Abstract
Background
Radiation therapy (RT) for breast cancer increases risk of coronary artery disease (CAD). Women treated for left- vs right-sided breast cancer receive greater heart radiation exposure, which may further increase this risk. The risk of radiation-associated CAD specifically among younger breast cancer survivors is not well defined.
Objectives
The purpose of this study was to report CAD risk among participants in the Women’s Environmental Cancer and Radiation Epidemiology Study.
Methods
A total of 1,583 women who were <55 years of age when diagnosed with breast cancer between 1985 and 2008 completed a cardiovascular health questionnaire. Risk of radiation-associated CAD was evaluated by comparing women treated with left-sided RT with women treated with right-sided RT using multivariable Cox proportional hazards models. Effect modification by treatment and cardiovascular risk factors was examined.
Results
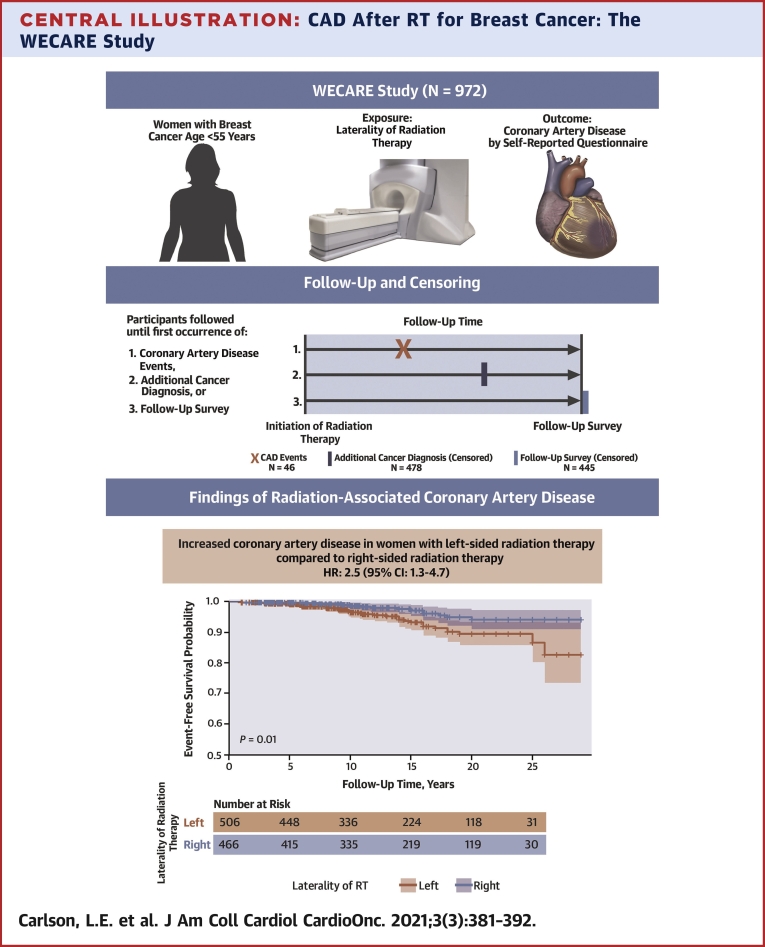
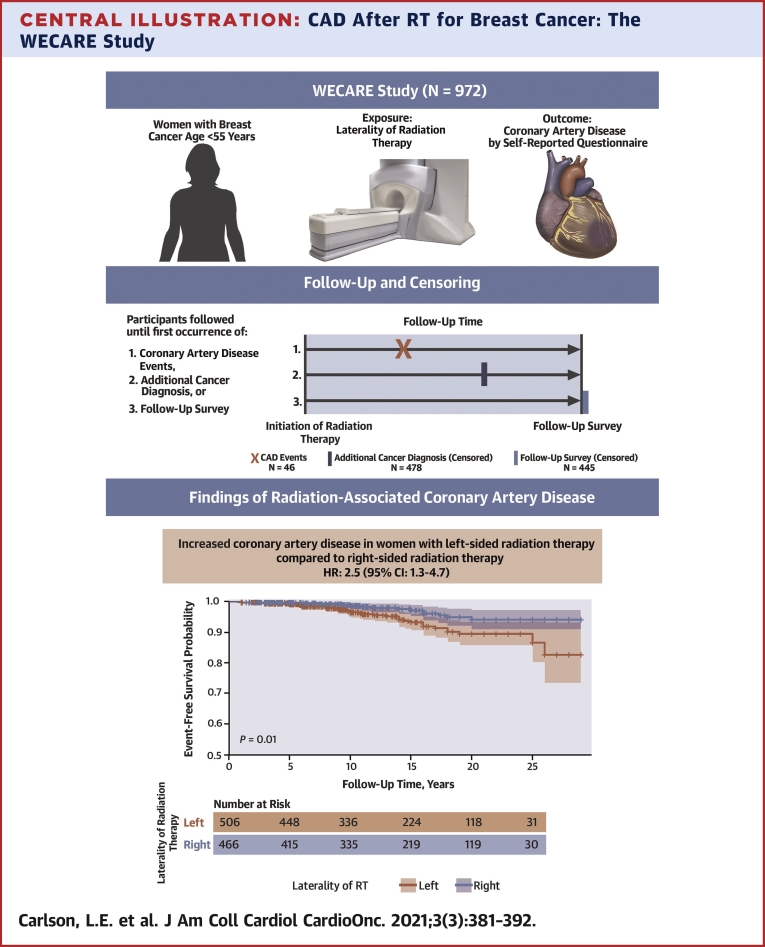
In total, 517 women who did not receive RT and 94 women who had a pre-existing cardiovascular disease diagnosis were excluded, leaving 972 women eligible for analysis. Their median follow-up time was 14 years (range 1-29 years). The 27.5-year cumulative incidences of CAD for women receiving left- vs right-sided RT were 10.5% and 5.8%, respectively (P = 0.010). The corresponding HR of CAD for left- vs right-sided RT in the multivariable Cox model was 2.5 (95% CI: 1.3-4.7). There was no statistically significant effect modification by any factor evaluated.
Conclusions
Young women treated with RT for left-sided breast cancer had over twice the risk of CAD compared with women treated with RT for right-sided breast cancer. Laterality of RT is independently associated with an increased risk of CAD and should be considered in survivorship care of younger breast cancer patients.
