Abstract
Aim
We developed tailored axillary surgery (TAS) to reduce the axillary tumor volume in patients with clinically node-positive breast cancer to the point where radiotherapy can control it. The aim of this study was to quantify the extent of tumor load reduction achieved by TAS.
Methods
International multicenter prospective study embedded in a randomized trial. TAS is a novel pragmatic concept for axillary surgery de-escalation that combines palpation-guided removal of suspicious nodes with the sentinel procedure and, optionally, imaging-guided localization. Pre-specified study endpoints quantified surgical extent and reduction of tumor load.
Results
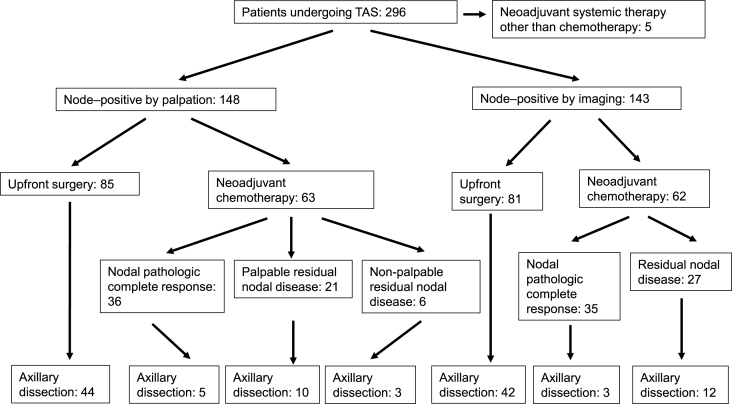
A total of 296 patients were included at 28 sites in four European countries, 125 (42.2%) of whom underwent neoadjuvant chemotherapy (NACT) and 71 (24.0%) achieved nodal pathologic complete response. Axillary metastases were detectable only by imaging in 145 (49.0%) patients. They were palpable in 151 (51.0%) patients, of whom 63 underwent NACT and 21 had residual palpable disease after NACT. TAS removed the biopsied and clipped node in 279 (94.3%) patients. In 225 patients with nodal disease at the time of surgery, TAS removed a median of five (IQR 3–7) nodes, two (IQR 1–4) of which were positive. Of these 225 patients, 100 underwent ALND after TAS, which removed a median of 14 (IQR 10–17) additional nodes and revealed additional positive nodes in 70/100 (70%) of patients. False-negative rate of TAS in patients who underwent subsequent ALND was 2.6%.
Conclusions
TAS selectively reduced the tumor load in the axilla and remained much less radical than ALND.
Keywords: Breast cancer, Breast surgery, Axillary dissection, Sentinel lymph node procedure, Axillary staging
Highlights
-
•
Tailored axillary surgery is a novel concept for clinically node-positive breast cancer
-
•
Tailored axillary surgery selectively removes positive lymph nodes
-
•
Tailored axillary surgery is much less radical than axillary dissection
-
•
Tailored axillary surgery removes the clipped node in the vast majority of patients
1. Introduction
Over the last decade, axillary surgery has been de-escalated in selected clinically node-negative patients with positive sentinel lymph nodes (SLNs) [[1], [2], [3], [4], [5], [6]]. In recent years, this trend also involved clinically node-positive patients with nodal pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT) [[7], [8], [9], [10], [11], [12]]. Axillary lymph node dissection (ALND) remains standard of care in the upfront surgery setting in most patients with palpable nodal disease. Patients with non-palpable axillary disease detected by preoperative imaging were eligible for the axillary surgery de-escalation landmark trials ACOSOG Z0011 and EORTC-AMAROS [1,2]. However, a series of observational studies consistently showed that patients with imaging-detected and biopsy-confirmed metastases have a higher burden of nodal involvement than patients with SLN-detected metastases, thereby questioning the routine omission of ALND in these patients [[13], [14], [15], [16], [17], [18], [19]]. Moreover, ALND is indicated in most patients with residual disease after NACT [20].
We developed a novel approach called tailored axillary surgery (TAS) for patients with clinically node-positive breast cancer during upfront surgery and after NACT. The concept of TAS is to turn a clinically positive axilla into clinically negative primarily by palpation-guided selective removal of obvious nodal disease, thereby tailoring the extent of axillary surgery to the extent of axillary disease. The concept also includes the sentinel lymph node (SLN) procedure to reduce the volume of microscopic disease. The aim of TAS is to decrease the tumor load in the axilla to the point where adjuvant regional nodal irradiation (RNI) can control it. The ongoing international TAXIS trial (SAKK 23/16/IBCSG 57-18/ABCSG-53/GBG 101; ClinicalTrials.gov Identifier: NCT03513614, see supplementary protocol) [21] will determine if TAS in combination with RNI is oncologically non-inferior and associated with improved quality of life (QoL) compared to ALND. Most surgeons consider it impossible to determine positive lymph nodes by clinical palpation alone, particularly in the neoadjuvant setting. Therefore, we pre-specified the present subproject during early stage of patient accrual in TAXIS [21] to study the difference in surgical extent between TAS and ALND and to quantify the extent of tumor load reduction by TAS.
2. Materials and methods
2.1. Study design and patient population
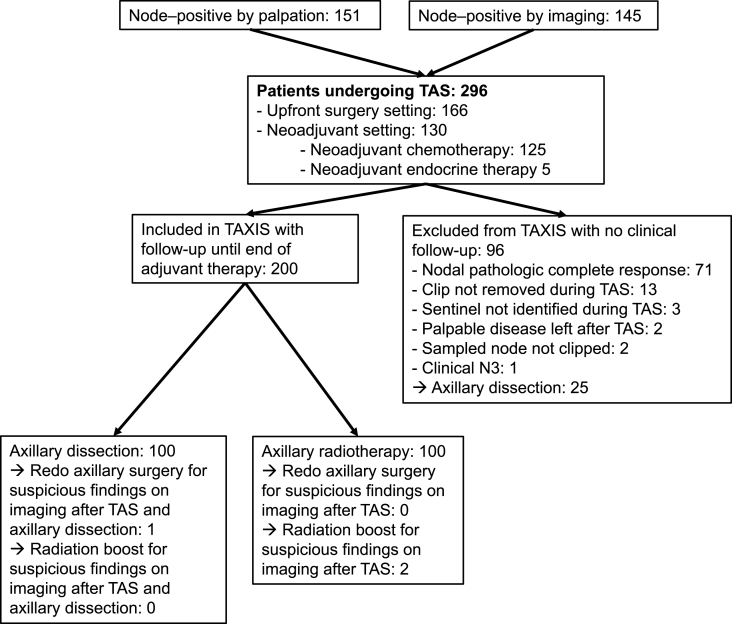
This was an international multicenter prospective study embedded in the randomized TAXIS [21] trial. Patients with clinically node-positive breast cancer were included, defined as nodal disease detected by palpation or imaging at the time of initial diagnosis. Histologic or cytologic confirmation of breast cancer was required both in primary tumor and lymph node. Patients were included in both the upfront surgery setting and in case of residual nodal disease after NACT. Patients with stage IV, cN3c or cN2b breast cancer, contralateral or other tumor malignancy within 3 years, prior axillary surgery (except SLN) or prior axillary radiotherapy were considered ineligible. The patient population included the first 200 consecutive TAXIS patients and all patients (n = 96) that were screening failures during the same period due to a) absence of clip in specimen radiography, b) palpable disease left behind in the axilla after TAS, c) failure to identify the SLN, and d) nodal pCR after NACT (see appendices, page 30, Figure A1: Pre-specified prospective study population embedded in TAXIS trial). These 296 patients were treated between August 07, 2018, and April 02, 2020.
