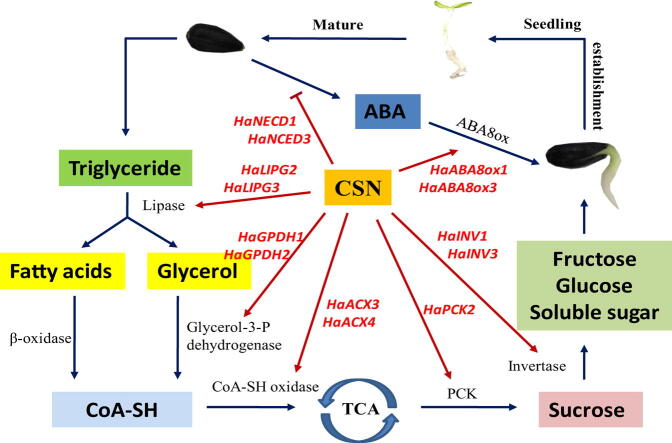
Graphical abstract
Keywords: Abscisic acid, Aged seeds, CSN, Fatty acid, Seed vigor, Sunflower
Abstract
Introduction
Sunflower seeds possess higher oil content than do cereal crop seeds. Storage of sunflower seeds is accompanied by loss of seed vigor and oxidation of storage and membrane lipids.
Objectives
This study first reported that compound sodium nitrophenolate (CSN), a new plant growth modulator, improved the germination and seedling emergence of aged sunflower seeds. The present study provide a future reference as to the potential applications of CSN and the regulation mechanism of exogenous substances in increasing aged crop seed vigor.
Methods
Phenotypic analysis was performed to investigate the effect of CSN on germination and seedling emergence from naturally- and artificially-aged sunflower seeds. The biochemical and enzyme activity analysis were conducted to test the CSN-induced effect on glycometabolism, fatty acid and abscisic acid metabolism. Meanwhile, gene expression analysis was carried out to detect the changes in the transcription level of sunflower seeds during early germination period after CSN treatment.
Results
CSN application significantly increased the germination rate and seedling emergence rate of sunflower seeds under natural and artificial aging. Biochemical analysis indicated that, CSN treatment significantly enhanced the sucrose and fructose contents in aged sunflower seeds during early germination period. Moreover, the contents of several different fatty acids in CSN-treated sunflower seeds also significantly increased. Enzyme activity analysis revealed that CSN treatment remarkably up-regulated the activities of several critical enzymes related to triacylglycerol hydrolysis. Consequently, the transcription levels of the above key enzymes-related synthetic genes were also significantly up-regulated in CSN treatment. Furthermore, CSN treatment significantly decreased abscisic acid (ABA) content through the regulation of the gene expressions and activities of metabolism related-enzymes.
Conclusion
Taken together, the contribution of CSN to the improvement of aged sunflower seed germination and seedling emergence might be closely related to the fatty acid, glycometabolism, and ABA metabolism.
Introduction
Sunflower (Helianthus annuus L.) originated in North America, is one of the vital oilseed crops globally [1]. According to the statistical data from the Food and Agriculture Organization of the United Nations (UNAFO) in 2015, the global sunflower planting area is approximately 25.4 million hm2. Additionally, sunflower is also the third-largest oil crop in China, with the planting area of up to 1.15 million hm2 and the total output of 299 t, only second to oilseed rape and peanut [2]. Sunflower has come under the spotlight in recent years due to its high ornamental, edible and oil values. Sunflower seed is rich in unsaturated fatty acids (UFAs), multiple vitamins and trace elements, which can suppress the cholesterol synthesis in human body. Currently, sunflower oil is the preferred cooking oil in developed countries [3]. The sunflower production in China shows an increasing trend on the whole, which increases from 278,000 tons to 2,899,700 tons from 1978 to 2016 [2].
Seeds are the foundation of agricultural production. The successful seed germination and seedling emergence are of paramount importance to the plant growth and yield formation of crops [4]. Seed germination is a complicated process controlled jointly by a variety of endogenous and exogenous factors [5]. Seed vigor stands for an integrative seed characteristic determining the rapid and neat seed emergence capacity in the complicated field environment [6]. As an important part in seed vigor, seed longevity is determined by seed genetic and physiological protection potentials, as well s the storage conditions [7]. High vigor seed is closely related to the high filed emergence and productivity, while low vigor seed always leads to a decrease of yields. The early seedling emergence from germinated seed is another critical development stage. Before the seedling obtains photosynthetic capacity, both seed germination and seedling emergence are powered by the energy from the storage material stored in the seed itself [8], [9].
There are abundant nutrients in the seeds, mainly including macromolecules like starch, protein and fat. These macromolecules are gradually decomposed and utilized during the germination process [10]. Triacylglycerol is one of the major storage substances in oilseeds, and the successful decomposition of triacylglycerol is of crucial importance to the germination of oilseeds like sunflower seed. During seed germination, the triacylglycerol is directly hydrolyzed into fatty acids and glycerin by the lipase. Fatty acids produce CoA-SH through the β-oxidation pathway, which then forms the oxaloacetic acid through the tricarboxylic acid cycle or the glyoxylate cycle and finally reversely converts into sucrose through glycolysis [11]. Glycerin, the other triacylglycerol hydrolysis product, forms the phosphoglyceride under the catalysis of glycerol-3-P dehydrogenase, and subsequently produces dihydroxyacetone phosphate (DHAP) via dehydrogenation and converts into glucose for the use of seed germination [12]. Sunflower seeds contain much higher contents of triacylglycerol and fatty acid as compared with cereal crop seeds [13], [14]. During the seed storage process, seeds continuously respire to decompose biological molecules such as saccharides and triacylglycerol, thus reducing seed longevity and seed vigor. Seed storage process significantly suppresses the germination and seedling emergence of sunflower seeds, and the reductions increase with the increases in storage time, environmental temperature and humidity [15], [16]. The oxidation of the unsaturated fatty acids can easily cause cell membrane permeability in oilseeds. Meanwhile, the free radicals and peroxides produced by oxidation will damage the protein, membrane structure, cell tissue and DNA, thus causing seed vigor loss [17]. Zhou et al [18] discovered that, the fatty acids catabolism was hindered at the early stage of aged soybean seed germination, which was the primary cause for the declined soybean seed vigor after storage. In the traditional agricultural production, the improper seed storage method or the excessively long storage time significantly decreased the sunflower seed vigor, which results in poor field seedling emergence, thus severely reducing the sunflower yield and quality [19]. Therefore, it is necessary to develop an effective approach to improve the germination and seedling emergence abilities of the aged sunflower seeds. Besides, further interpreting the molecular mechanisms during the germination and seedling emergence processes of aged seeds sheds more lights on the scientific research of sunflower.
Compound sodium nitrophenolate (CSN) is a novel artificially synthesized modulator for plant growth, which has been extensively used in agricultural production on various crops, including tomato [20], Chinese chive [21], cotton [22], and Rape [23]. CSN can promote cellular protoplasmic streaming, break seed dormancy, accelerate rooting and sprouting, improve fruit quality, and prevent flower and fruit drop [24], [25]. However, so far, there are few studies on the role of CSN in seed vigor regulation, in particular for seed germination as well as seedling emergence of aged sunflower seeds. The present study found that CSN had the function on seed vigor improvement in naturally and artificially aged sunflower seeds by regulating fatty acid, glycometabolism, and abscisic acid metabolism. CSN application boosted the triacylglycerol hydrolysis, promoted the conversion of fatty acids to sugars, and decreased the abscisic acid content during imbibitions of aged sunflower seeds, and resulted in the improvement of germination and seedling emergence of aged sunflower seeds. The present research might provide a future reference as to the potential applications of CSN and the regulation mechanism of exogenous substances in increasing aged crop seed vigor.
Materials and methods
Experimental materials
The sunflower (Helianthus annuus L.) seeds were employed in this study. Hybrid sunflower seeds were produced from parental inbreds through synchronous hand cross-pollination in the experimental farm of Zhejiang Academy of Agricultural Science (Hangzhou, China). The filled sunflower seeds were harvested to store for 1, 6, 12, 18 and 24 months, respectively. The sunflower seeds were stored in the dry airtight container under ambient temperature.
Accelerated aging test
The accelerated aging test (AAT) was carried out according to the method of Missaoui and Hill [26] with small modifications. Sunflower seeds stored for 1 month were used for the AAT. Briefly, 100 seeds of sunflower were put in each seed aged box and incubated at 45 °C and 100% relative humidity for 48 h (the accelerated aging test condition were determined by preliminary experiments), and then those processed seeds were subjected to 2 days of drying under ambient temperature prior to germination tests.
