Graphical abstract
Keywords: Endometriosis, Biomarker candidates, Gut microbiome, Genital microbiome, Glycan biomarker
Abstract
Background
Endometriosis is a chronic gynaecological disease whose aetiology is still unknown. Despite its prevalence among women of reproductive age, the pathology of the disease has not yet been elucidated and only symptomatic treatment is available. Endometriosis has high latency and diagnostic methods are both limited and invasive.
Aim of review
The aim of this review is to summarise minimally invasive or non-invasive diagnostic methods for endometriosis and their diagnostic efficiencies. Furthermore, we discuss the identification and diagnostic potential of novel disease biomarkers of microbial or glycan origin.
Key scientific concepts of review
Great efforts have been made to develop minimally invasive or non-invasive diagnostic methods in endometriosis. The problem with most potential biomarker candidates is that they have high accuracy only in cases of severe disease. Therefore, it is necessary to examine other potential biomarkers more closely. Associations between gastrointestinal and genital tract microbial health and endometriosis have been identified. For instance, irritable bowel syndrome is more common in women with endometriosis, and hormonal imbalance has a negative impact on the microbiome of both the genital tract and the gastrointestinal system. Further interrogation of these associations may have potential diagnostic significance and may identify novel therapeutic avenues. Glycomics may also be a potent source of biomarkers of endometriosis, with a number of glyco-biomarkers already approved by the FDA. Endometriosis-associated microbial and glycomic profiles may represent viable targets for development of innovative diagnostics in this debilitating disease.
Introduction
Endometriosis (EMS) is a chronic gynaecological condition which affects at least 176 million women worldwide, around 6–10% of women of childbearing age. EMS is characterized by the growth of endometrial-like tissue outside the uterine cavity, and is associated with pelvic pain, dysmenorrhea and infertility. Despite its prevalence, however, the aetiology of the disease is still poorly understood [1]. In addition to fertility problems and reduced quality of life, this enigmatic disease also has serious economic consequences [2]. Direct healthcare costs for women with EMS are more than twice as high as women without the disease [3]. Furthermore, the EMS-related burden of illness cost has been valued as high as 54 million euros per year in European Union countries, representing a serious burden for society. This amount also includes additional costs beyond hospitalisation of the disease e.g. lost days at work, layoffs, having to change jobs, sick leave, time off for having surgery [4].
There are several hypotheses regarding the origin of the disease, which can be divided into the following four major groups: transport, coelomic metaplasia, embryonic cell rests, and immunological theories [5], [6], [7]. The theory of coelomic metaplasia can provide an explanation for the phenomenon of EMS described in men, while immunological theories give an answer to the persistence of the disease [8]. Based on our current knowledge, we assume that the development and persistence of the disease depend on several co-existing factors. Indeed it is likely that EMS is a condition of multifactorial aetiology; involving genetic predisposition, prenatal exposure to endocrine-disrupting chemicals, the microbiome, the immune system and sex hormones [9].
Diagnosis of EMS remains challenging, due in large part to the wide range spectrum of symptoms associated with the disease [10]. Definitive diagnosis is invasive, requiring laparoscopic surgery. Numerous attempts have been made to develop an effective and less invasive diagnostic method to date. The emerging discipline of glycomics holds promise as a new ‘omics’ approach to understanding complex diseases. As a result of the development of separation techniques, we are increasingly aware of the importance of glycosylation. Glycomics is a discipline for the study of carbohydrates and indirectly provides an opportunity to discover new glyco-biomarkers [11]. In this review, we present efforts to diagnose EMS and outline a microbiome and glycosylation profile-based approach as a potential new source of biomarkers.
Background information on endometriosis
Symptoms and presentation of disease
There are many symptoms of EMS, but the most common are severe dysmenorrhea, deep dyspareunia, ovulation pain, irregular uterine bleeding, infertility, chronic fatigue, pelvic tenderness and chronic pelvic pain; although none of these symptoms are specific for EMS. Endometriosis is strongly associated with infertility, with a 35–50% prevalence of EMS in women presenting with infertility [12]. However, evidence for the effect of EMS on likelihood of pregnancy has been conflicting [13], [14]. Many women with EMS pursue pregnancy via Assisted Reproduction Technologies (ART) which is considered an effective treatment option for women with EMS [15]. However, even with the availability of ART, a negative impact can be seen on many parameters of in-virto fertilization in women with EMS [16]. The likely mechanism for the impact of EMS on fertility, whether related to oocyte and subsequent embryo quality, or implantation, has also been debated. Some have found no difference in number or quality of embryos from patients with EMS compared to those without [17]. A reduced ongoing pregnancy rate in those with EMS was noted, suggesting an altered endometrial receptivity. Others have noted a reduction in oocyte quality, fertilitisation rates and embryo quality [18], [19]. It is likely that EMS impairs fertility through multiple pathways [20].
Diagnosis
The diagnostic time for EMS is 4–11 years (average time ~ 7 years), in part due to the nonspecific disease symptoms and highly limited tools of diagnosis [21], [22]. Diagnostic techniques such as two-, or three-dimensional ultrasound, magnetic resonance imaging and other imaging techniques may be effective to diagnose ovarian and deep infiltrating EMS [23], [24]. These aforementioned imaging methods are only suitable for detecting severe and extended or clearly visible EMS, but histological confirmation is still required. The gold standard for confirmatory diagnosis of EMS is laparoscopic surgery with histologic examination after biopsy [25]. However, the surgical diagnosis has multiple drawbacks, such as risks inherent to the procedure and anaesthetic complications.
Pathogenesis and risk factors
Neither the exact pathophysiology of EMS nor the risk factors are fully elucidated, but a number of factors have been scientifically investigated [26], [27]. The most widely accepted theory of the pathogenesis of EMS is that of retrograde menstruation, wherein endometrial tissue is expelled into the peritoneal cavity during menstruation [28]. Evidence for this theory is based on the increased incidence of EMS seen in women with outflow obstruction, such as cervical stenosis and uterine anomalies [29], [30]. However, as 90% of women experience retrograde menstruation, it is likely that the eutopic endometrium itself in EMS is abnormal, predisposing to the formation of ectopic deposits [31]. A link between EMS and pelvic infection has been suggested. A retrospective study of data from over 14,000 individuals suggested that the risk of developing EMS was three times higher in those with pelvic inflammatory disease [32]. A growing number of studies suggest a link between EMS and other chronic and autoimmune diseases [33], [34]. The incidence of EMS is over 2-fold higher among women whose mothers also suffered from the disease, however, genetic predisposition is not the only contributing factor for the development of EMS [35], [36]. Additionally, lower birth weight, early age at menarche and shorter menstruation cycles (<26 days) have been associated with a higher risk of EMS [37], [38], [39]. Decreased pregnancy rates and nulliparity as a result of modern lifestyle contribute to an elevated incidence of EMS. However, pregnancy cannot be a strategy for managing symptoms and reducing the progression of the EMS, because there is a poor connection between the positive effects of pregnancy and EMS [40]. There is clear association between environmental toxins like polychlorinated biphenyl and dioxin [41], [42] and other adulthood exposure (e.g. alcohol and caffeine intake) and higher risk of EMS [43], [44], [45]. There are many other indirect risk factors of EMS, like skin sensitivity, night shift work and certain dietary factors, but further investigations are needed to uncover clear associations [27], [46].
Biomarkers
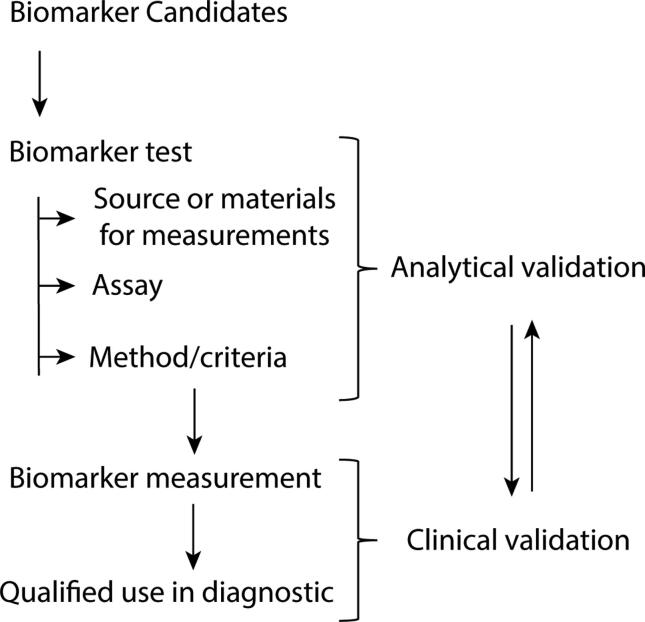
Given the severe and debilitating outcomes of EMS, there is a high demand for a less invasive diagnostic biomarker. First, we need to understand what a biomarker is and its significance. A biomarker is defined as a specific attribute that is measured as an indicator of normal biological processes, pathogenic processes, or response to exposure or intervention, including therapeutic interventions [47]. A diagnostic biomarker is a characteristic that can be used to detect or confirm disease or condition, or to identify individuals with a subtype of the disease. All available evidence must be gathered from a biomarker candidate and the potential benefits and risks of use must be presented objectively [48]. Clinical and analytical validation are distinct processes; however, these two parts of the validation process are interrelated (Fig. 1). A reliable measurement method must be developed and analytically validated before determining a cut-off value. These circumspect measures improve the biomarker candidate's clinical validation success in the clinical trial phase [48]. To choose biomarker candidates, it is very important to select an adequate control group. Most candidates perform well as a potential marker when compared to a healthy control group. Endometriosis is a disease with a very heterogeneous appearance and as such, it can be misdiagnosed as a number of other inflammatory gynaecological or urological conditions. Therefore, it is important to select a heterogeneous control group that meets the criteria of the highly specific diagnosis of EMS [48]. Cochrane studies have concluded that there are currently no non-invasive biomarker candidates that can replace invasive laparoscopic surgery in clinical practice [49]. In light of this landscape, we will herein briefly summarize the scientific information to date and outline how microbial and glycosylation patterns may hold promise as novel biomarkers of EMS.
Fig. 1.
Biomarker selection and validation approach. Clinical and analytical validation are distinct processes; however, these two parts of the validation process are connected.
Putative non-invasive candidate biomarkers
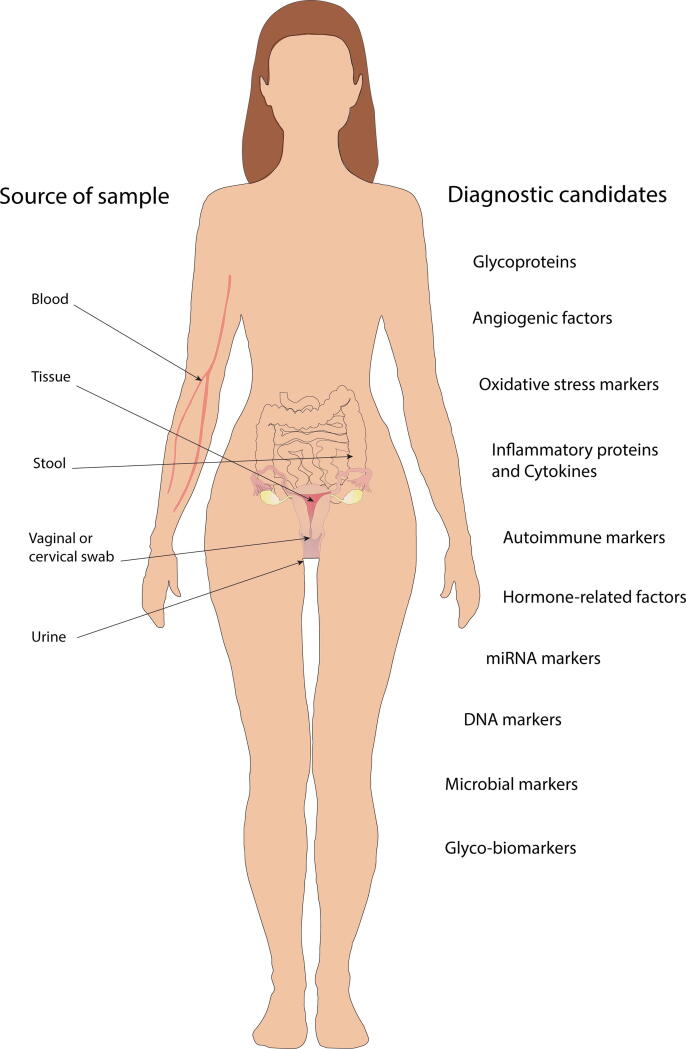
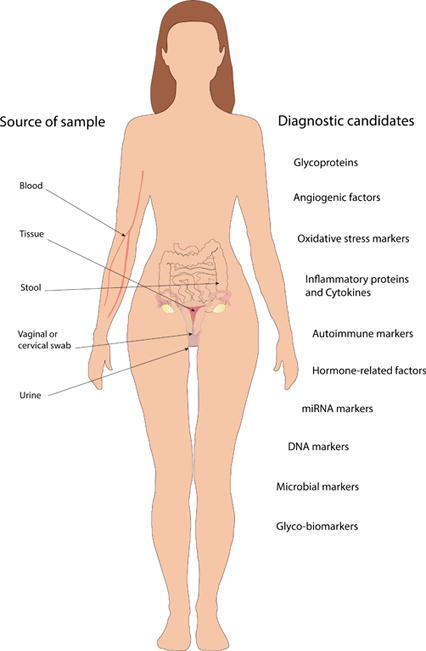
In recent years, many studies have targeted the pathomechanism of EMS adopting mostly molecular biological studies of endometrial lesions and healthy endometrial tissues. Another major area of research is the search for potential new biomarkers and several blood-derived candidates have been tested (Fig. 2). Below we present promising minimally or non-invasive biomarker candidates and evaluate their suitability as a potential replacement for laparoscopy diagnosis.
