Abstract
Trikafta, the combination of elexacaftor (VX-445), tezacaftor (VX-661) and ivacaftor (VX-770), was approved for therapy of cystic fibrosis (CF) patients with at least one allele of the CFTR mutation F508del. While the corrector function of VX-445 is well established, here we investigated the putative potentiator activity of VX-445 alone and in combination with VX-770. Acute addition of VX-445 increased the VX-770-potentiated F508del- and G551D-CFTR current by ~24% and >70%, respectively, in human bronchial and nasal epithelia. Combinatorial profiling and cluster analysis of G551D- and G1244E-CFTR channel activation to potentiator pairs indicated a distinct VX-445 mechanism of action that is, at least, additive to previously identified potentiator classes, including the VX-770. Since VX-770 only partially normalizes the G551D-CFTR channel function and adult G551D patients still experience progressive loss of lung function, VX-445+VX-770 combination therapy could provide clinical benefit to CF patients with the G551D and other dual potentiator responsive mutants.
1. Introduction
Cystic fibrosis (CF) is caused by loss-of-function mutations in the CF transmembrane conductance regulator (CFTR) gene, which lead to multiorgan pathology with lung disease being responsible for the majority of morbidity and mortality in CF [1]. More than 2000 mutations with varying disease liability have been identified that can be classified according to their cellular phenotype into expression (class I), folding (class II), gating (class III), conductance (class IV), quantity (class V), peripheral stability defect (class VI) associated mutants or combinations thereof [2].
The recent introduction of highly effective modulator therapy resulted in unprecedented clinical benefit for most CF patients. Many class III mutants, present in ~10% of CF patients on at least one allele, respond to treatment with the gating potentiator ivacaftor (VX-770), the first and so far only approved drug in this CFTR modulator class [3]. F508del-CFTR, the most common class II mutations present in ~80% of CF patients on at least one allele, and possibly other folding mutants are highly corrected by Trikafta, the combination of the correctors tezacaftor (VX-661) + elexacaftor (VX-445) and VX-770 [4–6].
Mechanistic understanding of the CFTR modulator effect can guide the combination of modulators and rationalize the implementation of modulator therapy for rare mutants [2,7]. Initial mechanistic studies revealed that VX-445 binds to and partially suppresses the unfolding of the isolated F508del nucleotide binding domain 1 (NBD1) of CFTR, consistent with a type III corrector mechanism [6]. Thus VX-445 in combination with type I or II correctors that target the NBD1-membrane spanning domains (MSDs) and NBD2 interfaces [8] synergistically restored F508del-CFTR processing and functional expression [6]. While the current report was in preparation, Laselva et al. [9] reported that the VX-445 has potentiator activity on class II CF mutations. In the current study, we focused our investigation to interrogate the utility and efficacy of VX-445 as a novel co-potentiator not only for F508del but also class III gating mutants. The additive potentiation ability of VX-445 with VX-770 of class III mutants provides a new therapeutic possibility for CF patients carrying VX-770 resistant or poorly responding CF mutations.
2. Material and Methods
Genotyping confirmed human nasal epithelia (HNE) carrying G551D-CFTR mutations were a kind gift from W. Finkbeiner (University of California, San Francisco) or were purchased from the Cystic Fibrosis Canada-Sick Kids Program in Individual CF Therapy [10]. Genotyping confirmed homozygous F508del-CFTR human bronchial epithelia (HBE) were purchased from the Cystic Fibrosis Translational Research center (CFTRc) at McGill University.
HNE were conditionally reprogrammed, expanded and differentiated on filter supports as described previously [11]. The generation and culture of CFBE41o- (CFBE) cell lines harboring the inducible expression of CFTR mutants and CFTR copy number determination by qPCR have been described [7,12]. PM density determination [12], short-circuit current (Isc) measurements [7] and the halide-sensitive YFP quenching assay [7] were performed as described previously. Isc measurements were performed in presence of 100 μM amiloride and the trans-epithelial resistance for CFBE, HBE and HNE was 1024 ± 94 (n = 6), 505 ± 46 (n = 3) and 837 ± 295 (n = 3) Ω*cm2, respectively. The fractional PM activity, an estimate of the mean channel open probability relative to that of the WT, was measured by determining mutants CFTRs forskolin-activated Isc normalized for their cell surface density as described previously [11] and is independent of the CFTR mRNA expression level.
