Abstract
Growing evidence suggests that there are similar pathological mechanisms and closely related pathogenic risk factors for inflammatory bowel disease (IBD) and Parkinson's disease (PD). However, the epidemiological features of these two diseases are different. This review systematically evaluated the relationship between inflammatory bowel diseases and Parkinson's disease risk. We searched PubMed, Embase, and Cochrane databases to retrieve observational studies of IBD and PD published from inception to October 2019. Nine observational studies, involving 12,177,520 patients, were included in the final analysis. None of the studies had Newcastle–Ottawa Scale scores that suggested a high risk of bias. After adjusting for confounders and excluding heterogeneous studies, the overall risk of PD was significantly higher in IBD patients than in the general population (adjusted risk ratio [RR] = 1.24, 95% confidence interval [CI]: 1.15–1.34, P < 0.001). A meta-analysis of the temporal relationship revealed that the incidence of IBD was significantly increased before (adjusted hazard ratio [HR] = 1.26, 95% CI: 1.18–1.35, P < 0.001) and after (adjusted RR = 1.40, 95% CI: 1.20–1.80, P < 0.001) PD diagnosis. After excluding a heterogeneous study, the pooled risk of PD development in patients with ulcerative colitis (adjusted HR = 1.25, 95% CI: 1.13–1.38, P < 0.001) or Crohn's disease (adjusted HR = 1.33, 95% CI: 1.21–1.45, P < 0.01) was significantly increased. Subgroup analysis revealed no significant differences in risk between men (adjusted HR = 1.23, 95% CI: 1.10–1.39) and women (adjusted HR = 1.26, 95% CI: 1.10–1.43); however, older (> 65 years old) IBD patients (adjusted HR = 1.32, 95% CI: 1.17–1.48) may have a higher risk than younger (≤ 65 years old) patients (adjusted HR = 1.24, 95% CI: 1.08–1.42). Patients with IBD who were not treated with anti-tumor necrosis factor-α or azathioprine had significantly higher PD risk (adjusted HR = 1.6, 95% CI: 1.2–2.2). Thus, our meta-analysis indicates a certain correlation between IBD and PD, and suggests that IBD may moderately increase PD risk regardless of sex, especially in patients over 65 years of age. Moreover, early anti-inflammatory therapies for IBD might reduce the risk of developing PD. Our findings suggest an urgent need for an individualized screening strategy for patients with IBD. However, most studies included in this paper were observational, and more randomized controlled trials are needed to confirm the precise association between IBD and PD.
Key Words: brain-gut axis, central nervous system, Crohn's disease, inflammatory bowel diseases, meta-analysis, neurodegenerative disease, neuroinflammation, Parkinson's disease, systematic review, ulcerative colitis
Chinese Library Classification No. R452; R574; R741.04
Introduction
Parkinson's disease (PD) is the most common neurodegenerative motor disorder (Park et al., 2020; Thomas Broome et al., 2020), with symptoms that include resting tremor, dyskinesia, ankylosis, and postural instability. Non-motor symptoms (e.g., dementia, depression, and sleep disorders) also occur, and can arise even before a motor diagnosis is made. The pathology of PD is associated with a profound loss of dopaminergic neurons in the substantia nigra pars compacta, which is accompanied by filamentous protein inclusions, termed Lewy bodies, in the surviving neurons. Lewy bodies are mainly composed of alpha-synuclein (α-syn), and these inclusions are the hallmark pathological feature of PD. However, their pathogenic relevance remains under debate (Kalinderi et al., 2016; Obergasteiger et al., 2018). Much evidence suggests that PD pathology starts from chronic low-level inflammation in the gastrointestinal tract, thus inducing misfolded α-syn, which then propagates to the brain via a dysfunctional blood-brain barrier (BBB) or the vagus nerve. This has been widely investigated in studies regarding the association between PD and alterations of the intestinal environment (Houser and Tansey, 2017; Lionnet et al., 2018; Spielman et al., 2018).
In recent years, the relationship between the enteric nervous system (ENS) and the central nervous system (CNS), also known as the gut-brain axis, has become the subject of active investigation (Houser and Tansey, 2017). Inflammatory bowel disease (IBD) mainly includes ulcerative colitis (UC) and Crohn's disease (CD) (Huang et al., 2020). IBD is a chronic disease with the pathological characteristics of intestinal inflammation; moreover, a large amount of biological evidence suggests that it can cause neuroinflammation. A common shared risk of genetic variants between IBD and PD was identified in a recent genome-wide pleiotropic analysis (Witoelar et al., 2017), revealing a potential genetic basis of the clinical co-occurrence of these two diseases. Additionally, Hui et al. (2018) identified commonly shared variants of LRRK2 (the gene encoding leucine-rich repeat serine/threonine-protein kinase 2) between CD and PD. Furthermore, an earlier epidemiological study examined the possible co-occurrence of IBD and PD based on clinical populations, and reported that the prevalence of PD in populations with IBD is significantly higher than that in general populations (Johnson et al., 2014). In addition, a Taiwanese retrospective cohort study reported an elevated association between IBD and the risk of PD prevalence (Lin et al., 2016). These findings suggest the need for a retrospective study that explores the association between IBD and PD.
Several high-quality clinical studies with large samples have also investigated the association between IBD and PD, with both positive (Lin et al., 2016) and negative (Escamilla-Sevilla et al., 2016; Fujioka et al., 2017; Camacho-Soto et al., 2018) results, which has reignited strong debates. The contradictory results of these studies might be caused by the different study designs and methods used to investigate the association between IBD and PD (Escamilla-Sevilla et al., 2016; Fujioka et al., 2017; Camacho-Soto et al., 2018). Zhu et al. (2019) conducted a recent meta-analysis that revealed that IBD increases the risk of PD, but their analysis included the results of only four studies. Although the inclusion criteria were cohort studies and case-control studies, the types of studies that were included were not described separately in the results, and they did not include important risk factors of PD, such as family history or drug exposure; thus, doubts remain about the conclusion of this analysis. Therefore, an updated systematic review and meta-analysis is needed to synthesize these studies and further elucidate the association between IBD and PD.
Data and Methods
Search strategy
This systematic review and meta-analysis was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al., 2009) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) (Stroup et al., 2000) guidelines. Two researchers (YZ and MY) systematically retrieved articles published in the PubMed, EMbase, and Cochrane database websites from their inception dates to October 2019. There were no language restrictions, but studies were restricted to observational studies of epidemiology. In addition, we manually searched in the references of articles to further identify studies suited to the meta-analysis. Articles were identified using the following search terms: “Parkinson's disease” or “Parkinsonism “and “Inflammatory bowel diseases” or “Crohn disease*” or “Ulcerative colitis.” The search strategy was as follows: ((Parkinson's disease [Title/Abstract]) OR (Parkinsonism [Title/Abstract])) AND ((Inflammatory bowel diseases [Title/Abstract]) OR (Crohn disease [Title/Abstract]) OR (Ulcerative colitis [Title/Abstract])). Medical subject heading (MeSH) terms were used in PubMed and Emtree terms were used in EMbase.
Selection criteria
The following studies were included: (1) Studies reporting an association between PD and IBD (UC or CD). (2) Studies including a longitudinal cohort, case-control, or cross-sectional design and published in a peer-reviewed journal. (3) Studies reporting unadjusted or adjusted effect estimates, such as the hazard ratio (HR), risk ratio (RR), odds ratio (OR), incidence rate ratio (IRR), or standardized incidence ratio (SIR) with corresponding 95% confidence interval (CI), or results that allowed the calculation of RRs or ORs. (4) Studies in which the definition or clinical diagnosis of PD and IBD conformed to the standard diagnostic criteria or the International Classification of Diseases (ICD)-10. (5) Cohort studies with study periods including follow-up for over 10 years.
Studies that included the following conditions were excluded: (1) Case reports, letter, reviews, and editorials. (2) A retrospective design without a control group, or genetic studies that reported risk alleles rather than studying the risk of incidence between PD and IBD. (3) Studies assessed by the Newcastle-Ottawa Scale (NOS) to have scores of less than 7.
Two investigators (YZ and MY) independently selected the studies, extracted the relevant information from the included studies, and assessed the risk of bias. Any discrepancies were resolved by consensus.
Data extraction
Two investigators (YZ and MY) independently extracted the relevant data from all eligible studies using the following data extraction strategies: study characteristics, including the author(s), publication year, study design, and study period. The study population characteristics were also extracted, such as the sample size, age of patients, ascertainment of exposure and outcome, risk estimates with 95% CI, case-finding methods, and adjustment factors. If required, we also contacted the corresponding authors to obtain additional information. Any discrepancies were resolved by consensus and arbitration by a panel of investigators within the review team (YZ, MY, FY, ZBX, and RSX).
Assessment of methodological quality
The NOS criteria were used to assess the risk of bias (Stang, 2010). This scale assesses the risk of bias in the following domains: the selection of study groups, the comparability of groups, and the ascertainment of exposure and outcome. Studies were considered to have a high, moderate, or low risk of bias with scores of less than 7, 7 or 8, or 9, respectively.
Outcome measures
The primary outcome measure was the overall risk of association between IBD and PD. Secondary outcome measures included the temporal relationship between IBD and PD, and different kinds of IBD [i.e., UC or CD, sex, age, and anti-tumor necrosis factor-α (TNF-α) or thiopurine exposure].
