Alzheimer's disease (AD) is a progressive and irreversible neurodegenerative disorder that is characterized by memory loss and a decline in activities of daily life. More than 50 million people worldwide are affected by AD, and this number will continue to rise over 100 million within next three decades. Its major pathological features are the extracellular plaque deposits of the β-amyloid peptide and the intracellular flame-shaped neurofibrillary tangle-aggregation of hyperphosphorylated tau-proteins (pTau). A number of descriptive hypotheses including amyloid hypothesis and tau propagation hypothesis have been proposed to understand the causes of AD (Liu et al., 2019). According to the tau propagation hypothesis, the balance of kinase and phosphatase activity is changed in AD, generating various hyperphosphorylated species of tau. These pTau can bind with each other to produce oligomers, then contributing to the formation of paired helical filaments, the primary content of neurofibrillary tangle (Gendron and Petrucelli, 2009). Based on postmortem findings in AD patients, tau pathobiology was propagated to distinct brain regions in a stereotypical manner, starting from the entorhinal cortex then distributing to the hippocampus and neocortex. However, the mechanisms underlying this spread of pathological tau are still poorly understood.
Recently, increasing lines of evidence suggested that pathological tau protein can be secreted to the extracellular space and then internalized into neighbor cells (Kfoury et al., 2012). The recruitment of endogenous naïve tau protein in the latter will generate new pathological tau seeds. However, the mechanisms of cellular release, internalization, processing, and subsequent seeding of naïve tau protein remain largely unknown. In order to understand the molecular mechanisms, several potential pathways have been proposed, including free form of tau secretion pathway and the extracellular vesicle (EV) mediated pathway (Ruan and Ikezu, 2019). On one hand, tau fibrils were shown to be taken up through lipid raft-dependent micropinocytosis, an endocytic pathway mediating nonselective fluid-phase uptake. Similarly, the injection of fibrillar tau isolated from human AD brain tissue could cause the tau pathology in wild type mice within a few months (Guo et al., 2016). On the other hand, the smallest EVs, known as exosomes, have been reported to be ferrying pathogenic forms of tau between cells. Exosomes are small membrane vesicles (50–150 nm) of endocytic origin that are released into the extracellular space on fusion of multivesicular bodies with the plasma membrane. The term exosome was first introduced as cellular waste product in the early 1980s. Currently, exosomes have been reported to be secreted by almost every cell, and they can carry proteins, RNA, and DNA from one cell to another. Its biological function has been well-explored in cancer biology including modulation of immune response and tumor microenvironment reprogramming (Azmi et al., 2013). Interestingly, microtubule-unbound tau was prone to be associated with intracellular vesicles. The vesicular association of tau was especially prevalent in cellular compartments with disorganized microtubules and pTau. Furthermore, the presence of tau protein has been documented in the exosome derived from interstitial fluid and cerebrospinal fluid or blood of transgenic tau rodent models and human AD, even in prodromal cases (Ruan and Ikezu, 2019). In addition, recombinant tau protein was found to be secreted in exosome by multiple non-neuronal and neuronal cell lines in vitro (Ruan and Ikezu, 2019). These findings encourage us to determine which pathway plays the leading role in the intercellular transfer of pathological tau in the progression of AD.
