Significance
Peatlands are sensitive ecosystems that store carbon and water and support biodiversity. Currently, European peatlands are threatened by climate change and exploitation. In this study, we show that many landscape settings may support both wetland ecosystems on thick peat soils and forest ecosystems on thin organic soils. Both ecosystems have distinctly different water–carbon dynamics that create internal positive feedbacks, allowing both ecosystems to coexist (bistability) but also to shift when critical limits are exceeded. With this new landscape perspective, we find that currently, 20% of European raised bogs are threatened by climate change and drainage. This study demonstrates that a landscape perspective including interactions between peatlands, forests, and rivers is essential to understand and steer the future of peatlands.
Keywords: peatlands, water–carbon feedbacks, resilience
Abstract
Northern peatlands store large amounts of carbon. Observations indicate that forests and peatlands in northern biomes can be alternative stable states for a range of landscape settings. Climatic and hydrological changes may reduce the resilience of peatlands and forests, induce persistent shifts between these states, and release the carbon stored in peatlands. Here, we present a dynamic simulation model constrained and validated by a wide set of observations to quantify how feedbacks in water and carbon cycling control resilience of both peatlands and forests in northern landscapes. Our results show that 34% of Europe (area) has a climate that can currently sustain existing rainwater-fed peatlands (raised bogs). However, raised bog initiation and restoration by water conservation measures after the original peat soil has disappeared is only possible in 10% of Europe where the climate allows raised bogs to initiate and outcompete forests. Moreover, in another 10% of Europe, existing raised bogs (concerning ∼20% of the European raised bogs) are already affected by ongoing climate change. Here, forests may overgrow peatlands, which could potentially release in the order of 4% (∼24 Pg carbon) of the European soil organic carbon pool. Our study demonstrates quantitatively that preserving and restoring peatlands requires looking beyond peatland-specific processes and taking into account wider landscape-scale feedbacks with forest ecosystems.
Northern soils (latitude >35° N) are estimated to contain over 65% of the global soil organic carbon pool (1–5). Here, wet soil conditions and low temperatures slow down the decay of plant material (6) and cause organic matter to accumulate in peat soils (2, 7). Soils rich in organic matter can hold more water and are often less permeable to water flow than the underlying mineral soils (8). In the European domain of this study, trees that may increase evaporation, dry the soil, and induce decay of organic matter (9–12) do not grow well under such wet and organic conditions (7, 13, 14). Hence, organic matter accumulation, which increases soil wetness, suppresses tree growth, and thus stimulates further organic matter accumulation, is a strong feedback sequence that reinforces the wet state of wetlands (7, 8, 13). This feedback sequence also operates in the opposite direction. It starts with tree initiation on a peat soil during prolonged dry periods. The growing trees increase water usage and thereby dry the soil further (15). The peat soil decomposes, which enhances tree growth until water shortages start to occur (16–18).
From these opposing feedback sequences, two dominant alternative ecosystem states emerge: relatively dry forests with high biomass, high water use, and a thin organic soil (from here on referred to by “forest”) versus peat-forming wetlands with few and/or small trees, relatively low biomass, low water use, and a thick organic soil (19–22) (from here on referred to by “peatland”). The interplay between climate, subsurface, and the local hydrological conditions controls which state occurs or whether both states are potentially stable for a particular landscape setting. In the latter situation, which we refer to by “forest–peatland bistability,” the past trajectory determines which state currently prevails. Under specific conditions, trees may also grow abundant on peatlands. This likely reflects a transitional state (both in space and/or in time) from peatland to forest (23, 24) or specific conditions that may create additional stable states such as frozen soils that allow organic matter accumulation despite the dryer conditions required for trees (25). Frozen soils are not considered in this study. The opposing feedback sequences operate at the location of a forest or peatland but also extend to their surroundings via surface and groundwater water flows (Fig. 1B). Such short- and long-range feedbacks typically underlie patterned landscapes (26, 27). The commonly observed patterned landscapes with sharp transitions between forests and peatlands (13, 22) (Fig. 1A) are thus consistent with the hypothesis of opposing feedback sequences creating bistability between forests and peatlands. Although bistability and patterning between trees and wetland vegetation is known from studies on individual peatlands (16, 27), we argue that much of the northern biome can be viewed as a patterned landscape where both forests and peatlands are potentially stable (19). With this landscape perspective, we aim to identify the European peatland regions most sensitive to tree growth and the implications of such ecosystem shifts for soil carbon storage.
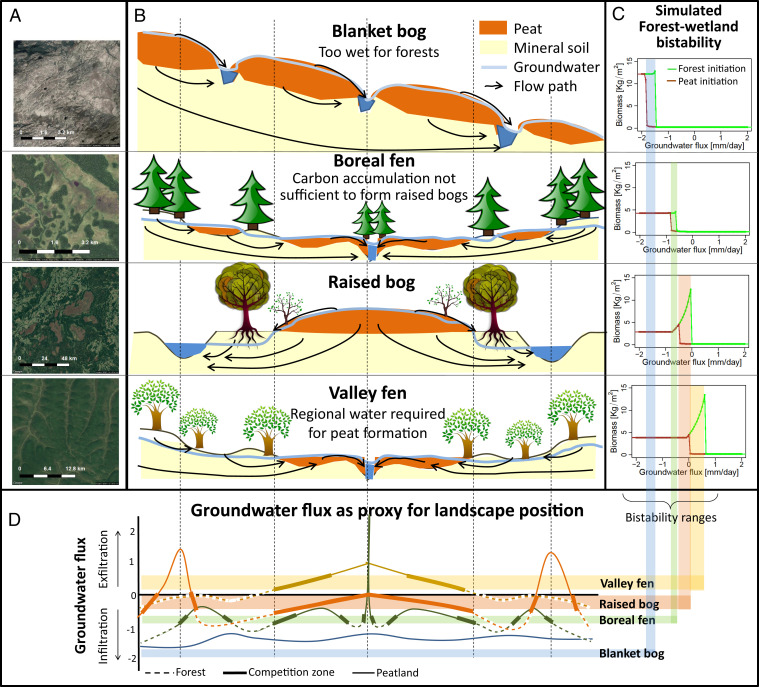
Fig. 1.
Landscape position and dominant hydrological controls on peatland type. Satellite images of typical spatial patterns between peatlands (brown or light green) and forests (dark green) for the four peatland types (Google Images, 2017) (A). Conceptual models of the dominant groundwater fluxes for each of the peatland types (i.e., the long-range feedbacks) (B). Examples of simulated landscape-scale biomass bistability diagrams for each peatland type as controlled by groundwater flux (Classification and SI Appendix, Fig. S2.1). The brown line represents the average aboveground biomass after 800 to 1,000 y for simulations that were initiated with a 2-m-thick peat layer, and the green line represents simulations that were initiated with a forest and a 30-cm-thin organic layer. The range on the x-axis where both lines deviate represents the bistability range (shaded colors). Note that the low biomass values are not zero but small value between 0 and 1 kg C/m2 (C). The groundwater flux (Drain or Drown: Ecosystem Bistability and Peatland Type) is a proxy for landscape position. The bistability range thus identifies the landscape zones where competition between peatland and forests may lead to shifts between peatland and forests under the current climate and where competition may induce spatial peatland–forest patterning (D).
