Abstract
Radiation therapy is a cornerstone of cancer therapy, with >50% of patients undergoing therapeutic radiation. As a result of widespread use and improved survival, there is increasing focus on the potential long-term effects of ionizing radiation, especially cardiovascular toxicity. Radiation therapy can lead to atherosclerosis of the vasculature as well as valvular, myocardial, and pericardial dysfunction. We present a consensus statement from the International Cardio-Oncology Society based on general principles of radiotherapy delivery and cardiovascular risk assessment and risk mitigation in this population. Anatomical-based recommendations for cardiovascular management and follow-up are provided, and a priority is given to the early detection of atherosclerotic vascular disease on imaging to help guide preventive therapy. Unique management considerations in radiation-induced cardiovascular disease are also discussed. Recommendations are based on the most current literature and represent a unanimous consensus by the multidisciplinary expert panel.
Key Words: cancer, cardiovascular disease, imaging, prevention, radiation therapy, screening
Abbreviations and Acronyms: aHR, adjusted hazard ratio; CABG, coronary artery bypass graft; CAC, coronary artery calcium; CAD, coronary artery disease; CI, confidence interval; CT, computed tomography; CTCA, computed tomography coronary angiography; CV, cardiovascular; DIBH, deep inspiratory breath hold; HF, heart failure; HL, Hodgkin lymphoma; HNC, head and neck cancer; HR, hazard ratio; LIMA, left internal mammary artery; MRI, magnetic resonance imaging; NT-proBNP, N-terminal pro–B-type natriuretic peptide; OR, odds ratio; PAD, peripheral arterial disease; RT, radiation therapy; SAVR, surgical aortic valve replacement; SVC, superior vena cava; TAVR, transcatheter aortic valve replacement; TTE, transthoracic echocardiogram
Central Illustration
Highlights
-
•
Radiation therapy leads to short- and long-term cardiovascular adverse effects of the vasculature and the heart, including valvular, myocardial, and pericardial disease.
-
•
Computed tomography scans conducted for radiation planning or cancer staging provide an available opportunity to detect asymptomatic atherosclerosis and direct preventive therapies.
-
•
Additional practical screening recommendations for cardiovascular disease based on anatomical exposure are provided.
-
•
There are unique considerations in the management of radiation-induced cardiovascular disease; contemporary percutaneous treatment is often preferred over surgical options.
Therapeutic radiation has become a cornerstone of cancer treatment since its first use in 1899, with >50% of cancer patients now receiving radiation therapy (RT). Despite its positive impact on cancer outcomes and survival, RT is associated with both short- and long-term adverse effects. Of particular note, studies have linked RT to an increased risk of long-term adverse cardiovascular (CV) effects, which can lead to increased morbidity and mortality in cancer survivors (1, 2, 3).
The spectrum of radiation-induced CV disease is broad. After thoracic RT, patients are at significantly increased risk of developing vascular disease (including coronary artery disease [CAD] and subclavian artery stenosis), valvular disease, constrictive pericarditis, restrictive cardiomyopathy, and heart failure (HF) (1,2,4) (Central Illustration). Similarly, after head and neck or whole brain RT, patients are at increased risk of carotid artery stenosis and cerebrovascular accidents (4, 5, 6, 7). Patients treated with abdominal or pelvic RT may develop aorto-iliac atherosclerosis and renal artery stenosis (8). The mechanisms of radiation-induced CV disease are complex, but key aspects are deoxyribonucleic acid damage, oxidative stress, and the release of inflammatory and profibrotic cytokines leading to vascular, myocardial, valvular, and pericardial fibrosis (9).
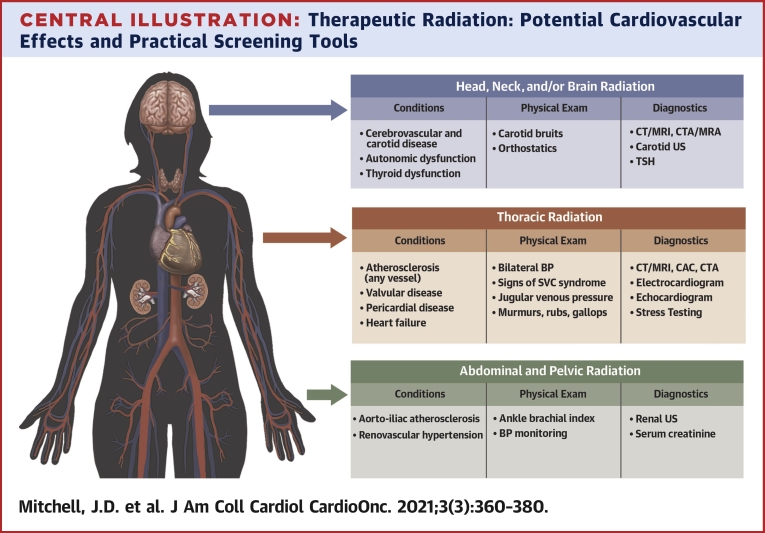
Central Illustration.
Therapeutic Radiation: Potential Cardiovascular Effects and Practical Screening Tools
The figure highlights the potential cardiovascular sequelae as well as physical examination findings and diagnostic tests that can aid in evaluation. BP = blood pressure; CAC = coronary artery calcium; CTA = computed tomography angiography; CT = computed tomography; MRA = magnetic resonance angiography; MRI = magnetic resonance imaging; TSH = thyroid-stimulating hormone; US = ultrasound.
Considering the overall context of RT benefits and risks, it is crucial that clinicians recognize the CV complications and incorporate appropriate screening, mitigation, and prevention strategies in their practice. Although guidelines and expert consensus statements have been published for the detection of RT-associated cardiac damage and dysfunction (10, 11, 12, 13, 14), screening recommendations for extracardiac vascular manifestations are limited. In addition, screening guidelines for survivors of thoracic RT have focused on the diagnosis of obstructive, but not nonobstructive, CAD. This limited focus fails to recognize the risk of myocardial infarction in nonobstructive CAD (15) and the importance of preventive medical therapy in these patients (16). Furthermore, current guidelines do not incorporate newer imaging modalities, such as coronary artery calcium (CAC) scanning and coronary computed tomography (CT) angiography, which can more comprehensively define a patient’s CV risk during and after RT (17).
The current consensus statement from the International Cardio-Oncology Society systematically reviews available data and provides comprehensive recommendations for prevention, screening, diagnosis, and management of CV disease in cancer survivors receiving or who have received RT to the head and neck, chest, or abdomen and pelvis. This document addresses general principles to mitigate CV disease after RT and provides guidance for the detection of specific vascular effects based on anatomical location (Figure 1).
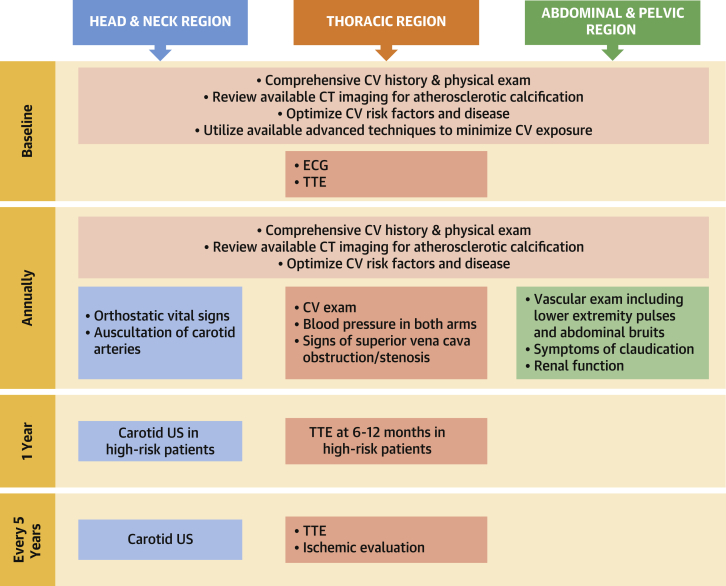
Figure 1.
Summary of Screening Guidelines
Summary of recommended monitoring intervals and evaluation procedures based on anatomical exposure of radiation therapy. CT = computed tomography; CV = cardiovascular; ECG = electrocardiogram; TTE = transthoracic echocardiogram; US = ultrasound.
Methods
In October 2019, the International Cardio-Oncology Society formed an international, multidisciplinary collaboration of experts in the field of medical oncology, radiation oncology, CV imaging, and cardio-oncology. An extensive literature review was performed through a search of the PubMed index of studies published in English from 1975 to the present incorporating text words and Medical Subject Headings, including radiation, cardiotoxicity, and CV abnormalities; the result was 2,999 manuscripts at the time of final search. The literature review was conducted initially in October 2019 and then updated before finalization of recommendations in December 2020. References of reviewed articles were also searched for salient titles. Priority was given first to evidence from randomized controlled trials or meta-analyses, followed by well-designed nonrandomized studies, other nonrandomized studies, and finally expert opinion or clinical experience. The authors also searched ClinicalTrials.gov for any relevant clinical trials.
The committee met using bimonthly webinars and accompanying teleconferences to review the literature and develop practical recommendations. In accordance with American College of Cardiology/American Heart Association consensus statements (18), recommendations are labeled with phrases such as “is recommended,” “can be useful,” and “can be considered.” Levels of Evidence are not provided due to limited randomized controlled trials specifically designed to address CV disease in RT survivors. These recommendations represent a consensus among the multidisciplinary, expert committee members. A summary of the recommendations, the underlying evidence, and important gaps and future directions are summarized in Table 1.
Table 1.
