Key Words: affective disorders, astrocytes, glial fibrillary acidic protein, ionized calcium binding adaptor molecule 1, memory, microglia, neurotrauma, spinal cord injury
Abstract
Evidence suggests that rapid changes to supporting glia may predispose individuals with spinal cord injury (SCI) to such comorbidities. Here, we interrogated the expression of astrocyte- and microglial-specific markers glial fibrillary acidic protein (GFAP) and ionized calcium binding adaptor molecule 1 (Iba1) in the rat brain in the first 24 hours following SCI. Female Sprague-Dawley rats underwent thoracic laminectomy; half of the rats received a mild contusion injury at the level of the T10 vertebral body (SCI group), the other half did not (Sham group). Twenty-four hours post-surgery the amygdala, periaqueductal grey, prefrontal cortex, hypothalamus, lateral thalamus, hippocampus (dorsal and ventral) in rats were collected. GFAP and Iba1 mRNA and protein levels were measured by real-time quantitative polymerase chain reaction and Western blot. In SCI rats, GFAP mRNA and protein expression increased in the amygdala and hypothalamus. In contrast, gene and protein expression decreased in the thalamus and dorsal hippocampus. Interestingly, Iba1 transcripts and proteins were significantly diminished only in the dorsal and ventral hippocampus, where gene expression diminished. These findings demonstrate that as early as 24 hours post-SCI there are region-specific disruptions of GFAP and Iba1 transcript and protein levels in higher brain regions. All procedures were approved by the University of Technology Sydney Institutional Animal Care and Ethics Committee (UTS ACEC13-0069).
Chinese Library Classification No. R446; R364; R741
Introduction
Spinal cord injuries (SCI) can result in long-term and permanent deafferentation of cortical circuits of the central nervous system (CNS) (Wall and Egger, 1971; Ziemann et al., 1998). Such changes can result in a substantial reorganization of cortical maps, exemplifying the plastic properties of the CNS (Aguilar et al., 2010). Sleep disturbance, anxiety, depression and cognitive dysfunction is highly prevalent in SCI patients (Davidoff et al., 1990; Kennedy and Rogers, 2000; Biering-Sørensen and Biering-Sørensen, 2001). This suggests strongly that following spinal cord trauma, in addition to changes in cortical circuits, other brain regions critical for the regulation of sleep, mood and cognition are also significantly impacted. For a complete understanding of the neurochemical bases of these changes in complex behaviors, it is essential to understand the changes in the brain triggered during the earliest stages of spinal cord injury, from which these long-term changes evolve.
Glial cells are the supporting cells of the CNS (He and Sun, 2007). Alterations within the astrocyte and microglia compartments play significant roles in the onset and progression of several pathophysiological processes that can lead to a spectrum of affective dysfunctions (Öngür and Heckers, 2004; Pav et al., 2008), as well as synaptic alterations (Honer et al., 1999; Coyle and Schwarcz, 2000; Cotter et al., 2001; Scholz and Woolf, 2007). Both astrocytes and microglia play major roles in shaping these CNS functions, and are likely to be the first cell populations primed following trauma, such as is associated with SCI. Glial fibrillary acidic protein (GFAP) is well established as the primary filament present in mature astrocytes within the CNS, where it is involved in modulating the structural stability, shape, and motility of the cells, as well as the cell-to-cell interactions with neurons (Eng, 1985; Eng et al., 2000; Li et al., 2020). Ionized calcium binding adaptor molecule 1 (Iba1), is expressed in the cells of several tissues, including brain, testis, spleen and, to a lesser extent, in the kidneys and lungs. In the brain, Iba1 is expressed uniquely by microglia (Ito et al., 2001; Hwang et al., 2008), where it elicits actin-bundling activity and participates in membrane ruffling and phagocytosis when the microglia are activated (Ohsawa et al., 2004).
