the events. Adverse Drug React. Toxicol. Rev. 2002;21(3):123–141. doi: 10.1007/BF03256188. [DOI] [PubMed] [Google Scholar]
- Kapoor Hema, Aqil Mohd, Imam Syed Sarim, Sultana Yasmin, Ali Asgar. Formulation of amlodipine nano lipid carrier: formulation design, physicochemical and transdermal absorption investigation. J. Drug Deliv. Sci. Technol. 2019;49(September):209–218. doi: 10.1016/j.jddst.2018.11.004. [DOI] [Google Scholar]
- Kelidari Hamid Reza, Saeedi Majid, Hajheydari Zohreh, Akbari Jafar, Morteza-Semnani Katayoun, Akhtari Javad, Valizadeh Hadi, Asare-Addo Kofi, Nokhodchi Ali. Spironolactone loaded nanostructured lipid carrier gel for effective treatment of mild and moderate acne vulgaris: a randomized, double-blind, prospective trial. Colloids Surf., B. 2016;146:47–53. doi: 10.1016/j.colsurfb.2016.05.042. [DOI] [PubMed] [Google Scholar]
- Khan Namrah, Shah Fawad Ali, Rana Isra, Ansari Muhammad Mohsin, Din Fakhar, Rizvi Syed Zaki Husain, Aman Waqar, Lee Gwan-Yeong, Lee Eun-Sun, Kim Jin-Ki. Nanostructured lipid carriers-mediated brain delivery of carbamazepine for improved in vivo anticonvulsant and anxiolytic activity. Int. J. Pharm. 2020:119033. doi: 10.1016/j.ijpharm.2020.119033. [DOI] [PubMed] [Google Scholar]
- Khan S., Shaharyar M., Fazil M., Hassan M.Q., Baboota S., Ali J. Tacrolimus-loaded nanostructured lipid carriers for oral delivery-in vivo bioavailability enhancement. Eur. J. Pharm. Biopharm. 2016;109:149–157. doi: 10.1016/j.ejpb.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Khosa A., Reddi S., Saha R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018;103:598–613. doi: 10.1016/j.biopha.2018.04.055. [DOI] [PubMed] [Google Scholar]
- Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv. Drug Deliv. Rev. 2014;71:2–14. doi: 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Lacatusu I., Istrati D., Bordei N., Popescu M., Seciu A.M., Panteli L.M., Badea N. Synergism of plant extract and vegetable oils-based lipid nanocarriers: emerging trends in development of advanced cosmetic prototype products. Mater. Sci. Eng. C. 2020;108(July):110412. doi: 10.1016/j.msec.2019.110412. [DOI] [PubMed] [Google Scholar]
- Lakhani P., Patil A., Kai-Wei W.u., Sweeney C., Tripathi S., Avula B., Taskar P., Khan S., Majumdar S. Optimization, stabilization, and characterization of amphotericin B loaded nanostructured lipid carriers for ocular drug delivery. Int. J. Pharm. 2019;572 doi: 10.1016/j.ijpharm.2019.118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landesman-Milo D., Peer D. Altering the immune response with lipid-based nanoparticles. J. Control. Release. 2012;161(2):600–608. doi: 10.1016/j.jconrel.2011.12.034. [DOI] [PubMed] [Google Scholar]
- Lauweryns J.M., Baert J.H. Alveolar clearance and the role of the pulmonary lymphatics. Am. Rev. Respir. Dis. 1977;115(4):625–683. doi: 10.1164/arrd.1977.115.4.625. [DOI] [PubMed] [Google Scholar]
- Li J., Tan G., Cheng B., Liu D., Pan W. Transport mechanism of chitosan-N-acetylcysteine, chitosan oligosaccharides or carboxymethyl chitosan decorated coumarin-6 loaded nanostructured lipid carriers across the rabbit ocular. Eur. J. Pharm. Biopharm. 2017;120:89–97. doi: 10.1016/j.ejpb.2017.08.013. [DOI] [PubMed] [Google Scholar]