Recommendations, Basis of Evidence, and Future Directions/Gaps for Treatment and Prevention of CV Disease During and After Therapeutic Radiation for Cancer
| Recommendation Summaries | Evidence Basis | Future Directions/Gaps |
|---|---|---|
| General principles to mitigate CV events | ||
| Recommendation A.1: In all patients receiving therapeutic radiation to the head and neck, chest, and/or abdomen/pelvis, a comprehensive baseline evaluation to screen for and optimize treatment of underlying CV risk factors and/or CV disease is recommended | Impact of CV risk modification on future CV events is extrapolated from large cohorts in the general population as well as cancer cohorts, most notably in survivors of childhood cancer | Current ASCVD risk scores are hypothesized to underestimate risk of CV events in cancer populations, and an optimized risk score, potentially including CAC, could be useful Future research should investigate optimal treatment goals (ie, blood pressure, LDL reduction) in patients post-RT |
| Recommendation A.2: Before RT delivery, review of available CT chest imaging for the presence of coronary and aortic calcifications to improve CV risk stratification and mitigation of future atherosclerotic cardiovascular events is recommended | CAC is diagnostic of underlying arterial disease and predicts future CV events in large cohorts in the general population and in patients included in lung cancer screening trials, as well as in smaller cohorts receiving RT | Presence of coronary calcifications on CT imaging identifies patients at risk for future CV events, but formal CAC screening protocols should be further evaluated It is unknown what CAC score threshold (>0, >10, >100) should prompt treatment in patients post-RT |
| Recommendation A.3: Efforts to maximally reduce radiation doses to CV structures without compromising cancer treatment are recommended | Radiation exposure should maintain tumor efficacy while minimizing dose to be consistent with therapeutic radiation guidelines | Future research should continue to evaluate the trade-off between tumor efficacy and CV morbidity and mortality |
| Head and neck RT | ||
| Recommendation B.1: In patients with prior neck irradiation, auscultation for carotid bruits during their routine physical examination is recommended | A low-risk examination technique can identify patients at increased risk for future stroke based on a meta-analysis in the general population | The presence of a bruit increases risk for significant atherosclerosis, but its absence does not rule out disease |
| Recommendation B.2: In patients with prior neck irradiation, carotid ultrasound to screen for development of asymptomatic atherosclerotic plaque is recommended. Initial evaluation as early as 1 y post-radiation in higher risk patients (determined by radiation dose and CV risk) with follow-up every 3 to 5 y can be useful to guide preventive therapy | Carotid ultrasound is low risk to the patient and, based on cohort studies in survivors of neck RT, can be used to identify asymptomatic disease | Further studies are needed to identify the optimal screening interval for ultrasound in conjunction with history and physical examination |
| Recommendation B.3: In patients with prior neck irradiation, reviewing available CT scans for carotid calcifications to aid in identification of asymptomatic atherosclerosis is recommended | Inferred from basic science, clinical trials, and population studies, numerous studies have established calcified plaque as a marker of atherosclerosis in various vascular beds Patients often undergo surveillance CT scans post-RT, which can be used to identify asymptomatic disease at no additional risk |
Although statin therapy has been shown to reduce carotid atherosclerosis and prevent stroke, no studies have directly investigated whether carotid calcifications should prompt statin therapy |
| Recommendation B.4: In patients with prior neck irradiation, screening for signs and symptoms of dysautonomia on follow-up physical examinations (including orthostatic vital signs) is recommended | This is based on expert opinion and small cohort studies in patients treated with RT The history and physical examination provide a low-risk procedure to identify patients with dysautonomia |
Optimal screening and treatment algorithms for dysautonomia in patients post-RT have not been established |
| Thoracic RT | ||
| Recommendation C.1: In patients with CV risk factors receiving radiation to mediastinal structures, including the heart, a baseline ECG and a comprehensive TTE can be useful | This is based on expert opinion. Current guidelines are mixed in their recommendation for baseline ECG and TTE evaluation of patients undergoing thoracic RT A baseline ECG and TTE are low-risk tools that provide the potential to identify asymptomatic disease and allow for CV optimization before RT |
Numerous long-term follow-up studies have shown the significant CV impact of RT, but a baseline ECG or echocardiogram to detect pre-existing disease has not been studied. Although identifying asymptomatic disease may help with risk stratification, there are no data to suggest that it should prevent a patient from receiving RT |
| RecommendationC.2: In patients with prior chest irradiation, review of available CT scans for coronary or aortic calcifications to guide therapy for asymptomatic atherosclerosis is recommended.The absence of coronary artery calcifications, particularly from a non-gated CT scan, cannot fully exclude the presence of CAD | Large cohort studies in the general population and small cohort studies in the cancer population have established the impact of CAC on future cardiac events Evaluating existing CT scans, which correlate with quantitative CAC scores, provides a tool to improve CV stratification and to help guide initiation of preventive therapy |
Although CAC on CT imaging identifies patients with underlying CAD, it is important to note that non–ECG-gated CT scans have a 9% false-negative rate and may not detect CAC in some patients with underlying CAD It is not known what threshold of qualitative CAC should prompt aspirin and/or statin use The impact of aspirin and statin therapy on identified atherosclerosis post-RT is not known |
| Recommendation C.3: In patients with prior chest irradiation without documented atherosclerosis on prior evaluations, further screening for CAD with CAC, coronary CT angiography, or functional stress testing during follow-up evaluations is recommended. Screening at 5-y intervals, depending on the patient’s overall CV risk, can be useful | Functional stress testing, CAC, and CT angiography can identify asymptomatic patients with underlying CAD based on large cohort studies in the general population and small cohort studies in patients post-RT | Optimal screening protocols have not been established. CTCA and CAC have the advantage of identifying nonobstructive CAD to help target preventive therapy, but there have not been comparison trials in patients post-thoracic RT. Further studies are needed to understand the relative prevalence of noncalcified and calcified plaque in patients post-RT by using historical and modern techniques |
| Recommendation C.4: In patients with prior chest irradiation who are at increased risk for cardiomyopathy, screening TTEor cardiac MRI after completion of cancer therapy are recommended | Prospective cohort studies have shown a high rate of cardiomyopathy and heart failure post-RT necessitating screening protocols | The optimal screening interval has not been established with historical or current radiation techniques and should be further evaluated with future studies |
| Recommendation C.5: The timing of the first echocardiogram post–chest RT can be guided by the individual patient risk, with echocardiography as early as 6-12 months after RT in high-risk patients. In all patients in whom the heart is in the radiation field, an echocardiogram within 5 y post-RT is recommended. Additional screening TTE and measurement of NT-proBNP levels every 5 y can be useful | The recommended timing of screening is based on the incidence of heart failure post-RT in cohort studies Recent cohort studies have suggested that cardiac biomarkers may also be an effective screening tool |
Additional research in the timing and measurement of biomarkers or cardiac imaging post-RT is needed |
| Recommendation C.6: In patients with prior RT with the heart in the radiation field, evaluation for subclinical valvular heart disease with TTE 5 y post-RT and then every 5 y thereafter is recommended | The recommended timing of screening is based on the incidence of valvular disease post-RT in cohort studies | The optimal screening interval has not been established with historical or current radiation techniques and should be further evaluated in future studies |
| Recommendation C.7: In patients with prior RT with the heart in the radiation field, evaluation for pericardial disease with TTE 5 y post-RT and then every 5 y thereafter is recommended | The recommended timing of screening is based on incidence of pericardial disease post-RT in cohort studies | The optimal screening interval has not been established with historical or current radiation techniques and should be further evaluated in future studies |
| Recommendation C.8: In patients who have received radiation involving the subclavian artery, bilateral BP on annual examination to screen for subclavian stenosis is recommended | A comprehensive physical examination with bilateral BP measurement allows for minimal-risk screening of patients for subclavian artery disease that can occur post- thoracic RT | No studies have evaluated screening protocols for subclavian disease post-thoracic RT |
| Recommendation C.9: In patients who have received radiation involving the subclavian artery and/or LIMA, evaluation with CT angiography or a comparable study before CABG is recommended | Case studies have shown that undiagnosed subclavian artery stenosis or LIMA atrophy can have significant consequences on surgical outcomes post-CABG | A complete angiographic assessment is recommended before planned bypass surgery to reduce risk of graft failure after surgery, but an optimal screening protocol has not been established |
| Abdominal and pelvic RT | ||
| Recommendation D.1: In patients with prior abdominal or pelvic irradiation, screening for symptoms of claudication, assessment of pedal pulses, and auscultation of aortic or renal artery bruits are recommended | A comprehensive physical examination can identify patients with underlying peripheral vascular disease to aid in management | No studies have evaluated screening protocols for peripheral arterial disease post-abdominal and pelvic RT. The prevalence of PAD post-abdominal and pelvic RT is not known |
| Recommendation D.2: In patients with prior abdominal or pelvic irradiation, reviewing available CT scans for aortic and iliofemoral calcifications to identify atherosclerosis can be useful | Aortic calcifications increase a patient’s mortality risk based on the Framingham Risk Study and in a lung cancer cohort receiving RT and can help risk-stratify patients. It is expert opinion that evaluating for calcifications post-RT can potentially help clinicians target preventive therapy | Screening for significant peripheral vascular disease post-abdominal and pelvic RT has not been adequately investigated |
| Recommendation D.3: In patients with prior abdominal or pelvic irradiation with worsening renal function and/or systemic hypertension, evaluation for radiation nephropathy and/or renal artery stenosis can be useful. | Based on case series, renal artery stenosis and radiation nephropathy are known complications of RT and may lead to hypertension. Identification of underlying renal artery stenosis and/or radiation nephropathy may help in disease and risk factor management | The incidence and prevalence of hypertension, renal artery stenosis, and radiation nephropathy in patients post-abdominal and pelvic RT are not established |
| CV disease prevention after RT | ||
| Recommendation E.1: In patients who have received RT, regular screening for and aggressive treatment of CV risk factors and CV disease are recommended. The interval of screening visits should be guided by the patient’s risk (patient and treatment factors). Screening at least annually can be useful | Modifiable CV risk factors significantly increase risk of future CV events post-RT, with the strongest evidence in cohorts of adult survivors of childhood RT In accordance with prevention guidelines and numerous studies in the general population, treatment of CV risk factors is indicated |
Screening intervals for CV risk factors post-RT remain to be clarified |
| CV management after RT | ||
| Recommendation F.1: In patients with prior chest RT, careful consideration before surgical versus percutaneous treatment for valvular or CAD is recommended due to their increased surgical risk. The percutaneous approach is often advantageous, especially in patients with higher radiation doses to the mediastinum or prior cardiac surgery | Small cohort studies have shown that survivors of thoracic RT are at increased risk for complications from chest surgery, and they may benefit from a percutaneous approach if technically feasible | Additional research is needed to determine the multidisciplinary strategy that is most successful for management of obstructive CAD and/or valvular disease post-RT |
| Recommendation F.2: In patients with prior chest RT and heart failure with preserved ejection fraction, consideration of both restrictive cardiomyopathy and constrictive pericarditis is recommended | This is based on expert opinion. The presentations of restrictive cardiomyopathy and constrictive pericarditis are similar, but management of the 2 conditions is vastly different. Distinguishing between the 2 is of major clinical importance | Diagnostic protocols vary between institutions and are not standardized |
| Recommendation F.3: In patients with prior chest RT and confirmed constrictive pericarditis who have failed initial medical management, pericardiectomy can be considered. Surgery is considered high risk, although timing surgery before progression to advanced disease can improve morbidity | This is based on expert opinion. Case series of patients with constriction have shown a high mortality with pericardiectomy but patients likely do better if they undergo surgery earlier in the disease process | Timing and best practices for pericardiectomy in patients with constriction post-thoracic RT needs further research |
ASCVD = atherosclerotic CV disease; BP = blood pressure; CABG = coronary artery bypass graft; CAC = coronary artery calcium; CAD = coronary artery disease; CT = computed tomography; CV = cardiovascular; ECG = electrocardiogram; LDL = low-density lipoprotein; LIMA = left internal mammary artery; MRI = magnetic resonance imaging; NT-proBNP = N-terminal pro–B-type natriuretic peptide; PAD = peripheral arterial disease; RT = radiation therapy; TTE = transthoracic echocardiogram.
A. General principles to mitigate CV events after therapeutic radiation
The 2 main determinants of RT-associated CV disease are a patient’s underlying CV risk (patient factors) and the dose of RT delivered to CV structures (treatment factors). Thus, the 2 main guiding principles in reducing CV sequelae of RT are to: 1) identify and optimize CV risk factors and CV disease; and 2) minimize RT delivered to the CV system without affecting cancer outcomes.
1. Identify and optimize baseline CV risk factors
Recommendation A.1: In all patients receiving therapeutic radiation to the head and neck, chest, and/or abdomen/pelvis, a comprehensive baseline evaluation to screen for and optimize treatment of underlying CV risk factors and/or CV diseaseis recommended.
Recommendation A.2: Before RT delivery, review of available CT chest imaging for the presence of coronary and aortic calcifications to improve CV risk stratification and mitigation of future atherosclerotic CV events is recommended.