Statistical analysis
Using the DerSimonian and Laird random-effects model (DerSimonian and Laird, 1986), we used extracted study-specific risk estimates to calculate the pooled RR with 95% CI. Risk estimates in terms of ORs, RRs, HRs, IRRs, and SIRs were considered equivalent. Heterogeneity was identified using Cochran's Q test (significance level at P < 0.1) and quantified using I2 values. Percentages of I2 of < 25%, 25–50%, 51–75%, and > 75% were taken as no, mild, moderate, and large heterogeneity, respectively (Higgins et al., 2003). Egger's linear regression test was used to evaluate publication bias for funnel plot asymmetry (Egger et al., 1997). To evaluate the stability of results, leave-one-out analyses were performed for sensitivity analyses.
We also performed subgroup analyses for the temporal relationship of onset between PD and IBD diagnoses (study design), age, and sex. To examine the sequence of incidence between PD and IBD, we performed a stratified analysis for PD, with exposure and outcomes taken separately.
For measures of risk effect that varied among study designs, we pooled RRs among cohort, case-control, and cross-sectional studies. For time-to-event cohort studies, we pooled risk effects, including HRs, RRs, ORs, IRRs, and SIRs, and reported them as HRs. Subsequent subgroup analyses only used studies that presented a low risk of bias or reported time-to-event data (HRs/IRRs). In each case, we examined the unadjusted and adjusted measures of effect separately. Statistical analysis was performed using STATA software (Version 14.0, StataCorp, College Station, TX, USA). Significance was set at P < 0.05.
This review was registered in the International Prospective Register of Systematic Reviews (PROSPERO; available at https://www.crd.york.ac.uk/prospero/, ID: CRD42012002291).
Results
Study characteristics and risk of bias
Our literature search yielded 1033 unique records, of which 996 were excluded after the initial screening of titles and abstracts. Thirty-seven full-text articles were assessed for their eligibility. Nine eligible studies, comprising 12,177,520 patients from five countries or regions, were included in the meta-analysis. Reasons for exclusion are summarized in Figure 1.
Figure 1.
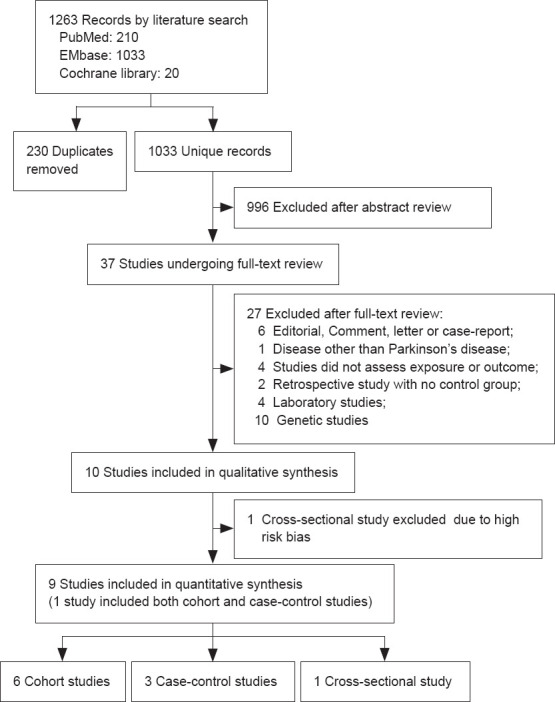
Flow chart of the search process and study selection.
Among the nine included studies (Rugbjerg et al., 2009; Li et al., 2012; Hsu et al., 2015; Lin et al., 2016; Bahler et al., 2017; Camacho-Soto et al., 2018; Peter et al., 2018; Villumsen et al., 2018; Weimers et al., 2019), one study was designed as both a cohort and case-control study based on the same population (Weimers et al., 2019). Thus, six cohort studies (Li et al., 2012; Hsu et al., 2015; Lin et al., 2016; Peter et al., 2018; Villumsen et al., 2018; Weimers et al., 2019), three case-control studies (Rugbjerg et al., 2009; Camachosoto et al., 2018; Weimers et al., 2019), and one cross-sectional study (Bahler et al., 2017) were included in this meta-analysis. Four of the six cohort studies (Lin et al., 2016; Peter et al., 2018; Villumsen et al., 2018; Weimers et al., 2019) and two of the case-control studies (Rugbjerg et al., 2009; Camacho-Soto et al., 2018) were assessed for the risk of PD development in patients with IBD, in which IBD was defined as exposed. Two cohort studies (Hsu et al., 2015; Weimers et al., 2019) evaluated IBD in patients with PD, in which IBD was considered as an outcome. Only a Swedish study (Weimers et al., 2019) and a Swiss cross-sectional study (Bahler et al., 2017) investigated the co-occurrence of PD and IBD. The Swedish study estimated the association between IBD and antecedent/subsequent PD in a case-control study and a cohort study, separately. We pooled the unadjusted and adjusted estimate effects separately when analyzing the overall relationships between IBD and PD. Few studies reported only unadjusted or adjusted measures of effect, and four cohort studies (Lin et al., 2016; Peter et al., 2018; Villumsen et al., 2018; Weimers et al., 2019) reported HRs/IRRs as time-to-event effects. Only five of the nine studies (Lin et al., 2016; Camacho-Soto et al., 2018; Peter et al., 2018; Villumsen et al., 2018; Weimers et al., 2019) were explicitly designed to assess the relationship between IBD and PD, whereas the other studies assessed autoimmune diseases as part of their analyses of PD or comorbidities of interest with IBD. All eligible studies assessed PD or IBD as an outcome event, which was based on ICD classification codes in eight studies (Rugbjerg et al., 2009; Li et al., 2012; Hsu et al., 2015; Lin et al., 2016; Camacho-Soto et al., 2018; Peter et al., 2018; Villumsen et al., 2018; Weimers et al., 2019) and on standard diagnostic criteria in one cross-sectional study (Bahler et al., 2017).
Population characteristics and Newcastle-Ottawa Quality Assessment Scale scores of the included studies are summarized in Tables 1 and 2. Quality scores ranged from 6 to 9. A Hispanic cross-sectional study based on the electronic prescribing system database was considered to have a high risk of bias; it identified patients who received antiparkinsonian drugs as “possible PD”, while those who received aminosalicylates as were identified as “possible IBD” (Escamilla-Sevilla et al., 2016). Four cohort studies (Lin et al., 2016; Peter et al., 2018; Villumsen et al., 2018; Weimers et al., 2019) that reported time-to-event data were assessed to have a low risk of bias. All of the case–control (Rugbjerg et al., 2009; Camacho-Soto et al., 2018) and cross-sectional (Bahler et al., 2017) studies were considered to have moderate risk of bias, as were two of the cohort studies. The Hispanic cross-sectional study (Escamilla-Sevilla et al., 2016) was excluded from this meta-analysis because of its high risk of bias.
Table 1.
