Abstract
Objective:
Cervical cancer survivors tend to smoke cigarettes at rates much higher than other cancer survivors and women in the general population. However, few studies take a deep dive into the smoking behavior of cervical cancer survivors and none focus on the barriers they experience related to smoking cessation. This study aimed to describe cervical cancer survivors’ tobacco use characteristics, quit attempts, and barriers to quit success.
Method:
In a concurrent mixed-method design, 50 cervical cancer survivors (94% White non-Hispanic) who were diagnosed in the past five years and were current smokers at diagnosis provided data via standardized questionnaire and semi-structured interview.
Results:
More than three-quarters of participants were current smokers at the time of study participation, 25.6% of whom also reported non-cigarette tobacco use (e.g., electronic cigarette, cigar, snus). Seventy percent of participants reported making at least one 24-hour quit attempt post-diagnosis, with 61.5% of current smokers preferring to quit without professional advice or counseling and 51.3% preferring to quit without medication assistance. Four themes emerged regarding barriers to smoking cessation: motivation and readiness; confidence and uncertainty; triggers; and social and environmental factors.
Conclusions:
The rate of smoking in cervical cancer survivors is remarkably high, which may partly be explained by negative attitudes toward and low use of evidence-based treatment as well as multi-level barriers to smoking cessation.
Keywords: cervical cancer, cancer survivors, qualitative research, smoking, smoking cessation
Cigarette smoking and other tobacco use have long been established as a chief cause of cancer (US Department of Health Education and Welfare, 1964). For this reason, the prevalence rates of current and former smoking in cancer survivors (i.e., individuals with a history of cancer diagnosis, regardless of time since diagnosis or treatment phase (Marzorati, Riva, & Pravettoni, 2017)) typically exceeds what is found in the general population (Gallaway et al., 2019; Swoboda, Walker, & Huerta, 2019; Tseng, Lin, Martin, Chen, & Partridge, 2010). As one example, a recent population-based US study found 52.6% of cancer survivors were current or former smokers compared to 42.1% of non-cancer controls (Swoboda et al., 2019). Notably, the prevalence of current smoking among cancer survivors is not equally distributed across disease sites. Some cancer survivors, based on their disease site alone, are at greater risk of continued smoking than others (Beesley, Eakin, Janda, & Battistutta, 2008; Mayer & Carlson, 2011; Swoboda et al., 2019; Tseng et al., 2010; Underwood et al., 2012; Westmaas, Alcaraz, Berg, & Stein, 2014).
For at least a decade, the results of US population-based surveys consistently show the highest prevalence rates for current smoking are among cervical cancer survivors (CCS). Data from large cross-sectional studies indicate the prevalence of current smoking in CCS ranges from 27 to 48% (Coups & Ostroff, 2005; Mayer & Carlson, 2011; Swoboda et al., 2019; Tseng et al., 2010; Underwood et al., 2012). This is more than twice the rate typically found in cancer survivors (9–18%) (Gallaway et al., 2019; Swoboda et al., 2019; Tseng et al., 2010; Underwood et al., 2012; Westmaas et al., 2014). It also rivals the rate found in a recent systematic literature review of smoking among lung and head/neck cancer survivors (33%) (Burris, Studts, DeRosa, & Ostroff, 2015), which are the disease sites most often studied in this area. Moreover, this rate is more than three times higher than what is observed among women in the general population (12%) (Creamer et al., 2019). Despite clear evidence of CCS being at profound risk for smoking - a behavior that is causally linked to all-cause mortality, cancer-specific mortality, and second primary cancer incidence (US Department of Health and Human Services, 2014) - there is a paucity of studies that focus on describing, explaining, or mitigating continued smoking in this group.
Albeit a very small literature (Hoover et al., 2019; Iyer et al., 2016; Schlumbrecht, Sun, Huang, Zandstra, & Bodurka, 2014; Waggoner, Darcy, Tian, & Lanciano, 2010), there are some important findings from prior studies of CCS’ smoking. Like most smokers who eventually develop a smoking-related health problem, CCS who smoke report a long history of smoking and high nicotine dependence (Hoover et al., 2019; Waggoner et al., 2010). To date, only one study has examined demographic, clinical, or psychosocial correlates of smoking after cervical cancer diagnosis (Waggoner et al., 2010), and with so little data to consider, no firm conclusions can be made. It is possible that correlates for CCS’ smoking behavior are largely consistent with what is found in other cancer survivor samples. If that literature is applied to CCS, younger age, lower socioeconomic status, lower levels of education, White race, and less healthcare access would be risk factors; stronger cancer-related risk perceptions, motivation to quit, and self-efficacy to quit would be protective factors; and gender, ethnicity, cancer stage, cancer treatment, time since cancer diagnosis, and emotional problems would show weak or inconsistent relationships (Little et al., 2018; Mayer & Carlson, 2011; Swoboda et al., 2019; Tseng et al., 2010; Waggoner et al., 2010).
Given the clinical significance of persistent smoking and successful quitting after cancer diagnosis (US Department of Health and Human Services, 2014, 2020), the current study aimed to more fully explore CCS’ smoking-related experiences. This mixed-methods study focuses on CCS who were current smokers at diagnosis, as prior work shows these individuals to be at an especially high risk of smoking in the weeks, months, and years after diagnosis (Burris et al., 2015). The study aim is to help fill a critical gap in the literature on smoking after cancer diagnosis by thoroughly describing CCS’ smoking and quitting behavior and beginning to pinpoint what this high-risk group of cancer survivors perceive as barriers to quitting.