Traditional CV risk factors
Baseline CV risk factors and CV disease have a substantial impact on the subsequent development of CV complications after RT (1, 2, 3). In a population-based case-control study of 2,168 women who underwent RT for breast cancer from 1958 to 2001 in Sweden or Denmark, women with baseline CV risk factors were at twice the risk for major CV events, and those with a history of CAD had a 6-fold higher risk (1). In a retrospective single-center review of 748 patients with locally advanced non–small cell lung cancer treated with RT, baseline CV disease (including coronary artery calcifications on CT imaging) conferred a 7-fold increased risk of major adverse cardiac events (adjusted hazard ratio [aHR]: 7.00; 95% confidence interval [CI]: 3.20-15.31), with a median time to first event of 18.5 months (3). In 3 cohorts of patients who received neck RT (n = 96, n = 125, and n = 290), baseline hyperlipidemia, diabetes, older age, smoking, and pre-existing heart disease were each associated with development of carotid atherosclerosis (19, 20, 21). In a retrospective cohort of 367 patients receiving RT for head and neck tumors, an increased risk of stroke was observed in patients with baseline hypertension (absolute excess risk: 9 per 1,000 patients per year) and diabetes (absolute excess risk: 13.9 per 1,000 patients per year) (7).
Vascular calcifications on CT imaging
Available CT imaging, routinely performed for cancer screening or staging, provides a readily available risk stratification tool to assess for vascular calcifications. Unlike other testing that may imply the presence of CAD, CAC on chest CT imaging directly correlates with a patient’s underlying atherosclerotic burden and is diagnostic of underlying CAD (22). The presence and severity of CAC accurately predict major adverse cardiac outcomes in addition to traditional risk factors in large population studies (23, 24, 25, 26) and in cohorts of patients specifically with cancer (27,28). In 1,130 participants in the Multi-Ethnic Study of Atherosclerosis at intermediate risk for CV events (Framingham risk score >5% and <20%), CAC significantly improved discrimination for future CV events compared with Framingham risk score alone (area under the receiver-operating characteristic curve increased from 0.627 to 0.752; P < 0.0001) and provided superior discrimination and risk reclassification to other risk markers (26). In a single-center cohort of 408 consecutive patients with breast cancer referred to cardio-oncology, CAC (but not Framingham risk score) was predictive of the composite endpoint of all-cause mortality and CV events (28). In the general population, a CAC score ≥100 also seems to select patients most likely to benefit from statin therapy (17). Given the accelerated nature of radiation-induced atherosclerosis, it is unclear if this same threshold applies to radiation survivors, or if a lower threshold may be appropriate.
CAC seen on radiation-planning CT scans, and other nongated, noncontrast CT scans, correlates with formal gated CAC scans and has significant predictive value (29,30). In a meta-analysis of 3 studies with 661 participants, the agreement in calcium score between nontriggered and electrocardiography-triggered CT scanning was 0.94 (95% CI: 0.89-0.97) (29). Across 5 studies with 34,028 participants, increasing calcium score categories on nongated CT imaging were consistently associated with increasing risk of CV death and events. With fewer image slices, however, there was a notable false-negative rate of 9% and an underestimation of high CAC scores in 19%.
When available, formal scoring of the baseline CT scan for CAC is likely the most robust, although the visual CAC-Data Reporting System or similar method can also be used (31). In cohorts of breast cancer patients with and without radiation exposure, cardiac events are associated with both the presence of CAC by formal Agatston CAC scoring (n = 939; HR: 4.95; 95% CI: 1.69-14.53) and the severity of CAC by visual assessment of CT scans (Figure 2) with or without contrast (n = 408; aHR: 4.90 for comparison of intermediate to high CAC vs no CAC; 95% CI: 1.95-12.29) (27,28).
Figure 2.
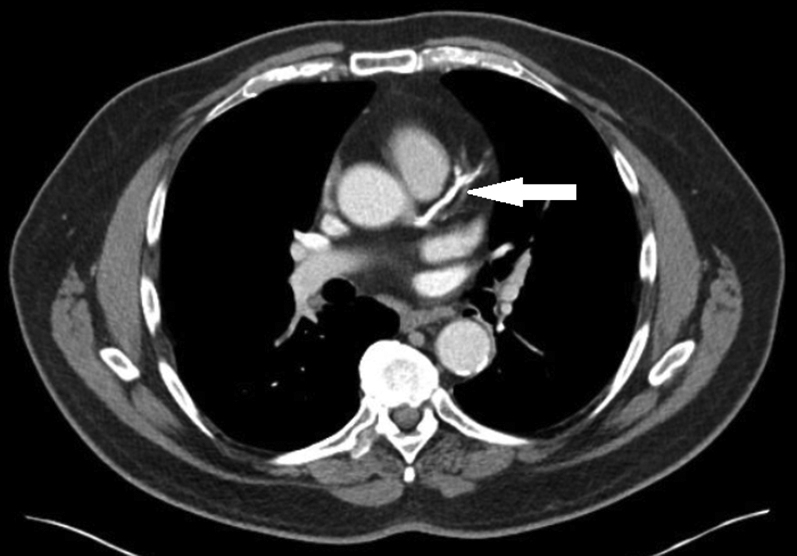
Coronary Artery Calcium on CT Imaging for Cancer Staging
A 68-year-old man was diagnosed with prostate cancer on biopsy and underwent computed tomography (CT) imaging for cancer staging. Severe coronary calcifications are noted extending from the left main coronary artery to the left anterior descending artery. He subsequently presented with unstable angina ∼1 year later and underwent bypass surgery.
Calcifications in other vascular beds on CT imaging may also aid in risk assessment. Over a median follow-up of 8 years in the Framingham Heart Study, presence of aortic calcifications was associated with CV events (n = 3,486; aHR: 2.71; 95% CI: 1.76-4.11), although this finding was no longer significant after adjusting for CAC (32). However, thoracic aortic calcifications were associated with all-cause mortality, even after CAC adjustment (aHR: 1.33; 95% CI: 1.10-1.61) (32). In a retrospective cohort of 334 patients with lung cancer treated with stereotactic body RT, the volume of thoracic calcifications on radiation-planning CT scanning was also modestly associated with all-cause mortality (aHR: 1.05 for each 1 cm3 increase; 95% CI: 1.00-1.11; P = 0.03) after adjusting for tumor volume, age, sex, and performance status (33). In summary, the presence of aortic calcifications, in addition to CAC, can guide risk assessment, although CAC is a more powerful predictor for CV outcomes when available based on large population studies.
There are no data to suggest that radiation planning should be altered based on the presence of pre-existing coronary artery calcifications or known CAD, although patients would be expected to derive benefit from optimal CV risk reduction.
2. Minimize radiation delivery to CV structures
Recommendation A.3: Efforts to maximally reduce radiation doses to CV structures without compromising cancer treatment are recommended.
Multiple studies have shown a linear dose-dependent relationship between the mean heart dose delivered and the risk of future cardiac events (1,2), with no minimum dose threshold identified. This relationship also holds true with other noncardiac vascular structures (5,6). Protocols for risk-adapted dose reduction and advances in planning and delivery techniques over the last 30 years have reduced radiation to nontarget structures without compromising cancer outcomes (Figure 3).
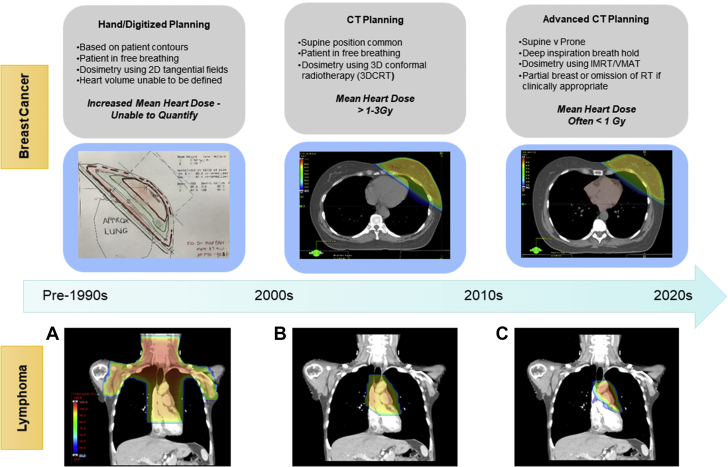
Figure 3.
Evolution of Cancer Radiation Techniques and Impact on Cardiac Dose
Advances in radiation techniques over the last 30 years have significantly decreased the mean heart dose during radiation therapy, especially in breast cancer (top) and lymphoma (bottom). In breast cancer, modern computed tomography (CT) planning and techniques such as deep inspiratory breath hold can help reduce the mean heart dose to <1 Gy. With the changes in systemic therapy and radiation treatment delivery techniques in lymphoma, modern radiation oncology practice has also evolved to reduce the size of historical mantle radiotherapy fields in a risk-adapted fashion to “involved site” or “involved nodal” fields, minimizing dose to surrounding organs while maintaining disease control. (A) Mantle radiotherapy. (B) Involved field radiotherapy. (C) Involved site radiotherapy. 2D = 2-dimensional; 3D = 3-dimensional; 3DCRT = 3-dimensional conformal radiation therapy; IMRT = intensity-modulated radiation therapy; RT = radiation therap; VMAT = volumetric modulated arc therapy.
Oncologic responses and overall survival were maintained in a randomized trial of 1,370 patients with early-stage Hodgkin lymphoma (HL) with reduced RT doses from 30 to 20 Gy (34). In one randomized study of patients with unresectable stage III non–small cell lung cancer, a reduction in total RT dose from 74 Gy to 60 Gy was associated with improved all-cause mortality, likely due to reduced cardiotoxicity (35). For any given RT dose, successful approaches to decrease radiation exposure to nontarget structures include radiation field reduction (eg, involved-node RT, accelerated partial breast irradiation) and modern RT planning and delivery techniques (eg, 3-dimensional conformal therapy, intensity modulated RT, respiratory gating, proton beam therapy).
In a small, retrospective single-center review of 29 adolescents and young adults with stage I to II HL, involved-node RT resulted in a mean heart dose of only 7.7 Gy compared with the 27.5 Gy that would have been delivered with mantle field RT, corresponding to an estimated reduction in the 25-year absolute excess risk of cardiac disease from 9.1% to 1.4% (36) (Figure 3). There is also ongoing interest in the use of intensity-modulated RT in early laryngeal cancer to reduce dose to the carotid arteries (37). Proton therapy, although not widely available, offers the potential to further decrease exposure to surrounding organs, such as the heart, in patients receiving RT (38).
Deep inspiratory breath hold (DIBH) has been shown to significantly decrease RT doses to the heart and surrounding tissues (39) and should be incorporated as standard of care for appropriate patients when the heart is potentially in the radiation field. During inspiration, flattening of the diaphragm and expansion of the lungs pull the heart toward the center of the chest, away from the breast/chest wall and the radiation beam (Figure 3). DIBH has been associated with significant improvements in both mean heart doses and mean left anterior descending artery doses in a number of studies, with respective decreases of 25% to 67% and 20% to 73%, respectively, using DIBH versus free breathing planning in the same patients.
Although vascular-sparing techniques are important in reducing vascular toxicity from RT, they do not eliminate the need for CV risk factor and disease screening. Even with modern RT, CV disease continues to develop at a faster rate after RT exposure. Moreover, there is an existent population of cancer survivors exposed to historical techniques.
B. CV effects of therapeutic radiation to the head, neck, and brain
Recommendation B.1: In patients with prior neck irradiation, auscultation for carotid bruits during their routine physical examination is recommended.
Recommendation B.2: In patients with prior neck irradiation, carotid ultrasound to screen for development of asymptomatic atherosclerotic plaque is recommended. Initial evaluation as early as 1-year post-radiation in higher risk patients (determined by radiation dose and CV risk) with follow-up every 3 to 5 years can be useful to guide preventive therapy .
Recommendation B.3: In patients with prior neck irradiation, reviewing available CT scans for carotid calcifications to aid in identification of asymptomatic atherosclerosis is recommended.