Characteristics of nine studies included in qualitative synthesis
| Study | Study population | Study period | Study design | Age at Inclusion (yr) | Effect Estimates | Crude risk | Adjusted risk | Adjustment variables | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Rugbjerg et al., 2009 | The Danish National Hospital Register, Denmark | 1986–2006 | Case-control | ≥ 35 | OR | IBD: 1.23 (0.91–1.67); CD: 1.06 (0.54–2.10); UC: 1.28 (0.91–1.80) |
NA | NA | 7 |
| Li et al., 2012 | The Primary Health Care Research Center-MigMed Database, Lund University, Sweden | 1964–2007 | Retrospective cohort study | > 0 | SIR | NA | UC: 1.23 (0.90–1.64); CD:0.62 (0.33–1.07) |
Age, period, socioeconomic status, region of residence, hospitalization of COPD, and alcoholism and alcohol-related liver disease | 8 |
| Hsu et al., 2015 | The National Health Research Institutes (NHRI), China (Taiwan) | 2000–2008 | Retrospective cohort study | > 0 | RR | IBD: 1.113 (0.78–1.60) | NA | NA | 7 |
| Lin et al., 2016 | The National Health Insurance--Longitudinal Health Insurance Database 2000 (LHID 2000), China (Taiwan) | 2000–2011 | Retrospective cohort study | ≥ 20 | HR | IBD: 1.43 (1.15–1.79); CD: 1.45 (1.15–1.83); UC: 1.25 (0.64–2.42); |
IBD: 1.35 (1.08–1.68); CD: 1.40 (1.11–1.77); UC:0.94 (0.49–1.84) |
Age, sex, and comorbidities of diabetes, hypertension, hyperlipidemia, stroke, CAD, head injury, depression, and CKD | 9 |
| Bählera et al., 2017 | The Helsana Insurance Group, Switzerland | 2014 | Cross-sectional study | > 1 | OR | NA | IBD:0.92 (0.67–1.27) | Age, sex, language area, type of insurance coverage, and urbanization | 7 |
| Camachosoto et al., 2018 | Medicare base file (BASF), USA | 2004–2009 | Case-control study | > 65 | OR | NA | IBD:0.85 (0.80–0.91); CD:0.83 (0.74–0.93); UC:0.88 (0.82–0.96) |
Age, race, sex, and probability of smoking, comorbidities | 7 |
| Peter et al., 2018 | The Truven Health MarketScan Commercial Database and the Medicare Supplemental Database, USA | 2000–2016 | Retrospective cohort study | ≥ 18 | IRR | IBD: 1.28 (1.14–1.44); CD: 1.26 (1.03–1.54); UC: 1.30 (1.13–1.5) |
IBD: 1.28 (1.14–1.44); CD: 1.26 (1.03–1.53); UC: 1.31 (1.14–1.51) |
Time-varying age group and sex, and offset by time | 9 |
| Villumsen et al., 2018 | The National Patient Registry (NPR), Denmark | 1977–2014 | Retrospective cohort study | ≥ 15 | HR | IBD: 1.24 (1.12–1.38) | IBD: 1.22 (1.09–1.35); CD: 1.35 (1.20–1.52); UC: 1.12 (0.89–1.40) |
Gender and age; comorbidity index | 9 |
| Weimers et al., 2019 | The National Patient Registry (NPR), Sweden | 2002–2014 | Retrospective cohort study | ≥ 18 | HR | NA | IBD: 1.3 (1.0–1.6) UC: 1.3 (1.0–1.7) CD: 1.1 (0.7–1.7) IBD-U: 1.7 (0.8–3.0) |
Sex, age, index date, and place of residency | 9 |
| The National Patient Registry (NPR), Sweden | 1964–2014 | Case-control study | ≥ 18 | OR | NA | IBD: 1.4 (1.2–1.8) UC: 1.4 (1.1–1.9) CD: 1.6 (1.1–2.3) IBD-U: 1.1 (0.5–2.3) |
Sex, age, index date, and place of residency | 9 | |
|
| |||||||||
| Study | Number of exposed cases (n) | Number of exposed cases with events (n) | Number of controls (n) | Number of controls with events (n) | Source of the controls | Descriptionof risk factor | Case finding methods | ||
|
| |||||||||
| Rugbjerg et al., 2009 | PD: 13695 | PD with IBD/CD/UC: 52/10/42 | Non-PD: 68445 | Non-PD with IBD/CD/UC: 211/47/164 | General population | NA | ICD | ||
| Li et al., 2012 | UC: 27881; CD: 22750; |
UC with PD: 46; CD with PD: 13 | NA | NA | Hospital settings | NA | ICD | ||
| Hsu et al., 2015 | PD: 1698; | PD with IBD: 37 | Non-PD: 6792 | Non-PD with IBD: 133 | General population | NA | ICD | ||
| Lin et al., 2016 | IBD: 8373; CD: 56507; UC: 84436; | IBD/CD/UC with PD: 106/97/9 | 33492 | with PD: 290 | General population | ≥ 60 years old; gender | ICD | ||
| Bählera et al., 2017 | IBD: 4791 | IBD: 72 | 575943 | with PD: 5184 | General population | NA | Medical record | ||
| Camachosoto et al., 2018 | PD: 89790 | PD with IBD/CD/UC: 2599/749/1583 | Non-PD: 118095 | Non-PD with IBD/CD/UC: 2381/708/1405 | General population | NA | ICD | ||
| Peter et al., 2018 | IBD: 144018; CD: 56507; UC: 84436 | IBD/CD/UC with PD: 371/122/243 | 720090 | Controls (IBD/CD/UC) with PD: 1425/480/913 | General population | Free medication; ≥ 60 years old | ICD | ||
| Villumsen et al., 2018 | IBD: 76477 | IBD with PD: 335 | 7548259 | with PD: 39784 | General population | ≥ 65 years old; gender | ICD | ||
| Weimers et al., 2019 | IBD: 39652; UC: 24422; CD: 11418; IBD-U: 3812; | IBD/CD/UC/IBD-U with PD: 103/23/69/11 (after IBD) | 396520 | with PD: 1556 | General population | Free Medication; ≥ 60 years old; gender | ICD | ||
| IBD: 39652; UC: 24422; CD: 11418; IBD-U: 3812 | IBD/CD/UC/IBD-U with PD: 102/30/64/8 (before IBD) | 396520 | with PD: 1556 | General population | ≥ 60 years old; gender | ICD | |||
CAD: Coronary Artery Disease; CD: Crohn’s disease; CKD: Chronic Kidney Disease; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; ICD: International Classification of Diseases; IRR: incidence rate ratio; OR: odds ratio; PD: Parkinson’s disease; RR: risk ratio; SIR: standardized incidence ratio; UC: ulcerative colitis.
Table 2.
Newcastle-Ottawa Quality Assessment Scale
| Study | Selection | Comparability | Outcome | Exposure | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 1 | 2 | 3 | |||
| Rugbjerg et al., 2009 | ★ | ☆ | ★ | ★ | ★☆ | ★ | ★ | ★ | 7 | |||
| Li et al., 2012 | ★ | ☆ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |||
| Hsu et al., 2015 | ★ | ★ | ★ | ★ | ★☆ | ★ | ☆ | ★ | 7 | |||
| Lin et al., 2016 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | |||
| Bählera et al., 2017 | ★ | ☆ | ☆ | ★ | ★★ | ★ | ★ | ★ | 7 | |||
| Villumsen et al., 2018 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | |||
| Peter et al., 2018 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | |||
| Camachosoto et al., 2018 | ★ | ☆ | ☆ | ★ | ★★ | ★ | ★ | ★ | 7 | |||
| Weimers et al., 2019 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | |||
Overall association between IBD and PD
Synthetic analyses of unadjusted estimates
The overall risk of PD in relation to IBD among eight studies (Rugbjerg et al., 2009; Li et al., 2012; Hsu et al., 2015; Lin et al., 2016; Bahler et al., 2017; Camacho-Soto et al., 2018; Peter et al., 2018; Villumsen et al., 2018; Weimers et al., 2019) reporting exposure and outcome events, calculated as an unadjusted RR, was significantly higher than that in general populations (pooled unadjusted RR = 1.35, 95% CI: 1.26–1.45, P < 0.001; I2= 45.9%, PQ = 0.07; Figure 2). Mild heterogeneity was observed when studies were pooled together; however, there was no presence of asymmetry in the funnel plot, and no small-study effects or publication bias were observed when assessed by Egger's test (P = 0.40). Sensitivity analysis revealed that the leave-one-out analysis results ranged from 1.31 (95% CI: 1.22–1.40) to 1.38 (95% CI: 1.29–1.48). Subgroup analysis demonstrated that the pooled unadjusted RR (95% CI) for the “IBD preceding PD diagnosis” group was 1.33 (1.23–1.43), revealing mild heterogeneity (I2= 45.5%, PQ = 0.10). In addition, the pooled unadjusted RR (95% CI) for the “PD diagnosis preceding IBD” group was 1.32 (1.04–1.66), revealing mild heterogeneity across studies (I2= 30.0%, PQ = 0.232), while that of the “Co-occurrence of PD and IBD” group was 1.46 (1.16–1.83), revealing moderate heterogeneity (Figure 3).
Figure 2.
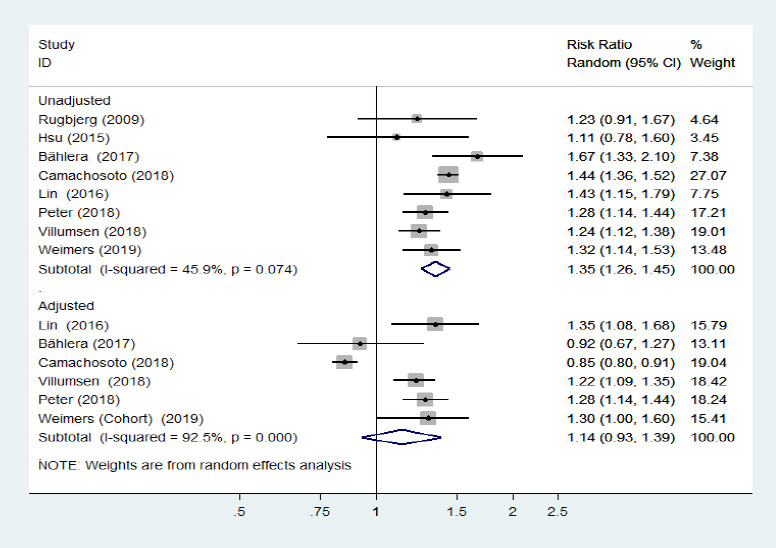
Forest plot of the overall risk of inflammatory bowel diseases in relation to Parkinson's disease.
The overall risk of Parkinson's disease was significantly higher in patients with inflammatory bowel diseases than in the general population. Confounding factors (age, sex, comorbidities, race, type of insurance, residence, smoking, and index date) were corrected for in the synthetic analysis of adjusted estimates. CI: Confidence interval.
Figure 3.
