Significance
During placentation, some trophoblast cells, termed spiral artery trophoblast giant cells (SpA-TGCs), invade the maternal decidua and replace arterial endothelial cells to increase blood supply to the placenta; how this directional trophoblast migration occurs remains unknown. We show here that planar cell polarity (PCP) signaling mediated by VANGL2 in SpA-TGCs plays a key role in trophoblast movement and successful placentation. Mice with SpA-TGC progenitors deficient in Vangl2 had pregnancy termination in mid-gestation due to increased random cell protrusion and compromised cell migration. We also found endocannabinoid signaling intersects with PCP signaling in directing trophoblast migration by direct association of CB1 and VANGL2. These results demonstrated that trophoblast invasion in placentation requires organized PCP.
Keywords: placentation, planar cell polarity, pregnancy, cannabinoid, Vangl2
Abstract
Directed trophoblast migration toward the maternal mesometrial pole is critical for placentation and pregnancy success. Trophoblasts replace maternal arterial endothelial cells to increase blood supply to the placenta. Inferior trophoblast invasion results in pregnancy complications including preeclampsia, intrauterine growth restriction, miscarriage, and preterm delivery. The maternal chemotactic factors that direct trophoblast migration and the mechanism by which trophoblasts respond to these factors are not clearly understood. Here, we show that invasive trophoblasts deficient in Vangl2, a core planar cell polarity (PCP) component, fail to invade in maternal decidua, and this deficiency results in middle-gestational fetal demise. Previously, we have shown that tightly regulated endocannabinoids via G protein–coupled cannabinoid receptor CB1 are critical to the invasion of trophoblasts called spiral artery trophoblast giant cells (SpA-TGCs). We find that CB1 directly interacts with VANGL2. Trophoblast stem cells devoid of Cnr1 and/or Vangl2 show compromised cell migration. To study roles of VANGL2 and CB1 in trophoblast invasion in vivo, we conditionally deleted Cnr1 (coding CB1) and Vangl2 in progenitors of SpA-TGCs using trophoblast-specific protein alpha (Tpbpa)-Cre. We observed that signaling mediated by VANGL2 and CB1 restrains trophoblasts from random migration by keeping small GTPases quiescent. Our results show that organized PCP in trophoblasts is indispensable for their directed movement and that CB1 exerts its function by direct interaction with membrane proteins other than its canonical G protein–coupled receptor role.
The placenta is a complex and highly vascularized organ that acts as an exchange platform for gases and nutrients between the maternal and fetal circulation (1, 2). Defects in placental development, therefore, have major consequences for the fetus and mother. Shallow trophoblast invasion results in pregnancy complications, including preeclampsia, intrauterine growth restriction, miscarriage, and preterm delivery. The outer epithelial layer of the blastocyst, termed trophectoderm, is the building block of the placenta in mice. Placental function is executed by differentiated trophoblast cells, originating from trophoblast stem (TS) cells. Factors regulating trophoblast invasion and the underlying mechanism are not clearly understood. We previously provided evidence for the role of cannabinoid signaling in placentation. Cannabinoids exert their effects via G protein–coupled cannabinoid receptors, CB1 and CB2.
During the initial stages of placentation, fetal trophoblast cells are separated from the maternal decidual zone by a single layer of parietal trophoblast giant cells (P-TGCs). Notably, most trophoblast cells reside within the placenta throughout pregnancy. Two phenotypes of invasive trophoblasts breach the boundary and invade the maternal decidual zone. Cells crawling between decidual stromal cells are termed interstitial invasive glycogen trophoblast cells (GlyTs), whereas cells moving along uterine spiral arteries are called spiral artery TGCs (SpA-TGCs). During migration, SpA-TGCs replace maternal endothelial cells; their invasion into the maternal decidual zone is indispensable for normal placentation, as ablation of SpA-TGCs in mice results in embryonic death. The movement of interstitial GlyTs differs from that of SpA-TGCs. In interstitial invasion, GlyTs dissociate and display elements of an epithelial to mesenchymal transformation as they penetrate the decidual stroma. By contrast, invasive SpA-TGCs maintain connectivity and exhibit an epithelial to endothelial-like transformation to replace endothelial cells. The mechanism that regulates this directional cell movement of SpA-TGCs remains elusive.
Cannabinoids can alter cell motility in a receptor-dependent or -independent manner (3–7). For example, the endocannabinoid 2-arachidonoylglycerol (2-AG) induces the migration of natural killer cells via CB2 (8). Activation of CB1 was reported to suppress prostate cell migration and induce growth cone collapse in developing GABAergic neurons (6, 9). In neurons, the stabilization of F-actin is diminished by CB1 agonists (10). Our current study focuses on Cnr1, since the expression of Cnr2 is not detectable in trophoblast cells (11). Considering transmembrane CB1 functions mainly through its coupling partners Gi or Gq (12) and their effectors cAMP and Ca2+, it is difficult to directly link CB1 and cell motility. Several reports have indicated CB1 influences activation of small GTPases and WAVE1 complex, which is known to be involved in actin nucleation (6, 7, 10). However, the mechanism by which CB1 regulates cell movement is still not clear. In addition, most of these studies, if not all, depended solely on pharmacological means to manipulate cannabinoid signaling. Given the inequity of agonists/antagonists’ specificity and efficacy, further studies using genetically modified cells or mouse models are necessary to preclude ambiguity.
Using mice deficient in CB1 and CB2, we have shown that endocannabinoid signaling plays various roles in implantation chamber formation and trophoblast migration (11, 13). In the absence of CB1 and CB2, implantation chambers show abnormal epithelial projections. Interestingly, our recent study also reveals that mice with uterine deletion of Vangl2, a core planar cell polarity (PCP) component, show similar phenotypes during implantation. The PCP pathway is best known for its role in cell polarization at the plane of a sheet of cells, and recent data suggest that it plays a key role in directed cell migration during development (reviewed in ref. 14). VANGL2’s localization in the cell membrane promotes our hypothesis that cannabinoid receptors coupled with VANGL2 regulate cell movement.
We found that VANGL2 physically associates with CB1, and deletion of either module compromises trophoblast cell migration in vitro. The in vitro data encourage us to study CB1 and VANGL2 interactions in vivo. VANGL2 is expressed in trophoblast cells located in the spongy layer where CB1 is also localized (13). Using a mouse line with Cre driven by Tpbpa (15), we deleted Vanlg2 and/or Cnr1 in trophoblast cells in this layer, where progenitor cells of invasive SpA-TGCs and GlyTs are located. We found that placentas deficient in spongy Vangl2 (Vangl2f/fTpbpacre/+; Vangl2d/d) are significantly compromised, resulting in termination of pregnancy of Vangl2d/d fetuses. Overall, we show that VANGL2 coupled with CB1 directs SpA-TGC and GlyT migration, significantly affecting placentation. This study reveals that G protein-coupled receptor (GPCR) CB1 influences cell migration by coupling with core components in the PCP pathway, illuminating a previously unrecognized avenue for CB1’s regulation of cytoskeleton reorganization. More importantly, our data suggest that PCP signaling is critical to trophoblast invasion in the maternal–fetal interface, potentially explaining the long-standing question as to why trophoblasts invade toward the mesometrial pole. Shallow endovascular invasion is associated with preeclampsia, intrauterine growth restriction, and preterm birth, which impact a significant global population each year (16).
Results
CB1 Is Physically Associated with Vangl2.
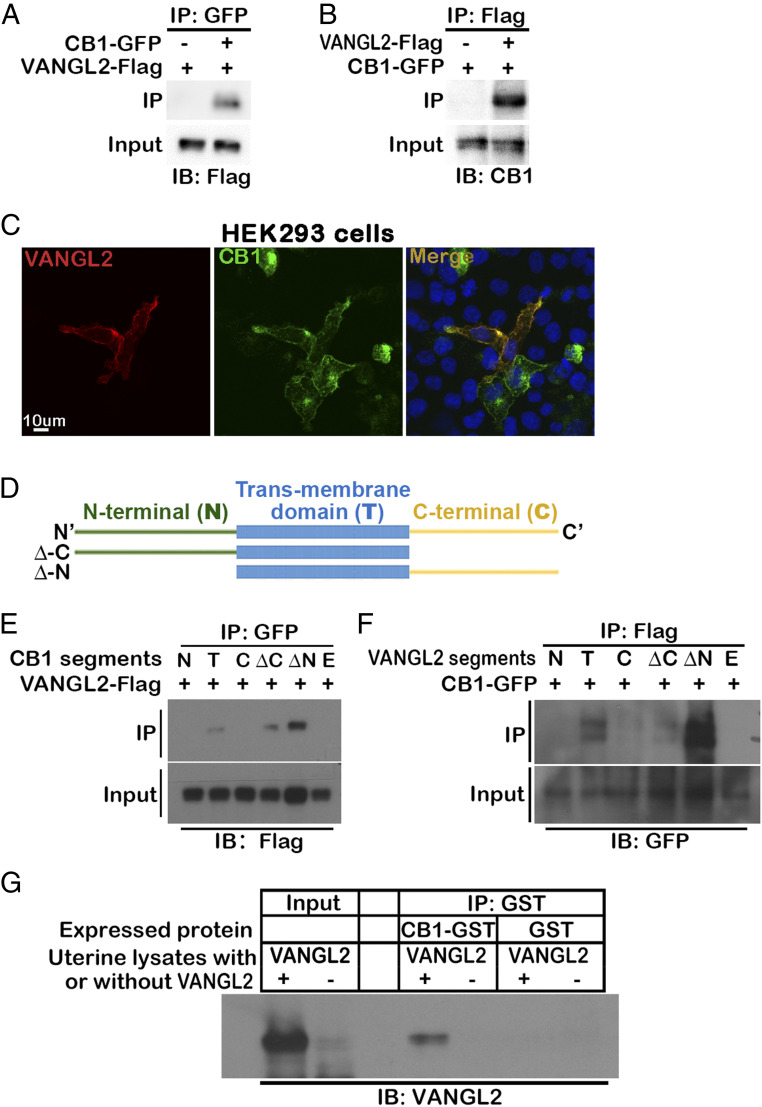
Given the similar phenotype of Vangl2f/fPgrcre/+ and Cnr1−/− uteri during mouse implantation (11, 17), we hypothesized that cannabinoid signaling via CB1 regulates cell migration by coupling with the VANGL2-mediated PCP signaling pathway and that this coupling is aided by their proximal membrane localization. To examine the physical interaction of CB1 and VANGL2, we generated constructs of CB1-GFP and VANGL2-Flag to cotransfect the HEK239T cells for coimmunoprecipitation (Co-IP) experiments. At 48 h after transfection, we were able to pull down VANGL2 by the tagged CB1 and vice versa (Fig. 1 A and B). These data are further supported by VANGL2 immunostaining in HEK239T cells transfected with CB1-GFP and VANGL2-Flag. VANGL2 signals colocalize with GFP signals linked to CB1 (Fig. 1C). To understand signal transduction between CB1 and VANGL2, we defined the domains in each protein that interact with each other.
