Abstract
Background
The prothrombotic phenotype has been extensively described in patients with acute coronavirus disease 2019 (COVID‐19). However, potential long‐term hemostatic abnormalities are unknown.
Objective
To evaluate the changes in routine hemostasis laboratory parameters and tissue‐type plasminogen activator (tPA) rotational thromboelastometry (ROTEM) 6 months after COVID‐19 intensive care unit (ICU) discharge in patients with and without venous thromboembolism (VTE) during admission.
Methods
Patients with COVID‐19 of the Maastricht Intensive Care COVID cohort with tPA ROTEM measurement at ICU and 6‐month follow‐up were included. TPA ROTEM is a whole blood viscoelastic assay that illustrates both clot development and fibrinolysis due to simultaneous addition of tissue factor and tPA. Analyzed ROTEM parameters include clotting time, maximum clot firmness (MCF), lysis onset time (LOT), and lysis time (LT).
Results
Twenty‐two patients with COVID‐19 were included and showed extensive hemostatic abnormalities before ICU discharge. TPA ROTEM MCF (75 mm [interquartile range, 68‐78]‐59 mm [49‐63]; P ≤ .001), LOT (3690 seconds [2963‐4418]‐1786 seconds [1465‐2650]; P ≤ .001), and LT (7200 seconds [6144‐7200]‐3138 seconds [2591‐4389]; P ≤ .001) normalized 6 months after ICU discharge. Of note, eight and four patients still had elevated fibrinogen and D‐dimer concentrations at follow‐up, respectively. In general, no difference in median hemostasis parameters at 6‐month follow‐up was observed between patients with (n=14) and without (n=8) VTE, although fibrinogen appeared to be lower in the VTE group (VTE–, 4.3 g/L [3.7‐4.7] vs VTE+, 3.4 g/L [3.2‐4.2]; P = .05).
Conclusions
Six months after COVID‐19 ICU discharge, no persisting hypercoagulable or hypofibrinolytic profile was detected by tPA ROTEM. Nevertheless, increased D‐dimer and fibrinogen concentrations persist up to 6 months in some patients, warranting further exploration of the role of hemostasis in long‐term morbidity after hospital discharge.
Keywords: COVID‐19, fibrinolysis, follow‐up studies, hemostasis, thromboelastography
Essentials.
Persistence of hemostatic abnormalities remains unclear in patients with coronavirus disease 2019.
Changes in hemostasis and fibrinolysis were analyzed at discharge and 6‐month follow‐up.
Viscoelastic parameters of clot formation normalized over the 6‐month follow‐up.
Nine (41%) patients still had elevated D‐dimer and/or fibrinogen at follow‐up.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19)–associated prothrombotic phenotype is characterized by elevated von Willebrand factor, D‐dimer, fibrinogen, and changes in global assays such as rotational thromboelastometry (ROTEM) and thromboelastography.1, 2, 3, 4, 5 ROTEM is a whole blood viscoelastic assay that monitors the dynamic properties of clot formation. The addition of tissue‐type plasminogen activator (tPA) to standard ROTEM reagents creates a novel assay (tPA ROTEM) able to both identify clot buildup and breakdown under fibrinolytic stimulus.6 The tPA ROTEM assay might better reflect the coagulopathy in vivo compared to routine hemostasis laboratory assays. Indeed, previous studies in patients with COVID‐19 demonstrated persisting hypercoagulability and impaired fibrinolysis during intensive care unit (ICU) admission, as measured with (tPA) ROTEM.4, 5
Abnormal hemostatic viscoelastic parameters often persist upon ICU discharge,4 and abnormalities in D‐dimer concentrations have been observed several months after COVID‐19 infection.7 The presence of a so‐called chronic COVID‐19 syndrome may potentially be driven by microvascular thrombosis, limiting recovery.8, 9 Although the prothrombotic phenotype during hospital admission has been extensively described, potential long‐term (micro)thrombotic morbidity following ICU discharge is currently unknown. Both routine hemostasis assays and the point‐of‐care tPA ROTEM quickly evaluate overall hemostasis and fibrinolysis, suitable for clinical decision making during a follow‐up appointment. We aimed to evaluate the changes over 6 months in routine hemostasis laboratory parameters and tPA ROTEM after COVID‐19 ICU discharge.
