Graphical abstract
Keywords: Nanomaterial, Enzyme Immobilization, Conjugate, Therapy, Biosensing, Drug delivery
Abstract
Background
Because enzymes can control several metabolic pathways and regulate the production of free radicals, their simultaneous use with nanoplatforms showing protective and combinational properties is of great interest in the development of therapeutic nano-based platforms. However, enzyme immobilization on nanomaterials is not straightforward due to the toxic and unpredictable properties of nanoparticles in medical practice.
Aim of review
In fact, because of the ability to load enzymes on nano-based supports and increase their renewability, scientific groups have been tempted to create potential therapeutic enzymes in this field. Therefore, this study not only pays attention to the therapeutic and diagnostic applications of diseases by enzyme–nanoparticle (NP) bio-conjugate (abbreviated as: ENB), but also considers the importance of nanoplatforms used based on their toxicity, ease of application and lack of significant adverse effects on loaded enzymes. In the following, based on the published reports, we explained that the immobilization of enzymes on polymers, inorganic metal oxide and hybrid compounds provide hopes for potential use of ENBs in medical activities. Then, the use of ENBs in bioassay activities such as paper-based or wearing biosensors and lab-on-chip/microfluidic biosensors were evaluated. Finally, this review addresses the current challenges and future perspective of ENBs in biomedical applications.
Key scientific concepts of review
This literature may provide useful information regarding the application of ENBs in biosensing and therapeutic platforms.
Introduction
Enzymes, based on catalytic activities, are considered as a key part of the development of health systems [1]. Indeed, the ability of the enzyme to perform catalytic reactions has made these compounds inevitably the most important cause of biological reactions over the past decades [1]. However, due to the lack of long- and medium-term stability in various environmental and biological conditions, the complexity of enzyme production processes, the presence of impurities, and the limited activity range (low rate of recovery and reproducibility) led the researchers to focus on producing alternative materials showing catalytic activity. Enzyme stabilization by covalent/non-covalent attachment on modified matrices using different chemical activation strategies [2] is surprisingly valuable because we will be able to reuse the enzymes after applying them during a particular process, and thus we can see a longer half-life and less degradability during the specific reactions [3], [4], [5]. Not only their diffusing and kinetic parameters are changed [6], [7], [8], but the rate, at which the reactions take place, as well as the onset of a series of reactions and their activation/inhibition, can be controlled, both in terms of time and type of complete reaction [9], [10], [11], [12]. Enzyme immobilization also prevents contamination of protein/enzyme substrates and other compounds [13], [14], which also reduces the cost of purification [15], [16]. Immobilization of the enzyme increases the stability and half-life of the enzyme [17], [18], [19], but at the same time allows the enzymes to operate on a larger scale and larger ecosystem ranges and possibly interact with other enzymes [20], [21], [22]. The stabilization of enzymes requires the formation of an environment in which the enzymatic activity in terms of temperature and the corresponding pH is similar to the initial environment of its function in the biological system [23], [24].
Recent advances in industrial and medical biotechnology have made the use of stabilized enzymes in a variety of biomedical and manufacturing applications [25], [26]. This increase in commercial applications of enzymes that are consistent with their stabilization has a wide variety of research areas in the field of enzyme loading on different solid supports [27]. Recently, special researches have been conducted on the development of more feasible and applicable methods for enzyme stabilization, which has led to the formation of more potential methods for the production or decomposition of a number of compounds by biocatalytic reactions [28], [29]. There are also several reports on the potential application of immobilized enzymes for target delivery [30], [31], anti-tumoral effect [32], [33] and biosensors [34], [35], [36].
Indeed, the development of enzyme immobilization in biotechnological [11], [37] and clinical platforms [38] has received a great deal of attention in many applications in the field of drug delivery [39] to various biological systems and anticancer activities [40], [41]. They are also used as sensors [42] to control some diseases like diabetes [43] and cancer [44]. Indeed, enzyme immobilization is widely used in the design and development of degradable biosensors [45].
With the development of nanotechnology, researchers are increasingly paying attention to the technology of enzyme–nanoparticle (NP) bio-conjugate (abbreviated as: ENB), and also development of nanozymes [46]. Although ENB is a potential strategy for application of enzymes in biomedical platforms [47], the complex processes of development, cost, and loss of enzymatic activity due to lack of proper interaction upon immobilization on nanomaterial-based matrixes have led to a limited development of this method compared to nanozymes [4], [48], [49], [50], [51], [52], [53], [54]. Of course, ENB has some potential advantages such as increased stability of different enzymes under harsh environmental and biological conditions, targeted enzyme transfer to tissues [55], [56], reduced inflammation induced by antioxidant activities of NPs [57], and induction of growth in tissues due to optimal effects of NPs [58], [59]. Given the importance of using enzymes with high stability and catalytic activity, this review article focuses on the development and application of ENB for therapeutic, drug delivery and biosensing approaches.
Different methods of ENB preparation
Despite the expanded immobilization of enzymes on nanomaterials in the industry, their use in biomedical applications encounters some limitations due to the toxic nature of some NPs. Nevertheless, the use of ENB for drug delivery and therapeutic applications is under development. In this regard, in addition to the toxicity of NPs, which can be moderated according to the type of coatings and materials used, enzyme activity with high efficiency is another major challenge. Advantages and disadvantages of the different methods of enzyme loading on the nano-based platforms are summarized in Table 1. It was revealed that the adsorption method can show the highest efficiency and the covalent or cross-linking methods provide the highest stability on the enzyme structure [23], [60]. Therefore, the type of target and materials used in the matrix are effective on the final efficiency of ENB development.
Table 1.
Advantages and disadvantages of different enzyme immobilization techniques.
| Immobilization methods | Advantages | Disadvantages |
|---|---|---|
| Adsorption | Simple production, no need for functionalization of support, inexpensive, lack of conformational changes of the enzyme, high catalytic activity, Minor changes of the active site of the enzyme. | The formation of weak bonds with solid support, low stability and high leakage. |
| Entrapment/ Encapsulation | High stability, minimal conformational changes of the enzyme, continuous reaction, easy downstream process, co-immobilization of different enzymes. | Low apparent activity of the enzyme, limitation of mass transfer, low loading percent, complicated experimental process. |
| Covalent attachment | Lack of enzyme leaching, strong interaction with the solid support, high stability, high operational consistency. | Mobility limitation of enzymes, low enzyme activity, structural restriction, most complicated and expensive, use of toxic chemicals. |
| Cross-linking | Strong bonding, lack of enzyme leakage, reusable, low release rate, | Decrease in enzymatic activity, decrease in diffusion rate, transfer limitations |
Material used in matrix
Despite the wide range of NPs, the applications of many of these supports are limited in biomedical activities for enzyme loading due to their toxicity and type of application. For example, despite providing sufficient space for enzymes loading on porous silica (SiO2) NPs [61] and carbon nanostructures [62], [63], they are not being widely used in biomedical applications because of the cytotoxicity stimulated via apoptosis. Hence, providing sufficient space for loading and improving the stability of enzymes is not the main criteria for application of NPs in therapeutic activities. The most important NPs used to transfer enzymes to target tissues, including synthetic and natural polymers, inorganic metal oxides and hybrid compounds.
Polymers
Each member of the polymers includes natural (collagen, fibrin, cellulose, chitin, chitosan, alginate, and creatine), synthesized [poly(ethylene glycol)(PEG), poly(lactic-co-glycolic acid) (PLGA), poly(lactic acid) (PLA), poly(vinyl pyrrolidone) (PVP), poly(acrylic acid) (PAA)and poly(caprolactone acid) (PCL)], and their combination have unique chemical and physical properties in biomedical activities that make them as potential and versatile agents in different biomedical applications such as drug delivery, tissue engineering, imaging, and diagnostic activities [24], [64]. These compounds show outstanding features such as biocompatibility, cost-effectiveness, biodegradability, safety, porosity, high surface area for enzymes loading, and finally reusability in multiple cycles [65], [66]. For instance, in a mice model, Salvalaio et al. [67] for the treatment of lysosomal storage disorders, one of the metabolic syndromes in the brain, using PLGA-NPs modified with albumin and loading lysosomal enzyme not only were able to pass the enzyme through the blood–brain barrier and improve disease by providing enzyme in the tissue, but also revealed that PLGA with significant biocompatibility and biodegradability in the target tissue had no side effect on cell viability. However, there are some disadvantages such as unwanted impurities in natural polymers that increase the immunogenicity at the time of decomposition, the highly variable mechanical structures due to the type of bonds and compounds in the polymers, and the production of acidic compounds during degradation [24], [68].
Inorganic metal oxidase
Metal and metal oxide NPs comprise a wide range of nanomaterials. These compounds are highly regarded because of their unique chemical or physical properties such as high stability, high availability, simple modifiability, optimal to excellent biocompatibility, crossing physiological barriers, imaging capability, and auxiliary activities such as photothermal therapy (PTT), antioxidant activities, tunable size and level [69], [70], [71], [72], [73], [74]. Moreover, high enzyme loading [75], unusual redox properties [76] and regulation of enzymatic activities through conformational induction [77], [78], [79] can be other prominent features of these NPs. However, these nanomaterials show some disadvantages that depend on their physical and chemical nature and methods selected for NPs production. For example, the toxicity of inorganic metal oxide NPs increases as the surface-to-volume ratio of NPs enhances, whereas the toxicity reduces as their dimensions decrease below 10–15 nm due to the detoxification of NPs [80]. While, Gao et al. [81] and Chen et al. [82] reported that iron oxide NPs (IONPs) and copper oxide (CuO) NPs inherently have peroxidase- and oxidase-like activities respectively, which by decreasing the size of NPs from 300 to 30 nm and 30 to 6 nm, their enzymatic-like activity increases. Therefore, the toxicity of the nanomaterials is consistent with their catalytic activities. Also, active sites on metal or metal oxide NPs that have inducible effects on cell death cause high toxicity through DNA degradation, destruction of mitochondrial electron transfer activities, reactive oxygen induction, denaturation of vital intracellular proteins, and increased expression of inflammatory proteins [83], [84]. In addition, the cost of manufacturing methods and high toxicity of NPs-forming materials are other challenges of this group [85]. Nevertheless, the toxicity reported in metal and metal oxide NPs are confusing and generally challenging due to the variety of cells used in the articles as well as the diverse conditions of the examined NPs. However, the most toxic inorganic metal oxide NPs appear to be CuO and zinc oxide (ZnO) and the most compatible NPs seem to be IONPs and titanium oxide (TiO2) [84], [86]. Among the metal NPs, gold (Au) and Fe exhibited the minimum toxicity [84], [87], [88].
Hybrid compounds
Sometimes the combination of polymer compounds with inorganic metal oxide is used to increase the efficiency of nanoplatforms. Each of the polymers and metal or metal oxide NPs show some merits and drawbacks that their optimization can reduce their side effects. Moreover, platforms produced can display properties that cannot be observed in individual materials. Synthesized hybrids are divided into three parts based on the type of ingredients: organic-organic hybrids, organic-metal oxide hybrids and metal oxide-metal oxide hybrids (Table 2). Combining an inorganic metal oxide with potential mechanical stability and the ability to perform auxiliary therapeutic activities with a biocompatible polymer produces a suitable ENB for enzymatic activities in medical application. In addition, by using different hydrophilic and hydrophobic compounds, a nanoplatform can be prepared which, in addition to increasing solubility and reducing immunogenicity in the physiological condition, increases the chemical bonds between the enzyme and the nano-based support and high enzyme efficiency under physiological states [89]. Also, Liu et al. [90] showed that the use of different polymers in the synthesis of nanoplatforms provides the possibility of creating higher porosity along with increasing the ability of chemical bonding. For example, Li et al. [91] by comparing the ENB and free enzyme with the platform derived from propyl-methyl ammonium chloride as a polymer and IONPs as a matrix, not only improved the lipase loading, but also increased the enzyme activity 1.5 fold and its recovery by 147.7%. Similarly, it was shown that the integration of poly-vinyl alcohol with IONPs increased the catalytic efficiency of the lipase and its stability compared to the individual state of the NPs and the free enzyme [92]. In addition to polymers, protein compounds such as amyloid fibrils can be used to produce hybrid NPs to further transfer NPs into the target cell or tissue [93].