Fig. 2.
Summary of sample sources and potential biomarker candidates for endometriosis diagnosis that could replace highly accurate but invasive laparoscopic surgery. The presented promising minimally or non-invasive biomarker candidates' suitability as a potential replacement for laparoscopy diagnosis are questionable. A large pool of biomarker sources (blood, tissue, urine, stool, vaginal or cervical swabs) and a glycomic or microbiology approach open up further perspectives for identifying new candidates.
Glycoproteins
Cancer antigen (CA)-125 is a membrane glycoprotein, member of the mucin family and a component of the epithelium of the female reproductive tract, which inhibits the adhesion of infectious agents to epithelial cells [50]. The FDA approved the measurement of CA-125, in combination with other factors, to estimate the risk of epithelial ovarian cancer and monitor response of the treatment [51], [52]. CA-125 had been identified as a very promising candidate in EMS [52] (Table 1), displaying menstrual levels almost 200% higher in women with EMS compared to healthy controls [53]. However, further clinical studies revealed that it is alone a poor diagnostic marker of EMS [54]. While CA-125 is effective for indicating inflammatory, benign, and malignant transformations of the genital tract, its ubiquitous presence renders it poorly specific as a marker for EMS. The efficacy of CA-125, CA 19–9, CA 72–4 and human epididymis protein 4 have been tested individually or in combination, but neither method has proven sensitive enough to be a viable biomarker in early EMS [55], [56], [57]. CA-125 has also been tested in combination with other potential candidates to promote higher accuracy, but these could be expensive or difficult to apply in clinical practice [58] (Table 1). The endometrial glands produce Glycodelin-A during the secretory phase, which has an immunosuppressive function, is involved in the regulation of angiogenesis and apoptosis and may play an important role in the fetomaternal protective mechanism [59], [60]. Glycodelin-A levels have been demonstrated to increase in the bloodstream of patients with EMS. It is an unsuitable biomarker candidate alone [61], [62]; but in combination with other factors may form an effective biomarker panel [63], [64]. While CA-125 and Glycodelin-A glycoproteins are promising biomarkers in severe EMS versus healthy controls, none are sufficiently sensitive enough to replace the gold standard of diagnostic surgery.
Table 1.
Reported sensitivity, specificity and other features of promising biomarker candidates in endometriosis.
| Analyte | Source of the analyte | Limitation | Sensitivity | Specificity | Cut-off value | Reference |
|---|---|---|---|---|---|---|
| CA-125 | serum | for diagnosis of moderate or severe EMS | 52% | 93% | ≥ 30 units/mL | Hirsch et al. 2016 |
| CA-125 + 50 IU/mL | peripherial blood | unknown | 92.2% | 81.6% | CCR1/HPRT + MCP-1 1.16 pg/mL 140 pg/mL |
Agic et al. 2008 |
| VEGF | serum | unknown | 74% | 80% | >3.88 pg/mL | Kressin et al. 2001 |
| sTNFR-I | serum | unknown | 60.7% | 75% | 351.22 pg/ml | Othman et al. 2016 |
| Galectin-9 | serum | only compared to healthy, normal pelvic control | 94% | 93.75% | 132 pg/mL | Brubel et al. 2017 |
| Urocortin | plasma | specific diagnosis only in ovarian EMS | 76% | 88% | 46 pg/mL | Pergialiotis et al. 2019 |
| let-7b + let-7c + let-7d + let-7e | serum | hormonal phase affects the result | 83.3% | 100.0% | 0.823 pg/mL | Cho et al. 2015 |
| miR-122 | serum | unknown | 95.6% | 91.4% | 3.24 pg/mL | Maged et al. 2018 |
| ccf nDNA and ccf mtDNA | peripheral blood | unknown | 70% | 87% | 416 ccf nDNA genome equivalent/ml | Zachariah et al. 2009 |
Angiogenic factors
Since angiogenesis has an essential role in the progression of ectopic lesions during EMS, vascular endothelial growth factor (VEGF) is the most studied pro-angiogenic factor in EMS [65]. It is widely accepted that VEGF is a major stimulus of angiogenesis and permeability in this disease [66] (Table 1). VEGF A, VEGF 121 and VEGF 189 factors were significantly overexpressed during menstruation in EMS patients compared to the control group, which may help to explain the pathomechanism of the disease and provide potential biomarkers, but further investigations are required [67]. Pigment epithelium-derived factor (PEDF) is a glycoprotein that is potentially involved in a variety of biological processes, possessing potent antiangiogenic, neuroprotective, anti-inflammatory and immunosuppressive properties. Previous studies found that the level of PEDF is decreased in peripheral blood of EMS patients [68] but further studies are needed to determine whether PEDF may be a suitable biomarker candidate. Research findings on angiogenic factors are encouraging, but more data are needed to estimate their specificity and sensitivity efficacy in EMS. In addition, studies should be performed in patients with gynaecological tumour disease, as these angiogenic factors are also of great significance in tumour development and a sensitive biomarker candidate has to distinguish between EMS from other gynaecological tumour.
Oxidative stress markers
Oxidative stress markers were found to be altered significantly in EMS patients and monitoring them was considered a promising avenue to identify new biomarkers. Blood levels of superoxide dismutase and glutathione peroxidase were measured with commercially available assay kits, but their combined sensitivity was 78%, which is not accurate enough for clinical use [69]. The marker soluble tumour necrosis factor-alpha receptor (sTNFR-I) was shown to detect early stage EMS with 75% specificity, which is notable as most candidates are only able to indicate an advanced stage of EMS [70] (Table 1). Research findings on these stress markers are encouraging, but larger clinical studies are necessary to estimate their actual efficacy as potential diagnostic markers of EMS.
Inflammatory proteins and cytokines
Immunomodulatory factors, such as Galectin-1, Galectin-3 and Galectin-9 have been investigated, but most of them require more data to assess usability and some have already proven to be inadequate biomarker candidates for EMS [71]. Galectins bind β-galactoside sugars and are involved in the regulation of apoptosis and they may play an indirect role in the abnormal survival of endometrial cells outside the uterus. The results of a Galectin-9 pilot study were very promising because of its high area under the curve (AUC) value, although this has yet to be repeated with a larger study cohort [72] (Table 1). Changes in several other immunomodulatory molecules have been reported in women with EMS; β1-integrin and other cell adhesion molecules [73], intercellular adhesion molecule-1 (ICAM-1) [74], E-cadherin [75] and matrix metalloproteinases (MMP) 2 and 9 [76]. C-reactive protein (CRP) is a general marker for inflammatory processes and correlates well with the presence of EMS, but its specificity is not high enough to be a potent biomarker for EMS. A more accurate method is to measure high-sensitivity C-reactive protein (hsCRP); however, hsCRP of plasma is not effective enough to make a definitive diagnosis of EMS [77], [78]. Due to the heterogeneous nature of this illness, none of the candidates or combinations mentioned herein is specific enough to make a definitive diagnosis. Inflammatory factors, as a panel of tumor necrosis factor-α, interleukin-1β, interleukin 6, interferon-γ and soluble ICAM-1, are not specific enough to diagnose early phase EMS, although there is ample evidence that they are involved in the pathomechanism of EMS [64], [79]. Many conditions, including EMS, can cause an increase in the level of inflammatory factors. Indeed, a number of these conditions can also co-present with EMS. Therefore, further studies are needed to prove the practical usage of these factors.
Autoimmune markers
Several autoimmune markers have been tested as potential biomarker candidates, but none have worked as expected except for a panel of anti-tropomodulin (TMOD)3b, anti-TMOD3c, anti-TMOD3d, anti-tropomyosin (TPM)3a, anti-TPM3c and anti-TPM3d. This panel showed an AUC of 0.869 with quite high specificity (80%), but further validation is required with a larger study group [80]. The contribution of dysregulated inflammatory processes to the pathomechanism of EMS is widely accepted, but none of the factors involved have proven thus far to be viable biomarkers.
Hormone-related factors to properly estimate efficacy
It is also well established that EMS is an estrogen-dependent condition, and the attempt to identify a hormone-based biomarker was, therefore, a logical approach. Urocortin (UCN) is a member of the corticotrophin-releasing hormone family and is expressed by eutopic and ectopic human endometrial tissue; its level can be measured in both tissue and blood. Serum UCN level in EMS patients suggested that it could be a novel biomarker of a subtype (endometrioma) of EMS, with sensitivity and specificity of 80%. Although UCN seems a promising candidate for endometrioma identification and follow-up, more studies are required to determine its efficacy [81] (Table 1). Activin A is a growth factor expressed by endometriotic tissue that participates in the regulation of the menstrual cycle and whose actions are controlled by the binding protein follistatin. Both proteins are traceable in serum and their concentrations increase in women with EMS. The AUC of activin A was 0.700, while AUC of follistatin was 0.620 (95% confidence interval: 0.510–0.730) for the diagnosis of ovarian endometrioma. However, the combination of activin A and follistatin did not improve their diagnostic accuracy, and provided no diagnostic value for peritoneal or deep infiltrating EMS [82]. In summary, hormone-related factors, such as UCN, have proven to be highly sensitive diagnostic candidates for ovarian endometrioma, however, they are less able to identify other manifestations of EMS.
Epigenetics: miRNA markers
One of the most promising areas of EMS diagnosis is genomics and epigenomics. In one study, women with EMS had significantly downregulated microRNA levels of miR-17-5p, miR-20a and miR-22 compared to the control group, although combined AUC value of these miRNAs was 0.74 which does not meet the biomarker accuracy criterion [83]. The miRNA let-7 is reportedly involved in abnormal endometrial growth and EMS [84]. Cho et al. quantified miRNA let-7a-f and miR-135a,b from 24 patient and 24 control blood samples. The combined AUC of miRNA let-7b, let-7c, let-7d and let-7e during the proliferative phase was 0.929. These results suggest let-7d may be a reliable candidate with high accuracy; nevertheless, the results should be treated with caution due to the small number of study participants [85] (Table 1). Other microRNAs are also dysregulated in EMS patients, including miR-122 and miR-199. Eighty women were enrolled in an Egyptian study that examined microRNAs extracted from blood samples. The results showed a positive correlation between miR-122 and interleukin 6 (IL-6) levels and accuracy of miR-122 was 93.75% (sensitivity 95.6% and a specificity of 91.4%). However, more validation studies are required with an extended case number [86]. It is clear that the miR-200-family has a different expression pattern in endometrial lesions compared to eutopic tissues, nevertheless only one study examined miR200-family member - miR-141* in EMS patients. MiR-200a, miR-200b and miR-141* were isolated from peripheral blood and the results indicated that the combined AUC value of the above-mentioned microRNAs was 0.76. Notably, however, this value was dependent on sampling time. All three miRNAs had lower levels in the blood samples collected in the morning which associated with the circadian clock. Other studies also concluded that sampling time is an important aspect of miR-200 levels. This important caveat should be taken into account in future studies and should be explored as a potential cause of fluctuation (e.g. circadian rhythm) [87]. The collection and processing of miRNA samples require expertise, and the conditions of a collection can affect the result, making practical application difficult.