2. MATERIAL AND METHODS
2.1. Cohort description
During the first European pandemic wave between March and May 2020, the Maastricht Intensive Care COVID (MaastrICCht) cohort included 94 patients.8, 10, 11 Of the 59 patients who survived COVID‐19 ICU admission, 42 patients (71.2%) were available for the cardiology outpatient follow‐up 6 months after ICU discharge. The missing patients died after ICU discharge (n = 7), attended follow‐up elsewhere (n = 7), declined to participate (n = 2), or emigrated to another country (n = 1). Of the 42 patients seen at 6‐month follow‐up, 22 (52%) had both a tPA ROTEM measurement during their ICU admission and at follow‐up and could be included in the current analysis. To address possible selection bias, patient characteristics and routine hemostatis laboratory parameters at ICU admission (prothrombin time [PT], activated partial thromboplastin time [aPTT], fibrinogen, and D‐dimer) were compared between our population (n=22) and the excluded patients with 6‐month follow‐up due to missing tPA ROTEM (n=20) and also all excluded ICU survivors (n = 37). The local institutional review board (Medische Ethische Toetsingscomissie) of the Maastricht University Medical Centre+ approved the study (2020‐1565/300523 and 2020‐2287); all patients or representatives gave consent.
2.2. Routine hemostasis laboratory panel
PT (Dade Innovin; Siemens, Marburg, Germany), aPTT (Dade Actin FSL; Siemens), fibrinogen concentration (Clauss method, Dade Thrombin Reagent; Siemens) and D‐dimer (Innovance; Siemens) were all measured on a Sysmex CS2500i (Sysmex Corporation, Kobe, Japan) in 3.2% citrated blood. D‐dimer cutoff was age dependent in patients aged >50 years and determined by the following formula: D‐dimer cutoff = 500 + (Age − 50)*10.12, 13
2.3. tPA ROTEM
The tPA ROTEM is a whole blood viscoelastic assay, where changes in viscoelastic properties during clot formation and fibrinolysis are measured. The tPA ROTEM assay was previously described and validated by Kuiper et al.6 In short, simultaneous addition of tissue factor (35 pM) and tPA (125 ng/mL) to the test cup results in coagulation succeeded by fibrinolysis of the clot. The maximum duration of the assay is 2 hours. The following tPA ROTEM parameters were analyzed: clotting time (CT; in seconds), maximum clot firmness (MCF; in mm), lysis onset time (LOT; time in seconds from CT until a 15% drop in MCF), and lysis time (LT; time in seconds from CT until a 90% drop in MCF). Prolonged LOT and LT suggest a hypofibrinolytic phenotype, while shortened LOT and LT illustrate hyperfibrinolysis. If 15% and/or 90% clot breakdown were not reached, LOT and LT were capped at 2 hours (7200 seconds).
2.4. Clinical characteristics
During ICU admission, patients had been screened for venous thromboembolism (VTE) upon clinical suspicion.14 Computed tomography pulmonary angiogram (CTPA) was performed to diagnose pulmonary embolism (PE) and deep vein thrombosis (DVT) was confirmed by compression ultrasonography. Patients with confirmed VTE during hospital admission were treated with anticoagulants for at least 3 months. After 3 months, specialist clinicians reviewed and (dis)continued the anticoagulant treatment based on laboratory and clinical characteristics. Patients without VTE did not receive standard thromboprophylaxis after hospital discharge, unless antithrombotic treatment was indicated by comorbidities (eg, atrial fibrillation).
2.5. Statistical analysis
Data analysis was performed using SPSS for Windows, version 25.0 (IBM, Armonk, NY, USA). Continuous data are presented as median (interquartile range [IQR]) and categorical data as counts (relative percentage). Routine hemostasis laboratory and tPA ROTEM measurements between time points were assessed by the dependent Wilcoxon signed‐rank test (continuous data) and McNemar test (categorical data). Subgroup comparison was performed by independent Mann‐Whitney U test and chi‐square test, respectively. P < .05 was considered statistically significant.
3. RESULTS AND DISCUSSION
In total, 22 patients with COVID‐19 had a tPA ROTEM measurement during ICU admission and at 6‐month follow‐up. A total of 17 patients were men (77.3%), the median [IQR] age was 61 [57‐69] years, and most were overweight (Table 1). The median length of ICU stay was 34 [22‐42] days, and the 6‐month follow‐up was performed 188 [171‐192] days after ICU discharge. We observed no significant differences between the 22 patients studied versus the other survivors in the MaastrICCht cohort, except for a longer length of stay in our analyzed ICU population.
