Abstract
Background
Currently, biotherapy is mainly administered to treat patients with severe asthma with the Type 2 (T2) inflammation phenotype. The variability of T2 inflammatory markers remains poorly understood.
Objective
We aimed to describe the individual distributions of different biomarkers at varying thresholds and their variation patterns in participants with severe asthma.
Methods
We retrospectively reviewed the data of participants who had completed 2 or more fraction of exhaled nitric oxide (FeNO) and peripheral blood eosinophil counts in our centre within 5 years. The individual distribution of biomarkers (blood or sputum eosinophils, FeNO, and serum total IgE) with repeated measurements at different thresholds was analysed. The varied patterns of biomarkers were analysed by cluster analysis.
Results
A total of 241 eligible participants were screened. Through long-term longitudinal multiple measurements, we found that approximately 50% of severe asthmatics had blood eosinophil levels fluctuating around the threshold defined by biological agents. FeNO persisted at levels >19.5 ppb or 25 ppb in more than half of patients; about 30% of participants crossed this threshold. In our centre, 47.4% of participants consistently exceeded sputum eosinophils >3%, and 47.4% of patients crossed this threshold. Approximately 66.7% of participants had more than 50% alterations of serum total IgE, and 98.1% of participants continued to have IgE levels greater than 30 IU/mL. We used cluster analysis to classify variability and levels of FeNO and blood eosinophils and identified 4 patient clusters. Cluster 1 can be summarised as T2 severe asthma with low blood eosinophil levels and stability. Cluster 2 can be summarised as asthma with continuous increase and small fluctuations in various T2 inflammatory markers. Cluster 3 can be summarised as a non/low-T2 inflammatory phenotype. Cluster 4 can be summarised as a stable, moderate T2 inflammatory phenotype.
Conclusion
We identified the distributions and variable patterns of the T2 inflammatory markers currently used to guide asthma biotherapy in clinical practice. The longitudinal comprehensive multiple assessments of T2 inflammatory markers provide support for initiating biologic therapy patients with severe asthma whose biomarker levels vary.
Keywords: Severe asthma, Biologic therapy, Biomarker, Variability, Type 2 inflammation
Introduction
Severe asthma generally refers to the patients who need step 4 or step 5 treatments, like combination inhaled/oral corticosteroids, rescue inhalers, and maintenance drugs, which may or may not control asthma symptoms.1 The mortality, hospitalisations, and exacerbations of these patients are higher than those of patients with mild to moderate asthma, which cause more significant social, economic, and psychological burden on patients.2, 3, 4, 5
In addition to conventional asthma control drugs, more and more biological agents are being used to treat severe asthma. IgE monoclonal antibody (mAb), interleukin 5/5Rα mAb, and IL4/13 mAb target different pathogenesis signalling pathways of severe asthma to reduce exacerbations and maintain asthma control.6,7 Currently, these biotherapies are mainly administered to the asthmatic population with Type 2 (T2) inflammation.8, 9, 10 A critical component of the successful clinical application of these drugs is finding suitable biomarkers to identify patients who may respond to treatment.11, 12, 13, 14
Peripheral blood eosinophil is a biomarker for evaluating and predicting the response to anti-IL-5 therapy, and high eosinophil counts suggest a better treatment response.13,15, 16, 17, 18 However, the absolute response thresholds for different treatments have not been determined, although such thresholds as 150 cells/μL (for mepolizumab), 300 cells/μL (for benralizumab), and 400 cells/μL (for reslizumab) have been studied.19 In addition to serum total IgE, it has been suggested that blood eosinophils >260 cells/μL and/or fraction of exhaled nitric oxide (FeNO) > 19.5 ppb should be used as predictors of omalizumab therapy response to help screen patients who may experience a more positive impact on exacerbations and lung function.9 IL-4 mAb dupilumab, on the other hand, may be more effective in severe asthma patients with blood eosinophils >150 cells/μL and FeNO >25 ppb.20,21
Sputum eosinophil is another clinical biomarker for asthma biotherapy. However, due to the lack of detection convenience, only a few studies currently use sputum cell detection as a biomarker to predict biotherapeutic responsiveness.22, 23, 24, 25 Sputum eosinophils ≥3% is the most commonly used threshold. The Global Initiative for Asthma (GINA) uses sputum eosinophils ≥2% as the threshold for T2 airway inflammatory when guiding biotherapy in severe asthma. In some ways, blood eosinophils, FeNO, and total IgE have gradually become pharmacodynamic, predictive biomarkers for the efficacy of biological agents. Future research may identify combinations of different biomarkers, rather than individual biomarkers, to act as predictors for individualised biotherapy.
If the above indicators are to become reliable biomarkers to guide severe asthma biological treatment, it is vital to clarify their stability, variability, and fluctuation. Currently, standardised measurements for biomarkers in patients with severe asthma remain poorly described. Each clinically established biomarker, like blood eosinophils, FeNO, and serum total IgE has various reported cut-off values, and biomarker combinations, with no standardised values. Furthermore, the stability of these biomarkers is not fully understood. If the variability and volatility of these inflammatory markers are not fully understood, it is difficult to accurately determine the correlation between clinical improvement and biomarker changes after biotherapy.26 Our centre has several long-term follow-up programmes for severe asthma, most of whom are potential biotherapy candidates. We sought to investigate whether multiple longitudinal measurements could assess the variability and variation patterns of T2 inflammatory biomarkers in patients with severe asthma, and whether such patterns could provide to support initiating biologic therapy.
Methods
Study population
This study is a retrospective study. Participants who met the GINA 2020 definition of severe asthma and visited the China-Japan Friendship Hospital from February 1, 2018 to February 1, 2019, were screened. As our study aimed to determine the dynamic changes of biomarkers in the severe asthma populations before the initiation of biotherapy, participants with a biotherapy history were excluded. Inclusion criteria were that participants need at least 2 blood eosinophil counts and FeNO examinations in the past 5 years. Written informed consent was obtained from each participant. The ethics committee approved this study.
Patient demographic information was obtained from electronic records at our hospital, which were searched for data on whole blood cell counts, percentage of sputum eosinophils, serum total IgE (enzymatic chemiluminescence, Beckmen Coulter, America), FeNO (NIOX VERO, Aerocrine AB, Sweden), and pulmonary function results. If multiple values were available within a month, we used the maximum value for that month.
