Physicists who study the behavior of galaxies posit the existence of invisible dark matter, which has mass but cannot be seen. The behavior of galaxies cannot be explained without the existence of something we cannot see (1). In this sense, asymptomatic infection is the dark matter of the current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. Asymptomatic infection is difficult to observe and characterize, by definition, as asymptomatic individuals are not sickened, do not present for care, and cannot be identified without testing. Nonetheless, the frequency with which such infection occurs is key to understanding the epidemiology of the pandemic. In PNAS, Sah et al. (2) provide a rigorous systematic review and metaanalysis of what we know about asymptomatic infection to date. Their review includes 390 studies—a testimonial to the intensity with which this question has been studied. Their results are important: Notwithstanding the virulence of SARS-CoV-2 infection, true asymptomatic infection is common (35%), and asymptomatic infection varies markedly by age, being far less common in older individuals (20%) than in children (47%), with symptomatic infection being more common in long-term care than other settings.
These authors (2) also address two important biases in the study of asymptomatic infection in their study and note that failure to address these biases distorts estimates of asymptomaticity. The first bias is an ascertainment effect associated with studies including symptomatic index cases in their estimates. The second bias is introduced when studies capture populations of infected individuals at a single time point, which means that presymptomatic individuals (symptomatic cases whose latent period has ended but who have not yet entered the symptomatic stage) are misclassified as asymptomatic. In their review, the authors find that failure to adjust for these biases results in a predictable underestimation of the frequency of asymptomatic infection in the former case, and overestimation of asymptomaticity in the latter.
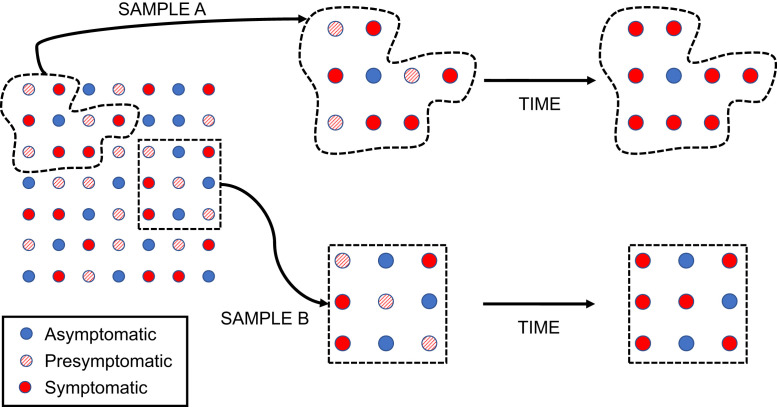
These biases, and their effects, are described in Fig. 1. The circles at the left-hand side of the figure make up a hypothetical population of infected individuals, with a true prevalence of asymptomatic infection (blue circles) of around 33%. If this population attracts notice as a result of an outbreak with notable illness, we may be more likely to sample symptomatic index cases, creating sample A. By contrast, if we are able to sample the population systematically, and obtain a representative sample of infectives, we will create sample B. If we ascertain the prevalence of symptoms at a single point in time, we will misclassify presymptomatic individuals (diagonally shaded circles) as asymptomatic. This will lead to overestimation of the prevalence of asymptomatic infection. In the diagram, 4/9 (44%) of the sample are “asymptomatic” at the first time point in sample A, while 6/9 (67%) are “asymptomatic” in sample B; both samples provide an overestimate of the true probability of asymptomatic infection. If we allow time to pass so that presymptomatic individuals become symptomatic, the probability of asymptomatic infection in sample A drops to 1/9 (11%), a marked underestimate. However, in sample B, the probability declines to 3/9 (33%), which reflects the true underlying probability of asymptomatic infection in the source population.
Fig. 1.
Biases encountered in the study of asymptomatic SARS-CoV-2 infection. Blue circles represent truly asymptomatic infections, while diagonally shaded circles represent presymptomatic infections. Red circles represent symptomatic infections. The nature and direction of biases in estimation of the percentage of infections that are truly asymptomatic, as represented in this figure, is described in the text.