In a number of studies examining mood change and cognitive dysfunction identical to that seen in individuals with SCI, there are reports of regionally specific reductions in glial cell populations and/or glial activities in the amygdala, prefrontal cortex, hippocampus and periaqueductal gray (Öngür et al., 1998; Bowley et al., 2002; Imbe et al., 2012). Examining changes in GFAP and Iba1 transcript and protein levels may provide important insights into the temporal and topographical responses of glial cells occurring in higher brain regions after spinal cord injury.
In this study, we evaluated the hypothesis that within the initial 24 hours after an injury, SCI leads to rapid mRNA and protein changes in glial cells of discrete brain regions critical for the regulation of mood/emotion, stress responsivity, memory and decision-making. To answer this question, we investigated the gene and protein expression of GFAP and Iba1 in the amygdala, periaqueductal gray, prefrontal cortex, hypothalamus, thalamus and dorsal and ventral hippocampus of female rats 24 hours after SCI.
Materials and Methods
Ethics statement
All procedures were carried out with the approval of the University of Technology Sydney Institutional Animal Care and Ethics Committee (UTS ACEC13-0069), according to the guidelines set out by the National Health and Medical Research Council code of conduct for the use of animals in research (Nguyen et al., 2017).
Animals
Six adult female Sprague-Dawley rats (9 weeks old, body weight 250–300 g) were acquired from the Animal Resource Centre (Perth, WA, Australia). Rats were housed in cages on a 12-hour dark-light cycle with unlimited access to food and water. Each cage was provided with environmental enrichment. Animals were assigned randomly to either; (1) mild contusion SCI group (SCI; n = 3), or (2) sham surgery group (Sham; n = 3) (Figure 1).
Figure 1.
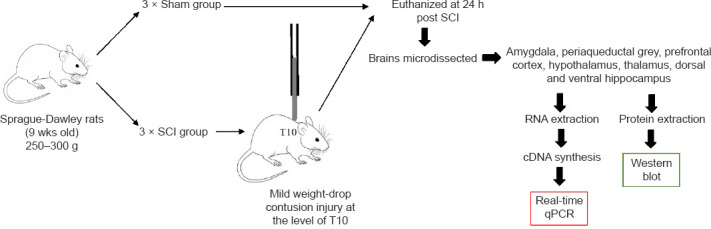
Flowchart of the experimental procedure.
Female, Sprague-Dawley rats, 9 weeks of age were divided into 2 groups: Sham (n= 3) and SCI (n= 3). A small incision was made in the thoracic region above the T10 vertebrae and a laminectomy performed (Krishna et al., 2013). The SCI group then received a weight drop contusion, by dropping a 10 g weight from 6.25 mm height and an impact head diameter of 2.5 mm onto the exposed dura of the spinal cord (Nguyen et al., 2017). Sham rats received the same surgical procedure but not the weight-drop procedure. All rats were euthanized 24 hours after surgery/injury and each brain was microdissected into the required identified regions. Each region was then processed for RNA and protein extraction and downstream real-time quantitative polymerase chain reaction (qPCR) and Western blot analyses. SCI: Spinal cord injury.
Surgery and euthanasia
Rats were anaesthetised with 2% isoflurane (Thermo Fisher Scientific, NSW, Australia) in O2 (flow rate of 1 L/min), once a surgical plane of anaesthesia was established, the fur above the thoracic region was shaved and iodine applied to the exposed skin. A subcutaneous injection of local anaesthetic (0.2 mL bupivacaine, Sigma-Aldrich, Castle Hill, NSW, Australia) was administered at the site of SCI or sham surgery. Each rat was given analgesics (buprenorphine hydrochloride -Temgesic 0.03 mg/kg, subcutaneously [s.c.]), antibiotics (cephazolin sodium 33 mg/kg, s.c.) and Hartman's replacement solution (compound sodium lactate 15 mL/kg, s.c.). A midline incision was made from the mid to lower thoracic region and subcutaneous tissues cleared from the spinous process of the T10 vertebral body. A bilateral laminectomy of the T10 vertebrae exposed the dorsal surface of the spinal cord. Using a NYU/MASCIS weight-drop impactor, the vertebral column of each rat was stabilised with clamps attached to the T9 and T11 vertebrae and the exposed spinal cord subjected to a mild weight-drop contusion injury (6.5 mm, 10 g, 2.5 mm impactor head diameter). The surgical incision was closed in layers and sutured, and the animals were returned to a warmed cage where they were observed closely during recovery. During the next 24 hours, each rat received two further doses of analgesics (buprenorphine hydrochloride -Temgesic 0.03 mg/kg, s.c.), antibiotics (cephazolin sodium 33 mg/kg, s.c.) and Hartman's replacement solution and underwent manual bladder expression (Nguyen et al., 2017).