Fig. 1.
VANGL2 physically associated with CB1 promotes TS migration. (A and B) CB1 is physically associated with VANGL2. Co-IP experiments are performed in HEK293T cells cotransfected with CB1-GFP and VANGL2-Flag vectors. VANGL2-Flag or CB1-GFP proteins are detected in the lysates immunoprecipitated by either GFP or Flag antibody, respectively. (C) Colocalization of CB1 and VANGL2 in HEK293T cells. HEK293T cells are cotransfected with CB1-GFP and VANGL2-Flag vectors, and signals are revealed by GFP and immunostaining of the Flag tag. (Scale bar, 10 μm.) (D) Illustration of different segments cloned for CB1 and VANGL2. (E) VANGL2 pulls down CB1 segments with transmembrane domains. Different segments of CB1 with GFP tags were cotransfected with VANGL2-Flag into HEK293T cells. (F) CB1 pulls down VANGL2 segments with transmembrane domains. Different segments of VANGL2 with tags were cotransfected with CB1-GFP into HEK293T cells. (G) The interaction of CB1 and VANGL2 is confirmed using CB1 purified from bacteria culture and VANGL2 in uterine lysates. CB1-GST purified from BL21 bacteria culture lysates is incubated with Vangl2f/fPgr+/+ (VANGL2+) uterine protein lysates from day 4 of pregnancy, and a GST antibody immunoprecipitates VANGL2. BL21 bacteria transformed with GST-only plasmids are used for negative control of CB1. Vangl2f/fPgrcre/+ (VANGL2-) uterine protein lysates serve as negative controls of VANGL2. IB, immunoblotting; N, N-terminal; T, transmembrane; C, C-terminal; ΔC, full length without C-terminal; ΔN, full length without N-terminal; F, full length; E, empty vector.
CB1 has a typical seven-transmembrane domain structure seen in GPCRs. We generated constructs expressing extracellular N-terminal (N), intracellular C-terminal (C), transmembrane (T), full-length without C-terminal (ΔC), and full-length without N-terminal (ΔN) domains of CB1 tagged with GFP (represented in Fig. 1D). All domains are successfully expressed in HEK293T cells (SI Appendix, Fig. 1A). Using these truncated fragments of CB1, we repeated the Co-IP experiments with VANGL2-Flag. The result showed that only segments containing the transmembrane domain of CB1 physically interact with VANGL2 (Fig. 1E). VANGL2 has a four-transmembrane domain, in which both N- and C-terminals are intracellularly embedded. Similarly, we generated segments of N-terminal (N), C-terminal (C), transmembrane (T), full-length without C-terminal (ΔC), and full-length without N-terminal (ΔN) domains of VANGL2 tagged with Flag (SI Appendix, Fig. 1B); only segments containing the transmembrane domain of VANGL2 physically interacted with CB1 (Fig. 1F).
We also examined the interaction using naturally expressed VANGL2 in uterine protein lysate on day 4 of pregnancy and purified mouse CB1 expressed in bacteria with a GST tag. We repeated the Co-IP experiments using purified CB1, and day-4 protein lysate from Vangl2f/fPgr+/+ and Vangl2f/fPgrcre/+ uteri served as the negative control (17). The results confirm the interaction of CB1 and VANGL2 (Fig. 1G). The results show that CB1 physically associates with VANGL2 via transmembrane domains.
Trophoblast stem cells deficient in Cnr1 or Vangl2 show compromised motility.
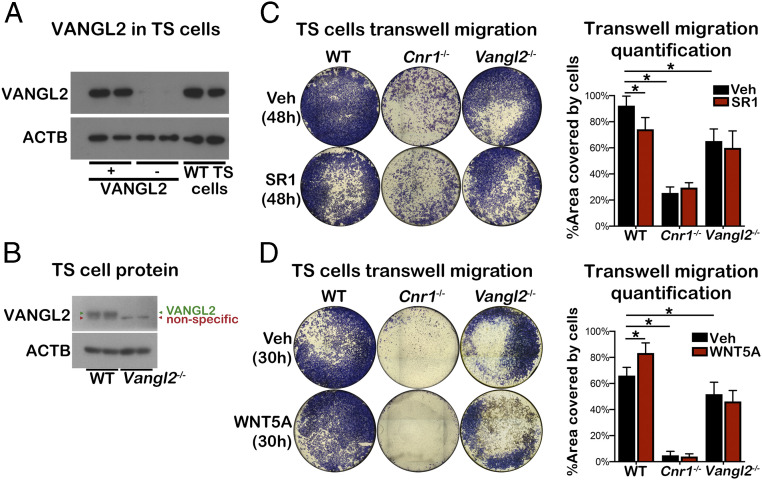
We previously showed that Cnr1−/− trophoblasts show impaired motility in vivo and in vitro (13). To test if trophoblasts can serve as a model to study the functional interaction of CB1 and VANGL2, we examined the expression of VANGL2 in TS cells. Protein lysates from Vangl2f/fPgr+/+ and Vangl2f/fPgrcre/+ uteri served as positive and negative controls, respectively. The result showed that VANGL2 is expressed in wild type (WT) TS cells (Fig. 2A). Given the presence of CB1 in WT TS cells (13), TS cells could serve as a good model to study the interplay between CB1 and VANGL2. Using the Cnr1−/− TS cell line (18), we showed that Cnr1−/− TS cells have compromised motility as compared to WT TS cells via a wound healing assay (SI Appendix, Fig. 2 A and B). We also generated Vangl2−/− TS cells using the CRISPR-Cas9 system (Fig. 2B). Detailed information on Vangl2−/− TS establishment and deletion confirmation is included in the Materials and Methods section. Vangl2−/− TS cells showed marginal decreases in cell motility as compared to WT TS cells in wound healing assays (SI Appendix, Fig. 2 C and D). Evidence suggests that WNT5A directs PCP pathways to activate cell migration and polarity during embryogenesis as a noncanonical signal (19, 20). WNT5A is expressed in first-trimester primary human villous and decidual stromal cells (21). Wnt5a is also expressed by decidual cells at the mesometrial side of implantation sites (SI Appendix, Fig. 3). We speculated that WNT5a would direct the migration of trophoblasts through the PCP pathway; thus, we added WNT5A in cell migration assays using WT and Vangl2−/− TS cells. Indeed, WNT5A significantly increases the motility of WT TS cells but not of Vangl2−/− TS cells (SI Appendix, Fig. 2 C and D).
Fig. 2.
Cnr1−/− and Vangl2−/− TS cells have compromised motility. (A) VANGL2 is expressed in TS cells. Vangl2f/fPgr+/+ (VANGL2+) and Vangl2f/fPgrcre/+ (VANGL2-) uterine protein lysates on day 4 of pregnancy serve as positive and negative controls respectively in Western blotting of VANGL2. (B) VANGL2 is successfully deleted in Vangl2−/− TS cells. Results of Western blotting of VANGL2 using WT and Vangl2−/− TS protein lysates were presented. VANGL2 and a nonspecific band are labeled. (C) Transwell cell migration assay in WT, Cnr1−/−, and Vangl2−/− TS cells with or without SR1 treatment. SR1 (0.5 μM) or vehicle were added to the upper and lower chambers. Cells were fixed 48 h after being seeded in the upper chambers. Each group had five replicate wells, and tiling images of representative wells in their entirety are presented. Cells migrating to the bottom surface of upper chambers were quantified on Right. *P < 0.05 (unpaired Student’s t test). (D) Transwell cell migration assay in WT, Cnr1−/−, and Vangl2−/− TS cells with or without WNT5A treatment. WNT5A (1.2 µg/mL) or vehicle were added to lower chambers. Cells were fixed 30 h after being seeded in the upper chambers. Each group had five replicate wells, and tiling images of representative wells in their entirety are presented. Cells migrating to the bottom surface of upper chambers were quantified on Right. *P < 0.05 (unpaired Student’s t test).