Method
Design and Participants
The methods of this embedded mixed-methods study, including the eligibility criteria, recruitment strategy, and enrollment procedures are described in detail elsewhere (Puleo, Borger, Montgomery, Rivera Rivera, & Burris, 2020) and are only summarized here. Briefly, the quantitative aspect of the study allowed comprehensive measurement of key behavioral variables that have yet to be fully described in the literature (e.g., cigarettes per day, quit attempts) while the qualitative aspect of the study involved open-ended questions meant to uncover what variables CCS believe contribute to smoking and undermine quitting. This study’s inclusion criteria were: 1) first primary cervical cancer diagnosis in the past five years with no other cancer diagnoses, 2) 30-day point prevalence current smoker at cervical cancer diagnosis, 3) 21 years old or older, 4) English literacy, 5) reliable phone access, and 6) Kentucky residence at cancer diagnosis. All 50 participants who completed the quantitative (questionnaire) portion of the study were invited to complete the qualitative (interview) portion and 21 did so. There were no significant differences in the demographic, clinical, or smoking-related characteristics of participants who did and did not complete the interview.
Procedures
Participants were recruited through either a statewide cancer registry that is part of the US Surveillance, Epidemiology, and End Results system and Centers for Disease Control and Prevention National Program of Cancer Registries (n=38) or statewide non-profit organization that provides uninsured and/or low-income adults with cancer-related services (n=12). Potentially eligible individuals were briefed about the study by the cancer registry/non-profit organization via phone and/or mail and could then opt-in to the release of their name and contact information to study staff. After study staff made contact with those who opted-in and determined eligibility for this study, participants provided informed written consent, completed a standardized questionnaire via phone or mail, and for some, completed a semi-structured interview via phone. Data collection occurred from September 2017 to October 2018. Participants received a US $45 check for completion of the questionnaire and a US $30 check for completion of the interview. All procedures were approved by the Institutional Review Board at the University of Kentucky (IRB# 44641) and conducted in compliance with the Belmont Report.
Measures
The cancer registry provided details about participants’ cancer diagnosis, recurrence status, and treatment. Participants responded to demographic (e.g., race, educational attainment) questions from a Behavioral Risk Factors Surveillance System survey (Centers for Disease Control and Prevention, 2016) as part of the questionnaire. Participants also responded to questions about their tobacco use and quit attempt history as part of the questionnaire. The specific questions were consistent with what is regularly used in research with the general population and cancer survivors specifically (Centers for Disease Control and Prevention, 2016; Land, Warren, et al., 2016). The outcomes captured in the questionnaire include: 30-day point prevalence of tobacco use (cigarettes separate from other products), cigarettes smoked per day in the past 7 days (via time-line follow-back procedures), level of nicotine dependence (via the Heaviness of Smoking Index (Heatherton, Kozlowski, Frecker, Rickert, & Robinson, 1989)), motivation and confidence to quit/maintain abstinence in the next 30 days (via Contemplation Ladders (Biener & Abrams, 1991) on a scale from 0=very definitely no/not at all confident to 10=very definitely yes/extremely confident), prevalence of 24-hour quit attempts across varying timespans (specifically, lifetime, past year, and since cancer diagnosis), and use of and preferences around evidence-based tobacco cessation treatment (e.g., nicotine replacement therapy). As part of the interview, participants were asked to describe barriers to making quit attempts and achieving long-term abstinence after cancer diagnosis. A standardized interview guide was used, with the nature and number of follow-up questions tailored to each participant’s response to a core set of open-ended questions. The pertinent core questions include: “What worked for you in your attempts to quit or cut back?”, “What hasn’t worked as well for you in your attempts to quit or cut back?”, and “What, or who, gets in the way of your desire to quit or cut back?”. Interviews were audio-recorded and transcribed verbatim.
Statistical Analysis
Questionnaire data analysis primarily involved descriptive statistics accomplished with the Statistical Package for the Social Sciences, version 25 (IBM Corp., 2017). Interview data analysis involved directed content analysis (Hsieh & Shannon, 2005), where prior research findings aided the initial identification of codes. This analysis involved: 1) reading and discussing a random selection of interviews (n=7); 2) identifying key concepts or variables as initial codes; 3) organizing those codes into a codebook and creating operational definitions for them; 4) double-coding the aforesaid interviews via line-by-line coding (i.e., independent coding by JLB and another co-author, with disagreements discussed and rectified); 5) finalizing the codebook, which necessitated “lumping” or “splitting” some of the initial codes and adding new codes; 6) revising the seven interviews coded previously to reflect the final codebook; 7) double-coding the remaining 14 interviews consistent with the final codebook; and 8) entering all coded interviews in ATLAS.ti 8 for Macintosh (Scientific Software Development GmbH, 2017).
Results
Sample Characteristics
Female participants were either White non-Hispanic (94.0%, n=47) or Black/African American (6.0%, n=3). Participants’ average age was 45.5 (SD=8.1). Nearly half (n=23) of participants were a member of a married or unmarried couple, with many being divorced, separated, or widowed (38.0%, n=19), and the rest being single, never married (14.0%, n=7); missing data: 2.0% (n=1). Participants’ educational attainment varied widely, with many not graduating high school (38.0%, n=19), some graduating high school (18.0%, n=9), and the remainder attending or graduating from college (36.0%, n=18 and 8.0%, n=4, respectively). Participants’ employment status also varied, with 20.0% (n=10) unemployed, 34.0% disabled (n =17), 36.0% (n=18) employed, and the remainder either retired or homemakers (10.0%, n=5). Reflecting their employment status, more than half of participants reported an annual household income under $20,000 (54.0%, n=27). Sixty percent (n=30) of participants described their place of residence as “rural”.
Average time since diagnosis was 2.7 years (SD=1.3). Most participants were diagnosed with Stage 1 disease (56.0%, n=28), with a fairly even split between Stages 2 and 3 (20.0%, n=10 and 22.0%, n=11, respectively), and a minority with Stage 4 disease (2.0%, n=1). Roughly one-third of participants received surgery alone (32.0%, n=16), with 28.0% (n=14) receiving chemotherapy and radiation, and 30.0% (n=15) receiving surgery, chemotherapy, and radiation; 10.0% (n=5) received another treatment regimen. Sixty-eight percent (n=34) of participants were disease-free at the time of recruitment.
