Significance
Identifying the conditions associated with a life history transition from cooperative colony life to exploitative social parasitism is important for understanding how changes in behavior contribute to speciation. To explore the evolutionary origins of social parasitism, we reconstructed the evolutionary history of Formica ants because half of all species are social parasites and all socially parasitic life history syndromes known from eusocial insects are represented in this genus. We demonstrate that social parasites evolved from an ancestor that lost the ability to establish new colonies independently and that highly specialized parasites can evolve from less complex social parasite syndromes. Our findings emphasize that social parasite syndromes readily originate in socially polymorphic organisms and evolved convergently across the ant phylogeny.
Keywords: brood parasitism, dulosis, Emery’s rule, Formicidae, inquilinism
Abstract
Studying the behavioral and life history transitions from a cooperative, eusocial life history to exploitative social parasitism allows for deciphering the conditions under which changes in behavior and social organization lead to diversification. The Holarctic ant genus Formica is ideally suited for studying the evolution of social parasitism because half of its 172 species are confirmed or suspected social parasites, which includes all three major classes of social parasitism known in ants. However, the life history transitions associated with the evolution of social parasitism in this genus are largely unexplored. To test competing hypotheses regarding the origins and evolution of social parasitism, we reconstructed a global phylogeny of Formica ants. The genus originated in the Old World ∼30 Ma ago and dispersed multiple times to the New World and back. Within Formica, obligate dependent colony-founding behavior arose once from a facultatively polygynous common ancestor practicing independent and facultative dependent colony foundation. Temporary social parasitism likely preceded or arose concurrently with obligate dependent colony founding, and dulotic social parasitism evolved once within the obligate dependent colony-founding clade. Permanent social parasitism evolved twice from temporary social parasitic ancestors that rarely practiced colony budding, demonstrating that obligate social parasitism can originate from a facultative parasitic background in socially polymorphic organisms. In contrast to permanently socially parasitic ants in other genera, the high parasite diversity in Formica likely originated via allopatric speciation, highlighting the diversity of convergent evolutionary trajectories resulting in nearly identical parasitic life history syndromes.
The complex societies of eusocial insects are vulnerable to exploitation by social parasites that depend on their host colonies for survival and reproduction without contributing to colony maintenance and brood care (1–4). Social parasitism is common among eusocial Hymenoptera and evolved independently in distantly related lineages, including bees, wasps, and ants (3–8). Many studies on social parasitism have focused on the evolution of cooperation and conflict in colonies of eusocial insects and on coevolutionary arms race dynamics between hosts and parasites (9–12). However, the evolutionary origins of social parasitism and the coevolutionary factors causing speciation and thereby contributing to the high diversity of social parasite species in eusocial insects are not well understood (13, 14). Comparative evolutionary studies of social parasites are promising, because they are expected to provide insights into the conditions associated with a behavioral change from cooperative eusociality to exploitative social parasitism as well as into the consequences of the life history transitions on speciation and biological diversification.
Social parasitism is a life history strategy that evolved at least 60 times in ants, and more than 400 socially parasitic species are known from six distantly related subfamilies (4). Despite the high diversity, three main life history strategies can be recognized across social parasites: 1) temporary, 2) dulotic, and 3) permanent social parasitism (1, 3, 15–21). The queens of temporary socially parasitic ant species invade the host nest and kill the resident queen(s), and the host workers raise the parasite’s offspring (16). In the absence of an egg-laying host queen, the host workforce is gradually replaced until the colony is composed solely of the temporary social parasite species. The queens of dulotic social parasites start their colony life cycle as temporary social parasites, and once sufficient parasitic workers have been reared, they conduct well-organized raids of nearby host nests to capture their brood (22). Some brood is eaten, but most workers eclose in the parasite’s nest and contribute to the workforce of the colony. By contrast, most permanent social parasite (i.e., inquiline) species are tolerant of the host queen, allowing her to continuously produce host workers, whereas the inquiline queens focus their reproductive effort on sexual offspring (1, 13). Inquilines obligately depend on their hosts and most inquiline species lost their worker caste entirely (1, 18, 19, 23).
The evolutionary origins of social parasitism have been debated since Darwin’s On the Origin of Species by Means of Natural Selection (24). Entomologists have long noticed that ant social parasites and their hosts are close relatives (15, 16, 25–29), an observation subsequently referred to as “Emery’s rule” (30). Strictly interpreted, Emery’s rule postulates a sister group relationship between host and parasite, whereas a less restrictive or “loose” interpretation signifies for example a congeneric, but not necessarily a sister taxon relationship (13, 31–33). Consequently, two competing hypotheses were developed for explaining the speciation mechanisms of social parasites: 1) The interspecific hypothesis proposes that host and social parasite evolved reproductive isolation in allopatry, whereas 2) the intraspecific hypothesis postulates that the social parasite evolved directly from its host in sympatry (3, 13, 18, 20, 21, 31–37). Empirical studies of temporary, dulotic, and host queen-intolerant workerless ant social parasites generally provide support for the interspecific hypothesis (14, 38–48), whereas recent phylogenetic studies lend support to the intraspecific hypothesis for queen-tolerant inquilines (33, 36, 49–51). In some cases, host shifts, secondary speciation events of hosts and/or parasites, and extinctions obscure the original evolutionary conditions under which social parasitism originated (52–54).
To explore the origin and evolution of diverse socially parasitic life histories in eusocial insects, we reconstructed the evolutionary history of the Holarctic ant genus Formica. Formica ants are ideally suited for comparative studies of social parasitism because the genus has the highest number of social parasite species in any ant genus (84 of 172; Table 1), and all socially parasitic life history traits known from eusocial insects evolved in Formica ants (Fig. 1 and Tables 1 and 2). In addition, colonies of Formica species vary significantly in colony-founding behavior as well as in nest and colony structures, providing an opportunity to explore the interplay between colony organization and life history at the origin of social parasitism. Some Formica species use independent colony foundation (ICF), when new colonies are started by a single queen (i.e., haplometrosis) or a group of cooperating queens (i.e., pleometrosis). Queens of other species rely on dependent colony founding (DCF), cooperating with groups of conspecific workers to found a new colony (i.e., budding) or invading an existing heterospecific colony as a temporary social parasite (TSP) or a permanent social parasite (PSP) (Table 2) (1, 56–62). In contrast to other studies (63), we regard TSP as a form of DCF because the socially parasitic queen relies on the social environment of the host for colony founding and rearing of the first brood. Furthermore, Formica colonies can have a single or multiple functional queens (monogyny vs. polygyny) and comprise one (monodomous) or multiple (polydomous) to thousands of interconnected physical nests covering a large area (supercolonial) (64).
Table 1.
Diversity of social parasites in the genus Formica compared to all other ants
| Temporary social parasites (%) | Dulotic social parasites (%) | Permanent social parasites (%) | Total social parasite diversity (%) | |
| All ants (n = 13,861) | 200 (1.4) | 80 (0.6) | 100 (0.7) | >400 (2.9) |
| Formica (n = 172) | 68 (39.5) | 14 (8.1) | 2 (1.2) | 84 (48.8) |
Socially parasitic life histories are significantly overrepresented in Formica ants, except for inquilinism. The total social parasite diversity in ants is higher than the sum of species in individual life history categories because the biology of numerous social parasites remains unknown. The data are derived from published sources (1, 3, 4, 55).
Fig. 1.
Diversity of life history traits in the formicine ants. In clockwise direction: (A) members of the F. fusca group practicing independent colony foundation; (B) F. obscuripes, representing the Formica integra group (Nearctic members of the paraphyletic “rufa” group), which practices dependent and temporary social parasitic colony founding; (C) Formica gynocrates, representing the facultatively dulotic species of the F. sanguinea group, with a worker of its neogagates group host species, Formica vinculans; (D) the highly modified worker of Polyergus mexicanus, representing the obligately dulotic formicine ants in the genera Polyergus and Rossomyrmex. All images courtesy of Alex Wild (www.alexanderwild.com).
Table 2.