Seed germination and seedling emergence tests
Seed germination was carried out after disinfection of 0.1% sodium hypochlorite solution for 15 min. Fifty seeds of sunflower were put in each rolled towels, and four duplicates were set. Rolled towels were immersed in 25 mg·L−1 CSN solution and purified water, respectively. Subsequently, the rolled towels were incubated at 25 °C in germination chamber under a diurnal cycle of 12 h of light and 12 h of darkness. The number of germinated seeds were counted every 12 h. Seeds samples were collected at diverse time points (0, 12, 24, 36, and 48 h) during imbibitions for further testing.
Fifty sunflower seeds were planted in soil within greenhouses at 25 °C and the 12 h light/dark cycle. At 7 days later, the seedling emergence rates, seedlings height, seedlings dry weight, and total chlorophyll content were determinate based on experimental requirements.
CSN content measurement
High performance liquid chromatography-mass spectrometry (HPLC-MS) was performed to determine CSN content of sunflower seeds by the methods of Zhang et al [27]. With mobile phase of methanol-10 mM ammonium acetate solution (60:40, v/v), the mass spectrometry was operated in the positive ion mode of multiple reaction monitoring.
Quantification of various sugars
Total soluble sugar content in sunflower seeds was determined through anthrone-H2SO4 colorimetry by the method of Zhu et al [28]. The resorcinol method was used to measure sucrose level and the absorbance (OD) value at 480 nm was adopted for sucrose content estimation [29]. The fructose determination was performed with the method of Cai et al [30]. HPLC was performed to determine glucose content in sunflower seed by the method of Bailly [31].
ATP and energy charge analysis
ATP and energy charge were determined by HPLC, as described by Emami and Kempken [32] with minor modifications. Data of ATP and energy charge analysis were expressed as means of four replicate determinations.
Fatty acid content measurement
The fatty acid extraction was performed with the method of Zhou et al [18] with small modification. Sunflower seeds were ground into powders and used to extract fatty acids. In brief, after adding 2 ml n-hexane into the grinded sunflower seeds (50 mg in each tube), the sample was subjected to ultrasonic extraction (40 kHz) for 15 min, then standing for 3 h under ambient temperature. Then the solution was centrifuged at 10,000 rpm and 4 °C for 10 min. Afterwards, supernatants were collected, followed by 30 s of vortex oscillation after the addition of 3 ml methanolic potassium hydroxide solution (Me-OH, 0.4 M). Subsequently, upper liquid layer was transferred into the 5 ml bottle and diluted with n-hexane until 5 ml. The extract was added to a gas chromatograph-mass spectrometer (GC–MS) system using the 0.22 μm filter (organic phase). Afterwards, fatty acids were identified and quantified by the method of Yang et al [33].
Assay of fatty acid, glycometabolism, abscisic acid and gibberellin metabolism-related enzymes activity
The activities of triacylglycerol lipase (LIPG), glycerol-3-P dehydrogenase (GPDH), CoA-SH oxidase (ACX), phosphoenolpyruvate carboxykinase (PCK), invertase (INV), 9-cis-epoxycarotenoid dioxygenase (NCED) activity, zeaxanthin epoxidase (ZEP), abscisic acid aldehyde oxidase (AAO), abscisic acid −8′-hydroxylases (ABA8ox), GA3-oxidase (GA3ox), GA20-oxidase (GA20ox), and GA2-oxidase (GA2ox) were detected with enzyme-linked immune kit (Mlbio, Shanghai, China) according to the protocol of manufacturer. Briefly, sunflower seeds were extracted in 1 ml of extraction buffer and then centrifuged at 15,000 rpm for 15 min. Add 10 μl supernatant of enzyme extraction and 40 μl Sample Dilution in each testing sample well and after incubation at 37 °C for 30 min, removed the solution. Afterwards, washed five times by filling each well with 50 μl Wash Buffer. Then 50 μl conjugate reagent was added and repeated the incubation and wash process. Subsequently, 50 μl Chromogen Solution A and 50 μl Chromogen Solution B were added to each well for chromogenic reaction. The color change was measured spectrophotometrically at a wavelength of 450 nm with enzyme mark instrument. The activities of fatty acid and glycometabolism metabolism-related enzymes in seeds were then measured by comparing the O.D. of the samples to the standard curve.
Abscisic acid and gibberellin determination
ABA and GA were extracted from sunflower seeds in accordance with the method of Huang et al [34] The HPLC system equipped with an ultraviolet detector and reverse-phase (C18) column (6.0 mm × 120 mm, 5 mm particle size, Shim-Pack CLC-ODS) was used for identification and quantification of ABA and GA levels in the extracting solution. The mobile phase consisted of methanol/water (64:36, v/v) was ran at the 1.0 ml·min−1 flow rate. The ABA and GA analysis were performed with four biological replications.
Real-Time Quantitative PCR
The RNA extraction and reverse-transcription of each seeds sample was carried out using PrimeScript™ RT reagent Kit (Vazyme, Nanjing, China). The qPCR protocol was performed on a CFX96 Touch Real-Time PCR instrument (Biorad) with the ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China). Primers applied in this study were listed in Supporting Information Table S1. 18srRNA was used as the internal control. Calculations of the fold change of expression (FC) were determined according to FC = EΔCt, where E corresponds to the mean value of the amplification efficacy of the gene, ΔCt is the difference between the mean Ct values of all of the biological replicates between the two samples that are being compared. All data were expressed as the mean SD after normalization.
Statistical analyses
The Statistical Analysis System (SAS) software was used for data statistical analysis by one-way analysis of variance (ANOVA). The data was test for normality and homoscedasticity before ANOVA. In addition, multiple comparisons were accomplished by the least significant difference of p < 0.05 (LSD0.05). The percentage data were subjected to arcsintrans formation prior to statistical comparison according to ŷ=arcsin [sqrt (x/100)].
Results
Natural aging remarkably inhibited sunflower seed germination and seedling emergence
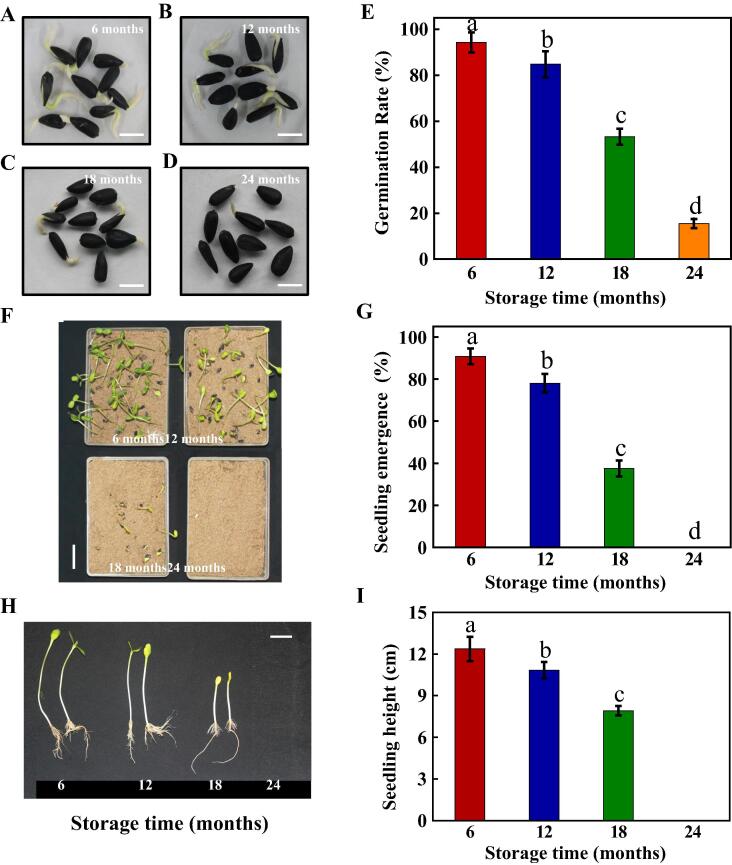
After storage for 6, 12, 18 and 24 months, the germination and seedling emergence capacities of sunflower seeds were determined (Fig. 1). The result showed that the germination rates of sunflower seeds in long-term storage (18 and 24 months) were remarkable lower than those in short-term storage. At 7 days of germination time, the germination rates of sunflower seeds stored for 6 and 12 months were 95% and 85%, respectively, while those after storage for 18 months was only 53%. After storage for 24 months, 85% of sunflower seeds lost the germination ability (Fig. 1A–E).
Fig. 1.
Natural aging remarkably suppressed sunflower seed germination and seedling emergence. (A–D) Typical images of naturally aged sunflower seeds in the imbibition process (48 h). Sunflower seeds after 6, 12, 18 and 24 months of storage were used for analysis. Scale bar, 10 mm. (E) The germination rates of different samples (A–D) (at 84 h post-sowing). (F) The seedling emergence phenotypes for different samples (A–D) after germination (at 5 days post-sowing). Scale bar, 600 mm. (G) The seedling emergence rates of different samples (A–D) (at 5 days post-sowing). (H) Typical images for sunflower seedling. Scale bar, 200 mm. (I) Quantitative analysis on the seedling height shown in (H). Four biological replicates each with 50 seeds for each treatment were set in seed germination and seedling emergence tests. The diverse lowercase(s) on top of the bars were indicative of significant differences (p < 0.01, Lsd) across treatments.