Smoking and Other Tobacco Use Behavior
Seventy-eight percent (n=39) of participants were current smokers. These participants reported smoking an average of 16.5 (SD=8.7) cigarettes per day, with 69.2% (n=27) smoking their first cigarette within 30 minutes of waking. Current smokers’ average level of nicotine dependence was 2.7 (SD=1.5), which is moderate. Twenty-five percent (n=10) of current smokers reported non-cigarette tobacco use in the past month, with 9 using two total tobacco products and 1 using five total tobacco products. Among current smokers, this translates to a prevalence rate of 15.4% (n=6) for electronic cigarettes or vapes, 12.8% (n=5) for cigars or pipes, 2.6% (n=1) for chew tobacco or moist snuff, and 2.6% (n=1) for Swedish-style snus; options not mutually exclusive. No former smoker reported alternative product use in the past month.
All but a few participants reported a lifetime history of at least one quit attempt (90.0%, n=45). After cancer diagnosis, 70.0% (n=35) of participants reported making at least one quit attempt, which reflects all former smokers (n=11) and 61.5% (n=24) of current smokers. Many current smokers preferred to quit without professional help (61.5%, n=24) and without the aid of medications like nicotine replacement therapy, varenicline, and bupropion (51.3%, n=20); options not mutually exclusive. For lifetime use of any evidence-based treatment, 64.1% (n=25) of current smokers and 45.5% (n=5) of former smokers responded in the affirmative. For current smokers who made a quit attempt in the past year (51.2%, n=20), treatment use was modest: nicotine replacement therapy (45.0%, n=9) and brief consultation with an oncologist (45.0%, n=9) were most popular, followed by brief consultation with another healthcare professional (40.0%, n=8), and finally very low rates of Quitline, varenicline, and bupropion use (each 10.0%, n=2); options not mutually exclusive.
Intention and confidence to quit were low among current smokers (M, SD = 3.5, 3.2 and 2.7, 3.1, respectively). Intention and confidence to maintain abstinence among former smokers, half of whom had been abstinent for over a year (45.5%, n=5), was moderate to high ((M, SD = 7.3, 4.7 and 9.8, 0.6, respectively).
Perceived Barriers to Smoking Cessation
In the interviews, participants were asked to discuss barriers to making a quit attempt and quitting for good (see Table 1). Four themes emerged from analysis of the interview data: motivation to quit, confidence to quit, triggers, and social/environmental factors. First, many current smokers described low motivation to quit or some ambivalence toward quitting:
“I know I should quit. I know I should. I mean, really, it’s not good for anybody, whether you’ve had cancer or not. But as far as my mindset is right now, I just don’t see it happening right now.”
(127, Current Smoker)
“I tried the Chantix, and I tried doin’ it by myself and it just…maybe I’m not ready. I don’t know. I mean, I don’t wanna get cancer again, and I don’t wanna die. I have two kids, but it’s just—I don’t know.”
(153, Current Smoker)
These and other statements by current smokers demonstrate low levels of intention, plans, and willingness to quit in the foreseeable future.
Table 1.
Cervical cancer survivors’ perceived barriers to smoking cessation
| Theme | Codes | Illustrative Quotesa |
|---|---|---|
| Motivation to quit | ||
| Readiness to quit: Motivation to quit, as it pertains to urgency or timeliness |
|
|
| Confidence to quit | ||
| Unsure how to quit: Unaware of best method for quitting in general or for oneself specifically |
|
|
| Belief “nothing works”: Belief that smoking cessation treatment is ineffective |
|
|
| Triggers | ||
| Triggers: Stimuli or situations that contribute to desire or urge to smoke |
|
|
| Social/environmental factors | ||
| Social network smokes: Family, friends, coworkers, etc. smoke |
|
|
| Negative social exchanges: Things said or done by members of one’s social network that undermine motivation or efforts to quit |
|
|
In parentheses is the participant’s identification number and smoking status
Many current smokers were also lacking in confidence they could quit if they tried. Some participants were unsure of the best approaches and treatment methods for smoking cessation, as illustrated by this quote: “…my doctor asked me yesterday, she said, ‘Is there anything that I can do to help you quit smoking?’, and I’m like, ‘I don’t know’. I’m not gonna lie.” (127, Current Smoker). Likewise, some participants held the belief that nothing could help them quit and/or smoking cessation treatment was ineffective: “And I had tried, I had tried in the past. In years past, I had tried the patches, the gum, you name it, I tried it. Anything over the counter to try, I had tried it, in the years past. But none of it, none of it had ever worked” (108, Former Smoker). Not knowing how to quit or believing that nothing helps can diminish self-efficacy and be an obstacle to smoking cessation.
Triggers that contributed to participants’ desire to smoke were numerous, and included specific activities, situations, places, people, and moods/emotional states:
“…when you hurt, and you don’t get out of your home or do anything and you’re just sittin’ here at home, yeah, you smoke a lot.”
(148, Current Smoker)
“Probably just the pressures of bein’ at a casino and bein’ in the—maybe even the smell or the chain reaction of somebody—it probably was the excitement of the casino and then somebody firin’ up one or bein’ in the environment maybe.”
(156, Former Smoker)
“I tried to quit, and it just, it was just a bad time to quit. ‘Cause with the stress level, it was not a good time to try.”
(131, Current Smoker)
Since many of the triggers described are unavoidable in everyday life (e.g., eating, situational stressors), participants’ widespread awareness of the link between those triggers and their smoking is commendable, even if few of them were also able to identify adequate coping strategies.
Participants’ social network and environment also played a role in their difficulties with smoking cessation. For current and former smokers alike, participants found it tough to quit while others in their lives continued to smoke. Not only did this serve as a trigger, but having what was viewed as an unsupportive social network compounded the stress in their lives, thereby making smoking cessation more difficult:
“I have no support system. I mean, everybody I know smokes.”
(121, Current Smoker)
“…if I hang out with my friends. I mean, they smoke like, they’re like—have you ever heard the phrase ‘chain smokers’?”