Diversity, taxonomy, life history, and evolutionary traits of Formica ants across currently recognized species groups, as well as of closely related formicine ants
| Formica species group or genus | No. of described of species | Estimated no. of new species | Colony-founding behavior | Colony organization | Nest organization | Socially parasitic life history | Phylogenetic information | Geographic distribution |
| F. dakotensis gr. | 2 | Unknown | TSP; budding at low frequency | Monogynous, polygynous | Monodomous, polydomous | TSP, PSP(?) | Monophyletic | Nearctic |
| F. difficilis gr. | 16 | 5 to 10 | TSP; budding at low frequency | Monogynous, polygynous | Monodomous | TSP, PSP | Monophyletic | Nearctic |
| F. exsecta gr. | 17 | Unknown | TSP; budding at high frequency | Monogynous, polygynous | Monodomous, polydomous, supercolonial | TSP | Monophyletic | Nearctic and Palearctic |
| “F. fusca gr.” | 76 | 1 to 15 | Haplo- and pleometrosis; budding rare, if present in a species, at low frequency | Monogynous, polygynous | Monodomous, polydomous, rarely supercolonial | Not socially parasitic | Paraphyletic | Nearctic and Palearctic |
| F. integra gr. | 20 | 2 to 3 | TSP; budding at low frequency | Monogynous, polygynous | Monodomous, polydomous, rarely supercolonial | TSP | Monophyletic | Nearctic |
| “F. neogagates gr.” | 8 | 2 to 3 | Haplometrosis | Monogynous, polygynous | Monodomous | Not socially parasitic | Paraphyletic | Nearctic |
| F. pallidefulva gr. | 5 | Unknown | Haplo- and pleometrosis | Monogynous,polygynous | Monodomous | Not socially parasitic | Monophyletic | Nearctic |
| F. rufa gr. | 13 | None | TSP; budding at high frequency | Monogynous, polygynous | Monodomous, polydomous, supercolonial | TSP | Monophyletic | Palearctic |
| F. sanguinea gr. | 14 | 3 to 5 | TSP | Monogynous, polygynous | Monodomous, rarely polydomous | TSP, facultative and obligate dulosis | Monophyletic | Nearctic and Palearctic |
| F. uralensis gr. | 1 | None | TSP; budding at high frequency | Monogynous, polygynous | Monodomous, polydomous,supercolonial | TSP | Monotypic | Palearctic |
| Iberoformica | 1 | None | Haplometrosis | Monogynous | Monodomous | Not socially parasitic | Monotypic | Palearctic |
| Polyergus | 14 | Unknown | TSP | Monogynous | Monodomous | Obligate TSP, dulosis dulosis | Monophyletic | Nearctic and Palearctic |
The former F. rufa group is divided into three clades, i.e., the dakotensis, integra, and rufa groups. The erstwhile microgyna group is properly referred to as the difficilis group based on name priority. Please refer to SI Appendix, Table S2 for a detailed list of traits for individual species and references to original research. Total number of Formica species does not add to 172 because of one valid, poorly described species (Formica gravelyi) of uncertain group affinity.
To infer the evolutionary origins of social parasitism and explore the behavioral transition from a social colony life to a socially parasitic life history, we reconstructed a global phylogeny for Formica ants and relevant outgroups from the formicine genera Iberoformica, Polyergus, Proformica, and Rossomyrmex, thus spanning the root node of the tribe Formicini (65). The comprehensive, time-calibrated phylogeny allows for 1) testing competing hypotheses regarding the origins and evolutionary transitions of social parasitism, 2) reconstructing the evolutionary and biogeographic history of the group, and 3) suggesting modifications to the internal classification of the genus.
Results and Discussion
Formica Originated in Eurasia during the Oligocene.
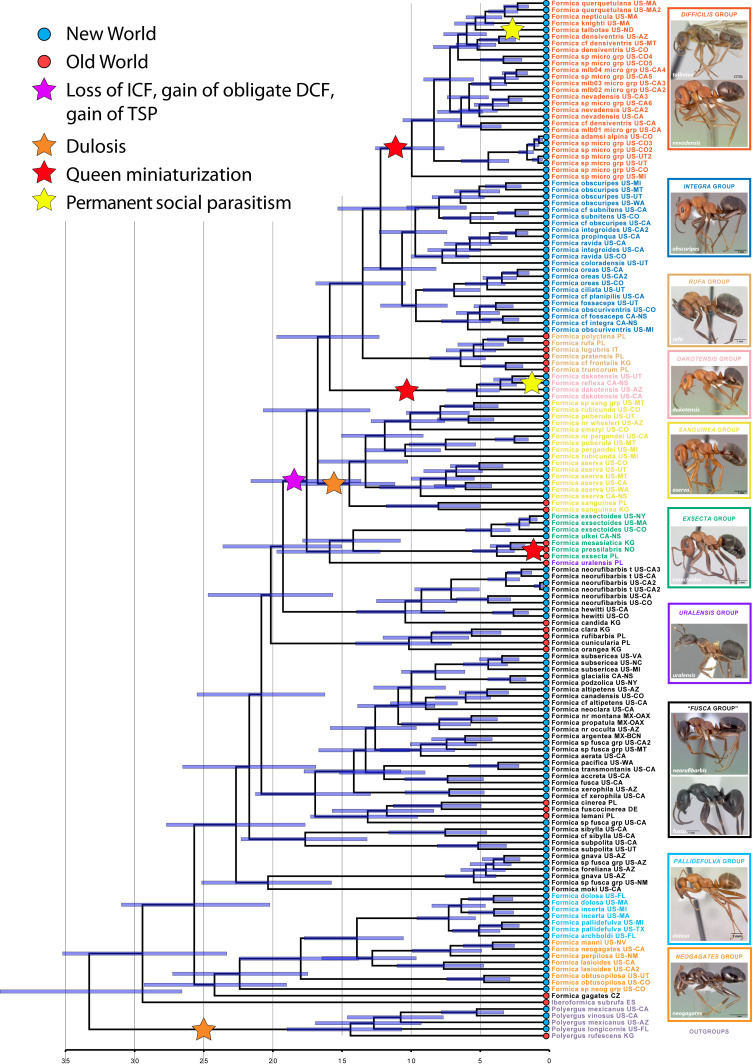
To infer the life history evolution of the diverse, Holarctic genus Formica, we inferred a comprehensive phylogeny for 101 Formica species representing all 10 currently recognized species groups (Table 2) across their wide geographic distribution in both the Old World (19 spp.) and the New World (82 spp.) and outgroups, using 2,242 ultraconserved element (UCE) loci per taxon. Our analyses recovered Formica as a strongly supported clade with the monotypic genus Iberoformica as its sister lineage (Fig. 2 and SI Appendix, Fig. S1). Formica and Iberoformica split from their sister genus Polyergus around 33 Ma ago (95% highest posterior density [HPD]: 27 to 39 Ma) and Formica diverged from a common ancestor with its sister lineage Iberoformica subrufa ∼30 Ma ago (95% HPD: 24 to 35 Ma). The crown group age of extant Formica ants is ∼26 Ma (95% HPD: 21 to 31 Ma). Therefore, modern Formica ants evolved recently and likely originated in Eurasia during the Oligocene after the global cooling following the Terminal Eocene Event (66). A similar evolutionary history was inferred for the species-rich Holarctic ant genus Myrmica (49).
Fig. 2.
A time-calibrated molecular phylogeny of Formica, Iberoformica, and Polyergus. Node bars indicate 95% highest posterior density. Scale is in millions of years. Abbreviations after taxa names indicate sample origin. Country codes follow the International Organization for Standardization (ISO) 3166: United States (US), Canada (CA), and Mexico (MX), in addition to noting state/province. Note single loss of independent colony foundation and gain of obligate DCF with inquilinism arising from within temporary parasites (yellow star). Taxon highlight colors signify species group membership; neogagates and fusca species groups are not monophyletic. Photographs are by April Nobile, Erin Prado, and Estella Ortega. Courtesy of https://www.antweb.org/.
Independent Colony Founding and Social Polymorphism Are Ancestral.
