Abstract
The healthy gut is achieved and maintained through a balanced relationship between the mucosal immune system, microbial communities resident in the lumen, and the intestinal epithelium. The intestinal epithelium plays an exceptionally important role in harmonizing the interaction between the host immunity and the luminal residents, as this selectively permeable barrier separates but also allows interchange between the 2 environments. Interleukin (IL)-10 has been well established to play an important role in maintaining gut homeostasis by imparting diverse effects on a variety of cell types in this relationship. In the intestine, the source and the target of IL-10 include leukocytes and epithelial cells. Given that both the epithelium and IL-10 are essential players in supporting homeostasis, we discuss the relationship between these 2 factors, focusing on epithelial sources of IL-10 and the effects of IL-10 on the intestinal epithelium. Insight into this relationship reveals an important aspect of the innate immune function of intestinal epithelial cells.
Keywords: intestinal epithelium, IL-10, gut homeostasis, inflammatory bowel disease
Abbreviations used in this paper: DSS, dextran sodium sulfate; ER, endoplasmic reticulum; IBD, inflammatory bowel disease; IEC, intestinal epithelial cell; IEL, intraepithelial lymphocyte; IFN-γ, interferon gamma; IL, interleukin; IL-10KO, interleukin-10 knockout; IL-10R, IL-10 receptor; mRNA, messenger RNA; SERT, serotonin transporter; Treg, T regulatory; TEER, transepithelial electrical resistance; TLR, toll-like receptor; TNF, tumor necrosis factor; WT, wild-type
Summary.
This review discusses the effect of interleukin-10 on the intestinal epithelium and the potential role of epithelial-derived interleukin-10 in gut homeostasis.
The intestinal epithelium, as a single contiguous layer of columnar cells, is the body’s largest mucosal surface.1 With the apical side facing the lumen and the basal side in contact with a basement membrane resting on the lamina propria, the epithelium is the bridge between lumen contents and the leukocytes of the lamina propria.2 In the small intestine, the epithelium is folded into crypts and villi, and is composed of immature dividing cells and mature cell types including enterocytes, enteroendocrine cells, Tuft cells, microfold cells (M cells), goblet cells, and Paneth cells.2 The large intestine lacks villi, but both undifferentiated and differentiated cell types are present, with the relative abundances of differentiated cell types differing between the small and large intestines.2 The epithelium is continually renewed by stem cells residing at the crypt bases.3 The epithelium limits macromolecules and microbial entry into the mucosa by forming an intercellular network of tight junctions between cells.4 Cells secrete mucus and antimicrobial products, and transport immunoglobulins into the mucus, limiting intestinal infiltration by microbes.5 Meanwhile, the adaptive immune system defaults to promote antigen tolerogenic responses. Thus, gut homeostasis depends on the interplay between innate and adaptive immunity, acting against the luminal microbiota.6,7 Much of this regulation is achieved through the secretion of soluble cytokines, including interleukin (IL)-10.
IL-10 in Immune Regulation and Homeostasis
IL-10, first reported as human cytokine synthesis inhibitory factor,8 is now a well-studied immunomodulator. As the descriptive name implies, the anti-inflammatory effects of IL-10 are attributed to suppressing secretion of many proinflammatory cytokines and chemokines, including interferon gamma (IFN-γ), tumor necrosis factor (TNF), IL-1α/β, and others, from a variety of cell types.9 IL-10 also induces secretion of anti-inflammatory molecules such as the IL-1 receptor antagonist, soluble TNF receptor, and IL-27, which contribute to an anti-inflammatory milieu.10 Besides manipulating the production of secreted products, IL-10 can exert direct effects on effector cells including preventing the stimulation of naïve CD4+ T cells, inhibiting maturation of dendritic cells and repressing activation and proliferation of macrophages.11 With such an array of direct and indirect impacts, IL-10 may well be the master regulator of the anti-inflammatory response in the intestines.
The pivotal role that IL-10 plays in balancing intestinal homeostasis was unequivocally demonstrated by the discovery that the lack of IL-10 predisposes to enterocolitis.12 This profound outcome was validated in humans, in which mutations in IL-10 or IL-10 receptor (IL-10R) resulted in inflammatory bowel disease (IBD).13,14 Noteworthy, the “cure” for patients was to restore expression in leukocytes through stem cell or bone marrow transplantation.15,16 Resolving the deficiency with leukocyte transplants not only confirms the importance of IL-10 in gut health, but also suggests that there is a significant population of IL-10–producing and IL-10–responsive leukocytes in the gut.
Sources of IL-10 in the Mucosa
IL-10 was first identified as a product of mouse CD4+ Th2 lymphocytes, regulating the secretion of cytokines by Th1 cells.8 Since that discovery, depending on the stimulation, IL-10 can be secreted by many leukocytes including subsets of T cells, B cells, macrophages/monocytes, innate lymphoid cells, and others.17,18 Most cells that produce IL-10 also possess the IL-10R, which suggests that IL-10 is likely acting in an autocrine fashion.19 In fact, systemically administered IL-10 has a very short half-life,20 suggesting that autocrine and paracrine roles are critical.
Early studies identified the major source of IL-10 in the healthy small intestine to be intraepithelial lymphocytes (IELs), specifically type 1 T regulatory (Treg) cells.21 However, in a recent study, a small subset of type 2 innate lymphoid cells, in the lamina propria, was shown to secrete IL-10, at steady state and following stimulation with IL-2, IL-4, IL-10, IL-27, and the neuropeptide neuromedin U.22 Contrasting the small intestine, colonic lamina propria T cells produce IL-10 upon activation, but few produce IL-10 constitutively, casting doubt on the magnitude of the contribution of lymphocytes during steady state.21, 22, 23, 24, 25 Unlike lymphocytes, IL-10 production from macrophages occurs in both small and large intestine.26,27 Importantly, it was also demonstrated that although IELs and type 2 innate lymphoid cells make IL-10 in the healthy gut, resident macrophages becomes the dominant source during acute inflammation of both intestines.26,27 The stimuli leading to macrophage secretion of IL-10 come from both endogenous (eg, secondary bile acids)28 and exogenous (eg, microbial metabolites) factors.29 Considering that the intestines contain the largest pool of IL-10–producing macrophages in the body,30 it is reasonable to presume this source is important in the regulation of intestinal homeostasis.
Mucosal leukocytes are not the only source of IL-10. There is evidence of constitutive and stimulated secretion of IL-10 from intestinal fibroblasts, thought to preserve mucosal T cell viability.31,32 Last, intestinal epithelial cells (IECs) also reportedly produce and secrete IL-10.