TABLE 1.
Patient characteristics
| Follow‐up cohort (n = 22) | |
|---|---|
| Age, y | 61.0 (56.5‐68.8) |
| Sex, male | 17 (77.3) |
| BMI, kg/m2 | 27.8 (26.2‐30.4) |
| Origin of admission | |
| Emergency department | 7 (31.8) |
| Hospital ward | 11 (50.0) |
| Transfer from other ICU | 4 (18.2) |
| Length of stay ICU, d | 34 (21‐42) |
| ECMO during admission | 3 (13.6) |
| RRT during admission | 2 (9.1) |
| Antithrombotic treatment before admission | |
| Antiplatelet agents | 3 (14) |
| Coumarin | 0 (0.0) |
| DOAC | 1 (4.5) |
| Disease severity score at ICU admission | |
| APACHE II | 15.5 (13.0‐18.0) |
| SAPS II | 37.5 (29.0‐43.0) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BMI, body mass index; DOAC, direct oral anticoagulant; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; RRT, renal replacement therapy; SAPS, Simplified Acute Physiology Score.
Data are presented as median (interquartile range) for continuous data and n (rel. %) for categorical data.
Routine hemostasis laboratory and tPA ROTEM measurements (last available tPA ROTEM measurement on ICU) are displayed in Table 2, confirming the presence of extensive hemostatic abnormalities during ICU admission. Both fibrinogen and D‐dimer concentrations were increased upon ICU discharge. TPA ROTEM parameters showed hypercoagulability (increased MCF) and hypofibrinolysis (prolonged LOT and LT). Overall, 6‐month follow‐up results for fibrinogen; D‐dimer; and tPA ROTEM MCF, LOT, and LT showed a significant reduction toward normal values (Table 2; Figure 1; P ≤ .001). Still, eight patients had elevated fibrinogen concentrations (38%), while four patients (18%) had elevated D‐dimer concentrations. Patients presented with isolated elevated fibrinogen (n = 5), D‐dimer (n = 1), or a combination of both (n = 3) at 6‐month follow‐up.
TABLE 2.
Routine hemostasis laboratory and tPA ROTEM measurement in 22 patients before ICU discharge and at 6‐month follow‐up
| Reference range | ICUa | 6‐month follow‐up | P value | |
|---|---|---|---|---|
| Days after ICU admission | 25 (18 to 40) | 213 (206 to 230) | ||
| Days after ICU discharge | −1 (−4 to 0) | 188 (171 to 192) | ||
| Laboratory parameters | ||||
| PT, s | 9.9–12.4 | 11.5 (11.0 to 11.8) | 10.9 (10.4 to 11.2)c | .01 |
| aPTT, s | 23–32 | 28 (27 to 33) | 29 (27 to 31)c | 0.37 |
| Fibrinogen, g/L | 1.7–4.0 | 6.0 (4.5 to 6.7)b | 3.7 (3.3 to 4.5)c | ≤0.001 |
| Fibrinogen above reference range | 17 (85.0) | 8 (38.1) | .02 | |
| D‐dimer, ng/mL | Age‐dependentd | 2270 (1538 to 4337) | 321 (218 to 559)c | ≤.001 |
| D‐dimer above reference range | 22 (100.0) | 4 (18.2) | ≤.001 | |
| tPA ROTEM | ||||
| Clotting time, s | 33–75 | 77 (68 to 89) | 80 (67 to 97) | .35 |
| Maximum clot firmness, mm | 47–68 | 75 (68 to 78) | 59 (49 to 63) | ≤.001 |
| Lysis onset time, s | 1560–2940 | 3690 (2963 to 4418) | 1786 (1465 to 2650) | ≤.001 |
| Lysis time, s | 2100–4620 | 7200 (6144 to 7200) | 3138 (2591 to 4389) | ≤.001 |
| Antithrombotic medication | ||||
| Heparin | 22 (100) | 0 (0.0) | ||
|
18 (82) | |||
|
2 (9.1) | |||
|
1 (4.5) | |||
|
1 (4.5) | |||
| Coumarin | 0 (0.0) | 1 (4.5) | ||
| DOAC | 0 (0.0) | 6 (27) | ||
|
5 (83) | |||
|
1 (17) | |||
| Antiplatelet agents | 3 (14) | 3 (14) | ||
Abbreviations: aPTT, activated partial thromboplastin time; DOAC, direct oral anticoagulation; ICU, intensive care unit; PT, prothrombin time; ROTEM, rotational thromboelastometry; tPA, tissue‐type plasminogen activator; UFH, unfractionated heparin.