To understand the evolution of social parasitism in Formica ants, it is necessary to recover the evolutionary origins of different life history strategies in the biologically diverse species groups. Character state reconstructions of life history traits including nest structure, social organization of colonies, and colony-founding behaviors (Table 2 and SI Appendix, Figs. S3–S5) based on our phylogenomic tree (Fig. 2) show that facultative polygyny and polydomy originated early during Formica evolution. Purely monogynous species groups are found only outside of Formica (Table 2). Within Formica, the deepest node marks the divergence between the independent colony-founding species in the “neogagates” and pallidefulva groups plus Formica gagates on one side and the independently colony-founding species in the paraphyletic grade of the Formica “fusca group” on the other side of the bifurcation (Fig. 2). Accordingly, facultative polygyny, independent colony founding, and polydomy are ancestral traits that were likely present in the most recent common ancestor (MRCA) of all extant Formica species. The fusca group arises as a paraphyletic grade nested between these early diverging lineages and all other Formica species and consists of at least five monophyletic groups (Fig. 2). Facultative DCF via budding has been documented in certain species of the fusca group (SI Appendix, Table S2). The nesting behavior transitioned repeatedly between constructing monodomous, polydomous, and even supercolonial nests in the fusca group, which has important implications for population structure and population density of those species.
ICF Was Lost Once, Leading to Obligate DCF.
In Formica, obligate dependent colony founding evolved ∼18 Ma ago (95% HPD: 14 to 21 Ma) (Fig. 2) when an ancestor practicing both independent and facultative dependent colony foundation via budding, as observed in some fusca group species, lost the capacity for independent founding. This loss was precipitated by or coincided with using heterospecific workers for dependent colony founding (TSP), which likely evolved from budding, a strategy involving the use of conspecific workers for colony founding. This is suggested by the fact that there are no confirmed obligate dependent founding species that practice budding only, without the capacity for TSP.
The clade with obligate DCF and capacity for TSP includes species of the exsecta, sanguinea, dakotensis, rufa, integra, and difficilis groups, as well as Formica uralensis, a species of uncertain taxonomic affiliation that is here inferred to be the sister lineage to the exsecta group. Recently, Romiguier et al. (48) also recovered a single loss of ICF among Palearctic Formica species including representatives of 4 of the 10 species groups. Our global phylogenetic analysis confirms and significantly expands on this earlier conclusion and adds a temporal scale showing that the MRCA of this clade lived around 18 Ma ago. Furthermore, our analysis reveals that clades of socially parasitic species have secondarily transitioned to other parasitic life histories. Evolutionary reversals from social parasitism to independent colony founding were not recovered, suggesting that a transition to a socially parasitic lifestyle is irreversible. A similar pattern was found in the ant genus Lasius, where temporary parasitism evolved twice but reversals to ICF are unknown (40).
Among the TSP species, two ecologically distinct life histories can be recognized. First, species in the dakotensis, difficilis, and integra groups are predominantly facultative temporary social parasites and practice colony budding at low frequency. Newly mated queens are unable to found new colonies independently via haplo- or pleometrosis, but instead, they seek adoption in heterospecific host colonies or readoption into conspecific colonies leading to secondary polygyny. In these species, colony budding seems to occur occasionally, which results in a characteristic population structure with smaller clusters of nests (usually less than five), whereas large, unicolonial populations are absent. In contrast, species in the exsecta and rufa groups are facultative temporary social parasites practicing colony budding at high frequency, which can result in highly polydomous and/or supercolonial populations (Fig. 2 and Table 2 and SI Appendix, Table S2).
The temporary social parasite species in the Formica difficilis, integra, rufa, and dakotensis groups shared a common ancestor ∼16 Ma ago (95% HPD: 13 to 19 Ma), and they constitute the sister group to the dulotic sanguinea group (Fig. 2). The difficilis group is also monophyletic and is sister to the integra group (Fig. 2). All species in the difficilis group have miniature queens not larger than their largest workers (Fig. 3 A and B), which is likely associated with the socially parasitic life history. For the difficilis group, we infer a single origin of queen miniaturization ∼10 Ma ago (95% HPD: 8 to 12 Ma). It is important to note that most difficilis group species are rare and our knowledge about their biology is fragmentary at best. Therefore, the temporary social parasitic behavior remains to be observed for most species. However, the few existing direct observations on nest founding behavior, which include F. difficilis (16), Formica densiventris (67), Formica impexa (67), Formica adamsi alpina (S.P.C., personal observation), and Formica new species (S.P.C., personal observation), confirm temporary social parasitism.
Fig. 3.
Convergent evolution of queen miniaturization in temporary social parasitic Formica ants. Queen miniaturization evolved in the F. difficilis (A and B) and dakotensis (C and D) species groups. In comparison, queens in the integra group (E and F) show a pronounced queen–worker dimorphism typical for Formica ants. (A and B) Queen (A) and worker (B) of a hitherto undescribed F. difficilis group species showing one of the most extreme cases of queen size reduction known in ants. (C and D) Queen (C) and worker (D) of F. reflexa representing a second, independent evolutionary origin of queen miniaturization, which is less extreme than in the difficilis group species. (E and F) Queen (E) and worker (F) of Formica ravida demonstrating a typically sized queen with morphological modifications related to wing bearing and reproduction that are absent from the worker. Note that the F. ravida individuals are significantly larger than all other ants depicted here. (Scale bars, 1 mm in A–D and 2 mm in E and F.) Specimen identifiers are as follows: (A) MCZ 574034, (B) MCZ 574022, (C) MCZ 525288, (D) MCZ 525283, (E) MCZ 552096, and (F) MCZ 575163. Photographs are by Patrick McCormack. Images copyright President and Fellows of Harvard University.
Interestingly, queen miniaturization evolved repeatedly in Formica, including in the exsecta group, where it is present in several Palearctic species (68), and in the Nearctic dakotensis group. Because our sampling includes only one of the Palearctic exsecta group species with miniature queens (Formica pressilabris), we cannot ascertain whether miniaturization evolved once or multiple times in this group. In the temporary social parasite species of the Nearctic Formica dakotensis clade (Fig. 3), both species (F. dakotensis and F. reflexa) have small queens. Formica dakotensis is a facultative temporary social parasite (55) and fully independent colonies are common. In contrast, Formica reflexa is rare and was found only in association with fusca group host workers (69, 70) (S.P.C., personal observation), suggesting a unique life history including parasitic colony founding and potentially a lifelong dependence on the host. Queen size reduction is frequently observed in inquiline social parasites (13, 71), but the independent origins of miniature queens in the difficilis, dakotensis, and exsecta groups imply that queen size reduction is adaptive for a temporary social parasitic life history syndrome in Formica ants.
Evolution of Dulosis.
The dulotic species of the Formica sanguinea group are monophyletic (Fig. 2), suggesting that dulotic behavior evolved once some time prior to its inferred crown group age of ∼14 Ma (95% HPD: 11 to 18 Ma). Thus, dulotic behavior and temporary social parasitism did not evolve simultaneously in Formica, but instead dulosis evolved secondarily from a temporary socially parasitic ancestor. The single origin of dulotic behavior in a diverse clade of temporary social parasite species supports the hypothesis that dulosis originates only under rare circumstances (3, 4, 22). In fact, the evolutionary origins of dulotic behavior in ants have been debated since Darwin’s On the Origin of Species by Means of Natural Selection (24) and three not mutually exclusive hypotheses have been proposed to explain the origins of this highly specialized behavior: 1) predation, 2) brood transport, and 3) territorial competition (1, 3, 20, 22, 24, 35, 72–76).
Our phylogenetic results and behavioral observations indicate that the predatory behavior of temporary social parasites could lead to the evolution of facultative dulosis in Formica. Brood stealing would be favored by natural selection if the aid of heterospecific workers increased the parasite’s fitness, although we are not aware of experimental studies demonstrating fitness benefits provided by stolen host workers to facultatively dulotic species. Additional biological factors that were associated with the evolutionary origins of dulosis, including polygyny, polydomy, brood transport, and territoriality (17, 22, 35, 77), can also be inferred for the common ancestor of the dulotic species in the F. sanguinea group.