Thereafter, the present study analyzed the effect of storage process on the seedling emergence of sunflower seeds. Consistently, the sunflower seedling emergence rate decreased with the increase in storage time. The seedling emergence rates of sunflower seeds stored for 6 and 12 months were 91% and 78%, respectively; while those after storage for 18 months was only 38%. After storage for 24 months, all the sunflower seeds lost the seedling emergence capacity (Fig. 1FG). Moreover, it should be pointed out that, the seedling emergence rate of sunflower seeds after storage for 6 months was lower than the germination rate, and a similar rule was also observed in sunflower seeds after storage for 12, 18 and 24 months. Particularly, after storage for 24 months, the seedling emergence rate of sunflower seeds was 0%, although its germination rate reached 15%. We also investigated seedling height and confirmed the inhibiting effect of natural aged process on sunflower seedling growth (Fig. 1HI). To sum up, the above results indicated that, the natural aging process apparently reduced the sunflower seed vigor and seedling emergence capacity.
CSN treatment promoted germination and seedling emergence of aged sunflower seeds
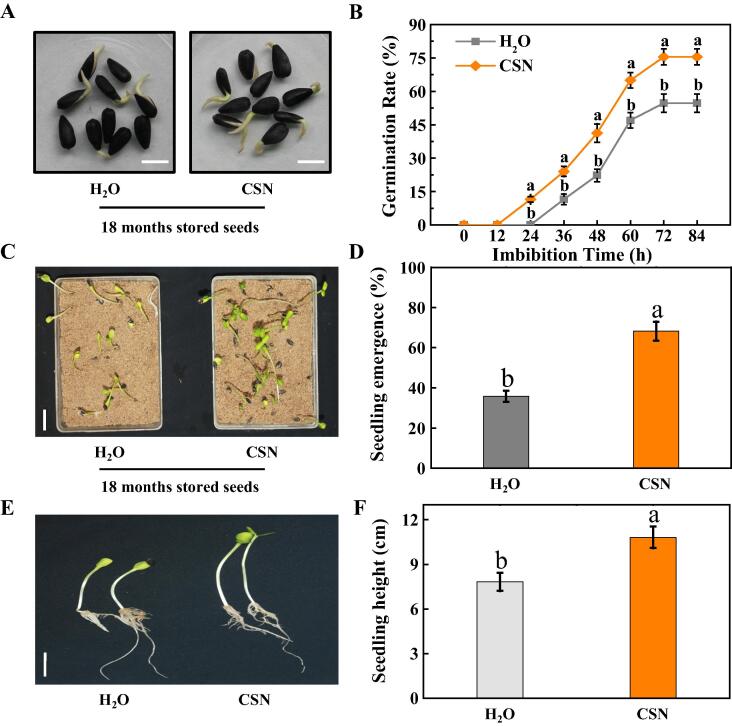
In the preliminary experiment, several extensively used plant growth regulators in agricultural production were screened, and their effects on improving the post-storage germination and seedling emergence capacities of sunflower seeds were determined. According to the results, compound sodium nitrophenolate (CSN) application improved the germination and seedling emergence capacities of the naturally aged sunflower seeds (Fig. 2). Exogenous CSN significantly enhanced the germination rate of sunflower seeds after storage for 18 months. At 7 days after germination, the germination rate of untreated sunflower seeds was only 55%, while those in CSN-treatment was as high as 79% (Fig. 2AB). Besides, CSN treatment also improved the seedling emergence of naturally aged sunflower seeds, for CSN-treated seeds, the seedling emergence rate was 68%, which doubled that of 34% for the untreated sunflower seeds (Fig. 2CD). The data on the effect of CSN on seedling height was consistent with the effect on seedling emergence (Fig. 2EF).
Fig. 2.
CSN enhanced seed germination and seedling emergence of naturally aged sunflower seeds. (A) Typical images of naturally aged sunflower seeds (after 18 months of storage) in imbibition process (48 h), in the presence or absence of CSN. Scale bar, 10 mm. (B) Time courses of germination rates of sunflower seeds stored for 18 months treated with CSN or not. (C) The seedling emergence phenotype from naturally aged seeds (after 18 months of storage) at 5 days post-sowing in the presence or absence of CSN treatment. Scale bar, 300 mm. (D) Seedling emergence rate for (C) was presented. (E) Typical images for sunflower seedling height. Scale bar, 200 mm. (F) Quantitative analysis on the plant height shown in (E). Four biological replicates each with 50 seeds for each treatment were set in seed germination and seedling emergence tests. The asterisk (*) or the diverse lowercase(s) on top of the bars were indicative of significant differences (p < 0.01, Lsd) across treatments. Exogenous CSN at 25 mg·L−1 was employed.
Given that CSN treatment promotes seed vigor of naturally aged sunflower seeds, the present study further analyzed the impact of CSN on the germination and seedling emergence of fresh sunflower seeds (storage for 1 month). The results indicated that CSN treatment had no significant effect on the germination and seedling emergence processes of fresh sunflower seeds (Supporting Information Fig. S1). Thus, it was suggested that the promotion effect of CSN on sunflower seed vigor was only reflected in the aged seeds, but not the fresh seeds.
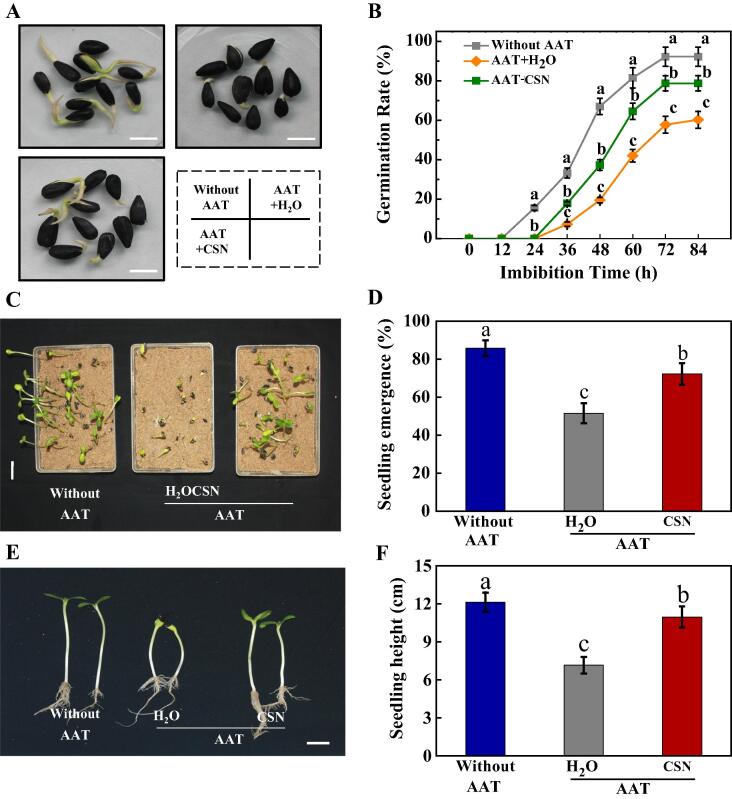
To further confirm the regulatory effect of CSN on the vigor of aged sunflower seeds, we adopted the accelerated aging test (AAT) to obtain the artificial aged sunflower seeds. The germination rate and seedling emergence rate of AAT-treated sunflower seeds were significantly lower than those of the non-aged seeds (Fig. 3). Consistent with the CSN effect on promoting germination of natural aged sunflower seeds, exogenous CSN also improved the germination and seedling emergence capacities of artificial aged sunflower seeds. The germination rate and seedling emergence rate of AAT + CSN treatment were 1.31 and 1.40 folds of those in AAT + H2O treatment, respectively (Fig. 3BD). Exogenous CSN also increased the seedling characteristic of AAT-aged seeds, with the seedling height of CSN-treated AAT-aged seeds being 1.5-fold that of AAT-aged seeds without CSN treatment (Fig. 3EF). To further verify the relationship between CSN treatment and improved sunflower seed vigor, the CSN content in imbibitions sunflower seeds was measured (Supporting Information Table S2). The results showed that CSN treatment significantly increased the CSN content in AAT-aged sunflower seeds during imbibitions time. After clarifying the role of CSN in promoting the germination and seedling emergence of naturally and artificially aged sunflower seeds, only artificially aged seeds were used in subsequent experiments.
Fig.3.