At the end of this 24-hour period, the rats were deeply anaesthetised and euthanized using pentobarbital sodium (Lethabarb, 1 mL/kg, intraperitoneally [i.p.]). The brain of each rat was carefully removed and transferred to HBSS buffer before being snap frozen in liquid nitrogen. The brains were stored at –80°C until microdissection.
Microdissections
The prefrontal cortex, amygdala, lateral thalamus, dorsal and ventral hippocampus, hypothalamus, and periaqueductal gray regions were microdissected using our previously described methods (Chiu et al., 2007; Castorina et al., 2019), and with reference to a stereotaxic atlas of the rat brain (Paxinos and Watson, 2006). The brain was sectioned into smaller tissue blocks by making, three complete coronal cuts at specific antero-posterior (AP) levels using a sterile, chilled razor blade, cleaned in “RNA-ase Away”. The first coronal section was made at the anterior border of the optic chiasm (+0.3 mm anterior to bregma), the second at the posterior border of the interpeduncular fossa, and the third immediately posterior to the inferior colliculi as the midbrain aqueduct opens in the fourth ventricle (approximately –4.6?mm to –7.8?mm caudal to bregma). Each section created a tissue block that included one or more of the regions of interest, as detailed below:
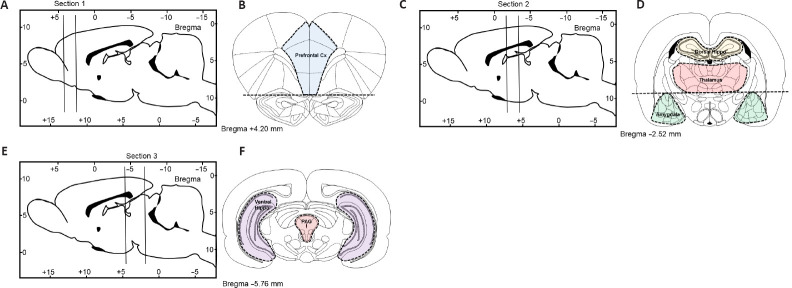
Prefrontal cortex: In the most anterior tissue block we isolated the medial prefrontal cortex, the first coronal section was cut between bregma levels +4.68 mm and +2.52 mm (Figure 2A). Thereafter, we removed the anterior olfactory nucleus which occupies the ventral 1.5 mm of this section (Figure 2B) and then made two parasagittal cuts approximately 1 mm lateral to the midline abutting the forceps minor.
Figure 2.
Schematic depicting rat brain microdissection procedures.
2-mm (sections 1 and 2) or 3-mm thick coronal brain sections (section 3) were cut from either sham-operated or spinal cord injury rats as indicated in (A, C and E) and tissue blocks containing the prefrontal cortex (B), the dorsal hippocampus, thalamus and amygdala (D), or the ventral hippocampus and periaqueductal gray (F) were microdissected under a stereoscopic microscope (magnification 10×) using the Paxinos and Watson rat brain atlas as a reference (Paxinos and Watson, 2006).