One approach to identifying constitutive cell sources of IL-10, whether mucosal or extraintestinal, is to examine germ-free animals or following antibiotic treatments. Studies indicate that IL-10 production in the intestines of germ-free mice and broad-spectrum antibiotic-treated mice is either similar or reduced compared with conventionally raised mice.33 It was also reported that Treg cells from germ-free mice expressed less IL-10 compared with cells from conventionally raised mice.34 Another noteworthy report showed that germ-free mice possess a population of splenic plasmacytes that secrete IL-10.35 The speculation was that these cells are a T cell–independent response by B cells, intended to regulate autoreactivity.35
In addition to constitutive or intrinsic secretion is the idea that microbes stimulate IL-10.26,36, 37, 38 Bacteroides fragilis polysaccharide A reportedly drives IL-10 production by CD4+ T cells at a level sufficient to protect mice from colitis.39 Colonization by clusters IV and XIVa of Clostridium-induced IL-10–secreting Treg cells and prevented colitis.40,41 The mechanism proved to be indirect, as Faecalibacterium prausnitzii (Clostridium IV) induced secretion of IL-10 among an array of Treg-polarizing molecules in dendritic cells, which in turn induced secretion by colonic Treg cells.42,43 Bifidobacterium breve had a similar effect.44 Clostridium butyricum, on the other hand, induced IL-10 production in macrophages, to an extent that attenuated colitis.29 Finally, the gut microbiota promoted expansion of IL-10–producing B cells in the colonic lamina propria.45 Collectively, these observations implicate gut microbes causally in the production of IL-10, through effects on multiple cell types, presumably contributing to homeostasis.
IL-10R Expression in IECs
The membrane-bound IL-10R is a tetramer consisting of 2 α (IL-10R1) and 2 β (IL-10R2) chains.46 IL-10R1 is specific to IL-10 while IL-10R2 has a lower affinity and shares roles in receptor complexes for other cytokines including IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, IL-29, and IFN-λ.47 Notwithstanding the plentiful evidence for IL-10 acting on leukocytes to achieve homeostasis, there is evidence that IL-10R is expressed in IECs. In mice, IL-10R messenger RNA (mRNA) for both subunits was detected from freshly isolated cells of murine intestines.48 These authors also reported surface expression detected by flow cytometry and labeled ligand binding in the murine small intestinal mode-K and colonic MCA-38 cell lines.48 Hasnain et al49 provided immunofluorescent data localizing IL-10R1 on goblet cells of the mouse distal colon; consequently, they pursued functional studies with the goblet cell-differentiated LS174T colon carcinoma cell line. Regarding expression in the human intestines, Bourreille et al50 detected IL-10R1 mRNA in healthy colonic epithelial cells and the human cell line SW1116 but surprisingly in neither HT-29 nor T84.50 On the other hand, IFN-γ was shown to induce apical IL-10R1 expression on T84 cells and apical expression was detected in the distal colon of dextran sodium sulfate (DSS)–inflamed mice, coincidental with peak IFN-γ levels.51 Mice with an IEC-specific IL-10R1 Cre/flox knockdown had more permeable intestines prior to DSS, and were more susceptible to the DSS, indicating that IL-10 acting on the colonic epithelium is critical to protection, although the study did not address whether the receptors were apical or otherwise.51 With regard to humans, apical IL-10R1 was reported only on IECs from IBD biopsies, inferring the expression was induced.51 Thus, at least in the colon, there is evidence that IEC apical IL-10R can be induced. Focusing on the possible stimuli for steady-state (including apical) expression, metabolites found in the gut were shown to induce the expression of the IL-10R on IECs.51, 52, 53, 54, 55 It is important that we understand the polarized expression of the IL-10R. Leukocyte-derived IL-10 presumably binds basolateral receptors (Figure 1) unless the leukocytes penetrate the epithelium (as in crypt abscesses). Otherwise, in the healthy gut, IL-10 that interacts with apically expressed IL-10R is more likely to come from IECs (Figure 2). Whether apical stimulation of IL-10R induces a different transcriptional response in IECs compared with stimulation through basolateral receptors has not been addressed.
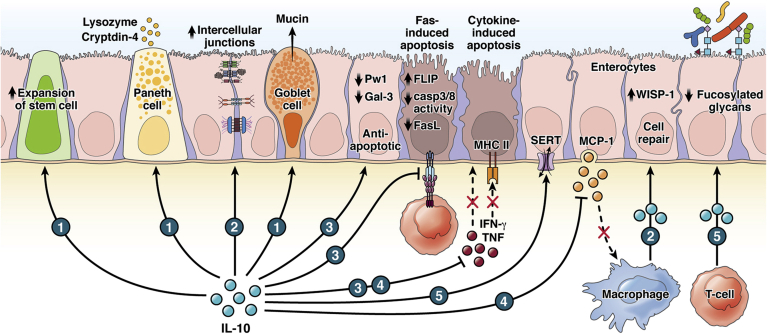
Figure 1.
Lamina propria IL-10 impacts the intestinal epithelium. (1) IL-10 supports the maturation and function of differentiated IECs such as Paneth cells and goblet cells while preserving stem cell renewal.56, 57, 58, 59, 60 (2) IL-10 sustains barrier integrity through regulating intercellular junctions and supports cell repair.38,52,53,57,61 (3) IL-10 exerts antiapoptotic effects on IECs by acting on IEC gene expression and extrinsic apoptosis-inducing factors.48,62,63,64 (4) IL-10 regulates the inflammatory response by inhibiting chemokine production by IEC65 and the IFN-γ–induced major histocompatibility complex class (MHC) II expression on IECs.48 (5) IL-10 upregulates SERT66 and downregulates fucosylated glycans,67 which shapes mucosal tolerance to microbes and gut homeostasis. casp, caspase; FasL, Fas ligand; FLIP, Fas-associated death domain IL-1–converting enzyme-like inhibitory protein; Gal-3, galectin-3; MCP-1, monocyte chemoattractant protein-1; Pw1, zinc finger transcription factor; WISP-1, WNT1-inducible-signaling pathway protein 1.
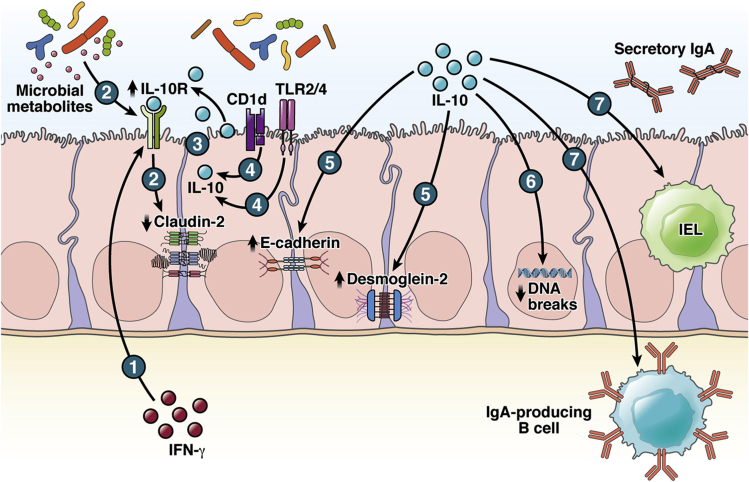
Figure 2.
Potential roles of epithelial-derived IL-10. (1) IL-10R was found dominantly expressed apically upon IFN-γ stimulation.51 (2) IL-10R on IECs can be induced by microbial metabolites,53, 54, 55 suppressing expression of “leaky” claudin-2.54 (3) Epithelial IL-10 was found in both steady-state and induced conditions.68, 69, 70,71,72,73,74 (4) Stimulation of IECs through TLR2, TLR4, or CD1d activation induces IL-10 secretion.72,73,74 In the absence of leukocyte-derived IL-10, experiments comparing WT vs IL-10KO IECs exposed the intrinsic differences between these 2 genotypes, suggesting potential roles of IL-10 in maintaining epithelial integrity by (5) regulating E-cadherin and desmoglein-261 while (6) reducing DNA breaks.75 (7) Overexpression of epithelial IL-10 enriches CD4+CD25+ IEL and IgA-producing B cell.76
The IL-10R2, partner in the IL-10R, might be expected to be more widespread, considering the polypeptide is found in multiple other receptors. IL-10R2 mRNA was detected in cell cultures and freshly isolated IECs.48 It is surprising then, that an immunofluorescent study of human stem cell-derived intestinal organoids observed IL-10R2 staining to be patchy and co-localized with a marker of enteroendocrine cells, a cell type that had not previously been reported to possess the IL-10R1.77 Considering that organoids lack interactions with the lamina propria and the microbiota, IL-10R2 expression might require nonepithelial sources of stimulation. Overall, despite limited and perhaps disputable characterization of IL-10R on IEC subsets, researchers nevertheless have investigated the biological effect of IL-10 on IECs, which infers the presence of the functional receptor.