Most European peatlands initiated and evolved during the Holocene (4, 28). However, given the substantial climate variability during the Holocene, it is likely that some of these peatlands formed under climate conditions that were more suited for peatland formation than the current climate (4, 29, 30). Such peatlands can persist under the current climate due to the described feedback mechanisms that enable these peatlands to maintain wet conditions under drier and warmer climates (7, 31). However, continued climate change and artificial drainage may reduce the resilience (32) of peatlands to cope with climate variability. When peatland resilience is exceeded (i.e., when peatlands experience a change that exceeds a critical threshold), shifts from peatland to forest, possibly amplified or initiated by peatland fires (33), and a subsequent release of large amounts of carbon to the atmosphere (3, 34) have been observed (23, 35). These interactions between peatland and forest ecosystems are not yet taken into account in current global peatland models and are a major source of uncertainty in their predictions (28, 36). Thus, to improve our understanding of the future of peatlands, it is essential to quantify the range of climatic and anthropogenic changes under which peatlands remain resilient to disturbances and under which conditions peatland resilience is exceeded, peatland ecosystems shift toward forest ecosystems, and recovery becomes exceedingly difficult (2, 3, 35, 37). In this study, we explore the hydrological and climatological conditions at which peatlands are likely to shift to forests and vice versa throughout Europe. These tipping points, the bistable range between both tipping points, and vegetation dynamics approaching the tipping points are related to peatland type, peatland resilience, and potential carbon release in Europe.
Results and Discussion
Drain or Drown: Ecosystem Bistability and Peatland Type.
We use a dynamic simulation model that links vegetation (living aboveground biomass), soil organic matter, and water and carbon uptake rates of both forests and peatlands to climate (Methods and Materials and SI Appendix, Fig. S1.1). This model is used to identify, for all of Europe south of the tree line, the range of landscape positions where both forests and peatlands are stable under current climatic conditions. The lateral flow of groundwater is assumed to be the main long-range feedback mechanism that regulates competition for favorable growing conditions between forests and peatlands in which forests tend to drain peatlands (8), and peatlands drown forests (7). This lateral flow of groundwater strongly depends on soil type, geology, and permeability of the subsurface and topography. To account for a wide range of landscape positions, we introduced a “groundwater flux” (conceptually illustrated in Fig. 1 B–D, and model results example shown in SI Appendix, Fig. S2.1), which collapses the two-dimensional landscape into a single variable (from Fig. 1D to the x-axis of Fig. 1C). Positive groundwater fluxes represent landscape positions where groundwater flow increases the water availability in the soil profile (referred to by “exfiltration sites”), which typically occur in river valleys or depressions. Negative groundwater fluxes correspond to infiltrating landscape positions (for example, in coarse upslope soils), while near-zero groundwater fluxes represent impermeable soils or flat, wet landscape positions with negligible infiltration. The model parameters and relationships were selected based on literature (SI Appendix, Model Setup) such that simulated equilibrium states (after 1,000 y of simulation) for both forests with optimal water supply and forests that cannot reach saturated groundwater closely envelope observed standing biomass, net primary productivity (NPP), and soil carbon stocks for the European climatic conditions (Fig. 2 A and B and SI Appendix, Water and Carbon Stores and Fluxes). Water availability is thus assumed to control the position of a forest within the envelope. For peatlands, the model is set up to yield realistic water and carbon fluxes (Fig. 2 C and D and SI Appendix, Water and Carbon Stores and Fluxes). The simulated long-term rates of carbon accumulation were close to observed values (root mean square error = 4.3 g/m2/y, red and yellow points in Fig. 2D), while the simulated net atmospheric exchange rates were within the range of the observed interannual variability (vertical and horizontal bars in Fig. 2D). The model setup for Europe was validated against similar Canadian forest and peatland datasets, which confirmed its robustness (SI Appendix, section S3.2).
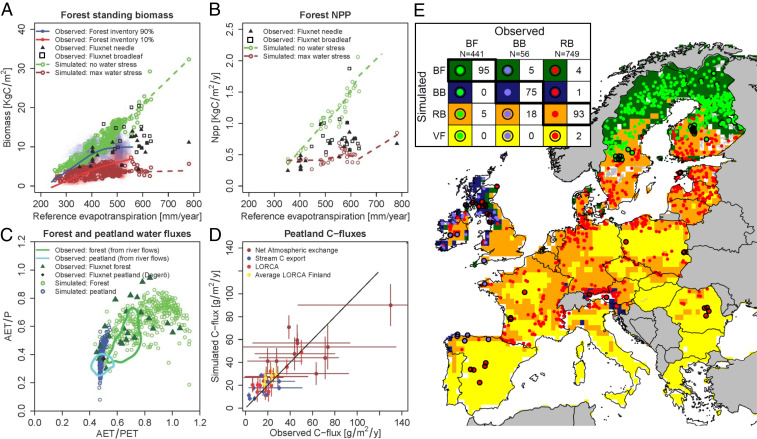
Fig. 2.
Observed versus simulated properties for forests and peatlands. Model comparison with observed relationships between reference evapotranspiration and forest biomass (A), forest net primary production (B), forest and peatland water fluxes (C), and peatland carbon fluxes (D) (refer to SI Appendix, Water and Carbon Stores and Fluxes for the locations of the sites and forest soil carbon). In A and B, we simulated forests that have an optimal water supply (green dots) and forests that cannot reach saturated groundwater (dry forests, brown dots). The brown and green dashed lines represent smoothed lines through the simulated values for the same locations. The percentages 90 and 10% in the legend of A refer to the 0.9 and 0.1 percentile biomass for forests older than 60 y (SI Appendix, Model Example). The green and blue solid lines (C) are estimates of peatland and forest water usage derived from river runoff in Sweden (11). The black solid line (D) is the 1:1 line, and the horizontal bars indicate year-to-year variability when multiple years are measured, while the vertical bars indicate the SD of the year-to-year modeled fluxes. (Refer to SI Appendix, Fig. S2.1 for additional validation with Canadian datasets). Validation and verification of potential peatland type classification (Classification) with recorded spatial distribution of peatland types (E) [>1,200 sites, Natura 2000 ecotypes (38); BF = boreal fen, BB = blanket bog, RB = raised bog, VF = valley fen]. Dots and grids represent observed and simulated peatland types, respectively. Black circled dots indicate wrongly simulated peatland types (wrong when distance between observed type and corresponding simulated type are >80 km). The color legend is given by colors in the confusion matrix (Inset in E). The numbers in the confusion matrix represent percentages of occurrence of that combination of observation and simulation (100% is perfect). All gray countries (E) are not covered by the Natura 2000 dataset.