It is important to note that dulosis evolved convergently and under different ecological conditions in distantly related, nonpredatory ants, such as the omnivorous, scavenging species in the genera Temnothorax and Tetramorium (12, 41, 42, 44, 46, 78). This pattern suggests that alternative factors, such as territoriality and brood transport, likely play an important role in the origin of dulotic behavior in nonpredatory ants. Across the ant tree of life, dulosis originated at least nine times convergently in distantly related clades (22, 47, 79), including three origins in the Formicini (65) and six origins in the Crematogastrini (41, 45, 46).
Evolution of Inquiline Social Parasitism.
The only confirmed workerless inquiline social parasite in the genus Formica is Formica talbotae (80, 81). Formica talbotae is phylogenetically nested within the difficilis clade (Fig. 2), suggesting that workerless permanent parasitism evolved once from a facultatively polygynous ancestor practicing temporary social parasitism. This is empirical evidence for an evolutionary transition from temporary to workerless inquiline social parasitism, a hypothesis earlier suggested by Wilson (18). Formica dirksi has also been repeatedly suggested to be a workerless social parasite of Formica subaenescens in Maine (3, 82, 83), but there are no natural history data substantiating this claim.
Formica talbotae is a distant relative of its integra group host, Formica obscuripes, with which it shared a common ancestor ∼12 Ma ago (95% HPD: 10 to 15 Ma). The host–parasite relationship of F. talbotae and F. obscuripes is consistent with the “loose” interpretation of Emery’s rule, where hosts and parasites can be congeners but not sister lineages, suggesting that F. talbotae evolved via the interspecific, allopatric route of social parasite evolution. This result contrasts with previous studies inferring workerless inquiline social parasites as directly evolving from free-living, closely related ancestors via the intraspecific, sympatric route of social parasite speciation (33, 36, 49, 50). However, and in contrast to many host queen tolerant inquiline social parasites, F. talbotae was found exclusively in queenless host colonies. It seems unlikely that the miniature F. talbotae queens assassinate the many and much larger host queens. Instead, it appears more likely that F. talbotae specializes on declining host colonies that lost their reproductive queen(s) (80, 81). Preferentially inhabiting queenless host colonies is a highly specialized and rare behavior that was described only for few social parasite species (84–87). It is important to note that the phylogenetic placement of F. talbotae was not unequivocal in our analysis, but it is important for understanding the evolution of workerless inquilinism in Formica ants. The museum specimens of F. talbotae available to us were collected in the 1950s, and from these specimens we recovered only fragments of 496 UCE loci (∼8% of nucleotides in the full data matrix). Coalescent-based species tree estimation is known to suffer from missing data (88), and to test for incongruencies, we performed coalescent-based species tree estimations (89–92) using a reduced data matrix that included only the 67 loci for which at least 50% of the F. talbotae sequences were present. This analysis recovered F. talbotae as the sister lineage to the difficilis group (SI Appendix, Fig. S8), and statistical support was low across the species tree. The phylogenetic position of F. talbotae differs from the nested position obtained in concatenation (Fig. 2 and SI Appendix, Fig. S1), but the topology is more similar to the concatenated tree than the species tree analysis of the full dataset (SI Appendix, Fig. S6), suggesting that missing data do have a negative impact on the species tree analysis. A quartet sampling analysis (93) revealed topological conflict at the same nodes where the concatenated tree differed from the species tree, but strongly supported monophyly of the difficilis group including F. talbotae nested within (SI Appendix, Fig. S9).
In agreement with morphological evidence, which places F. talbotae within the difficilis group (80, 81), we also consider F. talbotae a member of the difficilis group. We interpret the placement outside of the difficilis group by species tree methods as an artifact caused by missing data.
Historical Biogeography.
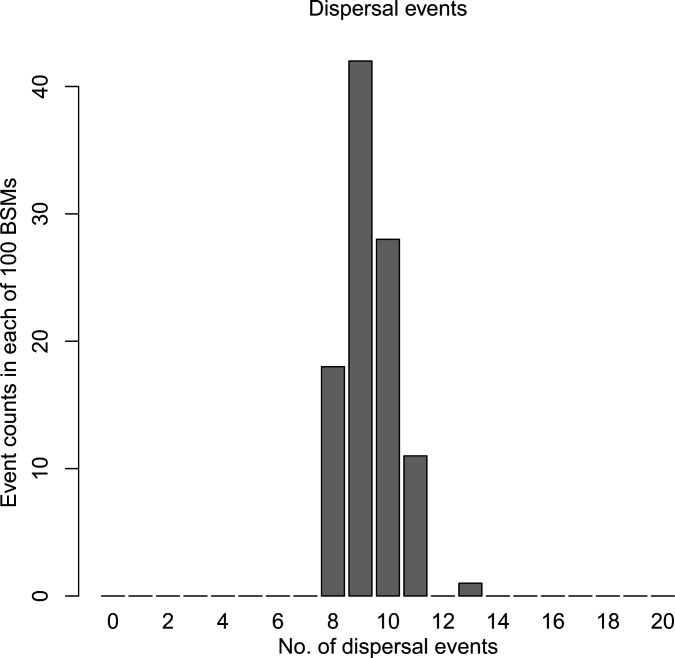
To infer the biogeographic conditions under which social parasitism evolved in the Formicini, we conducted a historic biogeography analysis inferring repeated dispersal between the Old and the New World in the genera Formica, Iberoformica, and Polyergus (Fig. 4 and SI Appendix, Fig. S2). According to our biogeographical stochastic mapping analysis (94), at least 8 and more likely 9 or 10 such dispersal events occurred in this genus group (Fig. 4). All other genera classified in the Formicini are confined to the Old World. Therefore, early stem lineages of the genus Formica almost certainly lived in the Old World and subsequently started dispersing into the New World and back, likely via Beringia, which connected Eurasia and the Nearctic throughout most of the Paleogene (95). Recent trans-Beringian dispersal was also demonstrated for several Holarctic ant species, which include Formica gagatoides (96).
Fig. 4.
Inferred dispersal events between the Old and the New World in Formica, Iberoformica, and Polyergus ants. Shown is a histogram of dispersal event counts from 100 biogeographic stochastic maps under the DEC+J model in BioGeoBEARS.
Nine fossil species of Formica are known from Eocene amber inclusions of Europe (47.8 to 33.9 Ma) (97–99). Several fossils have been compared to extant species in the fusca and rufa groups (100), and close examination suggests that this resemblance may be superficial (101, 102), which raises the question of whether the Eocene amber fossils indeed represent crown group Formica. Doubts about the correct identification of putative Formica fossils in Baltic amber currently limit the utility of Eocene amber fossils for calibrating divergence analyses in the genus.
Implications for Formica Taxonomy and Classification.
The internal classification of species-rich genera, such as Formica, is important because the affiliation with a species group (or subgenus) can provide first clues regarding the life history and general biology of an unknown species. Of the 10 presently recognized Formica species groups (Table 2), the pallidefulva (= Neoformica), exsecta, sanguinea (= Raptiformica), and difficilis groups were recovered as monophyletic. In contrast, species traditionally classified in three species groups (or subgenera), the fusca (= Serviformica), neogagates (= Proformica), and rufa (= Formica s. str.) groups, form nonmonophyletic assemblages. The Palearctic species F. gagates, traditionally placed in the fusca species group, was inferred as the sister species to the Nearctic clade comprising the neogagates and pallidefulva groups. The neogagates group itself forms a paraphyletic grade outside the pallidefulva group. The Holarctic exsecta group plus the problematic F. uralensis, placed in a monotypic group, are sisters to all other dependent colony-founding/temporary social parasite groups, instead of F. uralensis being part of either the fusca or the rufa group, as previously suggested (103, 104). The rest of species traditionally classified in the fusca group form a grade consisting of five clades progressively more closely related to the dependent colony-founding clade. Although our sampling did not include Formica fusca, prior work shows that it would be placed within the Palearctic clade containing Formica cinerea, Formica fuscocinerea, and Formica lemani (61). More thorough taxon sampling and a careful morphological study are necessary for a stable classification of those species. The Nearctic Formica obtusopilosa is closely related to Formica neogagates and not a member of the sanguinea group (105, 106), as treated by some authors (107, 108). This classification is consistent with the biology of F. obtusopilosa, which is not dulotic and its queens found colonies independently, like other species in the neogagates group (105, 107) (S.P.C., personal observation). Hence, the clypeal notch, long thought to be diagnostic of sanguinea group species, evolved convergently in F. obtusopilosa. The traditional rufa group is recovered as paraphyletic because the Old World rufa group species (now the true rufa species group) form a clade that is sister to the Nearctic integra and difficilis groups, and the distinctive F. dakotensis and F. reflexa form a clade (here called the dakotensis group) sister to rufa, integra, and difficilis groups. Finally, according to custom and for consistency we refer to the erstwhile microgyna group as the difficilis group, after the oldest constituent species name. The difficilis group species are monophyletic and sister to the integra group, not nested within it as previously suggested (109).