Data are presented as median (interquartile range) for continuous data and n (rel. %) for categorical data.
aICU measurement: last tPA ROTEM measurement on ICU.
bn = 2 missing.
cn = 1 missing.
dD‐dimer cutoff was age dependent in patients aged >50 years and determined by the following formula: D‐dimer cutoff =500 + (Age‐50)*10.
FIGURE 1.
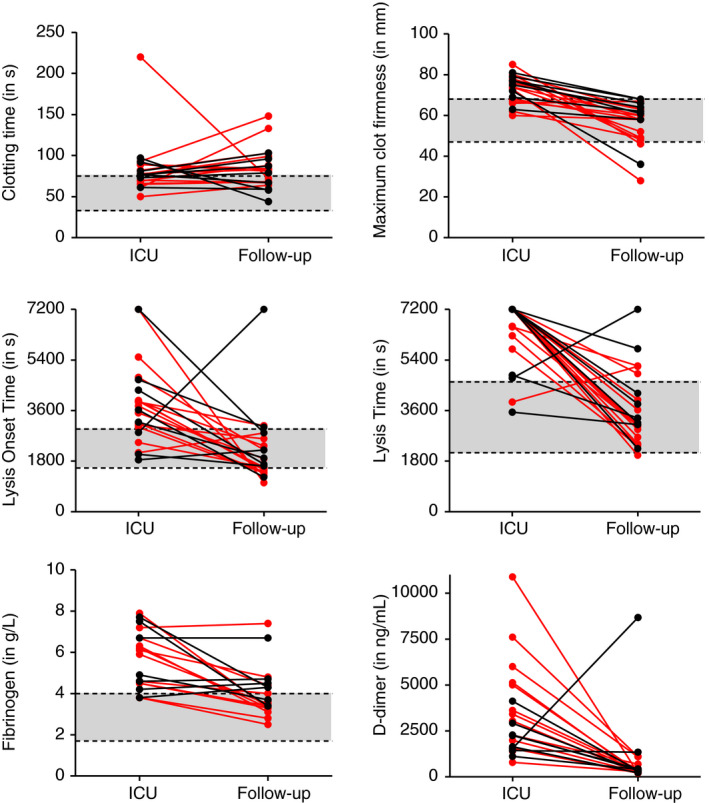
tPA ROTEM, fibrinogen and D‐dimer measurements at ICU admission and 6‐month follow‐up, stratified for VTE ( VTE+, ● VTE−). Grey areas illustrate reference ranges; tPA ROTEM reference ranges were previously determined by Kuiper et al.6 ICU, intensive care unit; tPA ROTEM, tissue‐type plasminogen activator; VTE, venous thromboembolism
VTE+, ● VTE−). Grey areas illustrate reference ranges; tPA ROTEM reference ranges were previously determined by Kuiper et al.6 ICU, intensive care unit; tPA ROTEM, tissue‐type plasminogen activator; VTE, venous thromboembolism
Fourteen patients (64%) developed VTE during ICU stay: 13 patients had CTPA‐confirmed PE, and one patient had compression ultrasonography–confirmed DVT. In five patients with VTE, anticoagulants were continued for up to 6 months. Two patients without VTE received anticoagulants for atrial fibrillation, which was diagnosed before or developed during ICU admission. Laboratory parameters at 6‐month follow‐up normalized irrespective of VTE during ICU stay (Table 3, Figure 1). Although not statistically significantly different between patients with and without VTE (3.4 [3.2‐4.2] vs 4.3 [3.7‐4.7]; P = .05), fibrinogen concentration at 6‐month follow‐up was higher in the non‐VTE group. This observation is consistent with the previous experience within the MaastrICCht cohort that low, rather than high, fibrinogen was associated with VTE development in ICU patients.15
TABLE 3.
