Abstract
Radiation therapy is an important component of cancer therapy for many malignancies. With improvements in cardiac-sparing techniques, radiation-induced cardiac dysfunction has decreased but remains a continued concern. In this review, we provide an overview of the evolution of radiotherapy techniques in thoracic cancers and associated reductions in cardiac risk. We also highlight data demonstrating that in some cases radiation doses to specific cardiac substructures correlate with cardiac toxicities and/or survival beyond mean heart dose alone. Advanced cardiac imaging, cardiovascular risk assessment, and potentially even biomarkers can help guide post-radiotherapy patient care. In addition, treatment of ventricular arrhythmias with the use of ablative radiotherapy may inform knowledge of radiation-induced cardiac dysfunction. Future efforts should explore further personalization of radiotherapy to minimize cardiac dysfunction by coupling knowledge derived from enhanced dosimetry to cardiac substructures, post-radiation regional dysfunction seen on advanced cardiac imaging, and more complete cardiac toxicity data.
Key Words: breast cancer, cancer survivorship, childhood cancer, esophageal cancer, imaging, lung cancer, lymphoma, radiation physics
Abbreviations and Acronyms: CAC, coronary artery calcium; CAD, coronary artery disease; CMRI, cardiac magnetic resonance imaging; CT, computed tomography; HL, Hodgkin lymphoma; LAD, left anterior descending artery; LV, left ventricular; MHD, mean heart dose; NSCLC, non–small cell lung cancer; RICD, radiation-induced cardiovascular disease; RT, radiation therapy; SBRT, stereotactic body radiation therapy
Central Illustration
Highlights
-
•
Despite improvements in radiation techniques, radiation-induced cardiac dysfunction can occur in those with thoracic cancers.
-
•
Newer studies have examined associations between cardiac substructure doses and cardiac outcomes and/or survival.
-
•
Advanced cardiac imaging allows assessment of regional dysfunction after cardiac radiation exposure.
-
•
Enhanced dose tracking, imaging, and comprehensive clinical data hold promise for personalized approaches to minimize cardiotoxicity.
Radiation therapy (RT) is a critical component of treatment for more than one-half of adult patients with cancer (1). Thoracic malignancies, including lung, esophageal, gastric, and breast cancers, as well as childhood cancers and lymphomas, often include RT in definitive curative treatment regimens. However, cardiac radiation exposure can lead to long-term adverse outcomes, including cardiac death. Although the heart was originally considered a relatively radiation resistant organ, data examining the association of cardiac radiation dose and adverse outcomes indicate that there can be a ∼4%-16% relative risk of heart disease and major cardiac events per Gray (Gy) of mean heart dose (MHD) of radiation (2, 3, 4), with no safe dose identified. Thus, even though advances in RT techniques have decreased incidental cardiac doses, late cardiac toxicity can still develop. Many studies in patients with breast cancer or lymphoma have demonstrated that radiation-induced cardiac disease (RICD) most often occurs a number of years after RT. However, data showing correlations between higher heart radiation exposure and cardiac events and survival in patients with lung or esophageal cancer have led to a reexamination of the severe cardiac effects that can occur within the first 2 years after RT (5, 6, 7, 8). In addition, an increasing number of studies have examined correlations between radiation doses to specific cardiac regions and cardiac morbidity and mortality (Central Illustration) (3,5,9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29). Although uniform standardized cardiovascular surveillance recommendations for cancer survivors with cardiac radiation exposure are lacking, modern cardiac imaging allows more sensitive determination of subclinical cardiac dysfunction in the heart, including individual cardiac regions. Together with data on cardiac substructure sensitivities, the knowledge gained from modern cardiac imaging techniques has the potential to ultimately guide RT treatments and post-RT surveillance. Distinct from RT for malignancies, over the past few years there have been increasing cohorts of noncancer patients receiving high-dose RT to the heart for treatment of refractory ventricular tachycardia (30). Data from the effects of such high-dose RT on the heart may also inform mechanisms and biomarkers of RICD in patients with cancer. Although RT exposure to areas outside of the thorax can also cause cardiovascular dysfunction (31), potentially through inflammation or other mechanisms, here we focus solely on thoracic RT-induced cardiotoxicity. In this review, we summarize clinical data on RICD in thoracic malignancies, correlations between doses in different cardiac regions and cardiac outcomes, and the utility of biomarkers and cardiac imaging in post-RT surveillance. We also discuss future directions toward more refined and personalized RT for patients, which can result in reduced RICD.
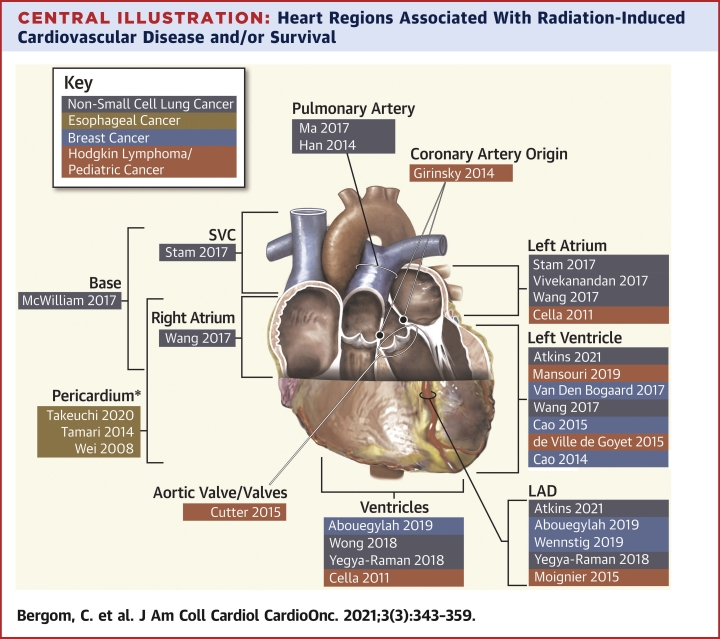
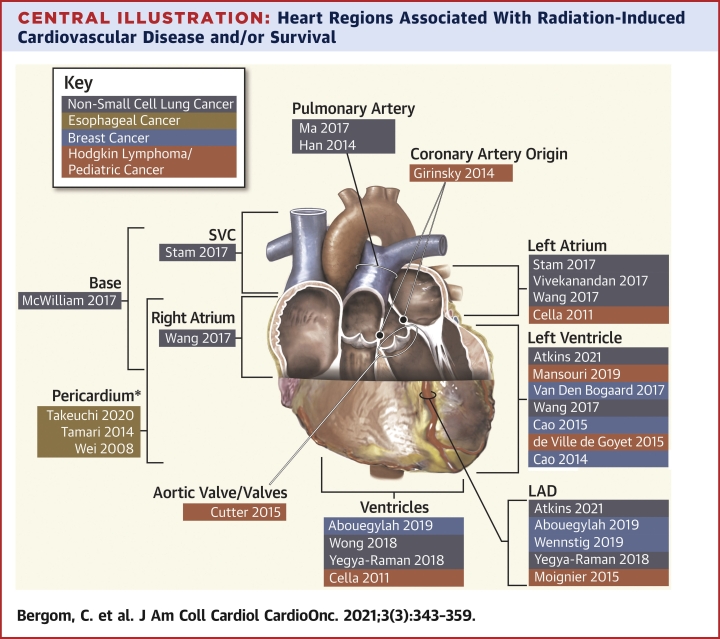
Central Illustration.
Heart Regions Associated With Radiation-Induced Cardiovascular Disease and/or Survival
First author and year of publication are listed. Highlighted colors indicate cancer type (see Key). Studies demonstrating associations between total heart doses and outcomes are not included. ∗Pericardium, not including the heart. LAD = left anterior descending artery; SVC = superior vena cava.
RICD in Thoracic Malignancies
Hodgkin lymphoma RT dose and field evolution over time
In the 1960s, pioneering work by Kaplan et al from Stanford University further advanced radiation oncology through the development of the linear accelerator, definition of RT fields and doses for curative treatment for early Hodgkin lymphoma (HL), and promotion of early randomized U.S. clinical trials (32). Subsequent studies reported on the relationship between mediastinal RT and the risk of fatal cardiovascular complications manifesting 5-10 years after treatment (33,34). Additional manifestations of RICD in HL survivors can include coronary artery disease (CAD), pericarditis, valvular dysfunction, arrhythmia, and heart failure, with risks persisting for up to 40 years (35,36). However, much of the data on RICD following RT for HL are based on patients treated decades ago with full-mantle fields to the standard curative RT dose of ≥40-44 Gy, resulting in high radiation exposure to the heart (Figure 1).
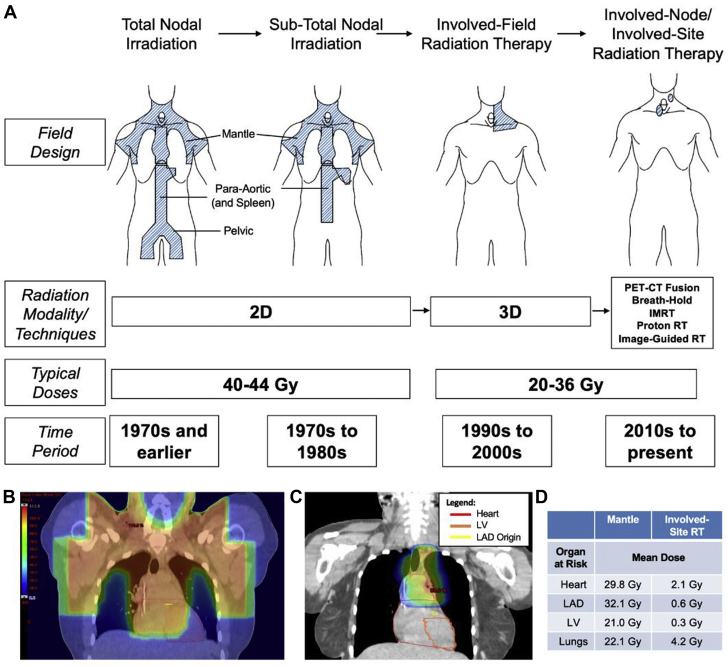
Figure 1.