For each position in Europe, 1,000 y of ecosystem development was simulated, initiated from both a forest and a peatland state under a large range of external groundwater fluxes. These results were summarized in biomass bistability diagrams (one per position, Fig. 1C shows examples; Methods and Materials). Most importantly, the bistability diagram quantifies the threshold groundwater flux of the tipping points from peatland to forest and from forest to peatland (TIP [threshold infiltration flux for peatlands] and TEF [threshold exfiltration flux for forests] in Table 1) and the range in groundwater fluxes where both states are potentially stable. These bistability diagrams are consistent with definitions of well-known peatland types (39) (Fig. 1) with each type related to specific carbon storage properties. The bistability diagrams are classified into potential peatland types (Methods and Materials). With “potential,” we mean that climate supports these peatland types but that topography, soil type, and geology may cause groundwater flows (both infiltration and exfiltration) that prevent these types from actually occurring. The distinction between groundwater-fed “valley fens” and rainwater-fed “raised bogs” is a fully physical one: a region is assigned “valley fen” when peat can only form with additional water supply. Such exfiltration conditions typically occur in valleys or depressions (Fig. 1B), which cover small fractions of the landscape [in Europe, on average eight NATURA2000 (38) peatlands per 104 km2; SI Appendix, Estimate of Potential Carbon Loss]. In contrast, in places where peat-forming wetlands can persist under infiltration conditions once they have established, they can grow into “raised bogs” of up to 12 m thick and cover large areas (39) (Fig. 1B). As such, raised bog regions represent large carbon stores (200 to 300 PgC; SI Appendix, Estimate of Potential Carbon Loss) with on average 18 peatlands per 104 km2 (38) (SI Appendix, Table S5.1). The high agreement between the simulated and observed position of the boundary between raised bogs and valley fens is a strong independent model validation (Fig. 2E).
Table 1.
Definitions, criteria, and reasoning used to classify the bistability diagrams into peatland types and raised bog sensitivity
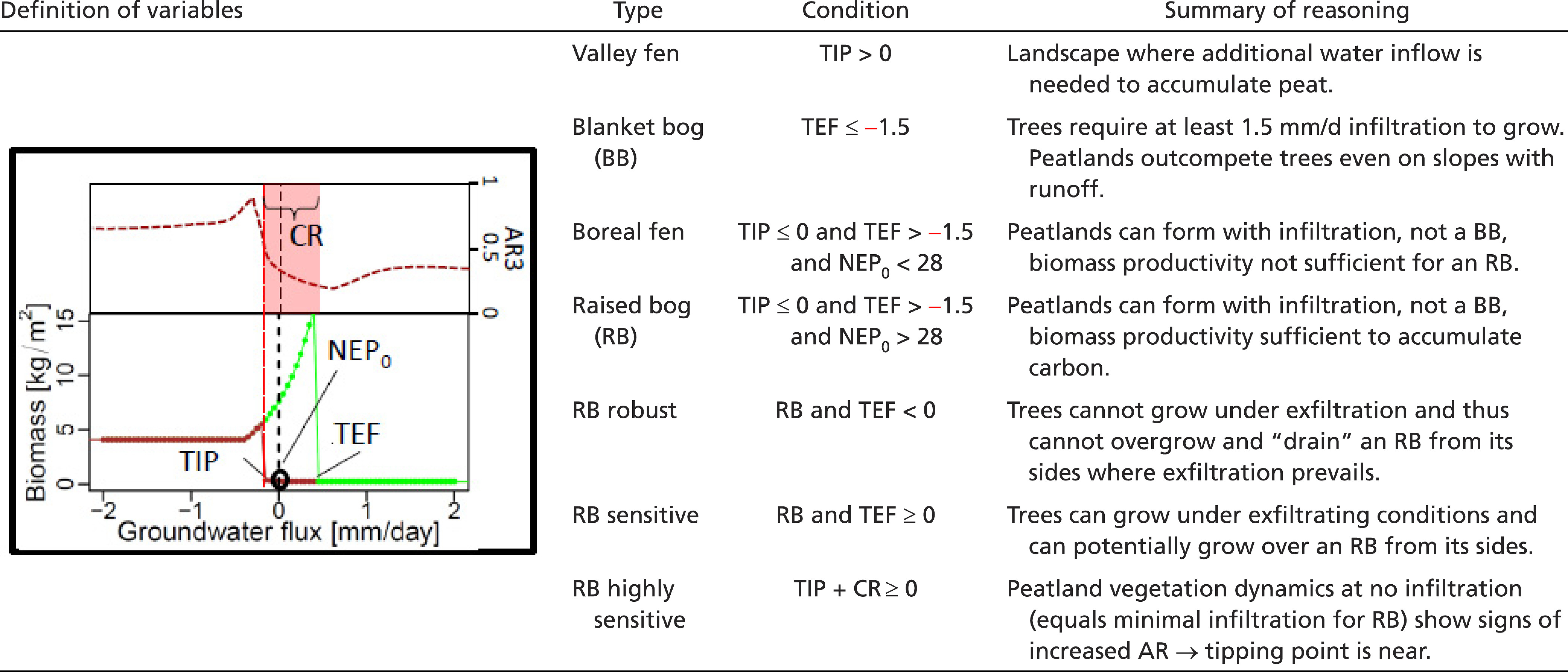
|
TIP, infiltration at tipping point from peatland to forest (mm/d); TEF, exfiltration at tipping point from forest to peatland (mm/d); NEP0, net ecosystem productivity of the peatland initiated run with 0 groundwater flux (gC/m2/y); CR, critical flux (mm/d).
The classifications of “boreal fens” and “blanket bogs” are additional conditions to the conditions required for “raised bogs.” These classes are based on a combination of physical reasoning and interpretation of the observed spatial pattern. At high latitudes (∼>60° N), peatlands do not tend to grow into raised bogs but remain confined to valleys and depressions even though enough water is available (boreal fens). We hypothesize that peat accumulation in boreal fens is too slow to overcome the temporary carbon loss associated with a species shift from more nutrient-rich groundwater-fed fens to nutrient-poor rainwater-fed raised bogs. By overlaying the observed spatial transition between raised bogs and boreal fens with our model results, we estimated this threshold net carbon accumulation (NEP) at 28 ± 3 gr C/m2/y (Fig. 2E). Boreal fens tend to occupy a significant fraction of the landscape (21 peatlands per 104 km2; SI Appendix, Estimate of Potential Carbon Loss), but remain thin compared to raised bogs (39). Given their large spatial extent, boreal fens represent a large carbon store (150 to 200 PgC; SI Appendix, Estimate of Potential Carbon Loss), which has been shown to be sensitive to continued and intensive drainage and forestry (9, 40).