Considering that all the traditional Formica subgenera are nested within Formica and that three of the four subgenera are paraphyletic, we suggest discontinuing the use of the subgeneric names and using species group names instead. While these results clarify the internal structure within the genus, much work remains to be done on the species level. The fusca, integra, difficilis, Nearctic sanguinea, and neogagates groups all need taxonomic revisions.
Conclusions
Our study provides a robust phylogenetic framework for studying the evolution of the diverse and ecologically important Holarctic ant genus Formica and allows for testing competing hypotheses regarding the origins and evolution of social parasitism in ants. We conclude that in the formicine genera Formica, Polyergus, and Rossomyrmex, social parasitism originated repeatedly and convergently. In the genus Formica, multiple transitions to increasingly more complex socially parasitic life histories evolved. First, the capacity for occasional DCF via budding evolved in facultatively polygynous species practicing ICF. Eventually the ability for ICF was lost in the ancestor of what is now a large clade of obligate dependent colony-founding species (clade marked by a purple star in Fig. 2). Temporary social parasitism either coincided with or preceded this loss of ICF and the transition to obligate DCF. Because all species of the obligate DCF clade appear capable of TSP, it is likely that the evolution of TSP precipitated the loss of ICF. Within this obligate DCF clade dulosis evolved once in the ancestor of the sanguinea group. Finally, the permanent social parasites, F. reflexa and the workerless F. talbotae, evolved independently from temporary social parasitic ancestors. Across species, Formica social parasites likely originated via the interspecific, allopatric speciation route of social parasite evolution, emphasizing that convergent evolutionary trajectories can lead to highly similar parasitic life history syndromes across eusocial insects.
The inferred sequence for the evolution of dulosis lends empirical support to Charles Darwin’s “predation hypothesis” for the origin of dulotic behavior in ants. Furthermore, our results suggest that the ancestor of the dulotic Formica species likely possessed all the traits associated with the evolution of dulotic behavior, namely territorial and predatory behavior, brood transport behavior among spatially distinct nests of polygynous colonies, and the capacity for parasitic and dependent colony founding. The origin of dulosis was then followed by secondary diversification into 14 species that today form the monophyletic sanguinea group.
Our study inferred the workerless social parasite F. talbotae as arising from a clade of temporary social parasites in the difficilis group, providing empirical evidence for a transition from temporary to workerless social parasitism. The example of F. talbotae underscores the importance of distinguishing between the different life history traits summarized under the umbrella term “inquiline social parasitism.” Taking the evolutionary origins into account is important because the majority of queen-tolerant workerless inquiline parasites likely speciated directly from free-living ancestors, whereas most queen-intolerant workerless social parasites apparently transitioned to the workerless state from a dulotic or temporary social parasitic ancestor. Formica talbotae can be regarded as a queen-intolerant obligate temporary social parasite that preferentially inhabits queenless host colonies and secondarily lost its worker caste. The distant relatedness to its host, F. obscuripes, and its distinct life history traits suggest that F. talbotae also evolved via the allopatric, interspecific route of social parasite evolution, contrasting with the sympatric origins of some queen-tolerant inquiline social parasites.
We show that Formica evolved during the early Oligocene, representing a relatively young ant genus that diversified rapidly into a diverse, ecologically dominant group. During its evolutionary history Formica ants dispersed several times between the Old and the New World.
Our study outlines the life history changes associated with the transition from a cooperative eusocial to exploitative socially parasitic life history. Given the high diversity of social parasite species in the genus Formica, and considering the high degree of morphological and behavioral specialization, socially parasitic Formica species appear to be an ideally suited study organism for investigating caste determination and for exploring the genetic basis underlying behavioral and life history evolution.
Materials and Methods
Taxon Sampling.
We newly sequenced 101 ingroup morphospecies from all 10 species groups of Formica ants that were recognized prior to our study and 8 outgroup species. Collection data associated with sequenced samples can be found in SI Appendix, Table S1 and detailed voucher information is on Zenodo.
Molecular Data Generation.
To obtain the genetic data we extracted DNA, prepared genomic libraries, and then enriched them using 9,446 custom-designed probes targeting 2,524 UCE loci in Hymenoptera (110). We submitted the enriched libraries to the University of Utah High Throughput Genomics Core Facility for sequencing on two Illumina HiSeq 125 Cycle Paired-End Sequencing v4 runs.
Data Processing.
We processed the resulting reads using the Phyluce bioinformatics pipeline (111). Following alignment and trimming, we retained only individual locus alignments that had 110 or more taxa (70% of total), resulting in 2,242 loci on average 667 nt long. The resulting concatenated matrix was 1,497,044 nt long and contained 17.58% of missing data and gaps.
Phylogenetics.
To infer the maximum-likelihood phylogeny, we used ModelFinder (112) as implemented in IQ-TREE (113) to select the best model for each UCE locus under the Akaike information criterion (AICc). These models were then used for by-locus partitioned analysis of the concatenated data matrix (114). To assess the robustness of this result to different analytics we performed an unpartitioned analysis and a quartet sampling analysis (93). In addition to concatenated analyses we performed coalescent-based species tree estimation using ASTRAL-III (92).
Divergence Time Analyses.
For divergence time analyses we used a node dating approach, as implemented in MCMCTree, a part of the PAML package, v4.9e (115). We constrained our root node with soft bounds around a conservative maximum age estimate of 79 Ma, which corresponds to the lower bound of the 95% highest posterior density interval for that split in a previous phylogenomic study (65).
Biogeography.
For biogeographic inference we used BioGeoBEARS (116). We discretized the distribution of Formica species into two regions, the New World and the Old World. We used 100 replicates of biogeographical stochastic maps (94) to estimate the number of times Formica dispersed between the Old and the New World.
Ancestral State Reconstruction.
To investigate the evolution of nest structure, colony structure, and mode of colony foundation, we used stochastic character mapping (117) as implemented in the R package Phytools (118). We compared and selected best-fitting models of character evolution using GEIGER (119) with a time-calibrated tree pruned from distant outgroups and intraspecific samples as input. We based our character coding for each species (SI Appendix, Table S2) on literature records and 80 y of cumulative field research by ourselves and colleagues.
Supplementary Material
Acknowledgments
This research was supported by the US National Science Foundation (NSF DEB-1456964, DEB-1654829, and NSF CAREER DEB-1943626). We gratefully acknowledge Philip Ward, James Trager, Matthew Prebus, Lech Borowiec, and André Francoeur for contributing important samples, as well as Jeffrey-Sosa Calvo, Benjamin Gerstner, and Cody Tipp for assisting with laboratory work and voucher specimen processing. Philip Ward and Jack Longino also contributed life history observations.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2026029118/-/DCSupplemental.
Data Availability
For detailed methods, see SI Appendix. Reads generates for this study are available at the National Center for Biotechnology Information (NCBI) Sequence Read Archive (BioProject ID PRJNA749764). Other files used in analyses are available on Zenodo (DOI: 10.5281/zenodo.4341310) (120).
