Due to the high degree of conservation of genes and mechanisms, the fruit fly Drosophila melanogaster is a powerful experimental in vivo tool to investigate complex diseases, thus complementing traditional vertebrate systems. Drosophila is also an advantageous system regarding the animal husbandry and the short generation time and lifespan. The growing interest in flies includes the establishment of easily manageable models to study visual degeneration which occur in humans (Gaspar et al., 2019).
A brief comparative view of Drosophila eye: The Drosophila compound eye is a complex structure of about 750–800 functional units known as ommatidia (Paulk et al., 2013) (Figure 1A). The cornea and the crystalline cone occupy the most distal part of each ommatidium, which together constitute the dioptric apparatus. Ommatidia form the retina and are made up of eight photoreceptor cells, R1–R8, that are highly polarized epithelial cells arranged in an hexagonal pattern, and eleven accessory cells. R1–R6 cells are located in the periphery of the ommatidium and are the outer photoreceptors. They surround the inner photoreceptors R7 and R8, which occupy the distal and the proximal portion of the center of the ommatidium, respectively. All photoreceptors have a stalk and a rhabdomere. Rhabdomere is the specialized visual organelle composed of thousands of microvilli and supported by an actin cytoskeleton (Figure 1B). Of notice, R7 and R8 share a common rhabdomere R7,8 (R7 distal, R8 proximal).
Figure 1.
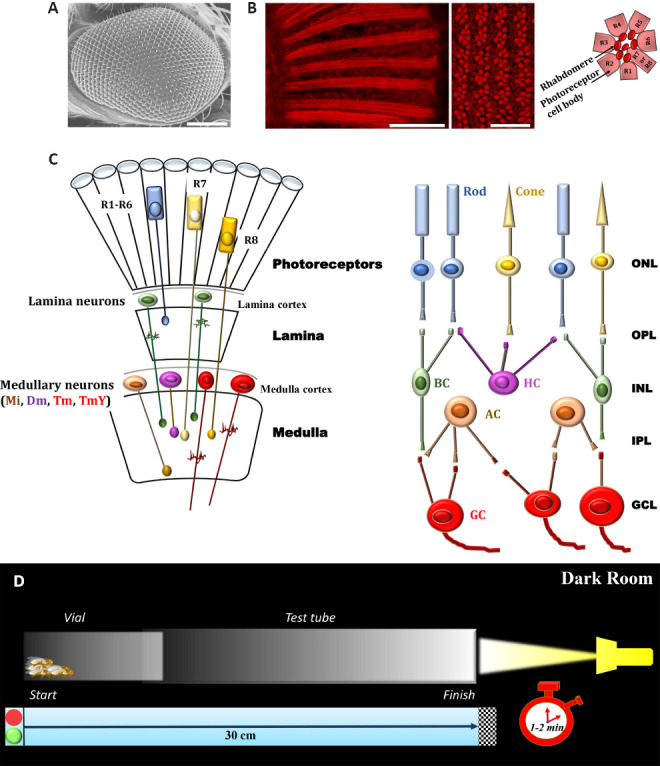
Basic structures underlying the early stages of visual processing of adult D. Melanogaster and experimental procedure for phototaxis assay.
(A) Scanning electron microscopy image of Drosophila eye (Oregon-R strain); scale bar: 100 µm (methods are in agreement with Catalani et al., 2021a, b). (B) Conventional (longitudinal section, left panel) and confocal (cross section, right panel) microscopy analysis of fly eyes stained with fluorescent phalloidin (F-actin staining) to detect rhabdomere morphology and the pattern of ommatidia/rhabdomeres, respectively (methods are in agreement with Catalani et al., 2021a, b). Scale bars: 20 μm (left) and 10 μm (right). Seven rhabdomeres are shown in any one given plane because R7 cell are placed atop the R8. The cartoon on the right depicts a transverse view of the ommatidial unit. Sourced from the authors’ unpublished data. (C) Schematic drawings of Drosophila (left) and vertebrate (right) retina. Main cell types are shown. Colored cells share similar functional characteristics. It should be noted that the complex retina (photoreceptor layer, ommatidial units)-lamina-medulla in flies is comparable to vertebrate retina circuitry in transfer of information through early stages of visual systems. (D) Experimental procedure to analyze the visual response of adult D. Melanogaster. A plastic vial (2.5 × 9.5 cm) is inserted and connected to a glass tube (3.0 × 23 cm) by transparent tape. The transparent apparatus (30 cm) is placed horizontal and perpendicular to the light source. The directional light source from one side, placed horizontal 15 cm away from the tube, acts as an attractant for the flies. In a dark room, flies are introduced independently in the apparatus and left separately for 30 minutes. This allow adaptation of the flies to darkness. The apparatus is then gently pounded down to place the flies at opposite end from the light. The light is then turned on and a timer started. A camera records fly behavior and their movement (horizontal walking) towards the light source during the experiment (1–2 minutes). Each trial was performed three times, at 1-minute intervals, and the results are averaged. Dm: Distal medulla intrinsic neurons; GCL: ganglion cell layer; INL: inner nuclear layer; IPL: inner plexiform layer; Mi: medulla intrinsic neurons; ONL: outer nuclear layer; OPL: outer plexiform layer; Tm and TmY: tangential medulla neurons (two classes, Tm neurons project to the lobula and TmY neurons to both the lobula and lobula plate).