To further examine the directed cell migration, transwell cell migration assays were performed using enriched fetal bovine serum (FBS) media as a chemoattractant. In contrast to the results from the wound healing assays, both Cnr1−/− and Vangl2−/− TS cells showed significantly compromised cell migration toward the chemoattractant (Fig. 2C). More interestingly, a CB1-selective antagonist, SR141716 (SR1), significantly inhibited WT TS cell migration but not that of Cnr1−/− and Vangl2−/− TS cells. To test whether WNT5A could attract TS cell migration, WNT5A was added to the lower culture chamber together with enriched FBS. WNT5A significantly increases WT TS cell migration but has little effect on Cnr1−/− and Vangl2−/− TS cells (Fig. 2D). These results suggest that both CB1 and VANGL2 play a role in TS cell migration. Furthermore, the results suggest not only a physical association but also a functional association between CB1 and VANGL2 and that WNT5A functions as a chemotactic signal to direct TS cell migration through PCP signaling.
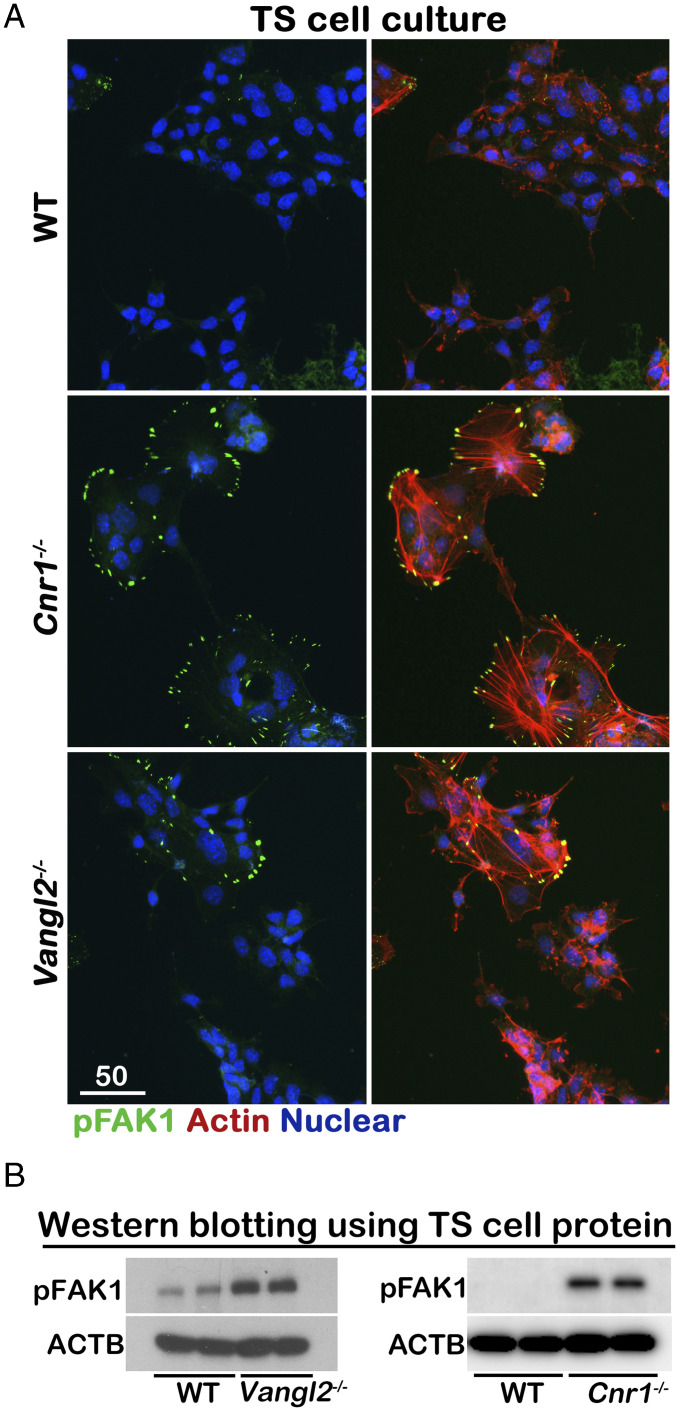
In directionally persistent cell migration, new protrusions are characteristically generated toward external chemotactic signals and thereafter from the preexisting leading edge. These actin-rich protrusions at the leading edge are called lamellipodia, whereas filopodia are cytoplasmic projections that extend beyond the leading edge of lamellipodia in migrating cells. This process restricts lateral protrusions. Cells with increased random protrusions will often reduce their directed migration. Focal adhesion kinase (FAK), a principal participant in focal adhesion dynamics, plays a key role in cell motility (22, 23). To compare cell directed migration, we tested the phosphorylation of FAK in the Cnr1−/− and Vangl2−/− TS cells. Phosphorylation of FAK1 is activated in Cnr1−/− and Vangl2−/− TS cells and concentrated at the tips of random protrusions around the cells (Fig. 3A). Elevated levels of FAK1 phosphorylation in Cnr1−/− and Vangl2−/− TS cells are confirmed by Western blotting (Fig. 3B). WT TS cells treated with SR1 showed increased FAK1 phosphorylation (SI Appendix, Fig. 4). These results suggest that CB1 and VANGL2 are critical to suppress random protrusion of filopodia in TS cells.
Fig. 3.
FAK1 is activated in Cnr1−/− and Vangl2−/− TS cells. (A) Activated FAK1 is concentrated at the tips of filopodia in Cnr1−/− and Vangl2−/− TS cells. (Scale bar, 50 μm.) The activation of FAK1 is confirmed by Western blotting in B.
Deletion of Vangl2 by Tpbpa-Cre expression compromises placenta development.
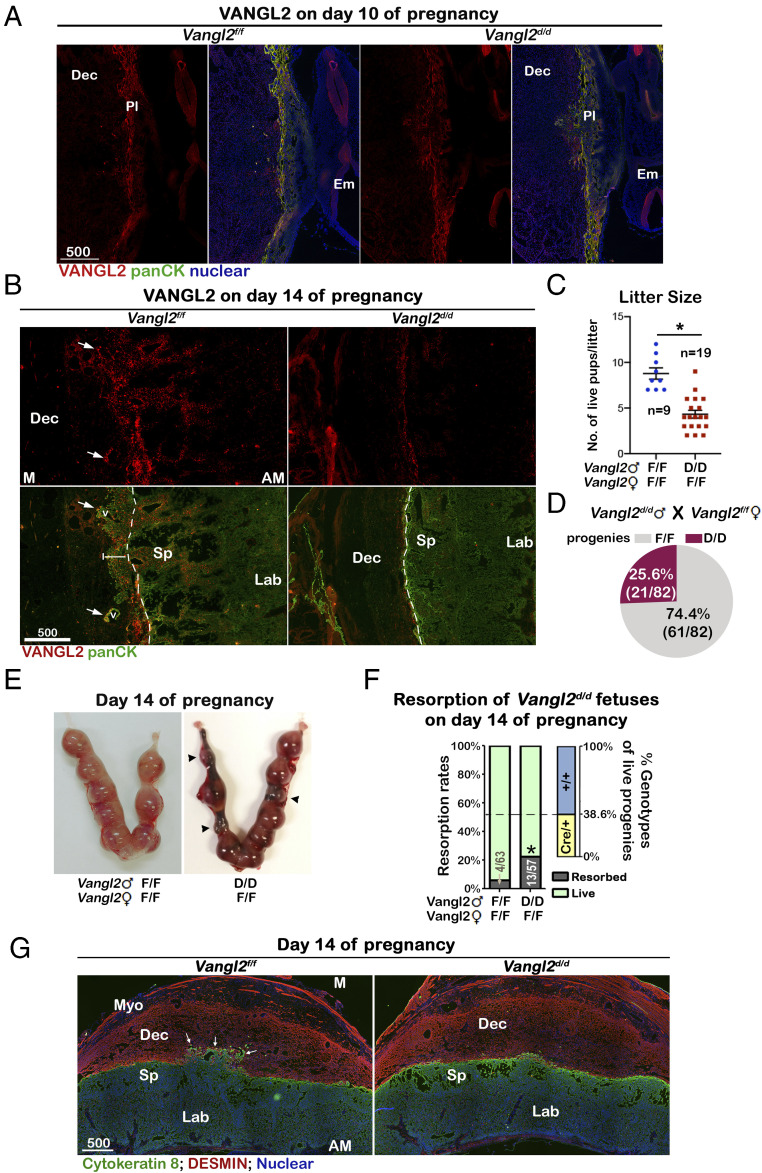
The role of VANGL2 in trophoblast migration in vitro studies encouraged us to investigate its role in vivo. In the mouse placenta, VANGL2 was expressed in most trophoblast cells with higher levels in P-TGCs and lower levels in trophoblasts in ectoplacental cones (EPC) on day 10 of pregnancy (Fig. 4A). On day 14 of pregnancy, VANGL2-positive signals were observed on trophoblasts located in the spongy layer and trophoblast cells invading the maternal tissue (Fig. 4B). All trophoblast cells in the spongy layer are positive for Tpbpa, which are normally initiated in trophoblast progenitors in the ectoplacental cone on days 8 to 9 of pregnancy (24). Tpbpa-positive cells are progenitors of many trophoblast subtypes, including spongiotrophoblasts, SpA-TGCs, and GlyTs. We obtained the mouse line with Cre activity driven by the Tpbpa promoter (24) and examined the CRE protein expression by immunostaining. CRE-positive signals were detected in trophoblasts in the spongy layer of day-12 and day-14 placentas; little or no CRE expression could be detected in day-10 placentas (SI Appendix, Fig. 5). To confirm the activity of CRE, Tpbpa-Cre (Tpbpacre/+) mice were crossed with RosamTmG/mTmG reporter mice (25), where GFP signals are turned on by active CRE activity. Similar to CRE expression patterns, CRE activity was detected in trophoblasts in the spongy layer on days 12 and 14 of pregnancy but little on day 10 (SI Appendix, Fig. 6). Little or no CRE expression/activity was detected in cells other than those in the spongy layer.