The TAXIS trial and the present prospective substudy were approved by the local ethics committees and were performed in accordance with the requirements of the national regulatory authorities. Written informed consent was obtained from all patients.
2.2. Surgical management
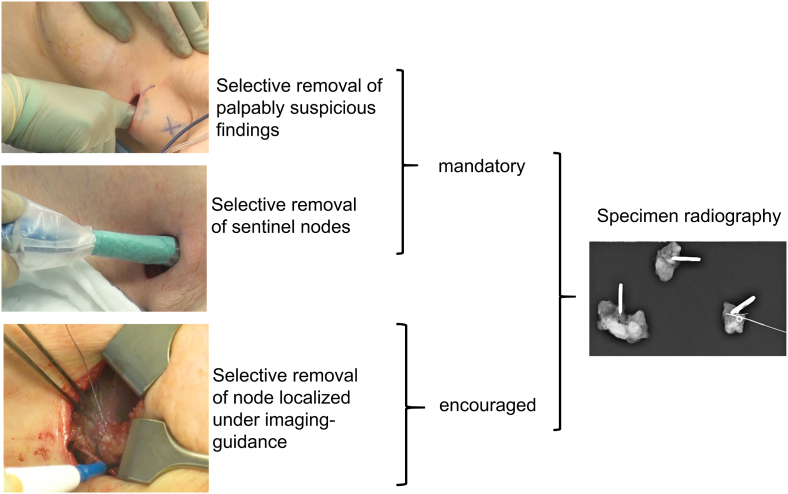
The initially sampled and histologically or cytologically positive node was marked with a clip. TAS was defined by palpation-guided selective removal of presumed nodal disease in combination with the SLN procedure, while the sequence of the individual steps was left to the surgeon's discretion. Imaging-guided localization of the clipped node and other suspicious nodes to facilitate surgical removal is conceptually encouraged in TAS, but not mandatory (Fig. 1).
Fig. 1.
The concept of tailored axillary surgery (TAS).
TAS was designed to turn a clinically node-positive axilla into clinically negative by removing all palpably obvious disease. Microscopic tumor volume is further reduced in the axilla by identifying and removing all lymph nodes with tracer uptake. We acknowledged that TAS cannot reflect a procedure as standardized as ALND, because it was designed to de-escalate axillary surgery in a personalized fashion. Therefore, we expected that the pragmatic concept of TAS will result in inter-surgeon variability. This sub-study was pre-specified to evaluate the translation of this concept into practice.
The SLN technique was left to the discretion of the operating surgeon, while dual mapping was recommended. ALND primarily cleared levels I and II. A full level III dissection was carried out only when there was gross nodal disease detected by palpation or imaging.
2.3. Pathologic and radiologic evaluation
Pathologic evaluation was not centralized and performed according to the lymph node processing protocol at the local pathology department. Nodal pathologic complete response was defined as absence of any nodal disease after NACT, including isolated tumor cells, which, however, were classified as ypN0 (i+) according to TNM staging in the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, eighth edition [22]. Specimen radiography was performed on all removed lymph nodes to document removal of the clip during surgery. Systemic radiologic staging was performed within two months before registration. Repeat staging after NACT was optional. Residual suspicious lymph nodes detected by imaging performed for radiotherapy treatment planning or re-staging before the end of adjuvant treatment neither demanded nor prohibited take back surgery for completion ALND or selective removal of these nodes or an additional radiotherapy boost.
3. Aims
The primary aim of the present study was to quantify the extent of residual disease after TAS. Therefore, we registered a) number of positive nodes removed by TAS, b) number of positive nodes removed by ALND after TAS, c) number of negative nodes removed by TAS, d) number of negative nodes removed by ALND after TAS, e) number of failed identification and removal of SLNs, f) number of patients taken back for surgery before start of radiotherapy for residual disease suspected by imaging, and g) number of patients receiving an extra radiation boost for residual disease suspected by imaging before end of adjuvant treatment. The following performance characteristics were added post-hoc: False-negative rate (FNR) was calculated as the number of patients with negative nodes during TAS who were found to have positive nodes by subsequent ALND, divided by the total number of patients with positive nodes detected by ALND and/or TAS. Negative predictive value (NPV) was defined as the number of true negative cases for TAS, divided by the total number of all pathologically negative cases detected by TAS. Diagnostic accuracy (DA) was defined as the number of true positive plus the number of true negative cases, divided by all cases.
3.1. Statistical analysis
This analysis includes the first 200 TAXIS patients as well as the 96 patients pre-registered but not randomized in TAXIS during this time period. Continuous endpoints were summarized using median and interquartile range (IQR). Categorical endpoints were summarized using frequency counts and percentages and compared between subgroups of interest using Fisher's exact test. Two-tailed tests with a significance level of 0.05 were used. No adjustment was made for multiple testing and all analyses are considered exploratory. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
4. Results
A total of 296 patients with a median age of 57 years (range: 25–88 years) were included at 28 breast centers in Switzerland, Hungary, Germany and Austria (Table 1).
Table 1.