Recommendation B.4: In patients with prior neck irradiation, screening for signs and symptoms of dysautonomia on follow-up physical examinations (including orthostatic vital signs) is recommended.
RT is an important component of curative-intent treatment for head and neck cancer (HNC), as well as an important treatment option for patients with primary or metastatic brain tumors. A 2017 Surveillance, Epidemiology, and End Results Program analysis reported that cardiac disease represented nearly one third of all competing causes of death for 64,598 patients with HNC (40).
Atherosclerosis
Given the close association of carotid sheath structures with lymphatic target structures in the neck (Figure 4), RT for HNC has been associated with increased risk of carotid artery disease, transient ischemic attack, and stroke (6). The 15-year cumulative risk of stroke was 12% in 367 patients with HNC post-RT (7), which was increased in patients with hypertension and diabetes. In a cohort of >14,000 patients with HNC, there was a 46% increase in the cause-specific hazard of stroke with RT compared with surgery alone (41). Patients with HNC traditionally have a high prevalence of pre-morbid CV risk factors that have also contributed to the development of their cancer (ie, smoking, heavy alcohol use) with a prevalence that is at least double that in age-matched control subjects (42). In contrast, there is also a growing recognition of a subgroup of patients with HNC who are human papillomavirus positive who are younger, nonsmokers, and have a more favorable oncologic prognosis (43). With improved survivorship, they are at risk of developing late effects from RT.
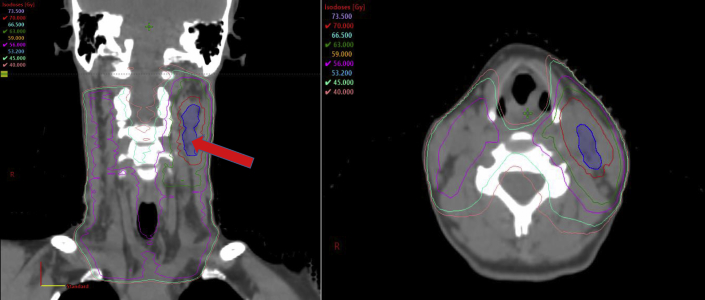
Figure 4.
RT Plan for Carcinoma of the Left Tonsil
Coronal and axial views of the radiation therapy (RT) plan used to treat a human papillomavirus–positive carcinoma of the left tonsil metastatic to the left neck lymph nodes (blue shaded region) in a 44-year-old woman. Isodose lines, in Gray (Gy), represent the dose distribution similar to topographical lines on a map. This patient was prescribed 70 Gy in 35 treatments to gross tumor (red line), with an intermediate-dose region of 63 Gy (green line) and a low-dose region of 56 Gy to the at-risk lymphatics (magenta line). One can appreciate the high radiation exposure to the carotid vessels, including the carotid bulb (red arrow).
In a cohort of 431 pediatric survivors of brain tumors, 61.5% of whom received radiation, the incident rate of neurovascular events was >100 times the general population. Among this high-risk population, neurovascular events were substantially further increased with any brain RT (HR: 8.0; 95% CI: 1.05-62.0) and especially with RT to the Circle of Willis (HR: 9.0; 95% CI: 1.2-70.0) (44). Radiation to the hypothalamic-pituitary axis was also associated with increased incidence of metabolic syndrome (odds ratio [OR]: 3.4; 95% CI: 1.01-11.8.0) in 142 survivors of childhood brain tumors (45). Several randomized trials have investigated the use of stereotactic radiosurgery to ablate metastases while largely sparing the rest of the brain, with variable success reported to date. In 1 randomized trial of 132 patients with 1 to 4 brain metastases, there was increased risk of intracranial relapse in the stereotactic radiosurgery group compared with the whole brain radiation arm, although the stereotactic radiosurgery arm had similar overall survival. Patients without intracranial relapse were also able to avoid whole brain radiation (46).
Autonomic dysfunction
Radiation to the neck can result in autonomic dysfunction many years after RT, secondary to injury and fibrosis of the carotid sinus. Autonomic dysfunction manifestations include inappropriate sinus tachycardia, orthostatic hypotension, postural orthostatic tachycardia syndrome, decreased heart rate variability, and a blunted blood pressure response to exercise (47, 48, 49). In a cohort of 89 patients with HNC compared with 48 matched control subjects, neck RT was associated with afferent baroreflex failure (sympathetic nervous system) manifested by an inappropriate heart rate response to the Valsalva maneuver, with relative sparing of the cardiovagal efferent pathway (parasympathetic nervous system) and a normal heart rate response to deep breathing (50). In a retrospective cohort of 263 HL survivors of RT compared with 526 matched control subjects who underwent exercise treadmill testing, RT was associated with increased resting heart rate (OR: 3.96; 95% CI: 2.52-6.23) and abnormal heart rate recovery (OR: 5.32; 95% CI: 2.94-9.65) after exercise. Abnormal heart rate recovery was independently associated with increased mortality (aHR: 4.60; 95% CI: 1.62-13.02). The prevalence of dysautonomia increased with radiation dose and time from RT (48).
Thyroid abnormalities
Notably, there is also a significant 20% to 30% prevalence of thyroid abnormalities within 5 years of curative RT to the neck regions (51). Although not a direct CV effect, derangements of the thyroid axis can have significant secondary effects on the CV system.
Head and neck: screening recommendations after therapeutic radiation
Physical examination for carotid bruits
In patients with prior neck irradiation, auscultation for carotid bruits can identify asymptomatic atherosclerosis and direct preventive therapy in addition to evaluating for carotid stenosis. The presence of a bruit had a 89% positive predictive value for carotid plaque in a multiethnic, community-based, asymptomatic prospective cohort (mean age: 68 years) and confers a 3 times higher risk of transient ischemic attack or stroke based on a meta-analysis of 17,913 patients and 28 prospective cohort studies in the general population (52,53). In ∼30% of noncancer patients with bruit, carotid stenosis >60% will be detected on carotid ultrasound (52).
Carotid ultrasound
Despite their positive predictive value for carotid plaque in the community-based cohort study, carotid bruits only had a 6% sensitivity for detecting plaque and 54% sensitivity for detecting carotid stenosis >60% (52). Thus, the panel believes that screening with carotid ultrasound is warranted to supplement the physical examination. Carotid atherosclerosis can be detected early after RT on ultrasound and should prompt appropriate risk factor management, as these findings correlate with risk of myocardial infarction and stroke in the general population, even after adjusting for traditional risk factors (54,55).
In a cohort of 96 patients, carotid ultrasound identified internal carotid stenosis of ≥30% in 28% of patients and critical stenosis in 13%, at a mean of 79.9 months from neck RT (20). In another cohort of 105 patients with nasopharyngeal carcinoma post-RT, carotid ultrasound detected carotid plaque in 38 patients (36%) at a median 48 months from radiation, with atherosclerosis severity increasingly linearly with time (56). Asymptomatic atherosclerosis can be seen as early as 1 year after RT (19).
CT neck
Surveillance CT scans should also be reviewed for development of carotid calcifications and atherosclerosis. In a series of 104 patients with laryngeal cancer with pre- and post-radiotherapy CT scans and without baseline atherosclerosis, there was a 48% incidence of new carotid atherosclerosis on follow-up contrast CT scans 4 years post-radiotherapy and a 17% incidence of new carotid calcifications (19). CT angiography and magnetic resonance imaging (MRI) are not routinely recommended for screening but can be used for further evaluation and characterization of carotid disease as clinically required.
Dysautonomia
Recommended screening for dysautonomia in patients with HNC post-RT focuses on clinical history and examination. In patients with signs or symptoms of dysautonomia, further evaluation and treatment should follow established recommendations (47,57).
C. CV effects of therapeutic radiation to the thorax
Recommendation C.1: In patients with CV risk factors receiving radiation to mediastinal structures, including the heart, a baseline electrocardiogram and a comprehensive transthoracic echocardiogram (TTE)can be useful.
Recommendation C.2: In patients with prior chest irradiation, review of available CT scans for coronary or aortic calcifications to guide therapy for asymptomatic atherosclerosis is recommended. The absence of coronary artery calcifications, particularly from a non-gated CT scan, cannot fully exclude the presence of CAD.
Recommendation C.3: In patients with prior chest irradiation without documented atherosclerosis on prior evaluations, further screening for CAD with CAC, coronary CT angiography, or functional stress testing during follow-up evaluations is recommended . Screening at 5-year intervals, depending on the patient’s overall CV risk, can be useful.
Recommendation C.4: In patients with prior chest irradiation who are at increased risk for cardiomyopathy, screening TTE or cardiac MRI after completion of cancer therapy is recommended .
Recommendation C.5 : The timing of the first TTE post-chest RT can be guided by individual patient risk, with echocardiography as early as 6 to 12 months after RT in high-risk patients. In all patients in whom the heart is in the radiation field, an echocardiogram within 5 years post-RT is recommended . Additional screening echocardiograms and measurement of N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels every 5 years can be useful .
Recommendation C.6: In patients with prior RT with the heart in the radiation field, evaluation for subclinical valvular heart disease with TTE 5 years post-RT and then every 5 years thereafter is recommended.
Recommendation C.7: In patients with prior RT with the heart in the radiation field, evaluation for pericardial disease with TTE 5 years post-RT and then every 5 years thereafter is recommended.
Recommendation C.8: In patients who have received radiation involving the subclavian artery, bilateral blood pressure on annual examination to screen for subclavian stenosis is recommended.
Recommendation C.9: In patients who have received radiation involving the subclavian artery and/or left internal mammary artery (LIMA), evaluation with CT angiography or a comparable study before coronary artery bypass graft (CABG)is recommended.
Thoracic RT is an integral part of the treatment for patients with lymphoma, lung cancer, and breast cancer. As a result of RT, survivors are at increased risk for developing CAD, cardiomyopathy, valvular dysfunction, pericardial disease, and/or disease of the aorta and great vessels depending on the structures involved in the radiation field and the magnitude of exposure. Advanced radiotherapy techniques have led to significant reductions in the mean heart dose, and lower incidence of CV events over time (58), although current screening recommendations should continue to consider the large number of survivors who have been previously treated with older RT techniques.
Patients at high-risk for radiation-associated cardiac disease have been defined as those with: 1) mediastinal radiotherapy ≥30 Gy with the heart in the treatment field; 2) lower dose radiotherapy (<30 Gy) with anthracycline exposure; 3) patients aged <50 years and longer time since RT; 4) high dose of radiation fractions (>2 Gy/d); 5) presence and extent of tumor in or next to the heart; 6) presence of CV risk factors; and 7) pre-existing CV disease (10,11,13). Mean cardiac dose correlates with risk, but notably no study has identified a safe RT dose that avoids CV complications.
Coronary artery disease
After thoracic RT, there is an increased risk of development and progression of CAD (Figure 5) and subsequent CV mortality (1,2,59, 60, 61). There was a 7% per Gray higher risk of major coronary events beginning within 4 years after RT in 2,168 breast cancer survivors who received RT between 1958 and 2001 in Sweden and Denmark (1). A >10% incidence of major cardiac events (CAD or HF) was observed in the initial 24 months after RT in 125 patients with non–small cell lung cancer across 4 trials from 2004 to 2013 (60).