EVs mediated tau propagation: In our most recent study, we have successfully isolated tau containing EVs from frozen human AD, prodromal AD and non-dementia control brain tissue (Ruan et al., 2020b). Those isolated EVs were highly enriched in the exosome fraction, ranging from 50 to 150 nm. After injected into the hippocampus of 18-month-old female wild-type mice, recipients of AD EVs showed more pTau in their hippocampus compared to recipients of prodromal or control EVs. Moreover, this pTau could be recognized by an antibody specific for paired helical filaments of tau and resisted against sarkosyl solubilization. It suggests AD EV inoculation induced accumulation of oligomeric and fibrillar tau in mouse brain. Notably, only 300 picograms of tau were contained in the injected AD EVs. This concentration was in the range of the extracellular tau concentration in mouse interstitial fluid of the central nervous system. In the previous studies, much larger amounts of vesicle-free tau fibrils, ranging from 1 to 8 μg, were needed to induce tau pathology in wild-type mice (Guo et al., 2016). To directly compare tau containing EVs with free tau, we injected 300 pg of vesicle-free tau (either oligomer or fibril-enriched fraction) isolated from the same human AD brain tissue into wild-type mice. No obvious pTau developed in these mice after 4.5 months. This is the first report of efficient tau propagation potency in EV-tau in comparison with vesicle-free tau, suggesting that EVs show higher transmissibility of tau than that of free tau. This AD EV-mediated tau propagation could serve as a novel non-genetically-altered tauopathy animal model. It may benefit in recreating the key molecular events occurring in the brain during human AD progression. Moreover, this data may reinforce the view that EV tau derived from central nervous system, or even blood of patient has the potential to be a biomarker for clinical diagnosis of AD.
Given that specific proteins enriched on the surface of AD EVs, it is important to determine that which subset of neuron those AD EVs may be transferred to. Based on fluorescence colocalization analysis, we unexpectedly found that AD EV-tau preferentially propagated in hippocampal GABAergic interneurons. This EV-mediated propagation of tau also caused dysfunction of GABAergic interneuron though the total number of GABAergic interneuron was not changed significantly. The reduced c-fos expression and whole-cell patch clamp recording also revealed a decrease in neuronal cell activity of interneuron. In line with this finding, accumulating evidence has supported the notion that the dysfunction of GABAergic interneurons could be one of the critical components in the early pathogenesis of AD. One fifth of patients with sporadic AD show at least one episode of seizure during the illness (Palop and Mucke, 2009), and administration of the anti-epileptic drug could improve cognitive function of AD patients to some extent. Taken together, these data demonstrates that EVs play a pivotal role in tau propagation to GABAergic interneurons, and opens avenues for further study since it is not clear which characteristics of GABAergic interneurons are responsible for this preferential targeting.
Although evidence suggests that small soluble tau oligomers may be more toxic than filamentous and fibrillar tau, the question that which form of tau is most toxic remains to be debated. In the present study, the specific pathological formation of tau protein carried by AD EVs may contribute to the robust tau propagation. Indeed, we found that EV containing tau from AD, but not prodromal or control, bound the tau-oligomer-specific antibody TOMA. Atomic force microscopy also showed oligomers in EVs from both AD and prodromal but not control brain, although the prodromal oligomers were sparse. Moreover, electron microscopy detected globular tau aggregates in both AD and prodromal EVs, but not in control EVs. Importantly, the aggregated tau from AD EVs resisted the membrane digestion induced by proteinase K, while tau from prodromal AD EVs did not. It suggested that both AD and prodromal EVs contain oligomeric tau, but more protofibrillar tau were inside AD EVs. Of note, most of these tau in AD EVs were sarkosyl-insoluble. Overall, these data indicated that pathological tau in AD brain could be incorporated in EVs and spread into the healthy neuronal cells in the form of EV. This may be the reason why EVs containing a physiological concentration of tau could enter cells better than free tau. In particular, EVs could represent a more physiological paradigm of tau spread in AD, by means of fusing with cell membranes and secreting their contents inside.
