Abstract
B cell activating factor of TNF family (BAFF) is a member of TNF ligand superfamily and plays a key role in B cell homeostasis, proliferation, maturation, and survival. In this study, we detected BAFF level, the expressions of BAFF receptors and important molecules in NF-κB pathway in rheumatoid arthritis (RA) patients and analyzed the correlation between BAFF level and clinical variables, laboratory parameters or X-ray scores in order to elucidate the roles of BAFF in RA. A total of 50 RA patients and 50 healthy controls (HCs) were enrolled. We showed that the serum BAFF level in RA patients was significantly higher than that of HCs, and the percentages of B cell subsets (CD19+ B cells, CD19+CD27+ B cells, CD19+CD20+CD27+ B cells, and CD19+CD20−CD27+ B cells) in the serum of RA patients were significantly increased compared with those of HCs. The percentages of CD19+BAFFR+ B cells, CD19+ BCMA+ B cells, and CD19+ TACI+ B cells in RA patients were significantly increased compared with those in HCs. The expression of important molecules in the NF-κB pathway (MKK3, MKK6, p-P38, p-P65, TRAF2, and p52) was significantly higher in RA patients than in HCs, but p100 level in RA patients was lower than that in HCs. The serum BAFF level was positively correlated with C-reactive protein, rheumatoid factor, disease activity score (in 28 joints), swollen joint counts, tender joint counts, and X-ray scores. When normal B cells were treated with BAFF in vitro, the percentages of the B cell subset and the expression of BAFF receptors were significantly upregulated. BAFF also promoted the expression of MKK3, MKK6, p-P38, p-P65, TRAF2, and p52. In conclusion, this study demonstrates that BAFF level is correlated with the disease activity and bone destruction of RA. BAFF is involved in the differentiation, proliferation, and activation of B cells in RA through NF-κB signaling pathway, suggesting that BAFF might be an ideal therapeutic target for RA.
Keywords: rheumatoid arthritis, B cell, BAFF, disease activity, correlation, biomarker
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by swelling, pain, and stiffness of joints, resulting in progressive joint destruction and loss of function [1–4]. RA is more common in women, and ~1% of adults aged 35 and 2% of adults aged 60 are affected. As an extremely disabling disease, RA limits mobility, hampers work, and reduces quality of life [5]. At present, the pathogenesis of RA is still unclear, although complex immune mechanisms contribute to RA. B cells have been considered to play a role in RA pathogenesis since the discovery of rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibodies [6].
Specificity surface markers, including various clusters of differentiation (CD) molecules, are expressed during B cell differentiation [7]. CD19 is a characteristic marker of B cells. Overexpression of CD19 leads to excessive activation of B cells after antigen stimulation [8]. CD20 is expressed on all B cell lineages except for pro-B cells and plasma cells and can significantly prolong B cell survival [6, 9]. CD27 is widely expressed on all memory B cells and plasma cells [10, 11]. B cells may drive autoimmune disorders through autoantibody-secreting plasma cells, the formation of immune complexes, the production of proinflammatory cytokines and immunoglobulins, acting as antigen-presenting cells, and the regulation of T cell differentiation and activation in autoimmune diseases [12, 13].
B cell activating factor of TNF family (BAFF) is a member of the TNF ligand superfamily and plays a key role in B cell homeostasis, proliferation, maturation, and survival [14]. The BAFF level might be an important factor in setting B cell thresholds to determine naive B cell selection [15]. BAFF also regulates T cell function by providing costimulatory signals [16]. Serum immunoglobulin levels in BAFF-transgenic mice were elevated [17]. High levels of serum BAFF and synovial fluid BAFF were detected in RA patients [14, 18, 19]. Elevated BAFF levels in early RA patients are related to autoantibody levels and synovitis [20].
BAFF has three receptors, including BAFF receptor (BAFF-R), transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), and B cell maturation antigen (BCMA). In humans, BAFF-R is widely expressed in all B cells. BAFF-R is a crucial receptor for mature B cells and regulates B cell proliferation and survival in CIA rats [21, 22]. TACI is expressed in CD27+ memory B cells, plasma cells, and certain subsets of naive and activated B cells. BCMA is expressed in tonsillar memory B cells, germinal center (GC) B cells, and plasma cells [15, 21, 23]. The expression of BAFF-R contributes to disease progression in RA. TACI is elevated since the first week of RA onset [19].
BAFF activates noncanonical and canonical NF-κB signaling pathways through binding to BAFF-R, which results in the expression of a series of downstream genes involved in B cell survival [24]. BAFF-R is specific for BAFF and is the primary receptor for transmitting BAFF-dependent B cell survival signals [25, 26]. BAFF-R upregulates CD19 expression in the noncanonical NF-κB pathway, which enhances BCR signaling and p100 production [23, 27]. Tumor necrosis factor receptor-associated factor 2 (TRAF2) is a multifunctional regulator of NF-κB activation [28]. TRAF2 and TRAF3 suppress p52 activation, gene expression, and survival in mature B cells in a cooperative manner. Binding of BAFF to BAFF-R reversed the suppression of B cell survival mediated by TRAF2-TRAF3 by triggering the depletion of TRAF3 protein, resulting in the activation of alternative NF-κB signaling pathways [29]. BAFF-R is a potent activator of the noncanonical NF-κB pathway and is responsible for the signaling process of p100/52 to activate p52 in B cells [22]. B cells lacking p52 or other critical upstream activators display greatly impaired survival and maturation in vivo [30]. In addition, BAFF-R binding to BAFF can lead to the phosphorylation of IκB, PI3K/AKT, and p38 [31, 32].
In the present study, we detected BAFF levels, the expression of BAFF-R and important molecules in the NF-κB pathway in RA patients and analyzed the correlation between BAFF levels and clinical variables, laboratory parameters or X-ray scores to demonstrate the roles of BAFF in RA.
Materials and methods
Study population
Fifty consecutive patients (forty-three women and seven men, mean age 57.87 ± 11.85 years) who fulfilled the American College of Rheumatology (ACR) 1987 revised criteria for RA were included [33]. All experiments were approved by the Ethics Review Committee for the Experimentation of the Institute of Clinical Pharmacology, Anhui Medical University. Participants were allowed to obtain baseline examinations during entry. Clinical evaluations included swollen joint counts (SJC) and tender joint counts (TJC), pain, patient’s global assessment of disease activity (PGA) and evaluator global assessment of disease activity (EGA), joint function, disease activity score in 28 joints (DAS28) and health assessment questionnaire score (HAQ). Laboratory parameters included erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), anti-CCP antibodies, RF, serum immunoglobulin (IgA, IgE, IgD, IgM, IgG1, IgG2, IgG3, IgG4) levels, and serum cytokine (BAFF, IL-1α, IL-1β, IL-6, IL-8, TNF-α, IFN-γ, MCP-1, IL-4, IL-10, IL-13) levels. Fifty healthy controls (HCs) were given as controls.
Pain, PGA, and EGA were evaluated by visual analog scale (VAS) from 0 to 10 cm (0 = good, 10 = bad). SJC and TJC were counted. Joint function was graded from 1 to 4: 1 = all kinds of activities are not limited; 2 = moderate activities are limited; 3 = movement is obviously restricted; 4 = cannot provide life care for oneself. X-ray was graded from 0 to 4: 1 = osteoporosis, or soft tissue swelling surrounding the affected joint, or joint space narrowing; 2 = osteoporosis, mild cartilage and bone erosion, and joint space narrowing; 3 = severity cartilage and bone erosion, and joint space narrowing, joint subluxation or dislocation; 4 = joint deformity and stiffness, and severity cartilage and bone erosion, joint space disappearance. HAQ was graded from 0 to 3: 0 = without difficulty; 1 = moderate difficulty, 2 = difficulty; 3 = cannot be performed. DAS28 was calculated using the following equation:
DAS28 = 0.56 × sqrt TJC + 0.28 × sqrt SJC + 0.7 × In (ESR) + 0.014 × PGA
Samples
Peripheral blood samples of RA patients and HCs were collected from the Department of Rheumatology and Immunology, First Affiliated Hospital, Anhui Medical University. All samples were centrifuged at 1600 × g for 15 min for serum.