(137, Current Smoker)
Many participants also described negative social interactions related to their smoking, which permeated all sorts of relationships (i.e., provider-patient, familial, romantic, platonic). For current smokers, some of these unhelpful or harmful exchanges centered around a “double standard” with regards to smoking: “Mainly he just tells me because of, I have cancer that I need to quit, and so I say ‘Well, you know, you need to quit, too’, and he’s like, ‘Yeah, but I’m not the one who has cancer!’” (128, Current Smoker). Other times the negativity came as expressions of doubt about participants’ commitment to smoking cessation and ability to quit for good, or lack of empathy about the difficulty of smoking cessation. Altogether, participants described a number of barriers that arose from their relationships and social environment.
Discussion
CCS have long been recognized as the subgroup of cancer survivors with the highest smoking rate (Mayer & Carlson, 2011; Swoboda et al., 2019; Tseng et al., 2010; Underwood et al., 2012; Westmaas et al., 2014), yet they are not well represented in observational studies of smoking after cancer diagnosis. Moreover, of the 20 plus clinical trials published in this area (Nayan, Gupta, Strychowsky, & Sommer, 2013; Nayan, Gupta, & Sommer, 2011; Sheeran et al., 2019), none have focused on CCS. The reason for the lack of scientific attention to CCS’ smoking is unclear, but it may be analogous to the lived experience of cervical and other gynecological cancer survivors, some of whom feel they have “the forgotten cancer” (Warren, Melrose, Brooker, & Burney, 2018; Wray, Markovic, & Manderson, 2007). In a departure from prior research, this is the first study to provide details about the smoking and quitting behavior of CCS in addition to the first to describe self-perceived barriers to smoking cessation after cervical cancer diagnosis.
This mixed-methods study with 50 CCS, all of whom were current smokers at diagnosis and many of whom were White non-Hispanic, lived in rural areas, and reported lower socioeconomic status, yielded four major findings. First, the risk of smoking and other tobacco use after cervical cancer diagnosis is unacceptably high. More than three-quarters of this sample reported smoking in the past month. Participants’ average number of cigarettes per day and time to first cigarette both indicate moderate nicotine dependence, consistent with prior, similar studies (Hoover et al., 2019; Waggoner et al., 2010). Furthermore, one-quarter of current smokers reported non-cigarette tobacco use in the past month, which means dual and poly tobacco use may well occur in CCS. This study did not ascertain the reason for non-cigarette tobacco use, but one could surmise that it is due to the belief these products might aid smoking cessation or be a helpful substitute in places or during times when smoking is prohibited (Popova & Ling, 2013; Shi, Cummins, & Zhu, 2017). Reports of electronic cigarette use in cancer survivors have grown in recent years (Akinboro et al., 2019; Kalkhoran et al., 2018), though research on other products remains rare (see Little et al., 2018 for an exception). Importantly, trial and adoption of electronic cigarettes and the like among cancer survivors who smoke may outpace the rate at which we accumulate empirical evidence of their safety profile and effectiveness for smoking cessation (Kalkhoran & Glantz, 2016; McRobbie, Bullen, Hartmann-Boyce, & Hajek, 2014). Given this, it is imperative future clinical trials of smoking cessation treatment target CCS and measure (if not try to intervene upon) all types of tobacco use (Burris et al., 2015; Land, Toll, et al., 2016).
Second, there is likely room for improvement in terms of CCS’ attitudes toward and use of evidence-based smoking cessation treatment. Motivation and confidence to quit were modest among current smokers in this study, a finding that was evident in both the qualitative and quantitative aspects of the study. Yet, many current smokers reported having made quit attempts since cancer diagnosis, with some having tried in the past year. Consistent with smokers in the general population (Babb, Malarcher, Schauer, Asman, & Jamal, 2017; Shiffman, Brockwell, Pillitteri, & Gitchell, 2008) and other cancer survivor samples (Gallaway et al., 2019; Hoover et al., 2019; Schlumbrecht et al., 2014; Wells et al., 2017), many of CCS’ prior quit attempts can be described as “unaided/unassisted” (i.e., sans medication or counseling), an approach fraught with difficulty. Furthermore, the preference for “unaided/unassisted” quit attempts is problematic, as little to no use of evidence-based treatment is a well-recognized reason for quit failure. The belief in “mind over matter” or that dogged perseverance and strong motivation are sufficient to combat nicotine dependence may be prevalent among CCS in the same way that other smokers espouse these beliefs, particularly those who live in rural areas (Morphett, Partridge, Gartner, Carter, & Hall, 2015; Rodriguez et al., 2017; Smith, Carter, Chapman, Dunlop, & Freeman, 2015; Wells et al., 2017). Consequently, healthcare providers who routinely encounter CCS who smoke should take every opportunity to not only advise tobacco cessation (Karam-Hage, Cinciripini, & Gritz, 2014), but to provide a description of and rationale for evidence-based tobacco cessation treatment, being careful to do so in a non-judgmental and empathic manner (Hoover et al., 2019).