Similarly, we found that blanket bogs occur under such wet climates that our model predicts that despite high drainage rates of more than 1.5 ± 0.3 mm/d (∼550 ± 110 mm/y; Fig. 2E), it is still too wet for tree growth. This means that peat can even form into hillside blankets on sloping terrain (Fig. 1B). Overall, our modeled peatland types correspond to 93% of the observed N2000 types (Fig. 2E) of which the simulated boundary between raised bogs and valley fens, which is the most important transition for this study, is completely independent of the observed N2000 types.
Peatland Resilience.
In Europe, the current boundary between landscapes with and without raised bogs (i.e., raised bogs versus valley fens) coincides closely with a yearly average dryness index (ratio of potential evapotranspiration to precipitation, PET/P) of 1 (Fig. 2E and see Fig. 4D), with only few exceptions. This is a strong indication that when the long-term dryness index rises above 1 due to climate change, raised bogs are likely to disappear completely. However, also the peatlands near the edge of their potential region may be at risk for disappearing due to being less resilient to extreme weather episodes and human disturbances or nonideal groundwater flows. In the context of our study, we define peatland resilience (32, 34) as the capacity of peatlands to absorb climate, natural, and artificial disturbances by reorganizing its biomass growth, species composition (41), peat decomposition, and water storage while maintaining its current functioning as carbon store. Resilience, often visualized with stability landscapes (19, 42) (examples in Fig. 3), entails many aspects (32, 43) and therefore cannot easily be fully quantified. From our model results, we selected three key resilience aspects, which we combined to identify the sensitivity of raised bogs in the next section:
-
1)
Threshold infiltration of peatlands (TIP): The groundwater flux at the tipping point from peatland to forest (Fig. 4A).
-
2)
Restoration resistance (TEF-TIP): The range in groundwater flux where both forests and peatlands are stable states (shaded bands in Fig. 1C, illustrated in model results of SI Appendix, Fig. S2.1 and in Fig. 3). When a peatland is artificially drained and is overgrown by forests, the restoration resistance indicates how much the groundwater flux needs to be restored to induce a shift back into peatland (Fig. 4B).
-
3)
Critical flux: When an ecosystem state approaches a tipping point, typically a “critical slowing down” is observed (43). Here, we quantified this critical slowing down by the increase in the 3-y temporal autocorrelation (AR3) (43) of the peatland biomass. For AR3, the contrast in autocorrelation between peatland and forest was found to be highest. The critical flux is the range in groundwater flux measured from the peatland-to-forest tipping point (TIP) over which this critical slowdown of the peatland biomass occurs (Figs. 4 C and D). For example, if for a certain location the TIP occurs at an infiltration rate of 0.5 mm/d and the AR3 starts to increase at an exfiltration rate of 0.4 mm/d, the critical flux is 0.9 mm/d.
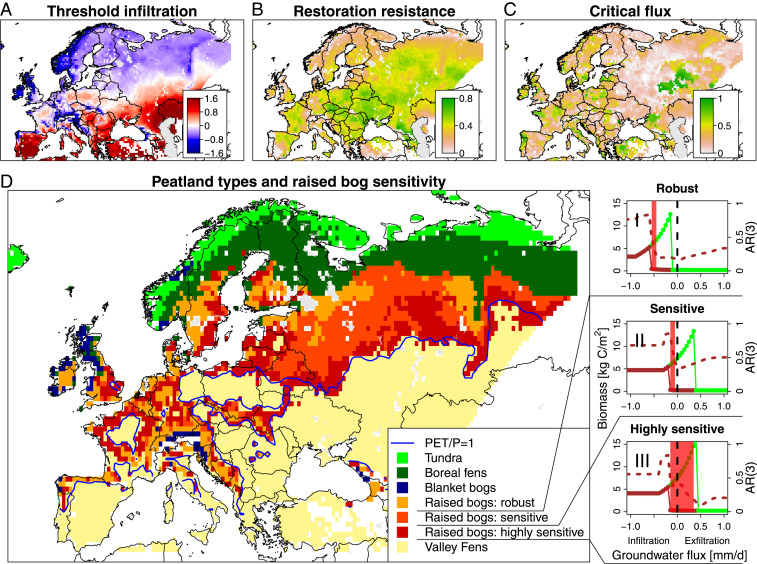
Fig. 4.
Resilience of potential raised bogs. The indicators (A–C) together identify regions with the most sensitive raised bogs (D). (I to III) The bistability diagrams for robust, sensitive, and highly sensitive raised bogs. The black dashed line indicates zero groundwater flux, which identifies potential raised bog sites (II), while the red shaded range indicates the groundwater fluxes over which “critical slowing down” is simulated [AR (3) brown dashed line]. Critical slowing down occurring from TIP into exfiltration conditions is defined highly sensitive raised bog (III). The blue line in D indicates a dryness index equal to 1.
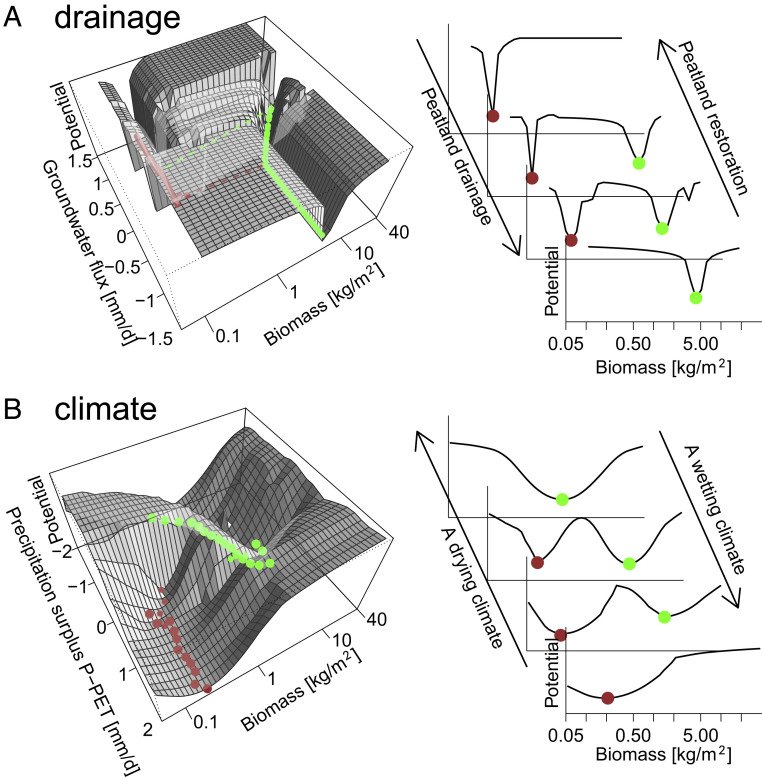
Fig. 3.