IL-10 and IEC Lineage
Schopf et al56 inferred that IL-10 impacted IEC differentiation fate by combining helminth infection with cytokine-deficient mouse strains. The study showed that during Trichurus muris infection, the epithelium of IL-10 knockout (IL-10KO) mice had fewer Paneth cells, no detectable goblet cells, disrupted villi, and reduced mucus production in the cecum in comparison to the infected wild-type (WT) mice.56 IL-10KO mice, without any inflammatory provocation, experience higher epithelial proliferation but reduced numbers of goblet cells and Paneth cells.57 This mature cell deficiency was confirmed in germ-free IL-10KO mice, which showed reduced mucin-2 compared with germ-free WT mice.58 With regard to Paneth cells specifically, aberrant granule content and less cryptdin-4 was found in Paneth cells of IL-10KO mice.59 These outcomes could be indirectly due to substantially higher levels of other mediators that affect IECs rather than a direct consequence of the lack of IL-10 responsiveness by IECs. However, there is direct evidence for IL-10 playing a role in intestinal stem cells, as Biton et al60 demonstrated that IL-10 promotes stem cell renewal, using small intestinal organoids from WT mice (Figure 1).60 Organoids may prove to be the model to determine whether IL-10 directly impacts IEC differentiation, as imbalanced sources of other factors may confound conclusions about deficiencies in phenotype and numbers in IL-10KO mice.
IL-10 and IEC Proteome
The impact of IL-10 at the subcellular level in IECs has also been reported. Proteomic studies revealed that IL-10R–reconstituted Mode-K cells stimulated with IL-10 synthesized proteins involved in cytoskeletal function, energy metabolism, proliferation, antigen presentation, and other cellular regulatory processes.62 (The need to transfect this cell line is at odds with the earlier report by Denning et al,48 who claimed the receptor was present.) Another study also compared proteomic profiles of IECs isolated from Eterococcus faecalis–mono-associated IL-10KO vs mono-associated WT mice.62 At an early stage of colonization, the 2 groups showed a number of differences in the proteins expressed. Later, during colonization, while WT IECs successfully returned most of the differentially expressed proteins to steady-state levels and restored homeostasis, IL-10KO IECs failed to normalize the bacterial-induced changes, inferring this deficit predisposed to ongoing acute or chronic inflammation.62 Thus, IL-10 orchestrates transcriptional events that protect IECs from injury, at least during inflammation.
In addition to levels of proteins, studies have reported on the effect of IL-10 on IEC proteostasis. Hasnain et al49 demonstrated that IL-10 is crucial in resolving protein misfolding and endoplasmic reticulum (ER) stress in goblet cells. Using both in vivo Winnie mice (mucin-2 missense mutation) and in vitro tunicamycin-treated LS174T cell (goblet cell–differentiated cell model) to simulate ER stress, their experiment showed a consistent reduction of ER stress upon IL-10 administration or exacerbation of ER stress upon IL-10 or IL-10R neutralization.49 Using primary IECs from inflamed IL-10KO mice and IBD patients, Shkoda et al78 demonstrated that IL-10 regulated ATF-6 (activating transcription factor 6) nuclear recruitment to the GRP78 gene promoter to inhibit the inflammation-induced ER stress response in IECs. Thus, IL-10 affects not only the quantity, but also the quality control of proteins to achieve a balanced and functional proteome in IECs.
IL-10 and IEC Apoptosis
IL-10 can protect IECs from apoptosis, though seemingly in an indirect manner. Neutralizing IL-10 allowed upregulation of IFN-γ, which resulted in a high level of epithelial apoptosis in vivo.79 Similarly, neutralizing IL-10 in rhesus macaque colon explants resulted in more apoptotic cells in the lamina propria and crypts, and goblet cells were observed with cytoplasmic vacuolar degeneration.63 Although no significant changes were observed in crypt length, anti-IL-10 treated explants had a more dilated crypt width.63 Working with explants, it cannot be assumed that the findings are the direct effects of IL-10 on the IECs, but rather are the consequence of the lack of IL-10 to dampen IFN-γ and TNF expression (Figure 1).63 On the other hand, there are reports of a direct effect of IL-10 on IEC apoptosis. Using Mode-K cells, Denning et al48 showed that IL-10 reversed the adverse effects of IFN-γ on cell growth and viability. Another study using Mode-K and IEC4.1 demonstrated that IL-10 protected cells from Fas-induced (ie, T cell–mediated) apoptosis.64 The mechanism was downregulation of Fas protein and caspase-3/8 activity, as well as the upregulation of Fas-associated death domain IL-1–converting enzyme-like inhibitory protein (Figure 1).64 Finally, in the proteomic study alluded to earlier, E. faecalis–mono-associated IL-10KO IECs had reduced galectin-3, which is typically associated with a significant increase in cleaved caspase-3, further indicating that IL-10 plays an antiapoptotic role.62 This study also observed nuclear factor kappa B–dependent upregulation of zinc finger protein Pw1 in infected IL-10KO IECs, which is also associated with p53-mediated apoptosis (Figure 1).62 Overall, there are diverse mechanisms whereby IL-10 exerts an antiapoptotic effect on IECs, either directly upon gene expression or through regulating extrinsic factors.
IL-10 and Epithelial Barrier Integrity
The gut epithelial barrier controls the selective permeability of nutrients, ions, water, macromolecules, and microbes.4 Early work by Madsen et al80 showed that exogenous IL-10 attenuated sodium and chloride transport and restored barrier integrity following disruption with IFN-γ in T84 cultures, a finding corroborated by Kominsky et al,51 who showed that IL-10 restored transepithelial electrical resistance (TEER) following disruption with IFN-γ. Another role of IL-10 in restoring barrier function was described by Quiros et al.38 These authors identified that macrophages were a significant source of IL-10 following an injury and showed that IL-10 acted by promoting the synthesis and secretion of epithelial Wnt1-inducible signaling protein-1, a connective tissue growth factor member that in turn induces pro-proliferative pathways (Figure 1).38 Similarly, Morhardt et al26 showed that IL-10 produced by macrophages was required to restore epithelial barrier from indomethacin-induced injury in the murine small intestine. Using a model of epithelial barrier dysfunction elicited by total parenteral nutrition in mice, Sun et al81 reported that IL-10 upregulated expression of tight junction proteins. Permeability and TEER were found significantly altered in IL-10KO colons compared with WT colons, possibly related to their finding that claudin-1 and occludin were lower in both mRNA and protein levels in the colons of IL-10KO mice (Figure 1).53 Others found similar differences in tight junction molecules, as well as decreased E-cadherin levels, in the IL-10KO mouse small intestine (Figure 1).57,61 IL-10 likely does not act alone to enhance barrier properties and while much remains to be understood about how combinations of mediators may work, a study conducted on TNF treated Caco-2 demonstrated that a combination of IL-10 plus glucocorticoids synergistically impacted TEER, as neither IL-10 nor glucocorticoids used alone were effective.82 Thus, combinations of drugs with epithelial protective properties are worth further investigation and hold promise to treat inflammatory gut diseases.