Evolution of RT in HL
(A) Schematic representation of field design, radiation therapy (RT) doses, and radiation techniques for adult Hodgkin lymphoma (HL) as they have evolved over time. (B, C) RT plan comparison of an HL patient treated with involved-site RT to the mediastinum including colorwash dose distribution. Recreated historical mantle field (B), anterior-posterior technique, treated to a dose of 40 Gy, versus involved-site RT using IMRT (C), with deep-inspiration breath hold (note expansion of lungs and elongation of mediastinal structures, displacing lungs and heart away from target volume) with arms down on inclined board (further displacement of heart inferiorly), treated to 30 Gy with a 6-Gy boost to a level 5 node. (D) Dosimetric comparison of plans in B and C. IMRT = intensity-modulated radiation therapy; LAD = left anterior descending artery; LV = left ventricle; PET-CT = positron emission tomography–computed tomography.
The relationship between radiation dose and the risk of heart disease was first demonstrated by Hancock et al, who found significantly increased cardiac mortality after HL in patients who received mediastinal RT doses >30 Gy (34). Other studies also have observed an association between mediastinal RT dose and risk of cardiac disease among childhood HL survivors (37, 38, 39). In those studies, the prescribed RT dose was used to estimate cardiac doses. More modern analyses use volume-based analysis of cardiac radiation doses to calculate RICD risks. A significant linear relationship was found between the estimated MHD and the risk of cardiovascular disease, with a 1.5%-7% increase in risk per 1 Gy MHD (40,41). A number of studies have found significant relationships between mean doses to the heart/cardiac substructures and the long-term cumulative incidence of cardiac events, including coronary heart disease, heart failure, and valvular disease (Central Illustration) (14,15,19,42).
As data on the late cardiac effects of HL accumulated, simultaneous efforts have improved cancer outcomes. More effective chemotherapy, advances in imaging, improved staging and response assessment, and patterns of relapse analyses have allowed the use of more limited RT fields and volumes, with resulting lower incidental cardiac radiation exposure. RT treatment fields for HL have evolved from extended-field to involved-field and then to involved-node/involved-site RT (Figure 1) (43,44). In conjunction with chemotherapy, RT doses have decreased from 40-44 Gy to as low as 20 Gy in selected patients (Figure 1). In addition, there have been significant technologic advancements in RT treatment planning and delivery. The prior 2-dimensional–based RT has been replaced by much more conformal approaches, such as 3-dimensional (3D) conformal RT, intensity-modulated RT, and proton beam therapy (45). The adoption of daily image guidance and respiratory motion management further improves conformal target coverage and normal-tissue sparing (45) (Table 1). A typical MHD from a mantle field is estimated to be 25-30 Gy, but with modern fields, doses, and techniques, an MHD of <10 Gy can often be achieved when targeting mediastinal disease (46) (Figure 1).
Table 1.
Cardiac-Sparing Radiation Therapy Techniques and Modalities
| Cardiac-Sparing Technology/Technique | Description | Cancers Where These Techniques May Be Used |
||||
|---|---|---|---|---|---|---|
| Lymphoma | Breast Cancer | Esophageal Cancer | Lung Cancer | Pediatric Cancer | ||
| Modality | ||||||
| 3-dimensional conformal radiation therapy | Photon beams designed using forward planning; minimize low dose bath; less conformality of high dose; can use a “heart block” to eliminate heart from the field using beam edge | ✓ | ✓ | ✓ | ✓ | ✓ |
| Intensity-modulated radiation therapy/volumetric-modulated arc therapy | Inverse planning using multiple photon beams or arcs to achieve conformality of high dose at the cost of increased low-dose exposure | ✓ | ✓ | ✓ | ✓ | ✓ |
| Proton therapy | Particle beam with Bragg peak phenomenon resulting in elimination of exit dose; enables both minimization of low dose and conformality of high dose | ✓ | ✓ | ✓ | ✓ | ✓ |
| Cardiac Displacement and motion management techniques | ||||||
| Deep-inspiration breath hold | Using a device to measure depth of inspiration, patient is guided to hold breath for 10-20 seconds to displace thoracic anatomy from the heart | ✓ | ✓ | |||
| Gating | Using an external abdominal device to gauge respiration, the beam is delivered during a set phase of the respiratory cycle when the heart is farthest from the target, and the beam shuts off when the patient exits this portion of the respiratory cycle | ✓ | ✓ | ✓ | ✓ | |
| Prone positioning | Displaces the target anatomy from the heart in many patients | ✓ | ||||
| Image-guided radiation therapy | ||||||
| Cone-beam computed tomography (CT) | Low-dose CT obtained in treatment vault permits alignment based on visualization of internal anatomy | ✓ | ✓ | ✓ | ✓ | ✓ |
| Surface alignment | Uses surface anatomy for patient positioning; cannot visualize internal anatomy; no radiation exposure for this alignment technique | ✓ | ||||
A number of dosimetric studies demonstrate significant reduction in cardiac RT doses with the use of involved-node/involved-site RT compared with historical treatment fields such as mantle or involved-field RT (Figure 1) (46,47). In a study evaluating HL treated with involved-node RT to 30-36 Gy, the authors constructed simulated mantle fields to a dose of 36 Gy in the same patients. Comparing the mantle RT and involved-node RT, mean doses to the heart were 27.5 Gy versus 7.7 Gy, respectively, and mean doses to the left anterior descending artery (LAD) were 23.1 Gy versus 8.9 Gy (48). Using estimates, the 25-year absolute excess risk of cardiovascular disease based on the estimated MHD demonstrated a significant risk reduction with the use of involved-node versus mantle RT (1.4% vs 9.1%; P < 0.001) (48). Deep-inspiration breath hold can further reduce heart dose by elongation of mediastinal structures and displacement of the heart outside of the RT beams (Figure 1, Table 1).
Most of the studies assessing the relationship between radiation dose and risk of RICD used reconstructed MHD owing to the challenges of retrospectively estimating doses to individual cardiac substructures. However, in the context of involved-site RT, the shape and distribution of the clinical target volume and its proximity to critical cardiac substructures can vary significantly from case to case. With the use of highly conformal techniques with a steep dose gradient, it is important to track doses to critical cardiac substructures including the coronary arteries, cardiac chambers, and valves to allow better individual risk assessment, as well as to further establish dose-volume effects of cardiac substructures on subsequent RICD to guide future treatment planning (49), as discussed below.
Pediatric malignancies and RICD
Many patients with HL are children or young adults. However, treatment of other pediatric malignancies can also result in significant cardiac radiation exposure, often concurrently administered with potentially cardiotoxic systemic treatment. In fact, cardiovascular events are the leading cause of noncancer death in childhood cancer survivors. Mulrooney et al (38) evaluated >14,000 children treated from 1970 to 1986 in the Childhood Cancer Survivor Study with the use of a questionnaire to assess for cardiac disease, with comparison to a sibling cohort. At 30 years after diagnosis, the incidence of heart failure, valvular abnormalities, and pericardial disease was 3%-4% each, with the cumulative incidence increasing over time and reaching 5-6 times that of the sibling cohort. Heart failure was associated with a cardiac dose of >15 Gy, and myocardial infarction was associated with a cardiac dose of >35 Gy (38). Updated data on >23,000 children diagnosed through 1999 demonstrated patients treated in the 1980s-90s had a lower risk of CAD than patients treated in the 1970s, likely owing to modifications in therapy intended to decrease cardiac toxicity, including decreased RT use and improved techniques. However, cardiac disease still occurred in the majority of patients, with 80% affected by a single cardiac condition and 19% by two or more conditions (50). Reconstructing the estimated cardiac radiation doses of children in the Childhood Cancer Survivor Study allowed evaluation of cardiac dose-volume relationships with outcomes. At 30 years after diagnosis, the cumulative incidence of cardiac disease was 4.8% (51). Both cardiac volume and radiation dose are important considerations in estimating the risk of cardiac toxicity. Doses of 5-19.9 Gy to ≥50% of the heart and doses ≥20 Gy to <30% of the heart were associated with an increased risk of cardiac disease (relative risks: 1.6 and 2.4, respectively). This dose-response relationship was amplified in those ≤13 years of age who received anthracyclines (51).
A French study also identified a dose-response relationship for left ventricle (LV) dose ≥30 Gy and heart failure in pediatric cancer survivors diagnosed from 1945 to 2000 (24). The cumulative incidence of heart failure was 2.5% at 30 years after diagnosis and 5.7% at 50 years, with 10% of those affected by cardiac disease requiring heart transplantation. The risk ratio increased from 3.6 to 24.6 as the volume of the LV receiving 30 Gy increased from <10% to ≥50%, with risk further increased with anthracycline use (24). In a study of 1,362 pediatric patients with cancer, the 30-year marginal cumulative incidences of a grade ≥3 cardiac event were 17%, 8% and 4.5% for those receiving anthracycline and cardiac RT, anthracycline without cardiac RT, and cardiac RT alone, respectively (52). In a study of ≥36,000 patients across Europe, patients who received both RT and chemotherapy compared with receipt of either or none had the highest rates and earliest onset of ischemic heart disease, with 1 in 18 survivors developing severe or fatal ischemia (53).
The American Heart Association has published an extensive scientific statement on the pathophysiology, surveillance, treatment, and prevention of cardiovascular toxicity for people treated for malignancy at a young age (54). Other groups also have published guidelines for surveillance of childhood cancer survivors for late cardiotoxicity, specifically after receipt of anthracycline chemotherapy, mediastinal RT, and neck RT (55,56).