Notwithstanding the virulence of SARS-CoV-2 infection, true asymptomatic infection is common (35%), and asymptomatic infection varies markedly by age, being far less common in older individuals (20%) than in children (47%), with symptomatic infection being more common in long-term care than other settings.
Why is asymptomatic infection so important? There are several reasons why we want to characterize this epidemiological attribute accurately.
-
1)
It has long been known that infectivity in individuals without symptoms makes communicable disease epidemics more difficult to control (3). Respiratory viral loads in asymptomatic individuals do not differ from viral loads in symptomatic infections (4), but asymptomatic individuals cannot by identified by symptom screens, and are less likely to modify their behavior (e.g., stay home, stay away from others) due to concern that they are sick.
-
2)
Failure to identify asymptomatic infections creates a distorted view of disease epidemiology; epidemic size is underestimated, and epidemic virulence is overestimated. Population groups that contribute disproportionately to epidemic spread but are asymptomatic (for example, younger individuals) may receive less focused attention than groups that suffer adverse outcomes (e.g., long-term care populations), ultimately resulting in a larger, more severe epidemic (5).
-
3)
Infectivity of asymptomatic individuals provides strong evidence for dominant aerosol transmission of SARS-CoV-2 infection (6). Large “ballistic” respiratory droplets travel short distances and are produced by respiratory activities like coughing and sneezing which are (by definition) absent in asymptomatic individuals (7). By contrast, aerosol production is a normal outcome of quiet breathing, and aerosol volume is markedly increased by activities like shouting and singing, which may explain the prominence of musical events as settings for superspreading events (8).
-
4)
The paradoxical nature of asymptomatic infection in a virulent disease with high infection fatality (9) is conceptually challenging, and may help contribute to misinformation and disinformation that have been a serious challenge in managing the pandemic (10).
Asymptomatic infection has been a signature of this pandemic from its earliest weeks. The outbreak on the Diamond Princess cruise ship, docked in Japan in February 2020, provided a (literally) captive, highly tested population in which the true prevalence of asymptomatic infection could be assessed. Mizumoto et al. (11) recognized the challenge of distinguishing presymptomatic infection from asymptomatic infection at that time, and estimated the true fraction of asymptomatic infection to be 18% in this older population, strikingly close to the estimate for older individuals presented by Sah et al. (2). Similarly, it is unsurprising that, when SARS-CoV-2 epidemics first emerged in Europe and North America, the sentinel for community spread was often the occurrence of high-mortality outbreaks in long-term care settings (12). The markedly increased virulence in long-term care populations (13), and lower likelihood of asymptomatic infection as noted by Sah et al., made long-term care outbreaks obvious sentinels for otherwise invisible community transmission. However, infected care workers with mild or asymptomatic infections are likely to have provided the mode of entry for the virus in many of these facilities (13), highlighting the importance of testing, particularly when care workers are unvaccinated.
The very high likelihood of asymptomatic infection in children poses a key challenge as Northern Hemisphere countries move toward autumn, and school reopening, in the context of emergence of variants of concern that are more virulent and transmissible. School closures have been associated with declines in epidemic growth throughout the pandemic (14), but, in the absence of systematic testing, the frequency of school outbreaks and the role of within-school transmission as a driver of community outbreaks have been challenging to quantify. Systematic testing in Israeli schools placed the frequency of asymptomatic infection in children at 51 to 70% (15), again, consistent with the findings of Sah et al. (2). These data suggest that safe school reopening requires consistent use of preventive measures, including masking, improved ventilation, and limitation of cohort sizes and high-risk activities such as indoor choir practices, even if outbreaks are not obviously occurring among children. Ideally, testing technologies that are acceptable for use in pediatric populations (such as saliva testing) would be helpful in maintaining situational awareness regarding the presence or absence of SARS-CoV-2 in school populations.