- Li N., Weng D., Wang S.-M., Zhang Y., Chen S.-S., Yin Z.-F., Zhai J., Scoble J., Williams C.C., Chen T., Qiu H., Wu Q., Zhao M.-M., Lu L.-Q., Mulet X., Li H.-P. Surfactant protein-A nanobody-conjugated liposomes loaded with methylprednisolone increase lung-targeting specificity and therapeutic effect for acute lung injury. Drug Delivery. 2017;24(1):1770–1781. doi: 10.1080/10717544.2017.1402217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Tian B., Zhang J., Li K., Wang L., Han J., Zimei W.u. Tumor-targeted polymeric nanostructured lipid carriers with precise ratiometric control over dual-drug loading for combination therapy in non-small-cell lung cancer. Int. J. Nanomed. 2017;12:1699. doi: 10.2147/IJN.S121262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.J., Duh Y.S. Nanostructured lipid carriers for transdermal delivery of acid labile lansoprazole. Eur. J. Pharm. Biopharm. 2016;108:297–303. doi: 10.1016/j.ejpb.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Lin Y.-K., Huang Z.-R., Zhuo R.-Z., Fang J.-Y. Combination of calcipotriol and methotrexate in nanostructured lipid carriers for topical delivery. Int. J. Nanomed. 2010;5:117. doi: 10.2147/ijn.s9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Liu Z., Zhang C., Zhang B. Nanostructured lipid carriers as novel ophthalmic delivery system for mangiferin: improving in vivo ocular bioavailability. J. Pharm. Sci. 2012;101(10):3833–3844. doi: 10.1002/jps.23251. [DOI] [PubMed] [Google Scholar]
- Martins I., Marques M.M., Antunes A. Protein adducts from carbamazepine-10, 11-epoxide, the major metabolite of the antiepileptic drug carbamazepine: possible biomarkers of toxicity. Toxicol. Lett. 2015;2(238):S359. [Google Scholar]
- Mehnert W., Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Deliv. Rev. 2012;64:83–101. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Mendes A.I., Silva A.C., Catita J.A.M., Cerqueira F., Gabriel C., Lopes C.M. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: improving antifungal activity. Colloids Surf., B. 2013;111:755–763. doi: 10.1016/j.colsurfb.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Mendes I.T., Ruela A.L.M., Carvalho F.C., Freitas J.T.J., Bonfilio R., Pereira G.R. Development and characterization of nanostructured lipid carrier-based gels for the transdermal delivery of donepezil. Colloids Surf., B. 2019;177(February):274–281. doi: 10.1016/j.colsurfb.2019.02.007. [DOI] [PubMed] [Google Scholar]
- Mendes L.P., Delgado J.M.F., Angela Daniela A., Costa M.S., Vieira P.L., Benfica E.M., Lima E.M., Valadares M.C. Biodegradable nanoparticles designed for drug delivery: the number of nanoparticles impacts on cytotoxicity. Toxicol. In Vitro. 2015;29(6):1268–1274. doi: 10.1016/j.tiv.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Muchow M., Schmitz E.I., Despatova N., Maincent P., Müller R.H. Omega-3 fatty acids-loaded lipid nanoparticles for patient-convenient oral bioavailability enhancement. Die Pharmazie-An Int. J. Pharm. Sci. 2009;64(8):499–504. [PubMed] [Google Scholar]
- Müller R.H., Petersen R.D., Hommoss A., Pardeike J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007;59(6):522–530. doi: 10.1016/j.addr.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Muller R.H., Radtke M., Wissing S.A. American Scientific Publishers; CA: 2004. Solid Lipid NPs and Nanostructured Lipid Carriers. Encyclopedia of Nanoscience and Nanotechnology. [Google Scholar]
- Müller R.H., Radtke M., Wissing S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002;54:S131–S155. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- Nafee Noha, Makled Shaimaa, Boraie Nabila. Nanostructured lipid carriers versus solid lipid nanoparticles for the potential treatment of pulmonary hypertension via nebulization. Eur. J. Pharm. Sci. 2018;125:151–162. doi: 10.1016/j.ejps.2018.10.003. [DOI] [PubMed] [Google Scholar]
- Nagaich Upendra, Gulati Neha. Nanostructured lipid carriers (NLC) based controlled release topical gel of clobetasol propionate: design and in vivo characterization. Drug Deliv. Transl. Res. 2016;6(3):289–298. doi: 10.1007/s13346-016-0291-1. [DOI] [PubMed] [Google Scholar]
