Introduction
Syphilis is a bacterial infection caused by inoculation by the spirochete Treponema pallidum subsp pallidum. Between 2014 and 2018, rates of syphilis have increased in most provinces and territories throughout Canada, with at least 8 provinces and territories experiencing outbreaks.1 Syphilis passes through several stages; the initial stage of primary syphilis is characterized by a painless skin ulcer or chancre at the site of inoculation, appearing approximately 3 weeks following exposure. Extragenital chancres are rare and can occur at any mucocutaneous site exposed to an infectious lesion. This case report presents an extremely rare presentation of an extragenital chancre, involving the neck, that mimicked a cutaneous neoplasm.
Case report
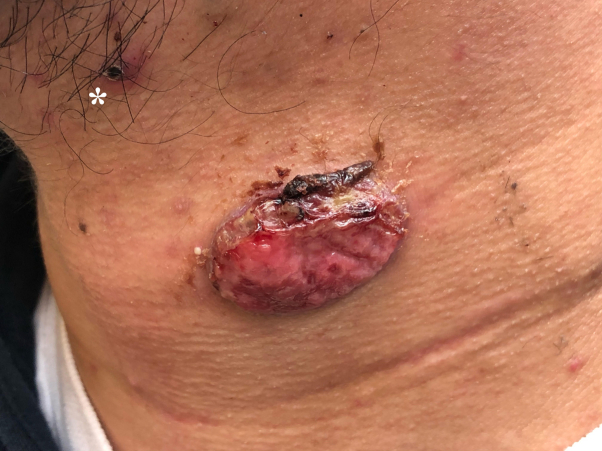
A 26-year-old man presented to an ambulatory care clinic in Saskatchewan, Canada, with a 3-month history of a gradually enlarging, fungating mass on the anterior aspect of the left side of his neck. Dermatologic examination performed by his primary care physician revealed a nontender, well-circumscribed, ulcerated, pink, ovoid mass measuring 3 × 2 cm with overlying scant serous exudate, surface crust, and a surrounding dull, erythematous border (Fig 1). Examination of the perilesional skin was significant for small, skin-colored papules as well as a dark, healed ulcer superior to the mass. The patient attributed the onset of the mass to localized trauma to the area caused by a cigarette burn and delayed seeking of medical attention. The patient denied constitutional symptoms. There were no oral lesions or regional lymphadenopathy present. The remainder of his medical history was unremarkable at the time of initial presentation, and there were no other pertinent findings on physical examination.
Fig 1.
A mass on the anterior aspect of the left side of the neck. Note the presence of an additional lesion (white asterisk) superior to the main chancre.
The patient was referred to a general surgeon, given the concerns for a cutaneous malignancy, and the mass was excised under local anesthesia for microscopic evaluation.
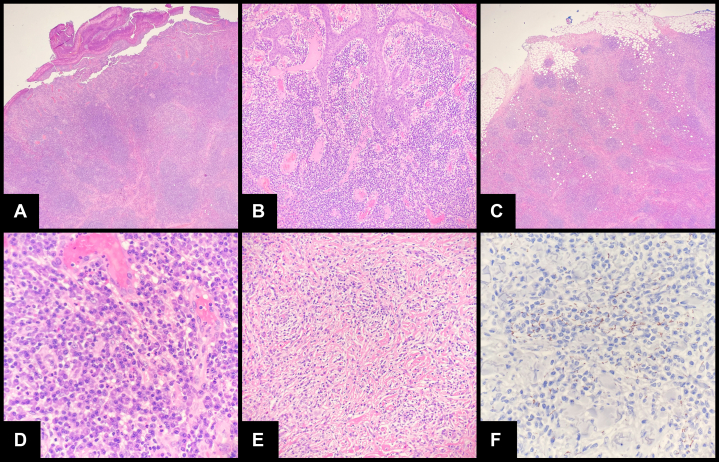
Histologically, sections (Fig 2, A to F) showed brisk papillary and reticular dermal inflammation (diffuse and nodular dermatitis pattern), including cytologically nonatypical lymphocytes, eosinophils, histiocytes, neutrophils, and numerous plasma cells extending from the papillary dermis into the subcutis. The entire mass was largely ulcerated; however, in areas of intact surface epithelium, long, slender retia were appreciated (highlighted using cytokeratin immunohistochemical stains [not shown]). The dermis was significant for hypervascularity in the papillary portion as well as established fibrosis in the reticular portion. There were also scattered, rare interstitial multinucleated giant cells and microabscesses in the papillary dermis (not shown).