Patient and tumor characteristics.
| Variable | No. (%) |
|---|---|
| No. of patients | 296 (100.0%) |
| Age, years | |
| Median (IQR) | 57 (46, 68.5) |
| Age, years | |
| ≤50 | 112 (37.8%) |
| >50 | 184 (62.2%) |
| Neoadjuvant chemotherapy | |
| No | 170 (57.4%) |
| Yes | 125 (42.2%) |
| Unknown | 1 (0.3%) |
| Nodal pCR rate after neoadjuvant therapy | 71 (24.0%) |
| Type of breast surgery | |
| Breast conserving | 179 (60.5%) |
| Mastectomy + - reconstruction | 116 (39.2) |
| Nonea | 1 (0.3%) |
| Tumor size at initial diagnosis, mm | |
| Median (IQR) | 28 (20, 40) |
| Clinical T stage at initial diagnosis | |
| T0 | 1 (0.3%) |
| T1 | 67 (22.6%) |
| T2 | 189 (63.9%) |
| T3 | 28 (9.5%) |
| T4 | 9 (3.0%) |
| Tis (DCIS) | 1 (0.3%) |
| Tx | 1 (0.3%) |
| Clinical N stage at initial diagnosis | |
| N1 by palpation | 137 (46.3%) |
| N1 by imaging | 136 (45.9%) |
| N2/3 | 23 (7.8%) |
| Postoperative N stage | |
| pN0 | 5 (1.7%) |
| pN1mi | 0 (0.0%) |
| pN1 | 102 (34.5%) |
| pN2 | 39 (13.2%) |
| pN3 | 20 (6.8%) |
| ypN0 | 71 (24.0%) |
| ypN0(ITC) | 2 (0.7%) |
| ypN1 | 46 (15.5%) |
| ypN2 | 9 (3.0%) |
| ypN3 | 2 (0.7%) |
| Histology | |
| Invasive ductal | 223 (75.3%) |
| Lobular | 27 (9.1%) |
| Other | 46 (15.5%) |
| Receptor status at initial diagnosis | |
| HR+/Her2- | 187 (63.2%) |
| HR+/Her2+ | 42 (14.2%) |
| HR-/Her2+ | 16 (5.4%) |
| HR-/Her2- | 35 (11.8%) |
| Missing/unknown | 16 (5.4%) |
| LVI | |
| Yes | 147 (49.7%) |
| No | 138 (46.6%) |
| Missing/unknown | 11 (3.7%) |
| Modified Bloom-Richardson score | |
| I | 11 (3.7%) |
| II | 162 (54.7%) |
| III | 116 (39.2%) |
| Missing/unknown | 7 (2.4%) |
Abbreviations: IQR, interquartile range; pCR, pathologic complete response; NST, no special type; LVI, lymphovascular invasion.
Primary tumor was inoperable and surgical treatment consisted exclusively of axillary surgery.
At time of initial diagnosis, lymph node metastases were palpable in 151 (51.0%) and detectable only by imaging in 145 (49.0%) patients (Fig. 2 and appendices, page 31, Table A1: Patient and tumor characteristics by palpable versus non-palpable nodal disease).
Fig. 2.
Study population by palpable versus non-palpable axillary disease.
According to the preferences of the treating physicians and institutions, 125 (42.2%) underwent NACT, of whom 71 (24.0%) achieved nodal pCR. The median age of patients who underwent NACT was 50 years (interquartile range [IQR] 44–58) compared to 61 years (IQR 50–73) in the upfront surgery setting (p < 0.001). In addition, patients undergoing NACT had higher clinical nodal stage at initial diagnosis, more Her-2 positive and triple negative disease, and higher tumor grade (all p < 0.001, Table 2).
Table 2.
Patient and tumor characteristics by use of neoadjuvant chemotherapy versus upfront surgery.
| Variable | Neoadjuvant therapy (N = 125) |
Upfront Surgery (N = 166) |
p-value |
||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Node positivity (categorized) | 0.89 | ||||
| Node-positivity detected by imaging and non-palpable ( | 62 | (49.6%) | 81 | (48.8%) | |
| Node-positivity palpable (cN1-3) | 63 | (50.4%) | 85 | (51.2%) | |
| Clinical N stage at initial diagnosis | 0.001 | ||||
| N1 by imaging | 56 | (44.8%) | 78 | (47.0%) | |
| N1 by palpation | 51 | (40.8%) | 83 | (50.0%) | |
| N2/3 | 18 | (14.4%) | 5 | (3.0%) | |
| Clinical T stage | 0.36 | ||||
| cT0 | 1 | (0.8%) | 0 | (0.0%) | |
| cT1b | 6 | (4.8%) | 5 | (3.0%) | |
| cT1c | 25 | (20.0%) | 30 | (18.1%) | |
| cT2 | 74 | (59.2%) | 112 | (67.5%) | |
| cT3 | 16 | (12.8%) | 11 | (6.6%) | |
| cT4a | 1 | (0.8%) | 0 | (0.0%) | |
| cT4b | 2 | (1.6%) | 5 | (3.0%) | |
| cT4c | 0 | (0.0%) | 1 | (0.6%) | |
| cTis (DCIS) | 0 | (0.0%) | 1 | (0.6%) | |
| cTx | 0 | (0.0%) | 1 | (0.6%) | |
| Postoperative N stage | <.001 | ||||
| pN0 | 0 | (0.0%) | 5 | (3.0%) | |
| pN1 | 0 | (0.0%) | 102 | (61.4%) | |
| pN2 | 0 | (0.0%) | 39 | (23.5%) | |
| pN3 | 0 | (0.0%) | 20 | (12.0%) | |
| ypN0 | 71 | (56.8%) | 0 | (0.0%) | |
| ypN0 (i+) | 2 | (1.6%) | 0 | (0.0%) | |
| ypN1 | 42 | (33.6%) | 0 | (0.0%) | |
| ypN2 | 8 | (6.4%) | 0 | (0.0%) | |
| ypN3 | 2 | (1.6%) | 0 | (0.0%) | |
| Tumor type | 0.07 | ||||
| Invasive ductal | 96 | (76.8%) | 123 | (74.1%) | |
| Invasive lobular | 6 | (4.8%) | 20 | (12.0%) | |
| Other | 23 | (18.4%) | 23 | (13.9%) | |
| Tumor receptor subtype | <.001 | ||||
| HR+/HER2+ | 33 | (26.4%) | 8 | (4.8%) | |
| HR+/HER2- | 44 | (35.2%) | 141 | (84.9%) | |
| HR-/HER2+ | 13 | (10.4%) | 2 | (1.2%) | |
| HR-/HER2- | 30 | (24.0%) | 5 | (3.0%) | |
| Missing | 5 | (4.0%) | 9 | (5.4%) | |
| Unknown | 0 | (0.0%) | 1 | (0.6%) | |
| Tumor grade | <.001 | ||||
| G1 | 3 | (2.4%) | 8 | (4.8%) | |
| G2 | 51 | (40.8%) | 107 | (64.5%) | |
| G3 | 65 | (52.0%) | 50 | (30.1%) | |
| Unknown | 6 | (4.8%) | 1 | (0.6%) | |
Note: Five patients who received neoadjuvant therapy other than chemotherapy are not shown here.