As alluded to previously, there are uncertainties regarding constitutive IL-10R1 in IECs, yet knockdown of IL-10R1 in T84 was found to directly impair the TEER.51 While this suggests that autocrine IL-10 is present and facilitating barrier function in the cell cultures, it was not confirmed in the study. Another study reported that the expression of IL-10R positively correlated with IEC barrier integrity, as microbe-derived butyrate downregulated “pore-forming” claudin-2 and enhanced epithelial barrier function in an IL-10R1–dependent manner (Figure 2).54 Similarly, Shi et al53 also demonstrated improvements in measures of barrier function by indole metabolites through IL-10R1 induction. Tryptophan metabolites reportedly act in a similar mechanism (Figure 2).55 Overall, the evidence weighs in favor of IL-10 promoting epithelial barrier function and therefore homeostasis, through direct and indirect mechanisms.
IL-10 and IEC-Secreted Mediators
IL-10 regulates cytokine production by multiple cell types in the intestines. One approach to demonstrate this point has been the use of ex vivo tissue cultures. Addition of IL-10 to cultured human colonic tissues inhibited the production of TNF and IL-1β,83 while depletion of IL-10 in human colonic mucosal explants correlated with the upregulation of IFN-γ, TNF, and IL-17.79 These findings suggested that similar proinflammatory cytokines may be elevated in the colon of IL-10KO mice without any inflammatory provocation, which was proven to be the case.84 The anti-inflammatory effects of IL-10 are not limited to activities on leukocytes, and can be measured directly on IECs. One example is the addition of IL-10 to Caco-2 cultures, or isolated human IECs, suppressing the production of monocyte chemoattractant protein-1, a chemokine for monocyte or macrophage recruitment in mice (Figure 1).65
In addition to modulating secretion of cytokines, IL-10 was also found to impact IEC membrane–bound proteins. Specifically, IL-10 was shown to inhibit the IFN-γ–induced increase of major histocompatibility complex class II on IEC (Figure 1).48 Another IEC product influenced by IL-10 is the serotonin transporter (SERT). SERT downregulation has been associated with several gut disorders. SERT upregulation in Caco-2 cells was induced by a high concentration of IL-10 (Figure 1).66 Fucosylated glycans, which serve as attachment receptors and nutrient sources for some microbial species, are also influenced by IL-10. Mutations of fucosylation genes were found to be associated with IBD, and T cell–derived IL-10 was found to downregulate fucosylated glycans on IECs (Figure 1).67 Through these direct effects, IL-10 affects the relationship between IECs, mucosal immunity, and microbes, shaping mucosal homeostasis and tolerance.
IL-10 Therapy to Treat IBD
The evidence that IL-10 works to build, preserve, and restore intestinal barrier integrity is near indisputable. Therefore, it was hopeful that IL-10 could be used directly as a therapy for IBD. Unfortunately, infused recombinant IL-10 therapy trials in humans had disappointing clinical outcomes, possibly owing to low mucosal bioavailability.85 As an alternative to systemic administration, recent approaches have used genetically modified bacteria to deliver IL-10 to the gut.86,87 Promising results were reported in mice and in early-phase clinical trials; however, no significant improvement was observed in phase II trials.88,89 The most recent approach was to deliver an IL-10–encoding plasmid directly to IECs, which protected mice from DSS colitis, although without a significant effect on TNF or IL-1β levels.90 This approach not only facilitates direct exposure of the epithelium to IL-10, but also allows eukaryotic expression of IL-10, which ensures the correct protein tertiary structure.91 Interestingly, this approach presumably promotes IEC production of IL-10, yet it is not entirely appreciated whether IEC-derived IL-10 can substitute for leukocyte-derived IL-10, or whether there is a role for vectorial secretion by IECs.
IECS as a Source of IL-10
The question of whether IECs secrete relevant amounts of IL-10 has not been rigorously investigated. Moreover, early studies did not localize production to particular cell types in the epithelium. IL-10 protein was detected in supernatants, and IL-10 mRNA from cells freshly isolated from human surgical specimens.68 A subsequent study localized IL-10 mRNA by in situ hybridization and IL-10 protein by immunohistochemistry to the epithelium of IBD and healthy controls.69 Multiple studies found IECs in explant cultures from healthy human colonic mucosa and isolated colonic IECs were IL-10–positive.79 Finally, using single-cell flow cytometry, a subset of IL-10–positive IECs were reported from jejunal and colon explants of rhesus macaques.70 Less evidence is available for the small intestine and these observations do not discern whether epithelial IL-10 expression is intrinsic or stimulated.
Early evidence that IEC expression of IL-10 may be stimulated is derived from cell culture experiments. Although IL-10 mRNA has been detected in unstimulated Caco-2 and SW620,92 most cell line experiments required stimulation or modification to the cells before detecting the mRNA. In one example, COLO 205 cells stimulated with cytokines from natural killer cells secreted IL-10.71 In another example, T84 transfected to express CD1d which was then crosslinked, showed induced IL-10 (Figure 2).72 Building on this finding, CD1d-stimulated IL-10 in vivo was shown to protect the gut from induced colitis in mice.93 In addition, it was observed that IL-10 was stimulated following toll-like receptor 4 (TLR4) activation by lipopolysaccharide, in the presence of mucosal macrophages, using cells isolated from patients with colon cancer and SW480-APC cells.73 Using Caco-2, as well as TLR-deficient mice, Latorre et al74 reported that IL-10 expression was influenced by activation through TLR2 and TLR4 differentially in the ileum and the colon (Figure 2).74 These data suggest that IL-10 expression in IECs (like the receptor, earlier) is influenced by cells and microorganisms in the intestines.
Regarding the putative roles for IEC-derived IL-10, knockdown of IEC-specific IL-10 resulted in exacerbation of oxazolone-induced colitis.81 Another approach using transgenic mice that overexpressed IL-10 in mature enterocytes reported that colitis was suppressed as a result of an increase in CD4+CD25+ IELs with characteristics of Treg cells, and IgA-producing B cells in the lamina propria.76 Although this study highlighted interesting tissue-specific epithelial-lymphocyte crosstalk through local cytokine production, the authors did not interrogate the impact on the epithelial monolayer.76 The recent advances in organoid cultures provide an opportunity to examine IECs without confounding cell types, and IL-10KO intestinal organoids were shown to have a higher level of double-stranded DNA breaks compared with WT organoids, which was reversible by adding IL-10.75 Moreover, IL-10KO colon organoids (colonoids) expressed an aberrant pattern of E-cadherin and a reduced level of desmoglein-2 (Figure 2).61 IL-10KO colonoids also showed differential expression of regulatory factors associated with epithelial barrier regulation pathways.61 The observation that IL-10KO organoids differ from WT organoids and that differences are correctable by added IL-10 implies the presence of endogenous IL-10 in WT organoids, although it was not proven. The advantage of using an organoid model is the preservation of the native cellular dynamics, since the organoid possesses all mature cell types found in the tissue, while lacking leukocytes.94 This unique characteristic of organoids allows more focused assessment of the functions of epithelial-sourced IL-10 confined to the epithelium.