Routine hemostasis laboratory and tPA ROTEM measurement at six‐month follow‐up, by VTE status
| Reference Range |
6‐month follow‐up n=22 |
VTE+ n=14 |
VTE− n=8 |
P value | |
|---|---|---|---|---|---|
| Days after ICU admission | 213 (206‐230) | 211 (209‐228) | 221 (199‐236) | ||
| Days after ICU discharge | 188 (171‐192) | 188 (170‐190) | 190 (181‐199) | ||
| Laboratory | |||||
| PT, s | 9.9‐12.4 | 10.9 (10.4‐11.2)a | 10.9 (10.4‐11.2)a | 10.8 (10.0‐11.3) | .80 |
| aPTT, s | 23‐32 | 29 (27‐31)a | 28 (26‐30)a | 30 (27‐38) | .16 |
| Fibrinogen, g/L | 1.7‐4.0 | 3.7 (3.3‐4.5)a | 3.4 (3.2‐4.2)a | 4.3 (3.7‐4.7) | .05 |
| Fibrinogen above reference range | 8 (38.1) | 3 (23.1) | 5 (62.5) | .071 | |
| D‐dimer, ng/mL | Age‐dependentb | 321 (218‐559)a | 287 (211‐559)a | 390 (323‐1113) | .10 |
| D‐dimer above reference range | 5 (23.8) | 2 (14.3) | 2 (25.0) | .60 | |
| tPA ROTEM | |||||
| Clotting time, s | 33‐75 | 80 (67‐97) | 82 (68‐100) | 73 (58‐94) | .24 |
| Maximum clot firmness, mm | 47‐68 | 59 (49‐63) | 58 (49‐61) | 63 (59‐68) | .05 |
| Lysis onset time, s | 1560‐2940 | 1786 (1465‐2650) | 1577 (1410‐2408) | 2054 (1640‐2959) | .24 |
| Lysis time, s | 2100‐4620 | 3138 (2591‐4389) | 3050 (2409‐4217) | 3584 (3114‐5388) | .19 |
| Medication | |||||
| Heparin | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Coumarin | 1 (4.5) | 0 (0.0) | 1 (12.5) | ||
| DOAC | 6 (27.3) | 5 (35.7) | 1 (12.5) | ||
|
5 (83.3) | 5 (100.0) | 0 (0.0) | ||
|
1 (16.7) | 0 (0.0) | 1 (100.0) | ||
| Antiplatelet agents | 3 (13.6) | 1 (7.1) | 2 (25.0) | ||
Abbreviations: aPTT, activated partial thromboplastin time; DOAC, direct oral anticoagulation; ICU, intensive care unit; PT, prothrombin time; ROTEM, rotational thromboelastometry; tPA, tissue plasminogen activator; VTE, venous thromboembolism.
Data are presented as median (interquartile range) for continuous data and n (rel. %) for categorical data.
an = 1 missing.
bD‐dimer cutoff was age dependent in patients aged >50 years and determined by the following formula: D‐dimer cutoff = 500 + (Age − 50)*10.
Most tPA ROTEM parameters were within normal values at 6‐month follow‐up. However, median CT was prolonged both before ICU discharge (77 seconds [68‐89]) and at 6‐month follow‐up (80 seconds [67‐97]), which may reflect the effect of anticoagulant treatment. Patients received standard low‐molecular‐weight heparin (LMWH) during ICU admission in line with national protocols. Indications for unfractioned heparin (UFH) administration were extracorporeal membrane oxygenation and renal replacement therapy. At 6‐month follow‐up, 32% of patients received anticoagulants, predominantly the direct oral anticoagulant (DOAC) apixaban (Table 2). In vitro spiking studies demonstrated that LMWH, UFH, and DOACs prolong ROTEM CT.16, 17 Unpublished data of patients using rivaroxaban and dabigatran show a concentration‐dependent effect of these DOACs on tPA ROTEM CT, whereas tPA MCF, LOT, and LT were unaffected by DOAC concentration (data not shown). Similarly, heparins had no clear effect on tPA ROTEM parameters besides CT prolongation.16 Taken together, the observed prolongation of CT during ICU admission and at 6‐month follow‐up can likely be attributed to the use of, respectively, heparins and DOACs. Indeed, patients treated with apixaban (n = 5) had significantly prolonged CT compared to patients not taking DOACs (n = 16; 99 seconds [78‐141] vs 70 seconds [65‐84] respectively; P = .03).