Third, there are several possible targets for intervention with CCS who smoke. Much of the research on correlates of smoking after cancer diagnosis evaluates demographic and clinical variables, and while this is informative insofar as one can identify those at greatest risk for continued smoking, its impact on treatment development is limited because most correlates identified are not modifiable. In this study, the use of semi-structured interviews allowed CCS to articulate their perceptions of barriers to smoking cessation for the first time - and all barriers described are modifiable. Results indicate that some potential obstacles to overcome are low motivation to quit and pessimistic beliefs about one’s ability to do so successfully. Other studies have found CCS and those with cervical dysplasia do not understand or appreciate fully the potential causal link between smoking and their (pre)cancerous condition or the risks of continued smoking on their future health (Costanzo, Lutgendorf, Bradley, Rose, & Anderson, 2005; Hoover et al., 2019; Puleo et al., 2020). In addition, there is evidence rural cancer survivors have lower levels of health literacy than their non-rural counterparts (McDougall et al., 2018), which might be a factor here given the high number of rural-dwelling CCS. While causal attributions and risk perceptions are not uniformly linked to motivation and health behavior change, it remains possible that greater knowledge of the impact of smoking on cervical cancer survivorship could spur motivation to quit (Alton et al., 2018; Hall et al., 2019; Hoover et al., 2019) and improvements in motivation might coincide with improvements in confidence (Dixon, 2008). Another barrier highlighted in this study is triggers. Just like any other smoker, CCS engaging in smoking cessation must learn to cope with triggers to smoke via evidence-based tools like distraction, replacement, self-monitoring, stress management, and problem solving. Finally, the social environment in which CCS live may be a novel and important treatment target. Current and former smokers described the smoking of those close to them as a barrier to their quitting in addition to the perception of limited social support due to harsh comments about their unsuccessful quit attempts in the past and pessimistic comments about their quitting in the future. Additionally, in some cases there was a perception of a “double standard” where CCS-but not their relatives, partners, friends, or anyone else in their social network-should quit smoking, which served to erode further the quality of social relationships. Consequently, interventions that include not only CCS, but also their primary sources of social support could prove beneficial in creating an environment that supports as opposed to undermines smoking cessation, especially if issues of acceptance, empathy, and communication are addressed and joint quit attempts are encouraged (Kane McDonnell, Hollen, Heath, & Andrews, 2015).
Finally, many of the CCS in this study must cope with the chronic stress of low socioeconomic status and/or the challenges to healthcare access that often comes with rural residence. These sociodemographic factors (namely, low socioeconomic status and rural residence) are important, as they are not only linked to smoking persistence at the population level (American Lung Association, 2012; Babb et al., 2017; Creamer et al., 2019; Jamal et al., 2016), but to other health risk behaviors and negative health outcomes, including early mortality (Douthit, Kiv, Dwolatzky, & Biswas, 2015; Nandi, Glymour, & Subramanian, 2014). It is therefore important to note that CCS often report poor adherence to health promotion guidelines around physical activity, fruit and vegetable consumption, and maintenance of a healthy weight (Beesley et al., 2008; Iyer et al., 2016). Health behaviors tend to cluster together, and in the case of some CCS, it may be that smoking is only one aspect of an overall unhealthy lifestyle. Issues of vulnerability and inequity play a role in US cervical cancer outcomes (Freeman & Wingrove, 2005), so women should not be blamed for their disease or any health behavior that may have contributed to it. Instead, there should be efforts to provide the highest quality cervical cancer survivorship care, which would promote stress management, health promotion, health literacy, financial wellbeing, and healthcare access.
To close, the results and implications of this study should be viewed in light of its limitations. First, the sample size is a limitation, though it is on par with similar studies (Hoover et al., 2019; Li, Chan, & Lam, 2014; Morphett et al., 2015). Second, the singular study location, recruitment methods, and sample characteristics limit generalizability to US CCS who smoked at diagnosis, and within that group, perhaps just those who are White non-Hispanic. The applicability of these findings to Black cervical cancer survivors with a smoking history is an open question, one that undoubtedly deserves investigation given Black-White disparities in quit rates among the general population (Kulak, Cornelius, Fong, & Giovino, 2016) and Black-White disparities in cervical cancer mortality (Benard et al., 2017). Third, the cross-sectional design precludes any statements about how cervical cancer diagnosis directly impacted participants’ smoking behavior. Finally, no inferential analysis or effect size estimates related to correlates of smoking after cervical cancer diagnosis was conducted, as the exploration of perceived barriers was limited to qualitative data. Given the importance of the issue, and the dearth of relevant research, it is recommended that future research on CCS’ tobacco use include larger, more racially and ethnically diverse samples that are followed over time and assess or intervene upon the multi-level barriers to tobacco cessation described in this and other research.