Considering that exacerbations are a specific sub-condition of asthmatic patients, current biotherapy studies mainly included participants with non-acute exacerbation states as the treatment population. Analysis of T2 inflammatory biomarker activity in non-acute exacerbation patients more closely resembles the clinical situations for biotherapy. Therefore, all asthma exacerbation data were excluded.
FeNO and blood eosinophils are currently simple and readily available biomarkers commonly used in clinical practice. While sputum eosinophil and serum total IgE measurements are strong predictors of potential improvement with biologic treatment, sputum eosinophils count is not readily available in some healthcare settings, and fewer participants in our study had IgE repeat measurements. Therefore, we selected the geometric mean and standard deviation (SD) of FeNO and blood eosinophils as variables to characterise the level and variability of inflammation in participants and performed cluster analysis.
Statistical analysis
The blood eosinophils, FeNO, and total IgE data with skewed distribution were logarithmically transformed, and the individual mean, SD, and coefficients of variation (CV) were calculated based on the repeated measurement data of each subject. We also used other methods to express the variation in these parameters (see Table 1).
Table 1.
Variability indicator of Type 2 inflammatory biomarkers
| Blood eos |
Formula |
| Blood eos geometric CV | SD of blood eos geometric mean/Blood eos geometric mean |
| ΔBlood eos | Blood eos (max-min) |
| %Blood eos | Δ Blood eos/multiple measurements mean |
| Blood eos (min/max %) |
Blood eos (min/max) |
| FeNO |
Formula |
| FeNO geometric CV | SD of FeNO geometric mean/FeNO geometric mean |
| ΔFeNO | FeNO (max-min) |
| %FeNO | ΔFeNO/multiple measurements mean |
| FeNO (min/max %) |
FeNO (min/max) |
| Sputum eos |
Formula |
| Sputum eos CV | SD of Sputum eos mean/Sputum eos mean |
| ΔSputum eos | Sputum eos (max-min) |
| %Sputum eos | ΔSputum eos/multiple measurements mean |
| Sputum eos (min/max %) |
Sputum eos (min/max) |
| IgE |
Formula |
| IgE geometric CV | SD of IgE geometric mean/IgE geometric mean |
| ΔIgE | IgE (max-min) |
| %IgE | ΔIgE/multiple measurements mean |
| IgE (min/max %) | IgE (min/max) |
CV, coefficient of variation. eos, eosinophils. Δ Blood eos, change in blood eos. FeNO, fraction of exhaled nitric oxide. Δ FeNO, change in FeNO. IgE, total immunoglobulin E. Δ IgE, change in IgE. max, maximum over measurements. min, minimum over measurements. min/max %: minimum as a percentage of maximum. SD, standard deviation. Sputum eos, percentage of sputum eosinophils. Δ Sputum eos, change in sputum eos
We attempted to cluster subjects using the K-means clustering algorithm, a non-hierarchical clustering method.27 First, we identified 4 separate cluster centres using cross-validation.28 The log mean and SD of blood eosinophils and FeNO were selected as variables to calculate the Euclidean distance of each subject from the centre of the cluster, to fall into categories according to the principle of the nearest distance, and to calculate various categories of new clustering centres. The procedure described above was continuously iterative until the centre of the cluster did not change in occurrence or reached our prescribed maximum number of iterations (100). Missing values were imputed to an unknown category. In short, the cluster analysis demonstrated that our participants naturally sorted into 4 separate clusters (Cluster 1, 2, 3, and 4), based on blood eosinophil and FeNO measurements. These clusters are further discussed below.
The normal distribution data were represented by mean ± SD and compared by one-way ANOVA. If there was a statistical difference, the Bonferroni method was used for pairwise comparison. Non-normally distributed data were compared by the Kruskal-Wallis test. We used a mixed-effect model of repeated measurements to analyse the effects of seasons on biomarker values. The categorical variables were expressed by frequency (composition ratio or percentage) and compared by the chi-square test. A two-tailed p-value of <0.05 was considered significant. All statistical analyses were performed with SPSS 20 and R software 3.5.
Results
Patient characteristics
A total of 241 eligible participants were screened. Table 2 shows the clinical characteristics of the participants. The average age of participants was 48.8 ± 13.1 years, the proportion of male participants was slightly higher (53.5%), most of the participants were overweight and obese (61.8%), only 28.6% of them had a history of smoking, and more than half of them had allergic rhinitis (54.4%). Long-acting muscarine anticholinergics were prescribed to 65.6% of participants, leukotriene receptor antagonists to 81.7%, and long-term oral glucocorticoids to 36.9%. The average level of forced expiratory volume in 1 s in multiple measurements was 2.2 ± 0.5 L, the mean SD of multiple measurements was 0.27, and the mean CV was 11.1%.
Table 2.
