Corresponding Author

Key Words: anthracycline, cardiomyopathy, heart failure
Since the introduction of anthracyclines in the 1960s, the risk of anthracycline-induced cardiotoxicity (AIC) has limited its use in both adult and pediatric cancers (1). Approximately 23% of long-term survivors of pediatric cancers have an abnormal left ventricular ejection fraction (LVEF) 4 to 20 years post-anthracycline exposure (2), suggesting that myocardial damage is long-lasting and persistent. Moreover, in a study of adult cancer survivors, despite the implementation of LVEF surveillance for early detection, the overall incidence of AIC was still 9%, among whom >70% did not fully recover left ventricular function (3). Further, adjunctive therapy administered with anthracyclines, such as radiotherapy and HER2-targeted therapy, can further increase the risk of AIC. Conventional heart failure therapies (ie, neurohormonal blockade), have failed to demonstrate a clinically tangible benefit (4), underscoring the urgent need for novel preventive and therapeutic measures against AIC.
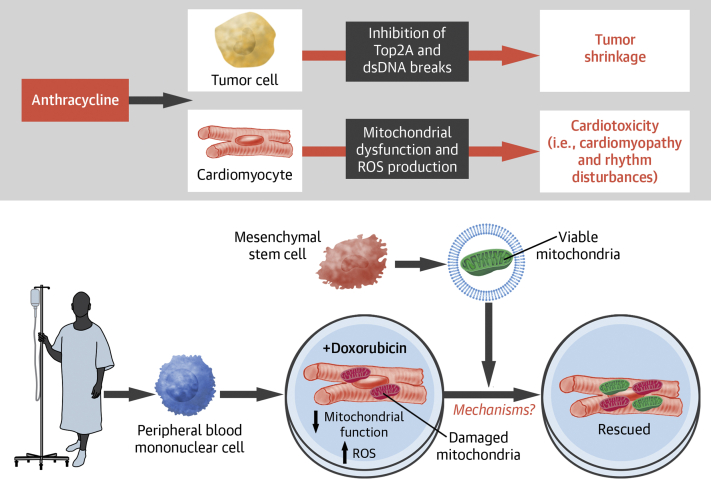
Anthracyclines exert their antitumor activity via inhibition of topoisomerase IIα, which induces double-stranded DNA breaks, halting DNA replication, transcription, and proliferation of cancer cells (5). In cardiomyocytes, which are terminally differentiated and do not replicate, anthracyclines appear to cause cell death via mitochondrial damage and inhibition of mitochondrial biogenesis (1). Moreover, doxorubicin preferentially accumulates in the mitochondria through direct binding to cardiolipin, a phospholipid abundant in the mitochondrial inner membrane, resulting in mitochondrial free-radical generation and disruption of mitophagy and mitochondrial fission and fusion (Figure 1) (5).
Figure 1.
AIC and an In Vitro Model of Mitochondrial Transfer as a Therapy for AIC
(Top) Anthracyclines inhibit tumor cell proliferation via inhibition of topoisomerase 2A (Top2a), which induces double-stranded DNA (dsDNA) breaks and halts cell division. In comparison, anthracyclines cause cardiotoxicity by inducing myocardial mitochondrial dysfunction and reactive oxygen species (ROS) production. (Bottom) Cardiomyocytes generated from patient peripheral blood mononuclear cell (PBMC)-derived induced pluripotent stem cells are injured by doxorubicin treatment, resulting in defective mitochondrial function and elevated ROS production. The transfer of vesicle-bound viable mitochondria derived from mesenchymal stem cells (MSCs) restores the mitochondrial functions of the injured cardiomyocytes. AIC = anthracycline-induced cardiotoxicity; iPSC = induced pluripotent stem cell.
In this issue of JACC: CardioOncology, O’Brien et al (6) explored the potential of stem cell therapy in AIC. The study recruited 3 patients from the SENECA trial (Phase I, First-in-Human, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Study of the Safety and Efficacy of Allogeneic Mesenchymal Stem Cells in Cancer Survivors with Anthracycline-Induced Cardiomyopathy) and generated induced pluripotent stem cells (iPSC) from their peripheral blood mononuclear cells. By exposing patient iPSC-derived cardiomyocytes (iCMs) to doxorubicin, the authors developed an in vitro AIC model and demonstrated that co-culture with mesenchymal stem cells (MSCs) alleviated cardiotoxicity. Interestingly, the beneficial effect was mediated via MSC-derived large extracellular vesicles (L-EVs) of size >200 nm. Diligent work by the authors also showed that respiring mitochondria inside L-EVs are required for benefit. Thus, the observation evokes the intriguing possibility that mitochondrial transfer between MSCs and cardiomyocytes could be therapeutic for AIC (Figure 1). This study is highly significant in several ways. First, it indicates tremendous potential to innovate the approach to AIC treatment. Moreover, it fuels the emerging interest in harnessing the mysterious power of mitochondrial transfer, the significance of which extends beyond cardio-oncology. Finally, it adds a new paracrine/endocrine mechanism to the arena of stem cell–based therapy.
In addition, the study reaffirms the utility of iCMs as a framework for cardiotoxicity assessment while abrogating the need for invasive endomyocardial biopsies. Although whether iCMs predict the risk for in vivo chemotherapy cardiotoxicity remains to be confirmed, this in vitro system demonstrates a model of precision medicine that uses patient-specific iCMs to assess for tolerance to anthracyclines and various treatment modalities. The design of many cardio-oncology trials to date has not been sufficiently targeted from the patient risk perspective or from the putative pathways of cardiotoxicity. In addition to providing a deeper comprehension of the mechanism of injury and the ability to test potential interventions to identify effective treatments, the current platform may help identify high-risk individuals with mitochondrial dysfunction and inform candidate selection for mitochondrial rescue. Beyond anthracyclines, similar experiments may be feasible across different classes of cancer drugs and exposures.