Fig. 4.
Vangl2d/d fetuses show significantly higher embryonic lethality. (A) On day 10 of pregnancy, VANGL2 is observed in most trophoblasts with higher levels in P-TGCs. (B) On day 14 of pregnancy, VANGL2 is expressed by trophoblasts localized in spongy layer and invasive trophoblasts in maternal decidual zone. All trophoblasts are demarcated by a Pan-cytokeratin (panCK) antibody. The arrows indicate spiral arteries (v). The dotted lines separate fetal and maternal zones, and a white solid line indicates the extent of WT trophoblast invasion. (C) Litter sizes in Vangl2f/f females mated with Vangl2f/f or Vangl2d/d males. The error bars represent means ± SEM; n numbers are numbers of litters. *P < 0.05. (D) Percentages of progeny genotypes from Vangl2f/f females mated with Vangl2d/d males. Percentages are calculated by dividing pup numbers in each genotype by total 82 pups examined. (E) Multiple resorption sites (arrowheads) are observed in Vangl2f/f females mated with Vangl2d/d males. (F) Resorption rates in Vangl2f/f females mated with Vangl2f/f or Vangl2d/d male mice on day 14. Numbers on bars are No. of resorptions/No. of total implantation sites. Percentages of live progeny genotypes with or w/o Cre in Vangl2f/f females mated with Vangl2d/d males are presented on Right. *P < 0.05 (Chi-square tests). (G) Vangl2f/f but not Vangl2d/d trophoblasts (positive of Cytokeratin 8) invade maternal decidua basalis (positive of Desmin). The arrows indicate invasive trophoblasts into maternal zone. Myo, myometrium; Dec, decidua basalis; Sp, spongy layer; Lab, labyrinth layer; Pl, placenta; Em, embryo; M, mesometrial side; AM; anti-mesometrial side. (Scale bars, 500 μm.)
To delete Vangl2 in trophoblasts in the spongy layer, we crossed Tpbpacre/+ mice with Vangl2f/f mice. Consistent with the temporal pattern of CRE, VANGL2 levels were comparable in Vangl2f/fTpbpacre/+ (Vangl2d/d) and Vangl2f/f placentas on day 10, but its expression is efficiently deleted in day-14 Vangl2d/d spongy layers (Fig. 4 A and B). To investigate VANGL2’s role in fetal trophoblast invasion, we mated Vangl2f/f females with Vangl2d/d or Vangl2f/f males. Litter sizes from Vangl2f/f females mated by Vangl2d/d males are significantly lower compared to those from Vangl2f/f females mated by Vangl2f/f males (Fig. 4C). The mating of Vangl2f/f females with Vangl2d/d males should generate Vangl2f/f and Vangl2d/d progenies with ∼1:1 ratio according to Mendel's law of inheritance. However, we found that the ratio of Vangl2f/f and Vangl2d/d progenies is significantly skewed ∼3:1 (Fig. 4D). The substantially reduced litter sizes of Vangl2f/f dams mated by Vangl2d/d males indicates a considerable loss of Vangl2d/d fetuses. To examine the timing and extent of fetal development, pregnant females on days 10 and 14 of pregnancy were examined. On day 10 of pregnancy, Vangl2f/f females crossed by Vangl2d/d males had comparable numbers of implantation sites with normal-looking morphology (SI Appendix, Fig. 7). However, by day 14 of pregnancy, the rate of resorption was significantly higher in Vangl2f/f dams mated by Vangl2d/d males as compared to dams in the control group (Fig. 4 E and F). We genotyped the remaining live embryos, and the results showed that more Vangl2f/f embryos survived (Fig. 4F). Interestingly, the survival rate of Vangl2f/f embryos in Vangl2f/f dams mated by Vangl2d/d males was comparable to Vangl2f/f embryos in Vangl2f/f dams mated with Vangl2f/f males, suggesting Vangl2d/d embryos undergo demise. To further examine the development of Vangl2d/d placentas, we immunostained all trophoblasts by a cytokeratin antibody on sections of Vangl2f/f and Vangl2d/d placentas on day 14 of pregnancy. Vangl2d/d placentas were collected from the remaining surviving fetuses, and all control Vangl2f/f placentas were collected from the same dams of Vangl2d/d fetuses to minimize any confounding maternal effects. The results revealed that Vangl2f/f trophoblasts invaded the maternal decidual basalis and replaced endothelial cells around spiral arteries, but Vangl2d/d trophoblast cells stopped at the border between maternal and fetal tissues (Fig. 4G). To confirm that invading trophoblasts surround maternal spiral arteries, blood vessels were demarcated by platelet endothelial cell adhesion molecule (PECAM) immunostaining on sections adjacent to the ones used in Figs. 4G and 5 (SI Appendix, Fig. 8). The results showed that deletion of Vangl2 in the spongy layer causes pregnancy termination of Vangl2d/d fetuses between days 10 and 14, and placental development is compromised in the surviving Vangl2d/d fetuses.
Fig. 5.
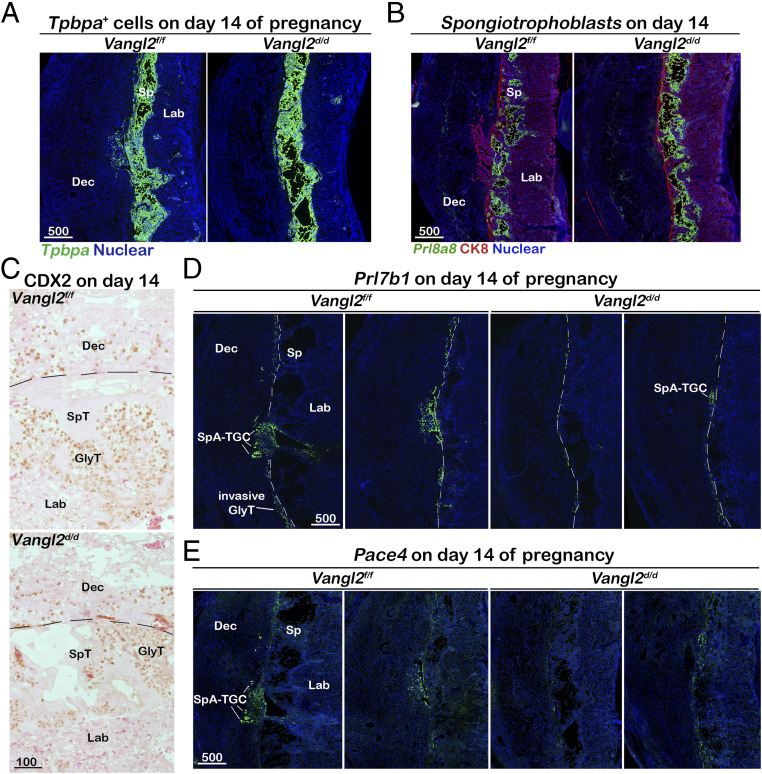
SpA-TGC invasion is compromised in Vangl2d/d fetuses. (A) Domains of Tpbpa+ trophoblasts are comparable in Vangl2f/f and Vangl2d/d placentas on day 14 as examined by in situ hybridization. (B) Spongiotrophoblasts in the spongy layer are outlined by in situ hybridization of Prl8a8. Overall trophoblasts are highlighted by CK8 immunostaining. (C) The patterns of GlyT cells are comparable in Vangl2f/f and Vangl2d/d placentas as examined by CDX2 immunostaining. (D and E) Significantly reduced Vangl2d/d SpA-TGC cells are spotted in decidua basalis. SpA-TGCs and invasive GlyTs are marked by Prl7b1 and Pace4 in situ hybridization. The dotted lines separate fetal and maternal zones. Dec, decidua basalis; Sp, spongy layer; Lab, labyrinth layer; GlyT, glycogen trophoblasts; SpT, spongiotrophoblasts. (Unit of scale bars, μm.)
SpA-TGC invasion is impaired in the absence of VANGL2.