We evaluated the changes over 6 months in routine hemostasis laboratory parameters and tPA ROTEM after COVID‐19 ICU discharge and observed that, with the exception of tPA CT, values generally normalized over time. However, nine (41%) patients still presented with abnormal D‐dimer or fibrinogen at 6‐month follow‐up. The long‐lasting physical and psychological sequelae observed in patients with COVID‐19 after hospital discharge are referred to as “long COVID syndrome.”18 Long‐term health consequences of COVID‐19 include fatigue, muscle weakness, reduced respiratory function, anxiety, and depression.19, 20, 21 The underlying pathophysiology, involved organ systems, and optimal follow‐up care remain largely unknown. Hospitalized patients with COVID‐19 are at increased risk for thrombotic complications; whether a prothrombotic tendency persists beyond discharge, putting recovering patients with COVID‐19 at continued risk for (micro)thrombi or VTE development remains uncertain. Several reports evaluated the thrombotic risk after COVID‐19 hospital discharge and found that the occurrence of VTE was low and similar to other acute illnesses.22, 23, 24, 25, 26
Two other studies assessed hemostasis laboratory assays after COVID‐19 hospital discharge. Von Meijenfeldt et al9 evaluated coagulation, fibrinolysis, and thrombin‐generation markers in 52 patients with COVID‐19 at admission and 4 months after hospital discharge. Mostly ward patients were included, in contrast to our population with only mechanically ventilated ICU patients. Patients with COVID‐19 had a sustained prothrombotic phenotype as evidenced by enhanced thrombin generation and decreased plasma fibrinolysis when compared to healthy volunteers.9 Though laboratory parameters remained elevated in recovering patients with COVID‐19 when compared to healthy controls, the prothrombotic phenotype was diminished at 4 months after discharge compared to hospital admission. Our results of elevated D‐dimer and fibrinogen concentrations at follow‐up are in line with the described prothrombotic phenotype 4 months after discharge. However, our tPA ROTEM does not illustrate a similar hypercoagulable and hypofibrinolytic phenotype, as MCF, LOT, and LT had generally reached normal values. A hypofibrinolytic phenotype at follow‐up as detected by Meijenfeldt et al, which we did not observe from our results, might suggest further normalization of fibrinolysis after 4 months. Alternatively, the discrepancy could arise from the different assays: Meijenfeldt et al evaluated fibrinolysis in platelet‐poor plasma, whereas we assessed whole blood tPA ROTEM. Townsend et al7 recently demonstrated in a cohort of 150 recovering patients with COVID‐19, both hospitalized and outpatients, that 25.3% had elevated D‐dimer concentrations 4 months after COVID‐19 infection. In the ICU subgroup (n = 16), six and five patients had elevated D‐dimer and fibrinogen concentrations at follow‐up, respectively. Both the D‐dimer and fibrinogen cutoffs differed, limiting comparison with our observations. Of note, our and other COVID‐19 follow‐up research mainly provides preliminary information concerning abnormalities in the hemostatic system. Since no baseline sample, before COVID‐19 infection, is available, we cannot rule out that D‐dimer and fibrinogen abnormalities that we associate with convalescent COVID‐19 reflect a previously existing chronic low‐grade inflammation that puts patients at higher risk for severe COVID‐19 disease.
Our study was embedded in a well‐defined cohort with systematic follow‐up protocols. Limitations to our study are the relatively small number of included patients and the single‐center approach limiting generalizability, single postdischarge measurement, and lack of a non–COVID‐19 control group. Additionally, patients with a short length of stay who were already discharged from the ICU before the start of ROTEM measurements could not be included in the current analysis.4 Nevertheless, the chance of selection bias is regarded as low, although not all survivors were included, as we observed no significant differences between the 22 patients studied versus the other patients at 6‐month follow‐up (n = 20) or all other patients discharged from ICU (n = 37), except for a longer length of stay in our ICU population (34 days [22‐42] vs 12 days [6‐21] and 13 days [8‐22], respectively; P < .001). At the least, our observations represent the most severe spectrum of COVID‐19 disease. Thereby, the occurrence of hemostatic disturbances, elevated D‐dimer and fibrinogen concentrations, might be overestimated compared to the general COVID‐19 ICU population. Nevertheless, our observations warrant further exploration to identify the pathophysiology provoking long‐term elevated D‐dimer and fibrinogen concentrations. Of interest are patient characteristics and confounders other than VTE possibly predisposing abnormal hemostatic markers at follow‐up that we could not identify, such as diabetes, chronic kidney disease, and smoking. Additionally, other fibrinolytic markers may provide a more elaborate overview of hemostasis in addition to the tPA ROTEM. Overall, studies assessing long‐term hemostatic abnormalities in patients with comparable pathologies are scarce. Yende et al27 evaluated hemostasis biomarkers, among others, in a septic population up to 1 year after admission and demonstrated increased D‐dimer levels in 31% of patients at 6‐month follow‐up. These results are somewhat comparable to our observations and may suggest a more universal pathophysiology underlying the elevations in D‐dimer and fibrinogen not specific to COVID‐19. Future follow‐up studies including both critically ill patients with COVID‐19 and similar pathologies alike (severe viral or bacterial infections) may confirm this hypothesis.