RICD in breast cancer
In the early decades of RT, field design was based on bony anatomic borders identified on X-rays. Measurements of target coverage and doses to adjacent organs were not possible in that 2-dimensional RT era. Data from those earlier radiation treatments in patients with breast cancer demonstrate significant efficacy of RT in disease control, but at a cost of heart and lung injury (57). The laterality of the breast cancer is also important, because the heart sits in closer proximity to the left breast. Thus, the risk of RICD is higher in patients with left-side breast cancer (58). For breast cancer RT, there was a slow adoption of 3D-contour–based planning. The earliest uses of contouring with the use of 3D-conformal RT in phase 3 cooperative group trials were the NSABP-B39/RTOG 0413 study partial breast irradiation arm (59) and RTOG 1005 (60), which opened to accrual in 2005 and 2011, respectively. A number of breast cancer contouring atlases have been published, with more recent atlases delineating cardiac substructures as well (61), allowing determination of associations between substructure doses and cardiac outcomes. Cardiac radiation exposure has decreased over time owing to improved techniques, although a number of factors influence the cardiac-sparing techniques used for patients with breast cancer (Figure 2, Table 1) (62).
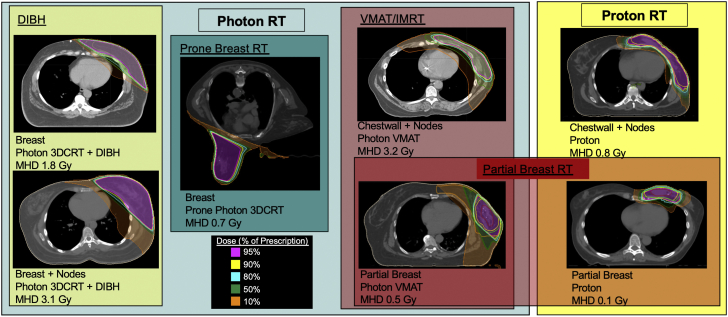
Figure 2.
Example Breast Plans Using Different RT Techniques and Modalities
Example plans using cardiac-sparing techniques and different target volumes are shown. The selection of techniques and modalities is complex, involving availability of the technology, patient anatomy, and target volumes. All plans shown prescribed 50 Gy in 2-Gy fractions, except partial breast RT was prescribed 30 Gy in 5 fractions. 3DCRT = 3-dimensional conformal radiation therapy; DIBH = deep-inspiration breath hold; IMRT = intensity-modulated radiation therapy; MHD = mean heart dose; RT = radiation therapy; VMAT = volumetric-modulated arc therapy.
MHD is the typical metric used to evaluate cardiac exposure in present-day clinical practice. Current cooperative group trials use a threshold of 3-5 Gy MHD to consider a plan acceptable (eg, NSABP-B51, Alliance-A221505). Data in breast cancer demonstrate a linear no-threshold model for cardiac radiation, with a relative risk of ischemic heart disease increased by 4%-16% per Gy of MHD (2, 3, 4). Jacobse et al (2) also demonstrated a linear relationship between MHD and subsequent myocardial infarction (63). However, the absolute increase in risk of major cardiac events from an MHD of 5 Gy is ∼1%, although this varies by age and cardiac risk factors. In most 3D-conformal RT plans for breast cancer, only the anterior aspect of the heart is exposed to radiation, and a large portion of the heart does not receive radiation (Figure 2). This uneven distribution of radiation dose to the heart begets the question of the relevance of the MHD metric for breast cancer. Duane et al, using 10 randomly selected computed tomography (CT) planning scans, evaluated doses to cardiac substructures in Scandinavian women with breast cancer treated from 1958 to 2001. Standard breast RT techniques were used to recreate dose on the CT scans, and the heart and cardiac substructures were contoured. A wide range of doses to segments within the LV and coronary arteries was seen, as well as a high correlation between doses to different substructures, such as the LV and the LAD, and dose to the whole heart (64). Emerging data have found associations between LAD and LV doses and cardiac outcomes in breast cancer (Central Illustration) (see discussion below) (3,20,21,25,29). These data have led to suggestions for dose-volume limits for the LV and LAD in breast cancer RT (65).
In modern radiotherapy, a range of options exist in addition to 3D-conformal RT to decrease heart dose for breast and other cancers (49,62,66,67) (Figure 2). These include prone positioning, respiratory management including deep-inspiration breath hold and gating, intensity-modulated RT (IMRT)/volumetric-modulated arc therapy (VMAT), and proton therapy (described in Table 1). Beam arrangements and beam energies are optimized for individual anatomy. In many cases, 3D techniques are optimal, but in others, IMRT/VMAT or proton therapy permits increased conformality of high doses (Figure 2). Current limitations to proton therapy include higher cost and accessibility. For selected women with early-stage breast cancer, the target volume can be decreased from the whole breast to partial breast, targeting the lumpectomy cavity with a margin of surrounding tissue to limit the exposure of intrathoracic organs. Partial breast irradiation can be delivered in several ways, including brachytherapy and external beam photon or proton therapy (59) (Figure 2). Ultimately, the choice of RT technique, treatment volumes, and modalities used must be personalized for each patient based on availability of technology, a patient’s anatomy, and cancer-specific variables.
Lung and esophageal cancer: RICD in the setting of higher cardiac radiation doses
Over the decades, data regarding the incidence of RICD after RT for intrathoracic malignancies have steadily increased following improvements in overall survival with novel therapeutics and treatment approaches. For esophageal cancer, some of the earliest RICD data came from Japan (68,69), where the incidence of esophageal cancer is among the highest worldwide. In more than 70 patients with esophageal squamous cell carcinoma treated with definitive chemoradiation therapy from 2002 to 2005, age ≥75 years was significantly associated with a higher 2-year cumulative incidence of grade ≥3 cardiopulmonary toxicities (∼30% for age ≥75 years vs 3% for age <75 years) (68). Because squamous cell esophageal cancers are predominately in the upper and/or middle esophagus, treatment fields would have included large mostly anterior-posterior mediastinal fields for lymph node coverage for the first 40 Gy, followed by a 20 Gy boost to the primary tumor with the use of oblique fields (68). Chemotherapy typically consisted of concurrent cisplatin and 5-fluorouracil, which on meta-analysis has a 1%-4% incidence of symptomatic cardiotoxicity (70). More recently, a systematic review examining the relationship between esophageal cancer treatment and RICD found the crude incidence of symptomatic cardiac toxicity, including pericardial effusions, ischemic heart disease, and heart failure, to be 10% (7). The majority of these events occurred within 2 years, and the most common finding was pericardial effusions. Although this timeline to symptomatic cardiac events appears shorter than that in many breast cancer or HL patients, esophageal and lung cancers often occur in patients with other confounding variables, including concurrent chemotherapy and significant smoking histories, and patients typically receive higher overall cardiac radiation exposure, which can influence the rates of cardiac events (6).
A number of esophageal cancer studies have found associations between whole-heart radiation doses and cardiac events and/or survival (69). In addition, studies have found associations between doses to the pericardial rim surrounding the heart predicted the risk of pericardial effusions, the most common manifestation of RICD in those patients (Central Illustration) (11, 12, 13). Similarly to anatomic concerns in esophageal cancer, RT fields for locally advanced non–small cell lung cancer (NSCLC) can span a large proportion of the mediastinum when covering NSCLC with ipsilateral mediastinal involvement and can lead to high-dose gradients in the heart. Correlations between cardiac doses in NSCLC and overall survival have been found in a number of studies (69).
For both esophageal and lung cancers, it can be difficult to balance limiting of both lung and cardiac doses. Typically, lung doses are prioritized because of the risk of severe radiation pneumonitis. For example, in the RTOG 0617 NSCLC study, the heart was the last-priority organ at risk, behind the spinal cord, lungs, esophagus, and brachial plexus, with acceptable criteria including 40 Gy to <100% of the heart (71). RTOG 0617 was a phase III randomized trial to evaluate the potential role of dose escalation for locally advanced NSCLC (60 Gy vs 74 Gy) (71). No benefit with RT dose escalation was found, and decreased survival occurred in the high-dose RT arm. On dosimetric analysis, the volume of heart receiving ≥40 Gy (V40) was significantly associated with worse overall survival (67). A recent combined analysis of prospective multicenter NSCLC trials showed a 2-year cumulative incidence of grade ≥3 cardiac events of 11%, with MHD independently associated with cardiac events and grade ≥3 cardiac events associated with decreased survival (6). Another pooled analysis of patients enrolled in locally advanced NSCLC trials demonstrated that 23% experienced a symptomatic cardiac event, with the rate of symptomatic cardiac events increasing with increasing MHD (72). However, MHD does not always correlate with outcomes and survival in NSCLC studies (69).
For early-stage lung cancer, stereotactic body RT (SBRT) has emerged as the gold standard for inoperable lung cancer. SBRT is characterized by high-dose per fraction treatments with very steep dose fall-off. While the treatment volumes are significantly smaller than locally advanced NSCLC (and without concurrent chemotherapy), the point doses to the heart and its substructures can be significantly higher than those seen with conventionally fractionated RT. When tumor targets are near cardiac substructures for SBRT, serious toxicities have been described, although there is not always a correlation between total heart doses and outcomes (73). However, others have reported an association between overall survival and maximum dose to the bilateral ventricles (27) or doses to the left atrium (10) or superior vena cava (10). Understanding the association between cardiac dose, cardiac toxicity, and overall survival has proven to be relatively more challenging than in locally advanced NSCLC, because patients receiving SBRT are often nonoperative candidates owing to cardiopulmonary comorbidities.
Data on the importance of location of radiation deposition in the heart
Linear relationships between MHD and cardiac outcomes have been identified in patients treated with RT for breast cancer (2, 3, 4), HL (40,41), and childhood cancers (38,51,74). However, for patients with lung cancers, the association between MHD and outcomes has not been as robust (69). This could be due, in part, to the variability in dose distributions across the heart for lung cancers, which are in contrast to the relatively uniform areas of the heart that receive radiation in tangential breast cancer RT or in mediastinal nodal treatments previously given for many lymphomas. Thus, correlations between heart doses and outcomes may be more evident when regions of the heart receiving RT are examined versus whole-heart doses. In addition, with decreasing cardiac doses in breast cancer over time, doses to certain cardiac structures may become more critical to predict the risk of cardiac outcomes.