CSN improved seed germination and seedling emergence of artificially aged sunflower seeds. (A) Typical images of different samples of sunflower seeds during imbibitions (48 h after sowing). Without AAT: healthy seeds without accelerated aging test (AAT); AAT + H2O: AAT seeds with H2O; AAT + CSN: AAT seeds with compound sodium nitrophenolate (CSN) treatment. Scale bar, 10 mm. (B) Time courses of germination rates of different samples (A) are presented. (C) Typical images of seedling emergence. Scale bar, 200 mm. (D) The seedling emergence rate for (C) was displayed. (E) Typical images of sunflower seeding height. Scale bar, 200 mm. (F) Quantitative analysis on the seedling height shown in (F). Four biological replicates each with 50 seeds for each treatment were set in seed germination and seedling emergence tests. The asterisk (*) or diverse lowercase(s) on top of the bars were indicative of significant differences (p < 0.01, Lsd) across treatments. Exogenous CSN at 25 mg·L−1 was employed.
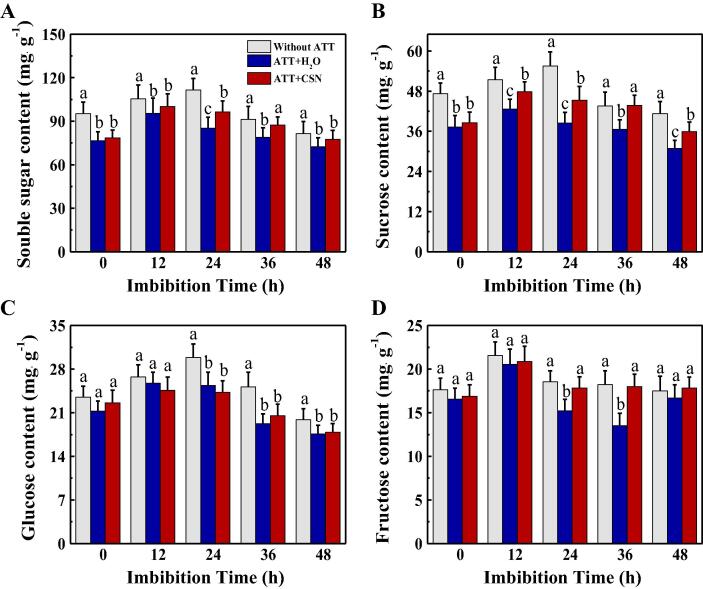
CSN treatment increased the contents of soluble sugars, ATP and energy charge in aged sunflower seeds during imbibitions time
The seed storage substances are the major sources of energy during early seed germination and seedling emergence, and sucrose, glucose and fructose are the main nutrients produced by the decomposition of storage substances. To further analyze the mechanism of CSN in promoting the germination and seedling emergence of aged sunflower seeds, this study determined the contents of soluble sugar, sucrose, glucose and fructose in sunflower seeds at the early germination stage (0–48 h) (Fig. 4). The results revealed that, CSN treatment remarkably improved the soluble sugar, sucrose and fructose contents in the AAT-treated sunflower seeds at the early germination stage, especially at 12 and 24 h after germination. While no significant difference in glucose content was detected between AAT + H2O and AAT + CSN treatments (Fig. 4). Furthermore, significant increased ATP content and energy charge were observed in AAT + CSN sunflower seeds as compared with AAT + H2O treatment (Supporting Information Fig. S2). The above findings revealed that CSN accelerated aged sunflower seed germination and seedling emergence through elevating soluble sugar and ATP levels.
Fig. 4.
Exogenous CSN increased the levels of soluble sugars (A), sucrose (B), glucose (C) and fructose (D) of sunflower seeds during imbibitions time. Without AAT: healthy seeds without accelerated aging test (AAT); AAT + H2O: AAT seeds with H2O; AAT + CSN: AAT seeds with compound sodium nitrophenolate (CSN) treatment. Four biological replicates for each treatment were set in sugars quantification. Diverse lowercase(s) on top of the bars were indicative of significant differences (p < 0.01, Lsd) across treatments. Exogenous CSN at 25 mg·L−1 was employed.
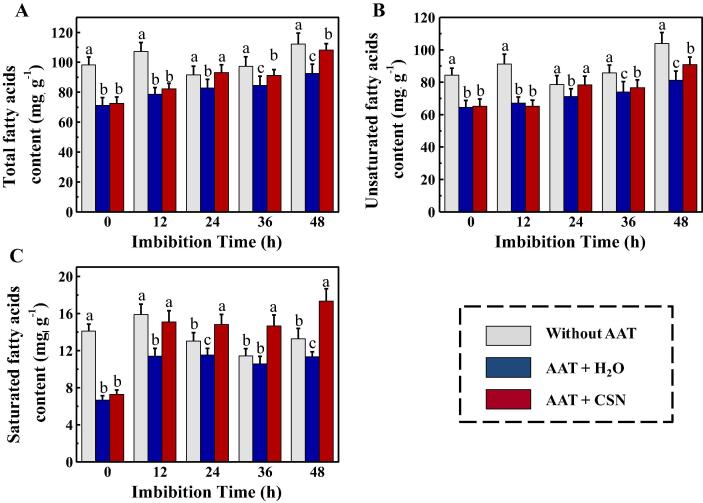
CSN increased several fatty acids contents in aged sunflower seeds during imbibitions
As is well-known, in the seed germination and early seedling emergence processes of oilseed crop, hydrolysis of triacylglycerol (oil) produced glycerol and fatty acids, while they are thus converted into diverse soluble sugars through gluconeogenesis [35], [36]. For understanding the reasons responsible for the increased soluble sugar levels in AAT-aged sunflower seeds after CSN treatment, we measured the contents of several fatty acids during sunflower seed imbibitions.
As suggested GA-MS analysis, the AAT treatment evidently decreased the total fatty acid, unsaturated fatty acid and saturated fatty acid contents. It was worth noting that the content of total, saturated, and unsaturated fatty acids were significantly increased in AAT + CSN seeds compared with AAT + H2O seeds at 24 h and 48 h of imbibitions time (Fig. 5). Subsequently, we test the changes in the main fatty acid content in sunflower seeds, such as the stearic, palmitic, oleic, linolenic and linoleic acids (Supporting Information Fig. S3). The results showed that CSN treatment increased the contents of oleic and linoleic in aged sunflower seeds during imbibitions. By contrast, the palmitic content of AAT + CSN seeds was significant lower than those in AAT + H2O seeds. While no significant difference in stearic acid and linolenic acids contents were detected between AAT + CSN and AAT + H2O treatments.
Fig. 5.
Exogenous CSN increased the contents of total fatty acids (A), unsaturated fatty acids (B) and saturated fatty acids (C) of sunflower seeds during imbibitions time. Without AAT: healthy seeds without accelerated aging test (AAT); AAT + H2O: AAT seeds with H2O; AAT + CSN: AAT seeds with compound sodium nitrophenolate (CSN) treatment. Four biological replicates for each treatment were set in fatty acids determination. Diverse lowercase(s) on top of the bars were indicative of significant differences (p < 0.01, Lsd) across treatments. Exogenous CSN at 25 mg·L−1 was employed.
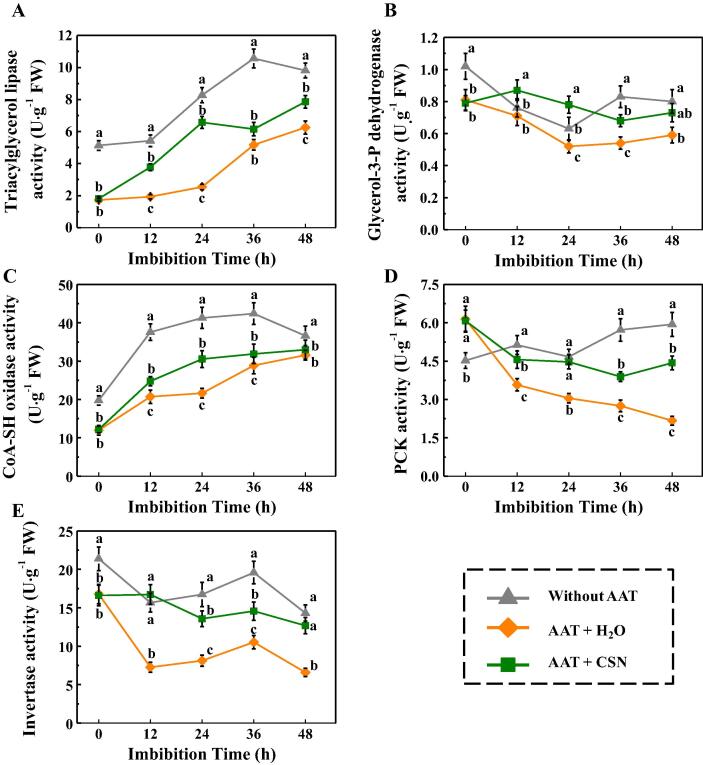
CSN increased the activities of several key enzymes which are involved in fatty acid and glycometabolism metabolism in aged sunflower seeds during imbibitions time
Given that CSN application increased levels of soluble sugars and fatty acids during aged sunflower seeds imbibitions, the CSN function in activities of several critical enzymes related to triacylglycerol conversion into sugars and fatty acids was examined (Fig. 6). AAT treatment significantly lowered the activities of triacylglycerol lipase (LIPG), glycerol-3-P dehydrogenase (GPDH), CoA-SH oxidase (ACX), phosphoenolpyruvate carboxykinase (PCK) and invertase (INV) in sunflower seeds during early germination time. By contrast, remarkably up-regulated activities of triacylglycerol lipase, glycerol-3-P dehydrogenase, and PCK were detected in AAT + CSN seeds compared with AAT + H2O treatment at 12 h, 24 h, 36 h, and 48 h of imbibitions time. Moreover, CSN application also enhanced the CoA-SH oxidase activity in AAT-treated seeds at 12 h, 24 h, and 36 h of imbibitions time. These results were in consistent with the positive effect of CSN on contents of fatty acid and soluble sugar in AAT-treated sunflower seeds during imbibitions.