For laboratory measurements, anti-CCP antibodies were measured by an enzyme-linked immunosorbent assay (ELISA) using commercially available test systems, with positive anti-CCP value defined above 12 IU/mL. The RF was measured by ELISA using commercially available test systems and considered positive above a cutoff value of 20 IU/mL [34]. CRP was tested by the Latex-Enhanced Turbidimetric Assay using a CRP test kit, and the result was considered positive at a cutoff level above 10 mg/L. ESR was tested by Westergren Assay and considered positive above 20 mm/h.
Serum levels of BAFF
The levels of BAFF in the serum of RA patients and HCs were detected by using ELISA kits (R&D Systems) following the manufacturer’s instructions. Briefly, 96-well plates were coated with mouse anti-human BAFF (100 µL) at 4 °C overnight. Diluted serum samples (100 µL/well) were incubated at room temperature for 2 h. Then, 96-well plates were washed three times, and the detection antibody was added into the well and incubated for 2 h. Streptavidin-horseradish peroxidase (HRP) was added to the well and incubated for 20 min. Substrate solution was added into each well and incubated for 20 min, and then stop solution was added into each well. The optical density was measured at 450 nm. All samples were run in triplicate.
Serum levels of immunoglobulins and cytokines
Immunoglobulins (IgA, IgE, IgD, IgM, IgG1, IgG2, IgG3, IgG4) and cytokines (IL-1α, IL-1β, IL-6, IL-8, TNF-α, IFN-γ, MCP-1, IL-4, IL-10, IL-13) in serum were analyzed by a protein chip array (RayBiotech, USA). The procedure steps were as follows: (1) The glass chip was completely air dried at room temperature. (2) Cytokine dilution (100 μL) was added to each well and incubated at room temperature for 30 min to block the slides. Standard cytokines or samples (100 μL) were added to each well and incubated for 1–2 h. (3) Detection antibody cocktail (80 μL) was added into each well. The glass chip was incubated for 1–2 h. (4) Cy3 equivalent dye-conjugated streptavidin (80 μL) was added into each well. The device was covered with aluminum foil to avoid exposure to light or incubated in a dark room for 1 h. (4) The signals were visualized using fluorescence detection using a laser scanner equipped with a Cy3 wavelength, such as Axon GenePix. Quantitative data were analyzed by Quantibody® Q-Analyzer software. Each sample was run in quadruplicate. The total number of samples was fifty.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) from RA patients and HCs were isolated by Ficoll-Paque Plus (Dakewe Biotech Co., Ltd, China). A total of 1 × 106 cells were suspended and incubated in PBS with 10 µL of antibodies against APC-CD19, FITC-CD20, and PE-CD27 (Biolegend, USA) for 30 min on ice in the dark. Finally, CD19+ B cells, CD19+CD27+ B cells, CD19+CD20+CD27+ B cells and CD19+CD20−CD27+ B cells were acquired by FC 500 (Beckman Coulter Ltd., USA). The percentage and number of B cell subsets were analyzed by using CXP analysis software (Beckman Coulter Ltd., USA, version 2.0).
The above protocol was used to measure the expression of BAFFR, BCMA, and TACI on B cells of RA and HCs with the following antibodies: PE-CD19 (Miltenyi Biotec, Germany), APC-BAFFR, APC-CD19, PE-BCMA, and PE-TACI (Biolegend, USA).
For in vitro culture assays, B cells (1 × 106 cells/mL) from HCs were added into plates and incubated with recombinant human BAFF (Peprotech, USA) (100 ng/mL) at 37 °C under 5% CO2 for 48 h. The above protocol was used with the following antibodies: APC-CD19, FITC-CD20, and PE-CD27 (Biolegend, USA) for analyzing B cell subsets or PE-CD19 (Miltenyi Biotec, Germany), APC-BAFFR, APC-CD19, PE-BCMA, and PE-TACI (Biolegend, USA) for measuring the expression of BAFFR, BCMA, and TACI.
Isolation of B cells
B cells were isolated from PBMCs by positive selection using magnetic cell separation (MACS). For this purpose, PBMC suspensions from RA patients or HCs in MACS buffer (500 µL) were incubated with PE-CD19 for 20 min, followed by incubation with anti-PE beads for an additional 20 min. The stained cell suspension was added to an LS column (Mitenyi). After washing with buffer three times, labeled B cells were collected [27].
Western blotting assay
The expression levels of MKK3, MKK6, p-P38, p-P65, TRAF2, and p100/52 were measured by Western blotting assay. B cells of RA and HC were lysed in cell lysis buffer with PMSF 1 mM at 4 °C for 30 min, followed by centrifugation at 13622 × g for 10 min at 4 °C. The supernatants were diluted to 4 mg protein/mL and kept frozen at −80 °C until use. A total of 50 μg of denatured protein was separated by 10% SDS-PAGE and transferred onto polyvinylidene fluoride membranes (PVDF membrane, Millipore, USA) in an ice water environment. The membranes were blocked with blocking buffer (0.05% Tween 20-PBS with 5% nonfat milk) for 2 h at room temperature and then incubated with (1:500) of mouse monoclonal TRAF2 (Santa Cruz Biotechnology, Inc, USA) or rabbit monoclonal MMK3 (Santa Cruz Biotechnology, Inc, USA) or rabbit monoclonal MKK6 (Santa Cruz Biotechnology, Inc, USA) or rabbit monoclonal p-P38 (Thr180/Tyr182, Cell Signaling, USA) or rabbit monoclonal p-P65 (Ser536, Cell Signaling, USA) or rabbit monoclonal p100 (Ser866/870, Cell Signaling, USA) and mouse monoclonal anti-β-actin (1:500, Cell Signaling, USA) primary antibodies at 4 °C overnight. Then, the membranes were incubated with anti-rabbit or anti-mouse secondary antibodies conjugated with HRP (1:60000) for 2 h at 37 °C. Membrane detection was achieved by measuring the chemiluminescence of the blotting agent on films. Finally, the band densities were quantified with a computerized densitometer (ImageJ Launcher, Broken Symmetry Software). Equivalent protein load and transfer efficiency were verified by staining for β-actin [35, 36]. Western blotting data were obtained from ten RA patients and ten HCs, and three replicates were performed.
For in vitro culture assays, B cells (1 × 106 cells/mL) from HCs were added to plates and incubated with BAFF (100 ng/mL) at 37 °C under 5% CO2 for 48 h. The above protocol was used to analyze the expression of MKK3, MKK6, p-P38, p-P65, TRAF2, and p100/52 in B cells stimulated by BAFF. Three replicates were performed.
The correlations between BAFF level and clinical variables or laboratory parameters in RA
The correlations between BAFF level and clinical variables (VAS of pain, DAS28 TJC SJC, EGA, PGA, HAQ and join function) or laboratory parameters (RF, ESR, anti-CCP antibody, and CRP) were analyzed by linear regression analysis.
The correlations between bone damage and BAFF, RF, anti-CCP antibody or CRP level
To clarify autoimmunity from B cell involvement in bone remodeling, we analyzed the correlations between X-ray scores and BAFF, RF, anti-CCP antibody, or CRP levels by linear regression analysis.
Statistical analysis
All data are presented as the mean ± standard deviation (SD). Analysis of variance (ANOVA) (SPSS Software Products, USA) was used to determine significant differences between groups. “The correlations between BAFF level and clinical variables or laboratory parameters in RA” and “The correlations between bone damage and BAFF, RF, anti-CCP antibody or CRP level” were analyzed by linear regression analysis. P < 0.05 was considered significant in all experiments.