Fig 2.
Sections show a predominantly ulcerated mass (A) with some intact surface epithelium with long, slender rete appreciated at (B) 1 lateral edge. C and D, Brisk superficial and deep lymphoplasmacytic inflammation is observed with (E) well-established fibrosis at the deep edge. F, Immunohistochemical stain for Treponema pallidum showed numerous spirochetes throughout the entire specimen (perivascular location has been shown).
Initial histologic impressions included cutaneous Castleman disease, pseudolymphoma, and an unusual presentation of IgG4-related disease, given the number of plasma cells. Immunohistochemical staining for human herpesvirus 8 was negative (not shown), and immunohistochemical staining for CD20, CD3, CD5, BCL2, CD21, CD23, and CD1a (not shown) confirmed benign lymphoid hyperplasia (pseudolymphomatous). Given the numerous plasma cells, immunohistochemical staining for κ and λ light chains was also performed, indicating no evidence of light chain restriction (not shown), and there was no increase in IgG4 plasma cells (IgG and IgG4 immunohistochemical staining was performed [not shown]). Histochemical staining (including Ziehl-Neelsen, Fite, Grocott-Gomori methenamine–silver nitrate, and periodic acid–Schiff) for bacterial and fungal organisms was negative (not shown).
An infectious process was not suspected by the general surgeon at the time of specimen submission for pathologic examination based on the clinical history. However, immunohistochemistry for spirochetes was performed by the pathologist, and, unexpectedly, numerous spirochete organisms morphologically consistent with T pallidum were identified throughout the epidermis and underlying dermis both with and without areas of angiocentric and vasculopathic distribution. Warthin-Starry histochemical staining was also performed (not shown), and some spirochetes were appreciated; however, these were fewer than in the immunohistochemical stain. This result was expediently communicated to the patient's primary care physician as well as the surgeon, and consultation with an infectious disease specialist was arranged. Subsequent prompt laboratory investigations included reactive syphilis enzyme-linked immunosorbent assay, syphilis rapid plasma reagin (RPR) with a titer of 1:64, and reactive T pallidum particle agglutination assay. Complete blood cell count and renal panel were within normal limits. Tests for HIV and hepatitis B and C viruses were negative. The culture of a superficial wound swab revealed Streptococcus pyogenes. Follow-up testing 3 months following treatment with a single intramuscular dose of 2.4 million units of benzathine penicillin G revealed a subsequent decrease in syphilis RPR to a titer of 1:16.
Discussion
This case illustrates an extremely uncommon clinical presentation of primary syphilis. Namely, the rare features of this case include the extragenital location on the neck, the fungating chancre that mimicked a cutaneous neoplasm, and the paucity of other associated clinical findings that typically accompany extragenital chancres, including hosts with HIV or other immunocompromizing conditions.