Table 2.
Summary of hybrid nanoplatforms applied for enzymes immobilization.
| Nanoplatforms | Immobilization type | Binding group | Immobilize enzyme | Ref. |
|---|---|---|---|---|
| Graphene oxide-Fe3O4 | Covalent binding | –OH, C = O | Glucoamylase | [151] |
| Cellulose-Polyacrylic acid | Covalent binding | –OH, COOH | Horseradish peroxidase | [152] |
| Chitosan-Alginate | Entrapment | –NH2, –OH | Amyl glucosidase | [153] |
| ZnO-SiO2 | Cross-linking | –OH | Horseradish peroxidase | [154] |
| Silica-Lignin | Adsorption | –OH, C = O | glucose oxidase | [155] |
Applications of ENB in medical activities
Therapeutic platforms
Therapeutic enzymes like other enzymes can be loaded onto nanomaterials and applied as a platform in different applications (Table 3). In this regard, it was shown that a magnetic carrier could handover streptokinase to the arterial thrombosis of the dog with high efficiency and high concentration [94]. In another study, Kempe and Kempe [95] exhibited that with the covalent bonding of magnetic NPs (10–30 nm) to the plasminogen activator as a protease, in addition to enhancing the enzyme stability, successful treatment of anti-thrombosis could be performed. On the other hand, it has been shown that the use of liposomes coated with PEG for entrapping tissue plasminogen activator results in greater stability of the enzyme in the blood up to 9 h versus the free enzyme with one hour due to preventing plasma clearance and enhancing the activity of the enzyme [96]. Moreover, Piras et al. [97] developed the enriched urokinase loading onto polymeric NPs based on a hydrophobic absorption gradient with 83% initial activity of the enzyme for thrombolysis treatment.
Table 3.
Therapeutic enzymes immobilized on nanomaterials.
| Enzyme | Nanomaterials | Application | Ref. |
|---|---|---|---|
| Bilirubin oxidase | Albumin aggregate | Treatment of neonatal jaundice | [156] |
| Chymotrypsin | Magnetic | Pancreatic insufficiency | [157] |
| Serine endopeptidase | Antithrombotic therapy | Thrombolytic activities | [158] |
| Chymotrypsin | Electrospinning nanofibers | Pancreatic insufficiency | [159] |
| Beta-Galactosidase | Magnetic | Lactose intolerance | [160] |
| Tyrosinase | Treatment of melanoma cancer | Polylactic-acid nanocapsules | [103] |
| Lysozyme | Antimicrobial therapies | Nanofiber mats | [161] |
| Asparginase | PEGylated | Acute lymphoblastic leukemia | [162] |
| Glucose-6-phosphate dehydrogenase | SiO2-based matrix | Jaundice | [163] |
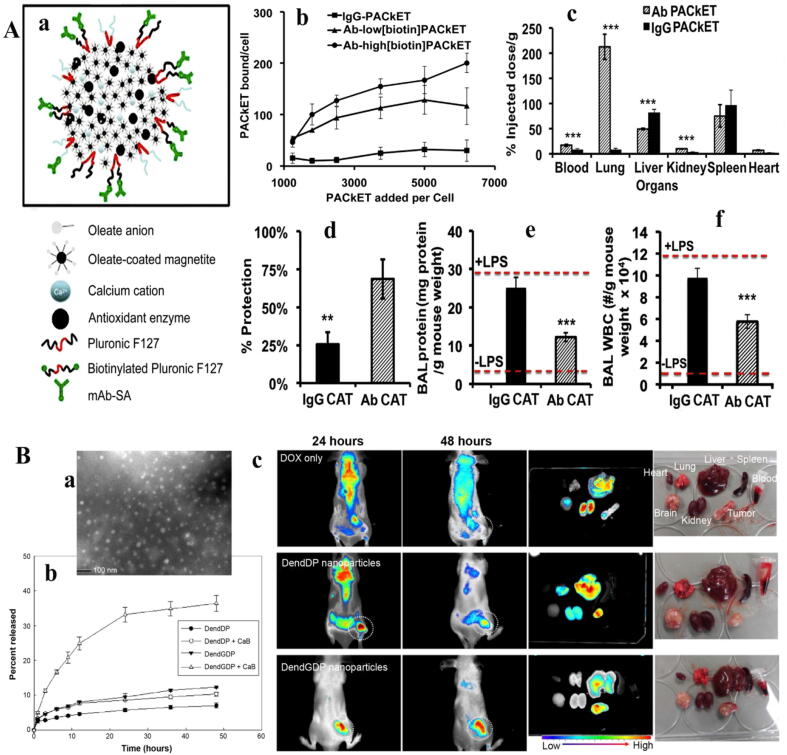
The use of microporous fibrin nanocomposites as a potential scaffold in entrapping the alkaline phosphatase with a covalent bond for bone regeneration resulted in a complete recovery of bone repair in vitro [58]. Researchers also used DNA and AuNP scaffolds to stabilize and improve the activity of alcohol oxidase for alcohol detoxification, which received reliable responses on clinical trials in vivo [98]. Furthermore, despite the high variation in the loading of the tyrosinase on nanomaterials in the medical field, such as ZnO nanorods [99], carbon nanotubes (CNTs) [100], magnetic nanobeads, [101] and chitosan [102], it has been recently shown that the nanocapsules of the polyhemoglobin–tyrosinase complex reduced the activity of the melanoma cells in the murine tumor model [103]. In another study, Yun et al. [104] were able to reduce the brain inflammation, reperfusion injury, and the possibility of stroke with the superoxide dismutase (SOD) encapsulated by liposome and polymer PLGA to increase stability, higher passage and higher enzyme accumulation in the damaged area. Meanwhile, the use of targeted polymeric NPs containing SOD and catalase after 30 min of injecting could provide strong protection from lung inflammation of the mouse due to the prevention of endotoxin production (Fig. 1A) [105]. Also, Martinez et al. [106] developed a dermal patch of the keratinase along with enrofloxacin antibiotic by the nano-gel from alcohol and pectin, which enzyme activity in this nano-composite was 100% of its initial enzyme activity with a higher stability and performance on the skin.
Fig. 1.
(A): a; Endothelial targeted antioxidant NPs formation scheme by controlled precipitation, b; Binding of Ab-PAC to cultured endothelial cells. 125I- labeled PACs incubated with cells at 37 °C, rinsed, lysed and measured for radioactivity, c; Tissue distribution of intravenous injected PACs into mice after 30 min circulation time. Protection by endothelial-targeted catalase PACs from oxidative stress in vitro (d) and in vivo based on brochoalveolar lavage (BAL) protein (e-f) [105]. (B): a; transmission electron micrograph of DendGDP NPs, b; Release of Dox from the NPs in the absence or presence of cathepsin (50 U), c; near-infrared fluorescence images for CT26-bearing male nude BALB/c mice. Dox itself or Dox-conjugated dendrimer NPs (5 mg/kg) were injected into the tail vein of each mouse. The major organs were removed from each mouse 48 h after injection [108].
Drug delivery
Enzymes can be used as agents in the drug delivery systems based on controlled release of drugs via enzyme reaction under specific conditions. In other words, targeting ligands in drug carriers are activated by enzymes to enter the selective cell to release the drug. The most important enzymes studied in this field are proteases, lipases and glycosidases. In this way, the NPs are assembled simultaneously with decomposed units, which, the drug is released after the digestion of the NP in the presence of the enzyme. This method can be very effective in reducing the secondary effects and toxicity due to the partial accumulation of drugs in non-target tissue due to the absence of required enzymes. Accordingly, Law et al. [107] designed a sequence of peptides that can diffuse the therapeutic factors by interaction with the protease.
In the cellular model, it was shown that with the use of dendrimers containing Gly-Phe-Leu-Gly peptide-sequences linked to doxorubicin (Dox), the cell death process increases with the presence of cathepsin B enzymes (Fig. 1B) [108]. Furthermore, Kang et al. [109] developed peptide nano-polymers for gene delivery that were activated in the presence of protein kinase or protease and induced drug transfer to the cells in vitro and in vivo. Similarly, it has been shown that the application of a peptide sequence containing a glycine-glycine bond attached to a nano-polymer (N-(2-hydroxypropyl) methacrylamide) and Dox that is degraded by protease, in addition to increasing the targeted release of drug in breast cancer, it can produce a higher stability and lower toxicity [110].
In the animal model, Singh et al. [111] and Liu et al. [112] reported that the Dox attached to the mesoporous SiO2 NP by peptide-sequence in response to the activity of the protease accumulated in the tumor tissue, led to cell apoptosis. In this line, the researchers showed that an antitumor ether lipids drug delivery based on the phospholipase A2 activity, which reduced drug toxicity, the increase of membrane permeability and drug performance [113]. In some cases, a linker susceptible to two or more enzymes can be established to improve the response of a developed platform to enzyme activity. For example, it was determined that the cyclodextrin caps of the cavities of the SiO2 NPs would be degraded by both lipase and amylase [114]. Since in the cancer cells, the emalase level is 85 times higher than that of normal cells [115], Ferguson and Duncan [116] described that sugar NPs provide drug delivery to the tumor site without cytotoxicity against normal cells. Also, this drug delivery system can be used as a potential platform in increasing drug stability by transporting drugs to cancer cells as well as decomposing glycosidic linkages by the amylase.
Biological assays
The real time monitoring of the biological events and simultaneously developing therapeutic approaches is known to be possible via examining chemical compounds such as glucose or urea [117]. Therefore, the use of enzymes as biosensors has received a great deal of interest in the control of diseases or physiological activities [118]. The use of ENB as a biosensor for diagnostic and therapeutic activities not only requires complete assurance of the biosensors' functional parameters, it is important to ensure that the sensor system does not present a hazard to the patient. Nevertheless, in vivo biosensors based on ENBs have attracted a number of researchers because of their important role in controlling some diseases, especially diabetes which is a global problem [119]. For instance, Chen et al. [120] in a short-term (21-d) and Luo et al. [121] in a long-term (295-d) period by designing a glucose oxidase (GOx)-based biosensing platform loaded on poly[2-(dimethylamino)ethyl methacrylate] containing insulin were able to control blood glucose levels in the both non-fasting and fasting models by implanting the designed nanobiosensor in the animal's arm. As blood glucose increases, the GOx is activated in ENB which converts peripheral glucose to glucuronic acid. Changes in environmental acidity caused by glucuronic acid accumulation alter the structure of the polymer and result in release of insulin [120]. Likewise, Chu et al. [122] by providing an albumin-based membrane containing GOx, catalase, and manganese NPs were able to control glucose in blood based on insulin release in diabetic rats over a 7-day period by altering the environmental acidity via GOx activity. Moreover, in the animal model, Gu et al. [123] designed a nano-network of alginate and chitosan containing insulin capsules which insulin released by increasing blood glucose levels due to the activity of the GOx in the network and the bond breaking of polymer and insulin.