- Neves Ana Rute, Lúcio Marlene, Martins Susana, Lima José Luís Costa, Reis Salette. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013;8:177. doi: 10.2147/IJN.S37840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor Norhayati Mohamed, Sheikh Khalid, Somavarapu Satyanarayana, Taylor Kevin M.G. Preparation and characterization of dutasteride-loaded nanostructured lipid carriers coated with stearic acid-chitosan oligomer for topical delivery. Eur. J. Pharm. Biopharm. 2017;117:372–384. doi: 10.1016/j.ejpb.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Pardeike Jana, Hommoss Aiman, Müller Rainer H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009;366(1–2):170–184. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Pardeike Jana, Schwabe Kay, Müller Rainer H. Influence of nanostructured lipid carriers (NLC) on the physical properties of the cutanova nanorepair Q10 cream and the in vivo skin hydration effect. Int. J. Pharm. 2010;396(1-2):166–173. doi: 10.1016/j.ijpharm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Pardeike J., Weber S., Haber T., Wagner J., Zarfl H.P., Plank H., Zimmer A. Development of an itraconazole-loaded nanostructured lipid carrier (NLC) formulation for pulmonary application. Int. J. Pharm. 2011;419(1-2):329–338. doi: 10.1016/j.ijpharm.2011.07.040. [DOI] [PubMed] [Google Scholar]
- Pardeike Jana, Weber Sabrina, Zarfl Hans Peter, Pagitz Maximilian, Zimmer Andreas. Itraconazole-loaded nanostructured lipid carriers (NLC) for pulmonary treatment of aspergillosis in falcons. Eur. J. Pharm. Biopharm. 2016;108:269–276. doi: 10.1016/j.ejpb.2016.07.018. [DOI] [PubMed] [Google Scholar]
- Patel Apurva R., Chougule Mahavir B., Townley I., Patlolla Ram, Wang Guangdi, Singh Mandip. Efficacy of aerosolized celecoxib encapsulated nanostructured lipid carrier in non-small cell lung cancer in combination with docetaxel. Pharm. Res. 2013;30(5):1435–1446. doi: 10.1007/s11095-013-0984-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak Kamla, Raghuvanshi Smita. Oral bioavailability: issues and solutions via nanoformulations. Clin. Pharmacokinet. 2015;54(4):325–357. doi: 10.1007/s40262-015-0242-x. [DOI] [PubMed] [Google Scholar]
- Patil-Gadhe Arpana, Pokharkar Varsha. Montelukast-loaded nanostructured lipid carriers: Part I oral bioavailability improvement. Eur. J. Pharm. Biopharm. 2014;88(1):160–168. doi: 10.1016/j.ejpb.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Patlolla Ram R., Chougule Mahavir, Patel Apurva R., Jackson Tanise, Tata Prasad N.V., Singh Mandip. Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J. Control. Release. 2010;144(2):233–241. doi: 10.1016/j.jconrel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokharkar Varsha, Patil-Gadhe Arpana, Kaur Gursheen. Physicochemical and pharmacokinetic evaluation of rosuvastatin loaded nanostructured lipid carriers: influence of long-and medium-chain fatty acid mixture. J. Pharm. Invest. 2018;48(4):465–476. [Google Scholar]
- del Pozo-Rodríguez A., Solinís M.A., Gascón A.R., Pedraz J.L. Short-and long-term stability study of lyophilized solid lipid nanoparticles for gene therapy. Eur. J. Pharm. Biopharm. 2009;71(2):181–189. doi: 10.1016/j.ejpb.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Pradhan Madhulika, Singh Deependra, Murthy S. Narasimha, Singh Manju Rawat. Design, characterization and skin permeating potential of fluocinolone acetonide loaded nanostructured lipid carriers for topical treatment of psoriasis. Steroids. 2015;101:56–63. doi: 10.1016/j.steroids.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Puglia Carmelo, Blasi Paolo, Rizza Luisa, Schoubben Aurélie, Bonina Francesco, Rossi Carlo, Ricci Maurizio. Lipid nanoparticles for prolonged topical delivery: an in vitro and in vivo investigation. Int. J. Pharm. 2008;357(1-2):295–304. doi: 10.1016/j.ijpharm.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Radtke Magdalene, Souto Eliana B, Müller R.H. Nanostructured lipid carriers: a novel generation of solid lipid drug carriers. Pharm. Technol. Eur. 2005;17(4):45–50. [Google Scholar]