TAS successfully removed the clipped node in 279 (94.3%) patients. The clipped node corresponded to a node with SLN tracer uptake in 197 (66.6%) of patients. It was localized under imaging-guidance in 183 (61.8%) and was considered palpably obviously suspicious by surgeons in 139 (47%). In the entire patient population that included patients with nodal pCR, the median number of lymph nodes removed by TAS was four (IQR 3–7), one (IQR 0–3) of which was positive (see appendices, page 33, Table A2: Characteristics of tailored axillary surgery and axillary lymph node dissection). Surgeons estimated to have removed and labeled a median of 2 (IQR 1–3) nodes with SLN tracer uptake that corresponded to a median of 3 (IQR 2–4) nodes when counted by the pathologists, one of which was positive (IQR 0–2). Presumed palpable disease could not be removed by TAS in two (0.7%) patients. Three patients (1.0%) had no tracer uptake in palpable and non-palpable nodes.
FNR of TAS in patients who underwent subsequent ALND was 1.8%, NPV was 95.5%, and DA was 98.3%. Clip removal rate, FNR, NPV and DA for the overall cohort and by upfront surgery versus NACT are shown in Table 3.
Table 3.
Clip removal rate, FNR, NPV and DA for the overall cohort and by upfront surgery versus NACT.
| Overall | Upfront Surgery | Neoadjuvant chemotherapy | |
|---|---|---|---|
| Clip removal rate | 94.3% | 96.4% | 91.2% |
| FNR | 1.8% | 2.4% | 0% |
| NPV | 95.5% | 92.3% | 100% |
| DA | 98.3% | 97.6% | 100% |
In 225 patients with nodal disease at the time of surgery -in case of upfront surgery or residual disease after NACT- TAS removed a median of five (IQR 3–7) nodes, two (IQR 1–4) of which were positive. Two [IQR 1–3] of these nodes were considered palpably obviously suspicious by surgeons. Three [IQR 2–4] of these nodes were radioactive and/or blue. Of 100 patients with confirmed nodal disease at the time of surgery who underwent ALND following TAS in the TAXIS trial, only 6 (6%) underwent axillary radiation. A median number of 14 (IQR 10–17) additional lymph nodes were removed by ALND and a median of 1.5 (IQR 0–5.5) were positive. Additional positive nodes were removed in a total of 70 (70%) patients. Of 100 patients with confirmed nodal disease at the time of surgery who underwent TAS without ALND in the TAXIS trial, 93 (93%) underwent axillary radiation. No patient underwent axillary redo surgery and two patients received a radiation boost for residual suspicious findings in the axilla on imaging before the end of adjuvant treatment.
Imaging-guided localization was attempted in 257 patients (86.8%) and was successful in 242 (81.8%). Various types of clips and imaging-guided localization techniques were used (Table 4).
Table 4.
Marking of sampled node with clip and imaging-guided localization.
| Variable | No. (%) |
|---|---|
| Imaging modality used to clip the node | N = 296 |
| Ultrasound | 293 (99.0%) |
| Type of clip used to mark the positive node | N = 296 |
| Direct magseed | 16 (5.4%) |
| Direct radioactive seed | 1 (0.3%) |
| Nitinol ring marker (nickel titanium alloy) | 91 (30.7%) |
| Titanium or stainless steel marker with gel | 92 (31.1%) |
| Titanium or stainless steel marker without gel | 88 (29.7%) |
| Imaging-guided localization of the clipped node: attempted | N = 296 |
| Yes | 257 (86.8%) |
| No | 39 (13.2%) |
| Imaging-guided localization of the clipped node: successful | N = 257 |
| Yes | 242 (94.2%) |
| Unsure | 7 (2.7%) |
| No | 8 (3.1%) |
| Reason for failure | N = 257 |
| Clip not visible | 6 (2.3%) |
| Wire missed target | 2 (0.8%) |
| Localization performed before surgery | 185/257 (72.0%) |
| Imaging modality used to localize the clipped node (before surgery) | |
| Ultrasound | 180 (97.3%) |
| Computed tomography | 2 (1.1%) |
| Type of localization used (before surgery) | |
| Magseed | 5 (2.7%) |
| ROLL | 52 (28.1%) |
| Radioactive seed | 21 (11.4%) |
| Tattoo | 4 (2.2%) |
| Wire | 93 (50.3%) |
| Other | 10 (5.4%) |
| Localization performed during surgery: | 72/257 (28.0%) |
| Type of localization used (during surgery) | N = 72 |
| Tattoo | 2 (2.8%) |
| Wire | 43 (59.7%) |
| Ultrasound alone | 21 (29.2%) |
| Other | 6 (8.3%) |
There was no significant difference in the rate of clip removal by use of imaging-guided localization (94.6% (243/257) with vs. 92.3% (36/39; p = 0.47) without), but a trend toward lower rate of clip removal after NACT (91.2% (114/125) with vs. 96.5% (164/170) without NACT (p = 0.075). There was a trend toward a lower median number of lymph nodes removed during TAS when imaging-guided localization was performed (4 [IQR 3–7] vs 6 [IQR 4–7]; p = 0.09). Type of clip was not associated with successful surgical removal of the clipped node in patients with nodal disease at the time of surgery (p = 0.197) and in patients with nodal pCR (p = 0.875; appendices, page 35, table A3: Successful surgical removal of clipped node by type of clip).
5. Discussion
The study showed that TAS removed the clipped node in 94.3% of patients and that TAS was much less radical than ALND in terms of the number of nodes removed. After omission of ALND after TAS, only two patients received an extra radiotherapy boost and no patient underwent axillary redo surgery for suspected residual disease. More than half of patients underwent upfront surgery, 85% of whom had estrogen receptor positive and Her-2 negative disease, which reflects current clinical practice at the 28 participating sites from four European countries.
TAS is not a novel surgical procedure, but a new concept that combines several surgical techniques to achieve tumor load reduction in a population of patients where ALND is still standard care. The individual steps, however, are either identical or related to known procedures. Targeted removal of palpable lymph nodes, for example, is inspired by a procedure called axillary node sampling [23,24]. During TAS, however, palpation is used to identify clearly abnormal nodes, with the limitations described above. The SLN procedure was defined in line with previous landmark studies in patients with biopsy-proven node-positive breast cancer [7,25]. While palpably suspicious findings are mostly not encountered during a SLN procedure outside of this experimental setting-because they are considered one of its contraindications when detected before surgery-they are routinely expected and targeted during TAS. Another difference to standard SLN techniques refers to the localization of the clipped node with use of modern-day imaging.