3. Results
To assess the biochemical and functional correction of F508del-CFTR by VX-661+VX-445 in relation to that of the WT-CFTR, the corrected F508del plasma membrane (PM) density and function were determined by PM ELISA and short-circuit current (Isc) measurement, respectively. In the CFBE41o-bronchial epithelial cells, the mRNA normalized F508del-CFTR PM density and function after VX-661+VX-445 correction attained ~45% and ~90% of the WT level, respectively (figure 1a). The functional correction was monitored upon acute forskolin and VX-770 addition. The fractional PM activity, an estimate of the mean channel open probability relative to that of the WT, reached ~200% (figure 1a). To test whether the hyperactivation of the mutant fractional PM activity was due to the potentiator activity of VX-445, the forskolin activated Isc of VX-661-corrected F508del-CFTR was determined upon consecutive acute addition of VX-770 and VX-445. As expected VX-770 increased the activity of phosphorylated F508del-CFTR. The VX-770-potentiated F508del current was further increased by ~45% upon acute VX-445 addition (figure 1b). Interestingly, when administered on its own, the VX-445-mediated potentiation was significantly reduced, which may indicate that the VX-770-mediated conformational change of F508del-CFTR is permissive for the VX-445 potentiator activity and VX-445 acts as co-potentiator for VX-770 (figure 1b). In differentiated human bronchial epithelia (HBE), derived from F508del-CFTR homozygous patients, acute addition of VX-445 increased the activated and VX-770-potentiated Isc by 24%, which represents ~50% additional enhancement of the potentiator elicited Isc (figure 1c). These results are in agreement with the VX-445 activity as single potentiator for F508del and some rare folding mutants [9].
Figure 1. VX-445 (elexacaftor) potentiates the activity of F508del and gating mutants.
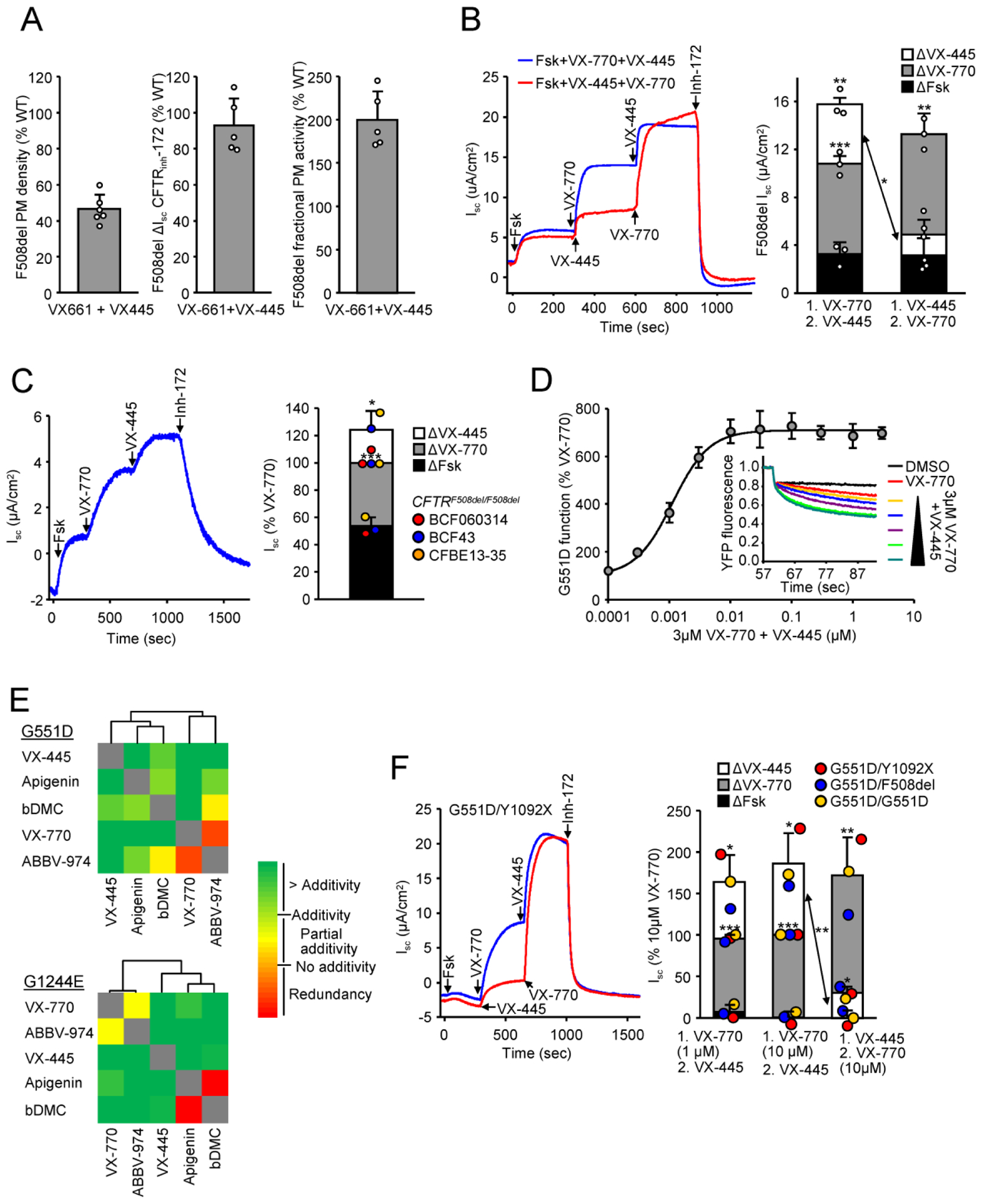
A) Fractional PM activity of VX-661+VX-445 corrected (3 μM VX-661, 2 μM VX-445, 24 hours, 37°C) F508del-CFTR calculated from PM density (left, n = 6 independent experiments) and short-circuit current (Isc, middle, n = 5 independent experiments) expressed as percentage of WT-CFTR expressing CFBE. Isc was measured after sequential addition of 20 μM forskolin and 10 μM VX-770, followed by CFTR inhibition with 20 μM CFTRinh-172. The fractional PM