- Rahman Heshu Sulaiman, Rasedee Abdullah, Othman Hemn Hassan, Chartrand Max Stanley, Namvar Farideh, Yeap Swee Keong, Samad Nozlena Abdul, Andas Reena Joys, Nadzri Nabilah Muhammad, Anasamy Theebaa. Acute toxicity study of zerumbone-loaded nanostructured lipid carrier on BALB/c mice model. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/563930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Lígia N.M., Breitkreitz Márcia C., Guilherme Viviane A., da Silva Gustavo H.R., Couto Verônica M., Castro Simone R., de Paula Bárbara O., Machado Daisy, de Paula Eneida. Natural lipids-based NLC containing lidocaine: from pre-formulation to in vivo studies. Eur. J. Pharm. Sci. 2017;106(May):102–112. doi: 10.1016/j.ejps.2017.05.060. [DOI] [PubMed] [Google Scholar]
- Salvi Vedanti R., Pawar Pravin. Nanostructured lipid carriers (NLC) system: a novel drug targeting carrier. J. Drug Delivery Sci. Technol. 2019;51(990):255–267. doi: 10.1016/j.jddst.2019.02.017. [DOI] [Google Scholar]
- Sánchez-López E., Espina M., Doktorovova S., Souto E.B., García M.L. Lipid Nanoparticles (SLN, NLC): overcoming the anatomical and physiological barriers of the eye-part I-barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017;110:70–75. doi: 10.1016/j.ejpb.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Sanna Vanna, Caria Giuseppe, Mariani Alberto. Effect of lipid nanoparticles containing fatty alcohols having different chain length on the ex vivo skin permeability of econazole nitrate. Powder Technol. 2010;201(1):32–36. [Google Scholar]
- Shah Nirmal V., Seth Avinash K., Balaraman R., Aundhia Chintan J., Maheshwari Rajesh A., Parmar Ghanshyam R. Nanostructured lipid carriers for oral bioavailability enhancement of raloxifene: design and in vivo study. J. Adv. Res. 2016;7(3):423–434. doi: 10.1016/j.jare.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahgaldian Patrick, Da Silva Eric, Coleman Anthony W, Rather Beth, Zaworotko Michael J. Para-acyl-calix-arene based solid lipid nanoparticles (SLNs): a detailed study of preparation and stability parameters. Int. J. Pharm. 2003;253(1-2):23–38. doi: 10.1016/s0378-5173(02)00639-7. [DOI] [PubMed] [Google Scholar]
- Sharma Kritika, Hallan Supandeep Singh, Lal Bharat, Bhardwaj Ankur, Mishra Neeraj. Development and characterization of floating spheroids of atorvastatin calcium loaded NLC for enhancement of oral bioavailability. Artif. Cells Nanomed. Biotechnol. 2016;44(6):1448–1456. doi: 10.3109/21691401.2015.1041637. [DOI] [PubMed] [Google Scholar]
- Shen Jie, Wang Yu, Ping Qineng, Xiao Yanyu, Huang Xin. Mucoadhesive effect of thiolated PEG stearate and its modified NLC for ocular drug delivery. J. Control. Release. 2009;137(3):217–223. doi: 10.1016/j.jconrel.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Shete Harshad, Chatterjee Sushmita, De Abhijit, Patravale Vandana. Long chain lipid based tamoxifen NLC. Part II: Pharmacokinetic, biodistribution and in vitro anticancer efficacy studies. Int. J. Pharm. 2013;454(1):584–592. doi: 10.1016/j.ijpharm.2013.03.036. [DOI] [PubMed] [Google Scholar]
- Shevalkar Ganesh, Vavia Pradeep. Solidified nanostructured lipid carrier (S-NLC) for enhancing the oral bioavailability of ezetimibe. J. Drug Delivery Sci. Technol. 2019;53(April) doi: 10.1016/j.jddst.2019.101211. [DOI] [Google Scholar]
- Sinhmar Gurpreet Kaur, Shah Neel N., Chokshi Nimitt V., Khatri Hiren N., Patel Mayur M. Process, optimization, and characterization of budesonide-loaded nanostructured lipid carriers for the treatment of inflammatory bowel disease. Drug Dev. Ind. Pharm. 2018;44(7):1078–1089. doi: 10.1080/03639045.2018.1434194. [DOI] [PubMed] [Google Scholar]