Results
Characteristics of RA patients and HCs
A total of 50 patients who met the revised ACR criteria for RA diagnosis and 50 HCs were enrolled in the study. The basic characteristics of the 50 RA patients and 50 HCs are shown in Table 1. Females were the majority of the 50 RA patients. The HC group had a lower proportion of women than the RA group (P < 0.01). The levels of BAFF, ESR, CRP, RF, and anti-CCP antibody in RA patients were higher than those in the HCs.
Table 1.
Clinical and demographic characteristics of RA patients and healthy controls.
| Characteristic | Healthy controls | RA | p |
|---|---|---|---|
| Number of subjects | 50 | 50 | P = 1 |
| Age (year) | 52 ± 5 | 57.87 ± 11.85 | P > 0.05 |
| Female/male ratio | 20:30 | 43:7 | P < 0.01 |
| BAFF (pg/mL) | 1920 ± 91 | 2700 ± 241 | P < 0.01 |
| ESR (mm/h) | 15.21 ± 4.28 | 40.67 ± 34.28 | P < 0.01 |
| CRP (mg/L) | 6.39 ± 4.31 | 46.69 ± 26.31 | P < 0.01 |
| RF (IU/mL) | 13.23 ± 11.11 | 138.99 ± 113.11 | P < 0.01 |
| Anti-CCP (IU/mL) | 8.14 ± 6.35 | 581.14 ± 489.39 | P < 0.01 |
| Scores of joints | – | 3.38 ± 1.86 | – |
| Scores of X-ray | – | 2.71 ± 1.20 | – |
| Pain (VAS) | – | 3.36 ± 1.17 | – |
| PGA (VAS) | – | 4.69 ± 2.58 | – |
| EGA (VAS) | – | 3.56 ± 2.32 | – |
| HAQ | – | 0.77 ± 0.36 | – |
| DAS28 | – | 4.01 ± 1.83 | – |
Anti-CCP anti-cyclic citrullinated peptide, BAFF B cell activating factor of TNF family, CRP C-reactive protein, DAS28 disease activity score in 28 joints, EGA evaluator global assessment of disease activity, ESR erythrocyte sedimentation rate, HAQ health assessment questionnaire, PGA patient’s global assessment of disease activity, RF rheumatoid factor, VAS visual analogue scale.
Baseline characteristics of RA patients
At study entry, laboratory variables (ESR, CRP, RF, anti-CCP antibody) and clinical indexes (scores of joints, scores of X-ray of joints, pain, PGA, EGA, HAQ, DAS28) of RA patients were measured or evaluated. Clinical variables and laboratory parameters in RA patients were abnormal. In Table 2, the median DAS28, joint score and X-ray score were 4.0, 4.0, and 3.0, respectively. The median values of RF and anti-CCP were 115 and 564.5 IU/mL, respectively. Among the 50 patients, 44 cases were RF positive, and the abnormity rate was 88%. Forty-two cases were anti-CCP antibody positive, and the abnormal rate was 84%. Thirty-three cases were ESR positive, and the abnormal rate was 66%. Sixteen cases were CRP positive, and the abnormal rate was 32% (Table 3).
Table 2.
Baseline clinical characteristics of RA patients.
| Variables | Mean ± SD | Min | Median | Max |
|---|---|---|---|---|
| Scores of joints | 3.38 ± 1.86 | 0 | 4 | 5 |
| Scores of X- ray | 2.71 ± 1.20 | 0 | 3 | 4 |
| Pain (VAS) | 3.36 ± 1.17 | 0 | 2 | 9 |
| PGA (VAS) | 4.69 ± 2.58 | 0 | 5 | 10 |
| EGA (VAS) | 3.56 ± 2.32 | 0 | 3 | 8 |
| HAQ | 0.77 ± 0.36 | 0 | 0.51 | 2.75 |
| DAS28 | 4.01 ± 1.83 | 0.78 | 4.00 | 7.6 |
| ESR (mm/h) | 40.67 ± 34.28 | 2 | 28 | 140 |
| CRP (mg/L) | 46.69 ± 26.31 | 0 | 4.9 | 180.08 |
| RF (IU/mL) | 138.99 ± 113.11 | 9 | 115 | 492.8 |
| Anti-CCP (IU/mL) | 581.14 ± 489.39 | 6 | 564.5 | 1645 |
Clinical variables and laboratory parameters in RA patients were abnormal.
Anti-CCP anti-cyclic citrullinated peptide, CRP C-reactive protein, DAS28 disease activity score in 28 joints, EGA evaluator global assessment of disease activity, ESR erythrocyte sedimentation rate, HAQ health assessment questionnaire, PGA patient’s global assessment of disease activity, RF rheumatoid factor, VAS visual analogue scale.
Table 3.
Rates of abnormity (%) of ESR, CRP, RF, Anti-CCP in RA patients.
| Variables | Positive (n) | Negative (n) | Rate of abnormity (%) |
|---|---|---|---|
| RF (IU/mL) | 44 | 6 | 88 |
| Anti- CCP (IU/mL) | 42 | 8 | 84 |
| ESR (mm/h) | 33 | 17 | 66 |
| CRP (mg/L) | 16 | 34 | 32 |
Among the 50 patients, 44 cases were RF positive, abnormity rate was 88%. Forty-two cases were Anti-CCP antibody positive, the rate of abnormity was 84%. Thirty-three cases were ESR positive, abnormity rate was 66%. Sixteen cases were CRP positive, abnormity rate was 32%.
RF rheumatoid factor, Anti-CCP anti-cyclic citrullinated peptide, ESR erythrocyte sedimentation rate, CRP C-reactive protein.
RA patients had elevated levels of immunoglobulins and cytokines in serum
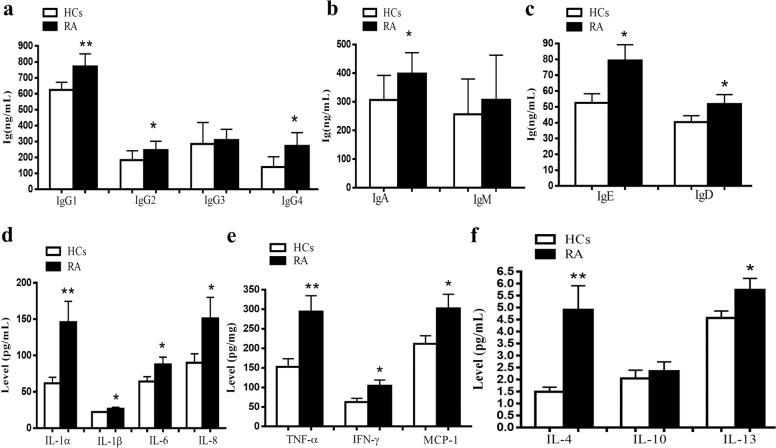
We found that the levels of IgG1, IgG2, IgG4, IgA, IgE, and IgD in RA patients were significantly higher those of the HCs, while there were no significant differences in IgG3 and IgM between RA patients and HCs (Fig. 1a–c). The levels of IL-1α, IL-1β, IL-6, IL-8, TNF-α, IFN-γ, MCP-1, IL-4, and IL-13 in RA patients were also more elevated in RA patients than those in HCs. In particular, the levels of IL-1α, IL-4, and TNF-α increased significantly. IL-10 levels were not different between RA patients and HCs (Fig. 1d–f).
Fig. 1. Levels of immunoglobins and cytokines in serum were compared between RA patients and HCs.