We systematically evaluated routine hemostasis laboratory parameters and tPA ROTEM during ICU admission and 6 months after ICU discharge in critically ill patients with COVID‐19. There was significant reduction toward normal values in almost all tPA ROTEM parameters when compared to ICU discharge and no clear hypercoagulable or hypofibrinolytic phenotype was detected at 6‐month follow‐up. However, our results may suggest long‐term involvement of hemostasis in some patients, evidenced by persistingly high D‐dimer and fibrinogen concentrations in a fifth and third of all patients, respectively. Further elucidation of the biological mechanisms associated with and clinical relevance of persisting D‐dimer and fibrinogen elevation is paramount for our understanding and adequate COVID‐19 follow‐up care.
4. RELATIONSHIP DISCLOSURES
CG‐D reports research support from Bayer, Abbot, Amgen, and Sanofi. HtC has received grants from Bayer and Pfizer, is a consultant for Alveron, and a shareholder of Coagulation Profile. RO has received research support and honoraria from Bayer, Pfizer/BMS, Leo Pharma, Portola, and Sanofi. YH reports that ROTEM reagents were previously provided for research and development. None of this was related to the submitted work. All other authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
AH, DCWB, RHO, HtC and YMCH contributed to the concept and design of the study. AH was the main author of the manuscript, and DCW B performed statistical analysis. BCTvB and ICCvdH developed the design of the MaastrICCht cohort. CG‐D and SvS coordinated the COVID‐19 follow‐up visits. SvS, JEM Sels, GJAJMK, ICCvdH, HtC, BCTvB, RHO, and YMCH critically reviewed the intellectual content of the manuscript. The manuscript was read and approved for submission to Research and Practice in Thrombosis and Haemostasis by all authors.
ACKNOWLEDGMENTS
We would like to thank all patients for participating in this research. We would also like to extend our gratitude to all the nurses, technicians, doctors, and medical students for their contribution to the MaastrICCht cohort.
Hulshof AM, Braeken DCW, Ghossein‐Doha C, et al. Hemostasis and fibrinolysis in COVID‐19 survivors 6 months after intensive care unit discharge. Res Pract Thromb Haemost. 2021;5:e12579. 10.1002/rth2.12579
Funding information
The author(s) received no funding or financial support for the current manuscript.
Contributor Information
Anne‐Marije Hulshof, Email: annemarije.hulshof@mumc.nl.
Chahinda Ghossein‐Doha, @ChahindaGhoss.
Iwan C. C. van der Horst, @iccvanderhorst.
Bas C. T. van Bussel, @BusselBas.