Ecosystem stability landscapes (42) show restoration resistance (range in groundwater fluxes between two clear minima) against drainage for a single location (A) and climate change based on model results for all Europe (B). Green dots indicate a high biomass forest state, while brown dots indicate a peatland state. Potential (y-axis) is calculated by log(1/probability)36, where probability represents the probability of a certain biomass during the last 200 y of simulation (for one site [A], for all sites and zero groundwater flux [B].
The TIP follows a clear north–south gradient with exceptions for high precipitation landscapes related to coastal zones and mountain ranges (Fig. 4A). Raised bogs, boreal fens, and blanket bogs are rainwater-fed peatland types and by their definition exist with some degree of infiltration and thus require a negative TIP. As such, the magnitude of the TIP is an important resilience indicator, signaling how close these peatland types minimally are to their tipping point. Low restoration resistances are simulated in several key peatland regions such as northern Sweden, Estonia, Scotland, northwest Europe, and north of the Alps, mostly related to a relatively high variability in precipitation between years (SI Appendix, Fig. S4.2). Here, peatlands and forest are expected to switch relatively easily and frequently with little hysteresis (i.e., restoration effort) in response to climate or artificial drainage. In contrast, the more stable continental climate in western Russia allows both forest and peatland states to fully adapt to its climate, which results in much larger restoration resistances (Fig. 4B and SI Appendix, Fig. S3.2). The smallest critical fluxes are found in a north–south band through Finland, western Russia, Belarus, and Ukraine (Fig. 4C). Here, disturbances in the groundwater flow by artificial drainage or an extreme climate episode outside the range of the input climate are least likely to induce shifts from peatland to forest. Both the input climate variability and additional infiltration do not seem to affect the dynamics of the peatland vegetation, even when the peatland is close to its tipping point. In contrast, high critical fluxes are found in Poland, parts of Sweden and France, and in parts of western Russia for a range of peatland types in which shifts are thus more likely to occur.
Raised Bogs, Ecosystem Shifts, and Potential Carbon Loss.
We focus on the transition from landscapes with raised bogs to forested landscapes with valley fens because this is a likely and ongoing transition (30) given the current trajectory of a warming climate and increased drainage and implies a significant carbon release (SI Appendix, Estimate of Potential Carbon Loss, largest decrease in average soil carbon storage). Moreover, for raised bogs, we showed that a small negative threshold infiltration flux (TIP) indicates that a tipping point is near. Boreal fens and valley fens might also be at risk for being overgrown by trees, but because these types occur under groundwater supply conditions, TIP is not a good indicator for the proximity of a tipping point. The tipping point proximity for these peatland types requires site-specific studies of local groundwater flows.
To quantify raised bog sensitivity, we combined the threshold infiltration, restoration resistance, and critical flux into “highly sensitive,” “sensitive,” and “robust” raised bogs (Fig. 4). Highly sensitive raised bogs are those peatlands that show “critical slowdown” due to a nearby tipping point under zero infiltration (and thus also under all negative groundwater fluxes required for raised bogs). We assumed that these raised bogs, that currently already show early warning signs (43) for a shift toward forest, are the most sensitive to artificial disturbances or extreme climate episodes that fall outside the input climate variability (Fig. 4 D, III). Restoration or growth of raised bogs without substantial supply of external rainwater, restoration of regional groundwater flows, and/or regional vegetation patterns is unlikely to be successful. Sensitive raised bog systems are defined based on the spatial interaction between forests and peatlands. We expect that, where forests can grow under exfiltration conditions, they can potentially overgrow and/or drain raised bogs from its edges where exfiltration conditions are common (Figs. 1B and 4 A, II). Restoring a raised bog from a forested state will only be successful if water storage measures are accompanied by continued deforestation. Raised bogs were classified robust when raised bogs do not show signs of critical slowing down at zero infiltration, and fully grown forests cannot exist under exfiltration conditions. Here, it is possible to restore raised bogs from a forested state by restoring the local hydrology (Fig. 4A).
Our analysis shows (Fig. 4D combined with NATURA2000 sites and soil carbon inventory; SI Appendix, Table S5.1) that under the current climate, raised bogs can potentially be present in 34% of European landscapes (area fraction between >35° N and <75° E). Of this 34%, only 10% of landscapes are locations where raised bogs can form from mineral soils under the current climate (i.e., robust). Another 14% of European landscapes is marked as sensitive to tree growth and will likely not form raised bogs from forests without extensive ecosystem management under the current climate. The last 10%, estimated to include >500 peatlands (SI Appendix, Table S5.1), is identified as “highly sensitive.” These are the most likely locations where transitions from a raised bog– to valley fen–dominated landscape can occur. Based on a detailed global soil carbon inventory (5) of the top 2 m, we conservatively (because raised bogs frequently are thicker than 2 m) estimated that a shift from raised bogs to valley fens (when raised bogs shift to forests, only valley fens remain) may release 4% (up to 24 Pg carbon) of the total European soil organic carbon pool into the atmosphere (SI Appendix, Estimate of Potential Carbon Loss).
To project the rate of this carbon loss, the size of individual raised bogs is likely important and not taken into account in this study. The larger a raised bog, the smaller the topographic gradients and infiltration, and the more surface ponding is expected. Moreover, raised bog size is also controlled by surface runoff, which leads to stream incision and subsequent drainage (44–46). Thus, raised bog size as a function of climate and runoff is likely an additional control on peatland resilience, which may both amplify or dampen the rate of peatland–forest transitions, especially in flat low-runoff regions such as western Siberia (47) and the Canadian boreal plain (10). In addition, in continental regions with cold winters, permafrost is likely to allow organic matter accumulation under drier conditions and therefore change the stable states between trees and peatlands (2, 36, 48). This expectedly is a key mechanism behind the formation of wooded bogs (20), a stable state that does not emerge in our model, as soil temperature is not incorporated, that are commonly found in colder regions outside Europe. The effect of permafrost on the interaction between forest and peatland ecosystems requires further study and is the main reason we confined our study to Europe, where permafrost is not widespread. Other model simplifications that were outside the scope of this study and require further study are the temporal and spatial dynamics of the groundwater flux, the effects of long-term trends and periodicity in past and future climates, and the role of species initiation, composition, and succession.