Clinical characteristics of the patients
| Overall n = 241 | Cluster 1 n = 46 | Cluster 2 n = 100 | Cluster 3 n = 16 | Cluster 4 n = 79 | pa | |
|---|---|---|---|---|---|---|
| Age, mean ± SD | 48.8 ± 13.1 | 47.4 ± 11.4 | 49.4 ± 12.5 | 44.9 ± 28.0 | 49.0 ± 10.6 | 0.798 |
| Sex, n (%) | 0.397 | |||||
| Male | 129 (53.5) | 31 (66.7) | 46 (46.0) | 8 (50.0) | 44 (56.1) | |
| Female | 112 (46.5) | 15 (33.3) | 54 (54.0) | 8 (50.0) | 35 (43.9) | |
| BMI, n (%) | 0.664 | |||||
| Normal (≤23.9 kg/m2) | 92 (38.2) | 21 (45.7) | 40 (40.0) | 6 (37.5) | 25 (31.6) | |
| Overweight (24.0–27.9 kg/m2) | 89 (36.9) | 112 (28.3) | 33 (33.0) | 8 (50.0) | 35 (44.3) | |
| Obese (≥28.0 kg/m2) | 60 (24.9) | 75 (26.1) | 27 (27.0) | 2 (12.5) | 19 (24.1) | |
| Smoke, n (%) | 69 (28.6) | 17 (37.5) | 27 (27.0) | 6 (37.5) | 19 (24.4) | 0.614 |
| Comorbidity, n (%) | ||||||
| Allergic rhinitis | 131 (54.4) | 27 (58.3) | 52 (52.0) | 10 (62.5) | 42 (53.7) | 0.919 |
| Rhinosinusitis | 42 (17.4) | 10 (20.8) | 17 (17.0) | 4 (25) | 11 (13.9) | 0.777 |
| Nasal polyp | 16 (6.6) | 4 (8.3) | 12 (12.0) | 0 (0) | 0 (0) | 0.093 |
| Lung function | ||||||
| Pre-BD FEV1 (L), mean ± SD | 2.2 ± 0.5 | 2.5 ± 0.8 | 2.1 ± 0.6 | 2.3 ± 0.7 | 2.2 ± 0.7 | 0.222 |
| Pre-BD FEV1 CV (%), median (IQR) | 11.1 (5.6, 17.5) | 9.3 (5.5, 16.3) | 11.6 (6.7, 21.7) | 7.8 (1.8, 15.4) | 8.5 (3.6, 13.4) | 0.222 |
| Pre-BD FEV1/FVC (%), mean ± SD | 63.1 ± 12.7 | 65.9 ± 12.5 | 63.1 ± 10.1 | 62.4 ± 15.7 | 62.2 ± 14.7 | 0.709 |
| Pre-BD FEV1/FVC CV (%), median (IQR) | 6.7 (2.8, 11.0) | 6.9 (5.2, 12.5) | 6.5 (2.9, 11.3) | 3.9 (2.9, 12.5) | 7.5 (2.4, 10.6) | 0.946 |
| Treatment, n (%) | ||||||
| ICS/LABA | 241 (100) | 46 (100) | 100 (100) | 16 (100) | 79 (100) | |
| LAMA | 158 (65.6) | 23 (50.0) | 73 (73.0) | 10 (62.5) | 52 (65.9) | 0.263 |
| LTRA | 197 (81.7) | 36 (79.2) | 87 (87.0) | 12 (75) | 62 (78) | 0.639 |
| Theophylline | 162 (67.2) | 40 (87.5) | 58 (58.0) | 12 (75) | 52 (65.9) | 0.066 |
| OCS | 89 (36.9) | 23 (50.0) | 33 (33.0) | 4 (25.0) | 29 (36.6) | 0.474 |
| ACT, mean ± SD | 22 ± 4 | 21 ± 4 | 22 ± 3 | 22 ± 3 | 22 ± 4 | 0.329 |
| acute exacerbations, n (%) | 181 (75.1) | 36 (79.2) | 81 (81.0) | 12 (75.0) | 52 (65.9) | 0.117 |
ACT, asthma control test. BMI, body mass index. CV, coefficient of variation. FEV1, forced expiratory volume in 1 s. FVC, forced vital capacity. ICS, inhaled corticosteroids. LABA, long-acting beta-agonists. LAMA, long-acting muscarine anticholinergic. LTRA, leukotriene receptor antagonists. OCS, oral corticosteroids. pre-BD, pre-bronchodilator. SD, standard deviation.
Cluster 1 vs Cluster 2 vs Cluster 3 vs Cluster4
Distribution of individual biomarkers
Distribution of individual blood eosinophil levels
The mean number of blood eosinophils measurements per patient was 5. The geometric mean of blood eosinophils was 209 cells/μL with a wide range (0–4610 cells/μL), and the average geometric CV was 19.36%. The scatter plot of blood eosinophils for each patient is shown in Fig. 1. To understand whether the participants met the criteria used by many trials for biologics, we determined how many participants had eosinophil values consistently above or below the thresholds of 150 cells/μL, 300 cells/μL, 400 cells/μL, and 500 cells/μL (Table 3).13,19,29 Our data indicate that, 33.9% of the participants continued to have blood eosinophil values greater than 150 cells/μL, and 57.6% exceeded this cut-off value at least once. When the threshold was 300 cells/μL, 22.0% of the participants continued to exceed the cut-off value, and 61.9% of the participants traversed the cut-off value. Further, 58.5% of participants crossed the threshold of 400 cells/μL. Only 5.1% of the participants demonstrated values greater than 500 cells/μL continuously, and more than half of the participants (56.8%) exceeded 500 cells/μL at some point.
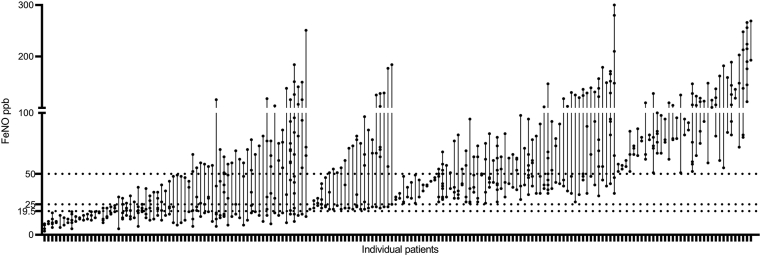
Fig. 1.
Scatter plot of blood eosinophils for each patient
Table 3.
Distribution of individual Type 2 inflammatory biomarkers
| Threshold | Always below threshold | Crossing the threshold | Persistently above threshold | |
|---|---|---|---|---|
| Blood eos | 150 cells/μL, n (%) | 20 (8.5) | 139 (57.6) | 82 (33.9) |
| 300 cells/μL, n (%) | 39 (16.1) | 149 (61.9) | 53 (22.0) | |
| 400 cells/μL, n (%) | 75 (31.3) | 141 (58.5) | 25 (10.2) | |
| 500 cells/μL, n (%) | 92 (38.1) | 137 (56.8) | 12 (5.1) | |
| FeNO | 19.5 ppb, n (%) | 26 (10.9) | 69 (28.6) | 146 (60.5) |
| 25 ppb, n (%) | 34 (14.3) | 85 (35.3) | 121 (50.4) | |
| 50 ppb, n (%) | 83 (34.5) | 117 (48.7) | 40 (16.8) | |
| Blood eos + FeNO | 19.5 ppb + 260 cells/μL, n (%) | 47 (19.5) | 166 (68.8) | 28 (11.7) |
| 25 ppb + 300 cells/μL, n (%) | 56 (23.4) | 162 (67.2) | 23 (9.4) | |
| Sputum eos | 3%, n (%) | 9 (5.2) | 82 (47.4) | 82 (47.4) |
| Serum total IgE | 30 IU/mL, n (%) | 0 (0) | 1 (1.9) | 61 (98.1) |
eos, eosinophils. FeNO, fraction of exhaled nitric oxide. IgE, total immunoglobulin E
Distribution of individual FeNO
The mean number of FeNO measurements per patient was 4. The scatter plot of FeNO values for each patient is shown in Fig. 2. The geometric mean of FeNO was 41 ppb with a wide range (3–300 ppb), and the mean geometric CV was 12.35%. When FeNO values were introduced to guide initial biotherapy in severe asthma, 19.5 ppb, 25 ppb, and 50 ppb became possible threshold levels in different biotherapy trials.20,30 In our centre, we found that 60.5% of participants had continuous measurements greater than 19.5 ppb, and 28.6% had at least 1 result greater than this cut-off value. Further, 50.4% of the participants continued to demonstrate FeNO values greater than 25 ppb, and 35.3% of the participants had levels that were repeatedly above 25 ppb. When the threshold level rose to 50 ppb, only 16.8% of participants consistently exceed 50 ppb, while 48.7% of the participants traversed the 50 ppb threshold at least once (Table 3).