Correlations between cardiac substructure doses and outcomes have been examined in a number of recent studies, with many associations identified (Central Illustration) (3,5,9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29). Studies have demonstrated associations between LAD radiation doses and cardiac and/or survival outcomes (20, 21, 22,28). LAD doses correlated with >10% decreases in LV ejection fraction (20) and coronary artery stenosis in patients with breast cancer (21) and coronary artery segment dose correlated with coronary artery stenosis in patients with HL (23). In patients with lung cancer, LAD doses correlated with acute coronary syndrome and heart failure (22) as well as major acute coronary events, especially in patients without CAD (28). Wang et al also reported that LV doses, as well as whole-heart doses, correlated with symptomatic cardiac events in patients with NSCLC (72). In addition, breast cancer studies have found that LV doses correlated with coronary toxicity (3,25). In studies of patients with childhood cancers, LV doses correlated with heart failure (24) and increased left atrial volumes on cardiac magnetic resonance imaging (CMRI) (26). Other studies have found that doses to the ventricles correlate with outcomes such as decreased LV ejection fraction, valvular defects, acute coronary syndrome, and/or survival (19,20,22,27). In lung cancer, doses to the base of heart and its structures (atria and proximal great vessels) have repeatedly been found to correlate with cardiac outcomes and survival. McWilliam et al used an unbiased approach to identify the base of heart as a region correlating with worse survival in >1,100 patients with lung cancer (9). Other studies have identified associations between cardiac toxicity/survival in patients with lung cancer and doses to the proximal pulmonary artery (16,17), proximal superior vena cava (10), and left atrium (5,10,18,19). In patients with HL, the coronary artery origin (14) and valvular doses (15) correlated with development of coronary stenosis and valvular heart disease. Taken together, studies such as those shown in the Central Illustration (3,5,9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29) are beginning to unveil specific areas of the heart for which radiation injury correlates directly with cardiac dysfunction and/or survival. Additional research and prospective studies will further refine our knowledge and ultimate allow better personalization of RT planning to minimize doses to the most critical cardiac structures.
Cardiac Risk Stratification, Screening, and Surveillance
Impact of cardiovascular risk factors
In addition to the risk caused by the radiation itself, a patient’s cumulative hazard of RICD is also directly related to underlying cardiovascular risk factors. Consistent with studies in the general population, both traditional risk factors (smoking, hypertension, diabetes mellitus) and imaging risk factors (coronary artery calcifications) have been linked to future cardiovascular events in survivors receiving cardiac radiation across multiple cancer types. In patients with breast cancer undergoing RT, the presence of cardiovascular risk factors doubles the risk of a major coronary event, and baseline ischemic heart disease carries >6-fold higher risk of a future event (2). Similarly, at a median follow-up of 18 years in 4,414 patients with breast cancer treated with and without RT, the combination of smoking and RT tripled the risk of myocardial infarction during follow-up (hazard ratio: 3.04; 95% confidence interval [CI]: 2.0-4.6; P = 0.039 for more than an additive effect) compared with nonsmokers who did not receive RT (75). Cholesterol levels also have been linked to development of coronary atherosclerosis in 43 irradiated Hodgkin lymphoma survivors (β = 308; 95% CI: 213-403) (76). In a cohort of 701 patients undergoing RT for locally advanced NSCLC, baseline coronary heart disease or the equivalent (peripheral vascular disease, stroke, or significant coronary calcifications) had a 25-fold increase in the hazard ratio of major adverse cardiovascular events, with hypertension carrying a nearly 3-fold increase on multivariable analysis (28). Even risk factors that develop during follow-up after RT, especially hypertension, have been found to add a synergistic and multiplicative increase in the risk for major adverse cardiovascular events in pediatric survivors of cancer (77).
Assessing for coronary artery calcifications can help to further refine a patient’s risk for future cardiovascular events after RT. Coronary artery calcium (CAC) on noncontrast CT is a direct measure of a patient’s atherosclerotic burden and has been shown to be the best predictor of future cardiovascular events in the general population (78). Significant coronary calcifications before RT have been independently associated with cardiac events after breast RT as well, even after adjustment for the MHD (79). CAC noted on a patient’s radiation planning CT or cancer surveillance or staging examinations correlate with more formal CAC assessments, although false negatives can exist owing to slice thickness and lack of gating (80).
Imaging modalities for screening for and diagnosis of cardiovascular toxicity
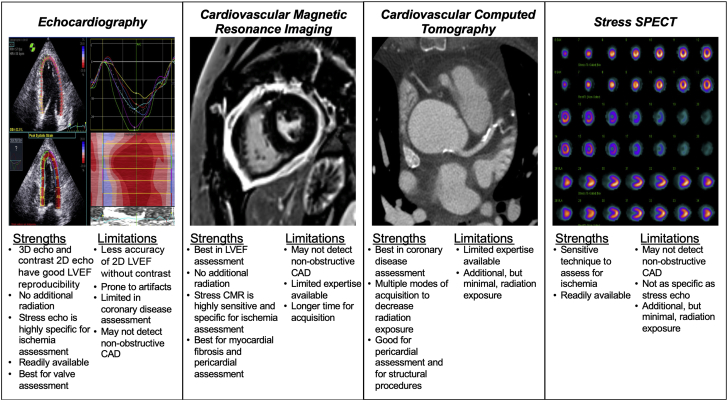
In survivors of mediastinal RT, several imaging tools have been used to screen for and diagnose cardiovascular toxicity. Echocardiography, cardiac magnetic resonance imaging, cardiovascular CT, and functional stress testing all have strengths and weaknesses in their sensitivity, specificity, and overall ability to detect and quantify cardiac damage (Figure 3) (81). Although clinical trials are lacking to guide screening intervals and recommendations for particular imaging modalities, a strong understanding of the relative benefits of each modality and research findings to date can help guide clinicians and future research in this area. In addition, expert consensus guidelines exist to help guide the clinician in screening intervals (56,81, 82, 83, 84, 85) (Table 2). The goal of screening is to detect heart injury before clinical symptoms develop, especially if preventive medicine can be used.
Figure 3.
Comparison of Cardiovascular Imaging Modalities for Assessment of Radiation-Induced Cardiovascular Disease
Major strengths and limitations of 4 major cardiovascular imaging modalities are shown. CAD = coronary artery disease; CMR = cardiac magnetic resonance imaging; echo = echocardiography; LVEF = left ventricular ejection fraction; SPECT = single-photon emission computed tomography.
Table 2.
Imaging Screening Guidelines and Consensus Recommendations by Major Societies Following Chest Radiation Therapy
| Organization(s) (Ref. #) | Year | Significant Risk Factors | Cardiac Structure and Function Screening | Coronary Ischemia Screening |
|---|---|---|---|---|
| European Association of Cardiovascular Imaging and American Society of Echocardiography (81) | 2013 | Anterior or left chest RT with ≥1 risk factor:
|
TTE 5 y after RT in patients at high risk, TTE 10 y after RT in patients not at high risk, TTE every 5 y thereafter | Stress testing 5 y after RT in patients at high risk, and every 5 y thereafter in patients without previous inducible ischemia |
| International Late Effects of Childhood Cancer Guideline Harmonization Group (56) | 2015 | Surveillance recommended in those with:
|
TTE no later than 2 y after completion of therapy in high-risk survivors, again at 5 y, and every 5 y thereafter | N/A |
| American Society of Clinical Oncology (82) | 2017 | High-dose RT (>30 Gy) where the heart is in the treatment field Lower-dose anthracycline (eg, 250 mg/m2 doxorubicin or 600 mg/m2 epirubicin) in combination with lower-dose RT (<30 Gy) where the heart is in the treatment field |
TTE 6-12 months after completion of therapy in patients at increased risk, no specific screening interval recommended thereafter | N/A |
| Children’s Oncology Group (85) | 2018 | Increased risk:
<5 y old at treatment, anteriorly weighted radiation field, lack of subcarinal shielding, longer time since treatment |
Annual physical and electrocardiography in patients with ≥15 Gy Echocardiography every 5 y:
|
Cardiology consultation 5-10 y after radiation in patients at highest risk |
| European Society of Medical Oncology (83) | 2020 | Mediastinal RT ± cardiotoxic chemotherapy, not further specified | Cardiac biomarkers and potentially cardiac imaging 6-12 months after therapy, 2 y after treatment, and possibly periodically thereafter | Evaluation for CAD and ischemia starting at 5 y after RT and every 3-5 y thereafter |
| International Cardio-Oncology Society (84) | 2021 | Reasserted previously defined high-risk groups:
|
Guided by individual patient risk: TTE as early as 6-12 months after RT in patients at high risk, and all patients should have TTE by 5 y after RT. Additional TTE and/or NT-proBNP every 5 y can be useful | Focus on diagnosing early CAD rather than ischemia for initiation of preventive therapy Review available CT scans for coronary calcifications In patients without known CAD, screening with stress testing, CAC, or CT angiography every 5 y |
CAC = coronary artery calcium; CAD = coronary artery disease; CT = computed tomography; NT-proBNP = N-terminal pro–B-type natriuretic peptide; RT = radiation therapy; TTE = transthoracic echocardiography.
Optimal employment of each imaging tool will depend on the individual patient’s comorbidities, RT exposure, and any previous treatment with anthracyclines or other cardiotoxic therapy. Patients at high risk of cardiotoxicity have been defined as those receiving >30 Gy with the heart in the treatment field, those who received both RT and anthracyclines, younger patients (<50 years of age), high-dose RT fractions (>2 Gy/day), presence and extent of tumor in or next to the heart, and cardiovascular risk factors (81,82). Significant radiation to specific structures in the heart, such as the LAD, should also be considered to confer higher risk (Central Illustration) (3,5,9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29), and special consideration should be given to patients who need to undergo surgery, especially cardiac surgery, after cardiac radiation exposure (84,86, 87, 88).