REFERENCES
- 1.Masi P, Hekimian G, Lejeune M, et al. Systemic inflammatory response syndrome is a major contributor to COVID‐19‐associated coagulopathy: insights from a prospective, single‐center cohort study. Circulation. 2020;142:611‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao D, Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID‐19: a retrospective cohort study. Lancet Haematol. 2020;7:e671‐e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright FL, Vogler TO, Moore EE, et al. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID‐19 infection. J Am Coll Surg. 2020;231:193‐203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hulshof AM, Bruggemann RAG, Mulder MMG, et al. Persisting Hypercoagulability and Hypofibrinolysis; Serial EXTEM, FIBTEM, and tPA Rotational Thromboelastometry Observations in the Maastricht Intensive Care COVID Cohort Frontiers in Cardiovascular Medicine. 2021. Accepted for publication. 10.3389/fcvm.2021.654174 [DOI] [PMC free article] [PubMed]
- 5.Nougier C, Benoit R, Simon M, et al. Hypofibrinolytic state and high thrombin generation may play a major role in SARS‐COV2 associated thrombosis. J Thromb Haemost. 2020;18:2215‐2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Poucke S, Stevens K, Wetzels R, et al. Early platelet recovery following cardiac surgery with cardiopulmonary bypass. Platelets. 2016;27:751‐757. [DOI] [PubMed] [Google Scholar]
- 7.Townsend L, Fogarty H, Dyer A, et al. Prolonged elevation of D‐dimer levels in convalescent COVID‐19 patients is independent of the acute phase response. J Thromb Haemost. 2021;19(4):1064‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Gassel RJJ, Bels JLM, Raafs A, et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID‐19. Am J Respir Crit Care Med. 2021;203:371‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Meijenfeldt FA, Havervall S, Adelmeijer J, et al. Sustained prothrombotic changes in COVID‐19 patients 4 months after hospital discharge. Blood Adv. 2021;5:756‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tas J, van Gassel RJJ, Heines SJH, et al. Serial measurements in COVID‐19‐induced acute respiratory disease to unravel heterogeneity of the disease course: design of the Maastricht Intensive Care COVID cohort; MaastrICCht. medRxiv. 2020: 2020.04.27.20080309. 10.1101/2020.04.27.20080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bels JLM, van Kuijk SMJ, Ghossein‐Doha C, et al. Decreased serial scores of severe organ failure assessments are associated with survival in mechanically ventilated patients; the prospective Maastricht Intensive Care COVID cohort. J Crit Care. 2020;62:38‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douma RA, le Gal G, Sohne M, et al. Potential of an age adjusted D‐dimer cut‐off value to improve the exclusion of pulmonary embolism in older patients: a retrospective analysis of three large cohorts. BMJ. 2010;340(mar30 3):c1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age‐adjusted D‐dimer cut‐off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167‐171. [DOI] [PubMed] [Google Scholar]
- 14.Bruggemann RAG, Spaetgens B, Gietema HA, et al. The prevalence of pulmonary embolism in patients with COVID‐19 and respiratory decline: a three‐setting comparison. Thromb Res. 2020;196:486‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulder MMG, Brandts L, Bruggemann RAG, et al. Serial markers of coagulation and inflammation and the occurrence of clinical pulmonary thromboembolism in mechanically ventilated patients with SARS‐CoV‐2 infection; the prospective Maastricht intensive care COVID cohort. Thromb J. 2021;19:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Berg TW, Hulshof AM, Nagy M, et al. Suggestions for global coagulation assays for the assessment of COVID‐19 associated hypercoagulability. Thromb Res [in press]. 2020;201:84‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seyve L, Richarme C, Polack B, Marlu R. Impact of four direct oral anticoagulants on rotational thromboelastometry (ROTEM). Int J Lab Hematol. 2018;40:84‐93. [DOI] [PubMed] [Google Scholar]
- 18.Baig AM. Chronic COVID syndrome: need for an appropriate medical terminology for long‐COVID and COVID long‐haulers. J Med Virol. 2020;93(5):2555‐2556. [DOI] [PubMed] [Google Scholar]
- 19.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: a cross‐sectional evaluation. J Med Virol. 2021;93:1013‐1022. [DOI] [PubMed] [Google Scholar]
- 20.Carfi A, Bernabei R, Landi F. Gemelli against C‐P‐ACSG: persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324:603‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts LN, Whyte MB, Georgiou L, et al. Postdischarge venous thromboembolism following hospital admission with COVID‐19. Blood. 2020;136:1347‐1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patell R, Bogue T, Koshy A, et al. Postdischarge thrombosis and hemorrhage in patients with COVID‐19. Blood. 2020;136:1342‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whyte MB, Barker R, Kelly PA, et al. Three‐month follow‐up of pulmonary embolism in patients with COVID‐19. Thromb Res. 2021;201:113‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelen MM, Vandenbriele C, Balthazar T, et al. Venous thromboembolism in patients discharged after COVID‐19 hospitalization. Semin Thromb Hemost. 2021;47:362‐371. [DOI] [PubMed] [Google Scholar]
- 26.Bourguignon A, Beaulieu C, Belkaid W, Desilets A, Blais N. Incidence of thrombotic outcomes for patients hospitalized and discharged after COVID‐19 infection. Thromb Res. 2020;196:491‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yende S, Kellum JA, Talisa VB, et al. Long‐term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open. 2019;2:e198686. [DOI] [PMC free article] [PubMed] [Google Scholar]