Figure 1.
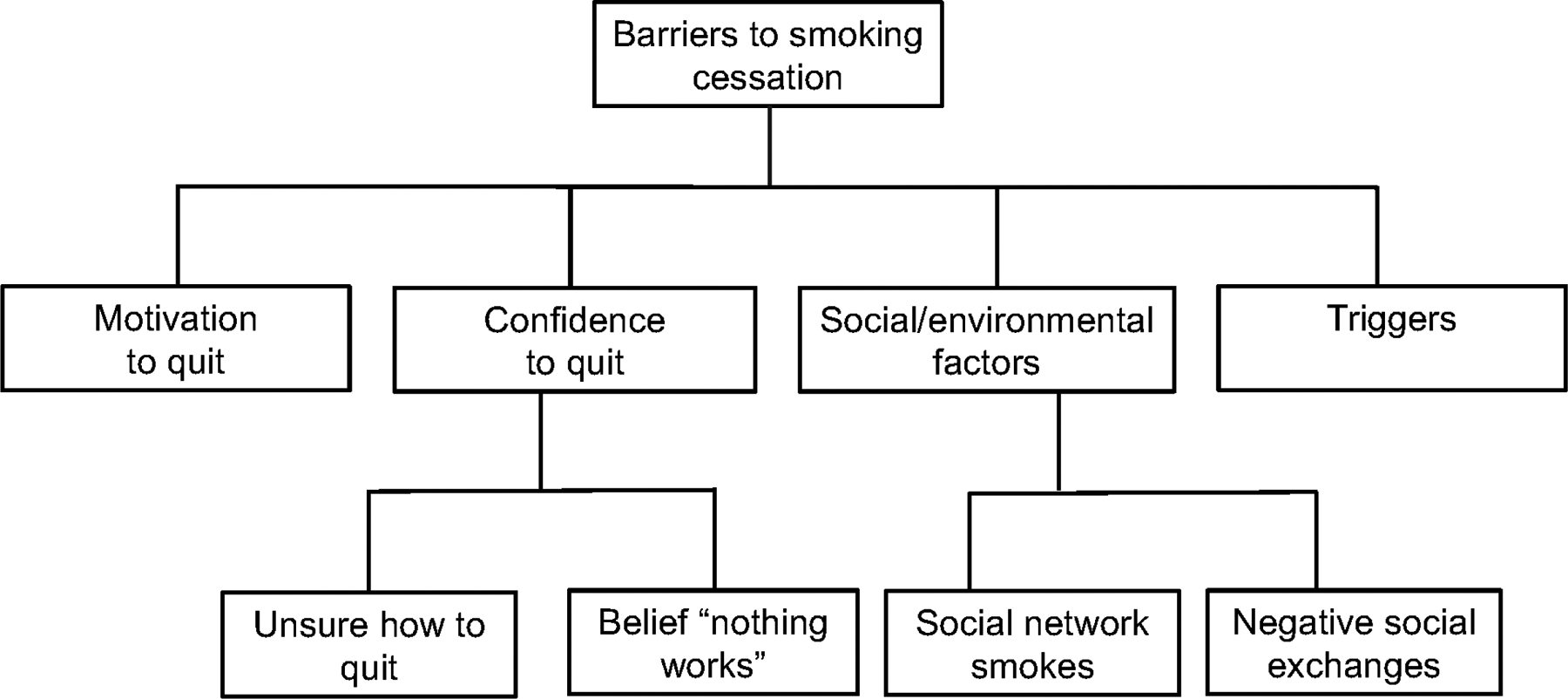
A coding tree of cervical cancer survivors’ perceived barriers to quitting
Public Health Significance Statement:
This study highlights a very high smoking rate among cervical cancer survivors as well as a multitude of barriers to their smoking cessation. As cervical cancer survivors are a vulnerable, high-risk group for smoking persistence, they could benefit from tailored, high-intensity interventions.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers K07 CA181351, P30 CA177558, and R25 CA221765, the National Institute of Drug Abuse of the National Institutes of Health under award number T32 DA035200, and the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1 TR000117. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors would like to acknowledge the support of Vicki Blevins-Booth and the entire Kentucky Cancer Link staff as this work would not have been possible without their assistance. The authors would also like to acknowledge the assistance of Jaclyn McDowell, DrPH and the rest of the Kentucky Cancer Registry staff. Data for this study was provided by the Kentucky Cancer Registry whose activities are supported by the National Cancer Institute’s Surveillance Epidemiology and End Results Program, the Centers for Disease Control and Prevention National Program of Cancer Registries, and the Commonwealth of Kentucky. The authors are also grateful for the assistance of Ashley Brown, Kelly Sudbrack, Jamie Ostroff, Elyse Shuke, and Nancy Schoenberg, each of whom contributed to the formulation or execution of this work.
Footnotes
The results of preliminary analysis of some of the data in this paper were presented as a poster presentation at the 2018 Society for Research on Nicotine and Tobacco - Europe Annual Conference and the 2019 Society for Research on Nicotine and Tobacco Annual Meeting.
We have no known conflicts of interest to declare.
References
- Akinboro O, Nwabudike S, Elias R, Balasire O, Ola O, & Ostroff JS (2019). Electronic cigarette use among survivors of smoking-related cancers in the United States. Cancer Epidemiology Biomarkers and Prevention, 28, 2087–2094. 10.1158/1055-9965.EPI-19-0105 [DOI] [PubMed] [Google Scholar]
- Alton D, Eng L, Lu L, Song Y, Su J, Farzanfar D, … Giuliani ME (2018). Perceptions of continued smoking and smoking cessation among patients with cancer. Journal of Oncology Practice, 14, e269–e279. 10.1200/jop.17.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Lung Association. (2012). Cutting tobacco’s rural roots. Washington, D.C.: American Lung Association. [Google Scholar]
- Babb S, Malarcher A, Schauer G, Asman K, & Jamal A (2017). Quitting smoking among adults-United States, 2000–2015. MMWR Morbidity and Mortality Weekly Report, 65, 1457–1464. 10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- Beesley VL, Eakin EG, Janda M, & Battistutta D (2008). Gynecological cancer survivors’ health behaviors and their associations with quality of life. Cancer Causes and Control, 19, 775–782. 10.1007/s10552-008-9140-y [DOI] [PubMed] [Google Scholar]
- Benard VB, Watson M, Saraiya M, Harewood R, Townsen JS, Stroup AM, … Allemani C (2017). Cervical cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 Study. Cancer, 123, 5119–5137. 10.1002/cncr.30906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, & Abrams DB (1991). The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology, 10, 360–365. 10.1037/0022-006X.70.3.494 [DOI] [PubMed] [Google Scholar]
- Borderud SP, Li Y, Burkhalter JE, Sheffer CE, & Ostroff JS (2014). Electronic cigarette use among patients with cancer: Characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer, 120, 3527–3535. 10.1002/cncr.28811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris JL, Studts JL, DeRosa AP, & Ostroff JS (2015). Systematic review of tobacco use after lung or head/neck cancer diagnosis: Results and recommendations for future research. Cancer Epidemiology, Biomarkers and Prevention, 24, 1450–1461. 10.1158/1055-9965.EPI-15-0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2016). 2017 Behavioral Risk Factor Surveillance System questionnaire. Atlanta, GA: Centers for Disease Control and Prevention,. Retrieved from https://www.cdc.gov/brfss/questionnaires/pdf-ques/2017_BRFSS_Pub_Ques_508_tagged.pdf [Google Scholar]
- Costanzo ES, Lutgendorf SK, Bradley SL, Rose SL, & Anderson B (2005). Cancer attributions, distress, and health practices among gynecologic cancer survivors. Psychosomatic Medicine, 67, 972–980. 10.1097/01.psy.0000188402.95398.c0 [DOI] [PubMed] [Google Scholar]
- Coups EJ, & Ostroff JS (2005). A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Preventive Medicine, 40, 702–711. 10.1016/j.ypmed.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Creamer MR, Wang TW, Babb S, Cullen KA, Day H, Willis G, … Neff L (2019). Tobacco product use and cessation among adults — United States, 2018. MMWR Morb Mortal Wkly Rep, 68, 1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon A (2008). Motivation and confidence: What does it take to change behaviour? King’s Fund. London: King’s Fund. Retrieved from https://www.kingsfund.org.uk/sites/files/kf/field/field_document/motivation-confidence-health-behavious-kicking-bad-habits-supporting-papers-anna-dixon.pdf [Google Scholar]