Based on the incidence of heart failure and other cardiac abnormalities after RT, current expert consensus guidelines generally recommend echocardiography to screen for cardiac abnormalities 5-10 years after RT and every 5 years thereafter (81,83,84). Patients at high risk for RICD (such as those receiving >30 Gy with the heart in the RT field or combination treatment with RT and anthracyclines), may benefit from echocardiography as early as 6-24 months after receiving RT (56,82). Functional stress testing to evaluate for ischemia and to detect CAD also has been recommended every 5 years beginning 5-10 years after RT (81,83). The recent International Cardio-Oncology Society (ICOS) recommendations place a higher value on detecting nonobstructive CAD with the use of CT imaging in order to start early preventive therapy (84). In patients who are ultimately diagnosed with obstructive CAD, severe valvular disease, or other RICD requiring intervention, additional consideration needs to be given to risks for radiation-induced fibrosis and scarring in the mediastinum that can affect surgical outcomes and may favor percutaneous intervention (84,87,88). As a particular example, in patients with breast cancer receiving regional nodal irradiation, the ipsilateral internal mammary artery is often in the target volume owing to treatment of the internal mammary lymph nodes (89), making evaluation of left internal mammary artery patency very important in candidates for coronary artery bypass grafting (84).
Echocardiography
Echocardiography is generally the initial imaging test to screen for RICD owing to its wide availability and accessibility, low risk profile, and ability to assess diastolic and systolic function, valvular pathophysiology, and pericardial disease. A comprehensive diastolic assessment is essential in RT survivors, because they are at particular risk of diastolic dysfunction due to RT-induced fibrosis and endothelial dysfunction (90). Resting wall-motion abnormalities can occur secondary to radiation damage directly to the myocardium, but their presence should also alert the treating physician to a high probability of underlying CAD (91).
Early post-RT subclinical changes on echocardiography can be seen 1 day after completing RT and include increased echodensity of the anterior cardiac segments (right ventricle wall and ventricular septum), as well as evidence of reduced right ventricular systolic function (92,93). However, these changes have not yet been correlated with future cardiovascular events. Global longitudinal strain measure via echocardiography may also identify subclinical cardiotoxicity in patients with breast cancer undergoing RT (94), and worse global longitudinal strain has been associated with increased mortality in patients undergoing cardiac surgery (95). LV dosimetry has been shown to be predictive of subclinical dysfunction-related changes in global longitudinal strain following RT (96). The aorto-mitral curtain thickness on transthoracic echocardiogram may also be used to prognosticate patients with RICD undergoing cardiac surgery, with a value of ≥0.6 cm predicting increased mortality (97).
Cardiac magnetic resonance imaging
CMRI is especially useful in patients with poor acoustic windows on echocardiography and for evaluation of myocardial fibrosis and/or pericardial constriction. CMRI is considered to be the reference standard in LV ejection fraction assessment (98), and it can identify myocardial edema and fibrotic changes in the myocardium (99,100). In addition, if transthoracic echocardiography is unable to assess aortic stenosis related to RICD because of technical limitations, CMRI can assess aortic valve area by means of planimetry and aortic valve velocities by means of phase-contrast velocity mapping (101). However, CMRI performs better in identifying regurgitant valvular lesions than in identifying stenotic lesions, and phase contrast may underestimate peak velocities owing to limitations in temporal resolution (101). Real-time cine and myocardial tagging can help in the diagnosis of constrictive pericarditis (102), which can occur as a late complication from RT (86). Myocardial fibrosis can develop secondary to RT (103), and techniques such as late gadolinium enhancement (100), T1 mapping, and extracellular volume fraction (100) allow for its identification. CMRI appears to be more sensitive than echocardiography in detecting LV systolic dysfunction in childhood survivors of cancer with and without previous RT (98). Earlier detection of cardiac dysfunction could allow for earlier intervention, although this approach still needs validation.
Functional stress testing
Patients are at risk for underlying CAD even with normal resting cardiac function. Stress echocardiography, stress single-photon emission CT (SPECT)/positron emission tomography (PET), and stress CMRI are appropriate functional tests to detect evidence of ischemia in survivors of RT. Exercise testing is preferred over pharmacologic studies when possible, given the additional prognostic information derived from a patient’s functional status. In deciding between two modalities with similar risk/benefit ratios, nonradiating techniques or techniques with lower additional radiation exposure may be advantageous. Stress PET has the unique ability of assessing coronary physiology noninvasively (104). However, its role in patients with history of RT has not yet been determined. Both stress echocardiography and radionucleotide perfusion imaging have been specifically shown to identify post-RT patients with obstructive CAD (91). In a cohort of 294 patients with HL and >35 Gy to the mediastinum, abnormal stress echocardiography had higher specificity for obstructive CAD than nuclear scintigraphy (89% vs 11%) with similar sensitivity (59% vs 65%) (91).
Perfusion abnormalities are relatively common in RT survivors. In a study by Hardenberg et al, 60% of patients with left breast cancer had resting perfusion defects at 6 months after RT (105). A larger follow-up study from the same group showed persistently high SPECT-perfusion defects, with 57%-71% incidence at 3-6 years after RT (106). In patients with distal esophageal cancer, 42% had LV inferior ischemia after RT according to stress SPECT (107). However, findings in stress SPECT in patients with lung or esophageal cancers receiving RT have not been predictive of future cardiac complications (108).
Cardiovascular computed tomography
Anatomic imaging has the advantage of detecting nonobstructive CAD early in the disease process before ischemia develops, thus allowing for potentially earlier use of preventive medications. Coronary computed tomographic angiography (CCTA) can noninvasively detect and quantify the burden of noncalcified plaque and coronary stenosis in symptomatic and asymptomatic patients, and it has been used to identify asymptomatic CAD in patients with a history of RT to the chest, even with normal stress imaging (109). CCTA notably also has a powerful negative predictive value in ruling out CAD, and the radiation exposure (1-4 mSv) is lower than nuclear perfusion or coronary angiography (109). CAC scans (noncontrast CTs) are able to evaluate for calcified, but not noncalcified plaque, and they have an advantage of even lower radiation exposure (1-1.5 mSv). CAC scans can identify the presence of coronary atherosclerosis secondary to RT (110,111), in addition to helping to risk-stratify asymptomatic CAD in patients with cancer before their RT treatment.
In patients with breast cancer, CAC on RT planning CTs correlated with future cardiac events (79), and CAC on surveillance CTs after RT were more predictive of cardiac events than Framingham risk scores (112). Without requiring further testing, reviewing available CT images may allow for identification of patients at higher risk of CAD and help to direct preventive therapy (113). In patients with NSCLC, coronary calcifications are often present at the time of cancer diagnosis, although unfortunately, an incidental finding of coronary calcifications on CT rarely results in prescription of preventive therapy (114). Small single-center studies suggest that baseline CAC might help to identify subclinical CAD and predict cardiovascular outcomes in those patients (115, 116, 117). There may also be a role for CAC surveillance in RT patients to assess RT-related CAD progression (110,111). Reviewing prior chest imaging may be important in establishing CV risk and can affect management. CCTA is also useful in planning transcatheter valve replacement in patients with RICD-related valve disease (109).
Biomarkers
Natriuretic peptides and troponin levels drawn early after RT have not shown any consistent ability to detect subclinical cardiotoxicity or future cardiomyopathy among several small, generally underpowered, studies across different cancer types. One of the more recent and larger studies examined N-terminal pro–B-type natriuretic peptide (NT-proBNP), high-sensitivity troponin T, placental growth factor (PlGF), and growth differentiation factor (GDF)-15 before and a median of 20 days after RT in 87 patients with cancer (60 breast cancers, 14 lymphomas, and 13 lung cancers) (118). There was no significant increase in troponin or NT-proBNP levels after RT. There was a significant increase in PIGF (P = 0.005) and GDF-15 (P = 0.006) in patients with lymphomas or lung cancer and a modest increase of borderline statistical significance (P = 0.054) in GDF-15 in patients with breast cancer. The study was not able to evaluate whether these biomarker changes correlated with subsequent LV dysfunction. In a small prospective cohort of 51 patients with breast cancer undergoing RT and 78 control subjects, Chalubinska-Fendler et al (119) found no difference in NT pro-BNP or troponin T between the patients with cancer or control subjects before or after RT. Levels of lipopolysaccharide-binding protein, however, drawn 24 hours and 1 month after RT, correlated with heart- and lung-associated dose volume. Higher levels were also associated with diastolic dysfunction (assessed by E/E′) measured by means of echocardiography 3 years later (24-hour level: β = 0.41; P = 0.032; 1 month level: β = 0.43; P = 0.028). Future research is warranted to further evaluate this novel biomarker. In survivors of childhood cancer more than 10 years after treatment, NT-proBNP was a useful tool to predict future occurrence of cardiomyopathy (120). Incorporating NT-proBNP into future screening algorithms for long-term survivors of RT is reasonable.
Therapeutic Applications of RT to the Heart in the Treatment of Ventricular Arrhythmias
Although radiation dose to healthy cardiac tissue can be associated with the development of RICD, as discussed in this review, there are now applications of RT to physiologically and structurally abnormal arrhythmogenic heart tissue that leverage RT-induced changes for clinical benefit. It had been previously theorized that an ablative RT dose to the electrophysiologic pathways of the heart could intentionally create a heart block. Preclinical work using SBRT on the normal cardiac conduction systems of animal models laid the groundwork in understanding the dose-response relationship and histologic changes of the target and nontarget heart tissue. Pulmonary vein and atrioventricular node radioablation in preclinical models was previously demonstrated with the use of 25 Gy SBRT (121). Lehmann et al also showed a median time to complete atrioventricular block of 11 weeks with no evidence of short-term toxicity in a large animal model (122).