Genetics: DNA markers
Screening of EMS-associated mitochondrial DNA (mtDNA) deletions may be an effective way to identify novel non-invasive markers. Mutation frequency in mtDNA is high and repair capability is limited, making mtDNAs an excellent source of biomarker candidates. Creed et al. selected the following genomic regions for PCR analysis: CO2 to ATP6 (1.0 kb deletion); ATP6 to ND3 (1.2 kb deletion); ATP8 to ND4 (2.4 kb deletion); ATP6 to ND5 (3.7 kb deletion); ATP8 to ND5 (5.0 kb deletion); CO1 to ND5 (6.5 kb deletion); and CO2 to CytB (7.7 kb deletion), two of these deletions showed moderate accuracy. The sensitivity of the 1.2 kb deletion assay was 81.8% and specificity was 72.2%, while the diagnostic value of the 3.7 kb deletion assay was less accurate with sensitivity and specificity of 85.1% and 57.9%. Interestingly, there is a minimal correlation between menstrual stage and mtDNA deletion, suggesting that menstrual phase should be taken into account in future expanded mtDNA genomic analyses [88]. Circulating cell-free DNA has offered novel possibilities for non-invasive diagnosis and monitoring of many diseases, including EMS. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) catalyses a key step in glycolysis and has recently been implicated in several non-metabolic processes, including transcription activation and initiation of apoptosis. A study reported that concentrations of circulating cell-free GAPDH gene sequence were significantly increased in plasma from EMS patient's sample [89] (Table 1).
Familial clustering of EMS suggests the importance of hereditary factors, but evidence indicates that genetic predisposition is multifactorial and not linked to a single mutation [90]. Mutations in the genes for DNA mismatch repair proteins, cell adhesion proteins and tumour suppressors have been identified in patients with EMS, but none of these mutations are specified as appropriate biomarkers in EMS [91]. Despite numerous studies conducted, none have yet identified clinically applicable non-invasive genetic or epigenetic biomarker candidates for EMS.
Overall, there are many different approaches for developing an effective and sensitive non-invasive diagnostic method. Although we sought to present the most promising candidates, it should be taken into account when evaluating the results that in the presented studies, samples from patients with severe EMS were compared with a healthy control group. Further sophisticated studies have indicated that these biomarker candidates may only be sensitive enough to identify severe EMS. Given the complex aetiology of EMS, it seems more likely that a panel of clinical markers will be necessary.
Microbiome in endometriosis: Diagnostic potential
In the study of the interaction between EMS and microbiome, there are two distinct but interacting microbial ecosystems: one in the gut and one in the female genital tract.
The presence of metabolically inactive, naturally cell-wall-deficient (CWD) or L-form bacteria, those which adopt a CWD state under stress [92], [93], [94], may play a significant role in the development of chronic diseases such as EMS [95]. These CWD and L-forms of pathogens (such as Chlamydia, Mycoplasma, Streptococcus, Enterococcus, and E. coli) are metabolically inactive, non-culturable forms of intracellular bacteria to allow them to survive non-conductive conditions for growth, characterized by slow growth and absence of cell wall [96]. They are able to evade the immune response and antibiotics, survive and persist long term in the host and then reactivate under more favourable conditions [95]. They integrate into host cells using the host pathways and their peptides then display structural similarity with self-peptides which can activate autoreactive T-cells and cause autoimmunity [95]. These pathogens are able to alter host energy metabolism and glucose balance, dysregulate autophagy, induce epigenetic modifications, and make changes in blood clotting and impaired wound healing to promote their spread [95]. In the developed infection the T-cells get exhausted and appropriate T-cell response is lost [95]. Immune cells could also be regulated by dopamine levels, which these bacteria use [95]. Some pathogens produce saccharide structures on their surface that mimic human carbohydrates which may help the bacteria to avoid recognition by the human immune system [95]. These factors all contribute to long term pro-inflammatory, autoimmune status in the host, which enables these pathogens to survive, and also alter the environment around them for their benefit.
In patients with EMS, several microbial abnormalities have been identified, such as higher amounts of Gardnerella, Streptococcus, Enterococci, and E. coli than in healthy women. This abnormality was associated with increases in vaginal pH and hormonal status [97], [98] (Table 2). These pathogens could be also attracted by the altered pH, which was originally generated by intracellular CWDs [95]. Hormones, especially estrogen, can regulate a number of anti-microbial peptides [95]. Bacteria can spread through endometrial influx in retrograde menstruation [95], when hormone levels are low. LPS/endotoxin from these bacteria was found to promote growth of EMS through TLR4 [99].
Table 2.
Microbiome changes in human female patients and animal models of endometriosis.
|
Genital tract microbiom |
Gut microbiome |
||||||
|---|---|---|---|---|---|---|---|
| Sample source/type | Detection technique | Results | Reference | Sample source/tpye | Detection technique | Results | Reference |
| sample of lower and upper genital tract/human | 16S rRNA amplicon sequencing | in relation to hysteromyoma Lactobacillus sp. were found to be more abundant in the samples of control group, while L. iners was more abundant in the patient group | Chen et al. 2017 | fresh stool samples/human | 16S rRNA gene amplifcation | Shigella and Escherichia dominant stool microbiome in severe EMS | Ata et al. 2019 |
| endometrial and cystic fluid samples/human | 16S rDNA and sequence analysis | Enterobacteriaceae and Streptococcus spp. were significantly overrepresented in EMS samples compared to the healthy controls | Khan et al. 2016 | faeces and peritoneal macrophage collection/mice | 16S V4 gene region amplification; hematoxylin-eosin and immunofluorescent staining | Firmicutes and Bacteroidetes ratio was elevated in EMS mice | Yuan et al. 2018 |
| vaginal fluid, eutopic endometrium and endometriotic lesion tissue samples/human | 16S rRNA amplicon sequencing | the microbial diversity of the endometriotic lesion had a higher diversity which had shifted towards Alishewanella, Enterococcus, Ureaplasma and Pseudomonas | Hernandes et al. 2020 | ||||
| rectal and vaginal samples/human | 16S rRNA amplicon sequencing | vaginal microbiome (e.g. Firmicutes/Bacteroidetes, Anaerococcus, Lactobacillus alterations) was predictive of endometriosis rASRM stages | Perrotta et al. 2020 | faeces sample/mice; eutopic endometrium and endometriotic lesions | 16S rRNA gene amplifcation; enzyme-linked immunosorbent assay | broad-spectrum antibiotic that reduced the size of endometriotic lesions; gut microbiome may promote inflammation in EMS | Chadchan et al. 2019 |
| vaginal and endometrial smear samples/human | bacterial vaginosis scores in Gram-stained vaginal samples, immunhistological test, measure of intra-vaginal pH | Lactobacillacae, Streptococcaceae, Staphylococaceae and Enterobacteriaceae were significantly increased in EMS samples compared to the control group of samples | Khan et al. 2014 | ||||
| vaginal and endocervical swab samples/human | 16S rRNA gene amplifcation | absence of Atopobium genus in the vaginal and cervical microbiota in severe EMS | Ata et al. 2019 | fresh faecal sample/Macaca mulatta | microflora cultivation on different types of agar | lowered Lactobacillus and higher Gram-negative bacteria ration in EMS samples | Bailey and Coe 2002 |
Therefore, EMS could be initiated and regulated by these pathogenic microbes.
The identification and treatment of microbial infections could be an effective treatment strategy for the symptoms of EMS. In this section, we present the available evidence for both gut and genital tract microbiome with regards to EMS.
Microbial composition of the genital tract
The relevance of the vaginal microbiota to health and homeostasis of the female genital tract is well known and its alteration has been observed in many gynaecological diseases including EMS [100], [101] (Table 2). The conventional theory of sterility of the uterus and upper reproductive tract, as well as the abnormal presence of the microbiome that can be detected there, are debatable in the light of new evidence and improved detection techniques [102], [103], [104]. Although there is a need to standardize and harmonize sample collection methods, it is clear that the microbiome is related to the functioning of the female genital tract [105]. There are several differences in the composition of the genital tract and gastrointestinal system microbiome between EMS and healthy female groups. These alterations and their potential effects are not well-characterized (Fig. 2), but they may help to further interrogate the underlying pathomechanism of this condition and to develop more efficient treatment guidelines. Dysbiosis of the upper genital tract microbiome has been described under inflammatory conditions such as EMS or adenomyosis [101] (Table 2) compared to healthy controls. The members of the control group are volunteers who meet pre-established criteria (e.g., members of a healthy population that do not have the particular disease to be analysed and meet the selection criteria) and serve as a reference point for evaluation. Microbial composition of endometrial swab samples was sequenced using next-generation sequencing techniques and significant alterations were identified between control and EMS groups. In this study, the proportion of Enterobacteriaceae and Streptococcus spp. were significantly overrepresented in EMS women compared to the healthy controls [106] (Table 2). 16 s rDNA sequencing of vaginal fluid, eutopic endometrium and EMS lesion samples were recently analysed, microbiome alpha and beta diversity did not change significantly with Lactobacillus, Gardnerella, Streptococcus and Prevotella dominating the vagina fluid. However, the microbiome of the endometriotic lesions had a higher diversity which had shifted towards Alishewanella, Enterococcus, Ureaplasma and Pseudomonas [107] (Table 2). An observational pilot study discovered that changes in the composition of the vaginal microbiome can predict the revised American Society for Reproductive Medicine (rASRM) stage of EMS patients [104]. This study was performed with a small patient group and the results are very promising, but further investigations are needed to confirm the previous findings [108] (Table 2). Endometriosis is an estrogen-dependent disease and a common choice of treatment is the use of Gonadotropin-Releasing Hormone agonist (GnRHa), which inhibits estrogen production thereby relieving symptoms. It should be noted that GnRHa treatment influences the microbial composition of the upper genital tract and ovaries, demonstrating the potential role for hormones in influencing the microbiome [106], [109]. The proportion of Lactobacillacae, Streptococcaceae, Staphylococaceae and Enterobacteriaceae were significantly increased in endometrial swabs and endometrioma/non-endometrioma cystic fluid EMS samples, compared to a control group of samples from women without EMS. This study further revealed an increase in endometrial colonization concurrent with endometritis in EMS. An intra-vaginal pH ≥ 4.5 was associated with endometritis in both the control and EMS groups, while treatment with GnRHa significantly increased prevalence of acute endometritis in both groups [98] (Table 2). The microbiota of stool samples, vaginal and endocervical swabs from women with severe EMS (stage 3 and 4) compared to healthy controls were analysed during an observational study. There was a not significant difference in microbiome diversity between control and patient’s samples but the proportion of some bacteria shifted in patient samples. The reduction of Atopobium spp. increased the proportion of Gardnerella in vaginal and cervical swab samples. Furthermore, the presence of Sneathia, Gardnerella, Streptococcus, Escherichia/Shigella and proportion of Ureaplasma were higher, while Alloprevotella was decreased in cervix microbiome. Fecal Escherichia and Shigella was also found to be higher in severe EMS patients compared to healthy controls [100], [110] (Table 2).
The scientific results available so far suggest that the composition of the genital microbiome changes during EMS. Perrotta et al. found that microbial alteration is associated with the severity of EMS (based on rASRM classification). Based on these findings, we believe that the further study of the genital microbiome is of great importance in future EMS treatment.