Patients in TAXIS are at the far end of the risk spectrum of node-positive breast cancer with the highest stages ever studied in axillary surgery de-escalation trials. It was not surprising that ALND removed additional positive nodes in 70% of patients after TAS. While all of these patients received axillary radiation to levels I-IV and mostly also to the internal mammary chain, it is important to wait for the results of TAXIS to confirm oncologic safety before replacing ALND by TAS in clinical practice. In Z0011, 27% of patients had additional positive nodes removed by ALND in the control group. Most patients in the SLN only group did not develop regional recurrence [2,3,26], even though available data on radiation fields suggest that many of them did not receive directed nodal irradiation [27]. Results were similar in EORTC AMAROS, where additional lymph nodes metastases were found in 33% of patients who underwent ALND [1]. Contemporary findings in patients with lymph nodes detected by either physical exam or imaging and treated with surgery first showed that more than 40% of breast cancer patients with clinically positive nodes had minimal nodal disease (pN1) at surgery [28]. Finally, even the old landmark trial NSABP B-04 showed no significant differences in disease-free and overall survival in women with palpable nodal disease among those who had the axilla irradiated as compared with those who had the lymph nodes removed [29]. These findings make us believe that outcomes will be favorable in the TAXIS trial.
In the present study, 51% of patients had palpable metastases at diagnosis and 57.4% underwent upfront surgery. Accordingly, the clipped node was considered to be palpably suspicious during surgery in almost half of patients (47%). While use of imaging-guided localization was associated with a trend toward less lymph nodes removed (p = 0.09), it did not improve the high detection rate of the clipped node by TAS. These are fundamental differences to the use of the SLN procedure or targeted axillary dissection (TAD) as diagnostic staging procedures to determine nodal pCR after NACT [[7], [8], [9], [10], [11], [12],30]. Such contemporary diagnostic concepts foresee further surgery when residual disease is found in the nodes. In addition, they are usually applied in the absence of palpably suspicious findings and TAD by definition requires imaging-guided localization. A recent prospective registry study of patients undergoing neoadjuvant systemic therapy at 50 German centers validated the performance of TAD in general, but showed that the clipped node is missed in a significant number of patients [31]. On a global scale, the SLN procedure is the most commonly performed procedure to determine nodal pCR and omit ALND in this setting, with several recent observational studies supporting its oncologic safety [[32], [33], [34], [35]]. Importantly, nodal pCR is not known at the time of surgery in many clinically node-positive breast cancer patients undergoing NACT, since modern day imaging is not capable of reliably detecting or excluding residual disease after NACT [36]. From a technical (as opposed to conceptual) point of view, therapeutic TAS can eventually turn out to be similar to diagnostic TAD in the subset of TAXIS patients with no palpably suspicious nodes when NACT is used and imaging to localize the clipped node. Therefore, it is reassuring to see the low FNR of 1.8% for TAS, which was comparable to the results from prospective studies on TAD [31,37].
In the neoadjuvant setting, Alliance A011202 (ClinicalTrials.gov Identifier: NCT01901094) overlaps with TAXIS in the patient population with palpable axillary disease that turns into a clinically negative axilla with residual disease in the SLN. A recent retrospective analysis of the National Cancer Database observed inferior survival associated with the omission of ALND in patients with residual nodal disease following NACT [38]. Nevertheless, accrual is high in both trials, implying limited skepticism among investigators in the US and in Europe to include patients into a trial where half of patients with residual disease do not undergo ALND. In fact, the 2021 St. Gallen consensus conference revealed substantial disagreement among experts when asked about the omission of ALND in the setting of micrometastatic residual disease [39]. Importantly, TAXIS also includes patients whose axillary disease remains palpable after NACT, which accounted for 7% of patients overall and to exactly one third (21 of 63) of patients with upfront palpable disease undergoing NACT (Fig. 2).
This study has several limitations, mainly due to the pragmatic concept of TAS. Firstly, we refrained from pre-defining and collecting enough variables to accurately assess the relative contribution of the individual steps of TAS to the reduction of tumor load in the axilla, which, in turn, makes it impossible to know if palpation-, SLN tracer-, or imaging-guided removal of nodes was most effective. For the same reason, we were not able to assess differences in number of removed nodes with SLN tracer uptake by use of single versus dual agent mapping. Secondly, while the resulting heterogeneity of the patient population will facilitate applicability and generalizability of the results, it complicates statistical analysis and interpretation due to numerous stratification factors and preplanned subgroup analyses.
6. Conclusions
In summary, TAS was feasible with removal of the sampled node, the SLNs and all palpably obvious disease in the vast majority of the 296 patients, with no further improvement by imaging-guided localization. TAS selectively removed positive lymph nodes and remained much less radical than ALND, but subsequent ALND removed additional positive nodes in 70% of patients after TAS. The ongoing TAXIS trial will determine whether axillary treatment by TAS and radiotherapy is oncologically non-inferior and associated with improved QoL compared to ALND in patients with clinically node-positive breast cancer.
Funding
The trial was supported by grants from the following organizations: Rising Tide Foundation for Clinical Cancer Research, Fond’Action, Krebsliga beider Basel, Claudia von Schilling Foundation for Breast Cancer Research, and Kaempf-Boetschi Foundation. It was also supported by the Swiss State Secretary for Education, Research and Innovation (SERI), Swiss Cancer Research Foundation (SCS), and Swiss Cancer League (SCL).
Contribution
Walter Paul Weber, M.D.; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Zoltan Matrai; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Stefanie Hayoz; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Christoph Tausch; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Guido Henke; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Daniel R. Zwahlen; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Günther Gruber; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Frank Zimmermann; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Stefanie Seiler; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Charlotte Maddox; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Thomas Ruhstaller; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Simone Muenst; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Markus Ackerknecht; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Sherko Kuemmel; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Vesna Bjelic-Radisic; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Christian Kurzeder; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Mihály Újhelyi; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Conny Vrieling; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Rok Satler; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Inna Meyer; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Charles Becciolini; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Susanne Bucher; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Colin Simonson; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Peter M. Fehr; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Natalie Gabriel; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Robert Maráz; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Dimitri Sarlos; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Konstantin J. Dedes; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Cornelia Leo; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Gilles Berclaz; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Peter Dubsky; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Ruth Exner; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Hisham Fansa; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Christopher Hager; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Klaus Reisenberger; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Christian F. Singer; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Roland Reitsamer; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Mattea Reinisch; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Jelena Winkler; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Giang Thanh Lam; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Mathias K. Fehr; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Tatiana Naydina; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Magdalena Kohlik; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Karine Clerc; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Valerijus Ostapenko; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Florian Fitzal; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Rahel Nussbaumer; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Nadia Maggi; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Alexandra Schulz; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Pagona Markellou; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Loïc Lelièvre; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Daniel Egle; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Jörg Heil; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published. Michael Knauer; Substantial contributions to conception and design, Substantial contributions to acquisition of data, Substantial contributions to analysis and interpretation of data, Participation in drafting the article or revising it critically for important intellectual content, Final approval of the version to be published.