Key Words: epidemiology, ischemic disease, prevention, risk factor, treatment, women’s oncology
Abbreviations and Acronyms: BMI, body mass index; CAD, coronary artery disease; RT, radiation therapy
Central Illustration
Breast cancer is the most common invasive nonskin cancer among women in the United States (1). The 10-year survival rate for women diagnosed before age 50 years is 93.4% among those with stage I disease and 76.1% among those with stage II disease (2). In 2021, an estimated 281,000 breast cancers will be diagnosed (1), and currently, 47% of women with stage I or II disease receive radiation therapy (RT) as part of breast-conserving therapy (3). However, RT has subsequent adverse effects on cardiovascular health. The radiation effects on the heart are approximately proportional to the mean radiation dose received to the heart, which is 3.7 Gy greater on average for left-sided compared with right-sided breast tumors (4).
Recent studies have suggested that irradiation of specific heart structures, along with the presence of cardiovascular risk factors, are important determinants of future radiation-induced cardiovascular disease (5,6). Prior studies, which have primarily focused on older populations (median diagnosis age >50 years), have shown that RT to the left breast is associated with a greater risk of developing cardiovascular disease, such as nonfatal myocardial infarction, valvular disease, and ischemic heart disease (4,7, 8, 9, 10, 11, 12). Additionally, radiation-associated cardiovascular disease has a latency of at least 5 years (13), requiring long-term follow-up of large numbers of breast cancer survivors to assess subsequent cardiovascular disease events. It is not established whether younger women receiving left-sided RT have a clinically significant increased risk of cardiovascular disease.
Adjuvant treatments for breast cancer, such as chemotherapy and hormone therapy, may interact with RT to further increase the risk of cardiovascular disease; anthracycline chemotherapy in combination with left-sided RT may further increase the risk of cardiovascular disease (14), whereas hormone therapy has shown no such interaction (8,15). Further assessment of the potential impact of these treatments in addition to RT on the risk of cardiovascular disease is needed.
Here, we report results from the WECARE (Women’s Environmental Cancer and Radiation Epidemiology) Follow-Up Study of self-reported incident cardiovascular disease. The WECARE Study is a population-based case-control study of women with contralateral and unilateral breast cancer where the first breast cancer was diagnosed before age 55 years. The WECARE Follow-Up Study was designed to assess late effects of treatment, including nonfatal incident cardiovascular disease following RT. We estimated the risk of RT-associated coronary artery disease (CAD) by comparing women receiving left- vs right-sided RT for breast cancer. In addition, we examined whether cardiovascular disease-associated lifestyle and treatment factors modified the association between RT and CAD risk.
Methods
Study population
The WECARE Study is a population-based study of women diagnosed with stage I or II invasive breast cancer before 55 years of age between 1985 and 2008. Recruitment and data collection for the WECARE Study were conducted in 2 phases, the WECARE I Study (2001-2004) and the WECARE II Study (2009-2012) (16,17). As part of the WECARE Study phases I and II, epidemiological risk factors, treatment details, laterality of RT, and tumor characteristics were determined by telephone interviews using structured questionnaires and detailed medical record abstraction (16,18).
The third phase, the WECARE Follow-Up Study, ascertained nonfatal incident cardiovascular disease events occurring after treatment. Living women recruited from 5 of the participating population-based cancer registries (3 from the U.S., 1 each from Canada and Denmark) were recontacted by study staff between 2013 and 2015 to identify cardiovascular disease events occurring before and since their breast cancer diagnosis. The study protocol was approved for participant recruitment by the Institutional Review Board at the University of Iowa (Iowa), the Cancer Prevention Institute of California (California), and the Fred Hutchinson Cancer Research Center (Seattle, Washington); the ethics committees at Mount Sinai Hospital (Ontario) and the Danish Cancer Society (Denmark); as well as the ethical committee system in Denmark. All other WECARE Study sites not involved in this study also received Institutional Review Board approval.
Participants completed the Follow-Up Study questionnaire either by mail or by structured telephone interview with study staff. Participants provided the age at which a doctor diagnosed them with cardiovascular disease (cardiomyopathy, myocardial infarction, coronary heart disease, angina pectoris requiring medication, arrhythmia, stiff or leaking heart valves), or the age at which they received any heart surgery. In addition to the cardiac diagnoses, participants reported the age at which they were diagnosed with a new primary cancer, breast cancer recurrence, or breast cancer metastasis, and their age at the time of the follow-up questionnaire. Participants also reported updated lifestyle risk factors, such as former or current smoking history. Self-reported events for the cardiovascular diagnoses were not confirmed by medical record review. However, a previously reported substudy of Danish WECARE Study participants was conducted to examine concordance of self-reported cardiovascular diseases with the National Patient Register (19).
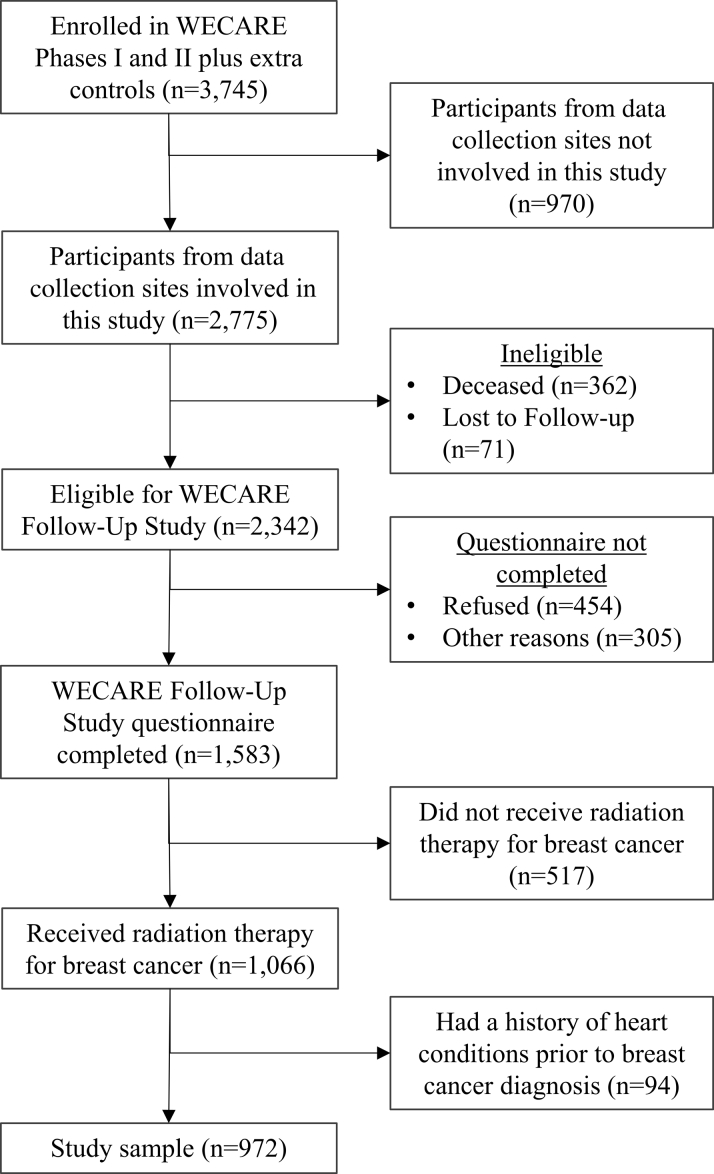
Of the 2,342 potentially eligible women who were recontacted, 1,583 (68%) completed the standardized follow-up questionnaire; 454 (19%) declined to participate in the WECARE Follow-Up Study, and 305 (13%) did not participate for other reasons (eg, were unable to complete the questionnaire in a timely manner). After excluding women who did not receive RT and women who had a personal history of cardiovascular disease or heart surgery before breast cancer diagnosis, the final study size was 972 women (Figure 1).