In order to identify molecular signatures of AD EVs, those EV samples have been applied with proteomics analysis (Muraoka et al., 2020). Interestingly, we found that lots of proteins derived from glial cells including microglia were significantly enriched in AD EVs compared to the non-AD control EVs. Indeed, it is now widely accepted that glial cells not only support many essential neuronal functions, but also actively communicate with neurons and each other. While numerous factors may be involved in this intercellular communication, EVs released from microglia provide an effective mean. This is also supported by the bulk of evidence that EV mediated communication between neural cells can propagate neuroinflammation and toxic protein aggregates, or even modulate regeneration (Holm et al., 2018). Furthermore, a previous study has shown that depletion of microglia or inhibition of EV biogenesis could suppress the tau propagation (Asai et al., 2015). More recently, we have also examined the hypothesis that suppression of EV secretion from glial cells may alleviate the tau pathology (Ruan et al., 2020a). After oral administration of P2RX7 inhibitor, pathological tau accumulation decreased along with the decrease in exosome release from microglia. Together, it suggests that EVs play systemic roles in the intercellular communication between neuron and glial cells especially in disease condition (Figure 1). In AD, EVs secreted from glial cells especially microglia, the primary immune cells of the brain, may be a key mediator for propagating the intercellular transfer of toxic tau aggregates.
Figure 1.
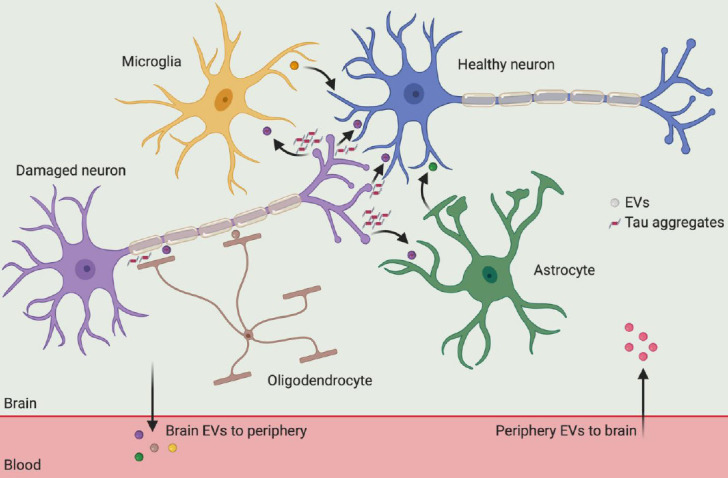
Cross-talk of extracellular vesicles among neural cells in the brain.
All major cell types (neuron, microglia, astrocyte, oligodendrocyte) in the brain send and receive extracellular vesicle (EV), which contain bioactive lipids, proteins, and nucleic acids. In Alzheimer's disease, EVs are involved in the propagation, spreading, and clearance of neurotoxic tau aggregates. In particular, tau aggregates can be secreted from damaged neuron and then taken up by glial cells, followed by incorporated with EVs to be internalized by healthy neuron again. Thus, those glial cell derived EVs may accelerate the intercellular transfer of tau aggregates in the progression of Alzheimer's disease. Additionally, EVs have also been shown to cross the blood-brain barrier under pathological conditions, enabling tau protein crosstalk between brain cells and the periphery.
Conclusion and future perspectives: With an increasing of ageing population, AD is becoming a major health crisis in the world. Although much progress in understanding the mechanisms of AD has been achieved during the past decades, it is still lack of efficient therapy against AD in clinical arena. A better understanding of the AD pathogenesis especially the spreading of tau pathology could accelerate the process of therapy development. In this respect, the EV mediated tau propagation provides novel and valuable information in understanding molecular mechanisms underlying the spread of pathogenic tau. Thus it will open avenues for mechanism study and identification of possible therapeutic targets.