Microbiome of the gastrointestinal tract
There is substantial evidence pointing to the role of the gut microbiome in modulating extra intestinal host health [111]. Indeed, changes in circadian rhythms and immune function have been linked to intestinal microbial processing and the resulting metabolites like short-chain fatty acids [112], [113]. Patients with EMS had reduced microbiome diversity and an increased proportion of potentially pathogenic microbes in both genital and gastrointestinal samples, compared to healthy women without EMS [100] (Table 2.). Composition of the gut microbiome was monitored in a mouse model of EMS and it was found that, 42 days after EMS induction, the Firmicutes and Bacteroidetes ratio in the stool with increased Ruminococcaceae, Bifidobacterium and Parasutterella generae suggesting that EMS could induce dysbiosis [114] (Table 2). Targeting of gut microbiota in EMS mice using a broad-spectrum antibiotic reduced the size of endometriotic lesions and inflammatory processes highlighting the microbial role in this disease. However, more in-depth studies are needed to understand how the gut microbiome contributes to pelvic inflammatory processes and EMS progression [115] (Table 2). Insight from in vivo mouse studies using transplantation of uterine tissue fragments does not suggest a significant impact on gut microbial composition, at least in the acute phase of EMS lesion formation [116]. Furthermore, lower Lactobacillus and higher Gram-negative microbial loads were identified in the intestinal microbiome of Rhesus macaque with EMS and subjects with EMS had an increased prevalence of gastrointestinal inflammation [117] (Table 2). This finding is consistent with clinical observations related to EMS; women with EMS are more likely to be diagnosed with irritable bowel syndrome, even after reaching a definitive diagnosis of EMS [118]. Estrobolome may play a role in EMS through β-glucuronidase secreted by gut microbiome. β-glucuronidase releases conjugated estrogen, thereby increasing the level of free estrogen, which may have a direct effect on EMS [109]. While further studies are required to fully delineate the relationship between the gut microbiome, the reproductive tract microbiome and EMS, this rapidly evolving field presents novel opportunities not only for diagnostic development, but also for therapeutic intervention using pro- or prebiotics [97], [119]. A double-blind, placebo-controlled clinical study found that Lactobacillus gasseri OLL2809 reduced menstrual pain and dysmenorrhea without side-effect in EMS patients [120]. A pilot placebo-controlled randomized clinical trial showed some positive influence of lactobacillus administration on endometriosis-related pain ease [121].
There is also evidence for changes in the intestinal microbiome in EMS and estrobolome may have a significant impact on EMS. In case of EMS affecting the intestinal tract, there may be a more direct interaction between the microbial composition of the intestinal and genital tract and EMS. Further investigations are needed to identify the importance of the microbiome in the pathomechanism and possible diagnostic or treatment usage of EMS. Nevertheless, it is likely that examination of the microbial composition of the intestinal and genital tracts as a complex system may yield the most effective results in the future.
Glycosylation and gynaecological diseases
Carbohydrates, also known as ‘glycans’, play a significant role in signal transduction and metabolic processes and are important in determining the properties of proteins and in immunological responses [11]. Glycosylation is one of the most common post-translational modifications of eukaryotic cells, which is important for normal biological functions. Only carbohydrates that have been studied in gynaecological research are discussed below.
Glycosaminoglycans (GAGs) are linear and heterogeneous sulphated glycans. Although GAGs are structurally complex, the backbones of these polysaccharides are simply made up of repeating disaccharide building blocks composed of alternating uronic acid and hexosamine units. Proteoglycans are made of GAGs covalently attached to the core proteins. They are found in all connective tissues, extracellular matrix and on the surfaces of many cell types [11], [122]. In mammals, there are two main types of glycosylation by which carbohydrates can attach to a protein. O-linked glycans are attached to proteins via the hydroxyl group of serine or threonine through post-translational modifications. N-glycans are attached to proteins via the asparagine residues due to co- and post-translational modifications [11]. Both N- and O-glycosylation have the same biological relevance in humans, but the study of O-glycosylation is hampered by a lack of specific enzymes. In the case of N-glycans, the availability of endo- and exoglycosidases has facilitated investigation [123], [124]. Many diseases are associated with impaired or deficient glycosylation such as galactosemia, congenital muscular dystrophies and rheumatoid arthritis or ovarian cancer [125]. Given that ovarian cancer has the highest mortality rate among gynaecological diseases and has limited early diagnostic tools, the glycomics approach is of great importance. Serum glycoproteins of sLex level were increased in both breast and ovarian cancer patients, with an increased proportion of fucosylated structures in the ovarian cancer group [126]. The degrees of galactosylation, sialylation, and the ratio of bisecting structures decrease in patients’ serum IgG, which directly affects inflammatory and antibody-dependent cellular cytotoxicity processes [127], [128], [129]. Alterations in glycosylation patterns and their potential importance as biomarkers in gynaecological diseases are supported by several studies. Serum mannose levels have been studied in women with polycystic ovary syndrome (PCOS) and it has been demonstrated that elevated mannose levels may be a good diagnostic marker. When mannose levels were combined with total testosterone levels, the accuracy increased to 83.3% compared to the original 72.5% [130]. The subtype of clear cell carcinoma of epithelial ovarian cancer is difficult to detect and is associated with poor prognosis. Studies aimed at enhancing the efficacy of the current diagnostic test have been performed, and have shown that the combination of Wisteria floribunda lectin analysis with the CA-125 marker may be an effective early biomarker of the disease [131]. Serum IgG glycosylation pattern is an effective marker in the early detection of breast cancer. Two glycan structures (m/z 1591 = FA2 (core fucosylated bintennary glycan) and 1794 = FA2B (core fucosylated biantennary bisected glycan)) are significantly altered in early phase breast cancer patients compared to healthy controls and their AUC values are 0.944 and 0.921, indicative of high accuracy [132].
In summary, glycans play an essential role in the functioning and regulation of living organisms. Under pathological conditions, in addition to changes at the proteomic, metabolic, and genetic levels, glycan structures are also modified. This finding has been validated in the case of gynaecological diseases by many studies, providing an opportunity to develop new less-invasive diagnostic methods based on the measurement of glycosylation changes.
Glycosylation and endometriosis
There is little published research on EMS and glycosylation, and most studies are conducted with a tissue or peritoneal liquid samples, collected by invasive means. As outlined above, Galectin-1 is an endogenous lectin expressed in human stromal and endothelial cells of EMS lesions and contributes to vascularization and growth of EMS lesions independently of other vascularization factors. Using a mouse EMS model, inhibition of Galectin-1 by monoclonal antibody decreased the size and vascularization area of EMS lesions and identified this lectin as a potential target of therapy [133] (Table 3). A study found that peritoneal dendritic cells of EMS tissue expressed significantly higher levels of mannose receptors than the healthy control tissue, which contribute to phagocytosis of dead EMS cell and participate in EMS lesion formation [134] (Table 3). The elevated mannosylation level of the patients’ sample also could be a possible target of a diagnostic test. Human endometriotic cyst stromal cells and normal endometrial stromal cells were analysed by Wisteria floribunda (WFA) agglutinin binding lectin microarray. The WFA-binding N-glycan level was decreased in human endometriotic cyst stromal cells and N-acetyl-galactose-aminyl transferase expression was repressed in these cells [135] (Table 3). Transforming growth factor-β1 cytokine increased the adhesion of endometrial cell to mesothelium due to the increased α2-6 sialylation; however, the inhibition of β-galactoside α2-6 sialyltransferase 1 and 2 decreased the establishment of EMS lesions [136] (Table 3). Another study analysed α2-6-sialyltransferase expression in eutopic and ectopic endometria of 102 women with EMS and 72 healthy women. Expression of α2-6-sialyltransferase was repressed in EMS lesions compared to the healthy control samples and the hyposialylated endometriotic cells might contribute to the formation of early EMS lesions [137] (Table 3). An accurate measurement of sialic acid level would be a possible diagnostic tool in the future. Experiments conducted with Dolichos biflorus agglutinin showed that EMS tissue glycosylation is different in an EMS patient group compared to controls and that was confirmed by gene expression study. Endometrial tissue shows delayed and abnormal differentiation mainly in Stage IV EMS patients, which may be directly associated with glycosylation and gene expression alterations. Deficiency of endometrial epithelial differentiation and glycosylation may also be related to EMS infertility and inappropriate endometrial receptivity [138], [139], [140] (Table 3). Glycodelin-A is expressed by endometrial tissue with different glycoforms and immune localization in women with or without EMS. Glycodelin-A expression significantly increased in luteal phase of EMS tissue and displayed a different N-glycan profile. These alterations may contribute to infertility in women with EMS, as blastocyst implantation is associated with the adequate epithelial glycocalyx of endometrial cells [141] (Table 3). Autoantibodies react with Thomsen-Friedenreich antigen-bearing proteins and behave as autoantigens in EMS, highlighting the association between glycosylation and EMS [142] (Table 3). Peritoneal haptoglobin, which is secreted by endometriotic tissues, is an analogue of hepatic haptoglobin and its glycosylation is different between women with or without EMS. The level of sialylation is decreased in peritoneal haptoglobin and this phenomenon can be explained by carbohydrate-deficient glycoprotein syndrome type I. Therefore, this glycoprotein may be a potential target for immunotherapy or a specific biomarker of EMS, as it is a tissue-specific factor and probably has an immune-modulating function similar to that of hepatic haptoglobin [143] (Table 3). There are very few articles regarding blood or urine glycosylation in EMS, although they would be ideal sources for diagnostic markers. Blood collection is minimally invasive and urine collection is a non-invasive and painless procedure. An Iraqi study draws attention to the importance of serum sialylation. Serum sialylation is dramatically changed in EMS patients after zoladex therapy, indicating that changes in serum sialylation may be a new biomarker of EMS [144] (Table 3). A study of plasma N-glycan profile revealed a significant decrease of monosialylated and an increase of tri- and tetra-antennary glycans in patients with mild to severe EMS, while a biantennary glycan structure significantly decreased only in deep infiltrating EMS compared to healthy controls. The authors were unable to reveal which changes in serum proteins caused the changes identified at the serum level, but suggested that glycosylation changes related to IgG and transferrin proteins contributed to the global change [145] (Table 3). While glycosylation of urine in EMS has not been studied so far, published work on urinary glycosylation in other gynaecological conditions can serve as a reference point for EMS studies. In a study of endometrial cancer, the urinary level of zinc alpha-2 glycoprotein and alpha 1-acid glycoprotein were significantly increased, while the fragment of nebulin and the CD59 levels were much lower in the cancer group compared to the control group. Western blot of O-glycan-binding champedac assay was used to investigate the O-glycan content of urinary proteins. Urine O-glycosylation was significantly different in endometrial cancer patients, compared with healthy controls. Six clusters of O-glycoproteins were identified in the urinary samples of both patient and control groups, but the O-glycosylated nebulin spot was well visualized only in control samples while it was barely visible in cancer patient samples [146] (Table 3). In addition, studies have confirmed that GAGs, proteoglycans and free oligosaccharides of urine can serve as potential biomarkers in many diseases [147], [148].
Table 3.