Despite incorporating only a few of the many feedback mechanisms acting in peatlands (8, 30, 49, 50), we quantitatively unified the essentials of water and carbon cycling at the timescale of decades to centuries for both forests and peatlands from a landscape perspective. Notwithstanding the strong simplifications, our results are well supported by multiple sources of data, including water and carbon cycling and the coverage of peatland types. Using well-established concepts of feedbacks, bistability, and resilience, we show that forest- and peat-forming wetlands can occupy the same landscape position for a range of hydrological conditions. The resulting interactions between forests and peatlands have shaped European landscapes and are likely to continue to affect these landscapes in the face of climate change and increasing human pressure. Our results urge for a change in the focus of research and conservation efforts toward a landscape perspective. Only then, peatlands and associated services including carbon storage, biodiversity, and water storage may be preserved or regenerated. This landscape perspective requires interactions between peatlands and their supporting landscape via groundwater, river morphology, and competing ecosystems states such as forests, in which neither of these interactions can be considered constant.
Methods and Materials
Model.
We formulated a one-dimensional (along a groundwater flux gradient) landscape-scale model that captures the key interactions between water and carbon in vegetation and soils (Eqs. 1–4). We simplified vegetation ranging from forest to peatland vegetation in a biomass continuum (B) with one set of parameters quantifying growth and mortality. This strong simplification compared to typical ecosystem models (16, 51) is possible because of the high contrast in living biomass between peatlands and forests.
Living biomass is assumed to grow (NPP) as a function of transpiration (T) via a biomass and stress-dependent water use efficiency (52). Wet conditions in peatlands reduce biomass production because of limited availability of oxygen (53) and CO2 (54). Also, the higher albedo of peatlands compared to forests is likely to reduce energy available for photosynthesis and thus biomass growth and lower evapotranspiration in peatlands compared to forests (55, 56). These processes are lumped by reducing the evapotranspiration from groundwater (the sum of evaporation and transpiration, Egr +Tgr) when the water table (H) is above or in the top of the rootzone. Furthermore, when rain and snowmelt (R) raise the groundwater table above the soil surface, runoff occurs (Q), and direct evaporation from soil or surface ponds becomes dominant over transpiration of the vegetation, which further reduces biomass growth under wet conditions.
Dying of biomass (M) is described as a fixed percentage of biomass and an additional die off under too wet and too dry conditions (Wd). All plant litter accumulates on the soil as soil organic carbon (O), where it raises the surface (S) and changes the soil specific yield (Sy). Soil organic carbon decomposes (D) with a rate depending on the yearly minimum groundwater table and yearly average temperature. Above this deepest groundwater table, the zone where most microbial activity takes place (acrotelm), decomposition is more than 1,000 times faster than the zone below the water table that remains saturated (catotelm, after ref. 57). Soil organic carbon removed by streams (Se) is incorporated to remove additional soil organic carbon under wet climates. All peat types have the same decomposition parameterization, and no special consideration is given to frozen soils. The interactions between the variables are described in detail in SI Appendix, Model Setup. Nutrient dynamics and species composition are not considered in this model. Although nutrient availability affects biomass growth of forests and peatlands substantially at short time and spatial scales (27), we assumed that over centennial timescales and the “forest versus peatland” focus of this study, the nutrient input and species composition is directly linked to groundwater inflow (i.e., rainwater-fed peatlands have a low nutrient status, and groundwater- or river water–fed peatlands have a higher nutrient status). The water fluxes, and groundwater levels are calculated with a daily time step to ensure a realistic partitioning of precipitation into discharge, recharge, and evapotranspiration (18), while all carbon fluxes and stores are simulated yearly. The simulation time is 1,000 y, and for each climate on a 0.5 × 0.5° grid across Europe, we varied the initial conditions. The landscape is represented by the groundwater flux (J), which we ranged from 2 mm/d exfiltration (positive) to 2 mm/d infiltration (negative) with steps of 0.05 mm/d, resembling a realistic range of possible landscape hydrological conditions. In reality, this groundwater flux is likely not a year round constant water flux but is expected to vary seasonally with landscape wetness. The typical dynamics of the vegetation was found to have a longer timescale of several years, allowing this simplification of a constant groundwater flux. The model was initiated with both representative forest conditions (high biomass, 8 kg C/m2; low soil organic carbon, 4 kg C/m2; groundwater table 1 m below surface) and peatland conditions (low biomass; 0.1 kg C/m2, high soil organic matter 100 kg C/m2, and groundwater at surface) on top of a sandy parent material.
| [1] |
| [2] |
| [3] |
| [4] |
An example model result for a specific climate (SI Appendix, Fig. S2.1) shows that for most groundwater fluxes, biomass reaches its new equilibrium within 100 y. However, for several groundwater fluxes, a shift from high to low biomass (forest to peatland) occurs after a sequence of wet years. As shown, this shift is not instantaneous because it may take several centuries to build up or reduce a peat layer. Note that these transitions rely on peat oxidation rates and forest mortality on which we did not include measured data and had to rely on previously derived parameter sets (36, 57). Moreover, spatial feedbacks within the peatland (8, 31) and temporal variability of landscape wetness conditions may considerably change the groundwater fluxes over time (here considered constant) and therefore affect the rate of forest–peatland transitions.
The average biomass in the period between 800 and 1,000 y after the start of the simulation for each groundwater flux shows that within a specific range of groundwater fluxes, it depends on the model initiation state (forest or peatland) for which state is simulated (SI Appendix, Fig. S2.1B). We identified this range as the restoration resistance, that is, the range in groundwater flux in which both a forest and a peatland are stable.
Classification.
Two classifications are used to classify the simulated biomass bistability diagrams into peatland type (Fig. 2C) and into raised bog sensitivity classes summarized in Table 1.
Model Forcing, Parameterization, and Validation.
The model was forced with the European Climate Assessment & Dataset (58) spanning Europe and western Russia for the period 1955 to 2015 (downloaded in 2017). For each 0.5 × 0.5° grid cell, 60 y of climate data were randomly resampled to yield 1,000 y of weather data. The model results should therefore be interpreted as the water–carbon interaction under the current climate conditions assuming no periodicity or trends that are outside the range that could be achieved by selecting 1,000 random years from the observed 60 y. By selecting 1,000 random years, still sequences of dry and wet, warm and cold years occur. Reference evapotranspiration is calculated using the Priesley–Taylor approach (59) (SI Appendix, section S3.2). Model results were discarded in a buffer <50 km around coastal zones and big lakes, as the Priestley–Taylor approach yielded unreliable values here.
The model parameters and equations were chosen from literature to match observed forest and peatland water and carbon uptake rates in Europe (Fig. 2 and SI Appendix, Model Setup and Fig. S2.1). Therefore, these observed relationships ultimately control model behavior, and we expect that different model setups describing the same relationships yield similar results. Model setup and parameters were validated against Canadian datasets (SI Appendix, section S3.1.2). Model parameter sensitivity analyses for Europe (SI Appendix, Sensitivity Analysis) revealed that the parameters describing evapotranspiration differences between forest and peatlands more strongly control the tipping points and bistability than the parameters describing water storage and soil type differences.