The levels of immunoglobins and cytokines in serum were analyzed by a protein chip array. a Levels of IgG1, IgG2, IgG3, and IgG4 in RA patients compared with HCs. b Levels of IgA and IgM in RA patients compared with HCs. c Levels of IgE and IgD in RA patients compared with HCs. d Levels of IL-1α, IL-1β, IL-6, and IL-8 in RA patients compared with HCs. e Levels of TNF-α, IFN-γ, and MCP-1 in RA patients compared with HCs. f Levels of IL-4, IL-10, and IL-13 in RA compared with HCs. Each sample was run in quadruplicate. The number of samples was 50. *P < 0.05, **P < 0.01 vs. HCs. HCs healthy controls.
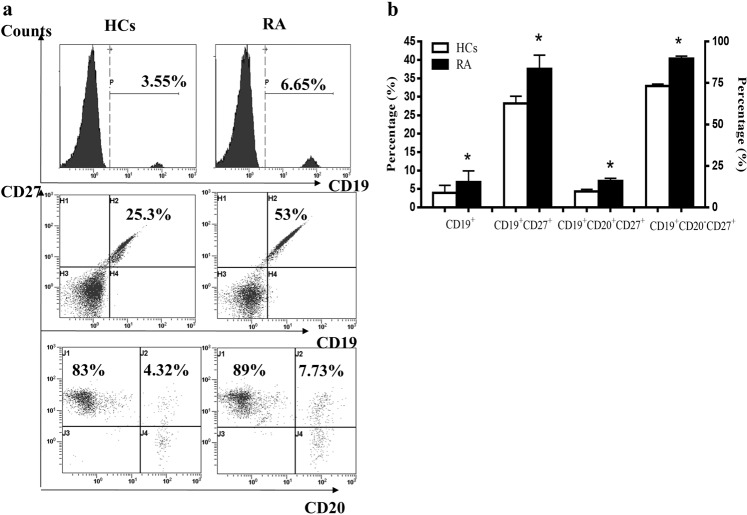
Activated B cell subpopulations in RA patients were increased
B cell subpopulations were analyzed by flow cytometry. The results showed that the percentages of CD19+ B cells, CD19+CD27+ B cells, CD19+CD20+CD27+ B cells, and CD19+CD20−CD27+ B cells were increased significantly in RA patients (Fig. 2a, b). The number of CD19+ B cells, CD19+CD27+ B cells, and CD19+CD20−CD27+ B cells in RA patients was higher than that in HCs. There were no significant differences between the two groups in the number of immature B cells (CD19+CD20+ CD27− B cells) and memory B cells (CD19+CD20+ CD27+ B cells) (data not shown).
Fig. 2. B cell subpopulations were compared between RA patients and HCs.
The percentages of B cell subpopulations (CD19+ B cells, CD19+CD27+ B cells, CD19+CD20+CD27+ B cells, and CD19+CD20−CD27+ B cells) were analyzed by flow cytometry. a Percentages of CD19+ B cells, CD19+CD27+ B cells, CD19+CD20+ CD27+ B cells, and CD19+CD20−CD27+ B cells in RA and HCs. b Percentages of B cell subpopulations were compared between RA and HCs. *P < 0.05 vs. HCs.
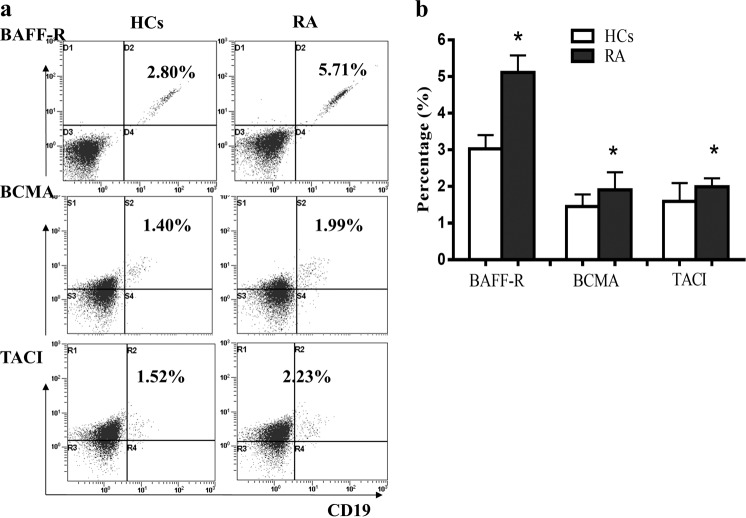
The expression of BAFFR, BCMA, and TACI on B cells in RA patients was elevated
The expression of BAFFR, BCMA, and TACI on B cells was measured by flow cytometry. Compared with HCs, the percentages of CD19+BAFFR+ B cells, CD19+BCMA+ B cells, and CD19+TACI+ B cells were increased significantly in RA patients. Among them, the percentage of CD19+BAFFR+ B cells (5.71%) was higher than that of CD19+BCMA+ B cells (1.99%) and CD19+TACI+ B cells (2.23%) in RA patients (Fig. 3a, b).
Fig. 3. The expression levels of BAFFR, BCMA, and TACI on B cells were compared between RA patients and HCs.
The expression of BAFF receptors (BAFFR, BCMA, and TACI) was analyzed by flow cytometry. a Percentages of CD19+BAFFR+ B cells, CD19+BCMA+ B cells, and CD19+TACI+ B cells in RA and HCs. b Percentages of CD19+BAFFR+ B cells, CD19+BCMA+ B cells, and CD19+TACI+ B cells were compared between RA and HCs. *P < 0.05 vs. HCs.
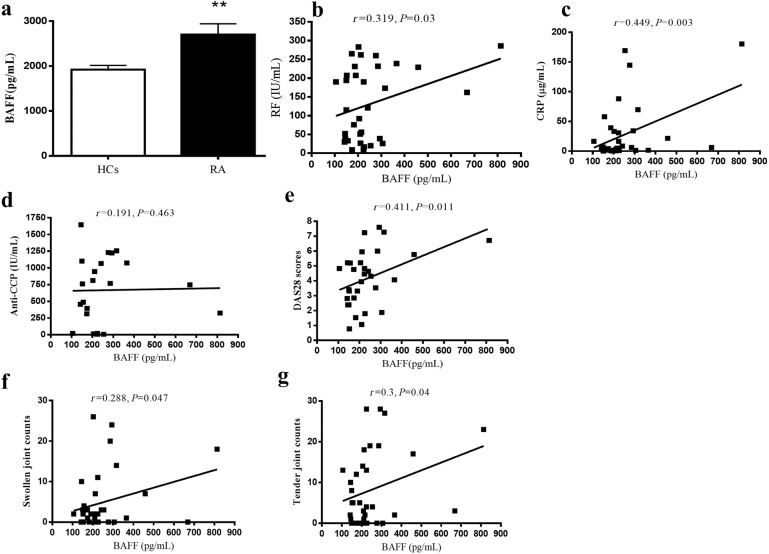
BAFF levels in RA were enhanced and associated with laboratory parameters or clinical variables in RA patients
BAFF levels in RA and HCs were analyzed by ELISA. Figure 4a shows that the BAFF level in RA patients was higher than that in HCs. The mean values of BAFF in RA patients and HCs were 2700 and 1920 pg/mL, respectively. There was a significant difference between the two groups (P = 0.008). There were significant correlations between BAFF level and the laboratory parameters of RF (P = 0.03) and CRP (P = 0.003) (Fig. 4b, c). An association was also shown between BAFF level and DAS28, SJC, or TJC (Fig. 4e–g). However, the BAFF level was not associated with the anti-CCP antibody (Fig. 4d).
Fig. 4. The level of BAFF in serum was compared between RA patients and HCs, and the correlation of BAFF levels with laboratory parameters and clinical variables in RA patients was determined.
a Level of BAFF in RA patients compared with HCs. b The association of BAFF with RF. c The association of BAFF with CRP. d The association of BAFF with anti-CCP. e The association of BAFF with DAS28. f The association of BAFF with swollen joint counts. g The association of BAFF with tender joint counts. **P < 0.01 vs. HCs. r, correlation coefficient; P, significant level.