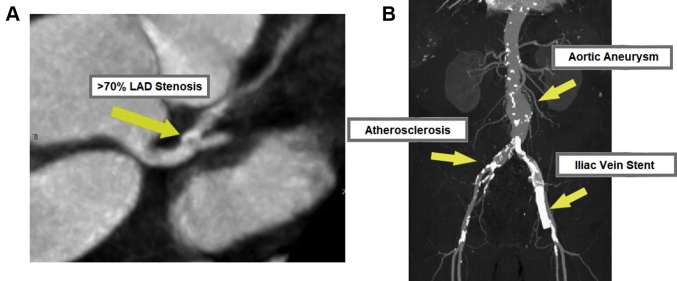
Figure 5.
Cases of Radiation-Induced Cardiovascular Disease
(A) Severe coronary artery disease on coronary computed tomography angiography. A 57-year-old woman underwent adjuvant chemotherapy and radiation therapy for left-sided breast cancer 14 years ago with normal coronary arteries on cardiac catheterization 7 years ago. She developed electrocardiography changes on ribociclib but was asymptomatic. A computed tomography angiogram showed >70% proximal left anterior descending artery (LAD) disease, and she was started on preventive therapy. A few months later, she began having angina and underwent percutaneous coronary intervention. (B) Iliac vein stenosis 1 year after pelvic radiation. A 70-year-old with prostate cancer developed iliac vein stenosis requiring stenting 1 year after radiation therapy. He notably also had baseline atherosclerotic disease manifesting as an abdominal aortic aneurysm and aorto-iliac calcifications before his radiation therapy.
Cardiomyopathy
After chest RT, patients are at risk for HF with reduced or preserved ejection fraction due to direct myocyte damage and subsequent fibrosis, ischemic heart disease, valvular heart disease, and pericardial disease. In a population case-control study of 170 breast cancer survivors in Olmstead County, Minnesota, post-RT, each increase in the log of the mean cardiac dose placed patients at a substantially higher risk of HF with preserved ejection fraction (OR: 16.9; 95% CI: 3.9-73.7) and a modest but nonsignificant increased risk of HF with reduced ejection fraction (OR: 3.2; 95% CI: 0.8-13.0) over an average of 6 years of follow-up (62).
Valvular heart disease
Valvular heart disease develops in a subgroup of patients who have received mediastinal RT, most commonly for lymphoma, with a latency of 10 to 20 years from radiation exposure. The historical prevalence of RT-associated valvular disease has ranged from 2% to 37% for patients treated for HL and 0.5% to 4.2% for patients treated for breast cancer (63). The 30-year cumulative risk is <2% in modern-day HL survivors treated with 20 to 30 Gy but significantly increases with >30 Gy mediastinal exposure (64).
Early RT-associated valve disease includes thickening of the valve leaflets and subvalvular apparatus. Calcification develops over time and can lead to restricted leaflet motion and leaflet retraction resulting in either stenosis or regurgitation. Calcification typically spares the tips of mitral valve leaflets and does not lead to commissural fusion (65). Calcification of the aorto-mitral curtain (intervalvular fibrosa) is common in patients treated with RT for HL. The extent of RT-associated damage often extends beyond the valve and affects the mitral annulus, aortic root, and other surrounding structures. Left-sided valves are more commonly affected, with the aortic valve being most commonly involved (66).
Pericardial disease
Acute pericarditis is an uncommon complication of RT in the modern treatment era. Chronic pericarditis may manifest years after RT, with an estimated prevalence of 7% to 20% reported for patients who received high-dose mantle RT for HL (67). A high incidence of pericardial effusion (36%) has also been reported in patients receiving high cardiac radiation doses, such as in a cohort of 214 patients receiving chemoradiation for esophageal cancer from 2001 to 2010 in Japan (68).
RT-associated latent pericardial disease can manifest as pericardial thickening and calcification, with some cases evolving to pericardial constriction. Patients are also at risk for myocardial fibrosis resulting in restrictive cardiomyopathy, and these 2 entities can be difficult to distinguish. Discerning between restriction and constriction often requires multimodality assessment, including echocardiography, cardiac CT/MRI, and invasive hemodynamic evaluation.
Aorta and the great vessels
Patients who have received mediastinal RT are at increased risk for disease of the aorta and great vessels, including subclavian stenosis (67) and superior vena cava (SVC) syndrome (69,70). The prevalence of subclavian stenosis or SVC syndrome after RT has not been well defined, however, and is largely based on case reports.
Baseline evaluation in patients undergoing thoracic RT
Consistent with the general principles, all patients should undergo history and physical examination to evaluate for symptoms and signs of CV risk factors (eg, hypertension, diabetes mellitus) or underlying CV disease. All patients undergoing thoracic RT will have, at minimum, a planning chest CT scan that can be reviewed for CAC to identify patients with CAD who would benefit from preventive therapy.
In addition, a TTE can be considered for all patients undergoing thoracic RT with the heart in the radiation field to ascertain baseline ventricular and valvular function. Global longitudinal strain may provide additional help in identifying subclinical left ventricular dysfunction at baseline (71). Patients with symptoms or signs of CV disease at baseline should undergo appropriate evaluation and management as for the general population (72,73). These recommendations are consistent with previous consensus statements (10, 11, 12, 13).
There is no definitive evidence supporting routine assessment of baseline cardiac biomarkers before RT. However, studies have shown that elevated levels of NT-proBNP in the general population without a baseline HF diagnosis are associated with increased mortality and a higher risk for subsequent clinical HF (74,75). Therefore, the panel believes that assessment of baseline NT-proBNP level may be considered in asymptomatic patients at risk for HF (age >40 years and the presence of CV risk factors or significant valvular heart disease).
Screening recommendations after thoracic RT
Coronary artery disease
In addition to a comprehensive examination and identification/optimization of CV risk factors, available surveillance CT or positron emission tomography/CT scans should be reviewed for development of CAC after thoracic RT. As with baseline screening, such data are readily available and provide useful information for guiding preventive therapy.
Prior guidelines have recommended functional noninvasive stress testing for CAD detection 5 to 10 years after exposure in asymptomatic high-risk patients, followed by reassessment every 5 years (10,13). These recommendations are based on nonrandomized studies showing that functional stress testing identified CV disease in patients with no symptoms and no abnormalities on cardiac testing at rest (76,77). However, nonobstructive CAD often goes undetected using this strategy, as functional testing requires ischemia to result in a positive test result. In one study of HL survivors treated with chest RT, 36% of patients with a negative stress echocardiogram and 78% of patients with a negative nuclear perfusion test result had >50% stenosis on invasive coronary angiography (77).
Even with single-vessel lesions, patients with nonobstructive CAD have twice the risk of myocardial infarction compared with patients with no CAD on angiography (15). In fact, most plaque ruptures that lead to acute myocardial infarction occur in patients with nonobstructive plaques (78,79). Thus, early identification of CAD and institution of preventive therapies are essential. Optimal medical therapy remains the foundation of treatment in patients with obstructive CAD and stable angina (16,80,81).
Chest CT imaging offers an opportunity to detect CAD earlier in the course and leads to better risk factor control (82). In a similar manner, the SCOT-Heart (Scottish Computed Tomography of the Heart) trial of patients with stable chest pain found that in patients who underwent CT coronary angiography (CTCA), CTCA clarified the diagnosis, allowed initiation of preventive therapies, and was associated with a lower incidence of death from CAD and nonfatal myocardial infarction at 5 years compared with those managed with standard care alone (83). Patients derived similar benefits from CTCA whether their presenting pain was or was not cardiac due to use of better preventive therapeutics. Data on the utility of CTCA in survivors of RT are limited but promising. In a series of 31 HL survivors a median of 24 years after chest RT, CTCA detected CAD in 12 patients and obstructive CAD in 3 patients, with only 1 of the patients being detected via functional stress testing (84). It is therefore reasonable to consider use of CAC and/or CTCA to screen for CAD and to help direct preventive therapy in survivors treated with thoracic RT.
The optimal timing of screening and indications/benefit of repeat screening are still unknown. In an asymptomatic patient already on optimal preventive therapy, repeated screening is unlikely to change management. Repeated evaluations also have to be weighed against radiation exposure imposed by CT imaging. In the noncancer population, a CAC of zero conferred a very low 5- to 15-year risk of CV mortality (85,86), and repeat screening is not indicated before 5 years. The appropriate interval in a patient after RT is not known. It is also uncertain whether there is a heightened risk of noncalcified plaque in radiation survivors. In that setting, CTCA would be preferred over CAC for screening. However, extrapolating from available data in noncancer patients, CAC is likely a sufficient screening test, and a 5-year interval seems reasonable in patients not currently on preventive therapies. Once atherosclerosis is diagnosed, further screening is not warranted, but the evaluation for progression to symptomatic CAD consistent with existing guidelines is indicated if patients develop angina or cardiomyopathy.
Cardiomyopathy
There is no prospective evidence regarding an optimal long-term screening strategy for cardiomyopathy post-RT. Screening echocardiograms 5 years after exposure is reasonable based on retrospective studies (12,63). Comprehensive assessment of diastolic function is of paramount importance given the relative predominance of HF with preserved ejection fraction in these patients (63). Cardiac MRI may be superior to TTE for screening in childhood survivors of cancer (87) and should be considered for patients with poor acoustic windows and/or suspected pericardial constriction. In survivors of RT without evidence of current cardiac dysfunction, NT-proBNP levels were associated with an increased risk of subsequent cardiomyopathy (88).
Valvular heart disease
TTE is the optimal imaging technique for valvular disease in the majority of patients with prior RT exposure. Transesophageal echocardiography can provide more detailed characterization of detected valvular abnormalities and may be indicated in the planning of potential interventions. Cardiac MRI imaging has a less established role in valvular disease assessment post-RT but may be useful in patients with suboptimal echocardiograms. The role of cardiac CT imaging is limited to the assessment of valvular calcifications and procedural planning (transcatheter aortic valve replacement [TAVR] and transcatheter mitral valve repair). The prevalence of moderate or severe valvular disease is low in the first 10 years after RT exposure (13,63,89). TTE is recommended as the primary screening test, commencing 5 years after the completion of RT and every 5 years thereafter. In patients with diagnosed radiation-induced valvular heart disease with valvular dysfunction, the frequency of follow-up TTE imaging is dictated by appropriate societal guidelines (90).
Pericardial disease
TTE often provides the initial evaluation of patients with suspected RT-associated pericardial disease, allowing evaluation of pericardial thickening, pericardial effusion, and functional assessment of constrictive physiology (90). However, the distinction between normal and thickened pericardium by TTE can be difficult, and other modalities such as cardiac MRI and/or CT imaging may be necessary. The timing of screening for pericardial disease aligns with that for valvular heart disease. The majority of patients will be evaluated by use of TTE at 5 years after the completion of RT and then in 5-year intervals. Cardiac MRI and CT imaging are not recommended for generalized screening.
Aorta and great vessels
Routine screening is not currently recommended for SVC syndrome given the lack of data; however, SVC syndrome should be considered in patients who present with any signs or symptoms such as sinus tachycardia, dyspnea, facial or upper extremity swelling, or headaches.
All patients who received RT to the subclavian territory should undergo bilateral, upper extremity blood pressure evaluations during their annual physical examination as a nominal screening for subclavian stenosis. In a series of 59 noncancer patients undergoing evaluation for coronary artery bypass surgery, an upper extremity systolic pressure difference between arms of ≥15 mm Hg identified all patients with ≥50% narrowing of the subclavian (91).
Signs of great vessel atherosclerosis or SVC stenosis may also be appreciated on available CT imaging or echocardiography. Patients being evaluated for CABG should undergo appropriate evaluation for left subclavian stenosis, as well as LIMA patency, if the LIMA is being contemplated as a graft. Notably, the internal mammary nodes are often included in regional nodal radiation in patients with breast cancer, with the internal mammary vessels (the LIMA in left-sided patients) in the first 3 intercostal spaces used as a surrogate target for these high-risk nodes (92).