Fig. 6.
Positive impacts of CSN on the activities of several key enzymes related to fatty acid metabolism and glycometabolism in sunflower seeds during imbibitions time. (A) Triacylglycerol lipase activity. (B) Glycerol-3-P dehydrogenase activity. (C) CoA-SH oxidase activity. (D) PCK activity. (E) Invertase activity. Without AAT: healthy seeds without accelerated aging test (AAT); AAT + H2O: AAT seeds with H2O; AAT + CSN: AAT seeds with compound sodium nitrophenolate (CSN) treatment. Four biological replicates for each treatment were set in enzymes activity assay. The asterisk (*) was indicative of significant differences (p < 0.01, Lsd) across treatments. Exogenous CSN at 25 mg·L−1 was employed.
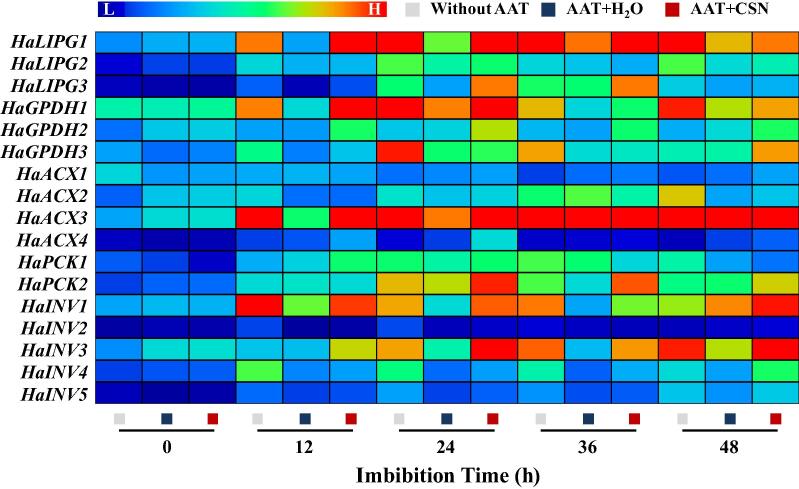
CSN increased the transcriptional levels of fatty acid and glycometabolism metabolism-related genes in aged sunflower seeds during imbibitions
The qPCR analysis revealed that the transcriptional levels of HaLIPG1, HaLIPG3, HaGPDH1, HaGPDH3, HaACX3, HaPCK2, HaINV1, HaINV2, and HaINV3 were decreased in AAT-treatment seeds as compared with seeds without AAT treatment. By contrast, CNS treatment significantly enhanced the transcriptional levels of HaLIPG1, HaLIPG3, HaGPDH1, and HaGPDH3. Similarly, the gene expressions of HaACX3 and HaPCK2 in AAT + CSN seeds were significantly higher than those in AAT + H2O. Besides, CSN application remarkably increased the transcriptional levels HaINV1 and HaINV3 in AAT-aged sunflower seeds (Fig. 7).
Fig. 7.
CSN treatment increased the transcriptional levels of fatty acid and glycometabolism metabolism-related genes in sunflower seeds during imbibitions. Without AAT: healthy seeds without accelerated aging test (AAT); AAT + H2O: AAT seeds with H2O; AAT + CSN: AAT seeds with compound sodium nitrophenolate (CSN) treatment; LIPG: triacylglycerol lipase; GPDH: glycerol-3-P dehydrogenase; ACX: acyl-CoA oxidase; PCK: phosphoenolpyruvate carboxykinase; INV: invertase. Real-time quantitative PCR was performed using three biological replications, and each was made in three technical replicates. The Illustrator software was used for creating the heat map. The gene levels from low (L) to high (H) indicated the lowest and highest levels in the whole database.
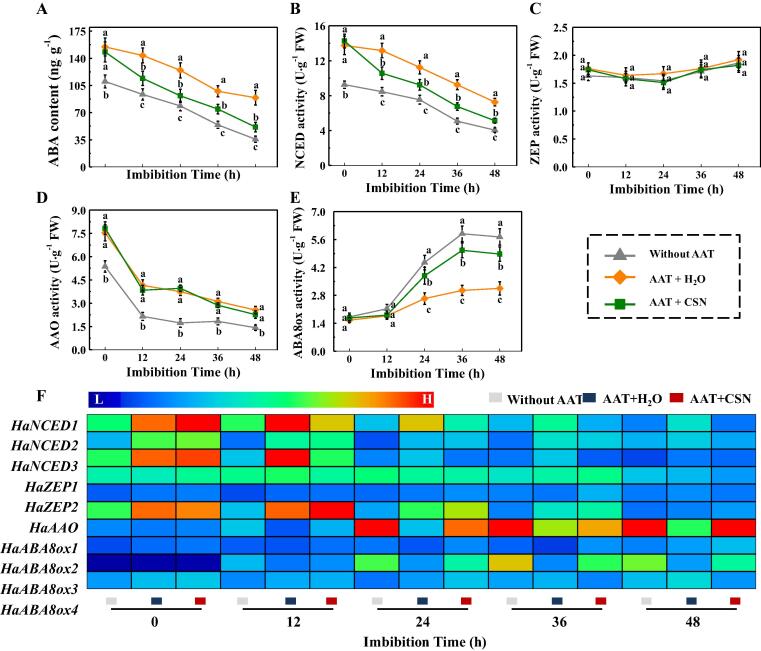
CSN decreased abscisic acid content in aged sunflower seeds during imbibitions
It was found that AAT treatment significantly enhanced the ABA content of sunflower seeds during early germination. CSN application significantly decreased the ABA content in AAT-aged sunflower seeds at 0–42 h of imbibitions time (Fig. 8A). Subsequently, we further test the effects of CSN on the activities of several key enzymes involved in the ABA metabolism, namely 9-cis-epoxycarotenoid dioxygenase (NCED), zeaxanthin epoxidase (ZEP), abscisic acid aldehyde oxidase (AAO), and ABA-8′-hydroxylases (ABA8ox). The enzyme activity analysis revealed that activities of NCED and AAO in AAT-aged sunflower seeds were significant lower than those in imbibed fresh seeds (Fig. 8BD). Besides, AAT treatment significantly decreased ABA8ox activity of sunflower seeds at 24 h, 36 h, and 48 h of imbibitions time (Fig. 8E). In contrast, CSN treatment markedly decreased NCED activity and increased ABA8ox activity in AAT-treated sunflower seeds at most of the time points (Fig. 8BE). While no significant difference in ZEP activity of sunflower seeds were observed among treatments during early imbibitions time (Fig. 8C).
Fig. 8.
The effect of CSN on the abscisic acid (ABA) metabolism in sunflower seeds during imbibitions time. (A) ABA content. (B) 9-cis-epoxycarotenoid dioxygenase (NCED) activity. (C) Zeaxanthin epoxidase (ZEP) activity. (D) Abscisic acid aldehyde oxidase (AAO) activity. (E) Abscisic acid −8′-hydroxylases (ABA8ox) activity. (F) The transcriptional levels of ABA metabolism-related genes. Without AAT: healthy seeds without accelerated aging test (AAT); AAT + H2O: AAT seeds with H2O; AAT + CSN: AAT seeds with compound sodium nitrophenolate (CSN) treatment. Four biological replicates for each treatment were set in ABA determination and enzymes activity assay. Real-time quantitative PCR was performed using three biological replications, and each was made in three technical replicates. The asterisk (*) was indicative of significant differences (p < 0.01, Lsd) across treatments. Exogenous CSN at 25 mg·L−1 was employed. The Illustrator software was used for creating the heat map. The gene levels from low (L) to high (H) indicated the lowest and highest levels in the whole database.
For GA, it was significantly lowered by AAT treatment during sunflower seed imbibitions time (Supporting Information Fig. S4). AAT treatment remarkably decreased the activities of GA3ox and GA20ox of sunflower seeds at 24 h, 36 h, and 48 h of imbibitions time. While exogenous CNS made no significant effect on GA content and activities of GA3ox, GA20ox, and GA2ox in AAT-treatment seeds during early germination time.