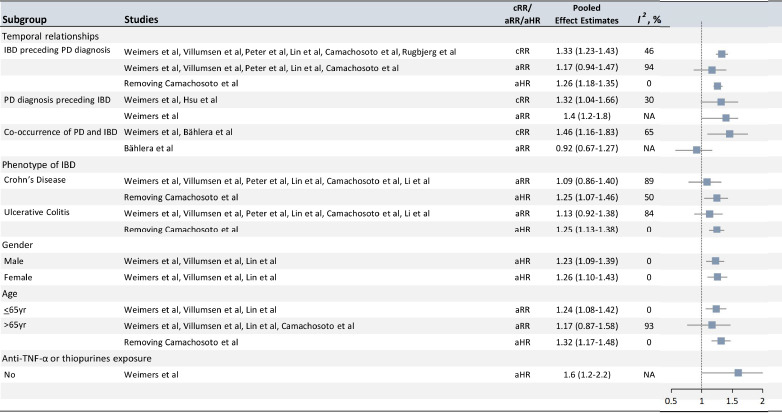
Analyses of subgroups relating IBD to PD.
Subgroup analyses by temporal relationship revealed that IBD occurrence was significantly higher before or after PD diagnosis. Crohn's disease and ulcerative colitis were both linked to elevated PD risk. There was no significant difference in risk in the sex-specific analysis, but older adult patients with IBD had a possibly increased PD risk compared with younger adult patients. IBD patients who were never exposed to anti-tumor necrosis factor-α (TNF-α) or thiopurines also had a possibly higher risk of developing PD compared with those who were exposed to these treatments. aHR: Adjusted hazard ratio; aRR: adjusted risk ratio; cRR: crude risk ratio; IBD: inflammatory bowel diseases; PD: Parkinson's disease.
Synthetic analysis of adjusted estimates
Four cohort studies (Lin et al., 2016; Peter et al., 2018; Villumsen et al., 2018; Weimers et al., 2019), one case-control study (Camacho-Soto et al., 2018), and one cross-sectional study (Bahler et al., 2017) reported adjusted risk estimates for the association between IBD and PD, which suggested no association (pooled adjusted RR = 1.14, 95% CI: 0.93–1.39, P = 0.22, I2= 92.5%; Figure 2) and revealed no evidence of publication bias (Egger's test: P = 0.20). However, when the study by Camachosoto et al. (2018) was removed from this analysis, the pooled adjusted RR increased to 1.24 (95% CI: 1.15–1.34, P < 0.001, I2= 9.9%) and the heterogeneity disappeared. A subgroup analysis of the “IBD preceding PD diagnosis” group revealed that the pooled adjusted RR was 1.17 (95% CI: 0.94–1.47, P = 0.16, I2= 94%). We therefore only included the four cohort studies that had a low risk of bias, and excluded the study by Camachosoto et al. (2018) from this subgroup analysis. In this analysis, the pooled adjusted HR was markedly stronger and the heterogeneity was completely eliminated (pooled adjusted HR = 1.26, 95% CI: 1.18–1.35, I2< 0.01%). For the subgroup analysis of “PD preceding IBD diagnosis”, only the Swedish case-control study revealed a risk of subsequent IBD incidence in patients with PD, of 1.4 (1.2–1.8) when reported as the adjusted OR (95% CI).
Analysis of PD development in CD/UC
Five cohort studies (Li et al., 2012; Lin et al., 2016; Peter et al., 2018; Villumsen et al., 2018; Weimers et al., 2019) and one case-control study (Camacho-Soto et al., 2018) examined the risk of subsequent PD development among patients with two specific phenotypes of IBD (CD/UC). The risk of PD development among CD and UC populations was not significantly different from that among general populations. The pooled adjusted RR was 1.09 (95% CI: 0.86–1.40, P = 0.48, I2= 88.9%; Figure 4) for CD, and the pooled adjusted RR was 1.13 (95% CI: 0.92–1.38, P = 0.25, I2= 83.8%; Figure 5) for UC. Large heterogeneity across the studies was observed. Removing the study by Camachosoto et al. (2018) revealed an increased risk of PD development among both CD (adjusted HR = 1.33, 95% CI: 1.21–1.45, I2= 50.4%; Figure 6) and UC (adjusted HR = 1.25, 95% CI: 1.13–1.38, I2< 0.01%; Figure 7), and markedly lower heterogeneity.
Figure 4.
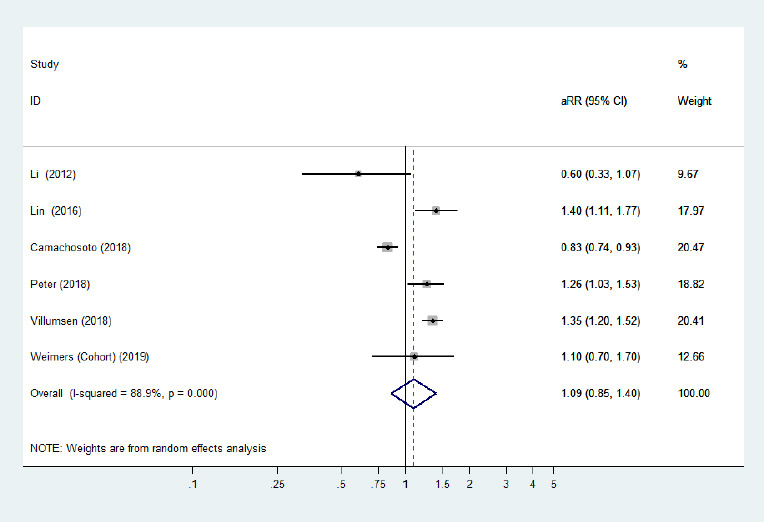
Forest plot of the associations between Crohn's disease and Parkinson's disease.
There was no significantly increased risk of Parkinson's disease in patients with Crohn's disease when all adjusted effect estimates were pooled. Confounding factors (age, sex, comorbidities, residence, alcoholism, smoking, and index date) were adjusted for. aRR: Adjusted risk ratio; CI: confidence interval.
Figure 5.
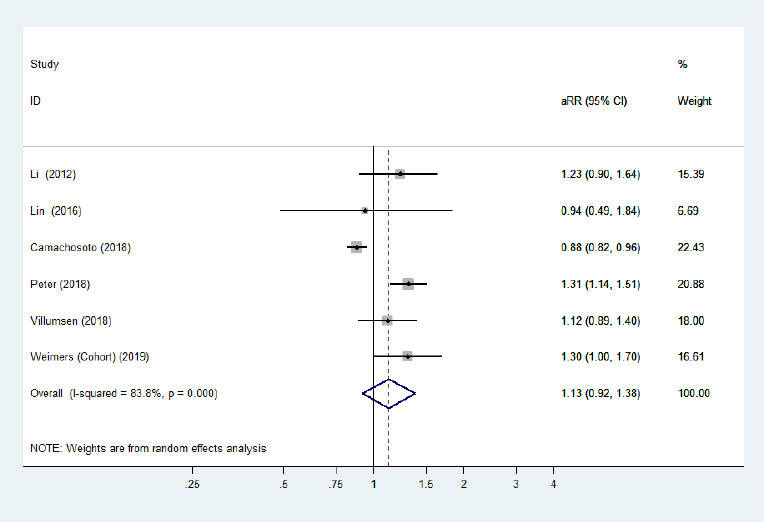
Forest plot of the associations between ulcerative colitis and Parkinson's disease.
There was no significantly increased risk of Parkinson's disease in patients with ulcerative colitis when all adjusted effect estimates were pooled. Confounding factors (age, sex, comorbidities, residence, alcoholism, smoking, and index date) were adjusted for. aRR: Adjusted risk ratio; CI: confidence interval.
Figure 6.
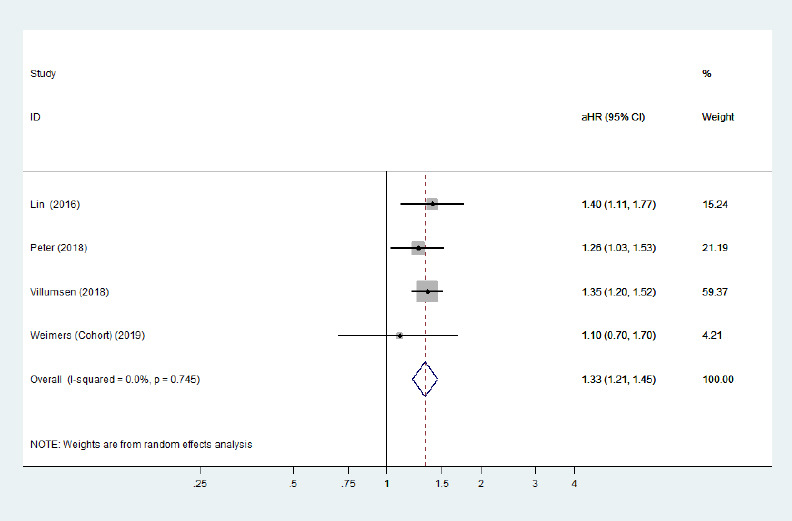
Forest plot of the associations between Crohn's disease and Parkinson's disease after removing the study with high heterogeneity.
There was a significantly increased risk of Parkinson's disease development among Crohn's disease patients after removing a heterogeneous study. Confounding factors (age, sex, comorbidities, residence, and index date) were adjusted for. aHR: Adjusted hazard ratio; CI: confidence interval.
Figure 7.