- Sinhmar Gurpreet Kaur, Shah Neel N, Rawal Shruti U, Chokshi Nimitt V, Khatri Hiren N, Patel Bhoomika M, Patel Mayur M. Surface engineered lipid nanoparticle-mediated site-specific drug delivery system for the treatment of inflammatory bowel disease. Artif. Cells Nanomed. Biotechnol. 2018;46(sup2):565–578. doi: 10.1080/21691401.2018.1463232. [DOI] [PubMed] [Google Scholar]
- Sloan K.B., Beall H.D., Taylor H.E., Getz J.J., Villaneuva R., Nipper R., Smith K. Transdermal delivery of theophylline from alcohol vehicles. Int. J. Pharm. 1998;171(2):185–193. [Google Scholar]
- Song Xu., Lin Qing, Guo Ling, Yao Fu., Han Jianfeng, Ke Huan, Sun Xun, Gong Tao, Zhang Zhirong. Rifampicin loaded mannosylated cationic nanostructured lipid carriers for alveolar macrophage-specific delivery. Pharm. Res. 2015;32(5):1741–1751. doi: 10.1007/s11095-014-1572-3. [DOI] [PubMed] [Google Scholar]
- Souto, Eliana B., Müller, Rainer H., 2010. Lipid Nanoparticles: effect on bioavailability and pharmacokinetic changes. In: Drug Delivery. Springer, pp. 115–41. [DOI] [PubMed]
- Souto Eliana B, Müller Rainer H, Alemieda Antonio J. Topical delivery of oily actives using solid lipid particles. Pharm. Technol. Europe. 2007;19(12) [Google Scholar]
- Sun Ming, Nie Shufang, Pan Xuan, Zhang Ruiwen, Fan Zhaoyang, Wang Shu. Quercetin-nanostructured lipid carriers: characteristics and anti-breast cancer activities in vitro. Colloids Surf., B. 2014;113:15–24. doi: 10.1016/j.colsurfb.2013.08.032. [DOI] [PubMed] [Google Scholar]
- Sung Jean C., Pulliam Brian L., Edwards David A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007;25(12):563–570. doi: 10.1016/j.tibtech.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Tazehjani, Del Aram Jafari, Farahpour, Mohammad Reza, Hamishehkar, Hamed, 2021. Effectiveness of topical caraway essential oil loaded into nanostructured lipid carrier as a promising platform for the treatment of infected wounds. Colloids Surf., A 610 (October2020): 125748. 10.1016/j.colsurfa.2020.125748. [DOI]
- Teeranachaideekul Veerawat, Müller Rainer Helmut, Junyaprasert Varaporn Buraphacheep. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)-effects of formulation parameters on physicochemical stability. Int. J. Pharm. 2007;340(1–2):198–206. doi: 10.1016/j.ijpharm.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Teeranachaideekul Veerawat, Souto Eliana B., Junyaprasert Varaporn B., Müller Rainer H. Cetyl palmitate-based NLC for topical delivery of coenzyme Q10–development, physicochemical characterization and in vitro release studies. Eur. J. Pharm. Biopharm. 2007;67(1):141–148. doi: 10.1016/j.ejpb.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Thapa Chhitij, Ahad Abdul, Aqil Mohd., Imam Syed Sarim, Sultana Yasmin. Formulation and optimization of nanostructured lipid carriers to enhance oral bioavailability of telmisartan using box-behnken design. J. Drug Delivery Sci. Technol. 2018;44:431–439. [Google Scholar]
- Tian Baocheng, Luo Qiuhua, Song Shuangshuang, Liu Dandan, Pan Hao, Zhang Wenji, He Ling, Ma Shilin, Yang Xinggang, Pan Weisan. Novel surface-modified nanostructured lipid carriers with partially deacetylated water-soluble chitosan for efficient ocular delivery. J. Pharm. Sci. 2012;101(3):1040–1049. doi: 10.1002/jps.22813. [DOI] [PubMed] [Google Scholar]
- Tian Zhiqiang, Yi Yueneng, Yuan Hailong, Han Jin, Zhang Xi, Xie Yunchang, Lu Yi, Qi Jianping, Wu Wei. Solidification of nanostructured lipid carriers (NLCs) onto pellets by fluid-bed coating: preparation, in vitro characterization and bioavailability in dogs. Powder Technol. 2013;247:120–127. [Google Scholar]