D. Cardiovascular effects of therapeutic radiation to the abdomen and pelvis
Recommendation D.1: In patients with prior abdominal or pelvic irradiation, screening for symptoms of claudication, assessment of pedal pulses, and auscultation of aortic or renal artery bruits are recommended.
Recommendation D.2: In patients with prior abdominal or pelvic irradiation, reviewing available CT scans for aortic and iliofemoral calcifications to identify atherosclerosis can be useful.
Recommendation D.3: In patients with prior abdominal or pelvic irradiation with worsening renal function and/or systemic hypertension, evaluation for radiation nephropathy and/or renal artery stenosis can be useful .
Therapeutic radiation is commonly used in the abdomen and pelvis to treat gynecologic malignancies, prostate cancer, sarcomas, and some lymphomas. In addition to direct vascular damage (8), abdominopelvic radiation is also associated with nephropathy and metabolic derangements that ultimately increase a patient’s overall CV risk (93, 94, 95). Unfortunately, there are limited techniques to spare the vasculature during RT of abdominopelvic malignancies due to the physical intimacy with the at-risk lymphatics.
Atherosclerosis
Based on available case reports and case series, therapeutic radiation to the abdomen and pelvis can lead to aortic and iliofemoral atherosclerosis (8) and stenosis of the renal artery (8), mesenteric artery (94), and iliac vein (96) (Figure 5). Due to an absence of systematic screening protocols, the true incidence of abdominopelvic atherosclerotic disease after RT is unknown. A higher likelihood of peripheral arterial disease (PAD) has been associated with a total radiation dose from 40 to 65 Gy and/or underlying CV risk factors, although the range of time to symptoms of PAD can be months to decades after exposure (8).
Hypertension and metabolic derangements
Abdominopelvic RT has also been associated with development of CV risk factors in adult survivors of cancer (94,94). Renovascular hypertension may result from renal artery stenosis (97,98) or direct radiation damage to the kidney (radiation nephropathy) (95). Previously described in patients with seminomas, radiation nephropathy has also been reported with total body irradiation even in the case of therapeutic radioisotopes that are filtered by the kidneys.
Abdomen and pelvis: screening recommendations after therapeutic radiation
Screening for PAD
Clinical history, review of symptoms, and physical examination help identify patients at risk for PAD (Table 2). Iliofemoral atherosclerosis can present as intermittent claudication and/or critical limb ischemia (99). Because PAD results in significant morbidity, mortality, and impairment of quality of life (100), early recognition is key and can potentially be identified by review of available CT imaging. Patients with signs or symptoms of PAD should undergo further evaluation and treatment per established guidelines.
Table 2.
Patients at Increased Risk for PADa
| Age ≥65 y |
| Age 50-64 y plus risk factors for atherosclerosis (eg, diabetes, hyperlipidemia, hypertension, history of tobacco use) or family history of PAD |
| Age <50 y but with diabetes mellitus and 1 additional risk factor for atherosclerosis |
| Individuals with known atherosclerotic disease in other vascular beds such as coronary arteries, carotid arteries, renal arteries, and mesenteric arteries |
| Additional: Patients with prior therapeutic radiation to the abdomen and pelvis as part of cancer treatment |
Prior therapeutic radiation to the abdomen and pelvis confers significantly increased risk for peripheral arterial disease (PAD) and should be considered in addition to other known risk factors.
Gerhard-Herman et al (100).
Renal artery stenosis and radiation nephropathy
In patients who develop hypertension after RT to the abdomen or pelvis, renal artery stenosis and radiation nephropathy should be considered. CV risk factors in survivors of RT are further addressed in Section E.
E. CV disease prevention after RT
Recommendation E.1: In patients who have received RT, regular screening for and aggressive treatment of CV risk factors and CV disease are recommended. The interval of screening visits should be guided by the patient’s risk (patient and treatment factors). Screening at least annually can be useful.
Treatment of modifiable CV risk factors
Modifiable CV risk factors are prevalent in middle-aged or older adult patients undergoing RT (3,42) as well as adult survivors of childhood RT (101). CV risk factors, especially hypertension, that develop after RT in adult survivors of childhood cancer have a sizeable and multiplicative impact on subsequent major cardiac events. Thus, aggressive preventive therapy is indicated, including lifestyle modifications. Exercise is specifically associated with improved mortality in childhood cancer survivors (102). Cardiac rehabilitation also reduces CV mortality in patients with coronary heart disease in the general population and is associated with improved cardiorespiratory fitness and quality of life in adult cancer survivors (103). Clinicians should consider calculating 10-year atherosclerotic CV disease risk score (or similar CV risk score) to risk-stratify patients who received RT; however, some data suggest that the pooled cohort equation underestimates the risk of atherosclerotic CV disease in this population (104).
Specific prevention and management strategies
Scientific studies, mostly in rodent models, have explored treatments to counteract the pathophysiological mechanisms involved in the development of radiation-induced vascular disease. Early evidence shows promise for statins, angiotensin-converting enzyme inhibitors, and antioxidants, but prospective studies in humans are still lacking (105). Statins have the strongest evidence to date. In-line with their known benefits in atherosclerosis, statins may reduce RT-related carotid artery injury by lowering cholesterol levels (106). Retrospective studies of patients with cancer and RT of the chest, neck, or head showed significantly reduced risk of stroke for those receiving statin therapy (107, 108, 109). Notably, trials of statins in young patients treated with RT are underway to study the primary prevention effect of statins against radiation-induced CV disease (110). In the absence of specific trials in radiation survivors, patients with established CV disease should be treated as per existing guidelines with aspirin, statins, or other medications as indicated.
F. Important considerations in CV management after RT
Recommendation F.1: In patients with prior chest RT, careful consideration before surgical versus percutaneous treatment for valvular heart disease or CAD is recommended due to their increased surgical risks. The percutaneous approach is often advantageous, especially in patients with higher radiation doses to the mediastinum or prior cardiac surgery.
Recommendation F.2: In patients with prior chest RT and HF with preserved ejection fraction, consideration of both restrictive cardiomyopathy and constrictive pericarditis is recommended.
Recommendation F.3. In patients with prior chest RT and confirmed constrictive pericarditis who have failed initial medical management, pericardiectomy can be considered . Surgery is considered high risk, although timing surgery before progression to advanced disease can improve morbidity.
Management of established radiation-induced CV disease can pose significant challenges due to radiation-induced alterations in surrounding tissues and the vasculature. After chest RT, the combination of scarring, fibrosis, and diffuse atherosclerosis of mediastinal structures increases surgical mortality from open heart surgery (111). Likewise, the risk of surgical treatment of carotid artery stenosis also increases due to inflammatory and fibrotic changes in the arterial wall and neck tissue, although carotid endarterectomy remains a viable strategy (112). There are limited data to guide management, with the available evidence restricted to small cohorts and case series.
Patients with prior chest RT and severe aortic stenosis have worse outcomes with any type of valve replacement than those without a radiation history. In retrospective cohorts (113,114), these patients seem to have improved outcomes with TAVR compared with surgical aortic valve replacement (SAVR), although a subgroup of patients at low surgical risk can potentially be managed well with SAVR. Importantly, the commonly used Society for Thoracic Surgery Score underestimates the 30-day mortality risk in RT survivors (114). The risk of SAVR seems to be further pronounced in those with prior cardiac surgery (115). A reasonable approach, pending further data, is to offer TAVR as the default for patients at intermediate or high risk and no technical TAVR reservations. SAVR can be considered in patients at lower surgical risk (such as younger age), especially those with technical/anatomical concerns for TAVR, or those who require additional surgical interventions such as pericardiectomy, multiple valve surgery, or CABG.
In terms of CAD management, the outcomes of percutaneous coronary intervention may be similar among those with or without RT (116), whereas CABG, as with any other open heart surgery, typically presents challenges. Subclavian artery stenosis and atresia of the LIMA can occur, limiting the availability of this bypass conduit that is a major contributor to the survival benefit of CABG versus percutaneous coronary intervention. Other bypass graft options are hampered if a severely calcified (“porcelain”) aorta is present. In these settings, a percutaneous coronary intervention approach is often the preferred option, unless there are other concomitant surgical needs.
Restrictive cardiomyopathy and constrictive pericarditis are challenging complications in survivors of chest RT, with similar manifestations but very different management. They develop independently but can also occur simultaneously, and cardiac catheterization is usually required in addition to cardiac imaging for proper assessment of restrictive versus constrictive hemodynamic profiles. Management of volume and blood pressure are key for both conditions, while surgical pericardiectomy is reserved for patients with constrictive pericarditis who have failed initial medical management. The outcomes of pericardiectomy are historically very poor in the general population, with nearly 20% mortality at 30 days in a single-center cohort of 97 patients from 1995 to 2012 (117), although improved outcomes were observed in another cohort of 99 patients when pericardiectomy was within 6 months after the onset of symptoms (118).
Future Directions
Although the recommendations in this document represent the expert panel’s consensus based on the available evidence, there are several areas that represent a gap in understanding or knowledge. For instance, there are no published papers addressing the incidence/prevalence of vascular disease after pelvic/abdominal radiation, and the available data rely on case series. We have summarized future directions for each recommendation in Table 1.
Conclusions
RT continues to be a major cancer treatment modality. Although modern delivery techniques have reduced the dose to healthy tissues, no dose of radiation is safe for CV structures, and there is an existent population of survivors of prior, high-dose RT. Clinicians should be aware of, and vigilant for, the potential cardiac and vascular complications of RT in patients with cancer at every level of practice. Optimal management of traditional CV risk factors, as well as the perceptive recognition and aggressive management of identified CV diseases, is essential.
The risks of CV radiation exposure are life-long, and education of patients and their care providers is an essential part of a successful survivorship plan. Patient awareness of potential symptoms and early reporting are as important as physician awareness and proactive management of risks and complications. Efforts to improve patient education through websites such as CardioSmart from the American College of Cardiology are ongoing (119). Regular vigorous exercise has also been shown to reduce overall mortality in cancer survivors and should be a cornerstone of the management strategy for all high-risk patients. Further research is needed to better understand the prevalence of CV disease with modern RT, especially to noncardiac structures, and to continue to improve prevention, screening, and management strategies.