CSN regulated the transcriptional levels of abscisic acid metabolism-related genes in aged sunflower seeds during imbibitions
The qPCR analysis revealed that the transcriptional levels of HaNCED1, HaNCED2, HaNCED3, and HaAAO were increased in AAT-treatment seeds as compared with seeds without AAT treatment (Fig. 8F). AAT treatment also down-regulated the transcriptional levels of HaABA8ox1 and HaABA8ox3 of sunflower seeds at 24–48 h of imbibitions time. It was worth noting that the effect of CNS application on ABA metabolism-related gene expression was opposite to that of AAT treatment. CNS treatment significantly decreased the expressions of HaNCED1, HaNCED3, and HaAAO; By contrast, CNS significantly up-regulated the expressions of HaABA8ox1 and HaABA8ox3 in AAT-treatment sunflower seeds during imbibitions time. Such result conformed to the CSN impact on the activities of AAO, NCED, and ABA8ox in AAT-aged sunflower seeds in the imbibitions process.
Discussion
Seed germination is a complicated process under the control of various exogenous and endogenous factors [5]. In the present study, the germination and seedling emergence were significantly inhibited in both natural aged and artificially aged sunflower seeds. Typically, the blocked triacylglycerol conversion into soluble sugars, along with the elevated ABA content, might be the important reason for the decrease of germination and seedling emergence in aged sunflower seeds. The germination test and phenotypic analysis revealed that the plant growth regulator CSN effectively alleviated the inhibition of naturally aging and artificially aging on germination and early seedling emergence of sunflower seeds. That CSN contributes to improved germination and seedling emergence of aged sunflower seeds might be related with the metabolism of fatty acid, glycometabolism, and abscisic acid.
CSN positively affected germination and early seedling emergence of aged sunflower seeds
Seeds are the foundation of agricultural production. The successful seed germination and seedling emergence are of paramount importance to the plant growth and yield formation of crops [37]. The reduced seed vigor showed positive correlation with the storage period [38], [39], [40]. In traditional agriculture, crop seeds are susceptible to aging due to poor preservation environment and long preservation time, thus affecting the agricultural production. Consequently, enhancing the germination and seedling emergence capacities of the aged seeds is a worthy objective of concern in agricultural production.
Hydrated graphene ribbon application boost aged wheat seed germination by enhancing the catabolism of starch and triglycerides [41]. Mahakham et al [42] developed a nanopriming technology for boosting seed germination of aged rice seeds using phytosynthesized silver nanoparticles. Besides, ultrasonic treatment was proved had the function on germination improvement in tall fescue and Russian wildrye aged seeds [43]. Interestingly, a biomaterial was recently developed on the basis of trehalose and silk fibroin, which effectively boosts seed germination and alleviates soil salinity [44]. However, most of these studies focused germination rather than seedling emergence of the aged seeds, and suggested that there were divergent mechanisms underlying the effects improving germination of aged seeds.
Compound sodium nitrophenolate (CSN) is a highly efficient plant growth regulator first discovered by Asahi Kagaku Kogyo Co., Ltd (Japan) in the 1960s. Its effective components are sodium ortho-nitrophenolate, sodium para-nitrophenol and 5-nitroguaiacol sodium salt. In 1997, CSN was approved by the US Environmental Protection Agency into the American Green Food Project, and it was designated as the Green Food Project-recommended plant growth regulator by the Food and Agriculture Organization of the United Nations (FAO). Several studies have showed that CSN has a wide range of biological functions on plant growth and development regulation, such as flower differentiation, fruit development, senescence, and seed germination [20], [21], [22], [23]. This work extended the actual applications of CSN, suggesting that CSN might alleviate the suppression of naturally and artificially aging on sunflower seed germination and seedling emergence.
CSN promoted the hydrolysis of triacylglycerol to sugars in aged sunflower seeds
As is well known, soluble sugars represent the major energy for plant seeds at the early germination stage, which mainly includes sucrose and fructose [12]. For oilseeds, such as soybean and sunflower, the catabolism of triglyceride plays an important role in providing energy for seed germination and seedling emergence. Triglyceride can be decomposed into fatty acid and glycerin under the catalysis of LIPG, and GPDH catalyzed the hydrolysis of glycerol to form CoA-SH [45]. SDP1 (Sugar-Dependent 1) encoding triacylglycerol lipase, and a blocked triacylglycerol hydrolysis and arrested germination phenotype was detected in sdp6 mutant seeds of Arabidopsis [46]. ACX is responsible for encoding the acyl-CoA oxidase, which participates in fatty acid β-oxidation process. PCK is in charge of catalyzing oxaloacetate conversion into phosphoenolpyruvate, which represents the rate-limiting step in the metabolic pathway [47], [48]. Fructose and sucrose were the end-products of triacylglycerol hydrolysis, which provided the energy and ATP necessary for supporting plant seed germination and seedling emergence [49], [50].
In the present research, natural aging and artificial accelerated aging significantly reduced the sunflower seed germination and seedling emergence capacities, while exogenous CSN effectively mitigated the suppression on sunflower seed vigor after the aging process. Subsequent analysis suggested that, AAT treatment significantly suppressed the activities of several key enzymes (LIPG, GPDH, ACX, PCK and INV) and abundance of related genes expressions involved in the triacylglycerol hydrolysis pathway. Consistently, the contents of fatty acids, soluble sugars and ATP in AAT-aged sunflower seeds significantly decreased. The inhibition of conversion of triacylglycerol into sucrose by AAT treatment as reported in this study was in agreement with the findings obtained by Zhou et al [18] who reported that the blockage of fatty acid catabolism was the main reason for the poor seed vigor in aged soybean seeds. In contrast, CSN treatment remarkably promoted the conversion of triacylglycerol into sucrose and eventually maintained the energy supply at the early seed germination process.
Moreover, it was worth noting that CSN treatment significantly decreased palmitic content in sunflower seeds during imbibitions. While the contents of oleic and linoleic were enhanced by CNS treatment. The relationship between seed fatty acid content and seed vigor has been studied in soybean and sweet pepper. Bachleda et al [51] reported that the oleic acid content was significantly and negatively correlated with seed vigor in soybean (Glycine max). Besides, a positive correlation between linoleic acid content and germination ability was detected in sweet pepper; while higher level of palmitoleic acid in seed leading to decreased seed vigor [48]. It was proposed that the changes of different fatty acids content might be related to the regulation of CSN on germination ability of aged sunflower seeds.
CSN decreased the abscisic acid content in aged sunflower seeds
Abscisic acid (ABA) and Gibberellin (GA) are two important phytohormones that play important roles during seed dormancy and germination [52], [53], [54]. For instance, GA can break seed dormancy and induce seed germination. It is shown that GA can activate the endogenous hydrolase activity of seeds to promote the decomposition of stored substances and weaken barrier tissues (such as endosperm and endosperm cap), thus inducing seed germination [55]. In contrast, high concentration of ABA induces seed dormancy that suppresses seed germination [56], [57]. Gibberellin (GA) and ABA metabolism is closely related to the seed aging process. Lin et al [58] reported that the natural aged process dramatically increased the ABA content and decreased the GA content in rice seeds. After storage, seeds with lower seed vigor had higher ABA content while lower GA content. However, whether the regulation of CSN on germination of aged-sunflower seeds is related to the GA and ABA metabolism has not been reported. In this study, AAT treatment significantly increased ABA content of sunflower seeds during imbibitions time, closely associated with the remarkably increased activities of ABA biosynthesis enzymes (NCED and AAO) and decreased activity of inactivation enzyme ABA8ox. Exogenous CSN weakened the induction of ABA content by AAT, notably, only associated with the regulation of NCED and ABA8ox enzymes. Besides, AAT treatment down-regulated the activities of GA biosynthesis enzymes (GA3ox and GA20ox), and thereby suppressed GA accumulation in sunflower seeds during imbibitions. However, CSN treatment made no significant effect on GA content in AAT-treated sunflower seeds during imbibitions time. It was proposed that GA metabolism might not play a crucial role in the regulation of CSN on sunflower seed germination.