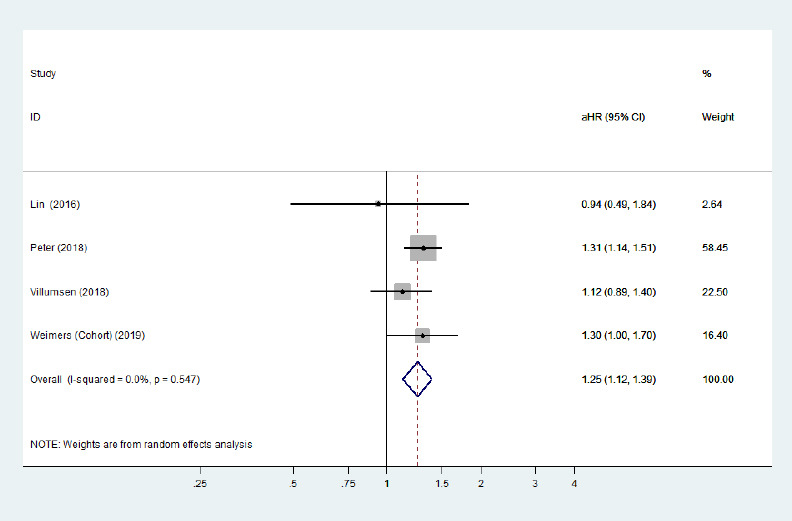
Forest plot of the associations between ulcerative colitis and Parkinson's disease after removing the study with high heterogeneity.
There was a significantly increased risk of Parkinson's disease development among ulcerative colitis patients after removing a heterogeneous study. Confounding factors (age, sex, comorbidities, residence, and index date) were adjusted for. aHR: Adjusted hazard ratio; CI: confidence interval.
Sex-specific analysis
In the subgroup analysis for sex, three cohort studies (Lin et al., 2016; Peter et al., 2018; Villumsen et al., 2018) with a low risk of bias investigated the subsequent risk of PD development among male and female IBD patients separately. The pooled adjusted HR of subsequent PD was 1.23 (95% CI: 1.10–1.39, P < 0.001, I2< 0.001%; Figure 8) in male IBD patients and 1.26 (95% CI: 1.10–1.43, P = 0.001, I2< 0.001%; Figure 9) in female IBD patients. Although Lin et al. (2016) reported that male patients with IBD had a higher risk of PD than female patients, our analysis revealed that the increased risk was present in both male and female patients; no sex differences were observed.
Figure 8.
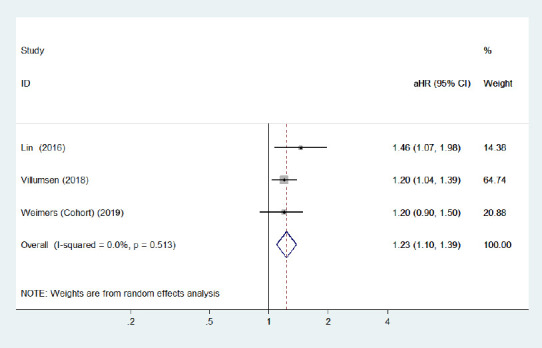
Forest plot of the associations between male patients with inflammatory bowel diseases and Parkinson's disease development.
There was a certain link between male inflammatory bowel diseases patients and higher Parkinson's disease risk. CI: Confidence interval; HR: hazard ratio.
Figure 9.
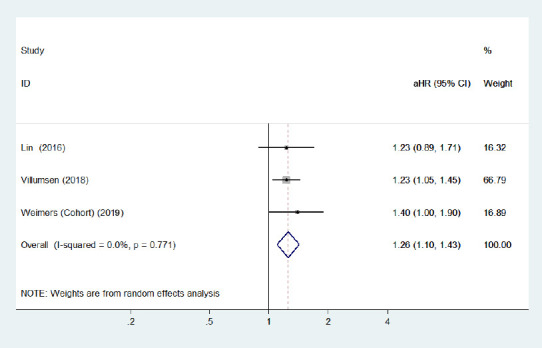
Forest plot of the associations between female patients with inflammatory bowel disease and Parkinson's disease development.
There was a certain link between female inflammatory bowel diseases patients and higher Parkinson's disease risk. CI: Confidence interval; HR: hazard ratio.
Age-specific analysis
Compared with younger IBD patients (age ≤ 65 years), three cohort studies (Lin et al., 2016; Peter et al., 2018; Villumsen et al., 2018) with a low risk of bias found that older (> 65 years) men with IBD were at a greater risk of PD incidence. Conversely, one case-control study (Camacho-Soto et al., 2018) reported that the risk of PD development was lower among IBD patients with older age. The pooled adjusted RR for older IBD patients was 1.17 (95% CI: 0.87–1.58, P = 0.29; Figure 10), and the pooled adjusted HR for younger patients was 1.24 (95% CI: 1.08–1.42, P = 0.002; Figure 11) in three cohort studies. No heterogeneity was observed and the pooled risk estimates were significantly increased for older patients after removing the study by Camachosoto et al. (2018) (adjusted HR = 1.32, 95% CI: 1.17–1.48; Figure 12). Our analysis suggests a slightly elevated risk of PD among older IBD patients compared with younger patients.
Figure 10.
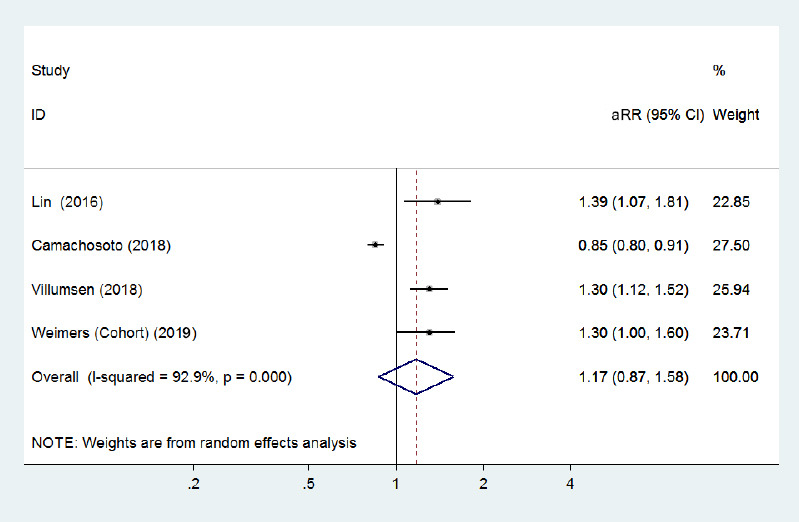
Forest plot of the associations between inflammatory bowel disease patients aged > 65 years and Parkinson's disease development.
There was no significantly increased risk of Parkinson's disease in inflammatory bowel disease patients aged > 65 years when all adjusted effect estimates were pooled. CI: Confidence interval; HR: hazard ratio.
Figure 11.
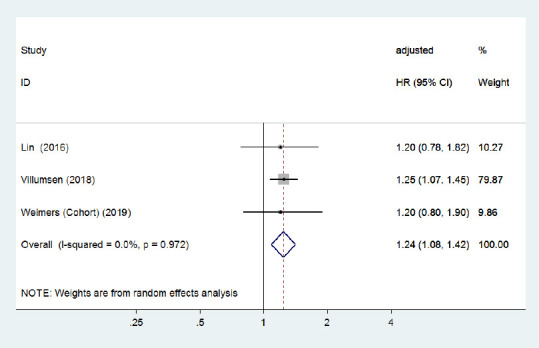
Forest plot of the associations between inflammatory bowel disease patients aged ≤ 65 years and Parkinson's disease development.
Younger inflammatory bowel disease patients (≤ 65 years) were associated with a higher risk of Parkinson's disease. Confounding factors (age, sex, comorbidities, residence, and index date) were adjusted for. CI: Confidence interval; HR: hazard ratio.
Figure 12.
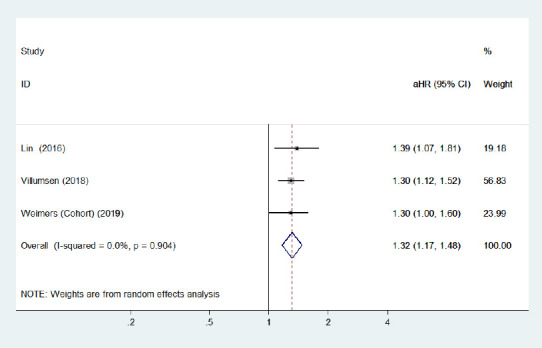
Forest plot of the associations between inflammatory bowel disease patients aged > 65 years and Parkinson's disease development after removing the study with high heterogeneity.
There was a significantly increased risk of Parkinson's disease development among inflammatory bowel disease patients aged > 65 years after removing a heterogeneous study. CI: Confidence interval; HR: hazard ratio.
Anti-inflammatory treatment-specific analysis
Two studies, performed by Peter et al. (2018) and Weimers et al. (2019), assessed the relationship between the risk of PD development and patients with IBD undergoing anti-TNF-α treatment. The results indicated that IBD patients who were prescribed anti-TNF-α therapy had a lower risk of developing PD compared with IBD individuals who were never treated with this therapy (adjusted IRR = 0.22, 95% CI: 0.05–0.88). Furthermore, IBD patients who were never treated with anti-TNF-α or thiopurines had a higher risk of developing PD than the general population (adjusted HR = 1.6, 95% CI: 1.2–2.2).