Funding Support and Author Disclosures
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002345 as well as by the National Institutes of Health grant R01 HL147884. Dr Mitchell has received research funding from Pfizer, Longer Life Foundation, and Children’s Discovery Institute; and is a consultant for Pfizer (modest). Dr Bergom has received research support from the Susan G. Komen Foundation and Innovation Pathways. Dr Ferencik has received research support from the National Institutes of Health and the American Heart Association; and is a consultant for Biograph, Inc (unrelated to current work). Dr Szmit has received personal fees from Amgen, Angelini, AstraZeneca, Bayer, Berlin-Chemie, Bristol Myers Squibb, Clinigen, Janssen-Cilag, Pfizer, Polpharma, Roche, and TEVA. Dr Zaha has received support from the Cancer Prevention Research Institute of Texas (RP180404). Dr Herrmann was supported by the National Cancer Institute of the National Institutes of Health (CA233610), the Miami Heart Foundation, and the Mayo Clinic. Dr Nohria has received research funding from Amgen, Inc; and is a consultant for Takeda Oncology and AstraZeneca. Dr Lenihan has received research funding from Myocardial Solutions; and is a consultant for Lilly, Prothena, AstraZeneca, Roche, Clementia, and Eidos (all consultancy renumeration is modest). Dr Dent has received research funding from Novartis; and is a consultant for Novartis and Eli Lilly. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Tochukwu M. Okwuosa, DO, served as the Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Darby S.C., Ewertz M., McGale P., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 2.van Nimwegen F.A., Schaapveld M., Cutter D.J., et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34:235–243. doi: 10.1200/JCO.2015.63.4444. [DOI] [PubMed] [Google Scholar]
- 3.Atkins K.M., Rawal B., Chaunzwa T.L., et al. Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. J Am Coll Cardiol. 2019;73:2976–2987. doi: 10.1016/j.jacc.2019.03.500. [DOI] [PubMed] [Google Scholar]
- 4.Hull M.C., Morris C.G., Pepine C.J., Mendenhall N.P. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–2837. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- 5.Xu J., Cao Y. Radiation-induced carotid artery stenosis: a comprehensive review of the literature. Interv Neurol. 2014;2:183–192. doi: 10.1159/000363068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plummer C., Henderson R.D., O'Sullivan J.D., Read S.J. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke. 2011;42:2410–2418. doi: 10.1161/STROKEAHA.111.615203. [DOI] [PubMed] [Google Scholar]
- 7.Dorresteijn L.D., Kappelle A.C., Boogerd W., et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20:282–288. doi: 10.1200/JCO.2002.20.1.282. [DOI] [PubMed] [Google Scholar]
- 8.Jurado J.A., Bashir R., Burket M.W. Radiation-induced peripheral artery disease. Catheter Cardiovasc Interv. 2008;72:563–568. doi: 10.1002/ccd.21681. [DOI] [PubMed] [Google Scholar]
- 9.Tapio S. Pathology and biology of radiation-induced cardiac disease. J Radiat Res. 2016;57:439–448. doi: 10.1093/jrr/rrw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai M.Y., Windecker S., Lancellotti P., et al. Prevention, diagnosis, and management of radiation-associated cardiac disease: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;74(7):905–927. doi: 10.1016/j.jacc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Armenian S.H., Lacchetti C., Barac A., et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 12.Curigliano G., Lenihan D., Fradley M., et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancellotti P., Nkomo V.T., Badano L.P., et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013–1032. doi: 10.1016/j.echo.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Iliescu C.A., Grines C.L., Herrmann J., et al. SCAI expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia intervencionista) Catheter Cardiovasc Interv. 2016;87:E202–E223. doi: 10.1002/ccd.26379. [DOI] [PubMed] [Google Scholar]
- 15.Maddox T.M., Stanislawski M.A., Grunwald G.K., et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–1763. doi: 10.1001/jama.2014.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell J.D., Brown D.L. Harmonizing the paradigm with the data in stable coronary artery disease: a review and viewpoint. J Am Heart Assoc. 2017;6(11) doi: 10.1161/JAHA.117.007006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell J.D., Fergestrom N., Gage B.F., et al. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol. 2018;72(25):3233–3242. doi: 10.1016/j.jacc.2018.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusumoto F.M., Calkins H., Boehmer J., et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. J Am Coll Cardiol. 2014;11(64):1143–1177. doi: 10.1016/j.jacc.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Kim B.J., Kang H.G., Lee S.W., et al. Changes in the common carotid artery after radiotherapy: wall thickness, calcification, and atherosclerosis. J Clin Neurol. 2018;14:35–42. doi: 10.3988/jcn.2018.14.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng S.W., Ting A.C., Lam L.K., Wei W.I. Carotid stenosis after radiotherapy for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126(4):517–521. doi: 10.1001/archotol.126.4.517. [DOI] [PubMed] [Google Scholar]
- 21.Chang Y.J., Chang T.C., Lee T.H., Ryu S.J. Predictors of carotid artery stenosis after radiotherapy for head and neck cancers. J Vasc Surg. 2009;50:280–285. doi: 10.1016/j.jvs.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 22.Sangiorgi G., Rumberger J.A., Severson A., et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 23.Detrano R., Guerci A.D., Carr J.J., et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell J., Paisley R., Moon P., Novak E., Villines T. Coronary artery calcium score and long-term risk of death, myocardial infarction and stroke: the Walter Reed Cohort Study. J Am Coll Cardiol Img. 2018;11(12):1799–1806. doi: 10.1016/j.jcmg.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Erbel R., Möhlenkamp S., Moebus S., et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2010;56:1397–1406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Yeboah J., McClelland R.L., Polonsky T.S., et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roos C.T.G., van den Bogaard V.A.B., Greuter M.J.W., et al. Is the coronary artery calcium score associated with acute coronary events in breast cancer patients treated with radiotherapy? Radiother Oncol. 2018;126:170–176. doi: 10.1016/j.radonc.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Phillips W.J., Johnson C., Law A., et al. Comparison of Framingham risk score and chest-CT identified coronary artery calcification in breast cancer patients to predict cardiovascular events. Int J Cardiol. 2019;289:138–143. doi: 10.1016/j.ijcard.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 29.Xie X., Zhao Y., de Bock G.H., et al. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: systematic review and meta-analysis. Circ Cardiovasc Imaging. 2013;6:514–521. doi: 10.1161/CIRCIMAGING.113.000092. [DOI] [PubMed] [Google Scholar]
- 30.Hecht H.S., Cronin P., Blaha M.J., et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr. 2017;11:74–84. doi: 10.1016/j.jcct.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Hecht H.S., Blaha M.J., Kazerooni E.A., et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT) J Cardiovasc Comput Tomogr. 2018;12:185–191. doi: 10.1016/j.jcct.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann U., Massaro J.M., D'Agostino R.B., Kathiresan S., Fox C.S., O'Donnell C.J. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2) doi: 10.1161/JAHA.115.003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abravan A., van Herk M., Brewster F., Faivre-Finn C., McWilliam A., Vasquez Osorio E.M. Predictive value of vascular calcification identified in 4D planning CT of lung cancer patients treated with stereotactic body radiation therapy. Phys Med. 2020;78:173–178. doi: 10.1016/j.ejmp.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Engert A., Plütschow A., Eich H.T., et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 35.Bradley J.D., Paulus R., Komaki R., et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maraldo M.V., Brodin N.P., Vogelius I.R., et al. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2012;83:1232–1237. doi: 10.1016/j.ijrobp.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Chera B.S., Amdur R.J., Morris C.G., Mendenhall W.M. Carotid-sparing intensity-modulated radiotherapy for early-stage squamous cell carcinoma of the true vocal cord. Int J Radiat Oncol Biol Phys. 2010;77:1380–1385. doi: 10.1016/j.ijrobp.2009.07.1687. [DOI] [PubMed] [Google Scholar]
- 38.Liao Z., Lee J.J., Komaki R., et al. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:1813–1822. doi: 10.1200/JCO.2017.74.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergom C., Currey A., Desai N., Tai A., Strauss J.B. Deep inspiration breath hold: techniques and advantages for cardiac sparing during breast cancer irradiation. Front Oncol. 2018;8:87. doi: 10.3389/fonc.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massa S.T., Osazuwa-Peters N., Christopher K.M., et al. Competing causes of death in the head and neck cancer population. Oral Oncol. 2017;65:8–15. doi: 10.1016/j.oraloncology.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Arthurs E., Hanna T.P., Zaza K., Peng Y., Hall S.F. Stroke after radiation therapy for head and neck cancer: what is the risk? Int J Radiat Oncol Biol Phys. 2016;96:589–596. doi: 10.1016/j.ijrobp.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Okoye C.C., Bucher J., Tatsuoka C., et al. Cardiovascular risk and prevention in patients with head and neck cancer treated with radiotherapy. Head Neck. 2017;39:527–532. doi: 10.1002/hed.24646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ang K.K., Harris J., Wheeler R., et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campen C.J., Kranick S.M., Kasner S.E., et al. Cranial irradiation increases risk of stroke in pediatric brain tumor survivors. Stroke. 2012;43:3035–3040. doi: 10.1161/STROKEAHA.112.661561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooksey R., Wu S.Y., Klesse L., et al. Metabolic syndrome is a sequela of radiation exposure in hypothalamic obesity among survivors of childhood brain tumors. J Investig Med. 2019;67:295–302. doi: 10.1136/jim-2018-000911. [DOI] [PubMed] [Google Scholar]
- 46.Aoyama H., Shirato H., Tago M., et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 47.Biaggioni I., Shibao C.A., Diedrich A., et al. Blood pressure management in afferent baroreflex failure: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:2939–2947. doi: 10.1016/j.jacc.2019.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groarke J.D., Tanguturi V.K., Hainer J., et al. Abnormal exercise response in long-term survivors of Hodgkin lymphoma treated with thoracic irradiation: evidence of cardiac autonomic dysfunction and impact on outcomes. J Am Coll Cardiol. 2015;65:573–583. doi: 10.1016/j.jacc.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 49.Adams M.J., Lipsitz S.R., Colan S.D., et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 50.Huang C.C., Huang T.L., Hsu H.C., et al. Long-term effects of neck irradiation on cardiovascular autonomic function: a study in nasopharyngeal carcinoma patients after radiotherapy. Muscle Nerve. 2013;47:344–350. doi: 10.1002/mus.23530. [DOI] [PubMed] [Google Scholar]
- 51.Jereczek-Fossa B.A., Alterio D., Jassem J., Gibelli B., Tradati N., Orecchia R. Radiotherapy-induced thyroid disorders. Cancer Treat Rev. 2004;30:369–384. doi: 10.1016/j.ctrv.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Ratchford E.V., Jin Z., Di Tullio M.R., et al. Carotid bruit for detection of hemodynamically significant carotid stenosis: the Northern Manhattan Study. Neurol Res. 2009;31:748–752. doi: 10.1179/174313209X382458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pickett C.A., Jackson J.L., Hemann B.A., Atwood J.E. Carotid bruits and cerebrovascular disease risk: a meta-analysis. Stroke. 2010;41:2295–2302. doi: 10.1161/STROKEAHA.110.585554. [DOI] [PubMed] [Google Scholar]
- 54.O'Leary D.H., Polak J.F., Kronmal R.A., Manolio T.A., Burke G.L., Wolfson S.K. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 55.Lorenz M.W., Markus H.S., Bots M.L., Rosvall M., Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 56.Huang T.L., Hsu H.C., Chen H.C., et al. Long-term effects on carotid intima-media thickness after radiotherapy in patients with nasopharyngeal carcinoma. Radiat Oncol. 2013;8:261. doi: 10.1186/1748-717X-8-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen W.K., Sheldon R.S., Benditt D.G., et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:620–663. doi: 10.1016/j.jacc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 58.Giordano S.H., Kuo Y.F., Freeman J.L., Buchholz T.A., Hortobagyi G.N., Goodwin J.S. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419–424. doi: 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carr Z.A., Land C.E., Kleinerman R.A., et al. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol Phys. 2005;61:842–850. doi: 10.1016/j.ijrobp.2004.07.708. [DOI] [PubMed] [Google Scholar]