activity (right) was calculated as the ratio of PM density and Isc, and normalized to WT. Data are means ± SD. B) Representative traces (left) of the Isc of VX-661 corrected (3 μM, 24 hours, 37°C) F508del in CFBE activated with forskolin (Fsk, 20 μM) and potentiated with VX-770 (10 μM) followed by VX-445 (2 μM) or in reverse order. At the end of the experiment CFTR was inhibited with CFTRinh-172 (20 μM). Quantification of the Isc (right, n = 3 independent experiments) shows the contribution of Fsk, VX-770 and VX-445 to the maximal current, depending on the order of potentiator addition. Data are means ± SD, *P < 0.05, **P < 0.01, ***P<0.001 by unpaired, two-tailed Student’s t-test. C) Representative traces (left) and quantification of the Isc (right) in HBE isolated from 3 patients homozygous for F508del-CFTR which were corrected with VX-661 (3 μM, 24 hours, 37°C), expressed as percentage of VX-770 induced currents. F508del was activated with forskolin (Fsk, 20 μM) and potentiated with VX-770 (10 μM) and VX-445 (2 μM) followed by inhibition with CFTRinh-172 (20 μM). Data are means ± SD, *P < 0.05, ***P<0.001 by unpaired, two-tailed Student’s t-test. D) Representative traces (insert) and dose-response of VX-445 for the phosphorylated G551D-CFTR potentiation in presence of 3 μM VX-770, measured by halide-sensitive YFP quenching assay in CFBE cells (n = 3 independent experiments). Values are expressed as percentage of the response to 3 μM VX-770. E) Combinatorial profiling and clustering of mechanistic classes of potentiators for G551D-CFTR (top) or G1244E-CFTR (bottom) in CFBE cells. Heat map of the combinatorial profiling was established by calculating the dual potentiator effect in relation to their theoretical additivity, determined by the halide sensitive YFP quenching assay (n = 3–5 independent experiments). Combinatorial profiles were subsequently used to cluster compounds. Clustering was performed by average linkage analysis followed by Euclidean distance determination. F) Representative traces (left) and quantification (right) of the effect of VX-770 (1 μM or 10 μM) and VX-445 (2 μM) on the forskolin-stimulated Isc in HNE from three patients, homozygous or heterozygous for the G551D mutation, expressed as percentage of 10 μM VX-770 induced currents. Data are means ± SD, *P < 0.05, **P < 0.01, ***P<0.001 by unpaired, two-tailed Student’s t-test.