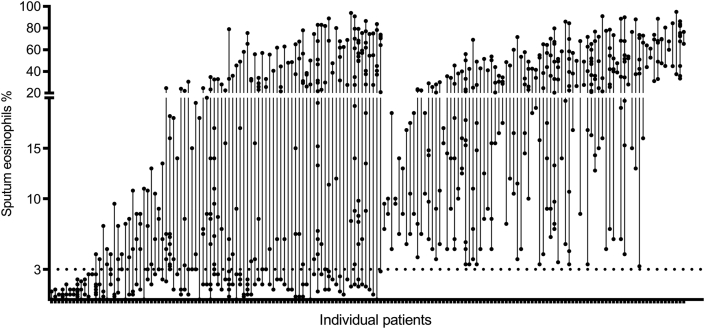
Fig. 2.
Scatter plot of sputum eosinophils for each patient
Distribution of blood eosinophil levels combined with FeNO
Several studies have investigated the biomarker potential of combined blood eosinophils with FeNO to predict response to biotherapy. When blood eosinophils of ≥260 cells/μL and FeNO of >19.5 ppb were used as the threshold values for initial omalizumab treatment,20,30 11.7% of participants had values persistently greater than this threshold, and 68.8% of participants demonstrated values greater than the combined threshold at least once. When the combined thresholds were blood eosinophils values of ≥300 cells/μL and FeNO values of ≥25 ppb,20,21 9.4% of participants’ values were consistently greater than this threshold, and 67.2% had at least 1 measurement that was greater than the combined thresholds at the same point (Table 3).
Distribution of sputum eosinophil level
A total of 172 participants had more than 2 sputum cell examinations and were measured an average of 5 times. The mean value of sputum eosinophils was 26% and ranged from 0% to 94%. The scatter plot of sputum eosinophils for each patient is shown in Fig. 3. A part of the anti-IL-5 trials used sputum eosinophils of ≥3% as a criterion to screen patients with eosinophilic asthma.18,23,25 In our centre, 47.4% of patients consistently exceeded this threshold (Table 3).
Fig. 3.
Scatter plot of FeNO for each patient. FeNO, fraction of exhaled nitric oxide
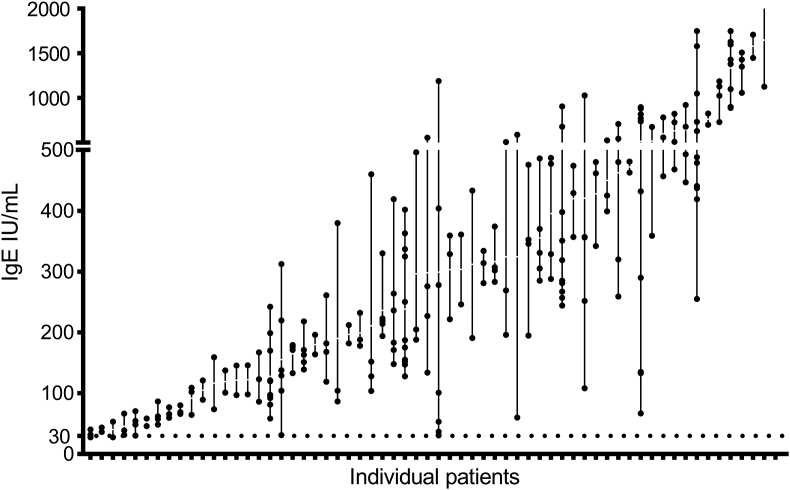
Distribution of individual total IgE levels
A total of 62 participants had more than 2 IgE examinations, with each of these participants measured an average of 4 times. The scatter plot of IgE for each patient is shown in Fig. 4. The geometric mean was 239.88 IU/mL, the range was 27.3–1750.0 IU/mL, and the mean geometric CV was 6%. In total, 66.7% of participants had more than 50% alternations of IgE. Currently, serum total IgE of ≥30 IU/mL is one of the criteria for omalizumab treatment in severe asthma,31 and we found that 98.1% of participants continued to demonstrate IgE values greater than this threshold (Table 3).
Fig. 4.
Scatter plot of total IgE for each patient
To determine whether the variability in all of the above T2 biomarkers was attributable to the effect of sampling time (month), we analysed the relationship between T2 biomarkers and month with a mixed-effects model for repeated measures. We found no significant relationship between blood or sputum eosinophils, FeNO, IgE, and time of year (data not shown).
Variability patterns and level of biomarkers
To further define patterns of variation in T2 inflammatory markers that could guide biotherapy in severe asthma, we performed cluster analysis of blood eosinophils and FeNO (see Methods), which are currently the simplest and most readily available inflammatory markers in the clinic. Cluster analysis sorted participants into 4 groups (Clusters 1, 2, 3, and 4) that demonstrated no significant differences in clinical characteristics that might influence T2 inflammatory levels and variability, such as age, sex, body mass index, smoking history, comorbidities, lung function, medication, asthma control test, and exacerbations (Table 2).