The visual systems of Drosophila and vertebrate share structural, developmental and functional features (Paulk et al., 2013). Both in vertebrate and fly retina there are specific neuronal cell types, which are arranged in parallel layers (Figure 1C). In vertebrates, cell bodies are distributed into three nuclear layers, that are the outer nuclear layer with photoreceptors, the inner nuclear layer that contains the interneurons (horizontal, bipolar and amacrine cells), and the ganglion cell layer, with retina ganglion cells and some amacrine cell bodies. The synapses of these cells form the outer and the inner plexiform layers. In flies, synapses are present in neuronal layers other than retina sensu stricto, since photoreceptors detect light and then transmit the signal to the optic lobe. The lobe comprises four layers of retinotopically-organized neuropiles called lamina, medulla, lobula and lobula plate (lobula and lobula plate constituting the lobula complex). Functionally, the outer Drosophila photoreceptors R1–R6 detect achromatic contrasts and mediate motion vision, similarly to vertebrate rods, while R7 and R8 are used primarily to detect color and polarization, being thus equivalent to vertebrate cones (Paulk et al., 2013). The visual information is processed in the optic lobe. In particular, photoreceptors synapse indirectly or directly in different layers of the medulla. The axons of R1–R6 cells project to the lamina in a single cartridge. In terms of network, the fly lamina is similar to the outer plexiform layer of vertebrate retina (Paulk et al., 2013). Neurons from a single cartridge in the lamina synapse to specific layers within a medulla column. R7–R8 neurons arborize directly in the medulla in such a way that the motion and color vision remain separate. Thus, the medulla elaborate a wide variety of visual information, such as colors, mobility detection and shape assessment, with an high degree of compartmentalization, like the vision system of vertebrates (Paulk et al., 2013).
Insights on Drosophila retina in health and disease: In a general point of view, vision represents a source of information on the environment in which animals live, including those required to recall food or nest. It has been demonstrated that D. melanogaster is capable of visual place learning and remembers the location of objects, being thus an useful system to study complex behaviors such as spatial memory (Ofstad et al., 2011).
Fly eye was extensively studied at functional level by electroretinogram recordings that measure electrical activities of photoreceptors, second order neurons and adjacent cells during first steps of vision process. However, an indirect approach could be also adopted to assess phototransduction performance. Indeed, Drosophila shows strong phototactic response since is attracted by light, avoiding shaded areas, when given a choice (Paulk et al., 2013). In this respect, phototaxis, in the absence of altered mobility and activity, is a robust and quick assay to reveal decreased fly responsiveness to the light because of vision defects. For instance, the phototactic responses of adult D. melanogaster can be measured easily utilizing a glass and transparent tube placed horizontal and perpendicular to a light source, in a dark room (Catalani et al., 2021a, b). During the test (light on) a camera records fly behavior and their movement (horizontal walking) towards the light source (Figure 1D). Animals are then analyzed at specific intervals in each part of the tube. Individuals that have vision defects do not reach the source as fast as the control, are not attracted by the light, are motionless, move perpendicular to the light or unbiased towards and away from the light. To note, phototaxis is helpful also to uncover neural function and networks such as visual perception since the assay may be related to the time of the day and the internal state of the flies. In addition, phototaxis may be used as an indicator of visual ability of flies. Indeed, phototactic behavior involves both chromatic and achromatic discrimination, it is not merely related to the intensity of light but also depends on the wavelength (Paulk et al., 2013). Taking advantages from the susceptibility to specific wavelengths of light, a programmable optical stimulator has been developed to induce retina degeneration in Drosophila, by exposing photoreceptors to blue but not red light (Chen et al., 2017).