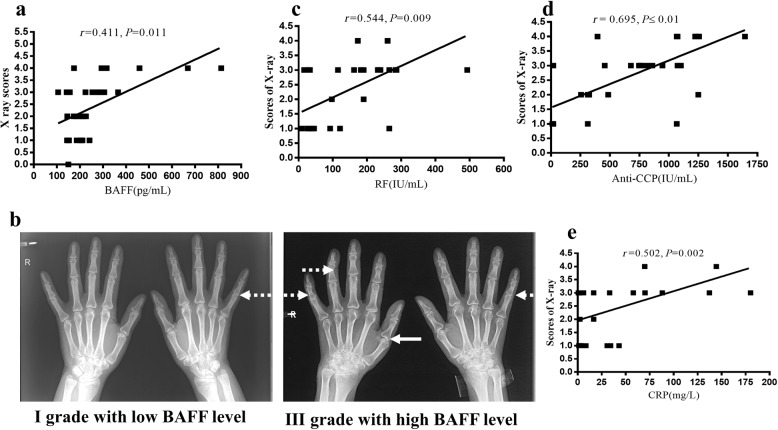
The X-ray score was correlated with high BAFF, anti-CCP antibody, RF, and CRP levels
Interestingly, the X-ray grades of RA patients with high BAFF levels were higher than those with low BAFF levels. The BAFF level was also associated with the X-ray grade (P = 0.011) (Fig. 5a). In Fig. 5b, grade I X-ray was found in RA patients with low BAFF levels. Soft tissue swelling around the affected joint and mild joint space stenosis were only observed in these patients. However, in RA patients with a high level of BAFF, grade II or III X-ray was found. Middle or severe joint space stenosis and joint subluxation were observed in these patients.
Fig. 5. The correlation of X-ray scores and BAFF, anti-CCP antibody, RF, and CRP levels.
a BAFF level was also associated with the grade of X-ray. b X-ray of hands9 of RA between low BAFF level and high BAFF level. Solid arrows show joint space stenosis, and dotted arrows show joint subluxation. c The association of X-ray with RF level. d The association of X-ray grade with anti-CCP antibody level. e The association of X-ray grade with CRP level. P values < 0.05 were considered significant. r, correlation coefficient; P, significant level.
In addition, we also analyzed the association between X-ray scores and autoantibodies (anti-CCP antibody and RF) or CRP levels. The results showed that a significant correlation of X-ray scores was found with serum RF, anti-CCP, or CRP levels (Fig. 5c–e).
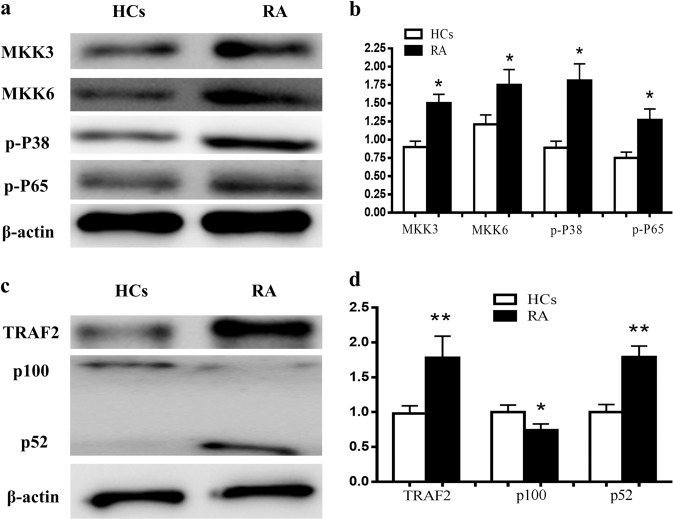
NF-κB pathway activation in B cells of RA patients
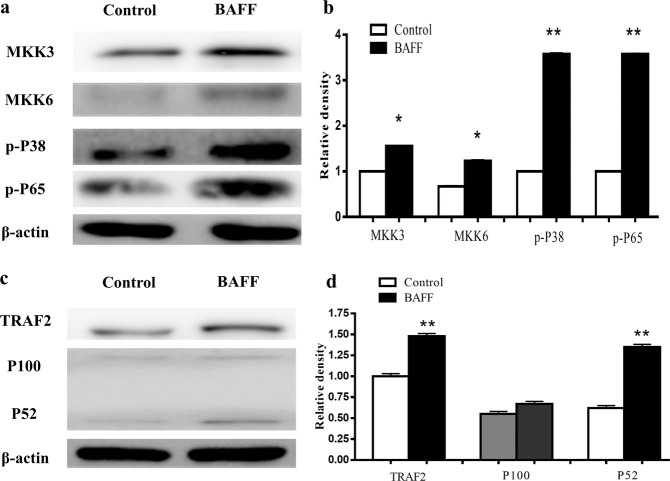
The protein expression levels of some molecules in the NF-κB pathway in B cells, such as MKK3, MKK6, p-p38 p-NF-κB65, TRAF2, and NF-κB100/52, were analyzed by Western blotting. The results showed that the expression of MKK3, MKK6, p-P38, and p-P65 in RA patients was enhanced (Fig. 6a, b). TRAF2 and p52 expression levels in RA patients were higher than that in HCs. p100 levels in RA were reduced (Fig. 6c, d).
Fig. 6. The expression levels of MKK3, MKK6, p-P38, p-P65, TRAF2, and p100/52 in the NF-κB pathway in B cells were compared between RA patients and HCs.
The protein expression levels of MKK3, MKK6, p-p38, p-P65, TRAF2, and p100/52 were analyzed by Western blotting. a Western blotting images of MKK3, MKK6, p-P38, and p-P65. b Expression of MKK3, MKK6, p-P38, and p-P65 in RA patients compared with HCs. c Western blotting images of TRAF2 and p100/52. d Expression of TRAF2 and p100/52 in RA compared with HCs. n = 10, three replicates were performed. *P < 0.05, **P < 0.01 vs. HCs.
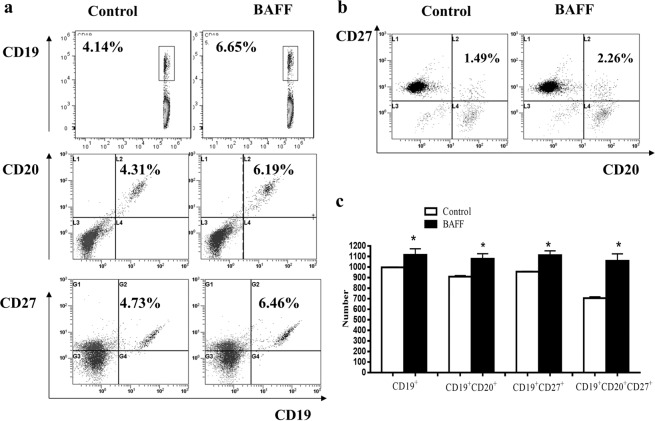
BAFF promoted the differentiation of B cells
In vitro, normal B cells were stimulated by BAFF. CD19+ B cells, CD19+CD27+ B cells, CD19+CD20+CD27+ B cells and CD19+CD20−CD27+ B cells were analyzed by flow cytometry. The results showed that the percentages of CD19+ B cells, CD19+CD27+ B cells, CD19+CD20+CD27+ B cells and CD19+CD20−CD27+ B cells were increased significantly in the BAFF stimulation group (Fig. 7a–c).