We next investigated whether CFTR gating mutants are responsive to VX-445. We choose G551D- and G1244E-CFTR mutants with an allelic frequency of 2,1% and 0.075% (CFTR2 project: http://cftr2.org), respectively, which display severe gating but not processing defects and can only be partially corrected by VX-770 [13,14]. In the presence of saturating VX-770 concentration, VX-445 increased the G551D-CFTR channel activity with an EC50 = 1.12 ± 0.08 nM (figure 1d), i.e. at a >100 fold lower concentration than its EC50 = 280 nM for F508del-CFTR corrector activity [6]. This observation is consistent with the possibility that VX-445 potentiator activity is mediated by a distinct binding site from that of the VX-770, identified by using cryo-EM microscopy on the WT CFTR [15]. VX-445 alone potentiated both G551D-and G1244E-CFTR and further increased the channel activity in combination with other investigational and preclinical potentiators (figure 1e).
To gain insight if VX-445 has a similar mechanism or exhibits and overlapping binding site with previously identified potentiator classes [13,14], combinatorial profiling was performed. VX-770 and ABBV-974, a potentiator under development by AbbVie, belong to the same potentiator class (figure 1e) [13]. The investigational potentiators bis-demethoxycurcumin and apigenin form a second cluster, as reported (figure 1e) [13]. VX-445 exhibited additivity or synergy with all other tested potentiators, suggesting a distinct mechanism of action (figure 1e). To confirm the VX-445 potentiator efficacy in patient-derived cells, human nasal epithelia (HNE) were isolated from three CF patients with the G551D mutation on at least one allele and differentiated as described [13]. Y1092X-CFTR, the mutant on the second allele of one patient, prevents detectable protein expression [11]. F508del, the mutant on the second allele of another patient, likely contributes < 5% to the maximal current in absence of correctors [7]. Acute VX-445 addition increased the phosphorylated and VX-770-potentiated G551D-CFTR current by ~70–85% in these HNE cells (figure 1f). Similar to F508del-CFTR, the presence of VX-770 was a permissive factor for the VX-445-mediated potentiation of G551D-CFTR (figure 1f).
4. Discussion
Several longitudinal studies report that the perpetual lung function decline is attenuated, but not prevented by VX-770 therapy in patients carrying the G551D mutation [16–18]. Since VX-770 could achieve only partial restoration of the gating activity for several gating mutants, including the G551D, we speculated that about half of the CF population with missense mutations approved for VX-770 (ivacaftor) treatment could benefit from the development of dual potentiator therapy [13]. Here we report that VX-445 acts as a co-potentiator, at least additively with VX-770, for the F508del-CFTR and two gating mutants, thus increases the functional correction efficacy. These results advocate for the rapid introduction of VX-770+VX-445 combination therapy for patients with dual potentiator-responsive gating mutants. Our data also suggests that the high F508del-CFTR functional correction efficacy of Trikafta is in part due to the potentiator action of VX-445. By inference, compounds exhibiting improved pharmaco-chaperone [7] or amplifier activity [19] could further increase the F508del channels copy number at the PM available for potentiation. Therefore, investments into the development of CFTR correctors could provide further benefit to low-responding CF patients, bearing F508del-CFTR or rare folding mutants that are modestly corrected by Trikafta administration.