It has also been shown that insulin can be used to control blood glucose based on solving insulin-containing polymeric vesicles due to the hydrophilic-hydrophobic balance change. In this regard, Hu et al. [124] were able to control the blood glucose of diabetic rats at 20, 30, and 50 min based on concentrations of 268, 330, and 450 mg/dL, respectively, by designing vesicles made of H2O2-sensitive copolymers [PEG and phenylboronic ester (PBE)-conjugated polyserine] containing insulin and GOx. With activation of the GOx and H2O2 generation, the vesicles components breakdown which releases insulin. Based on this structure and enzymatic activity, drugs can be delivered to the target tissue or released into the body. Recently, to accelerate responsiveness to a platform containing insulin, Mohammadpour et al. [125] using PLGA and chitosan polymers and loading of both catalase and GOx were able to respond rapidly to any changes in blood glucose based on copolymer degradation induced by gluconic acid accumulation to alter the platform acidity under diabetic rat skin. However, any response to blood glucose variation without the burst release of insulin from the nanoplatform is a major challenge. Indeed, the simultaneous use of two enzymes can provide a rapid response to any changes in blood glucose. While, the use of copolymers that provide greater rigidity of the platform can guarantee the duration of solid support integrity. In this regard, it was determined that the presence of catalase and GOx in the copolymer synthesized from dextran with a high cyclic acetyl content, not only alters the kinetics of insulin release in diabetic rats, but also enhances the rate of response to glucose variation by immobilized enzymes [126].
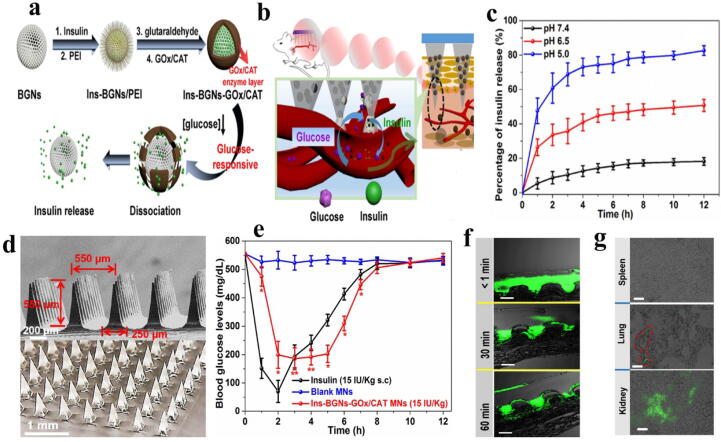
On the other hand, there are reports of the use of inorganic NPs such as SiO2 to increase the stability of the platform for regulating blood glucose level [127], [128], [129]. In this technique, mesoporous NPs containing GOx and catalase coated with polymers linked to micro-needles are generally sensitive to any changes in blood glucose. In this context, Jiang et al. [130] by designing ENB located on skin including polyethyleneimine with catalase and GOx on insulin-containing mesoporous bioactive glass NPs, were able to control the blood glucose changes of diabetic rats by prolonging polymer degradation induced by enzymes activation and insulin release (Fig. 2).
Fig. 2.
(A): a; Schematic representation of the glucose-sensitive insulin delivery system using glucose-sensitive BGNs, b; The glucose-sensitive insulin released from the MNs in vivo, c; Profiles of insulin release in different pH, d; SEM images of BGNs-GOx/CAT MNs, e; Profiles of blood glucose levels after Ins-BGNs-GOx/CAT MNs injection of insulin treated with diabetic rats, f; Fluorescence and bright-field histological of FITC-labeled insulin-loaded MNs attached on diabetes rats, g; Histological sections of spleen, lung, and kidney of diabetic rat after treated [130].
Taken together, these reports indicate that drug delivery systems based on biosensing activities of GOx or catalase is a promising strategy for the treatment of wide range diseases.
Enzymatic nanobiosensors
An enzymatic nanobiosensor is a valuable method for analyzing various types of biomaterials in medicine, pathology, pharmacy, and so on. In the clinical field, enzymatic nanobiosensors can accelerate laboratory processes at a lower cost [118], whereas a limited number of them are available in the laboratory. The enzymatic nanobiosensors are essentially designed based on the existence of a specific catalyst for binding to an analyte that can increase selectivity, sensitivity, and analytical signaling of the sensors [131]. Because of the wide variation in enzymatic nanobiosensors [132], [133], [134] and the presence of numerous reviews in this field [23], [24], [135], this paper focuses on the nanostructure of paper-based biosensors, wearable biosensing tools, and lab-on-a-chip or microfluidic instruments.
Paper-based enzymatic nanobiosensors for medical detection
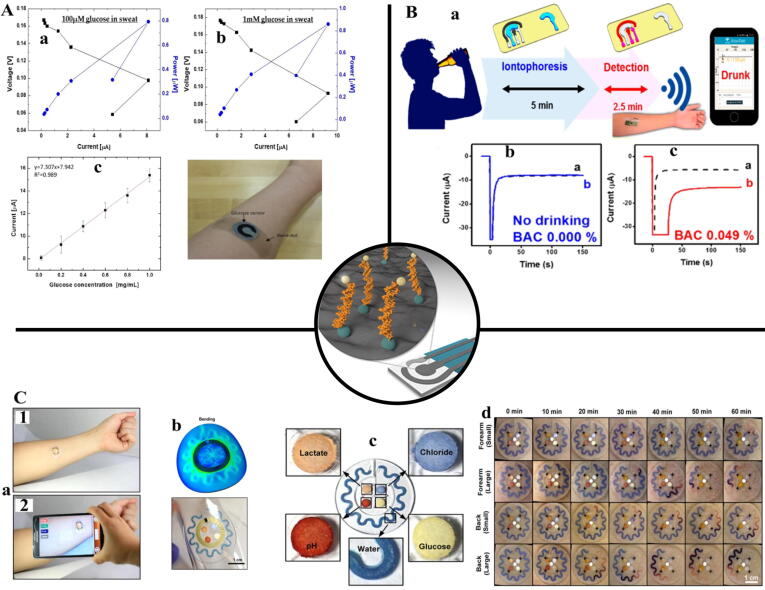
Paper-based enzymatic nanobiosensors that were introduced in 2007 are a powerful detection (Table 4) and portable instrument which greatly reduces production and maintenance costs. To increase the sensitivity and selectivity of paper sensors that are almost fabricated by photolithography, laser, and screen printing, the use of nanomaterials in its layers was also used. Enzymatic agents, cofactors and colorimetric dyes were also immobilized on nanomaterials in addition to the paper substrate. In this regard, Ornatska et al. [136] reported that ceria NPs along with the GOx onto filter paper in the presence of urinary glucose could change the color of the paper sensor from white to yellow. They declined the glucose limit of detection to 0.5 mM and linear range up to 100 mM. Analogously, Cho et al. [137] developed a paper enzymatic nanobiosensor with a GOx capable of detecting a level of glucose from a sweat with a range of 0.02–1.0 mg glucose mL−1 (Fig. 3A). Also, another study showed that electrochemical paper nanosensors based on AgNPs decorated boron-doped diamond onto paper with cholesterol oxidase could detect cholesterol [138]. In addition, with the application of GO in paper-based electrochemical nanobiosensors along with acetylthiocholine chloride, Panraksa et al. [139] provided diagnosis of acetylcholinesterase in the blood at a detection limit of 0.1 U/mL in range of 0.1–15 U/mL.
Table 4.
Paper-based enzymatic nanobiosensors.
| Enzyme | Nanostructure | Analyte | Method | Detection limit | Ref. |
|---|---|---|---|---|---|
| GOx | Tungsten disulfide nanosheets | Blood glucose | Colorimetric method | 2.9 µM | [164] |
| GOx | MFe2O4 (M = Mg, Ni, Cu) |
Glucose in the urine | Colorimetric biosensing | 4.5 × 10-7 M | [165] |
| Glucose dehydrogenase |
AuNPs | Dihydronicotinamide adenine dinucleotide |
Colorimetric readout | 12.5 µM | [166] |
| HRP | AuNPs | Nucleic acid | Lateral flow strip | 0.3 pM | [167] |
| Cholesterol oxidase |
AgNPs | Cholesterol | Amperometric detection | 0.25 mg/dL | [138] |
| Acetyl thiocholine esterase | AuNPs | acetylthiocholine | Colorimetric method | 0.5 µM | [168] |
| Acetylcholinesterase | CNTs | nerve agents | Lateral flow strip | 0.02 nM | [169] |
Fig. 3.
A: a-b; Power outputs and polarization curves with varying concentration of glucose in artificial sweat; (a) 0.02 mg/mL (~100 µM); (b) 0.2 mg/mL (~1 mM), c; The glucose levels in sweat were monitored immediately 30 min after the beginning of the exercise [137]. B: (a) Schematic diagram of a wireless operation of the iontophoretic-sensing tattoo device for transdermal alcohol sensing. In the diagrams of the tattoo-base device, blue and red highlights show the active zones during iontophoresis and amperometric detection, respectively, b; Control experiments without drinking, c; Experiments with consumption of 12 oz of beer measured from two different human subjects, before and after drinking alcohol beverage [145]. C: a; Pictures demonstrating NFC between a sweat monitoring device and a smartphone to launch software for image capture and analysis, b; Results of stress distribution associated with the devices on phantom skin and respective optical images under bending with 5 cm radius, c; colorimetric detection reservoirs that enable determination of total water loss and concentrations of lactate, glucose, creatinine, pH, and chloride ions in sweat, d; images of two different types of sweat patches (small and large harvesting areas) applied to the lower back and volar forearm collected at various times during the study [148].
Wearable biosensing devices
Integrating smart tools such as mobile phones, tablets, watches and flexible electronic devices with biosensing techniques can in addition to instantaneous medical monitoring and easy transport, provide quick collection of real-time biometric data at single way [140]. The best practical example in this scope is the continuous and extensive control of blood glucose levels among diabetics and athletes in periodic competitions. In this regard, Kudo et al. [141] and Iguchi et al. [142] produced a wearable enzymatic nanobiosensor with flexible oxygen and hydrogen peroxide electrodes, respectively, that could detect glucose level in tears with a range of 0.025–1.475 mM and a range of 0.06 to 2.00 mM. Similarly, in another study, a combination of sol–gel as a contact lens with GOx was used to determine the level of glucose with rapid rate and high precision (0.1–0.6 mM) [143]. Also, Jia et al. [144] demonstrated a wearable enzymatic nanobiosensor to determine the level of lactate with lactate oxidase in the sweat of cyclist sports with linearity up to 20 mM. Meanwhile, another study showed that the determination of alcohol level by alcohol dehydrogenase immobilized on the wearable nanobiosensor through sweat is possible (Fig. 3B) [145].
Lab-on-a-chip or microfluidic devices
Lab-on-a-chip and microfluidics are tools for analyzing samples with small volume, whereas designed to integrate multiple experiments. The main purpose of these tools is access to high precision and selection, and the lack of manual preparation of samples. In this line, Chikkaveeraiah et al. [146] identified the products of tissue culture by integrating cell culture into microfluidic reservoirs and using Horseradish peroxidase (HRP) deposited on AuNPs in canals. Furthermore, Wisitsoraat et al. [147] were able to measure the cholesterol levels of 60 specimens per hour by using cholesterol oxidase immobilized on CNTs based on amperometric sensors in microfluidic channels. In an experimental, the concentration of glucose, lactate, chloride, and pH of the secreted fluid derived from the sweat glands cultured in the microfluidic reservoirs was determined by immobilizing the enzymes like a peroxidase, Keratinase and oxidase in the channels (Fig. 3C) [148]. The change in the color of the fluid introduced into the channels caused by the enzyme activity indicated the concentration of the desired products. As well as, Backer et al. [149] developed a lab-on-a-chip microfluidic system based on amperometric sensor to measure glucose, glutamate and glutamine by inserting enzymes of GOx, glutamate oxidase and glutaminase onto platinum NPs with a detection limit of 0.05 mM for glucose and glutamate and 0.1 mM for glutamine. Besides, Ali et al. [150] using a microfluidic system based on lab on chip and cholesterol esterase and oxidase immobilized on nickel NPs and CNTs in canals, were able to detect cholesterol levels in body fluids with a sensitivity of 2.2 mA/mM/cm2.