References
- 1.Hölldobler B., Wilson E. O., The Ants (Harvard University Press, 1990). [Google Scholar]
- 2.Schmid-Hempel P., Parasites in Social Insects (Princeton University Press, 1998). [Google Scholar]
- 3.Buschinger A., Social parasitism among ants: A review (Hymenoptera: Formicidae). Myrmecol. News 12, 219–235 (2009). [Google Scholar]
- 4.Rabeling C., “Social parasitism” in Encyclopedia of Social Insects, Starr C. K., Ed. (Springer International Publishing, 2020), pp. 836–858. [Google Scholar]
- 5.Wcislo W.T., The roles of seasonality, host synchrony, and behaviour in the evolutions and distributions of nest parasites in Hymenoptera (Insecta). With special reference to bees (Apoidea). Biol. Rev. Camb. Philos. Soc. 62, 515–542 (1987). [Google Scholar]
- 6.Cervo R., Polistes wasps and their social parasites: An overview. Ann. Zool. Fenn. 43, 531–549 (2006). [Google Scholar]
- 7.Smith J. A., Chenoweth L. B., Tierney S. M., Schwarz M. P., Repeated origins of social parasitism in allodapine bees indicate that the weak form of Emery’s rule is widespread, yet sympatric speciation remains highly problematic. Biol. J. Linn. Soc. Lond. 109, 320–331 (2013). [Google Scholar]
- 8.Lhomme P., Hines H. M., Ecology and evolution of cuckoo bumble bees. Ann. Entomol. Soc. Am. 112, 122–140 (2019). [Google Scholar]
- 9.Foitzik S., DeHeer C. J., Hunjan D. N., Herbers J. M., Coevolution in host-parasite systems: Behavioural strategies of slave-making ants and their hosts. Proc. Biol. Sci. 268, 1139–1146 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandt M., Foitzik S., Fischer-Blass B., Heinze J., The coevolutionary dynamics of obligate ant social parasite systems–between prudence and antagonism. Biol. Rev. Camb. Philos. Soc. 80, 251–267 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Kilner R. M., Langmore N. E., Cuckoos versus hosts in insects and birds: Adaptations, counter-adaptations and outcomes. Biol. Rev. Camb. Philos. Soc. 86, 836–852 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Feldmeyer B., Elsner D., Alleman A., Foitzik S., Species-specific genes under selection characterize the co-evolution of slavemaker and host lifestyles. BMC Evol. Biol. 17, 237 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourke A. F. G., Franks N. R., Alternative adaptations, sympatric speciation and the evolution of parasitic, inquiline ants. Biol. J. Linn. Soc. Lond. 43, 157–178 (1991). [Google Scholar]
- 14.Huang M. H., Dornhaus A., A meta-analysis of ant social parasitism: Host characteristics of different parasitism types and a test of Emery’s rule. Ecol. Entomol. 33, 589–596 (2008). [Google Scholar]
- 15.Wasmann E., Die Zusammengesetzten Nester und Gemischten Kolonien der Ameisen. Ein Beitrag zur Biologie, Psychologie und Entwicklungsgeschichte der Ameisengesellschaften (Aschendorffsche Buchdruckerei, Münster, Germany, 1891).
- 16.Wheeler W. M., A new type of social parasitism among ants. Bull. Am. Mus. Nat. Hist. 20, 347–375 (1904). [Google Scholar]
- 17.Wheeler W. M., Ants: Their Structure, Development and Behavior (Columbia University Press, New York, 1910). [Google Scholar]
- 18.Wilson E. O., The Insect Societies (Harvard University Press, Cambridge, MA, 1971). [Google Scholar]
- 19.Kutter H., Die sozialparasitischen Ameisen der Schweiz. Neujahrsblatt Naturforschenden Gesellschaft Zürich 171, 1–62 (1968). [Google Scholar]
- 20.Buschinger A., Evolution of social parasitism in ants. Trends Ecol. Evol. 1, 155–160 (1986). [DOI] [PubMed] [Google Scholar]
- 21.Buschinger A., Sympatric speciation and radiative evolution of socially parasitic ants - heretic hypotheses and their factual background. J. Zool. Syst. Evol. Res. 28, 241–260 (1990). [Google Scholar]
- 22.D’Ettorre P., Heinze J., Sociobiology of slave-making ants. Acta Ethol. 3, 67–82 (2001). [Google Scholar]
- 23.Wilson E. O., Tropical social parasites in the ant genus Pheidole, with an analysis of the anatomical parasitic syndrome (Hymenoptera: Formicidae). Insectes Soc. 31, 316–334 (1984). [Google Scholar]
- 24.Darwin C., On the Origin of Species by Means of Natural Selection (Murray, London, 1859). [Google Scholar]
- 25.Wasmann E., Neues über die zusammengesetzten Nester und gemischten Kolonien der Ameisen. Allgemeine Zeitschrift für Entomologie 7, 1–5 (1902). [Google Scholar]
- 26.Wasmann E., Neues über die zusammengesetzten Nester und gemischten Kolonien der Ameisen. Allgemeine Zeitschrift für Entomologie 7, 167–173 (1902). [Google Scholar]
- 27.Emery C., Über den Ursprung der dulotischen, parasitischen und myrmekophilen Ameisen. Biologisches Centralblatt 29, 352–362 (1909). [Google Scholar]
- 28.Wheeler W. M., The compound and mixed nests of American ants. Part I. Observations on a new guest ant. Am. Nat. 35, 431–448 (1901). [Google Scholar]
- 29.Wheeler W. M., The compound and mixed nests of American ants. Part II. The known cases of social symbiosis among American ants. Am. Nat. 35, 513–539 (1901). [Google Scholar]
- 30.Le Masne G., Recherches sur les fourmis parasites. Plagiolepis grassei et l’évolution des Plagiolepis parasites. Comptes Rendus (Hebdomadaires) des Séances de l’Academie des Sciences 35, 1038–1041 (1956). [Google Scholar]
- 31.Ward P. S., “Genetic and social changes associated with ant speciation” in The Genetics of Social Evolution, Breed M. D., Page R. E., Eds. (Westview Press, Boulder, CO, 1989), pp. 123–148. [Google Scholar]
- 32.Ward P. S., A new workerless social parasite in the ant genus Pseudomyrmex (Hymenoptera: Formicidae), with a discussion of the origin of social parasitism in ants. Syst. Entomol. 21, 253–263 (1996). [Google Scholar]
- 33.Rabeling C., Schultz T. R., Pierce N. E., Bacci M. Jr, A social parasite evolved reproductive isolation from its fungus-growing ant host in sympatry. Curr. Biol. 24, 2047–2052 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Wheeler W. M., The parasitic Aculeata, a study in evolution. Proc. Am. Philos. Soc. 58, 1–40 (1919). [Google Scholar]
- 35.Buschinger A., Neue Vorstellungen zur Evolution des Sozialparasitismus und der Dulosis bei Ameisen (Hym., Formicidae). Biol. Zent. Bl. 89, 273–299 (1970). [Google Scholar]
- 36.Savolainen R., Vepsäläinen K., Sympatric speciation through intraspecific social parasitism. Proc. Natl. Acad. Sci. U.S.A. 100, 7169–7174 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Degueldre F., et al., Evolutionary history of inquiline social parasitism in Plagiolepis ants. Mol. Phylogenet. Evol. 155, 107016 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa E., Tinaut A., Ruano F., Molecular phylogeny of two slave-making ants: Rossomyrmex and Polyergus (Hymenoptera: Formicidae). Ann. Zool. Fenn. 39, 267–271 (2002). [Google Scholar]
- 39.Kronauer D. J. C., Gadau J., Hölldobler B., Genetic evidence for intra- and interspecific slavery in honey ants (genus Myrmecocystus). Proc. Biol. Sci. 270, 805–810 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janda M., Folková D., Zrzavý J., Phylogeny of Lasius ants based on mitochondrial DNA and morphology, and the evolution of social parasitism in the Lasiini (Hymenoptera: Formicidae). Mol. Phylogenet. Evol. 33, 595–614 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Beibl J., Stuart R. J., Heinze J., Foitzik S., Six origins of slavery in formicoxenine ants. Insectes Soc. 52, 291–297 (2005). [Google Scholar]
- 42.Beibl J., Buschinger A., Foitzik S., Heinze J., Phylogeny and phylogeography of the Mediterranean species of the parasitic ant genus Chalepoxenus and its Temnothorax hosts. Insectes Soc. 54, 189–199 (2007). [Google Scholar]