Compromised trophoblast invasion results in insufficient maternal blood vessel remodeling and defective pregnancy outcomes. To further investigate the underlying causes of the compromised Vangl2-deleted trophoblast invasion, we examined the subtypes of trophoblasts in the spongy layer. Considering Vangl2’s deletion in all Tpbpa-positive cells, we first studied whether the overall Tpbpa-positive cell population is changed by examining the expression of Tpbpa. In situ hybridization results show that Tpbpa-positive cells show no drastic changes in Vangl2f/f and Vangl2d/d placentas, although the area of positive signals shows modest individual variance (Fig. 5A). A major subtype of trophoblasts in the spongy layer is spongiotrophoblasts, in which Prl8a8 is primarily expressed (26). Indeed, spongiotrophoblasts are Prl8a8 positive, whereas GlyTs in between spongiotrophoblasts are negative for this signal (Fig. 5B). In Vangl2f/f placentas, cells positive for Prl8a8 are located within the spongy layer, but trophoblasts invading the maternal basalis are negative for Prl8a8 (Fig. 5B). The signal intensity of Prl8a8 is higher in Vangl2d/d placentas, suggesting more cells differentiate to spongiotrophoblasts. GlyT cells maintain CDX2 expression (27). Most GlyTs are located in the spongy layer, and some of them invade maternal decidua. A distinct nuclear CDX2 staining is observed in GlyTs (Fig. 5C). No significant changes in GlyT numbers in either the spongy layer or the maternal decidual zone in Vangl2f/f or Vangl2d/d placentas suggest that Vangl2f/f and Vangl2d/d GlyTs are comparably invasive. Prl7b1 expression is initially induced in a subset of cells within the EPC on day 9 of pregnancy, and invasive SpA-TGC and GlyTs still express it by day 15 of pregnancy (26). We examined the RNA signals of Prl7b1 in Vangl2f/f and Vangl2d/d placentas on day 14 of pregnancy. The results showed that sporadic GlyTs around the border of the maternal–fetal interface are positive for Prl7b1 (Fig. 5D). Numbers of GlyTs show no significant differences between Vangl2f/f and Vangl2d/d placentas. However, the Prl7b1-positive SpA-TGCs invading the maternal zone are only observed in Vangl2f/f placentas (Fig. 5D), whereas Prl7b1-positive SpA-TGCs were largely halted at the maternal–fetal border. These results suggest VANGL2 is critical for SpA-TGC invasion, further confirmed by the expression pattern of another invasive trophoblast marker, Pace4 (Fig. 5E) (28). In summary, deletion of Vangl2 in Tpbpa-positive cells does not greatly interfere with the differentiation of spongiotrophoblast and GlyT cells. GlyTs are able to invade the maternal zone in the absence of VANGL2, but invasion by SpA-TGCs deficient in Vangl2 is significantly compromised.
Cnr1d/dVangl2d/d SpA-TGCs have compromised invasiveness.
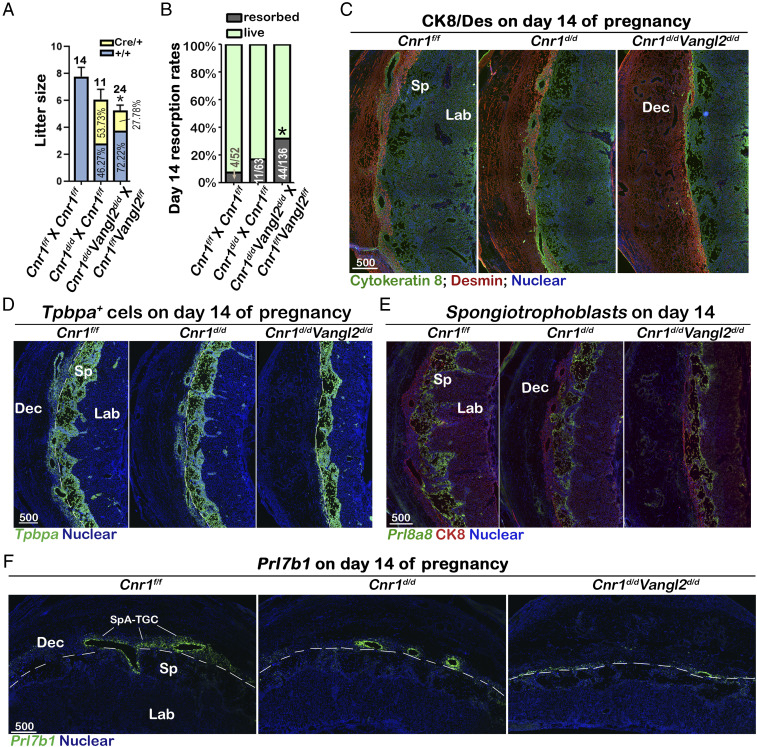
To further study the interaction of CB1 and VANGL2, we introduced floxed Cnr1 mice and created a Cnr1f/fVangl2f/fTpbpaCre/+ (Cnr1d/dVangl2d/d) mouse line with the Cnr1d/d mouse line as the control. Similar to the experimental design in Vangl2d/d mice, we first examined the litter sizes of Cnr1f/f females mated with Cnr1d/d males and Cnr1f/fVangl2f/f females mated with Cnr1d/dVangl2d/d males. Under these mating schemes, Cnr1f/fVangl2f/f females have a significant reduction in litter size and skewed genotypes of progenies, resembling the phenotype of Vangl2f/f females mated with Vangl2d/d males (Fig. 6A and SI Appendix, Table 1). The litter sizes of Cnr1f/f females show a modest reduction compared to WT females, and the ratio of progenies with Cre or without Cre is close to 1. An increased incidence of embryo resorption is observed in Cnr1f/fVangl2f/f females on day 14, whereas the resorption rate in Cnr1f/f females is much milder (Fig. 6B). These results suggest that CB1 complements VANGL2-mediated PCP signaling in Tpbpa-positive cells. Detailed examination of Tpbpa-positive cells and other subtypes of trophoblasts showed similar results as seen in Vangl2d/d placentas. Cnr1d/dVangl2d/d trophoblasts have limited invasion in the maternal decidual zone (Fig. 6C), although the overall Tpbpa-positive and spongiotrophoblast cells in the spongy layers are comparable in Cnr1d/dVangl2d/d and control placentas (Fig. 6 D and E). Prl7b1- and Pace4-positive Cnr1d/dVangl2d/d SpA-TGCs are absent in maternal decidua (Fig. 6F and SI Appendix, Fig. 9). All labeled SpA-TGCs are localized around maternal spiral arteries demarcated by PECAM immunostaining by sections adjacent to the ones used in Fig. 6F and SI Appendix, Fig. 9 (SI Appendix, Fig. 10). Taken together, these results suggest that CB1 and VANGL2 function together to regulate the SpA-TGC invasion and, consequently, pregnancy success.
Fig. 6.
Compromised placentation in Cnr1d/dVangl2d/d fetuses significantly increases embryonic lethality. (A) Cnr1d/dVangl2d/d fetuses show significantly reduced litter sizes in Cnr1f/fVangl2f/f females mated with Cnr1d/dVangl2d/d males. The numbers above bars are numbers of litters examined. Percentages of progeny genotypes are presented on bars. The error bars represent means ± SEM; n numbers are numbers of litters. *P < 0.05 (unpaired Student’s t test). (B) Resorption rates in pregnant females on day 14. Genotypes of breeding pairs are presented as male × female. The numbers on bars are No. of resorptions/No. of total implantation sites. *P < 0.05 (Chi-square tests). (C) Cnr1d/dVangl2d/d trophoblasts (positive of Cytokeratin 8) fail to invade maternal decidua basalis (positive of Desmin). Dec, decidua basalis; Sp, spongy layer; Lab, labyrinth layer. (D) Domains of Tpbpa+ trophoblasts are comparable in Cnr1f/f, Cnr1d/d, and Cnr1d/dVangl2d/d placentas on day 14 as examined by in situ hybridization of Tpbpa. (E) Spongiotrophoblasts in the spongy layer is outlined by in situ hybridizations of Prl8a8. All trophoblasts are highlighted by CK8 immunostaining. (F) Significantly reduced Cnr1d/dVangl2d/d SpA-TGCs are spotted in decidua basalis. SpA-TGCs and invasive GlyTs are marked by Prl7b1 in situ hybridization. The dotted lines outline maternal–fetal borders. Dec, decidua basalis; Sp, spongy layer; Lab, labyrinth layer. Unit of scale bars, μm.
CB1 and Vangl2 bind Rho family of GTPases.
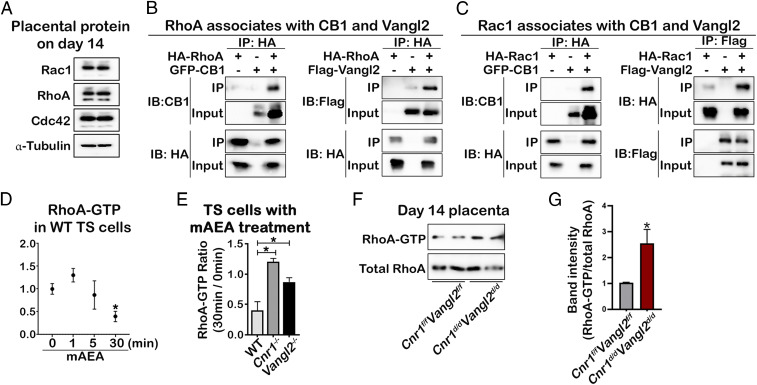
The members of the Rho family of small guanosine triphosphatase (GTPases), RhoA and Rac1, play crucial roles in a range of cellular functions, including the regulation of the actin cytoskeleton, cell polarity and gyration, gene expression, and cell proliferation. Specifically, these Rho GTPases play a role in cell migration and invasion through the regulation of these processes, acting at specific locations and times in cells (29, 30). Recent reports have shown that Rho GTPases mediate VANGL2’s regulation of the cytoskeleton (31, 32). Given the overactivated filopodia phenotype observed in Vangl2−/− and Cnr1−/− TS cells (Fig. 3A), we hypothesize that the VANGL2/CB1 complex regulates TS cell migration by coupling with Rho GTPase. We first confirmed that RhoA, Rac1, and Cdc42 are expressed in placentas of day 14, as well as WT, Vangl2−/−, and Cnr1−/− TS cells (Fig. 7A and SI Appendix, Fig. 11). To study the physical association of Rho GTPases with VANGL2 and CB1, HEK293T cells were transfected with VANGL2 or CB1 with the Rho family of GTPases. The results show that VANGL2 or CB1 interact physically with RhoA (Fig. 7B) and Rac1 (Fig. 7C). GTP-bound RhoA is active, and the activity is turned off after GTP is hydrolyzed to GDP (33). Activation of CB1 by methanandamide suppresses RhoA activity in WT TS cells (Fig. 7D). However, this suppression is impaired in Vangl2−/− and Cnr1−/− TS cells (Fig. 7E). The in vivo results from RhoA activity assays corroborate the in vitro results using TS cells. We examined RhoA activity using Cnr1d/dVangl2d/d and control placental protein lysates on day 14 of pregnancy. Placental samples without the labyrinth zone were collected in Cnr1f/fVangl2f/f and Cnr1d/dVangl2d/d placentas. Results show that RhoA is more active in Cnr1d/dVangl2d/d samples (Fig. 7 F and G). Together with previous data, these results suggest that CB1 and VANGL2 in SpA-TGCs are critical to suppress RhoA activity during placentation, which promotes random formation of filopodia in trophoblasts.