Challenges and future perspective
Despite the benefits of using ENB in biomedical activities, these compounds still face serious challenges, most notably:
-
1-
One of the major challenges in enzyme loading on porous NPs such as porous SiO2, scaffolds and even porous inorganic metal oxides, is the blockage of pores in tanks embedded in the platform. Increasing the enzymatic layers on the platform as well as the presence of enzymes that have not been fully incorporated into the tank can reduce the embedded gap diameter, which adversely affects the performance of the ENB. Moreover, because most designed ENBs are saturated with enzyme layer-by-layer aggregation, excessive enzyme accumulation on the support or part of it can impair the overall performance of the enzymes. Therefore, by creating specific sites on the NPs for binding to the enzyme, in addition to reducing the consumption of enzyme, it can prevent the reduction of the unwanted enzyme activity.
-
2-
Despite increasing enzyme stability and reproducibility upon ENB, a decrease in the content of enzyme loading is observed due to changes in the enzyme active site resulted from the conformational effects induced by the nanomaterials or the unfavorable binding of the enzyme to the nanomaterials. Since the developed NPs do not have a complete uniformity, so the size and shape of the NPs can strongly influence the structure of the enzymes. For this purpose, biological methods that produce more uniform NPs can be used as an alternative for fabrication of potential NPs.
-
3-
Another major challenge in development of ENB is the incompatibility of nanomaterials with biological activities that require surface modification. Surface modification sometimes reduces the performance of nanomaterials in enzyme transfer to the targeted tissue due to the lack of targeted ligands. On the other hand, the presence of ligands along with the enzymes can provide an unfavorable binding of the enzyme-ligand, which reduces the enzyme activity. Although targeting by entrapment/encapsulation may result only in partial structural changes of enzymes, it was found that enzyme function was reduced by creating rigid structures in the enzyme by NPs modification. The development of a promising strategy is difficult because of the lack of sufficient information on NPs-enzyme, enzyme-ligand and enzyme-enzyme interactions after immobilization of enzymes into the solid supports.
-
4-
Next challenge is the lack of confidence in the advantages of inorganic substrates such as inorganic metal oxide in biosensing activities in vivo, due to the long-term stability and also the unintended catalytic effects resulting from removal of the surface enzyme. According to the reported literature, the use of polymers or inorganic NPs less than 10 nm can greatly reduce this problem. However, the use of ENB in biosensing has generally been used in implants or skin attachments.
-
5-
Despite the widespread use of ENB in therapeutic activities and drug delivery to cancer cells due to the possibility of corona protein formation on inorganic NPs such as metal oxidize in blood and non-target cells, the risk of unwanted toxicity increases. However, with the change of nanomaterials used in the platform, the limitation caused by the presence of corona proteins has been partially controlled.
Taken together, the use of ENB instead of free enzyme in biomedical activities not only increases the targeting, sustainability, and reduces the therapeutic costs, but also enables the synchronization of therapeutic activities such as PTT or magnetic therapy based on the presence of metal oxide NPs.
Summary and outlook
Although free enzymes have higher enzymatic activity than enzymes loaded on objects, ENBs have received a great deal of attention in biomedical activities due to increased stability of enzymes and even improved enzymatic activity at inappropriate temperature and pH. Achieving the method of isolating and purifying proteins in solution proves that by using NPs, it is possible to remove certain enzymes from raw solutions. One of the future fields of research in the field of using NPs is the preparation of adsorbents with special functional groups to facilitate the specific or selective extraction of enzymes from different samples for biomedical applications. The use of ENB in the biomedical application can result in the development of simple, fast and sensitive methods for drug targeting, anticancer and biosensing platforms. This integration in the future can lead to a set-up of more potential and rapid methods for promising development of biomedical modalities based on immobilized enzymes. There are many challenges before the practical application of ENB in the biomedical applications. First, the potential dangers of ENB to human health must be fully assessed. Another issue is the use of coatings that do not have a health grade and can lead to health risks. Therefore, one of the important areas of research is the development of ENB that can be used in a safe aspect in the development of biomedical platforms.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgement
The authors gratefully acknowledge the China Postdoctoral Science Foundation research grant NO. 2020M672291; Henan Medical Science and Technolog Research Youth Project Co-Sponsored by the Province and Ministry in ChinaNO: SB201902020; Top Talent Fund of the Second Affiliated Hospital of Zhengzhou University, NO: 2020BJRCA03.
Biographies

Dr. Suliman Khan has obtained PhD degree from Chinese Academy of Sciences. He is working at the second affiliated hospital of Zhengzhou University as postdoctoral teacher. He has recently received two research grants from Chinese Postdoctoral Sciences foundation and the Second Affiliated hospital of Zhengzhou University. He has published more 60 papers in SCI journals including Scientific Data, Clinical Microbiology and Infection, Journal of Advanced Research, and Journal of Clinical Microbiology.

Mohammad Mahdi Nejadi Babadaei (Mahdi Nejadi) graduated from Isfahan University in 2012 with a B.Sc in Cellular and Molecular Biology- Microbiology and 2018 from Tehran University of Medical Science, Azad University with a M.Sc in Molecular Genetics. He is currently a PhD candidate in Molecular Genetics at Azad University, North Tehran Branch since 2018. He has many successful experiences on production of RT-diagnostic kits and recombinant proteins which are developed in Viragene Lab. He has more than 10 papers in the fields of nanomedicine, Covid-19, and cancer.

Dr. Anwarul Hasan is an Associate Professor in the Department of Mechanical and Industrial Engineering, and Biomedical Research Center at Qatar University. Earlier he worked as an Assistant Professor in the Department of Biomedical Engineering and Mechanical Engineering at American University of Beirut, Lebanon. He was also a visiting Assistant Professor during 2014 to 2017 and an NSERC Postdoctoral Fellow during 2012-2013 at the Harvard-MIT Division of Health Sciences and Technology at the Harvard Medical School and Massachusetts Institute of Technology in Boston, USA. Dr Hasan obtained his PhD from University of Alberta, Canada in 2010 and worked in industry in Canada during 2010-2011. Dr Hasan has more than 200 peer reviewed publications including over 150 journal articles, and more than 50 conference proceeding papers as well as two edited books on “Tissue Engineering for Artificial Organs”. He is a winner of more than sixteen national and international awards. In the latest ranking of world’s top 2% highly cited scientists’ list by Stanford University researchers published in October 2020, Dr Hasan has been ranked 320 out of 50331 top biomedical researchers in the world. Dr Hasan’s current research interests involve Biomaterials, Tissue Engineering, 3D Bioprinting and Organs on chips platforms and microneedle arrays for Diabetic wound healing, cancer biochips, Covid-19 diagnostics, and cardiovascular tissue engineering.

Dr. Zehra Edis has obtained BSc., MSc. and PhD. from the University of Cologne, Germany. She conducted her PhD. under the Scholarship of the University of Cologne. She worked as scientific employee at Akzo Nobel Chemicals and research Intern in Bayer AG, Leverkusen, Germany. She joined Ajman University and Sharjah University, UAE as lecturer. Since February 2014 she is Assistant Professor in Ajman University, UAE. She has published more than 31 papers and received from the Deanship of Graduate Studies and Research (DGSR), Ajman University, 6 research grants as Principal Investigator.

Dr. Farnoosh Attar has obtained Ph.D. and M.Sc. in Biochemistry from the Institute of Biochemistry and Biophysics (IBB) of Tehran University between 2002-2010. Since 2012, she is Assistant Professor at the Department of Food Toxicology, Research Center of Food Technology and Agricultural Products, Standard Research Institute (SRI), Karaj, Iran. Besides developing standards and standardization based on scientific and technical research to providing assurance of food quality and safety, Dr. Attar has collaborated with other scientists to publish more than 30 papers in the fields of nanozymes, nanoparticles-proteins interaction, and cancer.

Rabeea Siddique is a PhD student at the Department of cerebrovascular diseases at the Second affiliated hospital of Zhengzhou University. She received her master’s degree from HUST, Wuhan, China. She was an exchange student at Minnesota State University, USA during her undergraduate studies. Currently she is working on drugs development for neurological and oncological diseases. She has published over 25 papers in well reputed SCI/SCIE journals such as JARE, CMI, JCM, and frontiers in oncology.

Dr. Qian Bai has obtained her MD and PhD degrees from Zhengzhou University, China. She is currently working as deputy director of research and consultant at department of pain management in the second affiliated hospital of Zhengzhou University, She has received four funding grants from the Henan Medical Science and Technology Research Youth Project Co-Sponsored by the Province and Ministry in China, Top Talent Fund of the Second Affiliated Hospital of Zhengzhou University, Henan Middle-aged Youth Health Technology Innovation Talent Project, and National Natural Science Foundation of China Youth Project. She has published more than 15 papers in high impact factor SCI journals including “Brain”, “Molecular Neurobiology” and “Pain”.

Dr. Majid Sharifi has completed his scientific activities in the field of Animal Nutrition in the universities of Guilan (B.Sc.), Tehran (M.Sc.) and then Tabriz (Ph.D.) in 2003, 2006 and 2017. He re-educated in nanomedicine (M.Sc.) and graduated in 2019 due to his great interest in using nanomaterials for treating diseases and pathological disorders. He is Ph.D. student in Tissue Engineering at Shahroud University of Medical Science. He has implemented several projects in the fields of Tissue Engineering, Nano biosensing, Phototherapy therapy, and Drug delivery, and has published various articles in prestigious journals such as Biosensors and Bioelectronics, Controlled release, Advanced Research, Talanta, Scientific Reports, and Nanomedicine. Dr. Sharifi has recently received several international funding for the investigating the application of nanomaterials in theranostic, development of nanozymes, nanomaterials-mediated drug delivery for cancer therapy, and tissue engineering.

Dr. Mojtaba Falahati has completed his Bachelor Curriculum in the field of Biology at Ferdowsi University in 2004 (Mashhad, Iran). Due to his high interests in Biophysical mechanisms related to diseases and pathological disorders, he performed his master thesis in Biophysics in the area of nerve membrane with focusing on medicinal polymers for the treatment of spinal cord injury at the Institute of Biochemistry and Biophysics (IBB) between 2005-2007 (Tehran, Iran). Dr. Mojtaba Falahati received his PhD in Biophysics from IBB (Tehran, Iran) in 2011. During his PhD thesis he had a visit from Bremen, Jacob, Göttingen, and Tübingen Universities in Germany. His main research area during the PhD was the immobilization of enzyme into the nanoporous materials and uncovering the factors influencing the activity and stability of enzymes after interaction with nanoparticles. Since 2012, Dr. Falahati is an assistant professor at the Department of Nanotechnology, Faculty of Advanced Science and Technology, Tehran Medical Sciences, Azad University, Tehran, Iran. Dr. Falahati has recently received several national and international funding for the investigating the interaction of nanomaterials with proteins and cells, development of nanozymes, nanomaterials-mediated drug delivery for cancer therapy, and tissue engineering.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Qian Bai, Email: baiqian@zzu.edu.cn.
Majid Sharifi, Email: majidsharifi@tabrizu.ac.ir, sharifimajid@alumni.ut.ac.ir.
Mojtaba Falahati, Email: mojtaba.falahati@alumni.ut.ac.ir.