Figure 1.
Selection of Participants
Participants were selected from the WECARE (Women’s Environmental Cancer and Radiation Epidemiology) Study phases I and II. Participants must have been living at the time of the follow-up survey.
Statistical methods
Similar to previous studies, we restricted the analysis to participants receiving RT for their breast cancer treatment (20). Incident CAD was the cardiac event of interest, defined as the first diagnosis of myocardial infarction, coronary heart disease, or angina pectoris requiring medication. Participants were followed from breast cancer diagnosis until a diagnosis of CAD, with censoring at the earliest diagnosis of the following: 1) second primary contralateral breast cancer; 2) breast cancer recurrence or metastasis; 3) other primary cancer; or 4) end of follow-up at third phase interview. A participant’s follow-up time was defined as the time from breast cancer diagnosis until the first occurrence of a CAD event or censoring. The exposure of interest was left- vs right-sided treatment with RT for breast cancer. The overall follow-up time was compared between women with left- vs right-sided RT using the log-rank test, and the difference in cumulative incidence of CAD for women receiving left- vs right-sided RT was tested using the Fine and Gray method (21).
We used multivariable Cox proportional hazards models to estimate the association between left-sided RT and incident CAD using a cause-specific hazard function in the presence of competing risks (censoring due to cancer). Given that the laterality of breast cancer, and therefore the allocation of exposure (left- vs right-sided RT), is independent of cardiovascular risk factors, there were no anticipated confounders of the association between risk of CAD and left-sided RT (20). However, to account for possible selection bias incurred in the recruitment of each WECARE Study phase, and any temporal changes to RT treatment protocols, we adjusted for matching factors used in the WECARE Study design (age in years at breast cancer diagnosis, recruitment site, race/ethnicity, and WECARE Study phase) in the Cox proportional hazards models. Allowing for differences in delivery techniques and field design, the average radiation dose to the treated breast was 55 Gy (range 45-65 Gy), which did not differ by laterality.
We estimated HRs and corresponding 95% CIs for left-sided RT compared with right-sided RT in multivariable-adjusted Cox models. We evaluated effect modification by baseline lifestyle and cardiovascular risk factors on the association between RT and CAD by stratifying individual models by modifiers of interest (age at breast cancer diagnosis, year of breast cancer diagnosis, smoking history, body mass index [BMI] at breast cancer diagnosis, pre-existing hypertension or hypercholesterolemia at breast cancer diagnosis, adjuvant treatments received for breast cancer), and testing for heterogeneity of left-sided RT associations with CAD between strata. The proportional hazards assumption was confirmed by testing the linearity of Martingale residuals (22).
To evaluate the possible effect of internal mammary chain node radiation on CAD, we conducted a sensitivity analysis by restricting the results of the overall model to women with stage I breast cancer (for whom it was unlikely that a separate RT field was used to irradiate the internal mammary chain nodes) (23). To examine whether adjusting for study phase within the models was sufficient to control for any differences in breast cancer treatment protocols over time, an additional sensitivity analysis was conducted by substituting WECARE Study phase with year of breast cancer diagnosis in the overall model. Statistical tests were considered significant at P < 0.050, and all regression analyses were conducted using SAS Studio version 3.8 (SAS Institute Inc). Cumulative incidence analysis and graph generation were conducted using R version 4.0.4 (The R Foundation for Statistical Computing).
Results
Distribution of Risk Factors and CAD Outcomes
In general, clinical and lifestyle factors were equally distributed between women receiving left-sided RT (48% of women) compared with women receiving right-sided RT (52% of women) (Table 1). The median age of breast cancer diagnosis was 46 years, and the median length of follow-up was 14 years (range 1-29 years). Over 60% of participants received their breast cancer diagnosis between 1990 and 1999. Participants who completed the WECARE Study Follow-Up questionnaire did not differ from nonresponders regarding age at breast cancer diagnosis, stage of breast cancer diagnosis, laterality of breast cancer, or receipt of RT for breast cancer (Supplemental Table 1).
Table 1.