Fig. 7.
Signaling mediated by CB1/VANGL2 complex promotes SpA-TGC invasion by regulating Rho GTPase activity. (A) Rho GTPases are expressed in the placentas on day 14 of pregnancy. Western blotting of Rac1, RhoA, and Cdc42. α-Tubulin served as loading controls. (B) CB1 and VANGL2 are coimmunoprecipitated with RhoA. Co-IP experiments are performed in HEK293T cells transfected with HA-tagged RhoA as well as GFP-CB1 or Flag-VANGL2 vectors. (C) CB1 and VANGL2 are Co-IP with Rac1. Co-IP experiments are performed in HEK293T cells transfected with HA tagged Rac1 as well as GFP-CB1 or Flag-VANGL2 vectors. (D) Suppression of endocannabinoid signaling via CB1 decreases RhoA activity in WT TS cells. RhoA activity in WT TS cells decreases through time after mAEA treatment. (E) The decreases caused by mAEA treatment are abolished in Cnr1−/− and Vangl2−/− TS cells. The ratios of RhoA activity 30 min after treatment versus activity before treatment are presented. *P < 0.05, unpaired Student’s t tests. (F) Levels of RhoA-GTP are higher in Cnr1d/dVangl2d/d placentas on day 14. RhoA-GTP pulled down by rhotekin-RBD–coated beads is examined by immunoblotting with anti-RhoA antibodies. Total RhoA proteins serves as internal control. The ratios of RhoA-GTP versus total RhoA are quantified in (G), based on band intensity quantification. *P < 0.05 (unpaired Student’s t test).
Discussion
A long-standing goal in placentation studies is to identify signals directing fetal trophoblast migration toward maternal decidua, which is necessary for placental development and pregnancy success. The ectoplacental cone generates secondary trophoblast giant cells and highly invasive SpA-TGCs. SpA-TGCs that migrate toward the decidua surround, invade, and remodel the incoming maternal vasculature. GlyTs, specified within the early progenitor cell population in the spongy layer and at later stages, invade the maternal tissue. As noted, the process of placentation is complex, and this organ comprises multiple cell types, making its function vulnerable to internal and external factors. One common defect observed during placentation is insufficient blood supply, which causes maternal gestational hypertension and intrauterine embryo growth restriction. Blood supply to the placenta is considered to be closely related to the remodeling of maternal blood vessels by invasive SpA-TGCs. However, the maternal chemokines directing the migration of SpA-TGCs are elusive. During placentation, trophoblasts migrate toward the mesometrial side with higher levels of oxygen (34), which contradicts the findings that trophoblasts proliferate and invade more under hypoxic conditions (35, 36). Our current data suggests that PCP signaling in trophoblasts is critical to the SpA-TGCs invasion, which opens up a direction in the context of maternal cytokine cues.
PCP signaling provides directional information to control and coordinate polarized cell behavior, which is fundamental to tissue morphogenesis and organ formation (37). VANGL2, a core PCP component, mediates the chemotactic effects of WNT5A, a noncanonical WNT factor (38–40). There is evidence that WNT5A, as a noncanonical signaling effector, stimulates cell migration and polarity during embryogenesis via phosphorylation of VANGL2 (38). We have shown that decidual cells create WNT5A gradients, influencing uterine epithelial polarity via VANGL2 signaling (17). Furthermore, our preliminary data suggests that maternal decidual cells sustain Wnt5a expression at the midgestational stage (SI Appendix, Fig. 3), promoting the idea that the maternal WNT5A gradient directs fetal trophoblast invasion. Favoring this hypothesis, our data suggests that VANGL2-mediated WNT5A signaling accelerates TS cell migration in vitro (Fig. 2D). However, the in vivo role of WNT5A during placentation as a chemoattractant requires further studies.
Using multiple approaches, we demonstrate that PCP signaling, in cooperation with CB1, orchestrates the directed migration of trophoblasts in placentation. In vitro data suggest that Cnr1−/− and Vangl2−/− TS cells extend filopodia in random directions, which significantly reduces cell migration. This linkage is supported by the observed physical association between CB1 and VANGL2 (Fig. 1 A–F). We have previously shown that Cnr1−/− females have defective placentation and fetal development with increased resorption sites. Suppression of CB1-mediated endocannabinoid signaling causes premature differentiation in TS cells to TGCs and compromises TS invasiveness (13). Interestingly, the deletion of Cnr1 by Tpbpa-cre showed a much milder phenotype as compared to Cnr1−/− mice. One explanation is that cannabinoid signaling can influence mouse implantation and decidualization by activating CB1 (11, 41). In the current study, the outcome of PCP signaling supersedes that of the cannabinoid signaling. Our data suggests VANGL2 plays a dominant role in the regulation of SpA-TGC migration in vivo. This is consistent with the concept that endocannabinoid signaling is considered complementary in different biological processes (42). Either suppression or elevation of this signaling causes suboptimal outcomes but fails to completely inhibit specific biological processes, whereas disruption of PCP signaling often disrupts biological processes (43).
Our results suggest that compromised Vangl2d/d SpA-TGC invasion is a cause for resorption of implantation. Vangl2 is deleted by CRE activity driven by Tpbpa promoter. The cells with positive CRE signals and activity are detected on day 12 but not on day 10 of pregnancy, and signals are located within the spongy layer. Although we cannot exclude the adverse effects of Vangl2 deletion in other Tpbpa+ cells other than SpA-TGCs, like GlyTs and spongiotrophoblasts, our results in Fig. 5 suggest that the cell population of these two cell types are comparable to those in controls, while the reduction in SpA-TGCs in Vangl2d/d placenta is significant. Therefore, we reason that compromised SpA-TGC invasion between day 10 and day 14 is a cause for resorption of implantation.
FAK is a nonreceptor protein tyrosine kinase, expressed ubiquitously in mammalian tissues including brain, lymphocytes, and testes. A potential role of FAK in angiogenesis has also been suggested by a number of other studies. During mouse embryo development, FAK expression becomes increasingly restricted to the blood vessels. Increased endothelial cell migration into a wounded monolayer was correlated with increased tyrosine phosphorylation and kinase activity of FAK (44). In addition, activation of VEGF receptor-2 by VEGF-A induced association of FAK with PI3-kinase. CB1 or VANGL2 serves as the regulatory trigger for phosphorylation of FAK1, thereby supporting angiogenesis and migration. We found that CB1- or VANGL2-regulated phosphorylation of FAK1 is responsible for TS cell migration.
Furthermore, FAK works in concert with its partner proteins to create a giant regulatory protein complex, composed of p130Cas (p130 Crk-associated substrate), DOCK180 (Dedicator of cytokinesis 180), RhoA, and vinculin (and its associated partners such as Crk, R-ras, and Grb2), which is in turn associated with β1-integrin. Some studies reported that CB1 activation diminished RhoA activity but modestly increased Rac1 and Cdc42 activity in prostate carcinoma cells (6). Activated, GTP-bound RhoA promotes cell migration by activating Rho-associated kinase, which enhances myosin phosphorylation and the formation of actin/myosin microfilaments. The activated, GTP-bound forms of Rac1 and Cdc42 promote the formation of lamellipodia and filopodia, which define cell polarity and promote directional movement. Increased RhoA activity, which is often accompanied by increased cell migration and vision, can be induced by stimulating multiple GPCRs expressed by prostate carcinoma cells. In striking contrast, there are very few GPCRs, such as the angiotensin type II receptor, that have been demonstrated to inactivate RhoA. In contrast, the absence of CB1 reduced cell migration and promoted the growth of filopodia tips and lamellipodia edges in TS cells. The action of small GTPase may have a different role depending on cell type. We showed that PCP signaling in cooperation with CB1 regulates organized trophoblast migration in placentation, which is critical for pregnancy success; however, PCP signaling supersedes cannabinoid signaling, since Cnr double knockout mice show similar phenotypes as Vangl2f/f females.
This study is relevant to evolution of placentation in humans and other primates because both rodent and primate placentas are discoid and hemochorial in nature, and both CB1 and VANGL2 signaling are preserved across species. This investigation may stimulate further research into the role of stage-specific cannabinoid and PCP signaling in placentation across eutherian species.
Materials and Methods
Mice.