Summary of N- and O-glycosylation changes associated with endometriosis.
| Sample source/type | Detection technique | Results | Reference |
|---|---|---|---|
| endometriotic lesions and eutopic endometrium/human endometriotic lesions/mice |
immunofluorescence and immunohistochemistry of tissue samples | Galectin-1 inhibition with monoclonal antibody therapy decreased the size and vascularization area of EMS lesions in mice | Baston et al. 2014 |
| endometrial stromal cells, monocyte-derived dendritic cells from peritoneal fluid/human | isolation, culture, staining, and cell preparation of the cells; flow cytometry; RT-PCR |
rate of mannose receptor-positive myeloid dendritic cells was higher in EMS samples than in the control group; inhibition of mannose receptors reduced phagocytosis of dead endometrial stromal cells | Izumi et al. 2017 |
| endometriotic cyst stromal cells and normal endometrial stromal cells from endometrial tissue/human | protein extraction for lectin microarray; lectin histochemistry; western blot analysis; RT-PCR | Wisteria floribunda agglutinin binding glycans decreased in the stromal components of the ovarian endometriotic cysts; N-acetyl-galactose-aminyl transferaseswere downregulated | Hirakawa et al. 2014 |
| immortalized endometriotic epithelial cells, endometrial cells from adenocarcinoma, endometrial stromal cells, peritoneal mesothelial cells/human; in vivo endometriosis model/mice | cell adhesion assay; lectin blot analysis; lectin fluorescence-activated cell sorting analysis; western blot analysis; RT-PCR; gene knockdown with siRNA | transforming growth factor-β1 increased adhesion of endometrial cells to the mesothelium through induction of α2-6 sialylation | Choi et al. 2018 |
| endometrial and ectopic endometriotic cells from tissue and peritoneal fluid/human | enzyme-linked immunosorbent assay; RT-PCR; lectin immunoblot; transwell migration assay |
ST6GALNAC1 expression decreased and ST6GALNAC5 expression increased in EMS; reduced α-2,6 sialylation in the peritoneal fluid and endometriotic cells; higher migration capacities of desialylated eutopic endometrial stromal cells of EMS group |
Maignien et al. 2019 |
| endometrial tissue/human | electron microscopy; endometrial morphometry; lectin histochemistry; | lack of Dolichos biflorus agglutinin-binding glycans in severe EMS tissue; unusual ultrastructure of proliferative phase phenotype and delayed maturation | Jones et al. 2009, |
| endometrial tissue/human | lectin histochemistry; image analysis | Dolichos biflorus agglutinin-binding glycans significantly decreased in severe EMS tissue and moderated diminish of Vicia villosa agglutinin-binding glycans | Miller et al. 2010 |
| endometrial tissue/human | two-dimensional electrophoresis; western blot; immunofluorescence; | both epithelial and stromal cells produce Glycodelin-A with several glycoforms; abnormal expression of Glycodelin-A in EMS patients during the cycle | Focarelli et al. 2018 |
| serum, ectopic and eutopic endometrium/human | hematoxylin/eosin-staining; immunoblot; SDS-PAGE; modification of carbohydrate epitopes on glycoproteins | autoantibody responses in EMS tissue; lack of glycan moiety of antigens result in loss of antibody binding; autoantibodies react with other Thomsen-Friedenreich antigen-bearing proteins like IgA, haemopexin; autoimmune response may play a direct role in EMS | Lang et all. 2001 |
| serum, endometriotic lesions, non-affected serosal peritoneal tissue/human | recombinant gene over-expression; lectin binding assays; | peritoneal and hepatocellular haptoglobin deviate in N-glycan moiety and mainly in sialylation; increased interaction of peritoneal haptglobin with Maackia amurensis and Lotus tetragonolobuslectin | Piva et al. 2002 |
| serum/human | quantitative sandwich enzyme immunoassay | systemic level of inflammation with different sialylation status in EMS patients | Rasha Z. Jasim et al. 2014 |
| serum/human | HPLC N-glycan characterisation; collection of fasting glucose and epidemiological data | decrease of GP2 (A2B, A1G1, FA2) peak and increase of GP14 (A2BG1, A2G1, M4A1G1, FA2G1, FA2BG1, A1G1S1, FA2G1, M6D1), GP17 (A4F1G4S4, A4G4LacS4) and GP18 (A4F2G4S4) peaks in EMS patients; decrease of GP1 (A2) peak in deep infiltrating EMS samples | Berkes et al. 2013 |
| urine samples/human | two-dimensional electrophoresis with silver staining; on-membrane digestion; MALDI-ToF MS; western blot; CGB lectin affinity separation and LC-MS/MS | level of zinc alpha-2 glycoprotein and alpha 1-acid glycoprotein were significantly increased and the fragment of nebulin and the CD59 levels were decreased in the endometrial cancer group; O-glycosylation of nebulin was barely visible in patients | Mu et al. 2012 |
As is apparent from the above literature review, the number of studies that examine glycosylation changes during EMS is negligible. Most studies have examined tissue or peritoneal fluid glycosylation (in many cases in an animal model) to better understand the pathomechanism of the disease. Although we see examples of human serum and urine glycosylation assays, they were conducted with a low number of participants. The results are exciting and promising, but further research is needed on the subject. We have not found an example of an approach to glycans as a possible diagnostic tool; however, glycomics may be a promising source for potential biomarker candidates.
Discussion and future prospective
Efforts to discover possible EMS biomarkers have drawn from the diverse fields of genomics, transcriptomics, proteomics, and metabolomics. So far, none of the biomarker candidates have proven sensitive enough to compete with laparoscopic diagnosis. The importance of commensal microbes for human health and homeostasis has come to the fore in recent years. The microbiome of patients with EMS is different compared to healthy women, therefore changes in the genital and gut microbial profile may help to further illuminate the pathomechanism of the disease and provide potential biomarker candidates. The high sensitivity of analytical chemistry platforms supports the significant potential of glycomic research. The few glycosylation studies of EMS published to date have primarily used tissue-based approaches. Blood and urine tests for EMS biomarkers have extraordinary clinical diagnostic potential. Most importantly, these samples can be collected minimally or non-invasively and present abundant opportunities for identification of novel glycosylation patterns and biomarkers.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
CRediT authorship contribution statement
Zsuzsanna Kovács: Investigation, Writing - original draft, Visualization, Funding acquisition. Louise Glover: Writing - review & editing. Fiona Reidy: Writing - review & editing. John MacSharry: Writing - review & editing. Radka Saldova: Conceptualization, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 843862, H2020-MSCA-IF-2018.
Biographies

Dr Zsuzsanna Kovács is a young researcher who defended her PhD thesis (Identification of myeloma multiplex glyco-biomarkers by capillary electrophoresis, 2018) at Biomedical Sciences, Doctoral School of Molecular Medicine, the University of Debrecen. During her PhD, Zsuzsanna studied N-glycosylation of human diseases (such as lung cancer, multiple myeloma and other haematological diseases). Zsuzsanna was then employed as a Research Assistant in the Research Institute of Biomolecular and Chemical Engineering, University of Pannonia and described the N-glycosylation of the XIII subunit B haematological factor. Zsuzsanna was awarded by the New National Excellence Program' (ÚNKP) pre-doctoral (2017) and the National Higher Education Scholarship (2013). During her Erasmus Traineeship she studied N-glycosylation of DNA stabilizing proteins. Zsuzsanna has joined GlycoScience team in National Institute for Bioprocessing Research and Training (NIBRT), under Dr Radka Saldova’s supervision as a Postdoctoral Researcher on awarded EU Marie Currie Individual Researcher Fellowship on identification of non-invasive clinical markers for diagnosis of endometriosis (GLYCOMENDO, No 843862, H2020-MSCA-IF-2018). Zsuzsanna published 10 peer-reviewed publications (https://orcid.org/0000-0001-7319-0456).

Dr. Louise Glover Ph.D. is Clinical Research Officer in Merrion Fertility Clinic at the National Maternity Hospital, Dublin. She is also an adjunct Assistant Professor at both Trinity College Dublin and University College Dublin, two of Ireland’s leading academic research institutions. Dr. Glover completed postdoctoral training at Massachusetts General Hospital, Harvard Medical School and the University of Colorado, U.S.A. Dr. Glover has published over 50 peer-reviewed journal articles and has served in the role of Principle Investigator and Co-investigator on a number of international studies, including projects funded by the U.S. National Institutes of Health (NIH). She is a member of the European Society of Human Reproduction and Embryology (ESHRE) and the Health Research Board Clinical Research Coordination Ireland (CRCI). Dr. Glover’s primary research focus is reproductive immunology, in particular how immune pathways in the female reproductive tract are altered in infertility.

Dr Fiona Reidy graduated with Honours in Medicine from University College Dublin in 2010. Dr Reidy is completing a Clinical Research Fellowship and Doctorate of Medicine (MD) in Merrion Fertility Clinic and the National Maternity Hospital, with Professor Mary Wingfield and University College Dublin. Dr Reidy is a member of the Royal College of Physicians Ireland and of the Royal College of Obstetricians and Gynaecologists. Her research interests include endometrial innate immune pathways and the associated microbiome in endometriosis-associated infertility.

Dr John MacSharry is a researcher in microbial mucosal immunology with focus on the interactions of the host epithelium and microbes in the lung, gut and urinary tract. He has a B.Sc. in Microbiology and a Ph.D. in Mucosal Immunology from University College Cork. He has worked with Alimentary Health Ltd (now Precision Biotics) as Molecular Biology section head collaborating with several multinational research partners. John was also a Post-doctoral researcher with the joint APC-GlaxoSmithKline Host Response core. He was a guest researcher at the Meakins Christie Laboratories, McGill University in Montreal, Canada as part of an Science Foundation Ireland (SFI) fellowship in 2009 and 2010. In 2013 he was appointed as a Lecturer in Molecular Medical Microbiology and Deputy director of the GEM programme with the School of Medicine with an affiliation to the School of Microbiology. His research interests are in host-microbe interactions with particular focus on the immune sampling and response. John has active research projects with academic and industrial partners and currently has active collaborations funded by Nutricia, Danone and SFI. Recently John was funded by SFI to evaluate Saliva for screening COVID-19. E-mail:j.macsharry@ucc.ie

Dr Radka Saldova completed her PhD in Chemistry, specialization Glycobiology, at Institute of Chemical Technology in Prague, Czech Republic (2007) after obtaining her master’s degree from the same university. Radka has joined GlycoScience group under Prof PM Rudd supervision (2005) in Oxford University, UK and moved with the group to National Institute for Bioprocessing Research and Training (NIBRT), Ireland (2006). Radka currently works in NIBRT, where she became independent investigator at 2014. Radka became adjunct research fellow at University College Dublin at 2017 and CÚRAM investigator at 2018. Her research interests include glycobiomarker discovery, regulation and role of glycosylation in cancer and inflammatory diseases using multidisciplinary approach, and the development of high-throughput technologies for glycoanalysis. Radka has published 67 peer-reviewed publications (Google Scholar h-index:28, https://orcid.org/0000-0001-5085-5080) mainly in the field of glycan biomarkers in cancer diagnosis and progression, chronic inflammatory diseases, mental disorders and disorders of glycosylation. Radka has developed novel hypothesis about the origin of cancer and chronic inflammatory diseases, including Endometriosis, linking many fields together in a multidisciplinary approach (Saldova R., Dis Med, 2016, 22 (120):105-119.). It provides a framework for ongoing research and systems biology and has led to a deeper understanding of the interconnectedness of different systems.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.KS Adamson GD, Hummelshoj L. Creating solutions in endometriosis: global collaboration through the world endometriosis research foundation. SAGE Publications 2 (2010) 3-6 DOI: 10.1177/228402651000200102.