Participant Characteristics Stratified by Laterality of Breast Cancer in the WECARE Study
| Right (n = 466) | Left (n = 506) | |
|---|---|---|
| Age at breast cancer diagnosis, y | 46 (28-54) | 46 (25-54) |
| Length of follow-up time,a y | 14.00 (1-29) | 14.00 (1-29) |
| Race/ethnicity | ||
| Non-Hispanic white | 412 (88) | 456 (90) |
| Hispanic white | 24 (5) | 26 (5) |
| Black | 15 (3) | 13 (3) |
| Asian | 15 (3) | 11 (2) |
| Stage of breast cancer | ||
| Stage I | 306 (66) | 321 (64) |
| Stage II | 158 (34) | 178 (35) |
| Unknown | 2 (0) | 7 (1) |
| Year of breast cancer diagnosis | ||
| 1985-1989 | 56 (12) | 67 (13) |
| 1990-1994 | 162 (35) | 168 (33) |
| 1995-1999 | 153 (33) | 164 (32) |
| 2000-2004 | 91 (20) | 86 (17) |
| 2004-2008 | 4 (1) | 21 (4) |
| Chemotherapy for breast cancer | ||
| Yes | 278 (60) | 302 (60) |
| No | 187 (40) | 204 (40) |
| Anthracycline for breast cancer | ||
| Yes | 139 (30) | 150 (30) |
| No | 327 (70) | 356 (70) |
| Hormone therapy for breast cancer | ||
| Yes | 206 (44) | 221 (44) |
| No | 260 (56) | 285 (56) |
| BMI at breast cancer diagnosis, kg/m2 | ||
| Underweight (<18.5) | 13 (3) | 15 (3) |
| Normal weight (18.5-24.9) | 292 (63) | 310 (61) |
| Overweight (25-29.9) | 99 (21) | 122 (24) |
| Obese (≥30) | 62 (13) | 59 (12) |
| Lifetime smoking history | ||
| Ever | 175 (38) | 215 (42) |
| Never | 291 (62) | 291 (58) |
| Hypertension before breast cancer | ||
| Yes | 50 (11) | 43 (9) |
| No | 416 (89) | 463 (91) |
| Hypercholesterolemia before breast cancer | ||
| Yes | 22 (5) | 29 (6) |
| No | 444 (95) | 477 (94) |
| Recruitment site | ||
| Californiab | 163 (35) | 168 (33) |
| Denmarkc | 110 (24) | 115 (23) |
| Iowad | 65 (14) | 73 (14) |
| Ontarioe | 49 (11) | 57 (11) |
| Seattlef | 79 (17) | 93 (18) |
Values are median (range) or n (%).
WECARE = Women's Environmental Cancer and Radiation Epidemiology.
A woman's follow-up time began at the time of breast cancer diagnosis to the first to occur among the following: the diagnosis of a new primary cancer, the recurrence or metastasis of breast cancer, the diagnosis of coronary artery disease, or time of the follow-up survey.
Sacramento and Sierra Cancer Registries, The Cancer Surveillance Program of Orange County/San Diego, Greater San Francisco Bay Area Cancer Registry, Los Angeles County Cancer Surveillance Program.
The Danish Breast Cancer Cooperative Group Database, supplemented by the Danish Cancer Registry.
The State Health Registry of Iowa.
The Ontario Cancer Registry.
Cancer Surveillance System of the Fred Hutchinson Cancer Research Center.
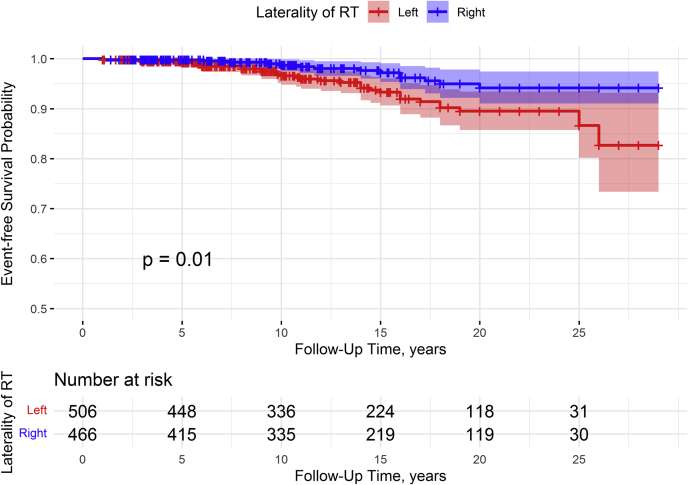
Table 2 shows the event-free survival for CAD in 5-year increments, stratified by laterality. Participants with right- or left-sided RT had equal event-free survival proportions for CAD before 5 years of follow-up (0.99), and participants with left-sided RT had a smaller event-free survival proportion for CAD at each subsequent time interval after 5 years of follow-up (P = 0.010) (Figure 2). A total of 46 participants reported a CAD diagnosis, and 91% of CAD diagnoses occurred more than 5 years after treatment with RT. The overall 27.5-year cumulative incidence of CAD for women who received left-sided RT was 10.5% (95% CI: 7.1-14.7), and the overall 27.5-year cumulative incidence of CAD for women who received right-sided RT was 5.8% (95% CI: 3.2-9.6) (P = 0.010) (Supplemental Figure 1). For women diagnosed with breast cancer at ages 25-39 years, the 27.5-year cumulative incidences of CAD were 5.9% for women receiving left-sided RT and 0% for women receiving right-sided RT; for women diagnosed with breast cancer at ages 40-54 years, the 27.5-year cumulative incidences of CAD were 18.7% for women receiving left-sided RT and 6.8% for women receiving right-sided RT. The absolute rate of CAD among women treated with left-sided RT was 4.7 events per 1,000 person-years, whereas the absolute rate of CAD among women treated with right-sided RT was 2.1 events per 1,000 person-years, conferring an absolute rate difference of 2.6 events per 1,000 person-years.
Table 2.
Event-Free Survival for CAD Diagnosis Among Women Receiving RT in the WECARE Study
| Follow-Up Timea Interval, y | Laterality of Breast Cancer | Participants Entering Interval, n | Participants With Event During Interval, n | Proportion of Event-Free Survival |
|---|---|---|---|---|
| 0 | Left | 506 | 0 | 1.00 |
| Right | 466 | 0 | 1.00 | |
| 1-4.9 | Left | 506 | 3 | 0.99 |
| Right | 466 | 1 | 0.99 | |
| 5-9.9 | Left | 448 | 10 | 0.97 |
| Right | 414 | 3 | 0.99 | |
| 10-14.9 | Left | 336 | 9 | 0.94 |
| Right | 335 | 4 | 0.98 | |
| 15-19.9 | Left | 224 | 8 | 0.90 |
| Right | 219 | 5 | 0.95 | |
| 20-24.9 | Left | 117 | 0 | 0.90 |
| Right | 119 | 1 | 0.94 | |
| 25-29 | Left | 30 | 2 | 0.83 |
| Right | 30 | 0 | 0.94 |
CAD = coronary artery disease; RT = radiation therapy; WECARE = Women's Environmental Cancer and Radiation Epidemiology
A woman's follow-up time is defined as the time from breast cancer diagnosis to the first to occur among the following: a new primary cancer, breast cancer recurrence/metastasis, diagnosis of coronary artery disease, or time of the follow-up survey.