While studies point to IECs as a source of IL-10, which specific cell type produces IL-10 is not commonly confirmed. Studies using freshly isolated IECs provide insights into mucosal IL-10 production but are limited in terms of longitudinal investigation into the potential role of IEC IL-10 in cellular function and development. IL-10 has been detected in transformed carcinoma cell lines, yet these lines lack mature differentiated epithelial cell types and of course have altered genomes and proteomes. Intestinal organoid model systems and monolayers derived from organoids are likely to be the model of choice to advance our understanding of the autocrine and paracrine roles of IL-10 within the epithelium.
Conclusion
The 3 decades since the discovery of IL-10 have seen tremendous advances in gut immunology due to the potent regulatory effects of this cytokine on the innate and adaptive immune systems. IL-10 has diverse cellular targets and functions, which generally results in the protection of organs and tissues against inflammation-induced damage. We now understand that IL-10 is critical to maintaining healthy epithelial homeostasis through supporting cellular function, the barrier, and mucosal tolerance. Considering that IECs produce IL-10 and express polarized IL-10R, more remains to be discovered regarding the autocrine roles within the epithelium.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Andrew W. Stadnyk is supported by grants from the Natural Sciences and Engineering Research Council of Canada and the W. Garfield Weston Foundation.
References
- 1.Helander H.F., Fändriks L. Surface area of the digestive tract-revisited. Scand J Gastroenterol. 2014;49:681–689. doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- 2.Allaire J.M., Crowley S.M., Law H.T., Chang S.-Y., Ko H.-J., Vallance B.A. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39:677–696. doi: 10.1016/j.it.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Krndija D., Marjou F. El, Guirao B., Richon S., Leroy O., Bellaiche Y., Hannezo E., Vignjevic D.M. Active cell migration is critical for steady-state epithelial turnover in the gut. Science. 2019;365:705–710. doi: 10.1126/science.aau3429. [DOI] [PubMed] [Google Scholar]
- 4.Buckley A., Turner J.R. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. 2018;10:a029314. doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thoo L., Noti M., Krebs P. Keep calm: the intestinal barrier at the interface of peace and war. Cell Death Dis. 2019;10:849. doi: 10.1038/s41419-019-2086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vivier E., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N.J., Mebius R.E., Powrie F., Spits H. Innate Lymphoid Cells: 10 years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L., Sonnenberg G.F. Essential immunological orchestrators of intestinal homeostasis. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aao1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorentino D.F., Bond M.W., Mosmann T.R. Two types of mouse t helper cell: IV. Th2 clones secrete a factor that inhibits cytokine production by Thl clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrams J., Figdor C.G., De Waal Malefyt R., Bennett B., De Vries J.E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akdis C.A., Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103:131–136. doi: 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minton K. Immune regulation: IL-10 targets macrophage metabolism. Nat Rev Immunol. 2017;17:345. doi: 10.1038/nri.2017.57. [DOI] [PubMed] [Google Scholar]
- 12.Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 13.Franke A., Balschun T., Karlsen T.H., Sventoraityte J., Nikolaus S., Mayr G., Domingues F.S., Albrecht M., Nothnagel M., Ellinghaus D., Sina C., Onnie C.M., Weersma R.K., Stokkers P.C.F., Wijmenga C., Gazouli M., Strachan D., McArdle W.L., Vermeire S., Rutgeerts P., Rosenstiel P., Krawczak M., Vatn M.H., Mathew C.G., Schreiber S. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 14.Engelhardt K.R., Shah N., Faizura-Yeop I., Kocacik Uygun D.F., Frede N., Muise A.M., Shteyer E., Filiz S., Chee R., Elawad M., Hartmann B., Arkwright P.D., Dvorak C., Klein C., Puck J.M., Grimbacher B., Glocker E.O. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2013;131:825–830. doi: 10.1016/j.jaci.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Kotlarz D., Beier R., Murugan D., Diestelhorst J., Jensen O., Boztug K., Pfeifer D., Kreipe H., Pfister E.D., Baumann U., Puchalka J., Bohne J., Egritas O., Dalgic B., Kolho K.L., Sauerbrey A., Buderus S., Güngör T., Enninger A., Koda Y.K.L., Guariso G., Weiss B., Corbacioglu S., Socha P., Uslu N., Metin A., Wahbeh G.T., Husain K., Ramadan D., Al-Herz W., Grimbacher B., Sauer M., Sykora K.W., Koletzko S., Klein C. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143:347–355. doi: 10.1053/j.gastro.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 16.Murugan D., Albert M.H., Langemeier J., Bohne J., Puchalka J., Järvinen P.M., Hauck F., Klenk A.K., Prell C., Schatz S., Diestelhorst J., Sciskala B., Kohistani N., Belohradsky B.H., Müller S., Kirchner T., Walter M.R., Bufler P., Muise A.M., Snapper S.B., Koletzko S., Klein C., Kotlarz D. Very early onset inflammatory bowel disease associated with aberrant trafficking of IL-10R1 and cure by T cell replete haploidentical bone marrow transplantation. J Clin Immunol. 2014;34:331–339. doi: 10.1007/s10875-014-9992-8. [DOI] [PubMed] [Google Scholar]
- 17.Moore K.W., O’Garra A., De Waal Malefyt R., Vieira P., Mosmann T.R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 18.Mosser D.M., Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shouval D.S., Ouahed J., Biswas A., Goettel J.A., Horwitz B.H., Klein C., Muise A.M., Snapper S.B. Interleukin 10 receptor signaling: master regulator of intestinal mucosal homeostasis in mice and humans. Adv Immunol. 2014;122:177–210. doi: 10.1016/B978-0-12-800267-4.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber S., Fedorak R.N., Nielsen O.H., Wild G., Williams C.N., Nikolaus S., Jacyna M., Lashner B.A., Gangl A., Rutgeerts P., Isaacs K., Van Deventer S.J.H., Koningsberger J.C., Cohard M., LeBeaut A., Hanauer S.B. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Gastroenterology. 2000;119:1461–1472. doi: 10.1053/gast.2000.20196. [DOI] [PubMed] [Google Scholar]