Conclusions
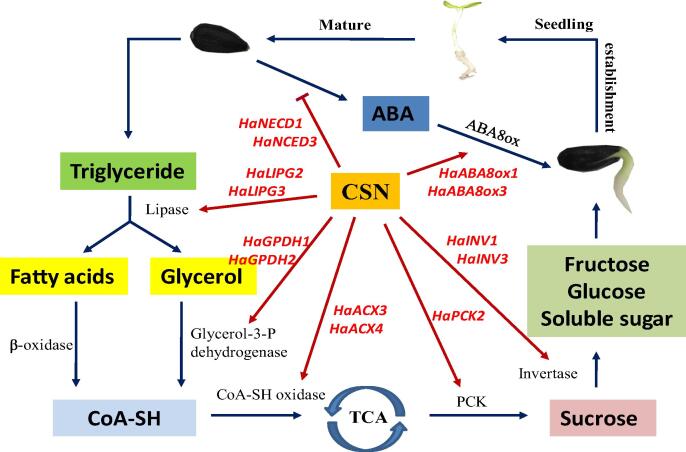
To sum up, this study revealed that, the CSN application could improve the germination and seedling emergence capacities of naturally and artificially aged sunflower seeds. During the germination process of aged sunflower seeds, exogenous CSN enhanced the hydrolysis rate of triacylglycerol into fatty acids and glycerin. Meanwhile, it promoted the conversion of fatty acids and glycerin into sucrose and fructose. Moreover, CSN treatment reduced the ABA content by regulating the activities and genes expressions of NCED and ABA8ox in aged sunflower seeds (Fig. 9). On the one hand, the present study revealed that the fatty acid, glycometabolism, and abscisic acid metabolism are crucial to the germination and seedling emergence of aged sunflower seeds. On the other hand, it provided theoretic and practical basis for using CSN to enhance safety production for sunflower.
Fig. 9.
Proposed schema for the role of CSN in promoting germination and seedling emergence of aged sunflower seeds. For aged sunflower seeds, the conversion from triacylglycerol to fatty acid and glycerol and finally to soluble sugars was blocked in the absence of CSN. The abscisic acid (ABA) content in aged seeds was significantly increased. Exogenous CSN application in aged sunflower seeds increased the activities of several key enzymes and transcription of related genes involved in this pathway. Besides, exogenous CSN decreased abscisic acid (ABA) content through the regulation of the gene expressions and activities of metabolism related-enzymes. In conclusion, this model suggested that CSN promoted aged sunflower seed germination and seedling emergence through modulating the metabolism of fatty acids, glycometabolism and ABA.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This study is supported by the Key Research and Development Program of Zhejiang Province (No. 2019C02004).
Author contributions
Huang Y designed and performed most of the experiments; Cai S performed the seed storage and germination test. Ruan X and Xu J contributed to the HPLC and qRT-PCR analysis. Cao D reviewed and edited the whole manuscript. All authors read and approved the final version of the manuscript.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.01.019.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Buti M., Giordani T., Cattonaro F., Cossu R.M., Pistelli L., Vukich M. Temporal dynamics in the evolution of the sunflower genome as revealed by sequencing and annotation of three large genomic regions. Theor. Appl. Genet. 2011;123(5):779–791. doi: 10.1007/s00122-011-1626-4. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y., Zhang W.L. Analysis of changes and trends in world sunflower production and trade structure. World Agr. 2018;9:119–126. [Google Scholar]
- 3.Celus M., Salvia-Trujillo L., Kyomugasho C., Maes I., Loey A.V., Grauwet T. Structurally modified pectin for targeted lipid antioxidant capacity in linseed/sunflower oil-in-water emulsions. Food Chem. 2018;241:86–96. doi: 10.1016/j.foodchem.2017.08.056. [DOI] [PubMed] [Google Scholar]
- 4.Nonogaki H., Bassel G.W., Bewley J.D. Germination-still a mystery. Plant Sci. 2010;179:574–581. [Google Scholar]
- 5.Rajjou L., Duval M., Gallardo K., Catusse J., Bally J., Job C. Seed germination and vigor. Annu. Rev. Plant. Biol. 2012;63(3):507–533. doi: 10.1146/annurev-arplant-042811-105550. [DOI] [PubMed] [Google Scholar]
- 6.Wang L., Hu W., Zahoor R., Yang X.N., Wang Y.H., Zhou Z.G. Cool temperature caused by late planting affects seed vigor via altering kernel biomass and antioxidant metabolism in cotton (Gossypium hirsutum L.) Field Crop Res. 2019;236:145–154. [Google Scholar]
- 7.Dang X.J., Thi T.G.T., Dong G.S., Wang H., Edzesi W.M., Hong D.L. Genetic diversity and association mapping of seed vigor in rice (Oryza sativa, L.) Planta. 2014;239:1309–1319. doi: 10.1007/s00425-014-2060-z. [DOI] [PubMed] [Google Scholar]
- 8.Chen M., Thelen J.J. The plastid isoform of triose phosphate isomerase is required for the postgerminative transition from heterotrophic to autotrophic growth in Arabidopsis. Plant Cell. 2010;22:77–90. doi: 10.1105/tpc.109.071837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theodoulou F.L., Eastmond P.J. Seed storage oil catabolism: a story of give and take. Curr. Opin. Plant. Biol. 2012;15:322–328. doi: 10.1016/j.pbi.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Y.F., Ren Y., Li W., Wu F.S., Yang W.J., Huang X.L. NF-YC12 is a key multi-functional regulator of accumulation of seed storage substances in rice. J. Exp. Bot. 2018;70:15. doi: 10.1093/jxb/erz168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goepfert S., Poirier Y. β-Oxidation in fatty acid degradation and beyond. Curr. Opin. Plant. Biol. 2007;10(3):245–251. doi: 10.1016/j.pbi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Quettier A.L., Shaw E., Eastmond P.J. SUGAR-DEPENDENT6 encodes a mitochondrial flavin adenine dinucleotide-dependent glycerol-3-P dehydrogenase, which is required for glycerol catabolism and postgerminative seedling growth in Arabidopsis. Plant Physiol. 2008;148(1):519–528. doi: 10.1104/pp.108.123703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morscher F., Kranner I., Arc E., Bailly C., Roach T. Glutathione redox state, tocochromanols, fatty acids, antioxidant enzymes and protein carbonylation in sunflower seed embryos associated with after-ripening and ageing. Ann Bot-London. 2015;116(4):669–678. doi: 10.1093/aob/mcv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-Mellado D., Salas J.J., Venegas-Calerón M., Perez A.J.M., Garcés R., Force E.M. Functional characterization and structural modelling of Helianthus annuus (sunflower) ketoacyl-CoA synthases and their role in seed oil composition. Planta. 2019;249(6):1–14. doi: 10.1007/s00425-019-03126-1. [DOI] [PubMed] [Google Scholar]
- 15.Faligowska A., Bartosspychala M., Panasiewicz K. The effect of storage period on sowing value and vigor of narrow-leaved lupin dressed seed. Prog. Plant. Prot. 2012;52(4):1151–1155. [Google Scholar]
- 16.Zhao S., Huang W., Jiang H., Sun J.Y., Yin L.Y., Li W. Hydrocharis dubia seeds maintain high seed vigor in ambient wet storage condition through scavenging hydrogen peroxide by antioxidant systems. Aquat Bot. 2017;143:18–24. [Google Scholar]
- 17.Chen X.J., Qi K.C., Pu H.M., Zhang J.F., Chen S., Gao J.Q. Effects of storage period and temperature on seed fatty acid and vigor of Brassica napus L. Chin. J. Oil. Crop. Sci. 2010;32(4):491–494. [Google Scholar]
- 18.Zhou W.G., Chen F., Zhao S.H., Yang C.Q., Meng Y.J., Shuai H.W. DA-6 promotes germination and seedling emergence from aged soybean seeds by mediating fatty acid metabolism and glycometabolism. J. Exp. Bot. 2019;70(1):101–114. doi: 10.1093/jxb/ery247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong D.Z., Tian Y.F., Cao Y.L., Liu Y.P. The physiological mechanisam study of ultradrying and aged sunflower seeds during germinating. Acta. Agric. Boreali-Sin. 2008;23(4):168–171. [Google Scholar]
- 20.Cui X.C., Hu J.L., Lin X.G., Xu J.B., Dai Y., Wang J.H. Application of arbuscular mycorrhizal fungi and compound sodium nitrophenolate in tomato seedling growth. Chin. J. Appl. Environ. Biol. 2012;18(5):843–846. [Google Scholar]
- 21.Li H.L., Wang J.L., Xue Z.J., Zhu E.C., Gao Z.K. Effects of compound sodium nitrophenolate on growth and nitrate reduction and assimilation in the leaves of Chinese Chive. Acta Bot. Boreal-Occident Sin. 2014;34(3):740–745. [Google Scholar]
- 22.Zhang Y.X., Xia S.N., Yang M.N., Li Y.Q., Zhang L.J., Xie Y.T. Plant growth promoter effects on the growth and yield of transplanted cotton in northern Jiangxi. Cotton Sci. 2019;31(3):233–241. [Google Scholar]
- 23.Li R.H., Xu D.B., Huang Q.W., Xu Y.C., Yang X.M., Shen Q.R. Effect of foliar fertilizer on growth of rape seed seedlings. J Nanjing Agr Univ. 2008;31(3):91–96. [Google Scholar]
- 24.Wang H.X., Lin M., Gong J.Q., Ke P.Z., Lin H.F. Effects of atonik on growth and fruit setting of Satsuma mandarin. J Fruit Sci. 2003;20(4):291–294. [Google Scholar]
- 25.Shen Y.D., Chen X.H., Yang J.Q. Effect of atonik SL on Feizixiao litchi. Chin. J. Trop. Agric. 2004;24(6):17–20. [Google Scholar]