- 2.Berkley K.J., Rapkin A.J., Papka R.E. The pains of endometriosis. Science. 2005;308(5728):1587–1589. doi: 10.1126/science.1111445. [DOI] [PubMed] [Google Scholar]
- 3.Soliman A.M., Surrey E.S., Bonafede M., Nelson J.K., Vora J.B., Agarwal S.K. Health care utilization and costs associated with endometriosis among women with medicaid insurance. J Manage Care Spec Pharm. 2019;25(5):566–572. doi: 10.18553/jmcp.2019.25.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianconi L., Hummelshoj L., Coccia M.E., Vigano P., Vittori G., Veit J. Recognizing endometriosis as a social disease: the European Union-encouraged Italian Senate approach. Fertil Steril. 2007;88(5):1285–1287. doi: 10.1016/j.fertnstert.2007.07.1324. [DOI] [PubMed] [Google Scholar]
- 5.Mok-Lin E.Y., Wolfberg A., Hollinquist H., Laufer M.R. Endometriosis in a patient with Mayer-Rokitansky-Kuster-Hauser syndrome and complete uterine agenesis: evidence to support the theory of coelomic metaplasia. J Pediatr Adolesc Gynecol. 2010;23(1):e35–e37. doi: 10.1016/j.jpag.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Sourial S., Tempest N., Hapangama D.K. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014 doi: 10.1155/2014/179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasson I.E., Taylor H.S. Stem cells and the pathogenesis of endometriosis. Ann NY Acad Sci. 2008;1127:106–115. doi: 10.1196/annals.1434.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn S.H., Monsanto S.P., Miller C., Singh S.S., Thomas R., Tayade C. Pathophysiology and immune dysfunction in endometriosis. Biomed Res Int. 2015;2015 doi: 10.1155/2015/795976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Penarrubia P., Ruiz-Alcaraz A.J., Martinez-Esparza M., Marin P., Machado-Linde F. Hypothetical roadmap towards endometriosis: prenatal endocrine-disrupting chemical pollutant exposure, anogenital distance, gut-genital microbiota and subclinical infections. Hum Reprod Update. 2020;26(2):214–246. doi: 10.1093/humupd/dmz044. [DOI] [PubMed] [Google Scholar]
- 10.von Theobald P., Cottenet J., Iacobelli S., Quantin C. Epidemiology of endometriosis in France: a large nation-wide study based on hospital discharge data. Biomed Res Int. 2016;2016:3260952. doi: 10.1155/2016/3260952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varki A. in: rd, Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH (Eds.). Essentials of Glycobiology, Cold Spring Harbor (NY); 2015. [PubMed]
- 12.Giudice L.C., Kao L.C. Endometriosis. Lancet (London, England) 2004;364(9447):1789–1799. doi: 10.1016/s0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 13.Paulson JD, Asmar P, Saffan DS. Mild and moderate endometriosis. Comparison of treatment modalities for infertile couples. J Reprod Med 36(3) (1991) 151–5. [PubMed]
- 14.Evers J.L. The pregnancy rate of the no-treatment group in randomized clinical trials of endometriosis therapy. Fertil Steril. 1989;52(6):906–907. doi: 10.1016/s0015-0282(16)53149-5. [DOI] [PubMed] [Google Scholar]
- 15.Dunselman G.A., Vermeulen N., Becker C., Calhaz-Jorge C., D'Hooghe T., De Bie B. European society of human, embryology, ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 16.Horton J., Sterrenburg M., Lane S., Maheshwari A., Li T.C., Cheong Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(5):592–632. doi: 10.1093/humupd/dmz012. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez A.M., Pagliardini L., Cermisoni G.C., Privitera L., Makieva S., Alteri A. Does endometriosis influence the embryo quality and/or development? Insights from a large retrospective matched cohort study. Diagnostics (Basel) 2020;10(2) doi: 10.3390/diagnostics10020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu B., Guo N., Zhang X.M., Shi W., Tong X.H., Iqbal F. Oocyte quality is decreased in women with minimal or mild endometriosis. Sci Rep. 2015;5:10779. doi: 10.1038/srep10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez A.M., Vanni V.S., Bartiromo L., Papaleo E., Zilberberg E., Candiani M. Is the oocyte quality affected by endometriosis? A review of the literature. J Ovarian Res. 2017;10(1):43. doi: 10.1186/s13048-017-0341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanbo T., Fedorcsak P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet Gynecol Scand. 2017;96(6):659–667. doi: 10.1111/aogs.13082. [DOI] [PubMed] [Google Scholar]
- 21.Arruda M.S., Petta C.A., Abrao M.S., Benetti-Pinto C.L. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756–759. doi: 10.1093/humrep/deg136. [DOI] [PubMed] [Google Scholar]
- 22.Balasch J., Creus M., Fabregues F., Carmona F., Ordi J., Martinez-Roman S. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod. 1996;11(2):387–391. doi: 10.1093/humrep/11.2.387. [DOI] [PubMed] [Google Scholar]
- 23.Exacoustos C., Zupi E., Piccione E. Ultrasound imaging for ovarian and deep infiltrating endometriosis. Semin Reprod Med. 2017;35(1):5–24. doi: 10.1055/s-0036-1597127. [DOI] [PubMed] [Google Scholar]
- 24.Saba L., Sulcis R., Melis G.B., de Cecco C.N., Laghi A., Piga M. Endometriosis: the role of magnetic resonance imaging. Acta Radiol. 2015;56(3):355–367. doi: 10.1177/0284185114526086. [DOI] [PubMed] [Google Scholar]
- 25.Duffy JM, Arambage K, Correa FJ, Olive D, Farquhar C, Garry R, Barlow DH, Jacobson TZ. Laparoscopic surgery for endometriosis, Cochrane Database Syst Rev (4) (2014) CD011031 DOI: 10.1002/14651858.CD011031.pub2. [DOI] [PubMed]
- 26.Burney R.O., Giudice L.C. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafrir A.L., Farland L.V., Shah D.K., Harris H.R., Kvaskoff M., Zondervan K. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1–15. doi: 10.1016/j.bpobgyn.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Vercellini P., Vigano P., Somigliana E., Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 29.Sanfilippo J.S., Wakim N.G., Schikler K.N., Yussman M.A. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154(1):39–43. doi: 10.1016/0002-9378(86)90389-3. [DOI] [PubMed] [Google Scholar]
- 30.Barbieri R.L. Stenosis of the external cervical os: an association with endometriosis in women with chronic pelvic pain. Fertil Steril. 1998;70(3):571–573. doi: 10.1016/s0015-0282(98)00189-7. [DOI] [PubMed] [Google Scholar]
- 31.Kao L.C., Germeyer A., Tulac S., Lobo S., Yang J.P., Taylor R.N. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144(7):2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 32.Tai F.W., Chang C.Y., Chiang J.H., Lin W.C., Wan L. Association of pelvic inflammatory disease with risk of endometriosis: a nationwide cohort study involving 141,460 individuals. J Clin Med. 2018;7(11) doi: 10.3390/jcm7110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kvaskoff M., Mu F., Terry K.L., Harris H.R., Poole E.M., Farland L. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update. 2015;21(4):500–516. doi: 10.1093/humupd/dmv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T., De Carolis C., Man G.C.W., Wang C.C. The link between immunity, autoimmunity and endometriosis: a literature update. Autoimmun Rev. 2018;17(10):945–955. doi: 10.1016/j.autrev.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Hadfield R.M., Mardon H.J., Barlow D.H., Kennedy S.H. Endometriosis in monozygotic twins. Fertil Steril. 1997;68(5):941–942. doi: 10.1016/s0015-0282(97)00359-2. [DOI] [PubMed] [Google Scholar]
- 36.Hansen K.A., Eyster K.M. Genetics and genomics of endometriosis. Clin Obstet Gynecol. 2010;53(2):403–412. doi: 10.1097/GRF.0b013e3181db7ca1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matalliotakis I.M., Cakmak H., Fragouli Y.G., Goumenou A.G., Mahutte N.G., Arici A. Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch Gynecol Obstet. 2008;277(5):389–393. doi: 10.1007/s00404-007-0479-1. [DOI] [PubMed] [Google Scholar]
- 38.Nnoaham K.E., Webster P., Kumbang J., Kennedy S.H., Zondervan K.T. Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies. Fertil Steril. 2012;98(3):702–712 e6. doi: 10.1016/j.fertnstert.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borghese B., Sibiude J., Santulli P., Lafay Pillet M.C., Marcellin L., Brosens I. Low birth weight is strongly associated with the risk of deep infiltrating endometriosis: results of a 743 case-control study. PLoS ONE. 2015;10(2) doi: 10.1371/journal.pone.0117387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leeners B., Damaso F., Ochsenbein-Kolble N., Farquhar C. The effect of pregnancy on endometriosis-facts or fiction? Hum Reprod Update. 2018;24(3):290–299. doi: 10.1093/humupd/dmy004. [DOI] [PubMed] [Google Scholar]
- 41.Rier S.E., Martin D.C., Bowman R.E., Dmowski W.P., Becker J.L. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam Appl Toxicol. 1993;21(4):433–441. doi: 10.1006/faat.1993.1119. [DOI] [PubMed] [Google Scholar]
- 42.Smarr MM, Kannan K, Buck Louis GM. Endocrine disrupting chemicals and endometriosis, Fertil Steril 106(4) (2016) 959-66 DOI: 10.1016/j.fertnstert.2016.06.034. [DOI] [PubMed]
- 43.Grodstein F., Goldman M.B., Cramer D.W. Infertility in women and moderate alcohol use. Am J Public Health. 1994;84(9):1429–1432. doi: 10.2105/ajph.84.9.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hemmings R., Rivard M., Olive D.L., Poliquin-Fleury J., Gagne D., Hugo P. Evaluation of risk factors associated with endometriosis. Fertil Steril. 2004;81(6):1513–1521. doi: 10.1016/j.fertnstert.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 45.Chiaffarino F., Bravi F., Cipriani S., Parazzini F., Ricci E., Vigano P. Coffee and caffeine intake and risk of endometriosis: a meta-analysis. Eur J Nutr. 2014;53(7):1573–1579. doi: 10.1007/s00394-014-0662-7. [DOI] [PubMed] [Google Scholar]
- 46.Jurkiewicz-Przondziono J., Lemm M., Kwiatkowska-Pamula A., Ziolko E., Wojtowicz M.K. Influence of diet on the risk of developing endometriosis. Ginekol Pol. 2017;88(2):96–102. doi: 10.5603/GP.a2017.0017. [DOI] [PubMed] [Google Scholar]
- 47.Strimbu K., Tavel J.A. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463–466. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.F.-N.B.W. Group, BEST (Biomarkers, EndpointS, and other Tools) Resource, Silver Spring (MD), 2016. [PubMed]
- 49.D. Gupta, M.L. Hull, I. Fraser, L. Miller, P.M. Bossuyt, N. Johnson, V. Nisenblat, Endometrial biomarkers for the non-invasive diagnosis of endometriosis, Cochrane Database Syst Rev 4 (2016) CD012165 DOI: 10.1002/14651858.CD012165. [DOI] [PMC free article] [PubMed]
- 50.Perez B.H., Gipson I.K. Focus on molecules: human mucin MUC16. Exp Eye Res. 2008;87(5):400–401. doi: 10.1016/j.exer.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bottoni P., Scatena R. The role of CA 125 as tumor marker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:229–244. doi: 10.1007/978-94-017-7215-0_14. [DOI] [PubMed] [Google Scholar]
- 52.Hirsch M., Duffy J., Davis C.J., Nieves M., Plana K.S., Khan O. International collaboration to harmonise, E. Measures for, diagnostic accuracy of cancer antigen 125 for endometriosis: a systematic review and meta-analysis. BJOG. 2016;123(11):1761–1768. doi: 10.1111/1471-0528.14055. [DOI] [PubMed] [Google Scholar]
- 53.Kafali H., Artuc H., Demir N. Use of CA125 fluctuation during the menstrual cycle as a tool in the clinical diagnosis of endometriosis; a preliminary report. Eur J Obstet Gynecol Reprod Biol. 2004;116(1):85–88. doi: 10.1016/j.ejogrb.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 54.Kitawaki J., Ishihara H., Koshiba H., Kiyomizu M., Teramoto M., Kitaoka Y. Usefulness and limits of CA-125 in diagnosis of endometriosis without associated ovarian endometriomas. Hum Reprod. 2005;20(7):1999–2003. doi: 10.1093/humrep/deh890. [DOI] [PubMed] [Google Scholar]