- 21.Kamanaka M., Kim S.T., Wan Y.Y., Sutterwala F.S., Lara-Tejero M., Galán J.E., Harhaj E., Flavell R.A. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Bando J.K., Gilfillan S., Di Luccia B., Fachi J.L., Sécca C., Cella M., Colonna M. ILC2s are the predominant source of intestinal ILC-derived IL-10. J Exp Med. 2020;217 doi: 10.1084/jem.20191520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melgar S., Yeung M.M.W., Bas A., Forsberg G., Suhr O., Öberg Å., Hammarström S., Danielsson Å., Hammarström M.L. Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin Exp Immunol. 2003;134:127–137. doi: 10.1046/j.1365-2249.2003.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braunstein J., Qiao L., Autschbach F., Schürmann G., Meuer S. T cells of the human intestinal lamina propria are high producers of interleukin-10. Gut. 1997;41:215–220. doi: 10.1136/gut.41.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebert E.C., Mehta V., Das K.M. Activation antigens on colonic T cells in inflammatory bowel disease: effects of IL-10. Clin Exp Immunol. 2005;140:157–165. doi: 10.1111/j.1365-2249.2005.02722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morhardt T.L., Hayashi A., Ochi T., Quirós M., Kitamoto S., Nagao-Kitamoto H., Kuffa P., Atarashi K., Honda K., Kao J.Y., Nusrat A., Kamada N. IL-10 produced by macrophages regulates epithelial integrity in the small intestine. Sci Rep. 2019;9:1223. doi: 10.1038/s41598-018-38125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krause P., Morris V., Greenbaum J.A., Park Y., Bjoerheden U., Mikulski Z., Muffley T., Shui J.W., Kim G., Cheroutre H., Liu Y.C., Peters B., Kronenberg M., Murai M. IL-10-producing intestinal macrophages prevent excessive antibacterial innate immunity by limiting IL-23 synthesis. Nat Commun. 2015;6:7055. doi: 10.1038/ncomms8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biagioli M., Carino A., Cipriani S., Francisci D., Marchianò S., Scarpelli P., Sorcini D., Zampella A., Fiorucci S. The bile acid receptor GPBAR1 regulates the M1/M2 phenotype of intestinal macrophages and activation of GPBAR1 rescues mice from murine colitis. J Immunol. 2017;199:718–733. doi: 10.4049/jimmunol.1700183. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi A., Sato T., Kamada N., Mikami Y., Matsuoka K., Hisamatsu T., Hibi T., Roers A., Yagita H., Ohteki T., Yoshimura A., Kanai T. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13:711–722. doi: 10.1016/j.chom.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Murray P.J. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Ina K., Kusugami K., Kawano Y., Nishiwaki T., Wen Z., Musso A., West G.A., Ohta M., Goto H., Fiocchi C. Intestinal fibroblast--derived IL-10 increases survival of mucosal T cells by inhibiting growth factor deprivation- and Fas-mediated apoptosis. J Immunol. 2005;175:2000–2009. doi: 10.4049/jimmunol.175.3.2000. [DOI] [PubMed] [Google Scholar]
- 32.Pang G., Couch L., Batey R., Clancy R., Cripps A. GM-CSF, IL-1α, IL-1β, IL-6, IL-8, IL-10, ICAM-1 and VCAM-1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL-1α and TNF-α. Clin Exp Immunol. 1994;96:437–443. doi: 10.1111/j.1365-2249.1994.tb06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy E.A., King K.Y., Baldridge M.T. Mouse microbiota models: Comparing germ-free mice and antibiotics treatment as tools for modifying gut bacteria. Front Physiol. 2018;9:1534. doi: 10.3389/fphys.2018.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strauch U.G., Obermeier F., Grunwald N., Gürster S., Dunger N., Schultz M., Griese D.P., Mähler M., Schölmerich J., Rath H.C. Influence of intestinal bacteria on induction of regulatory T cells: Lessons from a transfer model of colitis. Gut. 2005;54:1546–1552. doi: 10.1136/gut.2004.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fillatreau S. Natural regulatory plasma cells. Curr Opin Immunol. 2018;55:62–66. doi: 10.1016/j.coi.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutz S., Ouyang W. Regulation of interleukin-10 and interleukin-22 expression in T helper cells. Curr Opin Immunol. 2011;23:605–612. doi: 10.1016/j.coi.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 37.Ueda Y., Kayama H., Jeon S.G., Kusu T., Isaka Y., Rakugi H., Yamamoto M., Takeda K. Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. Int Immunol. 2010;22:953–962. doi: 10.1093/intimm/dxq449. [DOI] [PubMed] [Google Scholar]
- 38.Quiros M., Nishio H., Neumann P.A., Siuda D., Brazil J.C., Azcutia V., Hilgarth R., O’Leary M.N., Garcia-Hernandez V., Leoni G., Feng M., Bernal G., Williams H., Dedhia P.H., Gerner-Smidt C., Spence J., Parkos C.A., Denning T.L., Nusrat A. Macrophage-derived IL-10 mediates mucosal repair by epithelial WISP-1 signaling. J Clin Invest. 2017;127:3510–3520. doi: 10.1172/JCI90229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazmanian S.K., Round J.L., Kasper D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 40.Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y., Taniguchi T., Takeda K., Hori S., Ivanov I.I., Umesaki Y., Itoh K., Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., Takahashi M., Fukuda N.N., Murakami S., Miyauchi E., Hino S., Atarashi K., Onawa S., Fujimura Y., Lockett T., Clarke J.M., Topping D.L., Tomita M., Hori S., Ohara O., Morita T., Koseki H., Kikuchi J., Honda K., Hase K., Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 42.Alameddine J., Godefroy E., Papargyris L., Sarrabayrouse G., Tabiasco J., Bridonneau C., Yazdanbakhsh K., Sokol H., Altare F., Jotereau F. Faecalibacterium prausnitzii skews human DC to prime IL10-producing T cells through TLR2/6/JNK signaling and IL-10, IL-27, CD39, and IDO-1 induction. Front Immunol. 2019;10:143. doi: 10.3389/fimmu.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi O., Van Berkel L.A., Chain F., Tanweer Khan M., Taverne N., Sokol H., Duncan S.H., Flint H.J., Harmsen H.J.M., Langella P., Samsom J.N., Wells J.M. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci Rep. 2016;6:18507. doi: 10.1038/srep18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeon S.G., Kayama H., Ueda Y., Takahashi T., Asahara T., Tsuji H., Tsuji N.M., Kiyono H., Ma J.S., Kusu T., Okumura R., Hara H., Yoshida H., Yamamoto M., Nomoto K., Takeda K. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishima Y., Oka A., Liu B., Herzog J.W., Eun C.S., Fan T.J., Bulik-Sullivan E., Carroll I.M., Hansen J.J., Chen L., Wilson J.E., Fisher N.C., Ting J.P.Y., Nochi T., Wahl A., Victor Garcia J., Karp C.L., Balfour Sartor R. Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. J Clin Invest. 2019;129:3702–3716. doi: 10.1172/JCI93820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotenko S.V., Krause C.D., Izotova L.S., Pollack B.P., Wu W., Pestka S. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J. 1997;16:5894–5903. doi: 10.1093/emboj/16.19.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Commins S., Steinke J.W., Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol. 2008;121:1108–1111. doi: 10.1016/j.jaci.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 48.Denning T.L., Campbell N.A., Song F., Garofalo R.P., Klimpel G.R., Reyes V.E., Ernst P.B. Expression of IL-10 receptors on epithelial cells from the murine small and large intestine. Int Immunol. 2000;12:133–139. doi: 10.1093/intimm/12.2.133. [DOI] [PubMed] [Google Scholar]