With the demonstration of feasibility of electrical conduction blockade in nonhuman animal models with the use of a dose and fractionation that could be delivered with current RT approaches, the phase I/II ENCORE-VT (Electrophysiology-Guided Noninvasive Cardiac Radioablation for Ventricular Tachycardia) trial was developed to assess the safety and efficacy of this therapy for patients with refractory ventricular tachycardia. The primary end point of reduction in ventricular tachycardia events was achieved in 94% of trial patients. The treatment was well tolerated with a low rate of grade ≥3 toxicities (123).
A surprising finding from the ENCORE-VT trial was that the decrease in events emerged in the days following treatment, rather than on the orders of months seen in the preclinical models with normal cardiac substrate (123). These changes also occurred much sooner than subacute cardiac toxicities described in prospective NSCLC trials, where even the earliest cases of atrial fibrillation, heart block, or pericardial effusion occurred no sooner than 1 month after treatment (72). Because the antiarrhythmic effect of RT on the scar target tissue of ENCORE-VT participants occurred within the first 6 weeks, this suggested that the efficacy of RT is likely by a mechanism different from previously proposed myocyte cell death and creation of homogenous replacement fibrosis (turning a functional scar into a nonfunctional scar). In addition, while electrocardiographic (ECG) changes occurred in almost 90% of ENCORE-VT patients with complete ECG data, more than one-half of the changes occurred within the first 3 days. For RT patients who subsequently underwent heart transplantation or died from other causes, their donated heart specimens showed no significant difference in fibrosis levels between the targeted and nontargeted heart tissue (123).
As follow-up from ENCORE-VT continues, secondary analyses are underway to evaluate for long-term changes in cardiac conductivity and function. Although ECG changes were seen in the majority of patients, there were no clinically significant conduction changes apparent (such as atrioventricular block or dysrhythmias). In patients undergoing transthoracic echocardiography before treatment and >12 months after treatment, no LV ejection fraction changes were seen (124). A number of centers have now also treated ventricular tachycardia with SBRT, with similar efficacy in reducing ventricular tachycardia episodes and relatively rapid efficacy (30). Continued surveillance of patients who have received noninvasive cardiac radioablation will better define longer-term cardiac changes, and these findings may inform knowledge of cardiotoxicity following other thoracic RT approaches.
Summary and Conclusions
Increasingly advanced and complex RT techniques have significantly improved sparing of cardiac tissue during RT delivery. However, RICD remains a potentially significant toxicity for many patients with thoracic cancers. Improved knowledge of how the location of radiation deposition within the heart affects the risk of RICD will allow better personalization of RT in the future, including creation of dose constraints to specific cardiac substructures in appropriate patients. Long-term data from patients receiving focal high-dose RT for refractory VT may also enhance our knowledge of factors important in the development of RICD. Enhanced understanding of the pathophysiology of RICD can contribute to improvement in screening protocols to identify preclinical RICD and allow for earlier interventions. Together with aggressive cardiovascular risk modification, such efforts should further reduce development of symptomatic cardiac disease.
Funding Support and Author Disclosures
This work was supported by the National Institutes of Health (R01HL147884) and National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR002345). Dr Bergom has received research funding from the National Institutes of Health, Susan G. Komen Foundation, and Innovation Pathways. Dr Bradley has received an Ion Beam Application travel grant (April 2018) and funding from the Bankhead Coley Foundation and Ocala Royal Dames Foundation. Dr Ng has received funding from ViewRay. Dr Robinson has received research funding from the National Institutes of Health and the American Heart Association. Dr Mitchell has received research funding from Pfizer, Longer Life Foundation, and Children’s Discovery Institute and consultancy for Pfizer. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Delaney G., Jacob S., Featherstone C., Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–1137. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 2.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 3.van den Bogaard V.A.B., Ta B.D.P., van der Schaaf A. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol. 2017;35:1171–1178. doi: 10.1200/JCO.2016.69.8480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor C., Correa C., Duane F.K. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35:1641–1649. doi: 10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K., Pearlstein K.A., Patchett N.D. Heart dosimetric analysis of three types of cardiac toxicity in patients treated on dose-escalation trials for stage III nonsmall-cell lung cancer. Radiother Oncol. 2017;125:293–300. doi: 10.1016/j.radonc.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dess R.T., Sun Y., Matuszak M.M. Cardiac events after radiation therapy: combined analysis of prospective multicenter trials for locally advanced non–small-cell lung cancer. J Clin Oncol. 2017;35:1395–1402. doi: 10.1200/JCO.2016.71.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beukema J.C., van Luijk P., Widder J., Langendijk J.A., Muijs C.T. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother Oncol. 2015;114:85–90. doi: 10.1016/j.radonc.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 8.Atkins K.M., Rawal B., Chaunzwa T.L. Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. J Am Coll Cardiol. 2019;73:2976–2987. doi: 10.1016/j.jacc.2019.03.500. [DOI] [PubMed] [Google Scholar]
- 9.McWilliam A., Kennedy J., Hodgson C., Vasquez Osorio E., Faivre-Finn C., van Herk M. Radiation dose to heart base linked with poorer survival in lung cancer patients. Eur J Cancer. 2017;85:106–113. doi: 10.1016/j.ejca.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 10.Stam B., Peulen H., Guckenberger M. Dose to heart substructures is associated with noncancer death after SBRT in stage I-II NSCLC patients. Radiother Oncol. 2017;123:370–375. doi: 10.1016/j.radonc.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi Y., Murakami Y., Kameoka T. Analysis of cardiac toxicity after definitive chemoradiotherapy for esophageal cancer using a biological dose-volume histogram. J Radiat Res. 2020;61:298–306. doi: 10.1093/jrr/rraa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamari K., Isohashi F., Akino Y. Risk factors for pericardial effusion in patients with stage i esophageal cancer treated with chemoradiotherapy. Anticancer Res. 2014;34:7389–7393. [PubMed] [Google Scholar]
- 13.Wei X., Liu H.H., Tucker S.L. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2008;70:707–714. doi: 10.1016/j.ijrobp.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 14.Girinsky T., M’Kacher R., Lessard N. Prospective coronary heart disease screening in asymptomatic Hodgkin lymphoma patients using coronary computed tomography angiography: results and risk factor analysis. Int J Radiat Oncol Biol Phys. 2014;89:59–66. doi: 10.1016/j.ijrobp.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Cutter D.J., Schaapveld M., Darby S.C. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J.-T., Sun L., Sun X. Is pulmonary artery a dose-limiting organ at risk in nonsmall cell lung cancer patients treated with definitive radiotherapy? Radiat Oncol. 2017;12:34. doi: 10.1186/s13014-017-0772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han C.-B., Wang W.-L., Quint L. Pulmonary artery invasion, high-dose radiation, and overall survival in patients with nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;89:313–321. doi: 10.1016/j.ijrobp.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vivekanandan S., Landau D.B., Counsell N. The impact of cardiac radiation dosimetry on survival after radiation therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;99:51–60. doi: 10.1016/j.ijrobp.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cella L., Liuzzi R., Conson M. Dosimetric predictors of asymptomatic heart valvular dysfunction following mediastinal irradiation for Hodgkin’s lymphoma. Radiother Oncol. 2011;101:316–321. doi: 10.1016/j.radonc.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 20.Abouegylah M., Braunstein L.Z., Alm El-Din M.A. Evaluation of radiation-induced cardiac toxicity in breast cancer patients treated with trastuzumab-based chemotherapy. Breast Cancer Res Treat. 2019;174:179–185. doi: 10.1007/s10549-018-5053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wennstig A.-K., Garmo H., Isacsson U. The relationship between radiation doses to coronary arteries and location of coronary stenosis requiring intervention in breast cancer survivors. Radiat Oncol. 2019;14:40. doi: 10.1186/s13014-019-1242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yegya-Raman N., Wang K., Kim S. Dosimetric predictors of symptomatic cardiac events after conventional-dose chemoradiation therapy for inoperable NSCLC. J Thorac Oncol. 2018;13:1508–1518. doi: 10.1016/j.jtho.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moignier A., Broggio D., Derreumaux S. Coronary stenosis risk analysis following Hodgkin lymphoma radiotherapy: a study based on patient specific artery segments dose calculation. Radiother Oncol. 2015;117:467–472. doi: 10.1016/j.radonc.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 24.Mansouri I., Allodji R.S., Hill C. The role of irradiated heart and left ventricular volumes in heart failure occurrence after childhood cancer. Eur J Heart Fail. 2019;21:509–518. doi: 10.1002/ejhf.1376. [DOI] [PubMed] [Google Scholar]
- 25.Cao L., Hu W.G., Kirova Y.M. Potential impact of cardiac dose-volume on acute cardiac toxicity following concurrent trastuzumab and radiotherapy. Cancer Radiother. 2014;18:119–124. doi: 10.1016/j.canrad.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 26.de Ville de Goyet M., Brichard B., Robert A. Prospective cardiac MRI for the analysis of biventricular function in children undergoing cancer treatments. Pediatr Blood Cancer. 2015;62:867–874. doi: 10.1002/pbc.25381. [DOI] [PubMed] [Google Scholar]
- 27.Wong O.Y., Yau V., Kang J. Survival impact of cardiac dose following lung stereotactic body radiotherapy. Clin Lung Cancer. 2018;19:e241–e246. doi: 10.1016/j.cllc.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Atkins K.M., Chaunzwa T.L., Lamba N. Association of left anterior descending coronary artery radiation dose with major adverse cardiac events and mortality in patients with non–small cell lung cancer. JAMA Oncol. 2021;7:206–219. doi: 10.1001/jamaoncol.2020.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao L., Cai G., Chang C. Diastolic dysfunction occurs early in HER2-positive breast cancer patients treated concurrently with radiation therapy and trastuzumab. Oncologist. 2015;20:605–614. doi: 10.1634/theoncologist.2014-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Ree M.H., Blanck O., Limpens J. Cardiac radioablation—a systematic review. Heart Rhythm. 2020;17:1381–1392. doi: 10.1016/j.hrthm.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Haugnes H.S., Wethal T., Aass N. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol. 2010;28:4649–4657. doi: 10.1200/JCO.2010.29.9362. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan H.S. Evidence for a tumoricidal dose level in the radiotherapy of Hodgkin’s disease. Cancer Rese. 1966;26:5. [PubMed] [Google Scholar]