- 55.McKinnon B., Mueller M.D., Nirgianakis K., Bersinger N.A. Comparison of ovarian cancer markers in endometriosis favours HE4 over CA125. Mol Med Rep. 2015;12(4):5179–5184. doi: 10.3892/mmr.2015.4062. [DOI] [PubMed] [Google Scholar]
- 56.Harada T, Kubota T, Aso T. Usefulness of CA19-9 versus CA125 for the diagnosis of endometriosis, Fertil Steril 78(4) (2002) 733-9Fertil Steril DOI: 10.1016/s0015-0282(02)03328-9. [DOI] [PubMed]
- 57.Fassbender A, Burney RO, O DF, D'Hooghe T, Giudice L. Update on biomarkers for the detection of endometriosis. Biomed Res Int 2015 (2015) 130854 DOI: 10.1155/2015/130854. [DOI] [PMC free article] [PubMed]
- 58.Agic A., Djalali S., Wolfler M.M., Halis G., Diedrich K., Hornung D. Combination of CCR1 mRNA, MCP1, and CA125 measurements in peripheral blood as a diagnostic test for endometriosis. Reprod Sci. 2008;15(9):906–911. doi: 10.1177/1933719108318598. [DOI] [PubMed] [Google Scholar]
- 59.Seppala M., Taylor R.N., Koistinen H., Koistinen R., Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev. 2002;23(4):401–430. doi: 10.1210/er.2001-0026. [DOI] [PubMed] [Google Scholar]
- 60.Bolton A.E., Pockley A.G., Clough K.J., Mowles E.A., Stoker R.J., Westwood O.M. Identification of placental protein 14 as an immunosuppressive factor in human reproduction. Lancet. 1987;1(8533):593–595. doi: 10.1016/s0140-6736(87)90235-2. [DOI] [PubMed] [Google Scholar]
- 61.Koninckx P.R., Riittinen L., Seppala M., Cornillie F.J. CA-125 and placental protein 14 concentrations in plasma and peritoneal fluid of women with deeply infiltrating pelvic endometriosis. Fertil Steril. 1992;57(3):523–530. [PubMed] [Google Scholar]
- 62.Bersinger N.A., Birkhauser M.H., Yared M., Wunder D.M. Serum glycodelin pattern during the menstrual cycle in healthy young women. Acta Obstet Gynecol Scand. 2009;88(11):1215–1221. doi: 10.3109/00016340903294264. [DOI] [PubMed] [Google Scholar]
- 63.Drosdzol-Cop A., Skrzypulec-Plinta V. Selected cytokines and glycodelin A levels in serum and peritoneal fluid in girls with endometriosis. J Obstet Gynaecol Res. 2012;38(10):1245–1253. doi: 10.1111/j.1447-0756.2012.01860.x. [DOI] [PubMed] [Google Scholar]
- 64.Vodolazkaia A., El-Aalamat Y., Popovic D., Mihalyi A., Bossuyt X., Kyama C.M. Evaluation of a panel of 28 biomarkers for the non-invasive diagnosis of endometriosis. Hum Reprod. 2012;27(9):2698–2711. doi: 10.1093/humrep/des234. [DOI] [PubMed] [Google Scholar]
- 65.Vodolazkaia A., Yesilyurt B.T., Kyama C.M., Bokor A., Schols D., Huskens D. Vascular endothelial growth factor pathway in endometriosis: genetic variants and plasma biomarkers. Fertil Steril. 2016;105(4):988–996. doi: 10.1016/j.fertnstert.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 66.Kressin P., Wolber E.M., Wodrich H., Meyhofer-Malik A., Buchweitz O., Diedrich K. Vascular endothelial growth factor mRNA in eutopic and ectopic endometrium. Fertil Steril. 2001;76(6):1220–1224. doi: 10.1016/s0015-0282(01)02898-9. [DOI] [PubMed] [Google Scholar]
- 67.Mohamed M.L., El Behery M.M., Mansour S.A. Comparative study between VEGF-A and CA-125 in diagnosis and follow-up of advanced endometriosis after conservative laparoscopic surgery. Arch Gynecol Obstet. 2013;287(1):77–82. doi: 10.1007/s00404-012-2539-4. [DOI] [PubMed] [Google Scholar]
- 68.Chen L., Fan R., Huang X., Xu H., Zhang X. Reduced levels of serum pigment epithelium-derived factor in women with endometriosis. Reprod Sci. 2012;19(1):64–69. doi: 10.1177/1933719111413300. [DOI] [PubMed] [Google Scholar]
- 69.Ekarattanawong S., Tanprasertkul C., Somprasit C., Chamod P., Tiengtip R., Bhamarapravatana K. Possibility of using superoxide dismutase and glutathione peroxidase as endometriosis biomarkers. Int J Womens Health. 2017;9:711–716. doi: 10.2147/IJWH.S141021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Othman E.R., Hornung D., Hussein M., Abdelaal A.A., II, Sayed A.N., Fetih A. Al-Hendy. Soluble tumor necrosis factor-alpha receptors in the serum of endometriosis patients. Eur J Obstet Gynecol Reprod Biol. 2016;200:1–5. doi: 10.1016/j.ejogrb.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 71.Hisrich B.V., Young R.B., Sansone A.M., Bowens Z., Green L.J., Lessey B.A. Role of human galectins in inflammation and cancers associated with endometriosis. Biomolecules. 2020;10(2) doi: 10.3390/biom10020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brubel R., Bokor A., Pohl A., Schilli G.K., Szereday L., Bacher-Szamuel R. Serum galectin-9 as a noninvasive biomarker for the detection of endometriosis and pelvic pain or infertility-related gynecologic disorders. Fertil Steril. 2017;108(6):1016–1025 e2. doi: 10.1016/j.fertnstert.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 73.Regidor P.A., Vogel C., Regidor M., Schindler A.E., Winterhager E. Expression pattern of integrin adhesion molecules in endometriosis and human endometrium. Hum Reprod Update. 1998;4(5):710–718. doi: 10.1093/humupd/4.5.710. [DOI] [PubMed] [Google Scholar]
- 74.Pino M., Galleguillos C., Torres M., Sovino H., Fuentes A., Boric M.A. Association between MMP1 and MMP9 activities and ICAM1 cleavage induced by tumor necrosis factor in stromal cell cultures from eutopic endometria of women with endometriosis. Reproduction. 2009;138(5):837–847. doi: 10.1530/REP-09-0196. [DOI] [PubMed] [Google Scholar]
- 75.Matsuzaki S., Darcha C., Maleysson E., Canis M., Mage G. Impaired down-regulation of E-cadherin and beta-catenin protein expression in endometrial epithelial cells in the mid-secretory endometrium of infertile patients with endometriosis. J Clin Endocrinol Metab. 2010;95(7):3437–3445. doi: 10.1210/jc.2009-2713. [DOI] [PubMed] [Google Scholar]
- 76.Shaco-Levy R., Sharabi S., Benharroch D., Piura B., Sion-Vardy N. Matrix metalloproteinases 2 and 9, E-cadherin, and beta-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):226–232. doi: 10.1016/j.ejogrb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 77.Lermann J., Mueller A., Korber F., Oppelt P., Beckmann M.W., Dittrich R. Evaluation of high-sensitivity C-reactive protein in comparison with C-reactive protein as biochemical serum markers in women with endometriosis. Fertil Steril. 2010;93(7):2125–2129. doi: 10.1016/j.fertnstert.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 78.Thubert T, Santulli P, Marcellin L, Menard S, M'Baye M, Streuli I, Borghese B, de Ziegler D, Chapron C. Measurement of hs-CRP is irrelevant to diagnose and stage endometriosis: prospective study of 834 patients, Am J Obstet Gynecol 210(6) (2014) 533 e1-533 e10. DOI: 10.1016/j.ajog.2014.01.022. [DOI] [PubMed]
- 79.Symons L.K., Miller J.E., Kay V.R., Marks R.M., Liblik K., Koti M. The immunopathophysiology of endometriosis. Trends Mol Med. 2018;24(9):748–762. doi: 10.1016/j.molmed.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 80.Gajbhiye R., Bendigeri T., Ghuge A., Bhusane K., Begum S., Warty N. Panel of autoimmune markers for noninvasive diagnosis of minimal-mild endometriosis. Reprod Sci. 2017;24(3):413–420. doi: 10.1177/1933719116657190. [DOI] [PubMed] [Google Scholar]
- 81.Pergialiotis V., Tagkou N.M., Tsimpiktsioglou A., Klavdianou O., Neonaki A., Trompoukis P. Urocortin expression in endometriosis: a systematic review. Int J Fertil Steril. 2019;13(1):1–5. doi: 10.22074/ijfs.2019.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reis F.M., Luisi S., Abrao M.S., Rocha A.L., Vigano P., Rezende C.P. Diagnostic value of serum activin A and follistatin levels in women with peritoneal, ovarian and deep infiltrating endometriosis. Hum Reprod. 2012;27(5):1445–1450. doi: 10.1093/humrep/des055. [DOI] [PubMed] [Google Scholar]
- 83.Jia SZ, Yang Y, Lang J, Sun P, Leng J. Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis, Hum Reprod 28(2) (2013) 322-30Hum Reprod DOI: 10.1093/humrep/des413. [DOI] [PMC free article] [PubMed]
- 84.Johnson S.M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A. RAS is regulated by the let-7 microRNA family. Cell. 2005;120(5):635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 85.Cho S, Mutlu L, Grechukhina O, Taylor HS. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril 103(5) (2015) 1252–60 e1. DOI: 10.1016/j.fertnstert.2015.02.013. [DOI] [PMC free article] [PubMed]
- 86.Maged A.M., Deeb W.S., El Amir A., Zaki S.S., El Sawah H., Al Mohamady M. Diagnostic accuracy of serum miR-122 and miR-199a in women with endometriosis. Int J Gynaecol Obstet. 2018;141(1):14–19. doi: 10.1002/ijgo.12392. [DOI] [PubMed] [Google Scholar]
- 87.Rekker K., Saare M., Roost A.M., Kaart T., Soritsa D., Karro H. Circulating miR-200-family micro-RNAs have altered plasma levels in patients with endometriosis and vary with blood collection time. Fertil Steril. 2015;104(4):938–946 e2. doi: 10.1016/j.fertnstert.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 88.Creed J., Maggrah A., Reguly B., Harbottle A. Mitochondrial DNA deletions accurately detect endometriosis in symptomatic females of child-bearing age. Biomark Med. 2019;13(4):291–306. doi: 10.2217/bmm-2018-0419. [DOI] [PubMed] [Google Scholar]
- 89.Zachariah R., Schmid S., Radpour R., Buerki N., Fan A.X., Hahn S. Circulating cell-free DNA as a potential biomarker for minimal and mild endometriosis. Reprod Biomed Online. 2009;18(3):407–411. doi: 10.1016/s1472-6483(10)60100-9. [DOI] [PubMed] [Google Scholar]
- 90.Simpson JL, Bischoff FZ. Heritability and molecular genetic studies of endometriosis, Ann N Y Acad Sci 955 (2002) 239-51; discussion 293-5, 396-406. DOI: 10.1111/j.1749-6632.2002.tb02785.x. [DOI] [PubMed]
- 91.Montgomery G.W., Nyholt D.R., Zhao Z.Z., Treloar S.A., Painter J.N., Missmer S.A. The search for genes contributing to endometriosis risk. Hum Reprod Update. 2008;14(5):447–457. doi: 10.1093/humupd/dmn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mickiewicz K.M., Kawai Y., Drage L., Gomes M.C., Davison F., Pickard R. Possible role of L-form switching in recurrent urinary tract infection. Nat Commun. 2019;10(1):4379. doi: 10.1038/s41467-019-12359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kawai Y, Mickiewicz K, Errington J. Lysozyme counteracts beta-lactam antibiotics by promoting the emergence of L-form bacteria. Cell 172(5) (2018) 1038-1049 e10. DOI: 10.1016/j.cell.2018.01.021. [DOI] [PMC free article] [PubMed]
- 94.Domingue G.J. Demystifying pleomorphic forms in persistence and expression of disease: are they bacteria, and is peptidoglycan the solution? Discov Med. 2010;10(52):234–246. [PubMed] [Google Scholar]
- 95.Saldova R. Cause of cancer and chronic inflammatory diseases and the implications for treatment. Discov Med. 2016;22(120):105–119. [PubMed] [Google Scholar]
- 96.Onwuamaegbu M.E., Belcher R.A., Soare C. Cell wall-deficient bacteria as a cause of infections: a review of the clinical significance. J Int Med Res. 2005;33(1):1–20. doi: 10.1177/147323000503300101. [DOI] [PubMed] [Google Scholar]
- 97.Leonardi M, Hicks C, El-Assaad F, El-Omar E, Condous G. Endometriosis and the microbiome: a systematic review, BJOG 127(2) (2020) 239-249BJOG DOI: 10.1111/1471-0528.15916. [DOI] [PubMed]
- 98.Khan K.N., Fujishita A., Kitajima M., Hiraki K., Nakashima M., Masuzaki H. Intra-uterine microbial colonization and occurrence of endometritis in women with endometriosisdagger. Hum Reprod. 2014;29(11):2446–2456. doi: 10.1093/humrep/deu222. [DOI] [PubMed] [Google Scholar]
- 99.Khan K.N., Kitajima M., Hiraki K., Yamaguchi N., Katamine S., Matsuyama T. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil Steril. 2010;94(7) doi: 10.1016/j.fertnstert.2010.04.053. pp. 2860–3 e1–3. [DOI] [PubMed] [Google Scholar]