References
- 1.Chorny M., Hood E., Levy R.J., Muzykantov V.R. Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles. J Control Release. 2010;146(1):144–151. doi: 10.1016/j.jconrel.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharifi M., Robatjazi S.-M., Sadri M., Mosaabadi J.M. Immobilization of organophosphorus hydrolase enzyme by covalent attachment on modified cellulose microfibers using different chemical activation strategies: Characterization and stability studies. Chin J Chem Eng. 2019;27(1):191–199. [Google Scholar]
- 3.Kandimalla V.B., Tripathi V.S., Ju H. Immobilization of biomolecules in sol–gels: biological and analytical applications. Crit Rev Anal Chem. 2006;36(2):73–106. [Google Scholar]
- 4.Ansari S.A., Husain Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol Adv. 2012;30(3):512–523. doi: 10.1016/j.biotechadv.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Yang J., Wang Z., Lin Y., Ng T.B., Ye X., Lin J. Immobilized Cerrena sp. laccase: preparation, thermal inactivation, and operational stability in malachite green decolorization. Sci Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-16771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLouise L.A., Miller B.L. Enzyme immobilization in porous silicon: quantitative analysis of the kinetic parameters for glutathione-S-transferases. Anal Chem. 2005;77(7):1950–1956. doi: 10.1021/ac0486185. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed S.A., Saleh S.A., Abdel-Hameed S.A., Fayad A.M. Catalytic, kinetic and thermodynamic properties of free and immobilized caseinase on mica glass-ceramics. Heliyon. 2019;5(5) doi: 10.1016/j.heliyon.2019.e01674. e01674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Illanes A., Wilson L. Springer; Immobilization of Enzymes and Cells: 2020. Parameters for the Evaluation of Immobilized Enzymes Under Process Conditions; pp. 65–81. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Galan C., Berenguer-Murcia Á., Fernandez-Lafuente R., Rodrigues R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal. 2011;353(16):2885–2904. [Google Scholar]
- 10.Cirillo G., Nicoletta F.P., Curcio M., Spizzirri U.G., Picci N., Iemma F. Enzyme immobilization on smart polymers: Catalysis on demand. React Funct Polym. 2014;83:62–69. [Google Scholar]
- 11.Bilal M., Asgher M., Cheng H., Yan Y., Iqbal H.M. Multi-point enzyme immobilization, surface chemistry, and novel platforms: a paradigm shift in biocatalyst design. Crit Rev Biotechnol. 2019;39(2):202–219. doi: 10.1080/07388551.2018.1531822. [DOI] [PubMed] [Google Scholar]
- 12.Zdarta J., Meyer A.S., Jesionowski T., Pinelo M. Multi-faceted strategy based on enzyme immobilization with reactant adsorption and membrane technology for biocatalytic removal of pollutants: A critical review. Biotechnol Adv. 2019;37(7) doi: 10.1016/j.biotechadv.2019.05.007. 107401. [DOI] [PubMed] [Google Scholar]
- 13.Catana R., Eloy M., Rocha J., Ferreira B., Cabral J., Fernandes P. Stability evaluation of an immobilized enzyme system for inulin hydrolysis. Food Chem. 2007;101(1):260–266. [Google Scholar]
- 14.Yewale T., Singhal R.S., Vaidya A.A. Immobilization of inulinase from Aspergillus niger NCIM 945 on chitosan and its application in continuous inulin hydrolysis. Biocatalysis and Agricultural Biotechnology. 2013;2(2):96–101. [Google Scholar]
- 15.Wang W., Wang D.I., Li Z. Facile fabrication of recyclable and active nanobiocatalyst: purification and immobilization of enzyme in one pot with Ni-NTA functionalized magnetic nanoparticle. Chem Commun. 2011;47(28):8115–8117. doi: 10.1039/c1cc12685g. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh P., Ghosh U. Immobilization of Purified Fungal Laccase on Cost Effective Green Coconut Fiber and Study of its Physical and Kinetic Characteristics in Both Free and Immobilized Form. Current Biotechnology. 2019;8(1):3–14. [Google Scholar]
- 17.A.M. Azevedo, D.M. Prazeres, J.M. Cabral, L.s.P. Fonseca, Stability of free and immobilised peroxidase in aqueous–organic solvents mixtures, Journal of Molecular Catalysis B: Enzymatic 15(4-6) (2001) 147-153.
- 18.Wang P., Dai S., Waezsada S., Tsao A.Y., Davison B.H. Enzyme stabilization by covalent binding in nanoporous sol-gel glass for nonaqueous biocatalysis. Biotechnol Bioeng. 2001;74(3):249–255. doi: 10.1002/bit.1114. [DOI] [PubMed] [Google Scholar]
- 19.Mateo C., Abian O., Fernandez-Lorente G., Pessela B.C., Grazu V., Guisan J.M. Springer; Immobilization of Enzymes and Cells: 2020. Multi-Point Covalent Immobilization of Enzymes on Supports Activated with Epoxy Groups: Stabilization of Industrial Enzymes; pp. 109–117. [DOI] [PubMed] [Google Scholar]
- 20.Katchalski-Katzir E., Kraemer D.M. Eupergit® C, a carrier for immobilization of enzymes of industrial potential. J Mol Catal B Enzym. 2000;10(1–3):157–176. [Google Scholar]
- 21.Křenková J., Foret F. Immobilized microfluidic enzymatic reactors. Electrophoresis. 2004;25(21–22):3550–3563. doi: 10.1002/elps.200406096. [DOI] [PubMed] [Google Scholar]
- 22.Basso A., Serban S. Industrial applications of immobilized enzymes—A review. Molecular Catalysis. 2019;479 110607. [Google Scholar]
- 23.Sharifi M., Sohrabi M.J., Hosseinali S.H., Hasan A., Kani P.H., Talaei A.J. Enzyme immobilization onto the nanomaterials: Application in enzyme stability and prodrug-activated cancer therapy. Int J Biol Macromol. 2020;143:665–676. doi: 10.1016/j.ijbiomac.2019.12.064. [DOI] [PubMed] [Google Scholar]
- 24.M. Sharifi, A.Y. Karim, N. Mustafa Qadir Nanakali, A. Salihi, F. Mohammad Aziz, J. Hong, R. Hasan Khan, A. Akbar Saboury, A. Hasan, O.K. Abou-Zied, M. Falahati, Strategies for enzyme immobilization on nanomatrix supports and intracellular delivery of enzymes, Journal of Biomolecular Structure and Dynamics (2019) 1-28. [DOI] [PubMed]
- 25.Dwevedi A. Springer; 2016. Enzyme immobilization: advances in industry, agriculture, medicine, and the environment. [Google Scholar]
- 26.Lu Y., Lv Q., Liu B., Liu J. Immobilized Candida antarctica lipase B catalyzed synthesis of biodegradable polymers for biomedical applications. Biomater Sci. 2019;7(12):4963–4983. doi: 10.1039/c9bm00716d. [DOI] [PubMed] [Google Scholar]
- 27.De Rose S.A., Novak H., Dowd A., Singh S., Lang D.A., Littlechild J. Stabilization of a lipolytic enzyme for commercial application. Catalysts. 2017;7(3):91. [Google Scholar]
- 28.Bilal M., Zhao Y., Noreen S., Shah S.Z.H., Bharagava R.N., Iqbal H.M. Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal Biotransform. 2019;37(3):159–182. [Google Scholar]
- 29.de Andrades D., Graebin N.G., Ayub M.A., Fernandez-Lafuente R., Rodrigues R.C. Preparation of immobilized/stabilized biocatalysts of β-glucosidases from different sources: Importance of the support active groups and the immobilization protocol. Biotechnol Prog. 2019;35(6) doi: 10.1002/btpr.2890. e2890. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S., Jana A.K., Dhamija I., Maiti M. Chitosan-assisted immobilization of serratiopeptidase on magnetic nanoparticles, characterization and its target delivery. J Drug Target. 2014;22(2):123–137. doi: 10.3109/1061186X.2013.844157. [DOI] [PubMed] [Google Scholar]
- 31.Wu Z.-Y., Zhang H., Yang Y.-Y., Yang F.-Q. An online dual-enzyme co-immobilized microreactor based on capillary electrophoresis for enzyme kinetics assays and screening of dual-target inhibitors against thrombin and factor Xa. J Chromatogr A. 2020;460948 doi: 10.1016/j.chroma.2020.460948. [DOI] [PubMed] [Google Scholar]
- 32.Orhan H., Uygun D.A. Immobilization of L-Asparaginase on Magnetic Nanoparticles for Cancer Treatment. Appl Biochem Biotechnol. 2020:1–12. doi: 10.1007/s12010-020-03276-z. [DOI] [PubMed] [Google Scholar]
- 33.Chauhan P.S., Kumarasamy M., Sosnik A., Danino D. Enhanced Thermostability and Anticancer Activity in Breast Cancer Cells of Laccase Immobilized on Pluronic-Stabilized Nanoparticles. ACS Appl Mater Interfaces. 2019;11(43):39436–39448. doi: 10.1021/acsami.9b11877. [DOI] [PubMed] [Google Scholar]
- 34.Silva E.R., Nicolini J., Yamauchi L., Jr, Machado T., Curi M., Furtado J. Carbon-based electrode loaded with Y-doped SrTiO3 perovskite as support for enzyme immobilization in biosensors. Ceram Int. 2020;46(3):3592–3599. [Google Scholar]
- 35.Wang Z., Jinlong L., An Z., Kimura M., Ono T. Enzyme immobilization in completely packaged freestanding SU-8 microfluidic channel by electro click chemistry for compact thermal biosensor. Process Biochem. 2019;79:57–64. [Google Scholar]
- 36.Poghossian A., Jablonski M., Koch C., Bronder T.S., Rolka D., Wege C. Field-effect biosensor using virus particles as scaffolds for enzyme immobilization. Biosens Bioelectron. 2018;110:168–174. doi: 10.1016/j.bios.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 37.Verma M.L., Kumar S., Das A., Randhawa J.S., Chamundeeswari M. Enzyme immobilization on chitin and chitosan-based supports for biotechnological applications, Sustainable Agriculture Reviews. Springer. 2019;35:147–173. [Google Scholar]
- 38.Levashov P., Matolygina D., Ovchinnikova E., Adamova I.Y., Dmitrieva O., Pokrovsky N. A novel method of covalent lysozyme immobilization for the development of materials for medical applications. Russ J Bioorg Chem. 2019;45(2):101–106. [Google Scholar]
- 39.Jamwal S., Ram B., Ranote S., Dharela R., Chauhan G.S. New glucose oxidase-immobilized stimuli-responsive dextran nanoparticles for insulin delivery. Int J Biol Macromol. 2019;123:968–978. doi: 10.1016/j.ijbiomac.2018.11.147. [DOI] [PubMed] [Google Scholar]
- 40.Farag A.M., Hassan S.W., Beltagy E.A., El-Shenawy M.A. Optimization of production of anti-tumor l-asparaginase by free and immobilized marine Aspergillus terreus. The Egyptian Journal of Aquatic Research. 2015;41(4):295–302. [Google Scholar]
- 41.Bai J., Peng C., Guo L., Zhou M. Metal-Organic Framework-Integrated Enzymes as Bioreactor for Enhanced Therapy against Solid Tumor via a Cascade Catalytic Reaction. ACS Biomater Sci Eng. 2019;5(11):6207–6215. doi: 10.1021/acsbiomaterials.9b01200. [DOI] [PubMed] [Google Scholar]
- 42.Costoya A., Becerra L.E.V., Meléndez-Ortiz H.I., Díaz-Gómez L., Mayer C., Otero A. Immobilization of antimicrobial and anti-quorum sensing enzymes onto GMA-grafted poly (vinyl chloride) catheters. Int J Pharm. 2019;558:72–81. doi: 10.1016/j.ijpharm.2018.12.075. [DOI] [PubMed] [Google Scholar]
- 43.Kim K.B., Choi H., Jung H.J., Oh Y.-J., Cho C.-H., Min J.H. Mussel-inspired enzyme immobilization and dual real-time compensation algorithms for durable and accurate continuous glucose monitoring. Biosens Bioelectron. 2019;143 doi: 10.1016/j.bios.2019.111622. 111622. [DOI] [PubMed] [Google Scholar]