- 33.Boivin J.-F., Hutchison G.B., Lubin J.H., Mauch P. Coronary artery disease mortality in patients treated for Hodgkin’s disease. Cancer. 1992;69:1241–1247. doi: 10.1002/cncr.2820690528. [DOI] [PubMed] [Google Scholar]
- 34.Hancock S.L., Tucker M.A., Hoppe R.T. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA. 1993;270:1949–1955. [PubMed] [Google Scholar]
- 35.Galper S.L., Yu J.B., Mauch P.M. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117:412–418. doi: 10.1182/blood-2010-06-291328. [DOI] [PubMed] [Google Scholar]
- 36.van Nimwegen F.A., Schaapveld M., Janus C.P.M. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 37.Schellong G., Riepenhausen M., Bruch C. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr Blood Cancer. 2010;55:1145–1152. doi: 10.1002/pbc.22664. [DOI] [PubMed] [Google Scholar]
- 38.Mulrooney D.A., Yeazel M.W., Kawashima T. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhakta N., Liu Q., Yeo F. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol. 2016;17:1325–1334. doi: 10.1016/S1470-2045(16)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maraldo M.V., Giusti F., Vogelius I.R. Cardiovascular disease after treatment for Hodgkin’s lymphoma: an analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol. 2015;2:e492–e502. doi: 10.1016/S2352-3026(15)00153-2. [DOI] [PubMed] [Google Scholar]
- 41.van Nimwegen F.A., Schaapveld M., Cutter D.J. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34:235–243. doi: 10.1200/JCO.2015.63.4444. [DOI] [PubMed] [Google Scholar]
- 42.van Nimwegen F.A., Ntentas G., Darby S.C. Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood. 2017;129:2257–2265. doi: 10.1182/blood-2016-09-740332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Specht L., Yahalom J., Illidge T. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the International Lymphoma Radiation Oncology Group (ILROG) Int J Radiat Oncol Biol Phys. 2014;89:854–862. doi: 10.1016/j.ijrobp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Wirth A., Mikhaeel N.G., Aleman B.M.P. Involved site radiation therapy in adult lymphomas: an overview of International Lymphoma Radiation Oncology Group guidelines. Int J Radiat Oncol Biol Phys. 2020;107:909–933. doi: 10.1016/j.ijrobp.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Bortfeld T., Jeraj R. The physical basis and future of radiation therapy. Br J Radiol. 2011;84:485–498. doi: 10.1259/bjr/86221320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell B.A., Voss N., Pickles T. Involved-nodal radiation therapy as a component of combination therapy for limited-stage Hodgkin’s lymphoma: a question of field size. J Clin Oncol. 2008;26:5170–5174. doi: 10.1200/JCO.2007.15.1001. [DOI] [PubMed] [Google Scholar]
- 47.Koeck J., Abo-Madyan Y., Lohr F. Radiotherapy for early mediastinal Hodgkin lymphoma according to the German Hodgkin Study Group (GHSG): the roles of intensity-modulated radiotherapy and involved-node radiotherapy. Int J Radiat Oncol. Biol Phys. 2012;83:268–276. doi: 10.1016/j.ijrobp.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 48.Maraldo M.V., Brodin N.P., Vogelius I.R. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2012;83:1232–1237. doi: 10.1016/j.ijrobp.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 49.Voong K.R., McSpadden K., Pinnix C.C. Dosimetric advantages of a “butterfly” technique for intensity-modulated radiation therapy for young female patients with mediastinal Hodgkin’s lymphoma. Radiat Oncol. 2014;9:94. doi: 10.1186/1748-717X-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulrooney D.A., Hyun G., Ness K.K. Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: report from the Childhood Cancer Survivor Study cohort. BMJ. 2020;368:l6794. doi: 10.1136/bmj.l6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bates J.E., Howell R.M., Liu Q. Therapy-related cardiac risk in childhood cancer survivors: an analysis of the Childhood Cancer Survivor Study. J Clin Oncol. 2019;37:1090–1101. doi: 10.1200/JCO.18.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Pal H.J., van Dalen E.C., van Delden E. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30:1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 53.Feijen E.A.M., van Dalen E.C., van der Pal H.J.H. Increased risk of cardiac ischaemia in a pan-European cohort of 36 205 childhood cancer survivors: a PanCareSurFup study. Heart. 2021;107:33–40. doi: 10.1136/heartjnl-2020-316655. [DOI] [PubMed] [Google Scholar]
- 54.Lipshultz S.E., Adams M.J., Colan S.D. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 55.Shankar S.M., Marina N., Hudson M.M. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008;121:e387–e396. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 56.Armenian S.H., Hudson M.M., Mulder R.L. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16:e123–e136. doi: 10.1016/S1470-2045(14)70409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clarke M., Collins R., Darby S. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 58.Boekel N.B., Schaapveld M., Gietema J.A. Cardiovascular disease risk in a large, population-based cohort of breast cancer survivors. Int J Radiat Oncol Biol Phys. 2016;94:1061–1072. doi: 10.1016/j.ijrobp.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 59.Vicini F.A., Cecchini R.S., White J.R. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet. 2019;394:2155–2164. doi: 10.1016/S0140-6736(19)32514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.NRG Oncology RTOG 1005: a phase III trial of accelerated whole breast irradiation with hypofractionation plus concurrent boost versus standard whole breast irradiation plus sequential boost for early-stage breast cancer. https://www.nrgoncology.org/Clinical-Trials/Protocol/rtog-1005 Accessed January 25, 2021.
- 61.Gee H.E., Moses L., Stuart K. Contouring consensus guidelines in breast cancer radiotherapy: comparison and systematic review of patterns of failure. J Med Imaging. Radiat Oncol. 2019;63:102–115. doi: 10.1111/1754-9485.12804. [DOI] [PubMed] [Google Scholar]
- 62.Bradley J.A., Mendenhall N.P. Novel radiotherapy techniques for breast cancer. Annu Rev Med. 2018;69:277–288. doi: 10.1146/annurev-med-042716-103422. [DOI] [PubMed] [Google Scholar]
- 63.Jacobse J.N., Duane F.K., Boekel N.B. Radiation dose-response for risk of myocardial infarction in breast cancer survivors. Int J Radiat Oncol Biol Phys. 2019;103:595–604. doi: 10.1016/j.ijrobp.2018.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duane F.K., McGale P., Brønnum D. Cardiac structure doses in women irradiated for breast cancer in the past and their use in epidemiological studies. Pract Radiat Oncol. 2019;9:158–171. doi: 10.1016/j.prro.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piroth M.D., Baumann R., Budach W. Heart toxicity from breast cancer radiotherapy: current findings, assessment, and prevention. Strahlenther Onkol. 2019;195:1–12. doi: 10.1007/s00066-018-1378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Witt J.S., Jagodinsky J.C., Liu Y. Cardiac toxicity in operable esophageal cancer patients treated with or without chemoradiation. Am J Clin Oncol. 2019;42:662–667. doi: 10.1097/COC.0000000000000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chun S.G., Hu C., Choy H. Impact of intensity-modulated radiation therapy technique for locally advanced non–small-cell lung cancer: a secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35:56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morota M., Gomi K., Kozuka T. Late toxicity after definitive concurrent chemoradiotherapy for thoracic esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2009;75:122–128. doi: 10.1016/j.ijrobp.2008.10.075. [DOI] [PubMed] [Google Scholar]
- 69.Niska J.R., Thorpe C.S., Allen S.M. Radiation and the heart: systematic review of dosimetry and cardiac end points. Expert Rev Cardiovasc Ther. 2018;16:931–950. doi: 10.1080/14779072.2018.1538785. [DOI] [PubMed] [Google Scholar]
- 70.Polk A., Vaage-Nilsen M., Vistisen K., Nielsen D.L. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev. 2013;39:974–984. doi: 10.1016/j.ctrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Bradley J.D., Paulus R., Komaki R. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB nonsmall-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang K., Eblan M.J., Deal A.M. Cardiac toxicity after radiotherapy for stage III non–small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35:1387–1394. doi: 10.1200/JCO.2016.70.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reshko L.B., Kalman N.S., Hugo G.D., Weiss E. Cardiac radiation dose distribution, cardiac events and mortality in early-stage lung cancer treated with stereotactic body radiation therapy (SBRT) J Thorac Dis. 2018;10:2346–2356. doi: 10.21037/jtd.2018.04.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mulrooney D.A., Armstrong G.T., Huang S. Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: a cross-sectional study from the St. Jude Lifetime Cohort. Ann Intern Med. 2016;164:93–101. doi: 10.7326/M15-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hooning M.J., Botma A., Aleman B.M.P. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 76.Wethal T., Nedregaard B., Andersen R. Atherosclerotic lesions in lymphoma survivors treated with radiotherapy. Radiother Oncol. 2014;110:448–454. doi: 10.1016/j.radonc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 77.Armstrong G.T., Oeffinger K.C., Chen Y. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeboah J., McClelland R.L., Polonsky T.S. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roos C.T.G., van den Bogaard V.A.B., Greuter M.J.W. Is the coronary artery calcium score associated with acute coronary events in breast cancer patients treated with radiotherapy? Radiother Oncol. 2018;126:170–176. doi: 10.1016/j.radonc.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 80.Xie X., Zhao Y., de Bock G.H. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: systematic review and meta-analysis. Circ Cardiovasc Imaging. 2013;6:514–521. doi: 10.1161/CIRCIMAGING.113.000092. [DOI] [PubMed] [Google Scholar]
- 81.Lancellotti P., Nkomo V.T., Badano L.P. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:721–740. doi: 10.1093/ehjci/jet123. [DOI] [PubMed] [Google Scholar]
- 82.Armenian S.H., Lacchetti C., Barac A. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 83.Curigliano G., Lenihan D., Fradley M. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mitchell J.D., Cehic D.A., Morgia M. Cardiovascular manifestations from therapeutic radiation: a multidisciplinary expert consensus statement from the international cardio-oncology society. J Am Coll Cardiol CardioOnc. 2021;3:360–380. doi: 10.1016/j.jaccao.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Children’s Oncology Group Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers. Version 5.0, October 2018. www.survivorshipguidelines.org Accessed June 2, 2021.