Retina degeneration in mammals can be the consequence of failings in enzymatic visual cycle pathway that in the retina regenerates the chromophore after its release from light-activated rhodopsin. An enzymatic visual cycle for maintaining rhodopsin levels exists also in D. melanogaster retina, thus giving the opportunity to use it for exploring photoreceptor cycle defects (Wang et al., 2010). In this respect, genetic and molecular bases of phototransduction are greatly conserved in flies and vertebrates. Phototransduction is affected frequently in degenerative diseases and the use of Drosophila facilitates rapid genes screening (Gaspar et al., 2019). For instance, multiple genetic studies were performed over the years to clarify genetic mutations in different pathologies, including Retinitis pigmentosa (RP). Retinitis pigmentosa, also called rod-cone dystrophy, is a hereditary, genetically heterogeneous, clinically variable disease. It is characterized by progressive degeneration of light-sensing photoreceptors in the retina that lead to a gradual vision loss. The first mutation responsible for an autosomal dominant RP, RP4, was determined in the gene encoding opsin; the degeneration is due to impaired maturation of the rhodopsin. This was the first case described in which the fruit fly served as a model organism to unravel the human mechanisms leading to degeneration. Since then, over 3000 genetic mutations in approximately 70 genes have been found in humans, and the majority of them have orthologous in the Drosophila genome making the fly a valuable RP model (Gaspar et al., 2019).
Undeniably, the manipulation of target genes and the exploitation of genetic mutant animals represent a powerful instrument to study retina defects. Both mdx mouse (a mammalian model of Duchenne muscular dystrophy) and two dystrophic mutant strains of D. melanogaster have been used by us to explore retina homeostasis in the absence of functional full-length dystrophin (Catalani et al., 2021b). Retinas of mdx animals showed altered neuronal architecture compromising mainly the pre-synaptic photoreceptor terminals and their post-synaptic sites. Apoptotic neurons had an impaired autophagy that lead to the accumulation of autophagosomes. Of interest, a similar scenario, as for instance rhabdomere degeneration and structural damages in the retina/lamina, was observed in young adult fly mutants. They also demonstrated clear signs of vision defects and accumulated autophagosomes/apoptotic features in retina neurons. Of notice, the treatment of dystrophic Drosophila with the mTOR inhibitor rapamycin (as a booster for autophagy) re-activated autophagosome turnover, prevented neuronal cell death and structural damages, and ameliorated the vision, indicating that dystrophin is required for synapse stabilization and neuronal survival (Catalani et al., 2021b). Thus, proper autophagy seems a key prerequisite for physiological cell fate and visual properties in both fly and vertebrate retinas, counteracting neurodegenerative features.
Age-related neurodegenerative diseases in the eye, including macular degeneration and glaucoma, are functional diseases caused by aging but heavily influenced by lifestyle and genetic/environmental factors. In this respect, D. melanogaster may be used to study senescence-related visual pathologies in humans as flies share evolutionarily conserved genes affecting age-dependent decline in vision (Carbone et al., 2016). Among degenerative defects affecting retina, we have studied recently the outcome of high-sucrose-associated damages in D. melanogaster's visual system (Catalani et al., 2021a). In humans, diabetic retinopathy (DR) is the most common visual complication of hyperglycemia all over the world. Degeneration of retina neurons is appreciated before microvascular damages and clinical signs of DR. Restoring of retina neurons homeostasis in DR may thus reduce the damages caused by hyperglicemic insult (Simo et al., 2018). In our settings, adult flies were fed for 10 days with high-sucrose regimens. Diets were prepared changing sucrose independently while keeping the other components constant. Results revealed that flies had a normal locomotor ability/mobility and not obvious alterations of myofiber structure.Furthermore, the flies had normal body weight and size but higher whole-body glucose concentration and high level of phosphorylated Akt, a marker of insulin resistance, supporting the view that the increased availability of dietary sucrose in adult flies induced hyperglycemia and the initial stage of diabetes, with a low impact on the overall metabolic profile at short term. Most importantly, hyperglycemic flies showed a significant decrement in the responsiveness to the light assessed by phototaxis assay, thus clearly indicating vision defects. A detailed examination showed that hyperglycemia did not induce gross anatomical alteration of the external eye of Drosophila, whereas it led to a progressive and appreciable neurodegeneration of photosensitive components. Indeed, rhabdomere columns showed abnormal and disorganized morphology and ultrastructural analysis detected enlarged photoreceptors. At molecular level, hyperglycemic flies displayed typical retina neurodegenerative features, i.e. apoptosis, oxidative stress and dysfunctional autophagy flux. These molecular events primarily affected the internal network of the retina, where photoreceptors make the first synapse. Taken together, these data demonstrated that D. melanogaster fed with high-sugar diets was affected by visual impairment and neuroretina damage as occur, at least in part, at the early stages of DR. This system may thus parallel vertebrate tools in order to expand our knowledge on neuronal abnormalities and the strategies counteracting hyperglycemic insult in the eye (Catalani et al., 2021a). Comparable neuronal damages and altered apoptosis/autophagy machinery were observed in mouse retina models of early DR (Amato et al., 2018), further suggesting that apoptosis/autophagy crosstalk is a crucial point (and well conserved) in hyperglycemic impairment of visual system across species (Boya et al., 2016).