Declaration of interest statement
Outside the submitted work, W.P. Weber received research support from Takeda Pharmaceuticals International paid to the Swiss Group for Clinical Cancer Research (SAKK) and personal honoraria from Genomic Health, Inc., USA. Support for meetings was paid to his institution from Sandoz, Genomic Health, Medtronic, Novartis Oncology, Pfizer and Eli Lilly.
Hisham Fansa has no financial interest, and receives royalty payments from Springer-Nature publishers for two textbooks on breast surgery.
Florian Fitzal was editor of Oncoplastic Surgery part I and II (SPRINGER), received travel support and scientific support from COMESA (Mentor), NOVARTIS, ROCHE, ASTRA ZENECA, PFIZER, MYRIAD, NANOSTRING, BONDIMED (Polytech, Integra), Lilly, had an advisory role for PFIZER, ASTRA ZENECA, LILLY, ROCHE, and was founder of BREAST ANALYZING TOOL (BAT; breastanalyzing.com).
All other authors declare no competing interests relevant to this manuscript.
Footnotes
Part of this work was presented during spotlight discussion at the 2020 San Antonio Breast Cancer Symposium® and during on demand session at the 2021 St. Gallen International Breast Cancer Conference.
Appendix.
Fig. A.1Pre-specified prospective study population embedded in TAXIS trial.
Table A.1.
Patient and tumor characteristics by palpable versus non-palpable nodal disease
| Variable | Palpable nodal disease No. (%) |
Non-palpable nodal disease No. (%) |
|---|---|---|
| No. of patients | 151 | 145 |
| Age, years | ||
| Median (IQR) | 55 (45–69) | 57 (47–67) |
| Age, years | ||
| ≤50 | 62 (41.1%) | 50 (34.5%) |
| >50 | 89 (58.9%) | 95 (65.5%) |
| Neoadjuvant chemotherapy | ||
| No | 87 (57.6%) | 83 (57.2%) |
| Yes | 63 (41.7%) | 62 (42.8%) |
| Unknown | 1 (0.7%) | 0 (0.0%) |
| Nodal pCR rate after neoadjuvant therapy | 36 (23.8%) | 35 (24.1%) |
| Type of breast surgery | ||
| Breast conserving | 93 (61.6%) | 86 (59.3%) |
| Mastectomy + - reconstruction | 57 (37.7%) | 59 (40.7%) |
| None∗ | 1 (0.7%) | |
| Tumor size at initial diagnosis, mm | ||
| Median (IQR) | 28.5 (20–40) | 28 (21–40) |
| Clinical T stage at initial diagnosis | ||
| T0 | 0 (0.0%) | 1 (0.7%) |
| T1 | 37 (24.5%) | 30 (20.7%) |
| T2 | 93 (61.6%) | 96 (66.2%) |
| T3 | 15 (9.9%) | 13 (9.0%) |
| T4 | 4 (2.6%) | 5 (3.4%) |
| Tis (DCIS) | 1 (0.7%) | 0 (0.0%) |
| Tx | 1 (0.7%) | 0 (0.0%) |
| Clinical N stage at initial diagnosis | ||
| N1 by palpation | 137 (90.7%) | 0 (0.0%) |
| N1 by imaging | 0 (0.0%) | 136 (93.8%) |
| N2/3 | 14 (9.3%) | 9 (6.2%) |
| Postoperative N stage | ||
| pN0 | 3 (2.0%) | 2 (1.4%) |
| pN1mi | 0 (0.0%) | 0 (0.0%) |
| pN1 | 55 (36.4%) | 47 (32.4%) |
| pN2 | 15 (9.9%) | 24 (16.6%) |
| pN3 | 12 (7.9%) | 8 (5.5%) |
| ypN0 | 36 (23.8%) | 35 (24.1%) |
| ypN0(ITC) | 2 (1.3%) | 0 (0.0%) |
| ypN1 | 22 (14.6%) | 24 (16.6%) |
| ypN2 | 4 (2.6%) | 5 (3.4%) |
| ypN3 | 2 (1.3%) | 0 (0.0%) |
| Histology | ||
| Invasive ductal | 114 (75.5%) | 109 (75.2%) |
| Lobular | 18 (11.9%) | 9 (6.2%) |
| Other | 19 (12.6%) | 27 (18.6%) |
| Receptor status at initial diagnosis | ||
| HR+/Her2- | 91 (60.3%) | 96 (66.2%) |
| HR+/Her2+ | 18 (11.9%) | 24 (16.6%) |
| HR-/Her2+ | 7 (4.6%) | 9 (6.2%) |
| HR-/Her2- | 26 (17.2%) | 9 (6.2%) |
| Missing/unknown | 9 (6.0%) | 7 (4.8%) |
| LVI | ||
| Yes | 85 (56.3%) | 62 (42.8%) |
| No | 61 (40.4%) | 77 (53.1%) |
| Missing/unknown | 5 (3.3%) | 6 (4.1%) |
| Modified Bloom-Richardson score | ||
| I | 5 (3.3%) | 6 (4.1%) |
| II | 80 (53.0%) | 82 (56.6%) |
| III | 60 (39.7%) | 56 (38.6%) |
| Missing/unknown | 6 (4.0%) | 1 (0.7%) |
∗Primary tumor was inoperable and surgical treatment consisted exclusively of axillary surgery.
Table A.2.