- 86.Ganatra Sarju, Chatur Safia, Nohria Anju. How to diagnose and manage radiation cardiotoxicity. J Am Coll Cardiol CardioOnc. 2020;2:655–660. doi: 10.1016/j.jaccao.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Donnellan E., Masri A., Johnston D.R. Long-term outcomes of patients with mediastinal radiation-associated severe aortic stenosis and subsequent surgical aortic valve replacement: a matched cohort study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dolmaci O.B., Farag E.S., Boekholdt S.M., van Boven W.J.P., Kaya A. Outcomes of cardiac surgery after mediastinal radiation therapy: a single-center experience. J Card Surg. 2020;35:612–619. doi: 10.1111/jocs.14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whelan T.J., Olivotto I.A., Parulekar W.R. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307–316. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saiki H., Petersen I.A., Scott C.G. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation. 2017;135:1388–1396. doi: 10.1161/CIRCULATIONAHA.116.025434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heidenreich P.A., Schnittger I., Strauss H.W. Screening for coronary artery disease after mediastinal irradiation for Hodgkin’s disease. J Clin Oncol. 2007;25:43–49. doi: 10.1200/JCO.2006.07.0805. [DOI] [PubMed] [Google Scholar]
- 92.Tuohinen S.S., Skyttä T., Virtanen V. Detection of radiotherapy-induced myocardial changes by ultrasound tissue characterisation in patients with breast cancer. Int J Cardiovasc Imaging. 2016;32:767–776. doi: 10.1007/s10554-016-0837-9. [DOI] [PubMed] [Google Scholar]
- 93.Tuohinen S.S., Skyttä T., Virtanen V., Luukkaala T., Kellokumpu-Lehtinen P.-L., Raatikainen P. Early effects of adjuvant breast cancer radiotherapy on right ventricular systolic and diastolic function. Anticancer Res. 2015;35:2141–2147. [PubMed] [Google Scholar]
- 94.Trivedi S.J., Choudhary P., Lo Q. Persistent reduction in global longitudinal strain in the longer term after radiation therapy in patients with breast cancer. Radiother Oncol. 2019;132:148–154. doi: 10.1016/j.radonc.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 95.Chirakarnjanakorn S., Popović Z.B., Wu W. Impact of long-axis function on cardiac surgical outcomes in patients with radiation-associated heart disease. J Thorac Cardiovasc Surg. 2015;149:1643–1651.e2. doi: 10.1016/j.jtcvs.2015.01.045. [DOI] [PubMed] [Google Scholar]
- 96.Walker V., Lairez O., Fondard O. Early detection of subclinical left ventricular dysfunction after breast cancer radiation therapy using speckle-tracking echocardiography: association between cardiac exposure and longitudinal strain reduction (BACCARAT study) Radiat Oncol. 2019;14:204. doi: 10.1186/s13014-019-1408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Desai M.Y., Wu W., Masri A. Increased aorto-mitral curtain thickness independently predicts mortality in patients with radiation-associated cardiac disease undergoing cardiac surgery. Ann Thorac Surg. 2014;97:1348–1355. doi: 10.1016/j.athoracsur.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 98.Armstrong G.T., Plana J.C., Zhang N. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–2884. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim R.J., Wu E., Rafael A. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 100.Haaf P., Garg P., Messroghli D.R., Broadbent D.A., Greenwood J.P., Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reason. 2016;18:89. doi: 10.1186/s12968-016-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lopez-Mattei J.C., Shah D.J. The role of cardiac magnetic resonance in valvular heart disease. Methodist DeBakey Cardiovasc J. 2013;9:142–148. doi: 10.14797/mdcj-9-3-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ariyarajah V., Jassal D.S., Kirkpatrick I., Kwong R.Y. The utility of cardiovascular magnetic resonance in constrictive pericardial disease. Cardiol Rev. 2009;17:77–82. doi: 10.1097/CRD.0b013e318197e950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Veinot J.P., Edwards W.D. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27:766–773. doi: 10.1016/s0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 104.Gould K.L., Johnson N.P., Bateman T.M. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62:1639–1653. doi: 10.1016/j.jacc.2013.07.076. [DOI] [PubMed] [Google Scholar]
- 105.Hardenbergh P.H., Munley M.T., Bentel G.C. Cardiac perfusion changes in patients treated for breast cancer with radiation therapy and doxorubicin: preliminary results. Int J Radiat Oncol Biol Phy.s. 2001;49:1023–1028. doi: 10.1016/s0360-3016(00)01531-5. [DOI] [PubMed] [Google Scholar]
- 106.Prosnitz R.G., Hubbs J.L., Evans E.S. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: analysis of data 3 to 6 years after treatment. Cancer. 2007;110:1840–1850. doi: 10.1002/cncr.22965. [DOI] [PubMed] [Google Scholar]
- 107.Gayed I.W., Liu H.H., Yusuf S.W. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med. 2006;47:1756–1762. [PubMed] [Google Scholar]
- 108.Gayed I., Gohar S., Liao Z., McAleer M., Bassett R., Yusuf S.W. The clinical implications of myocardial perfusion abnormalities in patients with esophageal or lung cancer after chemoradiation therapy. Int J Cardiovasc Imaging. 2009;25:487–495. doi: 10.1007/s10554-009-9440-7. [DOI] [PubMed] [Google Scholar]
- 109.Layoun M.E., Yang E.H., Herrmann J. Applications of cardiac computed tomography in the cardio-oncology population. Curr Treat Options Oncol. 2019;20:47. doi: 10.1007/s11864-019-0645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Milgrom S.A., Varghese B., Gladish G.W. Coronary artery dose-volume parameters predict risk of calcification after radiation therapy. J. Cardiovasc. Imaging. 2019;27:268–279. doi: 10.4250/jcvi.2019.27.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yakupovich A., Davison M.A., Kharouta M.Z. Heart dose and coronary artery calcification in patients receiving thoracic irradiation for lung cancer. J Thorac Dis. 2020;12:223–231. doi: 10.21037/jtd.2020.01.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tomizawa N. Could coronary calcification identified at nongated chest CT be a predictor for cardiovascular events in breast cancer patients? Int J Cardiol. 2019;282:108–109. doi: 10.1016/j.ijcard.2019.01.103. [DOI] [PubMed] [Google Scholar]
- 113.Mitchell J.D., Fergestrom N., Gage B.F. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol. 2018;72:3233–3242. doi: 10.1016/j.jacc.2018.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cuddy S., Payne D.L., Murphy D.J. Incidental coronary artery calcification in cancer imaging. J Am Coll Cardiol CardioOnc. 2019;1:135–137. doi: 10.1016/j.jaccao.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Phillips W.J., Johnson C., Law A. Reporting of coronary artery calcification on chest CT studies in breast cancer patients at high risk of cancer therapy related cardiac events. Int J Cardiol Heart Vasc. 2018;18:12–16. doi: 10.1016/j.ijcha.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gernaat S.A.M., Išgum I., de Vos B.D. Automatic coronary artery calcium scoring on radiotherapy planning CT scans of breast cancer patients: reproducibility and association with traditional cardiovascular risk factors. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mast M.E., Heijenbrok M.W., Petoukhova A.L., Scholten A.N., Schreur J.H.M., Struikmans H. Preradiotherapy calcium scores of the coronary arteries in a cohort of women with early-stage breast cancer: a comparison with a cohort of healthy women. Int J Radiat Oncol Biol Phy.s. 2012;83:853–858. doi: 10.1016/j.ijrobp.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 118.Demissei B.G., Freedman G., Feigenberg S.J. Early changes in cardiovascular biomarkers with contemporary thoracic radiation therapy for breast cancer, lung cancer, and lymphoma. Int J Radiat Oncol Biol Phys. 2019;103:851–860. doi: 10.1016/j.ijrobp.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chalubinska-Fendler J., Graczyk L., Piotrowski G. Lipopolysaccharide-binding protein is an early biomarker of cardiac function after radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2019;104:1074–1083. doi: 10.1016/j.ijrobp.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 120.Dixon S.B., Howell C.R., Lu L. Cardiac biomarkers and association with subsequent cardiomyopathy and mortality among adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort. Cancer. 2021;127:458–466. doi: 10.1002/cncr.33292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zei P.C., Wong D., Gardner E., Fogarty T., Maguire P. Safety and efficacy of stereotactic radioablation targeting pulmonary vein tissues in an experimental model. Heart Rhythm. 2018;15:1420–1427. doi: 10.1016/j.hrthm.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 122.Lehmann H.I., Deisher A.J., Takami M. External arrhythmia ablation using photon beams: ablation of the atrioventricular junction in an intact animal model. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004304. [DOI] [PubMed] [Google Scholar]
- 123.Robinson C.G., Samson P.P., Moore K.M.S. Phase I/II trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation. 2019;139:313–321. doi: 10.1161/CIRCULATIONAHA.118.038261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hall D., Robinson C., Cuculich P.S. Cardiac function after noninvasive radioablation [abstract] Heart Rhythm Society Annual Meeting. 2019 [Google Scholar]