- 100.Ata B., Yildiz S., Turkgeldi E., Brocal V.P., Dinleyici E.C., Moya A. The endobiota study: comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Sci Rep. 2019;9(1):2204. doi: 10.1038/s41598-019-39700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen C., Song X., Wei W., Zhong H., Dai J., Lan Z. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun. 2017;8(1):875. doi: 10.1038/s41467-017-00901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lamont R.F., Sobel J.D., Akins R.A., Hassan S.S., Chaiworapongsa T., Kusanovic J.P. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG. 2011;118(5):533–549. doi: 10.1111/j.1471-0528.2010.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anahtar M.N., Byrne E.H., Doherty K.E., Bowman B.A., Yamamoto H.S., Soumillon M. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42(5):965–976. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rampersaud R., Randis T.M., Ratner A.J. Microbiota of the upper and lower genital tract. Semin Fetal Neonatal Med. 2012;17(1):51–57. doi: 10.1016/j.siny.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peric A., Weiss J., Vulliemoz N., Baud D., Stojanov M. Bacterial colonization of the female upper genital tract. Int J Mol Sci. 2019;20(14) doi: 10.3390/ijms20143405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khan K.N., Fujishita A., Masumoto H., Muto H., Kitajima M., Masuzaki H. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2016;199:69–75. doi: 10.1016/j.ejogrb.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 107.Hernandes C., Silveira P., Rodrigues Sereia A.F., Christoff A.P., Mendes H., Valter de Oliveira L.F. Microbiome profile of deep endometriosis patients: comparison of vaginal fluid. Endometrium and Lesion, Diagnostics (Basel) 2020;10(3) doi: 10.3390/diagnostics10030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perrotta A.R., Borrelli G.M., Martins C.O., Kallas E.G., Sanabani S.S., Griffith L.G. The vaginal microbiome as a tool to predict rASRM stage of disease in endometriosis: a pilot study. Reprod Sci. 2020 doi: 10.1007/s43032-019-00113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baker J.M., Al-Nakkash L., Herbst-Kralovetz M.M. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 110.Jung H., Ehlers M.M., Peters R.P.H., Lombaard H., Redelinghuys M.J., Bezuidenhoudt J.E. Growth forms of Gardnerella spp. and Lactobacillus spp. on vaginal cells. Front Cell Infect Microbiol. 2020;10:71. doi: 10.3389/fcimb.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Durack J, Lynch SV. The gut microbiome: Relationships with disease and opportunities for therapy. J Exp Med 216(1) (2019) 20-40J Exp Med DOI: 10.1084/jem.20180448. [DOI] [PMC free article] [PubMed]
- 112.Govindarajan K., MacSharry J., Casey P.G., Shanahan F., Joyce S.A., Gahan C.G. Unconjugated bile acids influence expression of circadian genes: a potential mechanism for microbe-host crosstalk. PLoS ONE. 2016;11(12) doi: 10.1371/journal.pone.0167319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Butler T.D., Gibbs J.E. Circadian host-microbiome interactions in immunity. Front Immunol. 2020;11:1783. doi: 10.3389/fimmu.2020.01783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yuan M., Li D., Zhang Z., Sun H., An M., Wang G. Endometriosis induces gut microbiota alterations in mice. Hum Reprod. 2018;33(4):607–616. doi: 10.1093/humrep/dex372. [DOI] [PubMed] [Google Scholar]
- 115.Chadchan S.B., Cheng M., Parnell L.A., Yin Y., Schriefer A., Mysorekar I.U. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: a potential role for gut microbiota. Hum Reprod. 2019;34(6):1106–1116. doi: 10.1093/humrep/dez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hantschel J., Weis S., Schafer K.H., Menger M.D., Kohl M., Egert M. Effect of endometriosis on the fecal bacteriota composition of mice during the acute phase of lesion formation. PLoS ONE. 2019;14(12) doi: 10.1371/journal.pone.0226835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bailey M.T., Coe C.L. Endometriosis is associated with an altered profile of intestinal microflora in female rhesus monkeys. Hum Reprod. 2002;17(7):1704–1708. doi: 10.1093/humrep/17.7.1704. [DOI] [PubMed] [Google Scholar]
- 118.Seaman H.E., Ballard K.D., Wright J.T., de Vries C.S. Endometriosis and its coexistence with irritable bowel syndrome and pelvic inflammatory disease: findings from a national case-control study–Part 2. BJOG. 2008;115(11):1392–1396. doi: 10.1111/j.1471-0528.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- 119.Kwa M., Plottel C.S., Blaser M.J., Adams S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J Natl Cancer Inst. 2016;108(8) doi: 10.1093/jnci/djw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Itoh H., Uchida M., Sashihara T., Ji Z.S., Li J., Tang Q. Lactobacillus gasseri OLL2809 is effective especially on the menstrual pain and dysmenorrhea in endometriosis patients: randomized, double-blind, placebo-controlled study. Cytotechnology. 2011;63(2):153–161. doi: 10.1007/s10616-010-9326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khodaverdi S., Mohammadbeigi R., Khaledi M., Mesdaghinia L., Sharifzadeh F., Nasiripour S. Beneficial effects of oral Lactobacillus on pain severity in women suffering from endometriosis: a pilot placebo-controlled randomized clinical trial. Int J Fertil Steril. 2019;13(3):178–183. doi: 10.22074/ijfs.2019.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pomin V.H., Mulloy B. Glycosaminoglycans and proteoglycans. Pharmaceuticals (Basel) 2018;11(1) doi: 10.3390/ph11010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Freeze HH, Kranz C. Endoglycosidase and glycoamidase release of N-linked glycans. Curr Protoc Mol Biol Chapter 17 (2010) Unit 17 13. DOI: 10.1002/0471142727.mb1713as89. [DOI] [PMC free article] [PubMed]
- 124.Zauner G., Kozak R.P., Gardner R.A., Fernandes D.L., Deelder A.M., Wuhrer M. Protein O-glycosylation analysis. Biol Chem. 2012;393(8):687–708. doi: 10.1515/hsz-2012-0144. [DOI] [PubMed] [Google Scholar]
- 125.Reily C., Stewart T.J., Renfrow M.B., Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15(6):346–366. doi: 10.1038/s41581-019-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saldova R., Wormald M.R., Dwek R.A., Rudd P.M. Glycosylation changes on serum glycoproteins in ovarian cancer may contribute to disease pathogenesis. Dis Markers. 2008;25(4–5):219–232. doi: 10.1155/2008/601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arnold J.N., Saldova R., Hamid U.M., Rudd P.M. Evaluation of the serum N-linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics. 2008;8(16):3284–3293. doi: 10.1002/pmic.200800163. [DOI] [PubMed] [Google Scholar]
- 128.Harbison A.M., Brosnan L.P., Fenlon K., Fadda E. Sequence-to-structure dependence of isolated IgG Fc complex biantennary N-glycans: a molecular dynamics study. Glycobiology. 2019;29(1):94–103. doi: 10.1093/glycob/cwy097. [DOI] [PubMed] [Google Scholar]
- 129.Quast I., Keller C.W., Maurer M.A., Giddens J.P., Tackenberg B., Wang L.X. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J Clin Invest. 2015;125(11):4160–4170. doi: 10.1172/JCI82695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Feng D., Shi B., Bi F., Sagnelli M., Sun X., Jiao J. Elevated Serum Mannose Levels as a Marker of Polycystic Ovary Syndrome. Front Endocrinol (Lausanne) 2019;10:711. doi: 10.3389/fendo.2019.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sogabe M., Nozaki H., Tanaka N., Kubota T., Kaji H., Kuno A. Novel glycobiomarker for ovarian cancer that detects clear cell carcinoma. J Proteome Res. 2014;13(3):1624–1635. doi: 10.1021/pr401109n. [DOI] [PubMed] [Google Scholar]
- 132.Gebrehiwot A.G., Melka D.S., Kassaye Y.M., Gemechu T., Lako W., Hinou H. Exploring serum and immunoglobulin G N-glycome as diagnostic biomarkers for early detection of breast cancer in Ethiopian women. BMC Cancer. 2019;19(1):588. doi: 10.1186/s12885-019-5817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baston J.I., Baranao R.I., Ricci A.G., Bilotas M.A., Olivares C.N., Singla J.J. Targeting galectin-1-induced angiogenesis mitigates the severity of endometriosis. J Pathol. 2014;234(3):329–337. doi: 10.1002/path.4397. [DOI] [PubMed] [Google Scholar]
- 134.Izumi G., Koga K., Takamura M., Makabe T., Nagai M., Urata Y. Mannose receptor is highly expressed by peritoneal dendritic cells in endometriosis. Fertil Steril. 2017;107(1):167–173 e2. doi: 10.1016/j.fertnstert.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 135.Hirakawa T., Nasu K., Kai K., Aoyagi Y., Ishii T., Uemura T. Wisteria floribunda agglutinin-binding glycan expression is decreased in endometriomata. Reprod Biol Endocrinol. 2014;12:100. doi: 10.1186/1477-7827-12-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Choi H.J., Chung T.W., Choi H.J., Han J.H., Choi J.H., Kim C.H. Increased alpha2-6 sialylation of endometrial cells contributes to the development of endometriosis. Exp Mol Med. 2018;50(12):1–12. doi: 10.1038/s12276-018-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maignien C., Santulli P., Chouzenoux S., Gonzalez-Foruria I., Marcellin L., Doridot L. Reduced alpha-2,6 sialylation regulates cell migration in endometriosis. Hum Reprod. 2019;34(3):479–490. doi: 10.1093/humrep/dey391. [DOI] [PubMed] [Google Scholar]
- 138.Jones C.J., Inuwa I.M., Nardo L.G., Litta P., Fazleabas A.T. Eutopic endometrium from women with endometriosis shows altered ultrastructure and glycosylation compared to that from healthy controls–a pilot observational study. Reprod Sci. 2009;16(6):559–572. doi: 10.1177/1933719109332825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Miller D.L., Jones C.J., Aplin J.D., Nardo L.G. Altered glycosylation in peri-implantation phase endometrium in women with stages III and IV endometriosis. Hum Reprod. 2010;25(2):406–411. doi: 10.1093/humrep/dep401. [DOI] [PubMed] [Google Scholar]
- 140.Burney R.O., Talbi S., Hamilton A.E., Vo K.C., Nyegaard M., Nezhat C.R. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 141.Focarelli R., Luddi A., De Leo V., Capaldo A., Stendardi A., Pavone V. Dysregulation of GdA expression in endometrium of women with endometriosis: implication for endometrial receptivity. Reprod Sci. 2018;25(4):579–586. doi: 10.1177/1933719117718276. [DOI] [PubMed] [Google Scholar]
- 142.Lang G.A., Yeaman G.R. Autoantibodies in endometriosis sera recognize a Thomsen-Friedenreich-like carbohydrate antigen. J Autoimmun. 2001;16(2):151–161. doi: 10.1006/jaut.2000.0465. [DOI] [PubMed] [Google Scholar]
- 143.Piva M., Moreno J.I., Sharpe-Timms K.L. Glycosylation and over-expression of endometriosis-associated peritoneal haptoglobin. Glycoconj J. 2002;19(1):33–41. doi: 10.1023/a:1022580813870. [DOI] [PubMed] [Google Scholar]
- 144.BHA, Jasim Rasha Z, Al-Mashhadany Zohair I. Sialic acid is a novel biochemical marker in sera of Iraqi endometriotic patients. J Nat Sci Res Vol.4, No.10, (2014).
- 145.Berkes E., Muzinic A., Rigo J., Jr., Tinneberg H.R., Oehmke F. The analysis of the human plasma N-glycome in endometriosis patients. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):107–115. doi: 10.1016/j.ejogrb.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 146.Mu A.K., Lim B.K., Hashim O.H., Shuib A.S. Detection of differential levels of proteins in the urine of patients with endometrial cancer: analysis using two-dimensional gel electrophoresis and o-glycan binding lectin. Int J Mol Sci. 2012;13(8):9489–9501. doi: 10.3390/ijms13089489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lepedda A.J., De Muro P., Capobianco G., Formato M. Significance of urinary glycosaminoglycans/proteoglycans in the evaluation of type 1 and type 2 diabetes complications. J Diabetes Complications. 2017;31(1):149–155. doi: 10.1016/j.jdiacomp.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 148.Alonzi D.S., Su Y.H., Butters T.D. Urinary glycan markers for disease. Biochem Soc Trans. 2011;39(1):393–398. doi: 10.1042/BST0390393. [DOI] [PubMed] [Google Scholar]