Highlights:
Acute treatment withVX-445 (elexacaftor) potentiates F508del and some gating mutants.
The mechanism of VX-445 is distinct from that of other potentiators, including VX-770.
VX-445 acts as co-potentiator, additive with VX-770, for F508del- and G551D-CFTR.
Funding
This work was supported by the Canadian Institutes of Health Research (MOP-142221 to G.L.L. and PJT-153095 and PJT-173342 to G.V. and G.L.L.), National Institute of Diabetes & Digestive & Kidney Diseases (5R01DK075302 to G.L.L.), the Cystic Fibrosis Foundation Therapeutics to G.L.L., as well as Cystic Fibrosis Canada to G.L.L. G.L.L. is a Canada Research Chair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
The authors have no conflicts to disclose.
References
- 1.Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015January;16(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veit G, Avramescu RG, Chiang AN, et al. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell. 2016February1;27(3):424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011November3;365(18):1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019November23;394(10212):1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middleton PG, Mall MA, Drevinek P, et al. Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N Engl J Med. 2019November7;381(19):1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veit G, Roldan A, Hancock MA, et al. Allosteric folding correction of F508del and rare CFTR mutants by elexacaftor-tezacaftor-ivacaftor (Trikafta) combination. JCI Insight. 2020September17;5(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veit G, Xu H, Dreano E, et al. Structure-guided combination therapy to potently improve the function of mutant CFTRs. Nat Med. 2018November;24(11):1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okiyoneda T, Veit G, Dekkers JF, et al. Mechanism-based corrector combination restores ΔF508-CFTR folding and function. Nat Chem Biol. 2013July;9(7):444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laselva O, Bartlett C, Gunawardena TNA, et al. Rescue of multiple class II CFTR mutations by elexacaftor+ tezacaftor+ivacaftor mediated in part by the dual activities of Elexacaftor as both corrector and potentiator. Eur Respir J. 2020December10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckford PDW, McCormack J, Munsie L, et al. The CF Canada-Sick Kids Program in individual CF therapy: A resource for the advancement of personalized medicine in CF. J Cyst Fibros. 2019January;18(1):35–43. [DOI] [PubMed] [Google Scholar]
- 11.Avramescu RG, Kai Y, Xu H, et al. Mutation-specific downregulation of CFTR2 variants by gating potentiators. Hum Mol Genet. 2017December15;26(24):4873–4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veit G, Bossard F, Goepp J, et al. Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol Biol Cell. 2012November;23(21):4188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veit G, Da Fonte DF, Avramescu RG, et al. Mutation-specific dual potentiators maximize rescue of CFTR gating mutants. J Cyst Fibros. 2020March;19(2):236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phuan PW, Tan JA, Rivera AA, et al. Nanomolar-potency ‘co-potentiator’ therapy for cystic fibrosis caused by a defined subset of minimal function CFTR mutants. Sci Rep. 2019November27;9(1):17640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F, Zhang Z, Levit A, et al. Structural identification of a hotspot on CFTR for potentiation. Science. 2019June21;364(6446):1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirwan L, Fletcher G, Harrington M, et al. Longitudinal Trends in Real-World Outcomes after Initiation of Ivacaftor. A Cohort Study from the Cystic Fibrosis Registry of Ireland. Ann Am Thorac Soc. 2019February;16(2):209–216. [DOI] [PubMed] [Google Scholar]
- 17.Sawicki GS, McKone EF, Pasta DJ, et al. Sustained Benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med. 2015October1;192(7):836–42. [DOI] [PubMed] [Google Scholar]
- 18.Guimbellot JS, Baines A, Paynter A, et al. Long term clinical effectiveness of ivacaftor in people with the G551D CFTR mutation. J Cyst Fibros. 2020November25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dukovski D, Villella A, Bastos C, et al. Amplifiers co-translationally enhance CFTR biosynthesis via PCBP1-mediated regulation of CFTR mRNA. J Cyst Fibros. 2020February14. [DOI] [PubMed] [Google Scholar]