Publication bias
There was no clear publishing bias in the synthetic analyses of unadjusted estimates (Egger's test: P = 0.40) or adjusted estimates (Egger's test: P = 0.20).
Discussion
Summary of evidence
The findings of our systematic review and meta-analysis suggested an association between IBD and PD, especially for CD and UC, and revealed a higher incidence of PD in IBD patients compared with the general population. The risk of PD development appeared similar in male and female IBD patients. However, there was a slightly higher risk of PD development among IBD patients over the age of 65, and early anti-inflammatory treatment in patients with IBD may reduce the risk of PD development.
Although the studies in our analysis had differences in their study design, all studies except for one, by Camachosoto et al. (2018), reported consistent results of a higher risk of PD development among IBD patients. Moreover, the study performed by Camachosoto et al. (2018) contributed to the considerable heterogeneity observed in this meta-analysis. The case-control study by Camachosoto et al. (2018), from the United States, was based on data from a Medicare database that only included PD patients aged > 65 years with newly diagnosed PD, and non-PD controls. These authors analyzed the association between IBD and the subsequent development of PD. However, the population in this study was not representative of general IBD populations. In addition, this study by Camachosoto et al. (2018) included data from 5 years prior to a PD diagnosis, and we noted that although this study reported a negative association between IBD and the subsequent development of PD, the prevalence of IBD in the PD patients (2.9%) was markedly higher than in the non-PD controls (2.0%). Furthermore, the majority of heterogeneity in our meta-analysis disappeared after removing the study by Camachosoto et al. (2018), and the consistency of the association between IBD and PD was enhanced. Although there was some heterogeneity across the other studies, the sensitivity analysis further confirmed the association between IBD and PD, and there was no evidence of publication bias.
Our findings provide evidence to suggest that IBD may be an important risk factor for PD development, and also indicate that PD might increase the relative risk of IBD development based on two studies (Hsu et al., 2015; Peter et al., 2018). Notably, all included studies were unidirectional except for a Swedish study (Peter et al., 2018), which attempted to investigate the explicit temporal relationship of onset between IBD and PD by bidirectional analysis. The majority of our included studies were based on Medicare databases or hospitalization registries for the estimated data of IBD and PD diagnoses; therefore, the data may have provided inaccurate insights into the temporal relationship of onset between IBD and PD. This means that it is difficult to accurately identify the early and late order of IBD and PD. Furthermore, the long incubation periods and atypical symptoms of both PD and IBD make it difficult to clarify the onset sequence. It is thus very difficult to conduct a bidirectional study of the relationship between IBD and PD.
Our meta-analysis suggested that populations with PD or IBD tend to have a higher risk of developing PD than populations without PD or IBD. The majority of included studies in our meta-analysis were focused on studying the association between IBD and the subsequent development of PD, and suggested that IBD might increase the risk of PD incidence Thus, the concept that IBD might be a potential biological mechanism of PD development was further identified. Although Zhu et al. (2019) concluded that IBD increases the risk of PD, they only included four articles in their meta-analysis. In addition, although their inclusion criteria were cohort studies and case-control studies, the different types of studies were not described separately in the results, and important risk factors of PD (such as family history or drug exposure) were not included, which has led to doubts about their findings. Our meta-analysis also revealed a correlation between IBD and PD, especially for CD and UC. The incidence of PD in patients with IBD was higher than that in the general population, regardless of sex. Early anti-inflammatory therapy in patients with IBD seemed to reduce this risk. This finding may be related to chronic pro-inflammatory immune activation of the intestine, because this feature is also thought to be associated with neurodegenerative diseases, including PD and multiple system atrophy. The characteristic pathological sign of PD is the aggregation of intracellular α-syn. Specific phosphorylated α-syn aggregation patterns can also be identified in the ENS of patients with PD, and is likely to be associated with inflammatory processes and intestinal barrier dysfunction. In addition, LRRK is the most common gene associated with both sporadic and familial PD and multiple system atrophy. Recent studies have shown that LRRK2 is also involved in regulating the inflammatory response as well as α-syn clearance, and that a congenital immune response deficiency may cause α-syn accumulation (Brudek, 2019; Villumsen et al., 2019).
Current evidence suggests that IBD increases the expression of proinflammatory cytokines, such as TNF-α, interferon gamma (IFN-γ), interleukin (IL)-6, and IL-1β, in the gut (Brudek, 2019; Villumsen et al., 2019) and injured intestinal tight junctions. This triggers a proinflammatory-initiated immune response that induces intestinal barrier dysfunction and increases intestinal permeability (Neurath, 2014; Olesen et al., 2016; Pazmandi et al., 2019). Recently, several studies have reported that PD shares similar intestinal alterations to IBD (Clairembault et al., 2015; Rocha et al., 2015; Qin et al., 2016; Houser et al., 2018; Perez-Pardo et al., 2019; Rutsch et al., 2020). In addition, some studies have suggested that patients with PD have a higher expression of proinflammatory cytokines (Rocha et al., 2015; Qin et al., 2016; Houser et al., 2018) and greater intestinal permeability compared with healthy controls (Forsyth et al., 2011; Clairembault et al., 2015; Rutsch et al., 2020). Moreover, a higher prevalence of small intestinal bacterial overgrowth has been observed in PD patients compared with controls without PD (Tan et al., 2014; Niu et al., 2016), and dysbiosis of the intestinal microbiome may also induce the intestinal inflammatory response (Spielman et al., 2018). The sustained intestinal inflammation of IBD can prompt a systemic immune response, subsequently resulting in systemic inflammation and increasing the permeability of the BBB (Pellegrini et al., 2018). Increased BBB permeability is commonly observed in PD patients (Rocha et al., 2015; Qin et al., 2016; Perez-Pardo et al., 2019). In addition, sustained systemic inflammation induces the overexpression and aggregation of α-syn (Griffin et al., 2006; Kelly et al., 2014) and then stimulates a proinflammatory response from immune cells, which prompts abnormal α-syn to propagate into the CNS via prion-like mechanisms (Lema et al., 2013). Furthermore, peripheral inflammation may transfer to the brain and aggravate the spread of α-syn to the CNS via BBB dysfunction (Lema et al., 2013). Ultimately, the intestinal inflammation, systemic inflammation, and aberrant α-syn pathology that is induced by IBD might accelerate neuroinflammation and lead to the specific neurodegenerative pathological features of PD (Lema et al., 2013). Notably, two previous studies (Lema et al., 2013; Holmqvist et al., 2014) have supported the results proposed by Braak et al. (2006), that synucleinopathy might initially occur in both the dorsal motor nucleus of the vagus (DMV) and the ENS in the earliest stages of PD (Braak et al., 2006). Therefore, another possible mechanism by which IBD might prompt the occurrence of PD is that intestinal synucleinopathy may be transmitted to the brain via the vagus nerve (Holmqvist et al., 2014), and then spread from the DMV to other regions of the brain (Lema et al., 2013; Holmqvist et al., 2014). A recent study reported a reduced risk of developing PD in patients who underwent vagotomy (Breen et al., 2019), and suggested that the resection of autonomic nerves may have neuroprotective effects in PD patients.
Another potential explanation for the association between IBD and PD is that both IBD and PD patients have shared genetic susceptibility, especially in patients with CD and PD (Derkinderen and Neunlist, 2018). A genome-wide pleiotropy analysis identified several shared loci between CD or UC and PD, such as LRRK2, HLA, MAPT, and TRIM10. Moreover, the strong genetic similarities observed between CD and PD indicate a common pathogenetic link between the two phenotypes of CD and PD (Witoelar et al., 2017). The CARD15/NOD2 gene is also associated with CD, and is overexpressed in patients with PD (Liu et al., 2015; Dudzińska et al., 2018). Variants of LRRK2 are considered to be a major susceptibility gene for both CD and PD; the protein encoded by LRRK2 regulates the inflammatory pathway and is involved in the incidence and/or progression of both CD and PD. Although strong genetic links have been observed between CD and PD in many studies, there was no significant difference in the risk of PD development between UC and CD in the present meta-analysis. Based on evidence regarding the similar pathological mechanisms of UC and CD (Khor et al., 2011), these two diseases might both increase the incidence of PD equally.
The sex-specific subgroup analysis revealed that the significantly increased risk of PD in IBD patients was similar between female and male patients. Although estrogen may have a neuroprotective role in modulating PD, especially in female patients (Yoo et al., 2020), it may only delay the progression of PD, but not avert its occurrence. Therefore, the relationship between sex and PD development remains uncertain (Kieburtz and Wunderle, 2013). We identified a slightly increased risk of PD in older patients with IBD compared with younger patients. One potential cause might be that PD is typically diagnosed in older men with a long latency period prior to their diagnosis. Furthermore, the time of use of anti-inflammatory drugs or immunosuppressants, such as anti-TNF-α agents, thiopurines, or nonsteroidal anti-inflammatory drugs (NSAIDs), is much shorter in older-onset IBD patients; this might be another underlying reason for the higher risk of PD development in older adult IBD patients.