Fig. 7. BAFF promoted the differentiation of B cells in vitro.
a The percentages of CD19+ B cells, CD19+CD27+ B cells, and CD19+CD20+ B cells were analyzed by flow cytometry. b The percentage of CD19+CD20+CD27+ B cells was analyzed by flow cytometry. c The percentages of CD19+ B cells, CD19+CD27+ B cells, CD19+CD20+ B cells, and CD19+CD20+CD27+ B cells were increased significantly in the BAFF stimulation group compared with the control group. *P < 0.05 vs. control.
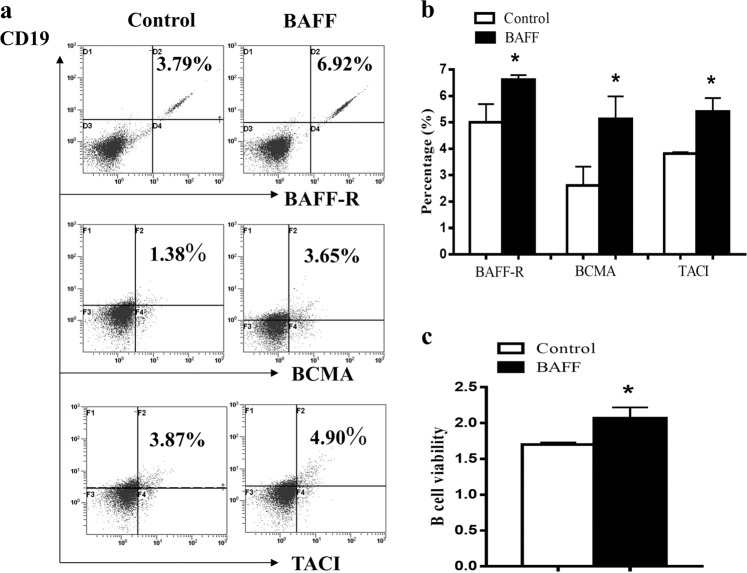
BAFF upregulated the expression of BAFFR, BCMA, and TACI on B cells and promoted the viability of B cells
In vitro, normal B cells were stimulated by BAFF, and CD19+BAFFR+ B cells, CD19+BCMA+ B cells, and CD19+TACI+ B cells were analyzed by flow cytometry. We found that the percentages of CD19+BAFFR+ B cells, CD19+BCMA+ B cells, and CD19+TACI+ B cells were increased significantly in the BAFF group compared with the control (Fig. 8a, b). Additionally, BAFF enhanced the viability of B cells (Fig. 8c).
Fig. 8. BAFF promoted the viability of B cells and upregulated the expression of BAFFR, BCMA, and TACI on B cells in vitro.
a CD19+BAFFR+ B cells, CD19+BCMA+ B cells, and CD19+TACI+ B cells were analyzed by flow cytometry. b The percentages of CD19+BAFFR+ B cells, CD19+BCMA+ B cells, and CD19+TACI+ B cells were increased significantly in the BAFF group compared with the control group. c BAFF enhanced the viability of B cells. *P < 0.05 vs. control.
BAFF might mediate canonical and noncanonical NF-κB pathways in B cells
In vitro, normal B cells were stimulated by BAFF, and the protein expression levels of MKK3, MKK6, p-P38 p-P65, TRAF2, and p100/52 in B cells stimulated by BAFF were analyzed by Western blotting. The results showed that the expression of MKK3, MKK6, p-P38, and p-P65 was enhanced in the BAFF group (Fig. 9a, b). TRAF2 and p52 were higher than those in the control. p100 was not changed significantly compared with the control (Fig. 9c, d).
Fig. 9. The protein expression levels of MKK3, MKK6, p-p38 p-P65, TRAF2, and p100/52 in B cells stimulated by BAFF.
a Western blotting images of MKK3, MKK6, p-P38, and p-P65. b The expression levels of MKK3, MKK6, p-P38, and p-P65 were compared between RA patients and the control group. c Western blotting images of TRAF2 and p100/52. d Expression of TRAF2 and p100/52 in RA was compared with the control. *P < 0.05, **P < 0.01 vs. control.
Discussion
The combined detection of anti-CCP antibodies and RF could increase the sensitivity and specificity of RA diagnosis. We showed that the levels of anti-CCP antibody and RF were high in RA patients with high DAS28 scores and joint function. These findings suggest that the humoral immune system involved in B cells is abnormal in RA pathological mechanisms. In addition, we found that the percentages of CD19+ B cells, activated CD19+CD27+ B cells, memory CD19+CD20+CD27+ B cells, and plasma CD19+CD20−CD27+ B cells were increased significantly in RA patients. Abnormal B cell subsets can lead to the loss of tolerance and autoantibody production and promote GC formation and memory B cell differentiation into plasma cells [37–40].
In addition to autoantibodies, high levels of immunoglobulins (IgA, IgE, IgD, IgG) produced by plasma cells are also observed in autoimmune disease [41]. In this study, the levels of IgA, IgE, IgD, IgG1, IgG2, and IgG4 were increased significantly in RA patients. These results might be related to the high percentage of plasma cells. IgD and IgM are coexpressed on the surface of the majority of mature B cells [41]. It was found first that IgD levels in the serum of RA patients were increased in our laboratory. The previous results in our laboratory showed that the levels of secretory IgD (sIgD), membrane IgD, and IgD receptor (IgDR) were significantly higher in RA patients than in HCs. The concentration of sIgD was positively correlated with soluble receptor activator of nuclear factor-κB ligand, RF and CRP in RA patients. IgD could promote the proliferation of PBMCs and induce IL-1α, IL-1β, TNF-α, IL-6, and IL-10 production from PBMCs. Furthermore, the expression of IgDR on T and B cells was significantly increased by treatment with IgD [42]. These findings suggest that IgD exerts some important immune functions and may contribute to RA pathogenesis.
BAFF plays an important role in the pathogenesis of several autoimmune diseases. BAFF has been identified as a potent survival factor for B cells and prevents spontaneous apoptosis of naive mature B cells in vivo [43]. Overexpression and high levels of BAFF were detected in RA patients [21, 44]. However, whether BAFF is associated with disease activity and bone damage in RA has not been clarified currently. In the present study, we characterized BAFF levels and analyzed the correlation between BAFF levels and clinical variables, laboratory parameters or X-ray scores in RA. We found that BAFF levels in RA were increased. In addition, we found that serum BAFF levels were correlated with RF, CRP, DAS28, SJC, TJC, and X-ray scores but not anti-CCP antibodies. Our findings were consistent with Geng’s report. Geng reported that plasma BAFF levels were correlated with DAS28, ESR, and CRP. Patients with more advanced changes in X-rays had high BAFF levels in serum [45]. This result indicated that BAFF levels may become a new index to evaluate disease activity and predict prognosis. The transcription factor NF-κB is a central mediator of immune and inflammatory responses. TRAFs are key regulatory proteins in NF-κB signaling pathways. TRAF2 and TRAF3 act cooperatively to regulate the survival and maturation signals mediated by the BAFF-R in NF-κB signaling pathways. Here, the expression of BAFF-R (BAFFR, BCMA, and TACI) and important molecules in the noncanonical and canonical NF-κB signaling pathways (MKK3, MKK6, p-P38, p-P65, TRAF2, p52) were also significantly increased in RA patients. These findings suggest that the abnormal subpopulations and functions of B cells might be associated with high expression of BAFF-R in RA. Noncanonical and canonical NF-κB signaling pathways in B cells of RA were activated, and high levels of BAFF and BAFF-R might mediate these abnormal signaling pathways. In conclusion, BAFF may play an important role in regulating immune homeostasis in RA.
B cell-mediated autoimmunity may have a causative role in bone remodeling in RA. Autoantibodies, such as anti-citrullinated protein antibody (ACPA) and RF, are major risk factors for articular bone destruction in RA. ACPA-positive RA patients have higher rates of joint damage progression. Systemic bone mineral density is reduced in RA patients with ACPA positivity and high RF levels [46, 47]. In this study, we analyzed the association between autoantibodies and bone destruction. The results showed that there is a significant positive correlation between serum anti-CCP and RF levels and X-ray scores, which indicated that high serum anti-CCP and RF can cause more severe bone destruction. Therefore, our findings are also consistent with a previous report [48].