Characteristics of tailored axillary surgery and axillary lymph node dissection
| Upfront Surgery N = 166 |
Neoadjuvant Chemotherapy N = 125 |
|||||
|---|---|---|---|---|---|---|
| Variable |
Residual Nodal disease (N = 54) |
Nodal Pathologic Complete Response (N = 71) |
||||
| TAS (n = 291∗) | Median | (IQR) | Median | (IQR) | Median | (IQR) |
| Total number of nodes removed by TAS | 5 | (3, 7) | 4 | (3, 5) | 4 | (2, 6) |
| Number of sentinel nodes | 3 | (2, 4) | 2 | (1, 4) | 3 | (2, 4) |
| Number of palpably suspicious nodes | 2 | (1, 4) | 1 | (0, 2) | 1 | (0, 2) |
| Number of positivea nodes | 2 | (1, 4) | 1 | (1, 2) | 0 | (0, 0) |
| Number of negative nodes | 2 | (1, 4) | 2 | (1, 4) | 4 | (3, 5) |
| n | (%) | n | (%) | n | (%) | |
| Largest sentinel node metastasis | ||||||
| Isolated tumor cells | 0 | (0.0%) | 1 | (1.9%) | 0 | (0.0%) |
| Micro | 4 | (2.5%) | 10 | (18.5%) | 0 | (0.0%) |
| Macro | 130 | (78.3%) | 36 | (66.7%) | 0 | (0.0%) |
| NA (no positive sentinels) | 22 | (13.3%) | 5 | (9.3%) | 71 | (100.0%) |
| Unknown | 10 | (6.0%) | 2 | (3.7%) | 0 | (0.0%) |
| Largest non-sentinel node metastasis | ||||||
| Isolated tumor cells | 0 | (0.0%) | 1 | (1.9%) | 0 | (0.0%) |
| Micro | 6 | (3.6%) | 2 | (3.7%) | 0 | (0.0%) |
| Macro | 54 | (32.5%) | 8 | (14.8%) | 0 | (0.0%) |
| NA (no positive non-sentinels) | 96 | (57.8%) | 43 | (79.6%) | 71 | (100.0%) |
| Unknown | 10 | (6.0%) | 0 | (0.0%) | 0 | (0.0%) |
| ALND (n = 123)∗∗ | Median | (IQR) | Median | (IQR) | Median | (IQR) |
| Number of additional lymph nodes removed by ALND | 14 | (9, 18) | 14 | (10, 16) | 12.5 | (8.5, 23) |
| Number of additional positivea lymph nodes removed by ALND | 2 | (0, 6) | 1 | (0, 3) | 0 | (0, 0) |
| n | (%) | n | (%) | n | (%) | |
| Number of patients with additional positivea nodes removed by ALND | ||||||
| No additional positive nodes | 25 | (29.1%) | 10 | (40.0%) | 8 | (100.0%) |
| One additional positive node | 17 | (19.8%) | 5 | (20.0%) | 0 | (0.0%) |
| Two additional positive nodes | 6 | (7.0%) | 2 | (8.0%) | 0 | (0.0%) |
| Three additional positive nodes | 6 | (7.0%) | 2 | (8.0%) | 0 | (0.0%) |
| Four additional positive nodes | 6 | (7.0%) | 2 | (8.0%) | 0 | (0.0%) |
| >four additional positive nodes | 26 | (30.2%) | 4 | (16.0%) | 0 | (0.0%) |
Abbreviations: IQR, interquartile range; TAS, tailored axillary surgery; ALND, axillary lymph node dissection; NA, not applicable.
Nodes with isolated tumor cells are counted as positive.
Five patients who received neoadjuvant therapy other than chemotherapy are not shown here.
Two patients who received neoadjuvant therapy other than chemotherapy are not shown here.
TABLE A.3.
Successful surgical removal of clipped node by type of clip
| Type of Clip |
||||||
|---|---|---|---|---|---|---|
| Direct Magseed | Direct Seed | Ring Marker | Marker With Gel | Marker Without Gel | P-Valueb | |
| Confirmed nodal disease at the time of surgery (n = 219) | N = 14 | N = 3 | N = 58 | N = 69 | N = 75 | 0.197 |
| Clip surgically removeda | 13 (92.9%) | 3 (100.0%) | 55 (94.8%) | 64 (92.8%) | 74 (98.7%) | |
| Clip not removed | 1 (7.1%) | 0 (0.0%) | 3 (5.2%) | 5 (7.2%) | 1 (1.3%) | |
| Nodal pathologic complete response (n = 71) | N = 2 | N = 0 | N = 33 | N = 23 | N = 13 | 0.875 |
| Clip surgically removeda | 2 (100.0%) | 0 (0.0%2) | 30 (90.9%) | 20 (87.0%) | 12 (92.3%) | |
| Clip not removed | 0 (0.0%) | 0 (0.0%) | 3 (9.1%) | 3 (13.0%) | 1 (7.7%) | |
As documented by specimen radiography.
Fisher's exact test, excluding the categories “direct magseed” and “direct seed” due to small sample size.
References
- 1.Donker M., van T.G., Straver M.E. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliano A.E., McCall L., Beitsch P. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–432. doi: 10.1097/SLA.0b013e3181f08f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giuliano A.E., Hunt K.K., Ballman K.V. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. J Am Med Assoc. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galimberti V., Cole B.F., Zurrida S. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savolt A., Peley G., Polgar C. Eight-year follow up result of the OTOASOR trial: the Optimal Treatment of the Axilla - surgery or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: a randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol. 2017;43:672–679. doi: 10.1016/j.ejso.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Weber W.P., Barry M., Stempel M.M. A 10-year trend analysis of sentinel lymph node frozen section and completion axillary dissection for breast cancer: are these procedures becoming obsolete? Ann Surg Oncol. 2012;19:225–232. doi: 10.1245/s10434-011-1823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boileau J.F., Poirier B., Basik M. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33:258–264. doi: 10.1200/JCO.2014.55.7827. [DOI] [PubMed] [Google Scholar]
- 8.Boughey J.C., Ballman K.V., Le-Petross H.T. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (alliance) Ann Surg. 2016 Apr;263(4):802–807. doi: 10.1097/SLA.0000000000001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuehn T., Bauerfeind I., Fehm T. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen T.T., Hoskin T.L., Day C.N. Decreasing use of axillary dissection in node-positive breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2018;25:2596–2602. doi: 10.1245/s10434-018-6637-9. [DOI] [PubMed] [Google Scholar]
- 11.Laws A., Hughes M.E., Hu J. Impact of residual nodal disease burden on technical outcomes of sentinel lymph node biopsy for node-positive (cN1) breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2019;26:3846–3855. doi: 10.1245/s10434-019-07515-4. [DOI] [PubMed] [Google Scholar]
- 12.Mamtani A., Barrio A.V., King T.A. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol. 2016;23:3467–3474. doi: 10.1245/s10434-016-5246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hieken T.J., Trull B.C., Boughey J.C. Preoperative axillary imaging with percutaneous lymph node biopsy is valuable in the contemporary management of patients with breast cancer. Surgery. 2013;154:831–838. doi: 10.1016/j.surg.2013.07.017. discussion 838-40. [DOI] [PubMed] [Google Scholar]
- 14.Caudle A.S., Kuerer H.M., Le-Petross H.T. Predicting the extent of nodal disease in early-stage breast cancer. Ann Surg Oncol. 2014;21:3440–3447. doi: 10.1245/s10434-014-3813-4. [DOI] [PubMed] [Google Scholar]