- 60.Dess R.T., Sun Y., Matuszak M.M., et al. Cardiac events after radiation therapy: combined analysis of prospective multicenter trials for locally advanced non-small-cell lung cancer. J Clin Oncol. 2017;35:1395–1402. doi: 10.1200/JCO.2016.71.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang K., Eblan M.J., Deal A.M., et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35:1387–1394. doi: 10.1200/JCO.2016.70.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saiki H., Petersen I.A., Scott C.G., et al. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation. 2017;135:1388–1396. doi: 10.1161/CIRCULATIONAHA.116.025434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gujral D.M., Lloyd G., Bhattacharyya S. Radiation-induced valvular heart disease. Heart. 2016;102:269–276. doi: 10.1136/heartjnl-2015-308765. [DOI] [PubMed] [Google Scholar]
- 64.Cutter D.J., Schaapveld M., Darby S.C., et al. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst. 2015;107(4):djv008. doi: 10.1093/jnci/djv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hering D., Faber L., Horstkotte D. Echocardiographic features of radiation-associated valvular disease. Am J Cardiol. 2003;92(2):226–230. doi: 10.1016/s0002-9149(03)00546-0. [DOI] [PubMed] [Google Scholar]
- 66.Heidenreich P.A., Hancock S.L., Lee B.K., Mariscal C.S., Schnittger I. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003;42(4):743–749. doi: 10.1016/s0735-1097(03)00759-9. [DOI] [PubMed] [Google Scholar]
- 67.Groarke J.D., Nguyen P.L., Nohria A., Ferrari R., Cheng S., Moslehi J. Cardiovascular complications of radiation therapy for thoracic malignancies: the role for non-invasive imaging for detection of cardiovascular disease. Eur Heart J. 2014;35:612–623. doi: 10.1093/eurheartj/eht114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fukada J., Shigematsu N., Takeuchi H., et al. Symptomatic pericardial effusion after chemoradiation therapy in esophageal cancer patients. Int J Radiat Oncol Biol Phys. 2013;87:487–493. doi: 10.1016/j.ijrobp.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 69.Van Putten J.W.G., Schlosser N.J.J., Vujaskovic Z., Leest A.H.D.V.D., Groen H.J.M. Superior vena cava obstruction caused by radiation induced venous fibrosis. Thorax. 2000;55:245–246. doi: 10.1136/thorax.55.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehta S.V., Koo D.J. Radiation-induced SVC syndrome. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2013-203446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Russo C., Jin Z., Elkind M.S., et al. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. 2014;16:1301–1309. doi: 10.1002/ejhf.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yancy C.W., Jessup M., Bozkurt B., et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 73.Fihn S.D., Gardin J.M., Abrams J., et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 74.Wang T.J., Larson M.G., Levy D., et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 75.Taylor C.J., Roalfe A.K., Iles R., Hobbs F.D.R. The potential role of NT-proBNP in screening for and predicting prognosis in heart failure: a survival analysis. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2013-004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirkham A.A., Virani S.A., Campbell K.L. The utility of cardiac stress testing for detection of cardiovascular disease in breast cancer survivors: a systematic review. Int J Womens Health. 2015;7:127–140. doi: 10.2147/IJWH.S68745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heidenreich P.A., Schnittger I., Strauss H.W., et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease. J Clin Oncol. 2007;25:43–49. doi: 10.1200/JCO.2006.07.0805. [DOI] [PubMed] [Google Scholar]
- 78.Libby P., Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 79.Little W.C., Constantinescu M., Applegate R.J., et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78:1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 80.Al-Lamee R., Thompson D., Dehbi H.M., et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. 2018;391:31–40. doi: 10.1016/S0140-6736(17)32714-9. [DOI] [PubMed] [Google Scholar]
- 81.Maron D.J., Hochman J.S., Reynolds H.R., et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382:1395–1407. doi: 10.1056/NEJMoa1915922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rozanski A., Gransar H., Shaw L.J., et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–1632. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Investigators S.-H., Newby D.E., Adamson P.D., et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379:924–933. doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 84.Mulrooney D.A., Nunnery S.E., Armstrong G.T., et al. Coronary artery disease detected by coronary computed tomography angiography in adult survivors of childhood Hodgkin lymphoma. Cancer. 2014;120:3536–3544. doi: 10.1002/cncr.28925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Valenti V., Oh B., Heo R., et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. J Am Coll Cardiol Img. 2015;8(8):900–909. doi: 10.1016/j.jcmg.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Silverman M.G., Blaha M.J., Krumholz H.M., et al. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2014;35:2232–2241. doi: 10.1093/eurheartj/eht508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Armstrong G.T., Plana J.C., Zhang N., et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–2884. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dixon S.B., Howell C.R., Lu L., et al. Cardiac biomarkers and association with subsequent cardiomyopathy and mortality among adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort. Cancer. 2021;127(3):458–466. doi: 10.1002/cncr.33292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Desai M.Y., Jellis C.L., Kotecha R., Johnston D.R., Griffin B.P. Radiation-associated cardiac disease: a practical approach to diagnosis and management. J Am Coll Cardiol Img. 2018;11(8):1132–1149. doi: 10.1016/j.jcmg.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 90.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:450–500. doi: 10.1016/j.jacc.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 91.Osborn L.A., Vernon S.M., Reynolds B., Timm T.C., Allen K. Screening for subclavian artery stenosis in patients who are candidates for coronary bypass surgery. Catheter Cardiovasc Interv. 2002;56:162–165. doi: 10.1002/ccd.10198. [DOI] [PubMed] [Google Scholar]
- 92.Whelan T.J., Olivotto I.A., Parulekar W.R., et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307–316. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Geenen M.M., Bakker P.J., Kremer L.C., Kastelein J.J., van Leeuwen F.E. Increased prevalence of risk factors for cardiovascular disease in long-term survivors of acute lymphoblastic leukemia and Wilms tumor treated with radiotherapy. Pediatr Blood Cancer. 2010;55:690–697. doi: 10.1002/pbc.22518. [DOI] [PubMed] [Google Scholar]
- 94.Haugnes H.S., Wethal T., Aass N., et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol. 2010;28:4649–4657. doi: 10.1200/JCO.2010.29.9362. [DOI] [PubMed] [Google Scholar]
- 95.Cohen E.P., Robbins M.E.C. Radiation nephropathy. Semin Nephrol. 2003;23:486–499. doi: 10.1016/s0270-9295(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 96.Lee S.J., Jeon G.S. Kissing stents for radiation-induced bilateral common iliac vein stenosis. Interact Cardiovasc Thorac Surg. 2018;27:617–618. doi: 10.1093/icvts/ivy146. [DOI] [PubMed] [Google Scholar]
- 97.Milutinovic J., Darcy M., Thompson K.A. Radiation-induced renovascular hypertension successfully treated with transluminal angioplasty: case report. Cardiovasc Intervent Radiol. 1990;13:29–31. doi: 10.1007/BF02576934. [DOI] [PubMed] [Google Scholar]
- 98.Saka B., Bilge A.K., Umman B., et al. Bilateral renal artery stenosis after abdominal radiotherapy for Hodgkin's disease. Int J Clin Pract. 2003;57:247–248. [PubMed] [Google Scholar]
- 99.Moutardier V., Christophe M., Lelong B., Houvenaeghel G., Delpero J.R. Iliac atherosclerotic occlusive disease complicating radiation therapy for cervix cancer: a case series. Gynecol Oncol. 2002;84:456–459. doi: 10.1006/gyno.2001.6525. [DOI] [PubMed] [Google Scholar]
- 100.Gerhard-Herman M.D., Gornik H.L., Barrett C., et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:1465–1508. doi: 10.1016/j.jacc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 101.Armstrong G.T., Oeffinger K.C., Chen Y., et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scott J.M., Li N., Liu Q., et al. Association of exercise with mortality in adult survivors of childhood cancer. JAMA Oncol. 2018;4:1352–1358. doi: 10.1001/jamaoncol.2018.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gilchrist S.C., Barac A., Ades P.A., et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019;139:e997–e1012. doi: 10.1161/CIR.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Addison D., Spahillari A., Rokicki A., et al. The pooled cohort risk equation markedly underestimates the risk of atherosclerotic cardiovascular events after radiation therapy. J Am Coll Cardiol. 2018;71(suppl 11):A1862. [Google Scholar]
- 105.Wang H., Wei J., Zheng Q., et al. Radiation-induced heart disease: a review of classification, mechanism and prevention. Int J Biol Sci. 2019;15:2128–2138. doi: 10.7150/ijbs.35460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pereira E.B., Gemignani T., Sposito A.C., Matos-Souza J.R., Nadruz W., Jr. Low-density lipoprotein cholesterol and radiotherapy-induced carotid atherosclerosis in subjects with head and neck cancer. Radiat Oncol. 2014;9:134. doi: 10.1186/1748-717X-9-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boulet J., Peña J., Hulten E.A., et al. Statin use and risk of vascular events among cancer patients after radiotherapy to the thorax, head, and neck. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.117.005996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Addison D., Lawler P.R., Emami H., et al. Incidental statin use and the risk of stroke or transient ischemic attack after radiotherapy for head and neck cancer. J Stroke. 2018;20:71. doi: 10.5853/jos.2017.01802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seicean S., Seicean A., Plana J.C., Budd G.T., Marwick T.H. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol. 2012;60:2384–2390. doi: 10.1016/j.jacc.2012.07.067. [DOI] [PubMed] [Google Scholar]
- 110.Marlatt K.L., Steinberger J., Rudser K.D., et al. The effect of atorvastatin on vascular function and structure in young adult survivors of childhood cancer: a randomized, placebo-controlled pilot clinical trial. J Adolesc Young Adult Oncol. 2019;8:442–450. doi: 10.1089/jayao.2017.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dolmaci O.B., Farag E.S., Boekholdt S.M., van Boven W.J.P., Kaya A. Outcomes of cardiac surgery after mediastinal radiation therapy: a single-center experience. J Card Surg. 2020;35:612–619. doi: 10.1111/jocs.14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fokkema M., den Hartog A.G., Bots M.L., van der Tweel I., Moll F.L., de Borst G.J. Stenting versus surgery in patients with carotid stenosis after previous cervical radiation therapy: systematic review and meta-analysis. Stroke. 2012;43:793–801. doi: 10.1161/STROKEAHA.111.633743. [DOI] [PubMed] [Google Scholar]
- 113.Donnellan E., Masri A., Johnston D.R., et al. Long-term outcomes of patients with mediastinal radiation-associated severe aortic stenosis and subsequent surgical aortic valve replacement: a matched cohort study. J Am Heart Assoc. 2017;6(5) doi: 10.1161/JAHA.116.005396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang D., Guo W., Al-Hijji M.A., et al. Outcomes of patients with severe symptomatic aortic valve stenosis after chest radiation: transcatheter versus surgical aortic valve replacement. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ejiofor J.I., Ramirez-Del Val F., Nohria A., et al. The risk of reoperative cardiac surgery in radiation-induced valvular disease. J Thorac Cardiovasc Surg. 2017;154:1883–1895. doi: 10.1016/j.jtcvs.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 116.Liang J.J., Sio T.T., Slusser J.P., et al. Outcomes after percutaneous coronary intervention with stents in patients treated with thoracic external beam radiation for cancer. J Am Coll Cardiol Intv. 2014;7(12):1412–1420. doi: 10.1016/j.jcin.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 117.Busch C., Penov K., Amorim P.A., et al. Risk factors for mortality after pericardiectomy for chronic constrictive pericarditis in a large single-centre cohort. Eur J Cardiothorac Surg. 2015;48:e110–e116. doi: 10.1093/ejcts/ezv322. [DOI] [PubMed] [Google Scholar]
- 118.Vistarini N., Chen C., Mazine A., et al. Pericardiectomy for constrictive pericarditis: 20 years of experience at the Montreal Heart Institute. Ann Thorac Surg. 2015;100:107–113. doi: 10.1016/j.athoracsur.2015.02.054. [DOI] [PubMed] [Google Scholar]
- 119.CardioSmart. American College of Cardiology. Available at https://www.cardiosmart.org/topics/cancer-treatment-and-your-heart/radiation-and-heart-disease. Accessed January 2021.