Syphilitic chancres have been reported to occur at many different extragenital locations in the literature over the last century.2 Fournier,3 in 1906, adamantly suggested that chancres have been observed from “head to toe” during times when the disease was not treatable. More recent estimates suggest that extragenital chancres do occur in the range of 5% to 10% of cases, and these predominantly involve the oral mucosa.4, 5, 6 Other rare anatomic locations have been reported to include the hands, arms, chest, breasts, abdomen, legs, and feet.4 Interestingly, the presence of a primary syphilitic chancre on the neck raises the question of how this could occur, as it is exceedingly rare.7, 8, 9, 10, 11, 12
Primary syphilis inoculation occurs via a broken skin barrier or intact mucosal surface.13 Previous reports of extragenital chancres have occurred on a mucosal surface or as a result of a compromised skin barrier. Examples include venereal and nonvenereal transmission to traumatized skin (eg, human bites, scratches, unprotected hands of health care providers, shared syringes).14, 15, 16, 17 This mechanism also explains the high incidence of oral chancres and anal chancres, specifically in patients who engage in anal sex.5 Additionally, the high rate of coinfection of syphilis and HIV can be attributed, at least in part, to the mucosal barrier disruption associated with chancre formation in addition to immunologic and behavioral factors.18 The patient in this case reported having sustained a cigarette burn on his neck during an evening of binge drinking 3 months prior to the presentation that progressed over time. Additional history gathered after excision of the mass revealed that the patient was a heterosexual man with a history of sexual contact with sex workers and alcohol use disorder. Therefore, in the setting of a primary chancre, there is a reason to postulate that the patient may have been infected at the site of the cigarette burn, perhaps by an oral lesion of an infected sexual partner.
A case of a primary pseudoneoplastic chancre has not been reported, to our knowledge. Microscopic examination and laboratory findings support that this case represents a primary syphilitic chancre of the neck that mimicked a neoplastic process clinically, with bacterial superinfection. Primary chancres usually resolve within a 3- to 6-week time period. However, chancre resolution is sometimes delayed in cases of immunosuppression (eg, HIV infection) and secondary bacterial infection (commonly anal chancres).19 Our patient was immunocompetent and experienced delayed chancre resolution due to secondary infection with S pyogenes confirmed with tissue culture and the accompanying exuberant pseudolymphomatous reaction that formed a mass on the neck. A gumma of tertiary syphilis was considered in the differential diagnosis for this case. However, the clinical and histopathologic data are not congruent with tertiary syphilis: (1) the patient denied a history of the signs and symptoms of secondary syphilis, (2) the patient has a documented negative RPR titer <2 years prior to this case presentation, (3) histologically, sections lacked granulomatous inflammation and caseous necrosis, hallmark features of gummas, and, finally, (4) the immunohistochemical staining pattern of spirochetes was the most consistent with primary syphilis, showing a mixed immunohistochemical staining pattern, including an epitheliotropic and vasculotropic staining pattern.20,21 The smaller lesion, superior to the primary mass, was not biopsied, and, therefore, we cannot conclude whether this represents an additional chancre. A report of multiple chancres at a rare extragenital site has not yet been previously described in the literature.
The patient's chancre was completely excised, and he was subsequently treated with intramuscular benzathine penicillin G with an observed decrease in syphilis RPR from 1:64 to 1:16 over a 3-month period.
This case represents a rare presentation of primary syphilis and highlights the remarkable variability in disease presentation. Specifically, this case was diagnostically challenging due to the noteworthy clinical presentation and paucity of clinical information at the initial presentation. However, clinical details, including patient's age, sexual history, and high local incidence of syphilis, are clues to inform a differential diagnosis of a large solitary extragenital chancre.
Conflicts of interest
None disclosed.