- 49.Hasnain S.Z., Tauro S., Das I., Tong H., Chen A.H., Jeffery P.L., McDonald V., Florin T.H., McGuckin M.A. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology. 2013;144:357–368. doi: 10.1053/j.gastro.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 50.Bourreille A., Segain J.P., Raingeard de la Blétière D., Siavoshian S., Vallette G., Galmiche J.P., Blottière H.M. Lack of interleukin 10 regulation of antigen presentation-associated molecules expressed on colonic epithelial cells. Eur J Clin Invest. 1999;29:48–55. doi: 10.1046/j.1365-2362.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 51.Kominsky D.J., Campbell E.L., Ehrentraut S.F., Wilson K.E., Kelly C.J., Glover L.E., Collins C.B., Bayless A.J., Saeedi B., Dobrinskikh E., Bowers B.E., MacManus C.F., Müller W., Colgan S.P., Bruder D. IFN-γ–mediated induction of an apical IL-10 receptor on polarized intestinal epithelia. J Immunol. 2014;192:1267–1276. doi: 10.4049/jimmunol.1301757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexeev E.E., Lanis J.M., Kao D.J., Campbell E.L., Kelly C.J., Battista K.D., Gerich M.E., Jenkins B.R., Walk S.T., Kominsky D.J., Colgan S.P. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am J Pathol. 2018;188:1183–1194. doi: 10.1016/j.ajpath.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi C.Z., Chen H.Q., Liang Y., Xia Y., Yang Y.Z., Yang J., Zhang J.D., Wang S.H., Liu J., Qin H.L. Combined probiotic bacteria promotes intestinal epithelial barrier function in interleukin-10-gene-deficient mice. World J Gastroenterol. 2014;20:4636–4647. doi: 10.3748/wjg.v20.i16.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng L., Kelly C.J., Battista K.D., Schaefer R., Lanis J.M., Alexeev E.E., Wang R.X., Onyiah J.C., Kominsky D.J., Colgan S.P. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor–dependent repression of Claudin-2. J Immunol. 2017;199:2976–2984. doi: 10.4049/jimmunol.1700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lanis J.M., Alexeev E.E., Curtis V.F., Kitzenberg D.A., Kao D.J., Battista K.D., Gerich M.E., Glover L.E., Kominsky D.J., Colgan S.P. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol. 2017;10:1133–1144. doi: 10.1038/mi.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schopf L.R., Hoffmann K.F., Cheever A.W., Urban J.F., Wynn T.A. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J Immunol. 2002;168:2383–2392. doi: 10.4049/jimmunol.168.5.2383. [DOI] [PubMed] [Google Scholar]
- 57.Xue Y., Zhang H., Sun X., Zhu M.J. Metformin improves ileal epithelial barrier function in interleukin-10 deficient mice. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwerbrock N.M.J., Makkink M.K., van der Sluis M., Büller H.A., Einerhand A.W.C., Sartor R.B., Dekker J. Interleukin 10-deficient mice exhibit defective colonic Muc2 synthesis before and after induction of colitis by commensal bacteria. Inflamm Bowel Dis. 2004;10:811–823. doi: 10.1097/00054725-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 59.Berkowitz L., Pardo-Roa C., Ramírez G., Vallejos O.P., Sebastián V.P., Riedel C.A., Álvarez-Lobos M., Bueno S.M. The absence of interleukin 10 affects the morphology, differentiation, granule content and the production of cryptidin-4 in Paneth cells in mice. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biton M., Haber A.L., Rogel N., Burgin G., Beyaz S., Schnell A., Ashenberg O., Su C.W., Smillie C., Shekhar K., Chen Z., Wu C., Ordovas-Montanes J., Alvarez D., Herbst R.H., Zhang M., Tirosh I., Dionne D., Nguyen L.T., Xifaras M.E., Shalek A.K., von Andrian U.H., Graham D.B., Rozenblatt-Rosen O., Shi H.N., Kuchroo V., Yilmaz O.H., Regev A., Xavier R.J. T Helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell. 2018;175:1307–1320.e22. doi: 10.1016/j.cell.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khare V., Krnjic A., Frick A., Gmainer C., Asboth M., Jimenez K., Lang M., Baumgartner M., Evstatiev R., Gasche C. Mesalamine and azathioprine modulate junctional complexes and restore epithelial barrier function in intestinal inflammation. Sci Rep. 2019;9:2842. doi: 10.1038/s41598-019-39401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Werner T., Shkoda A., Haller D. Intestinal epithelial cell proteome in IL-10 deficient mice and IL-10 receptor reconstituted epithelial cells: Impact on chronic inflammation. J Proteome Res. 2007;6:3691–3704. doi: 10.1021/pr070222x. [DOI] [PubMed] [Google Scholar]
- 63.Pan D., Das A., Lala W., Kenway-Lynch C.S., Liu D.X., Veazey R.S., Pahar B. Interleukin-10 prevents epithelial cell apoptosis by regulating IFNγ and TNFα expression in rhesus macaque colon explants. Cytokine. 2013;64:30–34. doi: 10.1016/j.cyto.2013.06.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bharhani M.S., Borojevic R., Basak S., Ho E., Zhou P., Croitoru K. IL-10 protects mouse intestinal epithelial cells from Fas-induced apoptosis via modulating Fas expression and altering caspase-8 and FLIP expression. Am J Physiol Gastrointest Liver Physiol. 2006;291:G820–G829. doi: 10.1152/ajpgi.00438.2005. [DOI] [PubMed] [Google Scholar]
- 65.Kucharzik T., Lügering N., Pauels H.G., Domschke W., Stoll R. IL-4, IL-10 and IL-13 down-regulate monocyte-chemoattracting protein-1 (MCP-1) production in activated intestinal epithelial cells. Clin Exp Immunol. 1998;111:152–157. doi: 10.1046/j.1365-2249.1998.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Latorre E., Mendoza C., Matheus N., Castro M., Grasa L., Mesonero J.E., Alcalde A.I. IL-10 modulates serotonin transporter activity and molecular expression in intestinal epithelial cells. Cytokine. 2013;61:778–784. doi: 10.1016/j.cyto.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 67.Goto Y., Obata T., Kunisawa J., Sato S., Ivanov I.I., Lamichhane A., Takeyama N., Kamioka M., Sakamoto M., Matsuki T., Setoyama H., Imaoka A., Uematsu S., Akira S., Domino S.E., Kulig P., Becher B., Renauld J.C., Sasakawa C., Umesaki Y., Benno Y., Kiyono H. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 2014;345:1254009. doi: 10.1126/science.1254009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panja A., Zhou Z., Mullin G.M.L. Secretion and regulation of IL-10 by intestinal epithelial cells. Gastroenterology. 1995;108:A890. [Google Scholar]