Figure 2.
Event-Free Survival Probability of Coronary Artery Disease
Young women under age 55 years receiving left-sided radiation therapy (RT) for breast cancer had a statistically significantly shorter follow-up time than women receiving right-sided RT for breast cancer (P = 0.01).
Multivariable-adjusted risk of CAD
Women receiving left-sided RT had greater risk of CAD compared with women receiving right-sided RT in the multivariable model (HR: 2.5; 95% CI: 1.3-4.7) (Table 3). We also report the association between CAD risk and left-sided RT stratified by baseline risk factors. None of the interactions by the baseline risk factors reached statistical significance. Never smokers showed an increased risk of CAD associated with left-sided RT (HR: 3.3; 95% CI: 1.3-8.6) whereas for former or current smokers, there was no statistically significant association between RT laterality and CAD (HR: 1.7; 95% CI: 0.7-4.0; P for heterogeneity = 0.53). Women with a BMI ≥25 kg/m2 at breast cancer diagnosis had increased risk of CAD associated with left-sided RT (HR: 2.8; 95% CI: 1.1-7.3), and a similar association was observed among those with a BMI <25 kg/m2 (HR: 2.4; 95% CI: 1.0-5.6; P for heterogeneity = 0.77). Given the low number of events among participants with pre-existing conditions, we combined the cardiovascular risk factors of smoking history, prior hypertension, and prior hypercholesterolemia into 1 group, considering the presence of at least 1 risk factor. Stratification by this combined grouping of pre-existing cardiovascular risk factors likewise did not reveal a statistically significant effect modification of the association between left-sided RT and CAD (p for heterogeneity = 0.66). Stratification by presence or absence of 2 or more of these risk factors also did not reveal statistically significant effect modification on the multiplicative or additive scale of the association between left-sided RT and CAD (P for heterogeneity = 0.45; 95% CI for relative excess risk due to interaction: –0.8 to 7.5).
Table 3.
Associations Between Left- vs Right-Sided RT for Breast Cancer and CAD, Stratified by Lifestyle Factors
| Laterality of RT | N | Total Cardiac Events | HRa (95% CI) | P Value for Heterogeneity | |
|---|---|---|---|---|---|
| Overallb | Right | 466 | 14 | Reference | |
| Left | 506 | 32 | 2.5 (1.3-4.7) | ||
| Age at breast cancer diagnosis, y | |||||
| <45 | Right | 182 | 7 | Reference | 0.24 |
| Left | 214 | 11 | 1.6 (0.6-4.2) | ||
| 45-54 | Right | 284 | 7 | Reference | |
| Left | 292 | 21 | 3.5 (1.5-8.3) | ||
| Lifetime smoking history | |||||
| Ever | Right | 175 | 8 | Reference | 0.53 |
| Left | 215 | 16 | 1.7 (0.7-4.0) | ||
| Never | Right | 291 | 6 | Reference | |
| Left | 291 | 16 | 3.3 (1.3-8.6) | ||
| BMI at breast cancer diagnosis, kg/m2 | |||||
| <25 | Right | 305 | 8 | Reference | 0.77 |
| Left | 325 | 16 | 2.4 (1.0-5.6) | ||
| ≥25 | Right | 161 | 6 | Reference | |
| Left | 181 | 16 | 2.8 (1.1-7.3) | ||
| Prior history of hypertension | |||||
| Present | Right | 50 | 3 | Reference | 0.94 |
| Left | 43 | 5 | 5.2 (0.9-28.3) | ||
| Absent | Right | 416 | 11 | Reference | |
| Left | 463 | 27 | 2.6 (1.3-5.2) | ||
| Prior history of hypercholesterolemia | |||||
| Present | Right | 22 | 2 | Reference | 0.67 |
| Left | 29 | 6 | 17.6 (1.7-187.7) | ||
| Absent | Right | 444 | 12 | Reference | |
| Left | 477 | 26 | 2.3 (1.2-4.6) | ||
| Any pre-existing risk factorc | |||||
| Present | Right | 208 | 10 | Reference | 0.66 |
| Left | 255 | 22 | 2.1 (0.9-4.4) | ||
| Absent | Right | 258 | 4 | Reference | |
| Left | 251 | 10 | 3.7 (1.1-12.2) | ||
| 2 or 3 pre-existing risk factorsc | |||||
| Present | Right | 34 | 3 | Reference | 0.45 |
| Left | 25 | 5 | 3.2 (0.3-30.9) | ||
| Absent | Right | 432 | 11 | Reference | |
| Left | 481 | 27 | 2.5 (1.2-5.1) | ||
| Year of breast cancer diagnosis | |||||
| 1985-1999 | Right | 371 | 12 | Reference | 0.93 |
| Left | 399 | 28 | 2.5 (1.2-4.9) | ||
| 2000-2008 | Right | 95 | 2 | Reference | |
| Left | 107 | 4 | 2.3 (0.4-12.6) | ||
| Follow-up time, yd | |||||
| 1-4.9 | Right | 51 | 1 | Reference | 0.94 |
| Left | 58 | 3 | 2.5 (0.2-26.8) | ||
| ≥5 | Right | 415 | 13 | Reference | |
| Left | 448 | 29 | 2.5 (1.3-4.9) | ||
BMI = body mass index; CAD = coronary artery disease; RT = radiation therapy.
HRs and 95% CIs are estimated in Cox proportional hazards regression.
Model is adjusted for age at breast cancer diagnosis, race/ethnicity, recruitment site, and WECARE Study phase. Participants were censored at age of diagnosis of a new primary cancer, breast cancer recurrence/metastasis, or the time of the cardiac survey.
Pre-existing risk factors include ever smoking history, prior hypertension, and prior hypercholesterolemia.
A woman's follow-up time is defined as the time from breast cancer diagnosis to the first to occur among the following: a new primary cancer, breast cancer recurrence/metastasis, diagnosis of coronary artery disease, or time of the follow-up survey.
CAD risk was not significantly modified by receipt of chemotherapy, hormone therapy for breast cancer, or anthracycline exposure (all P for heterogeneity >0.05) (Table 4). Among women who received chemotherapy, there was a statistically significant association between RT laterality and CAD (HR: 3.3; 95% CI: 1.3-8.5), whereas among women who did not receive chemotherapy, the association was nonsignificant (HR: 2.1; 95% CI: 0.9-5.0; P for heterogeneity = 0.62).