- Douthit N, Kiv S, Dwolatzky T, & Biswas S (2015). Exposing some important barriers to health care access in the rural USA. Public Health, 129, 611–620. 10.1016/j.puhe.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Freeman HP, & Wingrove BK (2005). Excess cervical cancer mortality: A marker for low access to health care in poor communities. NIH Publication No. 5–5282 Rockville, MD: National Cancer Institute,. [Google Scholar]
- Gallaway MS, Glover-Kudon R, Momin B, Puckett M, Lunsford NB, Ragan KR, … Babb S (2019). Smoking cessation attitudes and practices among cancer survivors - United States, 2015. Journal of Cancer Survivorship, 13, 66–74. 10.1007/s11764-018-0728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DL, Neil JM, Ostroff JS, Hawari S, O’Cleirigh C, & Park ER (2019). Perceived cancer-related benefits of quitting smoking and associations with quit intentions among recently diagnosed cancer patients. Journal of Health Psychology, 00, 1–12. 10.1177/1359105319845131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, & Robinson J (1989). Measuring the heaviness of smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Addiction, 84, 791–800. [DOI] [PubMed] [Google Scholar]
- Hoover DS, Spears CA, Vidrine DJ, Walker JL, Shih Y-CT, Wetter DW, … Vidrine JI (2019). Smoking cessation treatment needs of low SES cervical cancer survivors. American Journal of Health Behavior, 43, 606–620. 10.5993/ajhb.43.3.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HF, & Shannon SE (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15, 1277–1288. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- IBM Corp. (2017). IBM Statistical Package for the Social Sciences (SPSS) Statistics for Macintosh, version 25. Armonk, NY. [Google Scholar]
- Iyer NS, Osann K, Hsieh S, Tucker JA, Monk BJ, Nelson EL, & Wenzel L (2016). Health behaviors in cervical cancer survivors and associations with quality of life. Clinical Therapeutics, 38, 467–475. 10.1016/j.clinthera.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, & Graffunder CM (2016). Current cigarette smoking among adults — United States, 2005–2015. Morbidity and Mortality Weekly Report, 65, 1205–1211. 10.15585/mmwr.mm6544a2 [DOI] [PubMed] [Google Scholar]
- Kalkhoran S, & Glantz SA (2016). E-cigarettes and smoking cessation in real-world and clinical settings: A systematic review and meta-analysis. Lancet Respiratory Medicine, 4, 116–128. 10.1016/S2213-2600(15)00521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoran S, Kruse GR, Rigotti NA, Rabin J, Ostroff JS, & Park ER (2018). Electronic cigarette use patterns and reasons for use among smokers recently diagnosed with cancer. Cancer Medicine, 7, 3484–3491. 10.1002/cam4.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane McDonnell K, Hollen PJ, Heath J, & Andrews JO (2015). Recruiting family dyads facing thoracic cancer surgery: Challenges and lessons learned from a smoking cessation intervention. European Journal of Oncology Nursing, 20, 199–206. 10.1016/j.ejon.2015.08.006 [DOI] [PubMed] [Google Scholar]
- Karam-Hage M, Cinciripini PM, & Gritz ER (2014). Tobacco use and cessation for cancer survivors: An overview for clinicians. CA: A Cancer Journal for Clinicians, 65, 109–122. 10.3322/caac.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak JA, Cornelius ME, Fong GT, & Giovino GA (2016). Differences in quit attempts and cigarette smoking abstinence between Whites and African Americans in the United States: Literature review and results from the International Tobacco Control US Survey. Nicotine and Tobacco Research, 18 Suppl 1, S79–S87. 10.1093/ntr/ntv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land SR, Toll BA, Moinpour CM, Mitchell SA, Ostroff JS, Hatsukami DK, … Warren GW (2016). Research priorities, measures, and recommendations for assessment of tobacco use in clinical cancer research. Clinical Cancer Research, 22, 1907–1913. 10.1158/1078-0432.CCR-16-0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land SR, Warren GW, Crafts JL, Hatsukami DK, Ostroff JS, Willis GB, … Toll BA (2016). Cognitive testing of tobacco use items for administration to patients with cancer and cancer survivors in clinical research. Cancer, 122(11), 1728–1734. 10.1002/cncr.29964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WHC, Chan SSC, & Lam TH (2014). Helping cancer patients to quit smoking by understanding their risk perception, behavior, and attitudes related to smoking. Psycho-Oncology, 23, 870–877. 10.1002/pon.3486 [DOI] [PubMed] [Google Scholar]
- Little MA, Klesges RC, Bursac Z, Halbert JP, Ebbert J, Talcott GW, & Weksler B (2018). Correlates of smoking status in cancer survivors. Journal of Cancer Survivorship, 12, 828–834. 10.1007/s11764-018-0720-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzorati C, Riva S, & Pravettoni G (2017). Who is a cancer survivor? A systematic review of published definitions. Journal of Cancer Education, 32, 228–237. 10.1007/s13187-016-0997-2 [DOI] [PubMed] [Google Scholar]
- Mayer DK, & Carlson J (2011). Smoking patterns in cancer survivors. Nicotine and Tobacco Research, 13, 34–40. 10.1093/ntr/ntq199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall JA, Banegas MP, Wiggins CL, Chiu V, Rajput A, & Kinney AY (2018). Rural disparities in treatment-related financial hardship and adherence to surveillance colonoscopy in diverse colorectal cancer survivors. Cancer Epidemiology Biomarkers and Prevention, 27, 1275–1282. 10.1158/1055-9965.EPI-17-1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRobbie H, Bullen C, Hartmann-Boyce J, & Hajek P (2014). Electronic cigarettes for smoking cessation and reduction. Cochrane Database of Systematic Reviews, 12. 10.1002/14651858.CD010216.pub2 [DOI] [PubMed] [Google Scholar]