In addition, the results of the study showed that the levels of IL-1α, IL-1β, IL-6, IL-8, TNF-α, IFN-γ, MCP-1, IL-4, and IL-13 in RA patients were also elevated. IL-4 and IL-13 secreted by Th2 cells could induce IgG1, IgG4, and IgE production, promote the expression of MHCII, and participate in regulating the growth and differentiation of B cells in vivo [49]. Our findings were consistent with the studies from Pavlovic and Avdeev, where the levels of IL-4 and IL-13 were significantly high in patients with RA [50, 51]. These results suggest the dual functions of IL-4 and IL-13 in the pathological process of RA.
Conclusion
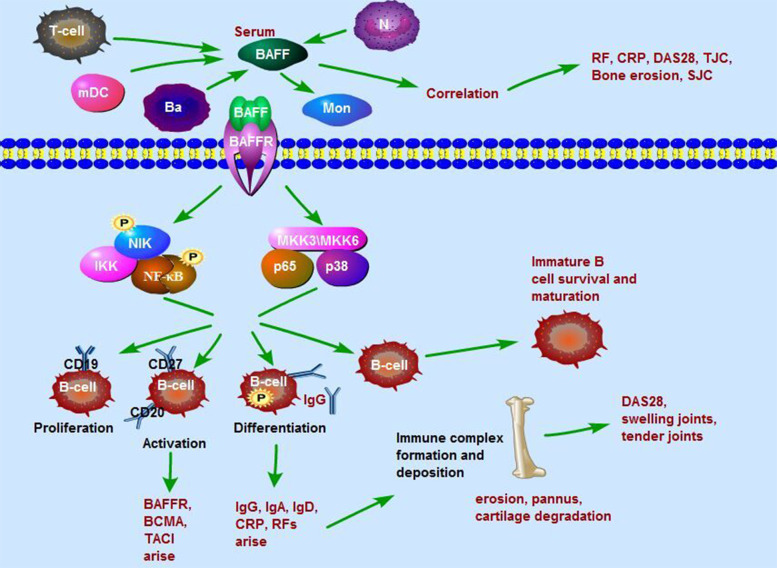
In conclusion, in this study, a high level of serum BAFF was found in RA, and the BAFF level was associated with RF, CRP, DAS28, SJC, TJC, and bone lesions. Abnormal subpopulations and functions of B cells might be associated with high expression of BAFF-R and high levels of BAFF in RA. BAFF activates alternative and canonical NF-κB pathways by binding to BAFF-R, thereby promoting the proliferation, activation, and differentiation of B cells as well as B cell survival and maturation, which in turn results in the production of autoantibodies, immunoglobulins and cytokines and BAFF-R expression. Immune complexes are formed by autoantibodies and deposited in joint synovium, which results in synovitis, pannus formation, bone erosion, and cartilage degradation (Fig. 10). This study allows us to further understand the pathological mechanisms of RA and provides clues that BAFF might be an ideal therapeutic target for RA.
Fig. 10. BAFF is involved in B cell differentiation and activation through the NF-κB signaling pathway in RA.
A high level of serum BAFF was found in RA, while the BAFF level was associated with RF, CRP, DAS28, SJC, TJC, and bone lesions. BAFF activates alternative and canonical NF-κB pathways by binding to BAFF receptors, thereby promoting the proliferation, activation, and differentiation of B cells as well as B cell survival and maturation, which in turn results in the production of autoantibodies, immunoglobulins and cytokines and BAFF receptor further expression. Immune complexes are formed by autoantibodies and deposit joint synovium, which results in synovitis, pannus formation, bone erosion, and cartilage degradation.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. U1803129, 81803538, 81302517 and 81330081).
Author contributions
LLZ designed the experiments and drafted the manuscript. JHX participated in the collection of samples and revised the manuscript. WW designed the experiments and drafted the manuscript. HX participated in the collection of samples and immunoassays. FZ carried out the ELISA studies. YJW participated in the flow cytometry analysis. JLS participated in the flow cytometry analysis. YL carried out Western blotting analysis. YT carried out ELISA studies and the collection of samples. SQX participated in the collection of samples. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Ling-ling Zhang, Hui Xiao, Feng Zhang
Contributor Information
Jian-hua Xu, Email: xujianhua86@aliyun.com.
Wei Wei, Email: wwei@ahmu.edu.cn.
References
- 1.Wei F, Chang Y, Wei W. The role of BAFF in the progression of rheumatoid arthritis. Cytokine. 2015;76:537–44.. doi: 10.1016/j.cyto.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Genovese MC, Fleischmann RM, Greenwald M, Satterwhite J, Veenhuizen M, Xie L, et al. Tabalumab, an anti-BAFF monoclonal antibody, in patients with active rheumatoid arthritis with an inadequate response to TNF inhibitors. Ann Rheum Dis. 2013;72:1461–8. doi: 10.1136/annrheumdis-2012-202775. [DOI] [PubMed] [Google Scholar]
- 3.Shetty A, Hanson R, Korsten P, Shawagfeh M, Arami S, Volkov S, et al. Tocilizumab in the treatment of rheumatoid arthritis and beyond. Drug Des Dev Ther. 2014;8:349–64. doi: 10.2147/DDDT.S41437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang LL, Wei W, Xiao F, Xu JH, Bao CD, Ni LQ, et al. A randomized, double-blind, multicenter, controlled clinical trial of chicken type II collagen in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59:905–10.. doi: 10.1002/art.23824. [DOI] [PubMed] [Google Scholar]
- 5.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SB. Targeting the B cell in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2010;24:553–63.. doi: 10.1016/j.berh.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Leandro MJ. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res Ther. 2013;15:S3. doi: 10.1186/ar3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nashi E, Wang Y, Diamond B. The role of B cells in lupus pathogenesis. Int J Biochem Cell Biol. 2010;42:543–50.. doi: 10.1016/j.biocel.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi JF. Targeted therapies in adult B-cell malignancies. BioMed Res Int. 2015;2015:217593. doi: 10.1155/2015/217593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fecteau JF, Cote G, Neron S. A new memory CD27-IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J Immunol. 2006;177:3728–36.. doi: 10.4049/jimmunol.177.6.3728. [DOI] [PubMed] [Google Scholar]
- 11.Berkowska MA, Driessen GJ, Bikos V, Grosserichter-Wagener C, Stamatopoulos K, Cerutti A, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118:2150–8. doi: 10.1182/blood-2011-04-345579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorner T, Lipsky PE. B cells: depletion or functional modulation in rheumatic diseases. Curr Opin Rheumatol. 2014;26:228–36.. doi: 10.1097/BOR.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 13.B cells and autoimmunity 2010. Proceedings of a conference. August 2010. Nara, Japan. Mol Immunol. 2011;48:1279–347. doi: 10.1016/j.molimm.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Morais SA, Vilas-Boas A, Isenberg DA. B-cell survival factors in autoimmune rheumatic disorders. Ther Adv Musculoskelet Dis. 2015;7:122–51.. doi: 10.1177/1759720X15586782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Davidson A. BAFF and selection of autoreactive B cells. Trends Immunol. 2011;32:388–94.. doi: 10.1016/j.it.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay F, Leung H. The role of the BAFF/APRIL system on T cell function. Semin Immunol. 2006;18:284–9. doi: 10.1016/j.smim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Khare SD, Sarosi I, Xia XZ, McCabe S, Miner K, Solovyev I, et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci USA. 2000;97:3370–5. doi: 10.1073/pnas.97.7.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–9. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Moura RA, Canhao H, Polido-Pereira J, Rodrigues AM, Navalho M, Mourao AF, et al. BAFF and TACI gene expression are increased in patients with untreated very early rheumatoid arthritis. J Rheumatol. 2013;40:1293–302.. doi: 10.3899/jrheum.121110. [DOI] [PubMed] [Google Scholar]