In order to explore the novel EV-based targets for treating AD, further studies on mechanisms underlying the EV secretion and uptake are needed. First of all, it is urgent to determine what kind of pathway is required for the internalization of tau containing EVs in the recipient cells. EV-mediated intercellular communication requires docking at the plasma membrane, followed by the activation of surface receptors, vesicle internalization or their fusion with target cells. These processes seem to be complex and may depend on both the origin of EVs and the identity of the recipient cells. In particular, the AD EVs preferentially propagated in the interneuron, making it as a unique event at the early stage of AD. Currently, primary interneuron cell culture from perinatal cortical or hippocampal rodent neurons is still a challenge. Rodent derived neuron may be also lack of the key features supporting the development of AD-like tau pathology. However, human stem cells may have promise to be differentiated into GABAergic interneuron with the unique molecular aspects that drive tau pathology. Secondly, pharmacological inhibition of exosome release from microglia would be a rational and innovative strategy. Proliferation and activation of microglia in the brain is a prominent feature of AD. Secretion of pathogens is one of the most direct response to microglia activation. Recently, the secretion of pathological tau has been reported to be an active secretion process in microglia. Thus, targeting the receptor, such as P2RX7, which induces the exosome secretion from microglia may serve as a potential anti-tau strategy. Last but not the least, directly targeting the biogenesis of exosome is another potential option. The biogenesis of exosome starts from sorting of extracellular or plasma membrane biomolecules to early endosomes. Then intraluminal vesicles will be generated within late endosomes including multivesicular bodies and finally released as exosomes. The most characterized and well-known biogenesis pathway is the endosomal sorting complexes required for transport (ESCRT) machinery. ESCRT machinery dependent pathway consists of protein complexes: ESCRT-0 (e.g., Hgs), -I (e.g., Tsg101), -II (e.g., Vps25), -III (e.g., Vps20), and associated ATPase Vps4. Thus, targeting the key step of this pathway may offer new directions for AD therapy.
Footnotes
C-Editors: Zhao M, Zhao LJ, Qiu Y; T-Editor: Jia Y
Copyright license agreement:The Copyright License Agreement has been signed by the author before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
References
- 1.Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kugler S, Ikezu T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gendron TF, Petrucelli L. The role of tau in neurodegeneration. Mol Neurodegener. 2009;4:13. doi: 10.1186/1750-1326-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo JL, Narasimhan S, Changolkar L, He ZH, Stieber A, Zhang B, Gathagan RJ, Iba M, McBride JD, Trojanowski JQ, Lee VMY. Unique pathological tau conformers from Alzheimer's brains transmit tau pathology in nontransgenic mice. J Exp Med. 2016;213:2635–2654. doi: 10.1084/jem.20160833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holm MM, Kaiser J, Schwab ME. Extracellular vesicles: multimodal envoys in neural maintenance and repair. Trends Neurosci. 2018;41:360–372. doi: 10.1016/j.tins.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem. 2012;287:19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu PP, Xie Y, Meng XY, Kang JS. History and progress of hypotheses and clinical trials for Alzheimer's disease. Signal Transduct Target Ther. 2019;4:29. doi: 10.1038/s41392-019-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muraoka S, DeLeo AM, Sethi MK, Yukawa-Takamatsu K, Yang Z, Ko J, Hogan JD, Ruan Z, You Y, Wang Y, Medalla M, Ikezu S, Chen M, Xia W, Gorantla S, Gendelman HE, Issadore D, Zaia J, Ikezu T. Proteomic and biological profiling of extracellular vesicles from Alzheimer's disease human brain tissues. Alzheimers Dement. 2020;16:896–907. doi: 10.1002/alz.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan Z, Ikezu T. Tau secretion. Adv Exp Med Biol. 2019;1184:123–134. doi: 10.1007/978-981-32-9358-8_11. [DOI] [PubMed] [Google Scholar]
- 11.Ruan Z, Delpech JC, Venkatesan Kalavai S, Van Enoo AA, Hu J, Ikezu S, Ikezu T. P2RX7 inhibitor suppresses exosome secretion and disease phenotype in P301S tau transgenic mice. Mol Neurodegener. 2020a;15:47. doi: 10.1186/s13024-020-00396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruan Z, Pathak D, Venkatesan Kalavai S, Yoshii-Kitahara A, Muraoka S, Bhatt N, Takamatsu-Yukawa K, Hu J, Wang Y, Hersh S, Ericsson M, Gorantla S, Gendelman HE, Kayed R, Ikezu S, Luebke JI, Ikezu T. Alzheimer's disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain. 2020b;144:288–309. doi: 10.1093/brain/awaa376. [DOI] [PMC free article] [PubMed] [Google Scholar]