- Tiwari Radheshyam, Pathak Kamla. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int. J. Pharm. 2011;415(1–2):232–243. doi: 10.1016/j.ijpharm.2011.05.044. [DOI] [PubMed] [Google Scholar]
- Tran Tuan Hiep, Ramasamy Thiruganesh, Truong Duy Hieu, Choi Han-Gon, Yong Chul Soon, Kim Jong Oh. Preparation and characterization of fenofibrate-loaded nanostructured lipid carriers for oral bioavailability enhancement. AAPS Pharmscitech. 2014;15(6):1509–1515. doi: 10.1208/s12249-014-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommer H., Neubert R.H.H. Overcoming the stratum corneum: the modulation of skin penetration. Skin Pharmacol. Physiol. 2006;19(2):106–121. doi: 10.1159/000091978. [DOI] [PubMed] [Google Scholar]
- Üner M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): their benefits as colloidal drug carrier systems. Die Pharmazie-an Int. J. Pharm. Sci. 2006;61(5):375–386. [PubMed] [Google Scholar]
- Vieira Alexandre C.C., Magalhães Joana, Rocha Sónia, Cardoso Marcos S., Santos Susana G., Borges Margarida, Pinheiro Marina, Reis Salette. Targeted macrophages delivery of rifampicin-loaded lipid nanoparticles to improve tuberculosis treatment. Nanomedicine. 2017;12(24):2721–2736. doi: 10.2217/nnm-2017-0248. [DOI] [PubMed] [Google Scholar]
- Wu Yumei, Song Xunan, Kebebe Dereje, Li Xinyue, Xue Zhifeng, Li Jiawei, Du Shouying, Pi Jiaxin, Liu Zhidong. Brain targeting of baicalin and salvianolic acid B combination by OX26 functionalized nanostructured lipid carriers. Int. J. Pharm. 2019;571:118754. doi: 10.1016/j.ijpharm.2019.118754. [DOI] [PubMed] [Google Scholar]
- Zhai Yingjie, Zhai Guangxi. Advances in lipid-based colloid systems as drug carrier for topic delivery. J. Control. Release. 2014;193:90–99. doi: 10.1016/j.jconrel.2014.05.054. [DOI] [PubMed] [Google Scholar]
- Zhang Tiecheng, Chen Jianian, Zhang Yi, Shen Qi, Pan Weisan. Characterization and evaluation of nanostructured lipid carrier as a vehicle for oral delivery of etoposide. Eur. J. Pharm. Sci. 2011;43(3):174–179. doi: 10.1016/j.ejps.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Zhao Chaoyue, Zhang Jiulong, Hu Haiyang, Qiao Mingxi, Chen Dawei, Zhao Xiuli, Yang Chunrong. Design of lactoferrin modified lipid nano-carriers for efficient brain-targeted delivery of nimodipine. Mater. Sci. Eng., C. 2018;92:1031–1040. doi: 10.1016/j.msec.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Zheng Kai, Zou Aihua, Yang Xiaomin, Liu Fang, Xia Qiang, Ye Ruqiang, Mu Bozhong. The effect of polymer-surfactant emulsifying agent on the formation and stability of α-lipoic acid loaded nanostructured lipid carriers (NLC) Food Hydrocolloids. 2013;32(1):72–78. [Google Scholar]
- Zhou Jun, Zhou Daxin. Improvement of oral bioavailability of lovastatin by using nanostructured lipid carriers. Drug Design, Devel. Therapy. 2015;9:5269. doi: 10.2147/DDDT.S90016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Lei, Chen Yan, Zhang Zhenhai, He Junjie, Meng Du., Qingqing Wu. Preparation of tripterine nanostructured lipid carriers and their absorption in rat intestine. Die Pharmazie-An Int. J. Pharm. Sci. 2012;67(4):304–310. [PubMed] [Google Scholar]
- Zhou Xiaotong, Zhang Xingwang, Ye Yanghuan, Zhang Tianpeng, Wang Huan, Ma Zhiguo, Wu Baojian. Nanostructured lipid carriers used for oral delivery of oridonin: an effect of ligand modification on absorption. Int. J. Pharm. 2015;479(2):391–398. doi: 10.1016/j.ijpharm.2014.12.068. [DOI] [PubMed] [Google Scholar]
- Zhuang Chun-Yang, Li Ning, Wang Mi, Zhang Xiao-Ning, Pan Wei-San, Peng Jun-Jie, Pan Yu-Sheng, Tang Xin. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int. J. Pharm. 2010;394(1-2):179–185. doi: 10.1016/j.ijpharm.2010.05.005. [DOI] [PubMed] [Google Scholar]