- 26.Missaoui A.M., Hill N.S. Use of accelerated aging as a surrogate phenotyping approach to improve endophyte survival during storage of tall fescue seed. Field Crop Res. 2015;183:43–49. [Google Scholar]
- 27.Zhang S.W., Gu Z.B., Wang X.M. Determination of compound sodium nitrophenolate residues in fruit by ultra performance liquid chromatography tandem mass spectrometry. Chem Anal Meter. 2015;24(1):68–70. [Google Scholar]
- 28.Zhu L., Cao D., Hu Q., Guan Y., Hu W., Nawaz A. Physiological changes and sHSPs genes relative transcription in relation to the acquisition of seed germination during maturation of hybrid rice seed. J Sci Food Agr. 2015;96:1–8. doi: 10.1002/jsfa.7283. [DOI] [PubMed] [Google Scholar]
- 29.Shi H., Wang B., Yang P., Li Y., Miao F. Differences in sugar accumulation and mobilization between sequential and non-sequential senescence wheat cultivars under natural and drought conditions. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0166155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai Y., Shao L., Li X., Liu G., Chen S. Gibberellin stimulates regrowth after defoliation of sheepgrass (Leymus chinensis) by regulating expression of fructan-related genes. J Plant Res. 2016;129:935–944. doi: 10.1007/s10265-016-0832-1. [DOI] [PubMed] [Google Scholar]
- 31.Bailly C., Audigier C., Ladonne F., Wagner M.H., Coste F., Corbineau F. Changes in oligosaccharide content and antioxidant enzyme activities in developing bean seeds as related to acquisition of drying tolerance and seed quality. J Exp Bot. 2010;52(357):701–708. doi: 10.1093/jexbot/52.357.701. [DOI] [PubMed] [Google Scholar]
- 32.Emami H., Kempken F. PRECOCIOUS1 (POCO1), a mitochondrial pentatricopeptide repeat protein affects flowering time in Arabidopsis thaliana. Plant J. 2019;100(2):265–278. doi: 10.1111/tpj.14441. [DOI] [PubMed] [Google Scholar]
- 33.Yang C., Iqbal N., Hu B., Zhang Q., Wu H., Liu X. Targeted metabolomics analysis of fatty acids in soybean seeds using GC-MS to reveal the metabolic manipulation of shading in the intercropping system. Anal Methods-UK. 2017;9:2144–2152. [Google Scholar]
- 34.Huang Y.T., Lin C., He F., Li Z., Guan Y.J., Hu Q.J. Exogenous spermidine improves seed germination of sweet corn via involvement in phytohormone interactions, H2O2 and relevant gene expression. BMC Plant Biol. 2017;17:1–16. doi: 10.1186/s12870-016-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eastmond P.J. MONODEHYROASCORBATE REDUCTASE4 is required for seed storage oil hydrolysis and postgerminative growth in Arabidopsis. Plant Cell. 2007;19:1376–1387. doi: 10.1105/tpc.106.043992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quettier A.L., Eastmond P.J. Storage oil hydrolysis during early seedling growth. Plant Physiol Bioch. 2009;47:485–490. doi: 10.1016/j.plaphy.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Zhang K.L., Yao L.J., Zhang Y., Baskin J.M., Baskin C.C., Xiong Z.M. A review of the seed biology of Paeonia species (Paeoniaceae), with particular reference to dormancy and germination. Planta. 2019;249:291–303. doi: 10.1007/s00425-018-3017-4. [DOI] [PubMed] [Google Scholar]
- 38.Yin G.K., Xin X., Xiao C.S., Chen L., Zhang J.M., Wu S.H. Activity levels and expression of antioxidant enzymes in the ascorbateglutathione cycle in artificially aged rice seed. Plant Physiol Bioch. 2014;80:1–9. doi: 10.1016/j.plaphy.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Yin X., He D., Gupta R., Yang P. Physiological and proteomic analyses on artificially aged Brassica napus seed. Front Plant Sci. 2015;6:112. doi: 10.3389/fpls.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleming M.B., Richards C.M., Walters C. Decline in RNA integrity of dry-stored soybean seeds correlates with loss of germination potential. J Exp Bot. 2017;68:2219–2230. doi: 10.1093/jxb/erx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu X., Zhou Q. Novel hydrated graphene ribbon unexpectedly promotes aged seed germination and root differentiation. Sci Rep-UK. 2014;4:3782. doi: 10.1038/srep03782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahakham W., Sarmah A.K., Maensiri S., Theerakulpisut P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci Rep-UK. 2017;7:8263. doi: 10.1038/s41598-017-08669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J., Wang Q., Karagić Đ., Liu X., Cui J., Gui J. Effects of ultrasonication on increased germination and improved seedling growth of aged grass seeds of tall fescue and Russian wildrye. Sci Rep-UK. 2016;6:22403. doi: 10.1038/srep22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zvinavashe A.T., Lim E., Sun H., Marelli B. A bioinspired approach to engineer seed microenvironment to boost germination and mitigate soil salinity. Proc. Natl. Acad. Sci. USA. 2019;116(51):25555–25561. doi: 10.1073/pnas.1915902116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li-Beisson Y., Shorrosh B., Beisson F., Andersson M.X., Arondel V., Bates P.D. Acyl-lipid metabolism. Arabidopsis Book. 2010;8 doi: 10.1199/tab.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eastmond P.J. SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell. 2006;18:665–675. doi: 10.1105/tpc.105.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Penfield S., Clements S., Bailey K.J., Gilday A.D., Leegood R.C., Gray J.E. Expression and manipulation of phosphoenolpyruvate carboxykinase 1 identifies a role for malate metabolism in stomatal closure. Plant J. 2012;69:679–688. doi: 10.1111/j.1365-313X.2011.04822.x. [DOI] [PubMed] [Google Scholar]
- 48.Eastmond P.J., Astley H.M., Parsley K., Aubry S., Williams B.P., Menard G.N. Arabidopsis uses two gluconeogenic gateways for organic acids to fuel seedling establishment. Nat Commun. 2015;6:6659. doi: 10.1038/ncomms7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaymak H. Potential effect of seed fatty acid profile of pepper (Capsicum annuum L.) cultivars on germination at various temperatures. Zemdirbyste. 2014;101:321–326. [Google Scholar]
- 50.Basnet R.K., Carpio D.P.D., Xiao D., Bucher J., Jin M., Boyle K. A systems genetics approach identifies gene regulatory networks associated with fatty acid composition in Brassica rapa seed. Plant Physiol. 2016;170(1):568–585. doi: 10.1104/pp.15.00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bachleda N., Grey T., Li Z. Effects of high oleic acid soybean on seed yield, protein and oil contents, and seed germination revealed by near-isogeneic lines. Plant Breeding. 2017;136:539–547. [Google Scholar]
- 52.An Y.Q., Lin L. Transcriptional regulatory programs underlying barley germination and regulatory functions of gibberellin and abscisic acid. BMC Plant Biol. 2011;11:105. doi: 10.1186/1471-2229-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boccaccini A., Lorrai R., Ruta V., Frey A., Mercey-Boutet S., Marion-Poll A. The DAG1 transcription factor negatively regulates the seed-to-seedling transition in Arabidopsis acting on ABA and GA levels. BMC Plant Biol. 2016;16(1):198. doi: 10.1186/s12870-016-0890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z.L., Ogawa M., Fleet C.M., Zentella R., Hu J.H., Heo J. SCARECROW-LIKE 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011;108:2160–2165. doi: 10.1073/pnas.1012232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X., Hu P., Huang M., Tang Y., Li Y., Li L. The NF-YC-RGL2 module integrates GA and ABA signaling to regulate seed germination in Arabidopsis. Nat Commun. 2016;7:12768. doi: 10.1038/ncomms12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arc E., Sechet J., Corbineau F., Rajjou L., Marion-Poll A. ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front Plant Sci. 2013;4:63. doi: 10.3389/fpls.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jia L., Wu Q., Ye N., Liu R., Shi L., Xu W. Proanthocyanidins inhibit seed germination by maintaining a high level of abscisic acid in Arabidopsis thaliana. J Integr Plant Biol. 2012;54:663–673. doi: 10.1111/j.1744-7909.2012.01142.x. [DOI] [PubMed] [Google Scholar]
- 58.Lin C., Shen H.Q., Guan Y.J., An J.Y., Hu W.M., Hu J. Changes of physiological, biochemistry and gene expression related to ABA and GA3 in hybrid rice seeds stored at different moisture contents and packing methods. Plant Physiol J. 2017;53(6):1077–1086. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.