The variability pattern analyses of T2 inflammatory markers are shown in Fig. 5. Cluster 1 had large variability in blood eosinophils and FeNO, with higher FeNO levels (geometric mean 58 ppb, 95% CI [49,69]), sputum eosinophils (median [IQR], 27.8% [13.8, 56.0] %), and IgE levels (geometric mean 251 IU/mL, 95% CI [158,417] IU/mL). Cluster 2 included the largest number of participants, with little variability in blood eosinophils and FeNO levels. However, participants in this cluster had the highest levels of blood eosinophils (geometric mean 513 cells/μL, 95% CI [447, 589] cells/μL), FeNO (geometric mean 62 ppb, 95% CI [54, 69]), sputum eosinophils (median [IQR], 34.8% [22.6, 42.0] %), and IgE (347 IU/mL, 95% CI [257, 468] IU/mL). Cluster 3 included the least number of participants, with large blood eosinophil variations but the lowest blood eosinophil counts, FeNO levels, sputum eosinophils, and IgE values. Cluster 4 had little variability in blood eosinophils, with a slightly higher geometric mean of blood eosinophils at 224 cells/μL (95% CI [191, 269]), and relatively lower FeNO levels of 21 ppb (95% CI [19, 25]). The sputum eosinophils and IgE levels in Cluster 4 were significantly lower than those in Cluster 2.
Fig. 5.
Variability patterns of Type 2 inflammatory biomarkers. Fig. 5A Variability patterns of blood eosinophils. (a1) Geometric mean of blood eos: C2 >C4 >C1 >C3 (p < 0.05); (a2) Geometric CV of blood eos: C1, C3 >C2, C4 (p < 0.05); (a3) %Blood eos: C1, C3 >C2, C4 (p < 0.05); (a4) ΔBlood eos: C1, C2 >C3 (p <0.05), C2 >C4 (p < 0.05); (a5) Blood eos (min/max %): C1, C3 <C2, C4 (p < 0.05). Fig. 5B Variability patterns of FeNO. (b1) Geometric mean of FeNO: C1, C2 >C3, C4 (p<0.05); (b2)Geometric CV of FeNO: C1, C3, C4 >C2 (p < 0.05); (b3) %FeNO: C1 >C2, C4; (b4) ΔFeNO: C1 >C2 >C3, C4 (p < 0.05); (b5) FeNO (min/max %): C1 <C2, C4 (p < 0.05). Fig. 5C Variability patterns of sputum eosinophils. (c1) Mean of sputum eos: C2 >C3, C4 (p < 0.05); (c2) CV of sputum eos; (c3) %Sputum eos; (c4) ΔSputum eos; (c5) Sputum eos (min/max %). Fig. 5D Variability patterns of serum total IgE. (d1) Geometric mean of IgE: C1, C2 >C3 (p <0.05), C2 >C4 (p < 0.05); (d2) Geometric CV of IgE; (d3) %IgE; (d4) ΔIgE: C2 >C4 (p < 0.05); (d5) IgE (min/max %). C1, Cluster1. C2, Cluster2. C3, Cluster3. C4, Cluster4. CV, coefficient of variation. eos, eosinophils. ΔBlood eos, change in blood eos. FeNO, fraction of exhaled nitric oxide. ΔFeNO, change in FeNO. ΔIgE, change in IgE. min/max %: minimum as a percentage of maximum. Sputum eos, percentage of sputum eosinophils. ΔSputum eos, change in sputum eos
Discussion
Biologic therapy has become an essential complement to T2 severe asthma treatment, and specific biomarkers have been used to guide biotherapy initiation. Previous studies have shown that T2 inflammatory markers often have significant variability and instability. Multiple measurements have been proposed to understand the variable patterns of biomarkers, to determine which biomarkers could diagnose asthma phenotypes and screen for more suitable biotherapy.32, 33, 34, 35 However, the current body of literature demonstrates various studies on biological agents, with different biomarkers and thresholds to guide treatment. Even the same biological agents (eg, IL-5/5Rα mAb mepolizumab and reslizumab) may have different therapeutic response thresholds.13,29 At present, most of the research data on these reactivity thresholds are from randomized controlled trial (RCT) studies, and the applicability of these thresholds in a range of severe asthma patients still needs to be determined. After all, some studies have shown that 80%–90% of severe asthma patients are excluded from RCT studies.36,37 Therefore, while the individual distribution and variation patterns of biomarkers under different thresholds are accurate in each study population, biological agents are applied in the real world, where population characteristics are not tightly controlled.
Previous studies have relied on blood eosinophil threshold levels (150 or 300 cells/uL) for biological treatment indication.22,32,38 Our study found that approximately 50% of severe asthmatics demonstrated blood eosinophil levels that fluctuated around this thresholds, mostly consistent with previous findings.22,32,38 However, our study extends these findings by effectively confirming the utility of thresholds 400/500 cells/uL. Only a small number of patients had blood eosinophil levels persistently greater than this threshold, and we could include more potential patients through multiple measurements.
In recent years, FeNO has become an increasingly used biomarker that can assess the response to and initiation of treatment with IgE or IL-4 mAb, but there are few studies on individual distribution and variability at different thresholds.39,40 Although FeNO levels persisted at values > 19.5 ppb or ≥25 ppb in more than half of participants in our study, about 30% of participants crossed this threshold. Our research shows that repeated measurements of FeNO can help identify patients with T2 asthma phenotypes. When using the combination of FeNO with blood eosinophils as a predictor of treatment response to biotherapy, only a small number of participants consistently demonstrated FeNO/blood eosinophil values greater than defined combination thresholds. Clinically, however, the majority of patients are potential beneficiaries of biologics through long-term longitudinal comprehensive assessments.
Sputum eosinophils are essential biomarkers for assessing the inflammatory phenotype of asthma. Biological agents improve airway eosinophilic inflammation, but there is no definite sputum eosinophilic threshold to guide their initial treatment.23,41,42 In this study, the sputum eosinophils in nearly half of severe asthma fluctuated around 3%, suggesting that repeated monitoring of sputum eosinophils helps define the phenotype of severe asthma. Moreover, thoroughly evaluating the changes of sputum eosinophils before biologic therapy is conducive to clarifying the relationship between clinical improvement and sputum eosinophils after treatment, which may positively affect determining a reasonable threshold of biologic therapy.
Of the 241 total participants, only 62 had any IgE data in their records, presumably because these tests are not repeatedly measurements in most clinical settings. Therefore, while these data are valuable and reported, they are not the focus of this study because of the lack of access across healthcare settings. IgE is also a variable indicator, and it changes with time.33 Our study found that IgE changed by more than 50% in most participants, and each individual's levels fluctuated about 6% around the geometric mean, which may lead to different doses of omalizumab in different periods. Whether this will affect the prognosis of patients is unknown. Further study is needed to select the highest, lowest, or average value of multiple measurements as the initial treatment threshold value.