- 15.Yoo T.K., Kang B.J., Kim S.H. Axillary lymph node dissection is not obligatory in breast cancer patients with biopsy-proven axillary lymph node metastasis. Breast Canc Res Treat. 2020;181:403–409. doi: 10.1007/s10549-020-05636-z. [DOI] [PubMed] [Google Scholar]
- 16.Lim G.H., Upadhyaya V.S., Acosta H.A. Preoperative predictors of high and low axillary nodal burden in Z0011 eligible breast cancer patients with a positive lymph node needle biopsy result. Eur J Surg Oncol. 2018;44:945–950. doi: 10.1016/j.ejso.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed M., Jozsa F., Baker R. Meta-analysis of tumour burden in pre-operative axillary ultrasound positive and negative breast cancer patients. Breast Canc Res Treat. 2017;166:329–336. doi: 10.1007/s10549-017-4405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verheuvel N.C., Voogd A.C., Tjan-Heijnen V.C.G. Different outcome in node-positive breast cancer patients found by axillary ultrasound or sentinel node procedure. Breast Canc Res Treat. 2017;165:555–563. doi: 10.1007/s10549-017-4342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilewskie M., Mautner S.K., Stempel M. Does a positive axillary lymph node needle biopsy result predict the need for an axillary lymph node dissection in clinically node-negative breast cancer patients in the ACOSOG Z0011 era? Ann Surg Oncol. 2016;23:1123–1128. doi: 10.1245/s10434-015-4944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network . 2020. Guidelines breast cancer.https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [Google Scholar]
- 21.Henke G., Knauer M., Ribi K. Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically node-positive breast cancer (TAXIS): study protocol for a multicenter, randomized phase-III trial. Trials. 2018;19 doi: 10.1186/s13063-018-3021-9. 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.TNM classification of malignant tumours. eighth ed. UICC); Jan 2017. [Google Scholar]
- 23.Bing A.U., Kerr G.R., Jack W. Pooled long-term outcomes from two randomized trials of axillary node sampling with axillary radiotherapy versus axillary node clearance in patients with operable node-positive breast cancer. Br J Surg. 2016;103:81–87. doi: 10.1002/bjs.9952. [DOI] [PubMed] [Google Scholar]
- 24.Ahlgren J., Holmberg L., Bergh J. Five-node biopsy of the axilla: an alternative to axillary dissection of levels I-II in operable breast cancer. Eur J Surg Oncol. 2002;28:97–102. doi: 10.1053/ejso.2001.1228. [DOI] [PubMed] [Google Scholar]
- 25.Boughey J.C., Suman V.J., Mittendorf E.A. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. J Am Med Assoc. 2013;310:1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuliano A.E., Ballman K.V., McCall L. Effect of axillary dissection vs No axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial. J Am Med Assoc. 2017;318:918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagsi R., Chadha M., Moni J. Radiation field design in the ACOSOG Z0011 (alliance) trial. J Clin Oncol. 2014;32:3600–3606. doi: 10.1200/JCO.2014.56.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angarita S., Ye L., Runger D. Assessing the burden of nodal disease for breast cancer patients with clinically positive nodes: hope for more limited axillary surgery. Ann Surg Oncol. 2021;28:2609–2618. doi: 10.1245/s10434-020-09228-5. [DOI] [PubMed] [Google Scholar]
- 29.Fisher B., Jeong J.H., Anderson S. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 30.Mittendorf E.A., Caudle A.S., Yang W. Implementation of the american college of surgeons oncology group z1071 trial data in clinical practice: is there a way forward for sentinel lymph node dissection in clinically node-positive breast cancer patients treated with neoadjuvant chemotherapy? Ann Surg Oncol. 2014;21:2468–2473. doi: 10.1245/s10434-014-3775-6. [DOI] [PubMed] [Google Scholar]
- 31.Kuemmel S., Heil J., Rueland A. A prospective, multicenter registry study to evaluate the clinical feasibility of targeted axillary dissection (TAD) in node-positive breast cancer patients. Ann Surg. 2020 Nov 4 doi: 10.1097/SLA.0000000000004572. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Wong S.M., Basik M., Florianova L. Oncologic safety of sentinel lymph node biopsy alone after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2021;28:2621–2629. doi: 10.1245/s10434-020-09211-0. [DOI] [PubMed] [Google Scholar]
- 33.Kahler-Ribeiro-Fontana S., Pagan E., Magnoni F. Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol. 2021;47:804–812. doi: 10.1016/j.ejso.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Damin A.P., Zancan M., Melo M.P. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with node-positive breast cancer: guiding a more selective axillary approach. Breast Canc Res Treat. 2021;186:527–534. doi: 10.1007/s10549-020-06011-8. [DOI] [PubMed] [Google Scholar]
- 35.Piltin M.A., Hoskin T.L., Day C.N. Oncologic outcomes of sentinel lymph node surgery after neoadjuvant chemotherapy for node-positive breast cancer. Ann Surg Oncol. 2020;27:4795–4801. doi: 10.1245/s10434-020-08900-0. [DOI] [PubMed] [Google Scholar]
- 36.Samiei S., de Mooij C.M., Lobbes M.B.I. Diagnostic performance of noninvasive imaging for assessment of axillary response after neoadjuvant systemic therapy in clinically node-positive breast cancer: a systematic review and meta-analysis. Ann Surg. 2021 Apr 1;273(4):694–700. doi: 10.1097/SLA.0000000000004356. [DOI] [PubMed] [Google Scholar]
- 37.Caudle A.S., Yang W.T., Krishnamurthy S. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016 Apr 1;34(10):1072–1078. doi: 10.1200/JCO.2015.64.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almahariq M.F., Levitin R., Quinn T.J. Omission of axillary lymph node dissection is associated with inferior survival in breast cancer patients with residual N1 nodal disease following neoadjuvant chemotherapy. Ann Surg Oncol. 2021;28:930–940. doi: 10.1245/s10434-020-08928-2. [DOI] [PubMed] [Google Scholar]
- 39.Burstein Gc H.J., Thürlimann B., Weber W.P., Poortmans P., Regan M., Senn H.J., Winer E.P. Customizing local and systemic therapies for women with early breast cancer: the St.Gallen International Consensus Guidelines for treatment of early breast cancer. 2021. M. Gnant and panelists of the St.Gallen consensus conference. [Google Scholar]