- Morphett K, Partridge B, Gartner C, Carter A, & Hall W (2015). Why don’t smokers want help to quit? A qualitative study of smokers’ attitudes towards assisted vs. unassisted quitting. International Journal of Environmental Research and Public Health, 12, 6591–6607. 10.3390/ijerph120606591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A, Glymour MM, & Subramanian SV (2014). Association among socioeconomic status, health behaviors, and all-cause mortality in the United States. Epidemiology, 25, 170–177. 10.1097/EDE.0000000000000038 [DOI] [PubMed] [Google Scholar]
- Nayan S, Gupta MK, Strychowsky JE, & Sommer DD (2013). Smoking cessation interventions and cessation rates in the oncology population. Otolaryngology-Head and Neck Surgery, 149, 200–211. 10.1177/0194599813490886 [DOI] [PubMed] [Google Scholar]
- Nayan S, Gupta M, & Sommer DD (2011). Evaluating smoking cessation interventions and cessation rates in cancer patients: A systematic review and meta-analysis. ISRN Oncology, 2011, 849023. 10.5402/2011/849023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L, & Ling PM (2013). Alternative tobacco product use and smoking cessation: A national study. American Journal of Public Health, 103, 923–930. 10.2105/AJPH.2012.301070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puleo GE, Borger TN, Montgomery D, Rivera Rivera JN, & Burris JL (2020). A qualitative study of smoking-related causal attributions and risk perceptions in cervical cancer survivors. Psycho-Oncology, 29, 500–506. 10.1002/pon.5291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EM, Twarozek AM, Erwin D, Widman C, Saad-Harfouche FG, Fox CH, … Mahoney MC (2017). Perspectives on smoking cessation in Northern Appalachia. Journal of Community Health, 41, 211–219. 10.1007/s10900-015-0084-3.Perspectives [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumbrecht MP, Sun CC, Huang MS, Zandstra F, & Bodurka DC (2014). Lifestyle modification in cervical cancer survivors: An ongoing need. International Journal of Gynecological Cancer, 24, 570–575. 10.1097/IGC.0000000000000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scientific Software Development GmbH. (2017). Atlas.ti 8. [Google Scholar]
- Sheeran P, Jones K, Avishai A, Symes YR, Abraham C, Miles E, … Ribisl KM (2019). What works in smoking cessation interventions for cancer survivors? A meta-analysis. Health Psychology. 10.1037/hea0000757 [DOI] [PubMed] [Google Scholar]
- Shi Y, Cummins SE, & Zhu S-H (2017). Use of electronic cigarettes in smoke-free environments. Tobacco Control, 26(E1), e19–e22. 10.1136/tobaccocontrol-2016-053118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, & Gitchell JG (2008). Use of smoking-cessation treatments in the United States. American Journal of Preventive Medicine, 34, 102–111. 10.1016/j.amepre.2007.09.033 [DOI] [PubMed] [Google Scholar]
- Smith AL, Carter SM, Chapman S, Dunlop SM, & Freeman B (2015). Why do smokers try to quit without medication or counselling? A qualitative study with exsmokers. BMJ Open, 5, e007301. 10.1136/bmjopen-2014-007301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoboda CM, Walker DM, & Huerta TR (2019). Likelihood of smoking among cancer survivors: An updated Health Information National Trends Survey analysis. Nicotine and Tobacco Research, 21, 1636–1643. 10.1093/ntr/ntz007 [DOI] [PubMed] [Google Scholar]
- Tseng TS, Lin HY, Martin MY, Chen T, & Partridge EE (2010). Disparities in smoking and cessation status among cancer survivors and non-cancer individuals: A population-based study from National Health and Nutrition Examination Survey. Journal of Cancer Survivorship, 4, 313–321. 10.1007/s11764-010-0127-9 [DOI] [PubMed] [Google Scholar]
- Underwood JM, Townsend JS, Tai E, White A, Davis SP, & Fairley TL (2012). Persistent cigarette smoking and other tobacco use after a tobacco-related cancer diagnosis. Journal of Cancer Survivorship, 6, 333–344. 10.1007/s11764-012-0230-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. (2014). The health consequences of smoking, 50 Years of Progress: A report of the Surgeon General, 2014. Washington, DC. [Google Scholar]
- US Department of Health and Human Services. (2020). Smoking cessation: A report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- US Department of Health Education and Welfare. (1964). Smoking and health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC. [Google Scholar]
- Waggoner SE, Darcy KM, Tian C, & Lanciano R (2010). Smoking behavior in women with locally advanced cervical carcinoma: A Gynecologic Oncology Group study. American Journal of Obstetrics and Gynecology, 202, 283.e1–283.e7. 10.1016/j.ajog.2009.10.884 [DOI] [PubMed] [Google Scholar]
- Warren N, Melrose DM, Brooker JE, & Burney S (2018). Psychosocial distress in women diagnosed with gynecological cancer. Journal of Health Psychology, 23, 893–904. 10.1177/1359105316640061 [DOI] [PubMed] [Google Scholar]
- Wells M, Aitchison P, Harris F, Ozakinci G, Radley A, Bauld L, … Williams B (2017). Barriers and facilitators to smoking cessation in a cancer context: A qualitative study of patient, family and professional views. BMC Cancer, 17, 348–362. 10.1186/s12885-017-3344-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmaas JL, Alcaraz KI, Berg CJ, & Stein KD (2014). Prevalence and correlates of smoking and cessation-related behavior among survivors of ten cancers: Findings from a nationwide survey nine years after diagnosis. Cancer Epidemiology Biomarkers and Prevention, 23, 1783–1792. 10.1158/1055-9965.EPI-14-0046 [DOI] [PubMed] [Google Scholar]
- Wray N, Markovic M, & Manderson L (2007). Discourses of normality and difference: Responses to diagnosis and treatment of gynaecological cancer of Australian women. Social Science and Medicine, 64, 2260–2271. 10.1016/j.socscimed.2007.02.034 [DOI] [PubMed] [Google Scholar]