- 43.Maruyama M., et al., A DNA and morphology based phylogenetic framework of the ant genus Lasius with hypotheses for the evolution of social parasitism and fungiculture. BMC Evol. Biol. 8, 237 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heinze J., Buschinger A., Poettinger T., Suefuji M., Multiple convergent origins of workerlessness and inbreeding in the socially parasitic ant genus Myrmoxenus. PLoS One 10, e0131023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward P. S., Brady S. G., Fisher B. L., Schultz T. R., The evolution of myrmicine ants: Phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Syst. Entomol. 40, 61–81 (2015). [Google Scholar]
- 46.Prebus M., Insights into the evolution, biogeography and natural history of the acorn ants, genus Temnothorax Mayr (hymenoptera: Formicidae). BMC Evol. Biol. 17, 250 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanllorente O., Lorite P., Ruano F., Palomeque T., Tinaut A., Phylogenetic relationships between the slave-making ants Rossomyrmex and their Proformica hosts in relation to other genera of the ant tribe Formicini (Hymenoptera: Formicidae). J. Zool. Syst. Evol. Res. 56, 48–60 (2018). [Google Scholar]
- 48.Romiguier J., Rolland J., Morandin C., Keller L., Phylogenomics of palearctic Formica species suggests a single origin of temporary parasitism and gives insights to the evolutionary pathway toward slave-making behaviour. BMC Evol. Biol. 18, 40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jansen G., Savolainen R., Vepsäläinen K., Phylogeny, divergence-time estimation, biogeography and social parasite-host relationships of the Holarctic ant genus Myrmica (Hymenoptera: Formicidae). Mol. Phylogenet. Evol. 56, 294–304 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Leppänen J., Seppä P., Vepsäläinen K., Savolainen R., Genetic divergence between the sympatric queen morphs of the ant Myrmica rubra. Mol. Ecol. 24, 2463–2476 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Nettel-Hernanz A., et al., Biogeography, cryptic diversity, and queen dimorphism evolution of the Neotropical ant genus Ectatomma Smith, 1958 (Formicidae, Ectatomminae). Org. Divers. Evol. 15, 543–553 (2015). [Google Scholar]
- 52.Parker J. D., Rissing S. W., Molecular evidence for the origin of workerless social parasites in the ant genus Pogonomyrmex. Evolution 56, 2017–2028 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Krieger M. J., Ross K. G., Molecular evolutionary analyses of the odorant-binding protein gene Gp-9 in fire ants and other Solenopsis species. Mol. Biol. Evol. 22, 2090–2103 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Shoemaker D. D., Ahrens M. E., Ross K. G., Molecular phylogeny of fire ants of the Solenopsis saevissima species-group based on mtDNA sequences. Mol. Phylogenet. Evol. 38, 200–215 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Bolton B., An online catalog of the ants of the world (2020). https://antcat.org/. Accessed 1 July 2021.
- 56.Creighton W. S., The ants of North America. Bull. Mus. Comp. Zool. 104, 1–585 (1950). [Google Scholar]
- 57.Letendre M., Huot L., Considérations préliminaires en vue de la revision taxonomique des fourmis du groupe microgyna, genre Formica (Hymenoptera: Formicidae). Annales Société Entomologique Québec 17, 117–132 (1972). [Google Scholar]
- 58.Savolainen R., Deslippe R. J., Facultative and obligate slavery in formicine ants: Frequency of slavery, and proportion and size of slaves. Biol. J. Linn. Soc. Lond. 57, 47–58 (1996). [Google Scholar]
- 59.Savolainen R., Deslippe R. J., Facultative and obligate slave making in Formica ants. Naturwissenschaften 88, 347–350 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Mori A., Grasso D. A., Visicchio R., Le Moli F., Comparison of reproductive strategies and raiding behaviour in facultative and obligatory slave-making ants: The case of Formica sanguinea and Polyergus rufescens. Insectes Soc. 48, 302–314 (2001). [Google Scholar]
- 61.Purcell J., Pellissier L., Chapuisat M., Social structure varies with elevation in an Alpine ant. Mol. Ecol. 24, 498–507 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Brelsford A., et al., An ancient and eroded social supergene is widespread across Formica ants. Curr. Biol. 30, 304–311.e4 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Cronin A. L., Molet M., Doums C., Monnin T., Peeters C., Recurrent evolution of dependent colony foundation across eusocial insects. Annu. Rev. Entomol. 58, 37–55 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Helanterä H., Strassmann J. E., Carrillo J., Queller D. C., Unicolonial ants: Where do they come from, what are they and where are they going? Trends Ecol. Evol. 24, 341–349 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Blaimer B. B., et al., Phylogenomic methods outperform traditional multi-locus approaches in resolving deep evolutionary history: A case study of formicine ants. BMC Evol. Biol. 15, e271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zachos J., Pagani M., Sloan L., Thomas E., Billups K., Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (2001). [DOI] [PubMed] [Google Scholar]
- 67.Wheeler W. M., A revision of the ants of the genus Formica (Linné) Mayr. Bull. Mus. Comp. Zool. 53, 379–565 (1913). [Google Scholar]
- 68.Seifert B., A taxonomic revision of the ant subgenus Coptoformica Mueller, 1923 (Hymenoptera, Formicidae). Zoosystema 22, 517–568 (2000). [Google Scholar]
- 69.Buren W. F., New ants from Minnesota, Iowa, and Wisconsin. Iowa State Coll. J. Sci. 16, 399–408 (1942). [Google Scholar]
- 70.King R. L., Sallee R. M., More mixed colonies in ants. Proc. Iowa Acad. Sci. 58, 487–489 (1951). [Google Scholar]
- 71.Aron S., Passera L., Keller L., Evolution of miniaturisation in inquiline parasitic ants: Timing of male elimination in Plagiolepis pygmaea, the host of Plagiolepis xene. Insectes Soc. 51, 395–399 (2004). [Google Scholar]
- 72.Wilson E. O., Slavery in ants. Evolution 29, 108–119 (1975). [DOI] [PubMed] [Google Scholar]
- 73.Alloway T. M., The origins of slavery in leptothoracine ants (Hymenoptera: Formicidae). Am. Nat. 115, 247–261 (1980). [Google Scholar]
- 74.Stuart R. J., Alloway T. M., Territoriality and the origin of slave raiding in leptothoracine ants. Science 215, 1262–1263 (1982). [DOI] [PubMed] [Google Scholar]
- 75.Stuart R. J., Alloway T. M., The slave-making ant, Harpagoxenus canadensis M. R. Smith, and its host-species, Leptothorax muscorum (Nylander): Slave raiding and territoriality. Behaviour 85, 58–90 (1983). [Google Scholar]
- 76.Pollock G. B., Rissing S. W., Intraspecific brood raiding, territoriality, and slavery in ants. Am. Nat. 133, 61–70 (1989). [Google Scholar]
- 77.Santschi F., A propos de moeurs parasitiques temporaires des fourmis du genre Bothriomyrmex. Ann. Soc. Entomol. Fr. 75, 363–392 (1906). [Google Scholar]
- 78.Alleman A., Feldmeyer B., Foitzik S., Comparative analyses of co-evolving host-parasite associations reveal unique gene expression patterns underlying slavemaker raiding and host defensive phenotypes. Sci. Rep. 8, 1951 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trager J. C., Global revision of the dulotic ant genus Polyergus (Hymenoptera: Formicidae, Formicinae, Formicini). Zootaxa 3722, 501–548 (2013). [DOI] [PubMed] [Google Scholar]
- 80.Talbot M., The natural history of the workerless ant parasite Formica talbotae. Psyche (Cambridge) 83, 282–288 (1977). [Google Scholar]
- 81.Wilson E. O., The first workerless parasite in the ant genus Formica (Hymenoptera: Formicidae). Psyche (Cambridge) 83, 277–281 (1977). [Google Scholar]