- 20.Bosello S, Youinou P, Daridon C, Tolusso B, Bendaoud B, Pietrapertosa D, et al. Concentrations of BAFF correlate with autoantibody levels, clinical disease activity, and response to treatment in early rheumatoid arthritis. J Rheumatol. 2008;35:1256–64. [PubMed] [Google Scholar]
- 21.Scholz JL, Crowley JE, Tomayko MM, Steinel N, O’Neill PJ, Quinn WJ, 3rd, et al. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci USA. 2008;105:15517–22.. doi: 10.1073/pnas.0807841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li PP, Liu DD, Liu YJ, Song SS, Wang QT, Chang Y, et al. BAFF/BAFF-R involved in antibodies production of rats with collagen-induced arthritis via PI3K-Akt-mTOR signaling and the regulation of paeoniflorin. J Ethnopharmacol. 2012;141:290–300. doi: 10.1016/j.jep.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 24.Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009;183:3561–7. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- 25.Ding H, Wang L, Wu X, Yan J, He Y, Ni B, et al. Blockade of B-cell-activating factor suppresses lupus-like syndrome in autoimmune BXSB mice. J Cell Mol Med. 2010;14:1717–25.. doi: 10.1111/j.1582-4934.2009.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, 3rd, et al. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9:1379–87.. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grech AP, Amesbury M, Chan T, Gardam S, Basten A, Brink R. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity. 2004;21:629–42.. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Grech AP, Amesbury M, Chan T, Gardam S, Basten A, Brink R. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity. 2004;21:629–42.. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Badr G, Borhis G, Lefevre EA, Chaoul N, Deshayes F, Dessirier V, et al. BAFF enhances chemotaxis of primary human B cells: a particular synergy between BAFF and CXCL13 on memory B cells. Blood. 2008;111:2744–54. doi: 10.1182/blood-2007-03-081232. [DOI] [PubMed] [Google Scholar]
- 31.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 32.Zheng N, Wang D, Ming H, Zhang H, Yu X. BAFF promotes proliferation of human mesangial cells through interaction with BAFF-R. BMC Nephrol. 2015;16:72. doi: 10.1186/s12882-015-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bridges SL. Update on autoantibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2004;6:343–50.. doi: 10.1007/s11926-004-0008-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhang F, Song SS, Shu JL, Li Y, Wu YJ, Wang QT, et al. BAFF upregulates CD28/B7 and CD40/CD154 expression and promotes mouse T and B cell interaction in vitro via BAFF receptor. Acta Pharmacol Sin. 2016;37:1101–9. doi: 10.1038/aps.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song SS, Yuan PF, Chen JY, Fu JJ, Wu HX, Lu JT, et al. TGF-beta favors bone marrow-derived dendritic cells to acquire tolerogenic properties. Immunol Invest. 2014;43:360–9. doi: 10.3109/08820139.2013.879172. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Sheng K, Chen J, Wu Y, Zhang F, Chang Y, et al. Regulation of PGE2 signaling pathways and TNF-alpha signaling pathways on the function of bone marrow-derived dendritic cells and the effects of CP-25. Eur J Pharmacol. 2015;769:8–21. doi: 10.1016/j.ejphar.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 37.Hiepe F, Radbruch A. Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat Rev Nephrol. 2016;12:232–40.. doi: 10.1038/nrneph.2016.20. [DOI] [PubMed] [Google Scholar]
- 38.Roll P, Palanichamy A, Kneitz C, Dorner T, Tony HP. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:2377–86.. doi: 10.1002/art.22019. [DOI] [PubMed] [Google Scholar]
- 39.Han BK, Olsen NJ, Bottaro A. The CD27-CD70 pathway and pathogenesis of autoimmune disease. Semin Arthritis Rheum. 2016;45:496–501. doi: 10.1016/j.semarthrit.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Xiao Y. HendriksJ, LangerakP, Jacobs H, Borst J. CD27 is acquired by primed B cells at the centroblast stage and promotes germinal center formation. J Immunol. 2004;172:7432–41.. doi: 10.4049/jimmunol.172.12.7432. [DOI] [PubMed] [Google Scholar]
- 41.Chen K, Cerutti A. The function and regulation of immunoglobulin D. Curr Opin Immunol. 2011;23:345–52.. doi: 10.1016/j.coi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Y, Chen W, Chen H, Zhang L, Chang Y, Yan S, et al. The elevated secreted immunoglobulin D enhanced the activation of peripheral blood mononuclear cells in rheumatoid arthritis. PLoS One. 2016;11:e0147788. doi: 10.1371/journal.pone.0147788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan TD, Gardam S, Gatto D, Turner VM, Silke J, Brink R. In vivo control of B-cell survival and antigen-specific B-cell responses. Immunol Rev. 2010;237:90–103. doi: 10.1111/j.1600-065X.2010.00942.x. [DOI] [PubMed] [Google Scholar]
- 44.Assi LK, Wong SH, Ludwig A, Raza K, Gordon C, Salmon M, et al. Tumor necrosis factor alpha activates release of B lymphocyte stimulator by neutrophils infiltrating the rheumatoid joint. Arthritis Rheum. 2007;56:1776–86.. doi: 10.1002/art.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geng Y, Zhang ZL. Comparative study on the level of B lymphocyte stimulator (BlyS) and frequency of lymphocytes between sero-negative and sero-positive rheumatoid arthritis patients. Int J Rheum Dis. 2012;15:478–85.. doi: 10.1111/j.1756-185X.2012.01814.x. [DOI] [PubMed] [Google Scholar]
- 46.van den Broek M, Dirven L, Klarenbeek NB, Molenaar TH, Han KH, Kerstens PJ, et al. The association of treatment response and joint damage with ACPA-status in recent-onset RA: a subanalysis of the 8-year follow-up of the BeSt study. Ann Rheum Dis. 2012;71:245–8. doi: 10.1136/annrheumdis-2011-200379. [DOI] [PubMed] [Google Scholar]
- 47.Bugatti S, Bogliolo L, Vitolo B, Manzo A, Montecucco C, Caporali R. Anti-citrullinated protein antibodies and high levels of rheumatoid factor are associated with systemic bone loss in patients with early untreated rheumatoid arthritis. Arthritis Res Ther. 2016;18:226. doi: 10.1186/s13075-016-1116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omar A, Abo-Elyoun I, Hussein H, Nabih M, Atwa H, Gad S, et al. Anti-cyclic citrullinated peptide (anti-CCP) antibody in juvenile idiopathic arthritis (JIA): correlations with disease activity and severity of joint damage (a multicenter trial) Joint Bone Spine. 2013;80:38–43. doi: 10.1016/j.jbspin.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Wu LC, Scheerens H. Targeting IgE production in mice and humans. Curr Opin Immunol. 2014;31:8–15. doi: 10.1016/j.coi.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Pavlovic V, Dimic A, Milenkovic S, Krtinic D. Serum levels of IL-17, IL-4, and INFgamma in Serbian patients with early rheumatoid arthritis. J Res Med Sci. 2014;19:18–22. [PMC free article] [PubMed] [Google Scholar]
- 51.Avdeev AS, Novikov AA, Aleksandrova EN, Panasiuk E, Nasonov EL. [The importance of cytokine profile characteristics for evaluating the therapeutic effectiveness of monoclonal antibodies against IL-6 receptors in patients with rheumatoid arthritis] Klin Med (Mosk) 2014;92:28–34. [PubMed] [Google Scholar]