Table 4.
Treatment Factors and Associations Between Left- Versus Right-Sided RT for Breast Cancer and CAD
| Laterality of RT | N | Total Cardiac Events | HRa (95% CI) | P Value for Heterogeneity | |
|---|---|---|---|---|---|
| Chemotherapy exposure | |||||
| Yes | Right | 279 | 6 | Reference | 0.62 |
| Left | 302 | 16 | 3.3 (1.3-8.5) | ||
| No | Right | 187 | 8 | Reference | |
| Left | 204 | 16 | 2.1 (0.9-5.0) | ||
| Hormone exposure | |||||
| Yes | Right | 206 | 6 | Reference | 0.69 |
| Left | 221 | 16 | 2.5 (0.9-6.5) | ||
| No | Right | 260 | 8 | Reference | |
| Left | 285 | 16 | 2.3 (0.9-5.4) | ||
| Anthracycline exposure | |||||
| Yes | Right | 139 | 4 | Reference | 0.66 |
| Left | 150 | 8 | 1.9 (0.6-6.5) | ||
| No | Right | 327 | 10 | Reference | |
| Left | 356 | 24 | 2.6 (1.2-5.4) |
Abbreviations as in Table 3.
HRs and 95% CIs are estimated in Cox proportional hazards regression model adjusted for age at breast cancer diagnosis, race/ethnicity, recruitment site, and WECARE Study phase.
Restricting the model to women with stage I breast cancer showed that the association between left-sided RT and risk of CAD was similar to the overall model (HR: 2.8; 95% CI: 1.3-6.1). Adjusting for year of breast cancer diagnosis rather than WECARE Study phase in the overall multivariable model showed that the association was also similar to the overall model (HR: 2.4; 95% CI: 1.3-4.6).
Discussion
This study shows that women receiving RT for left-sided breast cancer between 25 and 54 years of age and followed for a median of 14 years were more likely to experience subsequent CAD than women receiving RT for right-sided breast cancer (Central Illustration). Only 9% of CAD diagnoses were reported within the first 5 years of follow-up, supporting the need for long-term follow-up of younger breast cancer survivors to identify late cardiovascular disease as a result of RT. In the US population between 2015 and 2018, the risk of coronary heart disease among women aged 20-39 years was 0.9%, and among women aged 40-59 years the risk was 6.6% (24). For comparison, in our study, the risk of CAD among women who received right-sided RT is similar to that of the general population within similar age groups (0% for women between 25-39 years of age and 5.9% for women between 40-54 years of age, over 27.5 years of follow-up).
Central Illustration.
CAD After RT for Breast Cancer: The WECARE Study
Women <55 years of age at breast cancer diagnosis who were participants in the WECARE (Women’s Environmental and Radiation Epidemiology) Study were surveyed for coronary artery disease (CAD) outcomes following radiation therapy (RT). Participants were followed from the time of their breast cancer diagnosis until a CAD diagnosis or censoring event. Women who received left-sided RT were at more than twice the risk of a CAD event than women who received right-sided RT.
Our results add to the growing body of published reports suggesting that left-sided RT is an independent predictor of future cardiovascular disease risk (4,7, 8, 9, 10, 11, 12). In this study, we assessed incident CAD events among younger survivors (<55 years of age) of breast cancer, whom prior studies have not specifically evaluated. In addition, although most previous work relied on retrospective medical record reviews (4,7,9, 10, 11, 12), we prospectively surveyed a cohort of well-characterized breast cancer survivors for cardiovascular disease. Moreover, we are the first to evaluate detailed epidemiological and clinical information including BMI and smoking history as possible effect modifiers of radiation-associated cardiovascular disease.
Results of previous studies assessing the relationship between laterality of RT for breast cancer and cardiovascular disease have been inconsistent. Several studies found no evidence of a relationship between left-sided RT and cardiovascular disease risk (14,15,25), whereas others reported associations with varying effect sizes. A retrospective medical record review of 961 American women between 24 and 89 years of age with early-stage breast cancer and similar median follow-up time (12 years) showed an unadjusted association between left-sided RT and CAD that was similar to our study (incidence ratio: 2.7; 95% CI: 1.7-4.5) (7). Two large cancer registry-based studies of Scandinavian women drawing from similar registries (n > 19,000) reported small (incidence rate ratio <1.2), but statistically significant associations between left-sided RT and risk of broadly defined heart disease. The first study of 34,825 women assessed from the Danish Breast Cancer Cooperative Group and Stockholm and Umeå Breast Cancer Care Programs found an increased risk of ischemic heart disease after left-sided compared with right-sided RT for breast cancer (incidence rate ratio: 1.18; 95% CI: 1.07-1.30) (8), whereas the second study, assessing 19,464 women from the Danish Breast Cancer Cooperative Group only, also found an increased risk of heart disease in women treated with left- vs right-sided RT (incidence rate ratio: 1.10; 95% CI: 1.03-1.20) (10). The differences in magnitude of effect, or the finding of no statistically significant effect, between previously conducted studies and our study may be caused by differing ages at RT or differing follow-up times, variations in the definition of cardiovascular events, or country-specific differences in cardiovascular disease incidence rates and RT treatment doses over time. Despite these differences, there is evidence of an effect of treatment laterality on CAD risk among breast cancer survivors. Our study adds important information about CAD risk specifically among relatively young women receiving RT for breast cancer after controlling for clinical and lifestyle factors.
A previous study of 70,209 women followed for a median of 8.8 years found that when restricting the analysis to those diagnosed with breast cancer before age 50 years, the risk of a left- vs right-sided RT-associated cardiovascular event (subdistribution HR: 1.48; 95% CI: 1.07-2.04) was higher than the risk of a left- vs right-sided RT-associated cardiovascular event among women of all ages included in the study (subdistribution HR: 1.19; 95% CI: 1.04-1.36) (12). However, this study did not test heterogeneity of effects with women over 50 years of age. We did not find a statistically significant difference in the risk of CAD between participants 45-54 years of age and those below 45 years of age. However, the age distribution of participants in the WECARE Study is markedly younger than most studies of breast cancer survivors, confirming the importance of CAD risk assessment for women diagnosed with breast cancer under 55 years of age. Additionally, women in our study under 45 years of age at breast cancer diagnosis may require even longer follow-up to fully assess the risk of cardiovascular disease.