- 44.Hung B.-Y., Kuthati Y., Kankala R.K., Kankala S., Deng J.-P., Liu C.-L. Utilization of enzyme-immobilized mesoporous silica nanocontainers (IBN-4) in prodrug-activated cancer theranostics. Nanomaterials. 2015;5(4):2169–2191. doi: 10.3390/nano5042169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang L., Liang B., Yang G., Hu Y., Zhu Q., Ye X. Study of glucose biosensor lifetime improvement in 37 C serum based on PANI enzyme immobilization and PLGA biodegradable membrane. Biosens Bioelectron. 2014;56:91–96. doi: 10.1016/j.bios.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Callmann C.E., Barback C.V., Thompson M.P., Hall D.J., Mattrey R.F., Gianneschi N.C. Therapeutic Enzyme-Responsive Nanoparticles for Targeted Delivery and Accumulation in Tumors. Adv Mater. 2015;27(31):4611–4615. doi: 10.1002/adma.201501803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cormode D.P., Gao L., Koo H. Emerging biomedical applications of enzyme-like catalytic nanomaterials. Trends Biotechnol. 2018;36(1):15–29. doi: 10.1016/j.tibtech.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mateo C., Palomo J.M., Fernandez-Lorente G., Guisan J.M., Fernandez-Lafuente R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol. 2007;40(6):1451–1463. [Google Scholar]
- 49.Lee D.-G., Ponvel K.M., Kim M., Hwang S., Ahn I.-S., Lee C.-H. Immobilization of lipase on hydrophobic nano-sized magnetite particles. J Mol Catal B Enzym. 2009;57(1–4):62–66. [Google Scholar]
- 50.Adeel M., Bilal M., Rasheed T., Sharma A., Iqbal H.M. Graphene and graphene oxide: functionalization and nano-bio-catalytic system for enzyme immobilization and biotechnological perspective. Int J Biol Macromol. 2018 doi: 10.1016/j.ijbiomac.2018.09.144. [DOI] [PubMed] [Google Scholar]
- 51.Kumar A., Park G.D., Patel S.K.S., Kondaveeti S., Otari S., Anwar M.Z. SiO2 microparticles with carbon nanotube-derived mesopores as an efficient support for enzyme immobilization. Chem Eng J. 2019;359:1252–1264. [Google Scholar]
- 52.Sharifi M., Faryabi K., Talaei A.J., Shekha M.S., Ale-Ebrahim M., Salihi A. Antioxidant properties of gold nanozyme: A review. J Mol Liq. 2020;297 112004. [Google Scholar]
- 53.Sharifi M., Hosseinali S.H., Yousefvand P., Salihi A., Shekha M.S., Aziz F.M. Gold nanozyme: biosensing and therapeutic activities. Mater Sci Eng, C. 2020;108 doi: 10.1016/j.msec.2019.110422. 110422. [DOI] [PubMed] [Google Scholar]
- 54.Khan S., Sharifi M., Bloukh S.H., Edis Z., Siddique R., Falahati M. In vivo guiding inorganic nanozymes for biosensing and therapeutic potential in cancer, inflammation and microbial infections. Talanta. 2020;121805 doi: 10.1016/j.talanta.2020.121805. [DOI] [PubMed] [Google Scholar]
- 55.Bosio V.E., Islan G.A., Martínez Y.N., Durán N., Castro G.R. Nanodevices for the immobilization of therapeutic enzymes. Crit Rev Biotechnol. 2016;36(3):447–464. doi: 10.3109/07388551.2014.990414. [DOI] [PubMed] [Google Scholar]
- 56.Liang J.F., Li Y.T., Yang V.C. Biomedical application of immobilized enzymes. J Pharm Sci. 2000;89(8):979–990. doi: 10.1002/1520-6017(200008)89:8<979::aid-jps2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 57.Yang L., Yan S., Zhang Y., Hu X., Guo Q., Yuan Y. Novel enzyme formulations for improved pharmacokinetic properties and anti-inflammatory efficacies. Int J Pharm. 2018;537(1–2):268–277. doi: 10.1016/j.ijpharm.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 58.Osathanon T., Giachelli C.M., Somerman M.J. Immobilization of alkaline phosphatase on microporous nanofibrous fibrin scaffolds for bone tissue engineering. Biomaterials. 2009;30(27):4513–4521. doi: 10.1016/j.biomaterials.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teixeira L.S.M., Feijen J., van Blitterswijk C.A., Dijkstra P.J., Karperien M. Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials. 2012;33(5):1281–1290. doi: 10.1016/j.biomaterials.2011.10.067. [DOI] [PubMed] [Google Scholar]
- 60.M. Sharifi, A.Y. Karim, N. Mustafa Qadir Nanakali, A. Salihi, F.M. Aziz, J. Hong, R.H. Khan, A.A. Saboury, A. Hasan, O.K. Abou-Zied, M. Falahati, Strategies of enzyme immobilization on nanomatrix supports and their intracellular delivery, Journal of Biomolecular Structure and Dynamics (2019) 1-17. [DOI] [PubMed]
- 61.Li Z., Zhang Y., Feng N. Mesoporous silica nanoparticles: synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert opinion on drug delivery. 2019;16(3):219–237. doi: 10.1080/17425247.2019.1575806. [DOI] [PubMed] [Google Scholar]
- 62.Peng Z., Liu X., Zhang W., Zeng Z., Liu Z., Zhang C. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ Int. 2020;134 doi: 10.1016/j.envint.2019.105298. 105298. [DOI] [PubMed] [Google Scholar]
- 63.Yuan X., Zhang X., Sun L., Wei Y., Wei X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part Fibre Toxicol. 2019;16(1):18. doi: 10.1186/s12989-019-0299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.S.A. Varghese, S.M. Rangappa, S. Siengchin, J. Parameswaranpillai, Chapter 2 - Natural polymers and the hydrogels prepared from them, in: Y. Chen (Ed.), Hydrogels Based on Natural Polymers, Elsevier2020, pp. 17-47.
- 65.Ruiz-Rubio L., Pérez-Álvarez L., Vilas-Vilela J.L. In: Shape Memory Polymers, Blends and Composites: Advances and Applications. Parameswaranpillai J., Siengchin S., George J.J., Jose S., editors. Springer Singapore; Singapore: 2020. Biodegradable Shape-Memory Polymers; pp. 219–236. [Google Scholar]
- 66.Zhang Y., Qi Y., Ulrich S., Barboiu M., Ramström O. Dynamic covalent polymers for biomedical applications, Materials Chemistry. Frontiers. 2020 doi: 10.1039/c9qm00598f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salvalaio M., Rigon L., Belletti D., D’Avanzo F., Pederzoli F., Ruozi B. Targeted Polymeric Nanoparticles for Brain Delivery of High Molecular Weight Molecules in Lysosomal Storage Disorders. PLoS ONE. 2016;11(5) doi: 10.1371/journal.pone.0156452. e0156452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song R., Murphy M., Li C., Ting K., Soo C., Zheng Z. Current development of biodegradable polymeric materials for biomedical applications. Drug design, development and therapy. 2018;12:3117–3145. doi: 10.2147/DDDT.S165440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McNamara K., Tofail S.A.M. Nanoparticles in biomedical applications. Advances in Physics: X. 2017;2(1):54–88. [Google Scholar]
- 70.Rizwan M., Ali S., Qayyum M.F., Ok Y.S., Adrees M., Ibrahim M. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: a critical review. J Hazard Mater. 2017;322:2–16. doi: 10.1016/j.jhazmat.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 71.Sharifi M., Rezayat S.M., Akhtari K., Hasan A., Falahati M. Fabrication and evaluation of anti-cancer efficacy of lactoferrin-coated maghemite and magnetite nanoparticles. J Biomol Struct Dyn. 2020;38(10):2945–2954. doi: 10.1080/07391102.2019.1650114. [DOI] [PubMed] [Google Scholar]
- 72.Sharifi M., Jafari S., Hasan A., Paray B.A., Gong G., Zheng Y. Antimetastatic Activity of Lactoferrin-Coated Mesoporous Maghemite Nanoparticles in Breast Cancer Enabled by Combination Therapy. ACS Biomater Sci Eng. 2020 doi: 10.1021/acsbiomaterials.0c00086. [DOI] [PubMed] [Google Scholar]
- 73.Khan S., Hasan A., Attar F., Sharifi M., Siddique R., Mraiche F. Gold Nanoparticle-Based Platforms for Diagnosis and Treatment of Myocardial Infarction. ACS Biomater Sci Eng. 2020 doi: 10.1021/acsbiomaterials.0c00955. [DOI] [PubMed] [Google Scholar]
- 74.Sharifi M., Hasan A., Nanakali N.M.Q., Salihi A., Qadir F.A., Muhammad H.A. Combined chemo-magnetic field-photothermal breast cancer therapy based on porous magnetite nanospheres. Sci Rep. 2020;10(1):1–15. doi: 10.1038/s41598-020-62429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verma N., Kumar N., Upadhyay L.S.B., Sahu R., Dutt A. Fabrication and Characterization of Cysteine-Functionalized Zinc Oxide Nanoparticles for Enzyme Immobilization. Anal Lett. 2017;50(11):1839–1850. [Google Scholar]
- 76.Andreescu S., Ornatska M., Erlichman J.S., Estevez A., Leiter J. Springer; 2012. Biomedical applications of metal oxide nanoparticles, Fine Particles in Medicine and Pharmacy; pp. 57–100. [Google Scholar]
- 77.Ding S., Cargill A.A., Medintz I.L., Claussen J.C. Increasing the activity of immobilized enzymes with nanoparticle conjugation. Curr Opin Biotechnol. 2015;34:242–250. doi: 10.1016/j.copbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 78.Rabbani G., Khan M.J., Ahmad A., Maskat M.Y., Khan R.H. Effect of copper oxide nanoparticles on the conformation and activity of β-galactosidase. Colloids Surf B. 2014;123:96–105. doi: 10.1016/j.colsurfb.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 79.Khan S., Sharifi M., Hasan A., Attar F., Edis Z., Bai Q. Magnetic nanocatalysts as multifunctional platforms in cancer therapy through the synthesis of anticancer drugs and facilitated Fenton reaction. J Adv Res. 2020 doi: 10.1016/j.jare.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Longmire M., Choyke P.L., Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond) 2008;3(5):703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao L., Zhuang J., Nie L., Zhang J., Zhang Y., Gu N. Intrinsic peroxidase like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2(9):577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 82.Chen W., Chen J., Liu A.L., Wang L.M., Li G.W., Lin X.H. Peroxidase-like activity of cupric oxide nanoparticle. ChemCatChem. 2011;3(7):1151–1154. [Google Scholar]
- 83.Dizaj S.M., Lotfipour F., Barzegar-Jalali M., Zarrintan M.H., Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng, C. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 84.Sharifi M., Hosseinali S.H., Saboury A.A., Szegezdi E., Falahati M. Involvement of planned cell death of necroptosis in cancer treatment by nanomaterials: Recent advances and future perspectives. J Control Release. 2019;299:121–137. doi: 10.1016/j.jconrel.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 85.Djurišić A.B., Leung Y.H., Ng A.M., Xu X.Y., Lee P.K., Degger N. Toxicity of metal oxide nanoparticles: mechanisms, characterization, and avoiding experimental artefacts. Small. 2015;11(1):26–44. doi: 10.1002/smll.201303947. [DOI] [PubMed] [Google Scholar]
- 86.Seabra A., Durán N. Nanotoxicology of metal oxide nanoparticles. Metals. 2015;5(2):934–975. [Google Scholar]
- 87.Savliwala S., Chiu-Lam A., Unni M., Rivera-Rodriguez A., Fuller E., Sen K. Elsevier; Nanoparticles for Biomedical Applications: 2020. Magnetic nanoparticles; pp. 195–221. [Google Scholar]