At present, it is difficult to determine whether observed clinical improvement is proportional to the degree of change in specific biomarkers for biotherapy, due to the lack of understanding of the homeostatic changes in biomarkers at the individual level. The measurement of multiple biomarkers may be more informative, which may provide a more comprehensive and in-depth understanding of specific biologic regulatory pathways. In the study process, we found noticeable variability among individual distributions of biomarkers. We classified the variability and levels of FeNO and blood eosinophils by cluster analysis and identified 4 patterns.
Cluster 1 was a highly variable phenotype with high levels of T2 inflammation. However, the average level of blood eosinophils was low, and the variability was large, so in patients with Cluster 1 characteristics, it would be vital to select the right treatment timing if blood eosinophils were selected as the initial treatment biomarker. In other words, this group would not be considered the best potential beneficiaries of biologics if blood eosinophils were used as a biomarker and accordingly would be considered better suited for other biologics such as IgE mAb or IL-4/IL-13 mAb. When the biomarker variability for eosinophilic phenotypes is high, patients may reach treatment thresholds defined by different biologics at different periods, and thus be prescribed different biologics for treatment by a comprehensive longitudinal assessment of multiple T2 inflammation biomarkers, rather than if a single biomarker at a single time point is referenced.43
Cluster 2 was a low variability phenotype with high T2 inflammatory levels. This group would be considered beneficiaries of various currently approved biologics targeting eosinophilic phenotypes. What kind of biological agents would be prescribed, and whether the treatment response of different biological agents is consistent would be the main problem this group would face. Interestingly, there have been no head-to-head clinical trials for these biological agents, and most indirect comparative studies have not found significant differences in efficacy between these drugs.44, 45, 46
Cluster 3 was dominated by participants with a non-/low T2 inflammatory phenotype, and strictly speaking, these participants should be more accurately referred to as ‘having no prior evidence of an increased T2 inflammatory phenotype’. Although this group would not currently be considered potential beneficiaries of biologic therapy for eosinophilic phenotypes, we saw high variability in T2 inflammatory biomarkers in this cluster of participants, and whether this variability would be a criterion for the selection of subjects for biologic agents is unknown.
In Cluster 4, the level of T2 biomarkers fluctuated around the threshold of T2 inflammatory phenotype. Previous studies suggested that patients with elevated baseline eosinophils responded better to biotherapy, but patients with slightly higher eosinophil phenotypes still benefited from biotherapy.12,13 The phenotypes of Cluster 2 and Cluster 4 were stable, and the changes of multiple T2 inflammatory biomarkers in patients after biotherapy may be related to the treatment effect rather than the intrinsic variability. This class of phenotypically stable participants would readily find multiple sensitive biomarkers for assessing treatment response and their predictive thresholds.
Our clustering of biomarker variability adds a temporal dimension to the phenotype of severe asthma, and whether this could affect the prognosis of biotherapy is unknown. Future studies may add the dimension of time variability, rather than just considering absolute threshold levels, to explore criteria for initiating biological treatment in patients with severe asthma.
This study had limitations. Blood eosinophils and FeNO levels, the 2 most readily available biomarkers, were selected as indicators to assess eosinophilic inflammation, and the SD of the 2 was chosen as an indicator to evaluate their variability. While repeated IgE data are valuable and reported, only 62 participants had these data in their records, so we did not include them in the cluster analysis. Therefore, the variation pattern we analysed has a higher practical value, but its description of the variation pattern is not comprehensive enough. There is a linear correlation between T2 biomarkers, but there is also inconsistency. We used blood eosinophils and FeNO levels to assess eosinophilic inflammation, and the collinearity between them had little impact on our final analysis of T2 biomarker variation patterns. Cluster analysis can also be used to distinguish the populations with and without collinearity. For example, blood eosinophil levels in Cluster 1 were not correlated with FeNO levels, while there was no such inconsistency in Clusters 2–4.
Although data from the acute exacerbations were excluded from the study, it is difficult to confirm that fluctuations in these inflammatory biomarkers were not associated with changes in acute or maintenance therapy, because the data were derived from electronic records. Our study retrospectively analysed the variability of T2 inflammatory biomarkers in patients over 5 years. The mean number of each biomarker measurement was 4 or 5, consistent with previous studies that similarly used historical electronic medical records.32,47 Although the number of measurements is somewhat underpowered to assess the variability of T2 biomarkers, this is a real-world clinical assessment of T2 disease status in a severe asthma population. Previous studies have shown that season has little effect on the variability of eosinophils,32,34 and we also did not find the effect of time (month) on the variability of T2 biomarkers.
However, our study could not comprehensively assess patients' treatment adherence, inhalation technique, and control status at sampling time. We indiscriminately included all blood and sputum eosinophils, FeNO, and serum total IgE results from the past 5 years. Some of these tests are for asthma management purposes, such as daily or acute exacerbations assessment, while others are for physical examination, preoperative evaluation, and other tests that are not related to asthma management purposes. Patients with poorly controlled asthma tend to have elevated T2 inflammatory biomarkers, and different patients seek treatment at different thresholds: some patients may use self-management; others may undergo asthma-related tests under specialist guidance. Although all patients with severe asthma in the study were treated with high-dose ICS, some of the tests resulted from diagnosis or poor symptom control before optimizing asthma treatment.
The impact of patient behaviour, clinician behaviour, and medical process on a multidimensional longitudinal assessment of T2 inflammatory biomarkers means that any inference of potential mechanisms is purely hypothetical, but this form of sampling bias is not entirely undesirable. Purposeful sampling for clinical events implies that various tests, independent of the results, maybe significant in itself.48 Besides, these data represent clinical practice in the real world; so, the products described in this paper are highly transformative and relevant to the clinical setting.
In conclusion, our study found the distribution of different T2 inflammatory biomarkers and their combinations at different thresholds for assessing biological agent treatment for asthma. Simultaneously, a comprehensive longitudinal assessment of multiple T2 inflammatory biomarkers helped to reveal different variation patterns in the levels of currently used biomarkers blood and sputum eosinophils, FeNO levels. This may have important implications for identifying potential beneficiaries of biological therapies.