We did not observe effect modification by hormone therapy on the relationship between RT laterality and CAD risk. The absence of effect modification by hormone therapy confirms the results of previous studies, which showed no modification of heart disease risk by hormone exposure combined with RT (8,15).
The absence of radiation effect modification by anthracycline chemotherapy in this study contributes to the equivocal results from previous studies. Although one study of 19,464 women found no radiation-related difference in cardiac effects associated with laterality of RT for those treated with anthracyclines and those who were not (10), another study found a statistically significant increased risk of a cardiovascular event among women with left-sided RT treated with a cumulative doxorubicin equivalent dose of ≥250 mg/m2, but not among women treated with a lower dose. However, no association was seen when comparing left- to right-sided RT without consideration of chemotherapy received (14). Of note, during the years when WECARE Study participants were treated, chemotherapy regimens with ≥250 mg/m2 of anthracyclines were not used for adjuvant breast cancer therapy. Further research is needed to identify the types and doses of breast cancer chemotherapy that may increase the risk of RT-associated CAD.
Study strengths And limitations
The many strengths of this study included a long follow-up period, large numbers of breast cancer survivors treated with RT, and very detailed treatment and risk factor information. Nonetheless, there were some limitations. For example, we did not have detailed cardiac dosimetry and, therefore, used laterality of RT as a proxy for increased radiation dose received to the heart. Although there is variation in dose received to the heart, left-sided RT for breast cancer has been shown to have a significantly higher mean heart dose of radiation than right-sided RT (26). Therefore, laterality, while imperfect, is a reasonable indicator of relative dose. Further, while radiation dose to the internal mammary chain nodes delivers a high radiation dose to the heart (23), our sensitivity analysis demonstrating an effect of RT laterality even when the overall model was restricted to women with stage I breast cancer suggests that our results were not driven by effects of internal mammary chain node radiation; stage I breast cancer is not typically treated with RT to the mammary chain nodes. Cardiovascular disease was self-reported and was not verified overall through medical record review. However, in a validation study, self-reported cardiovascular disease events among Danish WECARE Study participants were compared with diagnoses recorded in the Danish National Patient Register, showing 80% agreement between self-reports and diagnoses in the National Patient Register (19). Further, any variation in CAD self-report is likely to be nondifferential with respect to RT laterality, which would bias our HR estimates toward the null. As with most follow-up studies, we cannot rule out the possibility of selection bias, especially regarding the radiation-associated CAD risk observed among women who participated in the WECARE Follow-Up Study. However, when we compared the characteristics of those who responded to the follow-up questionnaire with those who did not respond, we found no appreciable differences with respect to key risk factors (eg, age, stage, laterality, or receipt of RT), and all were diagnosed with stage I-II breast cancer. Last, we did not collect information on RT planning methods for cardiac dose sparing. Modern breast cancer RT planning includes cardiac dose sparing techniques such as prone positioning, breath hold techniques, and proton beam therapy, which reduce radiation dose to the heart (27). Therefore, women treated for breast cancer incorporating these contemporary RT techniques will likely have a lower risk of CAD compared with that seen in our study, as the majority of participants in our study were diagnosed with breast cancer before these techniques were introduced or commonly in use.
Implications
Our results expand our understanding of the late effects of RT for breast cancer and confirm that it may increase the risk of CAD among younger women (<55 years of age at breast cancer diagnosis) receiving RT to the left breast. Additionally, our study incorporated comprehensive clinical and epidemiological data in the analysis, allowing us to evaluate the multivariable-adjusted associations with risk of CAD in clinically relevant subgroups, some of which have not been previously studied as effect modifiers, including smoking history and BMI. Although we did not find evidence of effect modification by these factors, smoking history and BMI are important predictors of CAD risk that can be assessed during survival follow-up of women treated for breast cancer.
Future studies incorporating cardiac dosimetry and the use of newer cardiac dose sparing techniques may improve our understanding of the effects of differing RT treatment plans on the heart among younger women treated for breast cancer. Finally, studies of the risk of cardiovascular disease among women receiving both RT and chemotherapy as adjuvant treatment for breast cancer should be further investigated to determine the possible synergistic adverse effects of RT and chemotherapy treatments on the heart.
Conclusions
RT to the left breast was associated with increased risk of CAD compared with RT to the right breast among women treated for breast cancer between the ages of 25 and 54 years. CAD should be continually monitored in the years of survivorship following breast cancer diagnosis and treatment. As additional cardiovascular risk factors may emerge as younger women age during survivorship, these risk factors and resulting cardiovascular disease should also continue to be monitored and assessed in this population.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: RT to the left breast increases the risk of CAD among women diagnosed with breast cancer at <55 years of age. Given the latency period between RT and the development of CAD, long-term survivorship monitoring for cardiovascular disease in younger women treated with left-sided breast RT is warranted.
TRANSLATIONAL OUTLOOK: Future studies are needed to assess the additional risk that adjuvant chemotherapy may introduce in the relationship between left-sided breast RT and risk of CAD, specifically among younger women. Strategies to minimize radiation exposure to the heart and mitigate cardiovascular risk associated with breast RT are needed.
Funding Support and Author Disclosures
This work was supported by U01 CA083178, “Breast Cancer Radiation Exposure and the ATM Gene”; R01 CA097397, “Interaction of Radiation, BRCA 1/2, and Breast Cancer”; R01 CA114236, “Breast Cancer, Radiation and the ATM-Chek2 Pathway”; R01CA129639, “Genome-Wide Association Study of Radiation Exposure and Bilateral Breast Cancer”; CA008748, “Cancer Center Support Grant”; CA168339, “Mammographic Density and Risk of Contralateral Breast Cancer”; and CA206464, “Molecular pathoepidemiology of contralateral breast cancer.” Ms Carlson is employed by Bristol Myers Squibb, unrelated to this study. Dr Chow has received research funding from Abbott, unrelated to this study. Dr Yu has received consulting fees from Genentech and Ichnos Sciences. Dr Mellemkjӕr has an immediate family member employed at Novo Nordisk, and has an immediate family member who owns stocks in Novo Nordisk, unrelated to this study. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Mary Beth Terry for early guidance on this paper. In addition to the named authors, the WECARE Study Collaborative Group includes Memorial Sloan Kettering Cancer Center (Coordinating Center) investigators and staff of Jonine L. Bernstein (WECARE Study principal investigator), Marinela Capanu, Irene Orlow, and Mark Robson; collaborative site investigators Jørgen H. Olsen, Kathleen E. Malone, and Marilyn Stovall; and collaborative site staff Kristina Blackmore, Irene Harris, Rikke Langballe, Cecilia O’Brien, Rita Weathers, Michele West, Lisa Hunter, Judy Goldstein, and Elaine Ramos.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental figure and table, please see the online version of this paper.