Supplementary Material
Acknowledgments
Y.v.d.V. and J.J.N. gratefully acknowledge support for this study from the graduate school for Production Ecology and Resource Conservation of Wageningen University. Further, Y.v.d.V. and N.K. acknowledge support from the Irish Environmental Protection Agency (grant 2020-CCRP-MS.70). A.J.D. acknowledges support from the Netherlands Earth System Science Center (NESSC) through Gravitation (grant 024.002.001) from the Dutch Ministry for Education, Culture and Science. This work was carried out on the Dutch national e-infrastructure with the support of SURF Cooperative. We thank A. Beaudoin, M. Thurner, K. Webster, C. Williams, A. Gallego-Sala, and D. McKenney for providing datasets for model validation.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2101742118/-/DCSupplemental.
Data Availability
All presented datasets are openly available through the given references. Model scripts and model input to reproduce the results from this study are available at the DataverseNL repository, https://doi.org/10.34894/ZHUBQA (60). All other study data are included in the article and/or SI Appendix.
References
- 1.Hugelius G., et al., Large stocks of peatland carbon and nitrogen are vulnerable to permafrost thaw. Proc. Natl. Acad. Sci. U.S.A. 117, 20438–20446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Limpens J., et al., Peatlands and the carbon cycle: From local processes to global implications – a synthesis. Biogeosciences 5, 1475–1491 (2008). [Google Scholar]
- 3.Dise N. B., Peatland response to global change. Science 326, 810–811 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Yu Z., Holocene carbon flux histories of the world’s peatlands. Holocene 21, 761–774 (2011). [Google Scholar]
- 5.Hengl T., et al., SoilGrids1km—Global soil information based on automated mapping. PLoS One 9, e105992 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson E. A., Janssens I. A., Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440, 165–173 (2006). [DOI] [PubMed] [Google Scholar]
- 7.van Breemen N., How Sphagnum bogs down other plants. Trends Ecol. Evol. 10, 270–275 (1995). [DOI] [PubMed] [Google Scholar]
- 8.Waddington J. M., et al., Hydrological feedbacks in northern peatlands. Ecohydrology 8, 113–127 (2014). [Google Scholar]
- 9.Paavilainen E., Päivänen J., “Environmental effects of peatland forestry” in Peatland Forestry: Ecology and Principles, Lange O. L., Mooney H. A., Remmert H., Eds. (Springer-Verlag, 1995), pp. 191–199. [Google Scholar]
- 10.Devito K. J., et al., Landscape controls on long-term runoff in subhumid heterogeneous Boreal Plains catchments. Hydrol. Processes 31, 2737–2751 (2017). [Google Scholar]
- 11.van der Velde Y., Lyon S. W., Destouni G., Data-driven regionalization of river discharges and emergent land cover-evapotranspiration relationships across Sweden. J. Geophys. Res. Atmos. 118, 2576–2587 (2013). [Google Scholar]
- 12.Gogo S., Laggoun-Défarge F., Delarue F., Lottier N., Invasion of a Sphagnum-peatland by Betula spp. and Molinia caerulea impacts organic matter biochemistry. Implications for carbon and nutrient cycling. Biogeochemistry 106, 53–69 (2011). [Google Scholar]
- 13.Pluchon N., Hugelius G., Kuusinen N., Kuhry P., Recent paludification rates and effects on total ecosystem carbon storage in two boreal peatlands of Northeast European Russia. Holocene 24, 1126–1136 (2014). [Google Scholar]
- 14.Roebroek C. T. J., Melsen L. A., Van Dijke A. J. H., Fan Y., Teuling A. J., Global distribution of hydrologic controls on forest growth. Hydrol. Earth Syst. Sci. 24, 4625–4639 (2020). [Google Scholar]
- 15.Teuling A. J., Hoek van Dijke A. J., Forest age and water yield. Nature 578, E16–E18 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Heijmans M. M. P. D., van der Knaap Y. A. M., Holmgren M., Limpens J., Persistent versus transient tree encroachment of temperate peat bogs: Effects of climate warming and drought events. Glob. Change Biol. 19, 2240–2250 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Fan Y., Miguez-Macho G., Jobbágy E. G., Jackson R. B., Otero-Casal C., Hydrologic regulation of plant rooting depth. Proc. Natl. Acad. Sci. U.S.A. 114, 10572–10577 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laine A. M., Frolking S., Tahvanainen T., Tolvanen A., Tuittila E. S., Spring-season flooding is a primary control of vegetation succession trajectories in primary mires. Mires Peat 24, 1–8 (2019). [Google Scholar]
- 19.Scheffer M., Hirota M., Holmgren M., Van Nes E. H., Chapin F. S. III, Thresholds for boreal biome transitions. Proc. Natl. Acad. Sci. U.S.A. 109, 21384–21389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratcliffe J. L., et al., Ecological and environmental transition across the forested-to-open bog ecotone in a west Siberian peatland. Sci. Total Environ. 607-608, 816–828 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Hartshorn A. S., Southard R. J., Bledsoe C. S., Structure and function of peatland-forest ecotones in Southeastern Alaska. Soil Sci. Soc. Am. J. 67, 1572 (2003). [Google Scholar]
- 22.Dimitrov D. D., Bhatti J. S., Grant R. F., The transition zones (ecotone) between boreal forests and peatlands: Ecological controls on ecosystem productivity along a transition zone between upland black spruce forest and a poor forested fen in central Saskatchewan. Ecol. Modell. 291, 96–108 (2014). [Google Scholar]
- 23.Berg E. E., McDonnell Hillman K., Dial R., DeRuwe A., Recent woody invasion of wetlands on the Kenai Peninsula Lowlands, south-central Alaska: A major regime shift after 18 000 years of wet Sphagnum–sedge peat recruitment. Can. J. For. Res. 39, 2033–2046 (2009). [Google Scholar]
- 24.Linderholm H. W., Leine M., An assessment of twentieth century tree-cover changes on a southern swedish peatland combining dendrochronoloy and aerial photograph analysis. Wetlands 24, 357–363 (2004). [Google Scholar]
- 25.Munir T. M., Perkins M., Kaing E., Strack M., Carbon dioxide flux and net primary production of a boreal treed bog: Responses to warming and water-table-lowering simulations of climate change. Biogeosciences 12, 1091–1111 (2015). [Google Scholar]
- 26.Rietkerk M., Dekker S. C., Wassen M. J., Verkroost A. W. M., Bierkens M. F. P., A putative mechanism for bog patterning. Am. Nat. 163, 699–708 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Eppinga M. B., Rietkerk M., Wassen M. J., De Ruiter P. C., Linking habitat modification to catastrophic shifts and vegetation patterns in bogs. Plant Ecol. 200, 53–68 (2007). [Google Scholar]