Abbreviations
BMI: body mass index, CI: confidence interval, CV: coefficient of variation, eos: eosinophil, ΔBlood eos: change in blood eos, ΔSputum eos: change in sputum eos, FeNO: fraction of exhaled nitric oxide, ΔFeNO: change in FeNO, FEV1: forced expiratory volume in 1 s, FVC: forced vital capacity, ICS: inhaled corticosteroids, IL-4: Interleukin-4, IL-5: Interleukin-5, IL-13: Interleukin-13, IgE: Immunoglobulin E, ΔIgE: change in IgE, IQR: interquartile range, mAb: monoclonal antibody, max: maximum over measurements, min: minimum over measurements, min/max %: minimum as a percentage of maximum, LAMA: long-acting muscarine anticholinergic, LABA: long-acting beta-agonists, LTRA: leukotriene receptor antagonists, OCS: oral corticosteroids, pre-BD: pre-bronchodilator, RCT: randomized clinical trials, SD: standard deviation, T2: Type 2.
Funding
No funding to declare.
Ethics approval
The study protocol was approved by the Ethics Committee of China-Japan Friendship Hospital (approval number: 2018-19-k14).
Author contributions
Designed the study: Hongwen Li and Jiangtao Lin.
Acquisitions of data or analysis and interpretation of data: Qing Zhang, Chunxiao Li, Jianxin Wang, and Shuhua Zhang.
Statistical analysis: Shengnan Gao, Jingru Wang and Hongwen Li.
Drafting articles: Hongwen Li.
Revising the article critically for important intellectual content and final approval of the version to be published: all authors.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent for publication
The submission of the article and the publication of the article by World Allergy Organization Journal has been approved by all authors.
Submission declaration
We confirm that the manuscript is original, has not been published before, is not currently being considered for publication elsewhere, and has not been posted to a preprint server.
Declaration of competing interest
The authors declare that they have no relevant conflicts of interest.
Acknowledgements
None.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Global Initiative for Asthma . 2020. Global Strategy for Asthma Management and Prevention.http://www.ginasthma.org [Google Scholar]
- 2.Bourdin A., Fabry-Vendrand C., Ostinelli J. The burden of severe asthma in France: a case-control study using a medical claims database. J Allergy Clin Immunol Pract. 2019;7(5):1477–1487. doi: 10.1016/j.jaip.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Foster J.M., McDonald V.M., Guo M., Reddel H.K. "I have lost in every facet of my life": the hidden burden of severe asthma. Eur Respir J. 2017;50(3):1700765. doi: 10.1183/13993003.00765-2017. [DOI] [PubMed] [Google Scholar]
- 4.Teague W.G., Iqbal A., Ding Y., Chipps B.E., Zazzali J.L. The added burden of allergen sensitization among children with severe or poorly controlled asthma. J Allergy Clin Immunol Pract. 2020;S2213–2198(20):30949. doi: 10.1016/j.jaip.2020.08.063. 30941. [DOI] [PubMed] [Google Scholar]
- 5.Lin J., Xing B., Tang H. Hospitalization due to asthma exacerbation: a China asthma research network (CARN) retrospective study in 29 provinces across mainland China. Allergy Asthma Immunol Res. 2020;12(3):485–495. doi: 10.4168/aair.2020.12.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel S.S., Casale T.B., Cardet J.C. Biological therapies for eosinophilic asthma. Expet Opin Biol Ther. 2018;18(7):747–754. doi: 10.1080/14712598.2018.1492540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caminati M., Pham D.L., Bagnasco D., Canonica G.W. Type 2 immunity in asthma. World Allergy Organ J. 2018;11(1):13. doi: 10.1186/s40413-018-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fahy J.V. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanania N.A., Wenzel S., Rosén K. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–811. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 10.Busse W., Spector S., Rosén K., Wang Y., Alpan O. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunol. 2013;132(2):485–486. doi: 10.1016/j.jaci.2013.02.032. e411. [DOI] [PubMed] [Google Scholar]
- 11.Kroes J.A., Zielhuis S.W., van Roon E.N., Ten Brinke A. Prediction of response to biological treatment with monoclonal antibodies in severe asthma. Biochem Pharmacol. 2020;179:113978. doi: 10.1016/j.bcp.2020.113978. [DOI] [PubMed] [Google Scholar]
- 12.Casale T.B., Chipps B.E., Rosén K. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy. 2018;73(2):490–497. doi: 10.1111/all.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortega H.G., Yancey S.W., Mayer B. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549–556. doi: 10.1016/s2213-2600(16)30031-5. [DOI] [PubMed] [Google Scholar]
- 14.Bikov A., Oğuzülgen I.K., Baiardini I. Beliefs and preferences regarding biological treatments for severe asthma. World Allergy Organ J. 2020;13(7):100441. doi: 10.1016/j.waojou.2020.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yancey S.W., Keene O.N., Albers F.C. Biomarkers for severe eosinophilic asthma. J Allergy Clin Immunol. 2017;140(6):1509–1518. doi: 10.1016/j.jaci.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Albers F.C., Licskai C., Chanez P. Baseline blood eosinophil count as a predictor of treatment response to the licensed dose of mepolizumab in severe eosinophilic asthma. Respir Med. 2019;159:105806. doi: 10.1016/j.rmed.2019.105806. [DOI] [PubMed] [Google Scholar]
- 17.Bleecker E.R., FitzGerald J.M., Chanez P. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β(2)-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi: 10.1016/s0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 18.Castro M., Zangrilli J., Wechsler M.E. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–366. doi: 10.1016/s2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 19.Busse W., Chupp G., Nagase H. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol. 2019;143(1):190–200. doi: 10.1016/j.jaci.2018.08.031. e120. [DOI] [PubMed] [Google Scholar]
- 20.Shrimanker R., Keene O., Hynes G., Wenzel S., Yancey S., Pavord I.D. Prognostic and predictive value of blood eosinophil count, fractional exhaled nitric oxide, and their combination in severe asthma: a post hoc analysis. Am J Respir Crit Care Med. 2019;200(10):1308–1312. doi: 10.1164/rccm.201903-0599LE. [DOI] [PubMed] [Google Scholar]