Contributor Information
Gordon P. Watt, Email: wattg@mskcc.org.
WECARE Study Collaborative Group:
Jonine L. Bernstein, Marinela Capanu, Irene Orlow, Mark Robson, Jørgen H. Olsen, Kathleen E. Malone, Marilyn Stovall, Kristina Blackmore, Irene Harris, Rikke Langballe, Cecilia O’Brien, Rita Weathers, Michele West, Lisa Hunter, Judy Goldstein, and Elaine Ramos
Appendix
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.NIH National Cancer Institute . 2016. Breast Cancer SEER Survival Rates by Time Since Diagnosis, 2000-2016.https://seer.cancer.gov/explorer/application.html?site=55&data_type=4&graph_type=6&compareBy=age_range&chk_age_range_9=9&sex=3&race=1&stage=104&advopt_precision=1&advopt_display=2 Accessed June 2020. [Google Scholar]
- 3.Cancer Trends Progress Report: Breast Cancer Treatment. National Cancer Institute; 2020. https://progressreport.cancer.gov/treatment/breast_cancer Accessed June 2020. [Google Scholar]
- 4.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 5.Atkins K.M., Chaunzwa T.L., Lamba N. Association of left anterior descending coronary artery radiation dose with major adverse cardiac events and mortality in patients with non-small cell lung cancer. JAMA Oncol. 2021;7:206–219. doi: 10.1001/jamaoncol.2020.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergom C., Rayan D., Brown S.A. Predicting radiation-induced heart disease and survival-is location the key? JAMA Oncol. 2021;7:193–195. doi: 10.1001/jamaoncol.2020.6259. [DOI] [PubMed] [Google Scholar]
- 7.Harris E.E., Correa C., Hwang W.T. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 8.McGale P., Darby S.C., Hall P. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100:167–175. doi: 10.1016/j.radonc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Jagsi R., Griffith K.A., Koelling T., Roberts R., Pierce L.J. Rates of myocardial infarction and coronary artery disease and risk factors in patients treated with radiation therapy for early-stage breast cancer. Cancer. 2007;109:650–657. doi: 10.1002/cncr.22452. [DOI] [PubMed] [Google Scholar]
- 10.Rehammar J.C., Jensen M.B., McGale P. Risk of heart disease in relation to radiotherapy and chemotherapy with anthracyclines among 19,464 breast cancer patients in Denmark, 1977-2005. Radiother Oncol. 2017;123:299–305. doi: 10.1016/j.radonc.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa C.R., Litt H.I., Hwang W.T., Ferrari V.A., Solin L.J., Harris E.E. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol. 2007;25:3031–3037. doi: 10.1200/JCO.2006.08.6595. [DOI] [PubMed] [Google Scholar]
- 12.Boekel N.B., Schaapveld M., Gietema J.A. Cardiovascular disease risk in a large, population-based cohort of breast cancer survivors. Int J Radiat Oncol Biol Phys. 2016;94:1061–1072. doi: 10.1016/j.ijrobp.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 13.Handa N., McGregor C.G., Danielson G.K. Coronary artery bypass grafting in patients with previous mediastinal radiation therapy. J Thorac Cardiovasc Surg. 1999;117:1136–1142. doi: 10.1016/s0022-5223(99)70250-3. [DOI] [PubMed] [Google Scholar]
- 14.Kim D.Y., Youn J.C., Park M.S. Cardiovascular outcome of breast cancer patients with concomitant radiotherapy and chemotherapy: a 10-year multicenter cohort study. J Cardiol. 2019;74:175–181. doi: 10.1016/j.jjcc.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Geiger A.M., Chen W., Bernstein L. Myocardial infarction risk and tamoxifen therapy for breast cancer. Br J Cancer. 2005;92:1614–1620. doi: 10.1038/sj.bjc.6602562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein J.L., Langholz B., Haile R.W. Study design: evaluating gene-environment interactions in the etiology of breast cancer - the WECARE study. Breast Cancer Res. 2004;6:R199–R214. doi: 10.1186/bcr771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiner A.S., John E.M., Brooks J.D. Risk of asynchronous contralateral breast cancer in noncarriers of BRCA1 and BRCA2 mutations with a family history of breast cancer: a report from the Women's Environmental Cancer and Radiation Epidemiology Study. J Clin Oncol. 2013;31:433–439. doi: 10.1200/JCO.2012.43.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stovall M., Smith S.A., Langholz B.M. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int J Radiat Oncol Biol Phys. 2008;72:1021–1030. doi: 10.1016/j.ijrobp.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langballe R., John E.M., Malone K.E. Agreement between self-reported and register-based cardiovascular events among Danish breast cancer survivors. J Cancer Surviv. 2018;12:95–100. doi: 10.1007/s11764-017-0648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henson K.E., McGale P., Darby S.C., Parkin M., Wang Y., Taylor C.W. Cardiac mortality after radiotherapy, chemotherapy and endocrine therapy for breast cancer: cohort study of 2 million women from 57 cancer registries in 22 countries. Int J Cancer. 2020;147:1437–1449. doi: 10.1002/ijc.32908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22.Lin D.Y., Wei L.J., Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 23.Hooning M.J., Botma A., Aleman B.M. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 24.Virani S.S., Alonso A., Aparicio H.J. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 25.Wollschlager D., Merzenich H., Schwentner L. Self-reported long-term cardiac morbidity in breast cancer patients: a retrospective cohort study in Germany (PASSOS Heart Study) Breast Cancer Res Treat. 2017;163:595–604. doi: 10.1007/s10549-017-4215-7. [DOI] [PubMed] [Google Scholar]
- 26.Taylor C.W., Wang Z., Macaulay E., Jagsi R., Duane F., Darby S.C. Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys. 2015;93:845–853. doi: 10.1016/j.ijrobp.2015.07.2292. [DOI] [PubMed] [Google Scholar]
- 27.Shah C., Badiyan S., Berry S. Cardiac dose sparing and avoidance techniques in breast cancer radiotherapy. Radiother Oncol. 2014;112:9–16. doi: 10.1016/j.radonc.2014.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.