- 88.Sharifi M., Attar F., Saboury A.A., Akhtari K., Hooshmand N., Hasan A. Plasmonic gold nanoparticles: Optical manipulation, imaging, drug delivery and therapy. J Control Release. 2019;311–312:170–189. doi: 10.1016/j.jconrel.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 89.Piscitelli A., Pennacchio A., Longobardi S., Velotta R., Giardina P. Vmh2 hydrophobin as a tool for the development of “self-immobilizing” enzymes for biosensing. Biotechnol Bioeng. 2017;114(1):46–52. doi: 10.1002/bit.26049. [DOI] [PubMed] [Google Scholar]
- 90.Liu L., Qiao J., Zhang H., Qi L. Fabrication of a porous polymer membrane enzyme reactor and its enzymatic kinetics study in an artificial kidney model. Talanta. 2020;120963 doi: 10.1016/j.talanta.2020.120963. [DOI] [PubMed] [Google Scholar]
- 91.Li K., Fan Y., He Y., Zeng L., Han X., Yan Y. Burkholderia cepacia lipase immobilized on heterofunctional magnetic nanoparticles and its application in biodiesel synthesis. Sci Rep. 2017;7(1):16473. doi: 10.1038/s41598-017-16626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Y., Jing T., Xu G., Tian J., Dong M., Shao Q. 3-D magnetic graphene oxide-magnetite poly (vinyl alcohol) nanocomposite substrates for immobilizing enzyme. Polymer. 2018;149:13–22. [Google Scholar]
- 93.Chaves S., Pera L.M., Avila C.L., Romero C.M., Baigori M., Vieyra F.E.M. Towards efficient biocatalysts: photo-immobilization of a lipase on novel lysozyme amyloid-like nanofibrils. RSC Adv. 2016;6(11):8528–8538. [Google Scholar]
- 94.Torchilin V.P., Papisov M.I., Smirnov V.N. Magnetic sephadex as a carrier for enzyme immobilization and drug targeting. J Biomed Mater Res Part A. 1985;19(4):461–466. doi: 10.1002/jbm.820190410. [DOI] [PubMed] [Google Scholar]
- 95.Kempe H., Kempe M. The use of magnetite nanoparticles for implant-assisted magnetic drug targeting in thrombolytic therapy. Biomaterials. 2010;31(36):9499–9510. doi: 10.1016/j.biomaterials.2010.07.107. [DOI] [PubMed] [Google Scholar]
- 96.Kim J.Y., Kim J.K., Park J.S., Byun Y., Kim C.K. The use of PEGylated liposomes to prolong circulation lifetimes of tissue plasminogen activator. Biomaterials. 2009;30(2009):5751–5756. doi: 10.1016/j.biomaterials.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 97.Piras A.M., Chiellini F., Fiumi C., Bartoli C., Chiellini E., Fiorentino B. A new biocompatible nanoparticle delivery system for the release of fibrinolytic drugs. Int J Pharm. 2008;357(1–2):260–271. doi: 10.1016/j.ijpharm.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 98.Liu Y., Du J., Yan M., Lau M.Y., Hu J., Han H. Biomimetic enzyme nanocomplexes and their use as antidotes and preventive measures for alcohol intoxication. Nat Nanotechnol. 2013;8(2013):187–192. doi: 10.1038/nnano.2012.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gu X.B., Xu X.C., Zhu G.P., Liu S.Q., Chen L.Y., Li X.S. Tyrosinase immobilization on ZnO nanorods for phenol detection. J Phys Chem B. 2008;113(1):377–381. doi: 10.1021/jp808001c. [DOI] [PubMed] [Google Scholar]
- 100.Ozoner S.K., Yalvac M., Erhan E. Flow injection determination of catechol based on polypyrrole–carbon nanotube–tyrosinase biocomposite detector. Curr Appl Phys. 2010;10(1):323–328. [Google Scholar]
- 101.Sima V.H., Patris S., Aydogmus Z., Sarakbi A., Sandulescu R., Kauffmann J.M. Tyrosinase immobilized magnetic nanobeads for the amperometric assay of enzyme inhibitors: application to the skin whitening agents. Talanta. 2011;83(3):980–987. doi: 10.1016/j.talanta.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 102.Dincer A., Becerik S., Aydemir T. Immobilization of tyrosinase on chitosan–clay composite beads. Int J Biol Macromol. 2012;50(3):815–820. doi: 10.1016/j.ijbiomac.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y., Chang T.M.S. Nanobiotechnological nanocapsules containing polyhemoglobin-tyrosinase: effects on murine B16F10 melanoma cell proliferation and attachment. Journal of Skin Cancer. 2012;2012:1–9. doi: 10.1155/2012/673291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yun X., Maximov V.D., Yu J., Zhu H., Vertegel A.A., Kindy M.S. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J Cereb Blood Flow Metab. 2013;33(2013):583–592. doi: 10.1038/jcbfm.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hood E.D., Chorny M., Greineder C.F., Alferiev I.S., Levy R.J., Muzykantov V.R. Endothelial targeting of nanocarriers loaded with antioxidant enzymes for protection against vascular oxidative stress and inflammation. Biomaterials. 2014;35(11):3708–3715. doi: 10.1016/j.biomaterials.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martinez Y.N., Cavello I., Hours R., Sebastián C., Castro G.R. Immobilized keratinase and enrofloxacin loaded on pectin PVA cryogel patches for antimicrobial treatment. Bioresour Technol. 2013;145(2013):280–284. doi: 10.1016/j.biortech.2013.02.063. [DOI] [PubMed] [Google Scholar]
- 107.Law B., Weissleder R., Tung C.H. Peptide-based biomaterials for protease-enhanced drug delivery. Biomacromolecules. 2006;7(4):1261–1265. doi: 10.1021/bm050920f. [DOI] [PubMed] [Google Scholar]
- 108.Lee S.J., Jeong Y., Park H.K., Kang D.H., Oh J.C., Lee S.G. Enzyme-responsive doxorubicin release from dendrimer nanoparticles for anticancer drug delivery. Int J Nanomed. 2015;10(2015):5489–5503. doi: 10.2147/IJN.S87145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kang J.H., Asai D., Kim J.H., Mori T., Toita R., Tomiyama T. Design of polymeric carriers for cancer-specific gene targeting: Utilization of abnormal protein kinase Cα activation in cancer cells. J Am Chem Soc. 2008;130(45):14906–14907. doi: 10.1021/ja805364s. [DOI] [PubMed] [Google Scholar]
- 110.Vicent M.J., Greco F., Nicholson R.I., Paul A., Griffiths P.C., Duncan R. Polymer therapeutics designed for a combination therapy of hormone-dependent cancer. Angewandte Chemie International ed. in English. 2005;44(26):4061–4066. doi: 10.1002/anie.200462960. [DOI] [PubMed] [Google Scholar]
- 111.Singh N., Karambelkar A., Gu L., Lin K., Miller J.S., Chen C.S. Bioresponsive mesoporous silica nanoparticles for triggered drug release. J Am Chem Soc. 2011;133(49):19582–19585. doi: 10.1021/ja206998x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu Y., Ding X., Li J., Luo Z., Hu Y., Liu J. Enzyme responsive drug delivery system based on mesoporous silica nanoparticles for tumor therapy in vivo. Nanotechnology. 2015;26(14) doi: 10.1088/0957-4484/26/14/145102. 145102. [DOI] [PubMed] [Google Scholar]
- 113.Andresen T.L., Davidsen J., Begtrup M., Mouritsen O.G., Jørgensen K. Enzymatic release of antitumor ether lipids by specific phospholipase A2 activation of liposome-forming prodrugs. Journal of Medicicnal Chemistry. 2004;47(7):1694–1703. doi: 10.1021/jm031029r. [DOI] [PubMed] [Google Scholar]
- 114.Park C., Kim H., Kim S., Kim C. Enzyme responsive nanocontainerswith cyclodextrin gatekeepers and synergistic effects in release of guests. J Am Chem Soc. 2009;131(46):16614–16615. doi: 10.1021/ja9061085. [DOI] [PubMed] [Google Scholar]
- 115.Inaji H., Koyama H., Higashiyama M., Noguchi S., Yamamoto H., Ishikawa O. Immunohistochemical, ultrastructural and biochemical studies of an amylase-producing breast carcinoma. Virchows Archiv A. 1991;419(1):29–33. doi: 10.1007/BF01600149. [DOI] [PubMed] [Google Scholar]
- 116.Ferguson E.L., Duncan R. Dextrin-phospholipase A2: synthesis and evaluation as a bioresponsive anticancer conjugate. Biomacromolecules. 2009;10(6):1358–1364. doi: 10.1021/bm8013022. [DOI] [PubMed] [Google Scholar]
- 117.Akolpoglu M.B., Bozuyuk U., Erkoc P., Kizilel S. In: Inamuddin. Khan R., Mohammad A., Asiri A.M., editors. Elsevier; Advanced Biosensors for Health Care Applications: 2019. Chapter 9 - Biosensing-Drug Delivery Systems for In Vivo Applications; pp. 249–262. [Google Scholar]
- 118.M. Sharifi, A. Hasan, A. Taghizadeh, S. Haghighat, F. Attar, S.H. Bloukh, Z. Edis, M. Xue, S. Khan, M. Falahati, Rapid diagnostics of coronavirus disease 2019 in early stages using nanobiosensors: Challenges and opportunities, Talanta (2021) 121704. [DOI] [PMC free article] [PubMed]
- 119.Zhao L., Wang L., Zhang Y., Xiao S., Bi F., Zhao J. Glucose Oxidase-Based Glucose-Sensitive Drug Delivery for Diabetes Treatment. Polymers. 2017;9(7):255. doi: 10.3390/polym9070255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen X., Wu W., Guo Z., Xin J., Li J. Controlled insulin release from glucose-sensitive self-assembled multilayer films based on 21-arm star polymer. Biomaterials. 2011;32(6):1759–1766. doi: 10.1016/j.biomaterials.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 121.Luo J., Cao S., Chen X., Liu S., Tan H., Wu W. Super long-term glycemic control in diabetic rats by glucose-sensitive LbL films constructed of supramolecular insulin assembly. Biomaterials. 2012;33(33):8733–8742. doi: 10.1016/j.biomaterials.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 122.Chu M.K.L., Chen J., Gordijo C.R., Chiang S., Ivovic A., Koulajian K. In vitro and in vivo testing of glucose-responsive insulin-delivery microdevices in diabetic rats. Lab Chip. 2012;12(14):2533–2539. doi: 10.1039/c2lc40139h. [DOI] [PubMed] [Google Scholar]
- 123.Gu Z., Aimetti A.A., Wang Q., Dang T.T., Zhang Y., Veiseh O. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 2013;7(5):4194–4201. doi: 10.1021/nn400630x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hu X., Yu J., Qian C., Lu Y., Kahkoska A.R., Xie Z. H(2)O(2)-Responsive Vesicles Integrated with Transcutaneous Patches for Glucose-Mediated Insulin Delivery. ACS Nano. 2017;11(1):613–620. doi: 10.1021/acsnano.6b06892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mohammadpour F., Hadizadeh F., Tafaghodi M., Sadri K., Mohammadpour A.H., Kalani M.R. Preparation, in vitro and in vivo evaluation of PLGA/Chitosan based nano-complex as a novel insulin delivery formulation. Int J Pharm. 2019;572 doi: 10.1016/j.ijpharm.2019.118710. 118710. [DOI] [PubMed] [Google Scholar]
- 126.Volpatti L.R., Matranga M.A., Cortinas A.B., Delcassian D., Daniel K.B., Langer R. Glucose-Responsive Nanoparticles for Rapid and Extended Self-Regulated Insulin Delivery. ACS Nano. 2019 doi: 10.1021/acsnano.9b06395. [DOI] [PubMed] [Google Scholar]
- 127.Wang J., Wang Z., Yu J., Kahkoska A.R., Buse J.B., Gu Z. Glucose-Responsive Insulin and Delivery Systems: Innovation and Translation. Adv Mater. 2019 doi: 10.1002/adma.201902004. 1902004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jimenez-Falcao S., De Luis B., García-Fernández A., Llopis-Lorente A., Diez P., Sánchez A. Glucose-Responsive Enzyme-Controlled Mesoporous Nanomachine with a Layer-by-Layer Supramolecular Architecture. ACS Applied Bio Materials. 2019;2(8):3321–3328. doi: 10.1021/acsabm.9b00338. [DOI] [PubMed] [Google Scholar]