- 82.Wing M. W., A new Formica from northern Maine, with a discussion of its supposed type of social parasitism (Hymenoptera: Formicidae). Can. Entomol. 81, 13–17 (1949). [Google Scholar]
- 83.Ellison A. M., Gotelli N. J., Farnsworth E. J., Alpert G. D., A Field Guide to the Ants of New England (Yale University Press, 2012). [Google Scholar]
- 84.Bruch C., Estudios mirmecológicos. Anales Museo Nacional Historia Natural Buenos Aires 34, 341–360 (1928). [Google Scholar]
- 85.Rabeling C., Bacci M., A new workerless inquiline in the Lower Attini (Hymenoptera: Formicidae), with a discussion of social parasitism in fungus-growing ants. Syst. Entomol. 35, 379–392 (2010). [Google Scholar]
- 86.Schär S., Nash D. R., Evidence that microgynes of Myrmica rubra ants are social parasites that attack old host colonies. J. Evol. Biol. 27, 2396–2407 (2014). [DOI] [PubMed] [Google Scholar]
- 87.Rabeling C., et al., Acromyrmex fowleri: A new inquiline social parasite species of leaf-cutting ants from tropical South America. Insectes Soc. 66, 435–451 (2019). [Google Scholar]
- 88.Sayyari E., Whitfield J. B., Mirarab S., Fragmentary gene sequences negatively impact gene tree and species tree reconstruction. Mol. Biol. Evol. 34, 3279–3291 (2017). [DOI] [PubMed] [Google Scholar]
- 89.Edwards S. V., Liu L., Pearl D. K., High-resolution species trees without concatenation. Proc. Natl. Acad. Sci. U.S.A. 104, 5936–5941 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Edwards S. V., Is a new and general theory of molecular systematics emerging? Evolution 63, 1–19 (2009). [DOI] [PubMed] [Google Scholar]
- 91.Mirarab S., Bayzid M. S., Warnow T., Evaluating summary methods for multilocus species tree estimation in the presence of incomplete lineage sorting. Syst. Biol. 65, 366–380 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Zhang C., Rabiee M., Sayyari E., Mirarab S., ASTRAL-III: Polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19 (suppl. 6), 153 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pease J. B., Brown J. W., Walker J. F., Hinchliff C. E., Smith S. A., Quartet sampling distinguishes lack of support from conflicting support in the green plant tree of life. Am. J. Bot. 105, 385–403 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Dupin J., et al., Bayesian estimation of the global biogeographical history of the Solanaceae. J. Biogeogr. 44, 887–899 (2017). [Google Scholar]
- 95.McKenna M. C., “Cenozoic paleogeography of North Atlantic land bridges” in Structure and Development of the Greenland-Scotland Ridge, Bott M. H. P., Saxov S., Talwani M., Thiede J., Eds. (Springer US, 1983), pp. 351–399. [Google Scholar]
- 96.Schär S., et al., Do Holarctic ant species exist? Trans-Beringian dispersal and homoplasy in the Formicidae. J. Biogeogr. 45, 1917–1928 (2018). [Google Scholar]
- 97.Aleksandrova G. N., Zaporozhets N. I., Palynological characteristics of Upper Cretaceous and Paleogene deposits on the west of the Sambian Peninsula (Kaliningrad region), Part 1. Stratigr. Geol. Correl. 16, 295–316 (2008). [Google Scholar]
- 98.Aleksandrova G. N., Zaporozhets N. I., Palynological characteristics of Upper Cretaceous and Paleogene deposits on the west of the Sambian Peninsula (Kaliningrad region), Part 2. Stratigr. Geol. Correl. 16, 528–539 (2008). [Google Scholar]
- 99.Alekseev V. I., Alekseev P. I., New approaches for reconstruction of the ecosystem of an Eocene amber forest. Biol. Bull. 43, 75–86 (2016). [Google Scholar]
- 100.Wheeler W. M., The ants of the Baltic amber. Schriften der Physikalisch-Ökonomischen Gesellschaft zu Königsberg 55, 1–142 (1915). [Google Scholar]
- 101.Baroni Urbani C., Graeser S., REM-Analysen an einer pyritisierten Ameise aus Baltischem Bernstein. Stuttg. Beitr. Naturkd., B 133, 1–16 (1987). [Google Scholar]
- 102.Dlussky G. M., Ants of the tribe Formicini (Hymenoptera, Formicidae) from late Eocene amber of Europe. Paleontol. J. 42, 500–513 (2008). [Google Scholar]
- 103.Dlussky G. M., Ants of the Genus Formica (Hymenoptera, Formicidae, g. Formica) (Nauka Publishing House, Moskva, Russia, 1967) [In Russian.]. [Google Scholar]
- 104.Goropashnaya A. V., Fedorov V. B., Seifert B., Pamilo P., Phylogenetic relationships of Palaearctic Formica species (Hymenoptera, Formicidae) based on mitochondrial cytochrome B sequences. PLoS One 7, e41697 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilson E. O., Brown W. L. Jr., Revisionary notes on the sanguinea and neogagates groups of the ant genus Formica. Psyche (Cambridge) 62, 108–129 (1955). [Google Scholar]
- 106.Buren W. F., Some fundamental taxonomic problems in Formica (Hymenoptera: Formicidae). J. Georgia Entomol. Soc. 3, 25–40 (1968). [Google Scholar]
- 107.Gregg R. E., The Ants of Colorado, with Reference to Their Ecology, Taxonomy, and Geographic Distribution (University of Colorado Press, Boulder, CO, 1963). [Google Scholar]
- 108.Snelling R. R., Buren W. F., Description of a new species of slave-making ant in the Formica sanguinea group (Hymenoptera: Formicidae). Great Lakes Entomol. 18, 69–78 (1985). [Google Scholar]
- 109.Trager J. C., “Diversity, ecology and conservation of wood ants in North America” in Wood Ant Ecology and Conservation, Stockan J. A., Robinson E. J. H., Eds. (Cambridge University Press, 2016), pp. 221–237. [Google Scholar]
- 110.Branstetter M. G., Longino J. T., Ward P. S., Faircloth B. C., Enriching the ant tree of life: Enhanced UCE bait set for genome-scale phylogenetics of ants and other Hymenoptera. Methods Ecol. Evol. 9, 768–776 (2017). [Google Scholar]
- 111.Faircloth B. C., PHYLUCE is a software package for the analysis of conserved genomic loci. Bioinformatics 32, 786–788 (2016). [DOI] [PubMed] [Google Scholar]
- 112.Kalyaanamoorthy S., Minh B. Q., Wong T. K. F., von Haeseler A., Jermiin L. S., ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nguyen N.-P. D., Mirarab S., Kumar K., Warnow T., Ultra-large alignments using phylogeny-aware profiles. Genome Biol. 16, 124 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chernomor O., von Haeseler A., Minh B. Q., Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 65, 997–1008 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang Z., PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007). [DOI] [PubMed] [Google Scholar]
- 116.Matzke N. J., Probabilistic Historical Biogeography: New Models for Founder-Event Speciation, Imperfect Detection, and Fossils Allow Improved Accuracy and Model-Testing (University of California, Berkeley, CA, 2013). [Google Scholar]
- 117.Huelsenbeck J. P., Nielsen R., Bollback J. P., Stochastic mapping of morphological characters. Syst. Biol. 52, 131–158 (2003). [DOI] [PubMed] [Google Scholar]
- 118.Revell L. J., phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2011). [Google Scholar]
- 119.Harmon L. J., Weir J. T., Brock C. D., Glor R. E., Challenger W., GEIGER: Investigating evolutionary radiations. Bioinformatics 24, 129–131 (2008). [DOI] [PubMed] [Google Scholar]
- 120.Borowiec M. L., Cover S. P., Rabeling C., Data from publication “The evolution of social parasitism in Formica ants revealed by a global phylogeny.” Zenodo. https://zenodo.org/record/4341310. Deposited 15 February 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For detailed methods, see SI Appendix. Reads generates for this study are available at the National Center for Biotechnology Information (NCBI) Sequence Read Archive (BioProject ID PRJNA749764). Other files used in analyses are available on Zenodo (DOI: 10.5281/zenodo.4341310) (120).