Cnr1f/fTpbpacre/+ (Cnr1d/d), Vangl2f/fTpbpacre/+ (Vangl2d/d), and Cnr1f/fVangl2f/fTpbpacre/+ (Cnr1d/dVangl2d/d) mice were generated by mating Cnr1f/f (45) and Vangl2f/f (46) with Tpbpacre/+ males (15). RosamTmG/mTmG mice were obtained from JAX laboratory (Stock No. 7676). All genetically modified mice and WT controls were maintained on a C57BL6 mixed background and housed in the animal care facility at the Cincinnati Children’s Hospital Medical Center according to NIH and institutional guidelines for laboratory animals. All protocols of the present study were approved by the Cincinnati Children’s Hospital Research Foundation Institutional Animal Care and Use Committee. All mice were housed in wall-mount negative airflow polycarbonate cages with corn cob bedding. They were provided ad libitum with double-distilled autoclaved water and rodent diet (LabDiet 5010).
Female mice were mated with fertile males and checked for vaginal plug to confirm successful mating (vaginal plug = day 1 of pregnancy). To examine the postimplantation embryo development, mice were killed between 0900 and 1000 on a given day as described in the main text, and implantation sites were weighed, flash-frozen, or fixed in 10% neutral buffered formalin and embedded in paraffin for further analyses. Live or resorbed embryos were determined by the size of implantation sites. Resorbed embryos have a much smaller size with dark colors. Live embryos were dissected out for genotyping.
HEK293T Cell Culture and Transfection.
HEK293T cells were grown in high-glucose Dulbecco’s modified Eagle medium supplemented with 10% FBS, Penicillin-Streptomycin at 37 °C, and 5% CO2. HEK293T cells were transiently transfected using Lipofectamine 3000 (Invitrogen Life Technologies) according to the manufacture’s protocol. Co-IP was performed at 48 h posttransfection.
Western Blotting.
Protein extraction and Western blotting was performed as previously described (18). In brief, tissues were lysed in radioimmunoprecipitation assay (RIPA) lysis buffer including phosphatase and protease inhibitors. The protein extracts were boiled for 10 min before they were loaded and separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) gel except for CB1 detection, in which protein lysates were denatured at 65 °C for 10 min (47). The protein extracts were transferred onto the polyvinylidene fluoride (PVDF) membrane (Millipore). The membrane was blocked in 5% skimmed milk in tris-buffered saline containing 0.1% Tween 20 and incubated in a primary antibody at 4 °C for 16 h, followed by incubation in secondary antibodies at room temperature for 1 h. The signals were developed using Clarity Western ECL Blotting Substrates (Bio-Rad) and detected using an Amersham Iamger 680. The rat monoclonal antibody to VANGL2 (2G4) was generated previously (48). Other antibodies used in Western blotting are antibodies to CB1 (No. 93815, Cell signaling, 1:1,000), GFP (A11122, Invitrogen, 1: 1,000), Flag (No. 14793, Cell signaling, 1:1,000), Phosphor-FAK1 (No. 3283, Cell signaling, 1:2,000), HA (No. 3724, Cell signaling, 1:1,000), Rac1 (ARC03-A, Cytoskeleton Inc, 1:1,000), RhoA (ARH04, Cytoskeleton Inc, 1:1,000), Cdc42 (ACD03, Cytoskeleton Inc, 1:1,000). Actin (SC1615, Santa Cruz, 1:2,000), and α-Tubulin (No. 2144, Cell signaling, 1:2,000) served as loading controls.
Co-IP.
Co-IP was performed as previously described (49). Cells were lysed with IP buffer (10 mM Hepes, 142.5 mM KCl, 5 mM MgCl2, 1 mM EGTA, 0.5% Nonidet P-40, pH 7.5) containing phosphatase and protease inhibitors. Cell or tissue protein lysates obtained by centrifugation were incubated with antibody and magnetic beads (Invitrogen) for 16 h at 4 °C. The immunocomplexes were then washed with 10 mM Tris HCl and separated by SDS-PAGE gel as required. Target proteins were detected by standard Western blotting. Antibodies used for immunoprecipitation for GFP, Flag, and HA are the same as the ones used in Western blotting. The antibody to GST tag is from Cell Signaling (No. 2624). Cnr1 was cloned in pGEX-6P1 vector, and CB1 with a GST tag was expressed by Escherichia coli (BL21). CB1-GST was purified using Glutathione Sepharose 4 fast flow beads.
TS Cell Culture.
TS cells were maintained as previously described (18). TS cells were maintained in 70% embryonic mouse fibroblast cell–conditioned medium, 30% TS cell medium, 25 ng/mL FGF4, and 1 μg/mL heparin and incubated in a humidified tissue culture incubator at 37 °C saturated with 5% CO2 in air. WT and Cnr1−/− TS cells were generated from WT and Cnr1−/− blastocysts (18, 50) following the method described previously (51). Vangl2−/− TS cells were generated by deleting Vangl2 genes in WT TS cells using the Crispr/Cas9 system. Alt-R CRISPR-Cas9 system (IDT) was used to perform the deletion. Guidance RNAs (gRNAs) used are 5′-ATAGCCCGAGTACTGGGACT and 5′-CCCGCAGCTCCCGGAAGCAC. The two gRNAs are located right after the initial codon of Vangl2 protein coding sequence region (SI Appendix, Fig. 12A). A single clone was selected by genotyping using primers 5′-GGGTGACGGTTGACTTCCTAA-3′ and 5′-CTTAGAGCGGTGTCGGTCC-3′. The genotyping result is shown in SI Appendix, Fig. 12B. Deletion of Vangl2 was also confirmed by Western blotting of VANGL2 (Fig. 2B) and genome sequencing of Vangl2−/− TS cells. A 53-base pairs region of exon 3 in Vangl2 was deleted, which resulted in a translational frameshift and premature translation termination (SI Appendix, Fig. 12C).
Immunofluorescence.
Staining was performed as previously described (41). In brief, implantation sites from three individual animals in each experimental group were snap-frozen. Frozen sections (12 μm) of three implantation sites from different females in each group were mounted onto poly-L-lysine–coated slides and fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). Sections were blocked in 5% bovine serum albumin in PBS and incubated with primary antibody at 4 °C overnight, followed by incubation in secondary antibodies at room temperature for 1 h. Nuclear staining was performed using Hoechst 33342 (H1399, Molecular Probes, 2 µg/mL). Immunofluorescence was visualized under a confocal microscope (Nikon Eclipse TE2000). The images presented are representative of three independent experiments. Antibodies for VANGL2 (48), Cytokeratin 8 (TROMA-I, Developmental Studies Hybridoma Bank, 1:100), Pan-cytokeratin (Z0622, DAKO, 1:400), Desmin (sc7559, Santa Cruz, 1:200), PECAM (553370, BD, 1:300), CRE (15036, Cell signaling, 1:200), and pFAK1 (#3283, Cell signaling, 1:200) were used for immunofluorescence staining. Actin staining was performed using rhodamine phalloidin (R415, Invitrogen, 1:500). All fluorophore-conjugated secondary antibodies (used in 1:400 dilution) were from Jackson Immunoresearch.
Immunohistochemistry.
Immunostaining was performed in formalin-fixed, paraffin-embedded sections using specific antibodies to CDX2 (MU392A-UC, BioGenex, 1:400) as described previously (27). A Histostain-Plus (DAB) kit (Invitrogen) was used to visualize the antigen. The sections were counterstained with eosin. Tissue sections from Vangl2f/f and Vangl2d/d placentas on day 14 of pregnancy were processed on the same slide.
Fluorescence In Situ Hybridization.
Fluorescence in situ hybridization (FISH) was performed as previously described (52). In brief, implantation sites from three individual mice in each experimental group were collected. Frozen sections (12 μm) from three implantation sites from different females in each group were mounted onto poly-L-lysine–coated slides and fixed in 4% PFA in PBS. Following acetylation and permeabilization, slides were hybridized with the DIG-labeled Tpbpa, Prl8a8, Prl7b1, and Pace4 probes at 55 °C overnight. After hybridization, slides were washed, quenched in 3% H2O2, and blocked in 1% blocking buffer (Roche). Anti–Dig-peroxidase was applied onto hybridized slides and color was developed by Tyramide signal amplification Fluorescein according to the manufacturer’s instructions (PerkinElmer). Some slides were further stained with Cytokeratin 8 antibody. Images presented are representative of three independent experiments.
RhoA Pull-Down Activation Assay.
To determine the activity of RhoA, protein lysates from placenta were examined by a RhoA Activation Assay Biochem Kit (Cytoskeleton Inc.) following manufacturer's instructions. In brief, protein lysates (500 µg) were incubated with 50 µg Rhotekin-RBD beads for RhoA-GTP pull-down at 4 °C for 1 h. Protein–bead complexes were washed, and proteins were eluted by the sample buffer containing SDS. Protein samples were examined by Western blotting using the RhoA antibody in the kit.
Small GTPase Activation (G-LISA) Assay for RhoA.
The levels of RhoA-GTP were measured using RhoA G-LISA Activation Assay colorimetric kit (Cytoskeleton Inc.) following manufacturer's instructions. Briefly, cells were grown to 30% confluence and then serum starved for 6 h. Cells were stimulated by methanandamide (mAEA, 500 µM) for 30 min at 37 °C. Cells were washed twice by ice-cold PBS and lysed according to manufacturer’s protocol for 10 min on ice. Cell lysates were centrifuged at 14,000 × g for 1 min at 4 °C. Protein concentrations in supernatant were determined and adjusted to 0.5 µg/µL. A total of 25 µL equalized cell lysate was used to for each assay. Quantitative detection of RhoA-GTP was performed following manufacturer’s protocol in plates. Absorbance at 490 nm was detected using a microplate spectrophotometer (Synergy H1, BioTek).