- 129.Hei M., Wu H., Fu Y., Xu Y., Zhu W. Phenylboronic acid functionalized silica nanoparticles with enlarged ordered mesopores for efficient insulin loading and controlled release. J Drug Delivery Sci Technol. 2019;51:320–326. [Google Scholar]
- 130.Jiang G., Xu B., Zhu J., Zhang Y., Liu T., Song G. Polymer microneedles integrated with glucose-responsive mesoporous bioactive glass nanoparticles for transdermal delivery of insulin. Biomed Phys Eng Express. 2019;5(4) 045038. [Google Scholar]
- 131.Sapountzi E., Braiek M., Vocanson F., Chateaux J.F., Jaffrezic-Renault N., Lagarde F. Gold nanoparticles assembly on electrospun poly(vinylalcohol)/poly(ethyleneimine)/glucose oxidase nanofibers for ultra sensitive electrochemical glucose biosensing. Sens Actuators, B. 2017;238(2017):392–401. [Google Scholar]
- 132.Verma M.L. In: Nanoscience in Food and Agriculture 4. Ranjan S., Dasgupta N., Lichtfouse E., editors. Springer International Publishing; Cham: 2017. Enzymatic Nanobiosensors in the Agricultural and Food Industry; pp. 229–245. [Google Scholar]
- 133.Martín-Barreiro A., de Marcos S., Jesús M., Grazú V., Galbán J. Gold nanocluster fluorescence as an indicator for optical enzymatic nanobiosensors: choline and acetylcholine determination. Sens Actuators, B. 2018;277:261–270. [Google Scholar]
- 134.Chen L., Hwang E., Zhang J. Fluorescent Nanobiosensors for Sensing Glucose. Sensors. 2018;18(5):1440. doi: 10.3390/s18051440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Adhikary T., Nanda A., Thangapandi K., Roy S., Kumar S. Microbial Fermentation and Enzyme Technology; 2020. Trends in Biosensors and Role of Enzymes as Their Sensing Element for Healthcare Applications. [Google Scholar]
- 136.Ornatska M., Sharpe E., Andreescu D., Andreescu S. Paper bioassay based on ceria nanoparticles as colorimetric probes. Anal Chem. 2011;83(2011):4273–4280. doi: 10.1021/ac200697y. [DOI] [PubMed] [Google Scholar]
- 137.Cho E., Mohammadifar M., Choi S. A single-use, self-powered, paper-based sensor patch for detection of exercise-induced hypoglycemia. Micromachines. 2017;8(2017):265–266. doi: 10.3390/mi8090265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nantaphol S., Chailapakul O., Siangproh W. A novel paper-based device coupled with a silver nanoparticle-modified boron-doped diamond electrode for cholesterol detection. Anal Chim Acta. 2015;891(2015):136–143. doi: 10.1016/j.aca.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 139.Panraksa Y., Siangproh W., Khampieng T., Chailapakul O., Apilux A. Paper-based amperometric sensor for determination of acetylcholinesterase using screen-printed graphene electrode. Talanta. 2018;178(2018):1017–1023. doi: 10.1016/j.talanta.2017.08.096. [DOI] [PubMed] [Google Scholar]
- 140.Chai P.R. Wearable devices and biosensing: Future frontiers. J Med Toxicol. 2016;12(4):332–334. doi: 10.1007/s13181-016-0569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.H. Kudo, T. Sawada, E. Kazawa, H. Yoshida, Y. Iwasaki, K. Mitsubayashi, A flexible and wearable glucose sensor based on functional polymers with Soft-MEMS techniques, biosensors and Bioelectronic 22(4) (2006) 558-562. [DOI] [PubMed]
- 142.Iguchi S., Kudo H., Saito T., Ogawa M., Saito H., Otsuka K. A flexible and wearable biosensor for tear glucose measurement. Biomed Microdevices. 2007;9(2007):603–609. doi: 10.1007/s10544-007-9073-3. [DOI] [PubMed] [Google Scholar]
- 143.H. Yao, A.J. Shum, M. Cowan, I. La, B.A. Parviz, A contact lens with embedded sensor for monitoring tear glucose level, biosensors and Bioelectronic 26(7) (2011) 3290-3296. [DOI] [PMC free article] [PubMed]
- 144.Jia W., Bandodkar A.J., Valdes-Ramırez G., Windmiller J.R., Yang Z., Ramırez J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal Chem. 2013;14(2013):6553–6560. doi: 10.1021/ac401573r. [DOI] [PubMed] [Google Scholar]
- 145.Kim J., Jeerapan I., Imani S., Cho T.N., Bandodkar A., Cinti S. Noninvasive alcohol monitoring using a wearable tattoo-based iontophoretic-biosensing system. ACS Sensors. 2016;1(8):1011–1019. [Google Scholar]
- 146.Chikkaveeraiah B.V., Liu H.Y., Mani V., Papadimitrakopoulos F., Rusling J.F. A microfluidic electrochemical device for high sensitivity biosensing: detection of nanomolar hydrogen peroxide Electrochemical. Communications. 2009;11(4):819–822. doi: 10.1016/j.elecom.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wisitsoraat A., Sritongkham P., Karuwan C., Phokharatkul D., Maturos T., Tuantranont A. Fast cholesterol detection using flow injection microfluidic device with functionalized carbon nanotubes based electrochemical sensor biosensors and Bioelectronic. 2010;26(4):1514–1520. doi: 10.1016/j.bios.2010.07.101. [DOI] [PubMed] [Google Scholar]
- 148.Koh A., Kang D., Xue Y., Lee S., Pielak R.M., Kim J. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci Transl Med. 2016;8(366) doi: 10.1126/scitranslmed.aaf2593. 366ra165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Backer M., Rakowski D., Poghossian A., Biselli M., Wagner P., Schoning M.J. Chip-based amperometric enzyme sensor system for monitoring of bioprocesses by flow-injection analysis. J Biotechnol. 2013;163(4):371–376. doi: 10.1016/j.jbiotec.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 150.Ali M.A., Srivastava S., Solanki P.R., Reddy V., Agrawal V.V., Kim C. Highly efficient bienzyme functionalized nanocomposite-based microfluidics biosensor platform for biomedical application. Sci Rep. 2013;3(2013):2661. doi: 10.1038/srep02661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Amirbandeh M., Taheri-Kafrani A. Immobilization of glucoamylase on triazine-functionalized Fe3O4/graphene oxide nanocomposite: Improved stability and reusability. Int J Biol Macromol. 2016;93:1183–1191. doi: 10.1016/j.ijbiomac.2016.09.092. [DOI] [PubMed] [Google Scholar]
- 152.Riccardi C.M., Kasi R.M., Kumar C.V. Nanoarmoring of enzymes by interlocking in cellulose fibers with poly (acrylic acid) Methods in enzymology, Elsevier. 2017:475–500. doi: 10.1016/bs.mie.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 153.Pervez S., Aman A., Qader S.A.U. Role of two polysaccharide matrices on activity, stability and recycling efficiency of immobilized fungal amyloglucosidase of GH15 family. Int J Biol Macromol. 2017;96:70–77. doi: 10.1016/j.ijbiomac.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 154.Sun H., Jin X., Long N., Zhang R. Improved biodegradation of synthetic azo dye by horseradish peroxidase cross-linked on nano-composite support. Int J Biol Macromol. 2017;95:1049–1055. doi: 10.1016/j.ijbiomac.2016.10.093. [DOI] [PubMed] [Google Scholar]
- 155.Jędrzak A., Rębiś T., Klapiszewski Ł., Zdarta J., Milczarek G., Jesionowski T. Carbon paste electrode based on functional GOx/silica-lignin system to prepare an amperometric glucose biosensor. Sens Actuators, B. 2018;256:176–185. [Google Scholar]
- 156.Soltys P.J., Mullon C., Langer R. Oral treatment for jaundice using immobilized bilirubin oxidase. Artificial Organs Banner. 1992;16(4):331–335. doi: 10.1111/j.1525-1594.1992.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 157.Hegedus I., Nagy E. Improvement of chymotrypsin enzyme stability as single enzyme nanoparticles. Chem Eng Sci. 2009;64(5):1053–1060. [Google Scholar]
- 158.Ren L., Wang X., Wu H., Shang B., Wang J. Conjugation of nattokinase and lumbrukinase with magnetic nanoparticles for the assay of their thrombolytic activities. J Mol Catal B Enzym. 2010;62(2):190–196. [Google Scholar]
- 159.Medeiros S., Santos A., Fessi H., Elaissari A. Stimuli-responsive magnetic particles for biomedical applications. Int J Pharm. 2011;403(1–2):139–161. doi: 10.1016/j.ijpharm.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 160.Corchero J.L., Mendoza R., Ferrer-Miralles N., Montràs A., Martínez L.M., Villaverde A. Enzymatic characterization of highly stable human alpha-galactosidase A displayed on magnetic particles. Biochem Eng J. 2012;67(15):20–27. [Google Scholar]
- 161.Huang W., Li X., Xue Y., Huang R., Denga H.B., Mad Z.C. Antibacterial multilayer films fabricated by LBL immobilizing lysozyme and HTCC on nanofibrous mats. Int J Biol Macromol. 2013;53(2013):26–31. doi: 10.1016/j.ijbiomac.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 162.Wang R., Zhang Y., Lu D., Ge J., Liu Z., Zare R.N. Functional protein-organic/inorganic hybrid nanomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(4):320–328. doi: 10.1002/wnan.1210. [DOI] [PubMed] [Google Scholar]
- 163.Cumana S., Simonsen J., Liese A., Hilterhaus L., Smirnova I. Immobilization of glucose-6-phosphate dehydrogenase in silica-based hydrogels: a comparative study. J Mol Catal B Enzym. 2013;85–86(2013):220–228. [Google Scholar]
- 164.Lin T., Zhong L., Song Z., Guo L., Wu H., Guo Q. Visual detection of blood glucose based on peroxidase-like activity of WS2 nanosheets. Biosensors and Bioelectronic. 2014;62(2014):302–307. doi: 10.1016/j.bios.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 165.L. Su, W. Qin, H. Zhang, Z.U. Rahman, C. Ren, S. Ma, X. Chen, The peroxidase/catalase-like activities of MFe2O4 (M= Mg, Ni, Cu) MNPs and their application in colorimetric biosensing of glucose, biosensors and Bioelectronic 63(2015) (2015) 384-391. [DOI] [PubMed]
- 166.Liang P., Yu H., Guntupalli B., Xiao Y. Based device for rapid visualization of NADH based on dissolution of gold nanoparticles. ACS Appl Mater Interfaces. 2015;7(27):15023–15030. doi: 10.1021/acsami.5b04104. [DOI] [PubMed] [Google Scholar]
- 167.Y. He, S. Zhang, X. Zhang, M. Baloda, A.S. Gurung, H. Xu, X. Zhang, G. Liu, Ultrasensitive nucleic acid biosensor based on enzyme–gold nanoparticle dual label and lateral flow strip biosensor, biosensors and Bioelectronic 26(5) (2011) 2018-2024. [DOI] [PubMed]
- 168.Luckham R.E., Brennan J.D. Bioactive paper dipstick sensors for acetylcholinesterase inhibitors based on sol–gel/enzyme/gold nanoparticle composites. Analyst. 2010;135(2010):2028–2035. doi: 10.1039/c0an00283f. [DOI] [PubMed] [Google Scholar]
- 169.Du D., Wang J., Wang L., Lu D., Lin Y. Integrated lateral flow test strip with electrochemical sensor for quantification of phosphorylated cholinesterase: biomarker of exposure to organophosphorus agents. Analytic chemistry. 2012;84(3):1380–1385. doi: 10.1021/ac202391w. [DOI] [PubMed] [Google Scholar]