Remarks: There is a general agreement that performing functional studies in vivo is an invaluable tool since genes and proteins often have specific functions in organisms that are not always easily identifiable using in vitro systems. Many parallels can be drawn between D. melanogaster and human phenotypes, suggesting that relevant mechanisms involved in the homeostasis of retina neurons are well conserved. Drosophila models that mimic human diseases, in addition, are key tools for drug-discovery via target-identification (Papanikolopoulou et al., 2019). This is important for the translation of specific compounds in human therapeutics although the use of Drosophila is somewhat limited due to the evolutionary and functional distance from mammals. Therefore, before comparing fly studies to human physiology and pharmacology, data should be verified on more biologically complex organisms. Despite these limitations, a large body of evidence depicts the Drosophila visual system as a flexible in vivo model to investigate the pathophysiology of retina neurons, assaying easily different experimental parameters, molecular targets and treatments.
This work was supported by grants from “Departments of Excellence-2018” Program (Dipartimenti di Eccellenza) to DIBAF (University of Tuscia, Viterbo, Italy) (Project “Landscape 4.0 - food, wellbeing and environment”) (to DC).
Footnotes
P-Reviewer: Fernandez-Bueno I; C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open peer reviewer:Ivan Fernandez-Bueno, University of Valladolid, Spain.
References
- 1.Amato R, Catalani E, Dal Monte M, Cammalleri M, Di Renzo I, Perrotta C, Cervia D, Casini G. Autophagy-mediated neuroprotection induced by octreotide in an ex vivo model of early diabetic retinopathy. Pharmacol Res. 2018;128:167–178. doi: 10.1016/j.phrs.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Boya P, Esteban-Martinez L, Serrano-Puebla A, Gomez-Sintes R, Villarejo-Zori B. Autophagy in the eye: development, degeneration, and aging. Prog Retin Eye Res. 2016;55:206–245. doi: 10.1016/j.preteyeres.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Carbone MA, Yamamoto A, Huang W, Lyman RA, Meadors TB, Yamamoto R, Anholt RR, Mackay TF. Genetic architecture of natural variation in visual senescence in Drosophila. Proc Natl Acad Sci U S A. 2016;113:E6620–6629. doi: 10.1073/pnas.1613833113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalani E, Silvestri F, Bongiorni S, Taddei AR, Fanelli G, Rinalducci S, De Palma C, Perrotta C, Prantera G, Cervia D. Retinal damage in a new model of hyperglycemia induced by high-sucrose diets. Pharmacol Res. 2021a;166:105488. doi: 10.1016/j.phrs.2021.105488. [DOI] [PubMed] [Google Scholar]
- 5.Catalani E, Bongiorni S, Taddei AR, Mezzetti M, Silvestri F, Coazzoli M, Zecchini S, Giovarelli M, Perrotta C, De Palma C, Clementi E, Ceci M, Prantera G, Cervia D. Defects of full-length dystrophin trigger retinal neuron damage and synapse alterations by disrupting functional autophagy. Cell Mol Life Sci. 2021b;78:1615–1636. doi: 10.1007/s00018-020-03598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Leon-Salas WD, Zigon T, Ready DF, Weake VM. A programmable optical stimulator for the Drosophila eye. HardwareX. 2017;2:13–33. doi: 10.1016/j.ohx.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaspar P, Almudi I, Nunes MDS, McGregor AP. Human eye conditions: insights from the fly eye. Hum Genet. 2019;138:973–991. doi: 10.1007/s00439-018-1948-2. [DOI] [PubMed] [Google Scholar]
- 8.Ofstad TA, Zuker CS, Reiser MB. Visual place learning in Drosophila melanogaster. Nature. 2011;474:204–207. doi: 10.1038/nature10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papanikolopoulou K, Mudher A, Skoulakis E. An assessment of the translational relevance of Drosophila in drug discovery. Expert Opin Drug Discov. 2019;14:303–313. doi: 10.1080/17460441.2019.1569624. [DOI] [PubMed] [Google Scholar]
- 10.Paulk A, Millard SS, van Swinderen B. Vision in Drosophila: seeing the world through a model's eyes. Annu Rev Entomol. 2013;58:313–332. doi: 10.1146/annurev-ento-120811-153715. [DOI] [PubMed] [Google Scholar]
- 11.Simo R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61:1902–1912. doi: 10.1007/s00125-018-4692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Wang T, Jiao Y, von Lintig J, Montell C. Requirement for an enzymatic visual cycle in Drosophila. Curr Biol. 2010;20:93–102. doi: 10.1016/j.cub.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