- 69.Autschbach F., Braunstein J., Helmke B., Zuna I., Schürmann G., Niemir Z.I., Wallich R., Otto H.F., Meuer S.C. In situ expression of interleukin-10 in noninflamed human gut and in inflammatory bowel disease. Am J Pathol. 1998;153:121–130. doi: 10.1016/S0002-9440(10)65552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan D., Kenway-Lynch C.S., Lala W., Veazey R.S., Lackner A.A., Das A., Pahar B. Lack of interleukin-10-mediated anti-inflammatory signals and upregulated interferon gamma production are linked to increased intestinal epithelial cell apoptosis in pathogenic Simian Immunodeficiency Virus infection. J Virol. 2014;88:13015–13028. doi: 10.1128/JVI.01757-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cella M., Fuchs A., Vermi W., Facchetti F., Otero K., Lennerz J.K.M., Doherty J.M., Mills J.C., Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colgan S.P., Hershberg R.M., Furuta G.T., Blumberg R.S. Ligation of intestinal epithelial CD1d induces bioactive IL-10: critical role of the cytoplasmic tail in autocrine signaling. Proc Natl Acad Sci U S A. 1999;96:13938–13943. doi: 10.1073/pnas.96.24.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hyun J., Romero L., Riveron R., Flores C., Kanagavelu S., Chung K.D., Alonso A., Sotolongo J., Ruiz J., Manukyan A., Chun S., Singh G., Salas P., Targan S.R., Fukata M. Human intestinal epithelial cells express interleukin-10 through Toll-like receptor 4-mediated epithelial-macrophage crosstalk. J Innate Immun. 2015;7:87–101. doi: 10.1159/000365417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Latorre E., Layunta E., Grasa L., Pardo J., García S., Alcalde A.I., Mesonero J.E. Toll-like receptors 2 and 4 modulate intestinal IL-10 differently in ileum and colon. United Eur Gastroenterol J. 2018;6:446–453. doi: 10.1177/2050640617727180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frick A., Khare V., Paul G., Lang M., Ferk F., Knasmüller S., Beer A., Oberhuber G., Gasche C. Overt increase of oxidative stress and DNA damage in murine and human colitis and colitis-associated neoplasia. Mol Cancer Res. 2018;16:634–642. doi: 10.1158/1541-7786.MCR-17-0451. [DOI] [PubMed] [Google Scholar]
- 76.De Winter H., Elewaut D., Turovskaya O., Huflejt M., Shimeld C., Hagenbaugh A., Binder S., Takahashi I., Kronenberg M., Cheroutre H. Regulation of mucosal immune responses by recombinant interleukin 10 produced by intestinal epithelial cells in mice. Gastroenterology. 2002;122:1829–1841. doi: 10.1053/gast.2002.33655. [DOI] [PubMed] [Google Scholar]
- 77.Forbester J.L., Lees E.A., Goulding D., Forrest S., Yeung A., Speak A., Clare S., Coomber E.L., Mukhopadhyay S., Kraiczy J., Schreiber F., Lawley T.D., Hancock R.E.W., Uhlig H.H., Zilbauer M., Powrie F., Dougan G. Interleukin-22 promotes phagolysosomal fusion to induce protection against Salmonella enterica Typhimurium in human epithelial cells. Proc Natl Acad Sci U S A. 2018;115:10118–10123. doi: 10.1073/pnas.1811866115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shkoda A., Ruiz P.A., Daniel H., Kim S.C., Rogler G., Sartor R.B., Haller D. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 2007;132:190–207. doi: 10.1053/j.gastro.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 79.Jarry A., Bossard C., Bou-Hanna C., Masson D., Espaze E., Denis M.G., Laboisse C.L. Mucosal IL-10 and TGF-β play crucial roles in preventing LPS-driven, IFN-γ-mediated epithelial damage in human colon explants. J Clin Invest. 2008;118:1132–1142. doi: 10.1172/JCI32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Madsen K.L., Lewis S.A., Tavernini M.M., Hibbard J., Fedorak R.N. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology. 1997;113:151–159. doi: 10.1016/s0016-5085(97)70090-8. [DOI] [PubMed] [Google Scholar]
- 81.Sun X., Yang H., Nose K., Nose S., Haxhija E.Q., Koga H., Feng Y., Teitelbaum D.H. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2007;294:G139–G147. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- 82.Lorén V., Cabré E., Ojanguren I., Domènech E., Pedrosa E., García-Jaraquemada A., Mañosa M., Manyé J. Interleukin-10 enhances the intestinal epithelial barrier in the presence of corticosteroids through p38 MAPK activity in Caco-2 monolayers: a possible mechanism for steroid responsiveness in ulcerative colitis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomoyose M., Mitsuyama K., Ishida H., Toyonaga A., Tanikawa K. Role of interleukin-10 in a murine model of dextran sulfate sodium- induced colitis. Scand J Gastroenterol. 1998;33:435–440. doi: 10.1080/00365529850171080. [DOI] [PubMed] [Google Scholar]
- 84.Mazzon E., Cuzzocrea S. Role of iNOS in hepatocyte tight junction alteration in mouse model of experimental colitis. Cell Mol Biol (Noisy-le-grand) 2003;49:45–57. [PubMed] [Google Scholar]
- 85.Marlow G.J., van Gent D., Ferguson L.R. Why interleukin-10 supplementation does not work in Crohn’s disease patients. World J Gastroenterol. 2013;19:3931–3941. doi: 10.3748/wjg.v19.i25.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steidler L., Neirynck S., Huyghebaert N., Snoeck V., Vermeire A., Goddeeris B., Cox E., Remon J.P., Remaut E. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol. 2003;21:785–789. doi: 10.1038/nbt840. [DOI] [PubMed] [Google Scholar]
- 87.Luerce T.D., Gomes-Santos A.C., Rocha C.S., Moreira T.G., Cruz D.N., Lemos L., Sousa A.L., Pereira V.B., De Azevedo M., Moraes K., Cara D.C., Leblanc J.G., Azevedo V., Faria A.M.C., Miyoshi A. Anti-inflammatory effects of Lactococcus lactis NCDO 2118 during the remission period of chemically induced colitis. Gut Pathog. 2014;6:33. doi: 10.1186/1757-4749-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Braat H., Rottiers P., Hommes D.W., Huyghebaert N., Remaut E., Remon J.P., van Deventer S.J.H., Neirynck S., Peppelenbosch M.P., Steidler L. A Phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:754–759. doi: 10.1016/j.cgh.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 89.Vermeire S., Rutgeerts P.J., D’Haens G.R., De Vos M., Bressler B., Van der Aa A., Coulie B., Wong C.J., Feagan B.G. 46 A Phase 2a randomized placebo-controlled double-blind multi-center dose escalation study to evaluate the safety, tolerability, pharmacodynamics and efficacy of AG011 in patients with moderately active Ulcerative Colitis. Gastroenterology. 2010;138 S-9. [Google Scholar]
- 90.Zurita-Turk M., del Carmen S., Santos A.C.G., Pereira V.B., Cara D.C., Leclercq S.Y., de LeBlanc A.D.M., Azevedo V., Chatel J.M., LeBlanc J.G., Miyoshi A. Lactococcus lactis carrying the pValac DNA expression vector coding for IL-10 reduces inflammation in a murine model of experimental colitis. BMC Biotechnol. 2014;14:73. doi: 10.1186/1472-6750-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cook D.P., Gysemans C., Mathieu C. Lactococcus lactis as a versatile vehicle for tolerogenic immunotherapy. Front Physiol. 2018;8:1961. doi: 10.3389/fimmu.2017.01961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eckmann L., Jung H.C., Schürer-Maly C., Panja A., Morzycka-Wroblewska E., Kagnoff M.F. Differential cytokine expression by human intestinal epithelial cell lines: Regulated expression of interleukin 8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 93.Olszak T., Neves J.F., Dowds C.M., Baker K., Glickman J., Davidson N.O., Lin C.S., Jobin C., Brand S., Sotlar K., Wada K., Katayama K., Nakajima A., Mizuguchi H., Kawasaki K., Nagata K., Müller W., Snapper S.B., Schreiber S., Kaser A., Zeissig S., Blumberg R.S. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature. 2014;509:497–502. doi: 10.1038/nature13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.George M.M., Rahman M., Connors J., Stadnyk A.W. Opinion: Are organoids the end of model evolution for studying host intestinal epithelium/microbe interactions? Microorganisms. 2019;7:406. doi: 10.3390/microorganisms7100406. [DOI] [PMC free article] [PubMed] [Google Scholar]