Cell Migration Wound Healing Assay.
The migration assay was performed as previously described with some modifications (18). In brief, WT and mutant TS cells were seeded in 6-well plates. When reaching 100% confluence, TS cells were treated with mitomycin C (10 µg/mL) for 2 h. To induce cell migration, each well was scratched using a 200-µL pipette tip, and floating cells were removed by gentle wash. Fresh regular TS cell–maintaining media with or without WNT5A (1.2 μg/mL) was added. Each culture well was photographed at 0, 20, and 24 h after scratches. Images at 20 and 24 h after scratch were used to quantify rates of cell migration.
Transwell Cell Migration Assay.
The migration assay is based on previously published reports with some modifications (13). In brief, migration of TS cells was analyzed using corning 6.5 mm Transwell with 8.0-µm Pore Polyester Membrane Insert (No. 3464). Equal numbers of WT, Cnr1−/−, and Vangl2−/− TS cells were seeded in five individual chambers for each group. Regular TS cell–maintaining media with 1 or 10% FBS were used for upper and lower chambers. For SR1 treatment, SR1 (0.5 μM) or vehicle were added to the upper and lower chambers. Cells were fixed 48 h after being seeded in the upper chambers. In WNT5A-treatment experiments, WNT5A (1.2 µg/mL) or vehicle were added to lower chambers. Cells were fixed 30 h after being seeded in the upper chambers. Cells remaining on the top of the membrane were removed by cotton-tipped applicators. The migrated TS cells were then stained by crystal violet and photographed using an EVOS cell imaging system. Areas of membranes covered by migrated cells were quantified using ImageJ software.
Statistical Analysis.
All values represent the mean ± SEM. Statistical analyses were performed using the unpaired Student’s t tests, and P < 0.05 was considered statistically significant for more than three groups.
Supplementary Material
Acknowledgments
We thank Katie Gerhardt for her excellent editing of the manuscript. This work was supported in part by NIH Grant Nos. HD103475 and HD068524 to S.K.D.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2108201118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Hemberger M., Hanna C. W., Dean W., Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 21, 27–43 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Watson E. D., Cross J. C., Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 20, 180–193 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Vaccani A., Massi P., Colombo A., Rubino T., Parolaro D., Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br. J. Pharmacol. 144, 1032–1036 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oka S., et al., 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces the migration of EoL-1 human eosinophilic leukemia cells and human peripheral blood eosinophils. J. Leukoc. Biol. 76, 1002–1009 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Preet A., Ganju R. K., Groopman J. E., Delta9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene 27, 339–346 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Nithipatikom K., et al., Cannabinoid receptor type 1 (CB1) activation inhibits small GTPase RhoA activity and regulates motility of prostate carcinoma cells. Endocrinology 153, 29–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hohmann T., Feese K., Ghadban C., Dehghani F., Grabiec U., On the influence of cannabinoids on cell morphology and motility of glioblastoma cells. PLoS One 14, e0212037 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishimoto S., et al., Endogenous cannabinoid receptor ligand induces the migration of human natural killer cells. J. Biochem. 137, 217–223 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Berghuis P., et al., Hardwiring the brain: Endocannabinoids shape neuronal connectivity. Science 316, 1212–1216 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Njoo C., Agarwal N., Lutz B., Kuner R., The cannabinoid receptor CB1 interacts with the WAVE1 complex and plays a role in actin dynamics and structural plasticity in neurons. PLoS Biol. 13, e1002286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y., Bian F., Sun X., Dey S. K., Mice missing Cnr1 and Cnr2 show implantation defects. Endocrinology 160, 938–946 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howlett A. C., Cannabinoid receptor signaling. Handb. Exp. Pharmacol. 168, 53–79 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Sun X., et al., Endocannabinoid signaling directs differentiation of trophoblast cell lineages and placentation. Proc. Natl. Acad. Sci. U.S.A. 107, 16887–16892 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davey C. F., Moens C. B., Planar cell polarity in moving cells: Think globally, act locally. Development 144, 187–200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmons D. G., Fortier A. L., Cross J. C., Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev. Biol. 304, 567–578 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y., et al., Comparative analysis of maternal-fetal interface in preeclampsia and preterm labor. Cell Tissue Res. 329, 559–569 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Yuan J., et al., Planar cell polarity signaling in the uterus directs appropriate positioning of the crypt for embryo implantation. Proc. Natl. Acad. Sci. U.S.A. 113, E8079–E8088 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie H., et al., Silencing or amplification of endocannabinoid signaling in blastocysts via CB1 compromises trophoblast cell migration. J. Biol. Chem. 287, 32288–32297 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seifert J. R., Mlodzik M., Frizzled/PCP signalling: A conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 8, 126–138 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Kohn A. D., Moon R. T., Wnt and calcium signaling: Beta-catenin-independent pathways. Cell Calcium 38, 439–446 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Meinhardt G., et al., Wingless ligand 5a is a critical regulator of placental growth and survival. Sci. Rep. 6, 28127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishibe S., Joly D., Liu Z. X., Cantley L. G., Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol. Cell 16, 257–267 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Ilić D., et al., Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539–544 (1995). [DOI] [PubMed] [Google Scholar]
- 24.Hu D., Cross J. C., Ablation of Tpbpa-positive trophoblast precursors leads to defects in maternal spiral artery remodeling in the mouse placenta. Dev. Biol. 358, 231–239 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Muzumdar M. D., Tasic B., Miyamichi K., Li L., Luo L., A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Simmons D. G., Rawn S., Davies A., Hughes M., Cross J. C., Spatial and temporal expression of the 23 murine prolactin/placental lactogen-related genes is not associated with their position in the locus. BMC Genomics 9, 352 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H., et al., Stage-specific integration of maternal and embryonic peroxisome proliferator-activated receptor delta signaling is critical to pregnancy success. J. Biol. Chem. 282, 37770–37782 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Mould A., Morgan M. A., Li L., Bikoff E. K., Robertson E. J., Blimp1/Prdm1 governs terminal differentiation of endovascular trophoblast giant cells and defines multipotent progenitors in the developing placenta. Genes Dev. 26, 2063–2074 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaffe A. B., Hall A., Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Petrie R. J., Doyle A. D., Yamada K. M., Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol. 10, 538–549 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindqvist M., et al., Vang-like protein 2 and Rac1 interact to regulate adherens junctions. J. Cell Sci. 123, 472–483 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayes M. N., et al., Vangl2/RhoA signaling pathway regulates stem cell self-renewal programs and growth in rhabdomyosarcoma. Cell Stem Cell 22, 414–427.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bos J. L., Rehmann H., Wittinghofer A., GEFs and GAPs: Critical elements in the control of small G proteins. Cell 129, 865–877 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Jauniaux E., Watson A., Burton G., Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am. J. Obstet. Gynecol. 184, 998–1003 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Chakraborty D., et al., HIF-KDM3A-MMP12 regulatory circuit ensures trophoblast plasticity and placental adaptations to hypoxia. Proc. Natl. Acad. Sci. U.S.A. 113, E7212–E7221 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakraborty D., Rumi M. A., Konno T., Soares M. J., Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc. Natl. Acad. Sci. U.S.A. 108, 16295–16300 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butler M. T., Wallingford J. B., Planar cell polarity in development and disease. Nat. Rev. Mol. Cell Biol. 18, 375–388 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao B., et al., Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev. Cell 20, 163–176 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang W., et al., Wnt-induced Vangl2 phosphorylation is dose-dependently required for planar cell polarity in mammalian development. Cell Res. 27, 1466–1484 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang K., et al., A mammalian Wnt5a-Ror2-Vangl2 axis controls the cytoskeleton and confers cellular properties required for alveologenesis. eLife 9, e53688 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y., Dewar A., Kim Y. S., Dey S. K., Sun X., Pregnancy success in mice requires appropriate cannabinoid receptor signaling for primary decidua formation. eLife 9, e61762 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X., Dey S. K., Endocannabinoid signaling in female reproduction. ACS Chem. Neurosci. 3, 349–355 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kibar Z., et al., Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat. Genet. 28, 251–255 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Romer L. H., McLean N., Turner C. E., Burridge K., Tyrosine kinase activity, cytoskeletal organization, and motility in human vascular endothelial cells. Mol. Biol. Cell 5, 349–361 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marsicano G., et al., CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Song H., et al., Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature 466, 378–382 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wager-Miller J., Mackie K., Western blotting of the endocannabinoid system. Methods Mol. Biol. 1412, 247–254 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Belotti E., et al., Molecular characterisation of endogenous Vangl2/Vangl1 heteromeric protein complexes. PLoS One 7, e46213 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan J., et al., Tridimensional visualization reveals direct communication between the embryo and glands critical for implantation. Nat. Commun. 9, 603 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmer A., Zimmer A. M., Hohmann A. G., Herkenham M., Bonner T. I., Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 96, 5780–5785 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka S., Kunath T., Hadjantonakis A. K., Nagy A., Rossant J., Promotion of trophoblast stem cell proliferation by FGF4. Science 282, 2072–2075 (1998). [DOI] [PubMed] [Google Scholar]
- 52.Yuan J., et al., Primary decidual zone formation requires Scribble for pregnancy success in mice. Nat. Commun. 10, 5425 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.