The experiments showed that viable mitochondria taken up by the iCMs were incorporated into the existing mitochondrial network. Functionally, the injured iCMs receiving the MSC-derived mitochondria exhibited preserved viability and recovery of contractility, calcium flux, contraction rate, increased ATP production, and a reduction of cytoplasmic and mitochondrial reactive oxygen species (ROS). It is unclear if the beneficial effects were due to transferred mitochondria actively participating in aerobic respiration and ATP generation. If so, how would the transferred mitochondria silence the detrimental effects of damaged mitochondria, such as ROS production? Would the enhancement of ATP production be transient if the transferred mitochondria were also susceptible to anthracycline toxicity? Alternatively, mitochondrial transfer may have cured the existing damage caused by anthracyclines via unknown mechanisms. In that case, identification of these mechanisms could lead to ground-breaking discoveries.
The realization of mitochondrial transfer as a viable treatment for clinical AIC still faces many challenges. The delivery of viable mitochondria to the injured cardiomyocytes in vivo is not trivial. Intravenous delivery may result in the degradation of mitochondria by plasma phospholipase A2 IIA, to release the inflammation-inciting mitochondrial damage-associated molecular patterns (7,8), inadvertently inducing inflammation and further myocardial injury (8,9). Moreover, cardiac resident macrophages were recently shown to rapidly take up and degrade extracellular exophers containing mitochondria (10), an obstacle that the mitochondrial delivery system would need to bypass. In addition, there remains a concern for mitochondrial calcium overload because of the high Ca2+ concentrations in the blood and extracellular environment, which may limit the efficacy of viable mitochondrial transfer (11).
The optimal source of donor mitochondria is another unanswered question. It has not been tested whether MSC-derived vesicles are uniquely therapeutic. Mitochondria isolated from other cell types were shown to be capable of boosting cardiac muscle function, albeit also via unknown mechanisms (12). It remains to be determined if mitochondria from any cell types can be used to overcome AIC. Furthermore, the authors identified several limitations in the current approach. For example, the therapeutic effect derived from vesicle-bound mitochondria is detectable only when the doxorubicin dose is sufficiently low and the L-EV given within 24 hours after doxorubicin has been washed out, suggesting that there is a limited temporal therapeutic window. In the clinical setting where anthracyclines are typically given over multiple cycles, direct and/or repeated transfer of L-EV may not be practical or effective. Taken together, to move the field forward, mechanistic research is required to understand how the transfer of mitochondria alleviates toxicity and reduces cell death. Identification of end-effectors of mitochondria-containing L-EV will likely reveal new targets for the next generation of therapy.
Despite these challenges, the study poses promising opportunities for future endeavors to validate mitochondrial transfer as a viable therapy for AIC. If safely and sufficiently targeted, mitochondrial transfer may offer an attractive intervention to improve cardiac function that is relatively free of systemic adverse effects or risk for treatment-treatment interactions in oncology patients receiving ongoing cancer therapy.
Funding Support and Author Disclosures
The work is supported in part by the John L. Locke Jr. Charitable Trust (Dr Wang) and the National Institutes of Health grants HL110349, HL142628, and HL149695 (Dr Tian). Dr Tian has received compensation by serving on the scientific advisory committee of Cytokinetics, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Henriksen P.A. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart Br Card Soc. 2018;104(12):971–977. doi: 10.1136/heartjnl-2017-312103. [DOI] [PubMed] [Google Scholar]
- 2.Steinherz L.J. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266(12):1672–1677. [PubMed] [Google Scholar]
- 3.Cardinale D., Colombo A., Bacchiani G. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 4.Vaduganathan M., Hirji S.A., Qamar A. Efficacy of neurohormonal therapies in preventing cardiotoxicity in patients with cancer undergoing chemotherapy. J Am Coll Cardiol CardioOnc. 2019;1(1):54–65. doi: 10.1016/j.jaccao.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murabito A., Hirsch E., Ghigo A. Mechanisms of anthracycline-induced cardiotoxicity: is mitochondrial dysfunction the answer? Front Cardiovasc Med. 2020;7:35. doi: 10.3389/fcvm.2020.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien C., Ozen M., Ikeda G. Mitochondria rich extracellular vesicles rescue patient-specific cardiomyocytes from doxorubicin injury: insights into the SENECA trial. J Am Coll Cardiol CardioOnc. 2021;3:428–440. doi: 10.1016/j.jaccao.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambeau G., Gelb M.H. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 8.Boudreau L.H., Duchez A.-C., Cloutier N. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124(14):2173–2183. doi: 10.1182/blood-2014-05-573543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q., Raoof M., Chen Y. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolás-Ávila J.A., Lechuga-Vieco A.V., Esteban-Martínez L. A network of macrophages supports mitochondrial homeostasis in the heart. Cell. 2020;183(1):94–109.e23. doi: 10.1016/j.cell.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Bertero E., O’Rourke B., Maack C. Mitochondria do not survive calcium overload during transplantation. Circ Res. 2020;126(6):784–786. doi: 10.1161/CIRCRESAHA.119.316291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaza A.K., Wamala I., Friehs I. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J Thorac Cardiovasc Surg. 2017;153(4):934–943. doi: 10.1016/j.jtcvs.2016.10.077. [DOI] [PubMed] [Google Scholar]