Both anti-inflammatory drugs and immunosuppressants might suppress the peripheral inflammatory response, reduce neuroinflammation via the damaged BBB, and impede the propagation of abnormal α-syn, which highlights their potential for modulating PD risk in IBD patients (Kieburtz and Wunderle, 2013; Racette et al., 2018). Two large cohort studies that were included in our meta-analysis reported the same conclusion: that earlier treatment of IBD with anti-inflammatory drugs might reduce the risk of PD development (Peter et al., 2018; Weimers et al., 2019). Thus, we suggest that age, sex, and treatment with anti-inflammatory drugs or immunosuppressants are modified risk factors for the development of PD, and should be further evaluated in future studies.
Limitations
There were several factors that limit the interpretation of this study. First, the majority of the included studies were retrospective observational studies that only included data from Medicare databases or hospitalization registries. Thus, differences in the diagnostic criteria of PD or IBD may contribute to the ascertainment bias of this meta-analysis. Second, the unmeasured bias caused by combining various individual study designs may also affect the quality of this analysis. The study performed by Camachosoto et al. (2018) reported several limitations, and was considered to be the major source of heterogeneity in the pooled analysis. We have addressed this issue by evaluating cohort studies with a low risk of bias separately. Third, almost all of the studies included in this meta-analysis were subject to bias related to a lack of demographic information; these studies may have failed to consider potential confounders, such as potential risk factors for PD (e.g., tobacco use, caffeine intake, NSAIDs, or traumatic brain injury) (Kieburtz and Wunderle, 2013). Although most studies adjusted for the confounders of age and sex, they ignored other confounders, which might limit our ability to examine the association between IBD and the incidence of PD. Thus, the potential impact of secondary associations should be considered in the future. Fourth, despite few studies (Hsu et al., 2015; Weimers et al., 2019) reporting an increased risk of IBD development among PD patients, the interpretation of the subgroup analyses with limited numbers of studies should be be cautious, because they may be easily dominated by single studies. Similarly, a limited number of studies reported that anti-inflammatory treatment reduced the risk of PD, which was consistent with the previous findings of biological mechanisms; however, more epidemiological evidence is needed to further support this concept. Fifth, the pooled different effect measures may have resulted in some misrepresented results in this analysis. Hazard ratios were the most suitable effect estimate for investigating the relationship between IBD and PD, but they were only available for less than half of all studies. Because the rate of PD prevalence is relatively low among the general population or IBD patients, these different estimates are consistent overall, and combining varying estimates is therefore reasonable for this meta-analysis. Sixth, Weimers et al. (2019) suggested that the higher risk of subsequently developing PD is proportional to the number of healthcare visits, which may reflect surveillance bias (Haut and Pronovost, 2011). In contrast, Villumsen et al. (2019) were against this viewpoint in their study. Further research should therefore address this concern to clarify the association between IBD and PD. In general, further studies are required to collect more thorough data regarding patient demographics, including confounders such as smoking and caffeine intake. More well-designed epidemiological studies focusing on the association between IBD and PD are warranted.
Conclusions
The results of our meta-analysis suggest that there is a certain correlation between IBD and PD. In addition, IBD may moderately increase the risk of PD regardless of sex, especially in patients over the age of 65 years. Early treatment with anti-inflammatory therapies for IBD might reduce the risk of subsequently developing PD. However, because the studies included in this analysis were retrospective observational studies, more high-quality prospective cohort studies or randomized controlled trials are needed to confirm the precise association between IBD and PD.
Footnotes
C-Editor: Zhao M; S-Editor: Wang J, Li CH; L-Editors: Gardner B, Song LP; T-Editor: Jia Y
Conflicts of interest:The authors declare that there is no conflict of interest.
Financial support:None.
Reporting statement: This study followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of Nanchang University, China.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
References
- 1.Bahler C, Schoepfer AM, Vavricka SR, Brungger B, Reich O. Chronic comorbidities associated with inflammatory bowel disease: prevalence and impact on healthcare costs in Switzerland. Eur J Gastroenterol Hepatol. 2017;29:916–925. doi: 10.1097/MEG.0000000000000891. [DOI] [PubMed] [Google Scholar]
- 2.Benarroch EE, Schmeichel AM, Sandroni P, Low PA, Parisi JE. Involvement of vagal autonomic nuclei in multiple system atrophy and Lewy body disease. Neurology. 2006;66:378–383. doi: 10.1212/01.wnl.0000196638.98781.bb. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, de Vos RA, Bohl J, Del TK. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Breen David P, Halliday Glenda M, Lang Anthony E. Gut-brain axis and the spread of α-synuclein pathology: vagal highway or dead end? Mov Disord. 2019;34:307–316. doi: 10.1002/mds.27556. [DOI] [PubMed] [Google Scholar]
- 5.Brudek T. Inflammatory bowel diseases and parkinson's disease. J Parkinsons Dis. 2019;9:S331–S344. doi: 10.3233/JPD-191729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camacho-Soto A, Searles NS, Racette BA. Inflammatory bowel disease and risk of Parkinson's disease in medicare beneficiaries. Parkinsonism Relat Disord. 2018;57:77. doi: 10.1016/j.parkreldis.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Chao Y, Wong SC, Tan EK. Evidence of inflammatory system involvement in Parkinson's disease. Biomed Res Int. 2014;2014:308654. doi: 10.1155/2014/308654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clairembault T, Leclair-Visonneau L, Coron E, Bourreille A, Le Dily S, Vavasseur F, Heymann MF, Neunlist M, Derkinderen P. Structural alterations of the intestinal epithelial barrier in Parkinson's disease. Acta Neuropathol Commun. 2015;3:12. doi: 10.1186/s40478-015-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derkinderen P, Neunlist M. Crohn's and Parkinson disease: is LRRK2 lurking around the corner? Nat Rev Gastroenterol Hepatol. 2018;15:330–331. doi: 10.1038/s41575-018-0006-9. [DOI] [PubMed] [Google Scholar]
- 10.Der Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 11.Dudzińska E, Gryzinska M, Kocki J. Single nucleotide polymorphisms in selected genes in inflammatory bowel disease. Biomed Res Int. 2018;2018:1–5. doi: 10.1155/2018/6914346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escamilla-Sevilla F, Pinel-Ríos J, Madrid-Navarro CJ, Piñar-Morales R, Herrera-García JD, Pérez-Navarro MJ, Del CC, Cabello-Tapia MJ, Martín-Rodríguez MDM, Sánchez-Capilla D, Piña-Vera MJ, Aguilar-Muñoz A, Campos-Arillo V, Gutiérrez-García J, Chamorro-Santos CE, Gómez-García MR, Mínguez-Castellanos A. Are inflammatory bowel disease and/or treatment with aminosalicylates protective factors against Parkinson's disease? Movement Disord. 2016;31:S141. [Google Scholar]
- 14.Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujioka S, Curry SE, Kennelly KD, Tacik P, Heckman MG, Tsuboi Y, Strongosky AJ, van Gerpen JA, Uitti RJ, Ross OA, Ikezu T, Wszolek ZK. Occurrence of Crohn's disease with Parkinson's disease. Parkinsonism Relat Disord. 2017;37:116–117. doi: 10.1016/j.parkreldis.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin WS, Liu L, Li Y, Mrak RE, Barger SW. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation. 2006;3:5. doi: 10.1186/1742-2094-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haut ER, Pronovost PJ. Surveillance bias in outcomes reporting. JAMA. 2011;305:2462–2463. doi: 10.1001/jama.2011.822. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Bjorklund T, Wang ZY, Roybon L, Melki R, Li JY. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 20.Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson's disease pathogenesis? NPJ Parkinsons Dis. 2017;3:3. doi: 10.1038/s41531-016-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houser Madelyn C, Chang Jianjun, Factor Stewart A, Molho Eric S, Zabetian Cyrus P, Hill-Burns Erin M, Payami Haydeh, Hertzberg Vicki S, Tansey Malú G. Stool immune profiles evince gastrointestinal inflammation in Parkinson's disease. Mov Disord. 2018;33:793–804. doi: 10.1002/mds.27326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu YT, Liao CC, Chang SN, Yang YW, Tsai CH, Chen TL, Sung FC. Increased risk of depression in patients with parkinson disease: a nationwide cohort study. Am J Geriatr Psychiatry. 2015;23:934–940. doi: 10.1016/j.jagp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Huang WW, Li S, Hou ZL, Wang WJ. Pathogenesis of inflammatory bowel disease and mesenchymal stem cell therapy: therapeutic application and existing problems. Zhongguo Zuzhi Gongcheng Yanjiu. 2020;24:1138–1143. [Google Scholar]