Acknowledgments
We would like to acknowledge Reid McGonigle, MD, for providing historical details surrounding the initial presentation of this case.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Public Health Agency of Canada Infectious syphilis in Canada, 2018 (infographic) Can Commun Dis Rep. 2019;45(11):302. http://www.canada.ca/content/dam/phac-aspc/documents/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2019-45/issue-11-november-7-2019/ccdrv45i11a05-eng.pdf [Google Scholar]
- 2.Dourmishev L.A., Dourmishev A.L. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23(6):555–564. doi: 10.1016/j.clindermatol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Fournier A. The treatment and prophylaxis of syphilis. Rebman Limited. 1906;86:118. [Google Scholar]
- 4.Mindel A., Tovey S.J., Timmins D.J., Williams P. Primary and secondary syphilis, 20 years' experience. 2. Clinical features. Genitourin Med. 1989;65(1):1–3. doi: 10.1136/sti.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapel T.A., Prasad P., Chapel J., Lekas N. Extragenital syphilitic chancres. J Am Acad Dermatol. 1985;13(4):582–584. doi: 10.1016/s0190-9622(85)70200-9. [DOI] [PubMed] [Google Scholar]
- 6.Allison S.D. Extragenital syphilitic chancres. J Am Acad Dermatol. 1986;14(6):1094–1095. doi: 10.1016/s0190-9622(86)80197-9. [DOI] [PubMed] [Google Scholar]
- 7.Lewe I.A. Extragenital chancre; with report of a case of chancre of the neck. Ann West Med Surg. 1950;4(1):37–39. [PubMed] [Google Scholar]
- 8.Yarington C.T., Jr., Jensen O.C. Syphilis as a differential diagnosis of neck masses. Arch Otolaryngol. 1967;86(2):219–221. doi: 10.1001/archotol.1967.00760050221022. [DOI] [PubMed] [Google Scholar]
- 9.Fuehrer N.E., Furukawa B.J., Kowalewski C.L., Cadena-Zuluaga J., Becker L.E., Fernandez M.P. A 48-year-old man with a crusted plaque on the left side of the neck: challenge. Am J Dermatopathol. 2010;32(1):44–45. doi: 10.1097/DAD.0b013e3181ac52ff. 99-100. [DOI] [PubMed] [Google Scholar]
- 10.Iglesias-Plaza A., Arando M. Ulcerated nodule on the neck: syphilitic chancre. Med Clin (Barc) 2018;151(12):e73. doi: 10.1016/j.medcli.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Olsavszky V., Géraud C. Liaison leads to solitary syphilitic chancre on the neck. Lancet. 2018;392(10162):2397. doi: 10.1016/S0140-6736(18)32620-5. [DOI] [PubMed] [Google Scholar]
- 12.Ramoni S., Genovese G., Pastena A., Cusini M. Primary syphilis of the neck mimicking pyodermatitis. Sex Transm Dis. 2020;47(10):e45–e46. doi: 10.1097/OLQ.0000000000001210. [DOI] [PubMed] [Google Scholar]
- 13.Lafond R.E., Lukehart S.A. Biological basis for syphilis. Clin Microbiol Rev. 2006;19(1):29–49. doi: 10.1128/CMR.19.1.29-49.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrade P., Juliao M.J., Reis J.P., Figueiredo A. Secondary syphilis with persisting hard chancre on the forearm. Acta Derm Venereol. 2013;93(2):236–237. doi: 10.2340/00015555-1355. [DOI] [PubMed] [Google Scholar]
- 15.Han J.Y., Oh M.D., Lee S.H., Kim N.J., Kim C.W., Choe K.W. Extragenital primary syphilis acquired by scratching with the fingernails. Korean J Infect Dis. 2000;32(2):164–166. [Google Scholar]
- 16.De Koning G.A., Blog F.B., Stolz E. A patient with primary syphilis of the hand. Br J Vener Dis. 1977;53(6):386–388. doi: 10.1136/sti.53.6.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonci A., Di Lernia V., Lo Scocco G., Bisighini G. A patient with primary syphilis of the finger. Acta Derm Venereol. 2001;81(5):382–383. doi: 10.1080/000155501317140223. [DOI] [PubMed] [Google Scholar]
- 18.Solomon M.M., Mayer K.H. Evolution of the syphilis epidemic among men who have sex with men. Sex Health. 2015;12(2):96–102. doi: 10.1071/SH14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rompalo A.M., Joesoef M.R., O'Donnell J.A. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis. 2001;28(3):158–165. doi: 10.1097/00007435-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Moon J., Yu D.A., Yoon H.S., Cho S., Park H.S. Syphilitic gumma: a rare form of cutaneous tertiary syphilis. Ann Dermatol. 2018;30(6):749–751. doi: 10.5021/ad.2018.30.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martín-Ezquerra G., Fernandez-Casado A., Barco D. Treponema pallidum distribution patterns in mucocutaneous lesions of primary and secondary syphilis: an immunohistochemical and ultrastructural study. Hum Pathol. 2009;40(5):624–630. doi: 10.1016/j.humpath.2008.10.017. [DOI] [PubMed] [Google Scholar]