- 28.Chaudhary N., et al., Modelling past and future peatland carbon dynamics across the pan-Arctic. Glob. Change Biol. 26, 4119–4133 (2020). [DOI] [PubMed] [Google Scholar]
- 29.MacDonald G. M., et al., Rapid early development of circumarctic peatlands and atmospheric CH4 and CO2 variations. Science 314, 285–288 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Belyea L. R., Malmer N., Carbon sequestration in peatland: Patterns and mechanisms of response to climate change. Glob. Change Biol. 10, 1043–1052 (2004). [Google Scholar]
- 31.Dekker S. C., et al., Holocene peatland initiation in the Greater Everglades. J. Geophys. Res. Biogeosci. 120, 254–269 (2015). [Google Scholar]
- 32.Walker B. H., Holling C. S., Carpenter S. R., Kinzig A., Resilience, adaptability and transformability in social-ecological systems. Ecol. Soc. 9, 5 (2004). [Google Scholar]
- 33.Kettridge N., et al., Moderate drop in water table increases peatland vulnerability to post-fire regime shift. Sci. Rep. 5, 8063 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheffer M., Carpenter S., Foley J. A., Folke C., Walker B., Catastrophic shifts in ecosystems. Nature 413, 591–596 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Belyea L. R., Baird A. J., Beyond “the limit to peat bog browth”: Cross-scale feedback in peatland development. Ecol. Monogr. 79, 3–24 (2009). [Google Scholar]
- 36.Kleinen T., Brovkin V., Schuldt R. J., A dynamic model of wetland extent and peat accumulation: Results for the Holocene. Biogeosciences 9, 235–248 (2012). [Google Scholar]
- 37.Loisel J., et al., Expert assessment of future vulnerability of the global peatland carbon sink. Nat. Clim. Chang. 11, 70–77 (2020). Correction in: Nat. Clim. Chang. 11, 362 (2021). [Google Scholar]
- 38.European Environment Agency , Natura 2000 data—The European network of protected sites. https://www.eea.europa.eu/data-and-maps/data/natura-9. Accessed 29 November 2017.
- 39.Wheeler B. D., Proctor M. C. F., Ecological gradients, subdivisions and terminology of north-west European mires. J. Ecol. 88, 187–203 (2000). [Google Scholar]
- 40.Simola H., Pitkänen A., Turunen J., Carbon loss in drained forestry peatlands in Finland, estimated by re-sampling peatlands surveyed in the 1980s. Eur. J. Soil Sci. 63, 798–807 (2012). [Google Scholar]
- 41.Robroek B. J. M., et al., Taxonomic and functional turnover are decoupled in European peat bogs. Nat. Commun. 8, 1161 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirota M., Holmgren M., Van Nes E. H., Scheffer M., Global resilience of tropical forest and savanna to critical transitions. Science 334, 232–235 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Scheffer M., et al., Early-warning signals for critical transitions. Nature 461, 53–59 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Ingram H. A. P., Size and shape in raised mire ecosystems: A geophysical model. Nature 297, 300–303 (1982). [Google Scholar]
- 45.Larsen L. G., Harvey J. W., How vegetation and sediment transport feedbacks drive landscape change in the everglades and wetlands worldwide. Am. Nat. 176, E66–E79 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Oosterwoud M., van der Ploeg M., van der Schaaf S., van der Zee S., Variation in hydrologic connectivity as a result of microtopography explained by discharge to catchment size relationship. Hydrol. Processes 31, 2683–2699 (2017). [Google Scholar]
- 47.Eppinga M. B., et al., Resource contrast in patterned peatlands increases along a climatic gradient. Ecology 91, 2344–2355 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Johnson K. D., et al., Permafrost and organic layer interactions over a climate gradient in a discontinuous permafrost zone. Environ. Res. Lett. 8, 035028 (2013). [Google Scholar]
- 49.Nijp J. J., et al., Including hydrological self-regulating processes in peatland models: Effects on peatmoss drought projections. Sci. Total Environ. 580, 1389–1400 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Fenner N., Freeman C., Drought-induced carbon loss in peatlands. Nat. Geosci. 4, 895–900 (2011). [Google Scholar]
- 51.Sitch S., et al., Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Glob. Change Biol. 9, 161–185 (2003). [Google Scholar]
- 52.Tomassen H. B. M., Smolders A. J. P., Lamers L. P. M., Roelofs J. G. M., Stimulated growth of Betula pubescens and Molinia caerulea on ombrotrophic bogs: Role of high levels of atmospheric nitrogen deposition. J. Ecol. 91, 357–370 (2003). [Google Scholar]
- 53.Bartholomeus R. P., Witte J.-P. M., van Bodegom P. M., van Dam J. C., Aerts R., Critical soil conditions for oxygen stress to plant roots: Substituting the Feddes-function by a process-based model. J. Hydrol. (Amst.) 360, 147–165 (2008). [Google Scholar]
- 54.Williams T. G., Flanagan L. B., Effect of changes in water content on photosynthesis, transpiration and discrimination against 13CO2 and C18O16O in Pleurozium and Sphagnum. Oecologia 108, 38–46 (1996). [DOI] [PubMed] [Google Scholar]
- 55.Peichl M., et al., Energy exchange and water budget partitioning in a boreal minerogenic mire. J. Geophys. Res. Biogeosci. 118, 1–13 (2013). [Google Scholar]
- 56.Wu J., Kutzbach L., Jager D., Wille C., Wilmking M., Evapotranspiration dynamics in a boreal peatland and its impact on the water and energy balance. J. Geophys. Res. 115, G04038 (2010). [Google Scholar]
- 57.Clymo R. S., The limits to peat bog growth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 303, 605–654 (1984). [Google Scholar]
- 58.Klein Tank A. M. G., et al., Daily dataset of 20th-century surface air temperature and precipitation series for the European Climate Assessment. Int. J. Climatol. 22, 1441–1453 (2002). [Google Scholar]
- 59.Priestley C. H. B., Taylor R. J., On the assessment of surface heat flux and evaporation using large-scale parameters. Mon. Weather Rev. 100, 81–92 (1972). [Google Scholar]
- 60.van der Velde Y., Replication data for “Emerging forest-peatland bi-stability and resilience of European peatland carbon stores.” DataverseNL. 10.34894/ZHUBQA. Deposited 2 September 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All presented datasets are openly available through the given references. Model scripts and model input to reproduce the results from this study are available at the DataverseNL repository, https://doi.org/10.34894/ZHUBQA (60). All other study data are included in the article and/or SI Appendix.