- 24.Hui KY, Fernandez-Hernandez H, Hu J, Schaffner A, Pankratz N, Hsu NY, Chuang LS, Carmi S, Villaverde N, Li X, Rivas M, Levine AP, Bao X, Labrias PR, Haritunians T, Ruane D, Gettler K, Chen E, Li D, Schiff ER, et al. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn's disease and Parkinson's disease. Sci Transl Med. 2018;10:eaai7795. doi: 10.1126/scitranslmed.aai7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson M, Lithgo K, Price T. Is there an association between inflammatory bowel disease and Parkinson's disease? J Crohns Colitis. 2014;8:S324–S325. [Google Scholar]
- 26.Kalinderi K, Bostantjopoulou S, Fidani L. The genetic background of Parkinson's disease: current progress and future prospects. Acta Neurol Scand. 2016;134:314–326. doi: 10.1111/ane.12563. [DOI] [PubMed] [Google Scholar]
- 27.Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, Bakay RA, Kordower JH. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson's disease. Mov Disord. 2014;29:999–1009. doi: 10.1002/mds.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khor Bernard, Gardet Agnès, Xavier Ramnik J. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieburtz K, Wunderle KB. Parkinson's disease: evidence for environmental risk factors. Mov Disord. 2013;28:8–13. doi: 10.1002/mds.25150. [DOI] [PubMed] [Google Scholar]
- 30.Kingsbury AE, Bandopadhyay R, Silveira-Moriyama L, Ayling H, Kallis C, Sterlacci W, Maeir H, Poewe W, Lees AJ. Brain stem pathology in Parkinson's disease: an evaluation of the Braak staging model. Mov Disord. 2010;25:2508–2515. doi: 10.1002/mds.23305. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Pardo P, Dodiya HB, Engen PA, Forsyth CB, Huschens AM, Shaikh M, Voigt RM, Naqib A, Green SJ, Kordower JH, Shannon KM, Garssen J, Kraneveld AD, Keshavarzian A. Role of TLR4 in the gut-brain axis in Parkinson's disease: a translational study from men to mice. Gut. 2019;68:829–843. doi: 10.1136/gutjnl-2018-316844. [DOI] [PubMed] [Google Scholar]
- 32.Lema TC, Tyson T, Rey NL, Grathwohl S, Britschgi M, Brundin P. Inflammation and alpha-synuclein's prion-like behavior in Parkinson's disease-is there a link? Mol Neurobiol. 2013;47:561–574. doi: 10.1007/s12035-012-8267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Sundquist J, Sundquist K. Subsequent risks of Parkinson disease in patients with autoimmune and related disorders: a nationwide epidemiological study from Sweden. Neurodegener Dis. 2012;10:277–284. doi: 10.1159/000333222. [DOI] [PubMed] [Google Scholar]
- 34.Lin JC, Lin CS, Hsu CW, Lin CL, Kao CH. Association between Parkinson's disease and inflammatory bowel disease: a nationwide taiwanese retrospective cohort study. Inflamm Bowel Dis. 2016;22:1049–1055. doi: 10.1097/MIB.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 35.Lionnet A, Leclair-Visonneau L, Neunlist M, Murayama S, Takao M, Adler CH, Derkinderen P, Beach TG. Does Parkinson's disease start in the gut? Acta Neuropathol. 2018;135:1–12. doi: 10.1007/s00401-017-1777-8. [DOI] [PubMed] [Google Scholar]
- 36.Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shah T, Abedian S, Cheon JH, Cho J, Dayani NE, Franke L, Fuyuno Y, Hart A, Juyal RC, Juyal G, Kim WH, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979–986. doi: 10.1038/ng.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 39.Niu XL, Liu L, Song ZX, Li Q, Wang ZH, Zhang JL, Li HH. Prevalence of small intestinal bacterial overgrowth in Chinese patients with Parkinson's disease. J Neural Transm (Vienna) 2016;123:1381–1386. doi: 10.1007/s00702-016-1612-8. [DOI] [PubMed] [Google Scholar]
- 40.Obergasteiger J, Frapporti G, Pramstaller PP, Hicks AA, Volta M. A new hypothesis for Parkinson's disease pathogenesis: GTPase-p38 MAPK signaling and autophagy as convergence points of etiology and genomics. Mol Neurodegener. 2018;13:40. doi: 10.1186/s13024-018-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olesen CM, Coskun M, Peyrin-Biroulet L, Nielsen OH. Mechanisms behind efficacy of tumor necrosis factor inhibitors in inflammatory bowel diseases. Pharmacol Ther. 2016;159:110–119. doi: 10.1016/j.pharmthera.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Park HJ, Zhao TT, Kim SH, Lee CK, Hwang BY, Lee KE, Lee MK. Ethanol extract from Gynostemma pentaphyllum ameliorates dopaminergic neuronal cell death in transgenic mice expressing mutant A53T human alpha-synuclein. Neural Regen Res. 2020;15:361–368. doi: 10.4103/1673-5374.265557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pazmandi J, Kalinichenko A, Ardy RC, Boztug K. Early-onset inflammatory bowel disease as a model disease to identify key regulators of immune homeostasis mechanisms. Immunol Rev. 2019;287:162–185. doi: 10.1111/imr.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peter I, Dubinsky M, Bressman S, Park A, Lu C, Chen N, Wang A. Anti-tumor necrosis factor therapy and incidence of parkinson disease among patients with inflammatory bowel disease. JAMA Neurol. 2018;75:939–946. doi: 10.1001/jamaneurol.2018.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pellegrini C, Antonioli L, Colucci R, Blandizzi C, Fornai M. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathol. 2018;136:345–361. doi: 10.1007/s00401-018-1856-5. [DOI] [PubMed] [Google Scholar]
- 46.Qin XY, Zhang SP, Cao C, Loh YP, Cheng Y. Aberrations in peripheral inflammatory cytokine levels in parkinson disease: a systematic review and meta-analysis. JAMA Neurol. 2016;73:1316–1324. doi: 10.1001/jamaneurol.2016.2742. [DOI] [PubMed] [Google Scholar]
- 47.Racette BA, Gross A, Vouri SM, Camacho-Soto A, Willis AW, Searles NS. Immunosuppressants and risk of Parkinson disease. Ann Clin Transl Neurol. 2018;5:870–875. doi: 10.1002/acn3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocha NP, de Miranda AS, Teixeira AL. Insights into neuroinflammation in parkinson's disease: from biomarkers to anti-inflammatory based therapies. Biomed Res Int. 2015;2015:628192. doi: 10.1155/2015/628192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rugbjerg K, Friis S, Ritz B, Schernhammer ES, Korbo L, Olsen JH. Autoimmune disease and risk for Parkinson disease: a population-based case-control study. Neurology. 2009;73:1462–1468. doi: 10.1212/WNL.0b013e3181c06635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutsch A, Kantsjö JB, Ronchi F. The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front Immunol. 2020;11:604179. doi: 10.3389/fimmu.2020.604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spielman LJ, Gibson DL, Klegeris A. Unhealthy gut, unhealthy brain: the role of the intestinal microbiota in neurodegenerative diseases. Neurochem Int. 2018;120:149–163. doi: 10.1016/j.neuint.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 53.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 54.Tan AH, Mahadeva S, Thalha AM, Gibson PR, Kiew CK, Yeat CM, Ng SW, Ang SP, Chow SK, Tan CT, Yong HS, Marras C, Fox SH, Lim SY. Small intestinal bacterial overgrowth in Parkinson's disease. Parkinsonism Relat Disord. 2014;20:535–540. doi: 10.1016/j.parkreldis.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 55.Thomas Broome S, Louangaphay K, Keay KA, Leggio GM, Musumeci G, Castorina A. Dopamine: an immune transmitter. Neural Regen Res. 2020;15:2173–2185. doi: 10.4103/1673-5374.284976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Villumsen M, Aznar S, Pakkenberg B, Jess T, Brudek T. Inflammatory bowel disease increases the risk of Parkinson's disease: a Danish nationwide cohort study 1977–2014. GUT. 2018;68:18–24. doi: 10.1136/gutjnl-2017-315666. [DOI] [PubMed] [Google Scholar]
- 57.Villumsen M, Aznar S, Pakkenberg B, Brudek T, Jess T. Authors’ response: Association between IBD and Parkinson's disease: seek and you shall find? GUT. 2019;68:1722. doi: 10.1136/gutjnl-2018-317336. [DOI] [PubMed] [Google Scholar]
- 58.Weimers P, Halfvarson J, Sachs MC, Saunders-Pullman R, Ludvigsson JF, Peter I, Burisch J, Olen O. Inflammatory bowel disease and parkinson's disease: a nationwide swedish cohort study. Inflamm Bowel Dis. 2019;25:111–123. doi: 10.1093/ibd/izy190. [DOI] [PubMed] [Google Scholar]
- 59.Witoelar A, Jansen IE, Wang Y, Desikan RS, Gibbs JR, Blauwendraat C, Thompson WK, Hernandez DG, Djurovic S, Schork AJ, Bettella F, Ellinghaus D, Franke A, Lie BA, McEvoy LK, Karlsen TH, Lesage S, Morris HR, Brice A, Wood NW, et al. Genome-wide pleiotropy between parkinson disease and autoimmune diseases. JAMA Neurol. 2017;74:780–792. doi: 10.1001/jamaneurol.2017.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoo JE, Shin DW, Jang W, Han K, Kim D, Won HS, Park HS. Female reproductive factors and the risk of Parkinson's disease: a nationwide cohort study. Eur J Epidemiol. 2020;35:871–878. doi: 10.1007/s10654-020-00672-x. [DOI] [PubMed] [Google Scholar]
- 61.Zhu F, Li C, Gong J, Zhu W, Gu L, Li N. The risk of Parkinson's disease in inflammatory bowel disease: A systematic review and meta-analysis. Digest Liver Dis. 2019;51:38–42. doi: 10.1016/j.dld.2018.09.017. [DOI] [PubMed] [Google Scholar]