More broadly, the data presented by Sah et al. (2) reinforce the importance of widespread testing for maintenance of situational awareness and surveillance. Simply put, it is hard to fight an enemy you can’t see. Proof of concept on the impact of widespread use of testing has been seen at the level of nations, such as the remarkable impact of a testing blitz in Slovakia in late 2020 (16), and within organizations. Deep-pocketed professional sports teams have used frequent testing to keep players and coaching staff safe, and to maintain their playing schedules (17, 18). Given the economic damage inflicted by the pandemic to date (19), better epidemic control through widespread use of testing isn’t simply good public health practice, it likely makes economic sense as well.
Footnotes
Competing interest statement: D.N.F. serves on advisory boards of Seqirus, Sanofi Pasteur, Pfizer, and Astrazeneca vaccines, and consults for the Ontario Nurses Association, Elementary Teachers' Federation of Ontario, JP Morgan-Chase, WE Foundation, and Farallon Capital, outside the submitted work.
See companion article, “Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis,” 10.1073/pnas.2109229118.
References
- 1.CERN , Dark matter. https://home.cern/science/physics/dark-matter. Accessed 10 August 2021.
- 2.Sah P., et al., Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis. Proc. Natl. Acad. Sci. U.S.A. 118, e2109229118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser C., Riley S., Anderson R. M., Ferguson N. M., Factors that make an infectious disease outbreak controllable. Proc. Natl. Acad. Sci. U.S.A. 101, 6146–6151 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P. Z., et al., Heterogeneity in transmissibility and shedding SARS-CoV-2 via droplets and aerosols. eLife 10, 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisman D. N., et al., COVID-19 case-age distribution: Correction for differential testing by age. medRxiv [Preprint] (2020). 10.1101/2020.09.15.20193862. Accessed 5 July 2021. [DOI] [PMC free article] [PubMed]
- 6.Murray M. T., et al., Mitigating a COVID-19 outbreak among Major League Baseball players - United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 1542–1546 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenhalgh T., et al., Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet 397, 1603–1605 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller S. L., et al., Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air 31, 314–323 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin A. T., et al., Assessing the age specificity of infection fatality rates for COVID-19: Systematic review, meta-analysis, and public policy implications. Eur. J. Epidemiol. 35, 1123–1138 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West J. D., Bergstrom C. T., Misinformation in and about science. Proc. Natl. Acad. Sci. U.S.A. 118, e1912444117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizumoto K., Kagaya K., Zarebski A., Chowell G., Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance 25, 2000180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMichael T. M.et al.; Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team , Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N. Engl. J. Med. 382, 2005–2011 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisman D. N., Bogoch I., Lapointe-Shaw L., McCready J., Tuite A. R., Risk factors associated with mortality among residents with Coronavirus Disease 2019 (COVID-19) in long-term care facilities in Ontario, Canada. JAMA Netw. Open 3, e2015957 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haug N., et al., Ranking the effectiveness of worldwide COVID-19 government interventions. Nat. Hum. Behav. 4, 1303–1312 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Israeli Ministry of Health , Analysis of data on coronavirus morbidity in children. https://static1.squarespace.com/static/5e7b914b3b5f9a42199b3337/t/5fc59c993c02f22b9dcd92be/1606786208260/Schools+in+Israel.pdf. Accessed 12 August 2021.
- 16.Pavelka M.et al.; CMMID COVID-19 working group; Inštitút Zdravotných Analýz , The impact of population-wide rapid antigen testing on SARS-CoV-2 prevalence in Slovakia. Science 372, 635–641 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mack C. D., et al., Optimizing SARS-CoV-2 surveillance in the United States: Insights from the National Football League Occupational Health Program. Ann. Intern. Med. 174, 1081–1089 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mack C. D., et al., SARS-CoV-2 transmission risk among National Basketball Association players, staff, and vendors exposed to individuals with positive test results after COVID-19 recovery during the 2020 regular and postseason. JAMA Intern. Med. 181, 960–966 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson J. K., et al., Global economic effects of COVID-19. https://sgp.fas.org/crs/row/R46270.pdf. Accessed 11 August 2021.