- 21.Bourdin A., Papi A.A., Corren J. Dupilumab is effective in type 2-high asthma patients receiving high-dose inhaled corticosteroids at baseline. Allergy. 2021;76(1):269–280. doi: 10.1111/all.14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz L.E., Gleich G.J., Hartley B.F., Yancey S.W., Ortega H.G. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc. 2014;11(4):531–536. doi: 10.1513/AnnalsATS.201310-354OC. [DOI] [PubMed] [Google Scholar]
- 23.Castro M., Mathur S., Hargreave F. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125–1132. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee M., Aleman Paramo F., Kjarsgaard M. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197(1):38–46. doi: 10.1164/rccm.201707-1323OC. [DOI] [PubMed] [Google Scholar]
- 25.Nair P., Pizzichini M.M., Kjarsgaard M. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360(10):985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 26.Upham J.W., Jurak L.M. How do biologicals and other novel therapies effect clinically used biomarkers in severe asthma? Clin Exp Allergy. 2020;50(9):994–1006. doi: 10.1111/cea.13694. [DOI] [PubMed] [Google Scholar]
- 27.Khan S.S., Ahmad A. Cluster center initialization algorithm for K-means clustering. Pattern Recogn Letters. 2004;25:1293–1302. [Google Scholar]
- 28.Wong T.-T. Performance evaluation of classification algorithms by k-fold and leave-one-out cross validation. Pattern Recogn. 2015;48(9):2839–2846. [Google Scholar]
- 29.Corren J., Weinstein S., Janka L., Zangrilli J., Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150(4):799–810. doi: 10.1016/j.chest.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Holguin F., Cardet J.C., Chung K.F. Management of severe asthma: a European respiratory society/American thoracic society guideline. Eur Respir J. 2020;55(1):1900588. doi: 10.1183/13993003.00588-2019. [DOI] [PubMed] [Google Scholar]
- 31.Humbert M., Busse W., Hanania N.A. Omalizumab in asthma: an update on recent developments. J Allergy Clin Immunol Pract. 2014;2(5):525–536. doi: 10.1016/j.jaip.2014.03.010. e521. [DOI] [PubMed] [Google Scholar]
- 32.Rakowski E., Zhao S., Liu M. Variability of blood eosinophils in patients in a clinic for severe asthma. Clin Exp Allergy. 2019;49(2):163–170. doi: 10.1111/cea.13310. [DOI] [PubMed] [Google Scholar]
- 33.Hatipoğlu U., Subramanian A., Campbell T. Intrasubject variability in total IgE levels in patients with moderate to severe persistent allergic asthma over 1 year. J Allergy Clin Immunol Pract. 2016;4(4):691–696. doi: 10.1016/j.jaip.2016.02.007. e691. [DOI] [PubMed] [Google Scholar]
- 34.Mathur S.K., Fichtinger P.S., Evans M.D., Schwantes E.A., Jarjour N.N. Variability of blood eosinophil count as an asthma biomarker. Ann Allergy Asthma Immunol. 2016;117(5):551–553. doi: 10.1016/j.anai.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silkoff P.E., Laviolette M., Singh D. Longitudinal stability of asthma characteristics and biomarkers from the airways disease endotyping for personalized therapeutics (ADEPT) study. Respir Res. 2016;17:43. doi: 10.1186/s12931-016-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown T., Jones T., Gove K. Randomised controlled trials in severe asthma: selection by phenotype or stereotype. Eur Respir J. 2018;52(6):1801444. doi: 10.1183/13993003.01444-2018. [DOI] [PubMed] [Google Scholar]
- 37.Richards L.B., van Bragt J., Aarab R. Treatment eligibility of real-life mepolizumab-treated severe asthma patients. J Allergy Clin Immunol Pract. 2020;8(9):2999–3008. doi: 10.1016/j.jaip.2020.04.029. e2991. [DOI] [PubMed] [Google Scholar]
- 38.Ortega H., Gleich G., Mayer B., Yancey S. Reproducibility of a single blood eosinophil measurement as a biomarker in severe eosinophilic asthma. Ann Am Thorac Soc. 2015;12(12):1896–1897. doi: 10.1513/AnnalsATS.201507-443LE. [DOI] [PubMed] [Google Scholar]
- 39.Rolla G., Heffler E., Pizzimenti S., Michils A., Malinovschi A. An emerging role for exhaled nitric oxide in guiding biological treatment in severe asthma. Curr Med Chem. 2020;27(42):7159–7167. doi: 10.2174/0929867327666200713184659. [DOI] [PubMed] [Google Scholar]
- 40.Solidoro P., Patrucco F., de Blasio F. Predictors of reversible airway obstruction with omalizumab in severe asthma: a real-life study. Ther Adv Respir Dis. 2019;13 doi: 10.1177/1753466619841274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sehmi R., Lim H.F., Mukherjee M. Benralizumab attenuates airway eosinophilia in prednisone-dependent asthma. J Allergy Clin Immunol. 2018;141(4):1529–1532. doi: 10.1016/j.jaci.2018.01.008. e1528. [DOI] [PubMed] [Google Scholar]
- 42.Flood-Page P., Swenson C., Faiferman I. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176(11):1062–1071. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 43.Manka L.A., Wechsler M.E. Selecting the right biologic for your patients with severe asthma. Ann Allergy Asthma Immunol. 2018;121(4):406–413. doi: 10.1016/j.anai.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 44.Agache I., Beltran J., Akdis C. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1023–1042. doi: 10.1111/all.14221. [DOI] [PubMed] [Google Scholar]
- 45.Henriksen D.P., Bodtger U., Sidenius K. Efficacy, adverse events, and inter-drug comparison of mepolizumab and reslizumab anti-IL-5 treatments of severe asthma - a systematic review and meta-analysis. Eur Clin Respir J. 2018;5(1):1536097. doi: 10.1080/20018525.2018.1536097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cabon Y., Molinari N., Marin G. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect meta-analyses of randomized placebo-controlled trials. Clin Exp Allergy. 2017;47(1):129–138. doi: 10.1111/cea.12853. [DOI] [PubMed] [Google Scholar]
- 47.Azim A., Newell C., Barber C. Clinical evaluation of type 2 disease status in a real-world population of difficult to manage asthma using historic Electronic Health Care Records of Blood Eosinophil counts. Clin Exp Allergy. 2021 doi: 10.1111/cea.13841. [DOI] [PubMed] [Google Scholar]
- 48.Agniel D., Kohane I.S., Weber G.M. Biases in electronic health record data due to processes within the healthcare system: retrospective observational study. BMJ. 2018;361:k1479. doi: 10.1136/bmj.k1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.