Amygdala: Similar to the prefrontal cortex, a 2-mm thick coronal section was cut between –1.92 mm and –3.96 mm caudal to bregma (Figure 2C). To obtain a block that included the entire amygdaloid complex, we used the opening of the lateral ventricle as a reference point to further dissect the triangular shaped area of the amygdala from each side located approximately 4 mm from the midline (Figure 2D).
Thalamus: To obtain lateral thalamus samples, we used the same tissue blocks used to isolate the amygdala. The hypothalamus was isolated from the remaining tissue block and using the internal capsule as the lateral boundary and the dorsal opening of the 3rd ventricle as the upper boundary we micro-dissected the lateral thalamus from each side as two semi-rectangular tissue samples (~8 mm × ~2 mm) (Figure 2D).
Dorsal hippocampus: was isolated from the same tissue block used to isolate the amygdala and lateral thalamus. The right and left dorsal hippocampi are easily identified and isolated. The tissue was separated from the cortical layer above and the corpus callosum (Figure 2D).
Ventral hippocampus: The ventral hippocampus was dissected from the most caudal tissue block and was isolated from a smaller tissue block approximately –4.6?mm to –7.8 mm caudal to bregma (Figure 2E). The ventral hippocampus was isolated from the surrounding cortex under a dissecting microscope using the thin layer of white matter surrounding the lateral boundary for reference, the tissue was removed with Dumont #7 forceps (Fine Science Tools, North Vancouver BC, Canada) (Figure 2F).
Periaqueductal gray (PAG): The tissue block used to isolate the ventral hippocampus was used to isolate the PAG. The PAG is a tubular-shaped region of the midbrain surrounding the cerebral aqueduct which resulted in a tissue sample of about 2 mm diameter (Figure 2F).
Each of the brain regions were weighed and immediately snap-frozen in liquid nitrogen and stored at –80°C for subsequent RNA extraction.
RNA extraction and cDNA synthesis
Each of the brain regions obtained from the SCI and sham groups were processed for RNA extraction, following TRI reagent manufacturer's protocol, with minor modifications (Sigma-Aldrich). To extract RNA from samples we used 1 mL TRI reagent (Sigma-Aldrich) and 0.2 mL chloroform. We then precipitated the RNA with 0.5 mL 2-propanol at 12,000 × g for 15 minutes at 4°C (Castorina et al., 2014). We washed the pellet with 75% ethanol, left to air dry and re-dissolved in 30 µL milli-Q H2O. Final RNA concentration was measured with a spectrophotometer (Nanodrop ND-1000® spectrophotometer, Wilmington, DE, USA). To obtain single-stranded cDNAs we used the Tetro cDNA synthesis kit (Bioline, Sydney, NSW, Australia). We incubated 1?μg of total RNA with reverse transcriptase (200?U/μL); Oligo-(dT)18 primer (100?nM); 0.5'mM dNTP mix, RNase-inhibitor (10?U/μL) at 45°C for 40?minutes in a final volume of 20?μL. Temperature was finally increased to 85°C for 5'minutes to terminate the reaction.
Real time qPCR analysis
To analyze changes in steady-state levels of GFAP and Iba1 transcripts between SCI and Sham rats we used the CFX96 Touch™Real-Time PCR Detection System (BioRad, Gladesville, NSW, Australia). The ribosomal protein 18S was used as the housekeeping gene. qPCR experiments were carried out by following a modified protocol, adapted from our previous study (Castorina et al., 2013). 3 μL of diluted cDNA (10 ng/μL), 5 μL of SensiFAST SYBR®No-ROX master mix (Bioline), 0.8 μL of 5 μM forward primer, 0.8 μL of 5 μM reverse primer and 0.4 μL of MilliQH2O were added to a final volume of 10 µL per reaction. Differentially expressed genes were analysed using the ΔΔCt method and are expressed as mean fold change (Schmittgen and Livak, 2008). The ΔΔCt of each sample was obtained by subtracting the calibrator (Sham) ΔCt to the target sample ΔCt and then applying the formula 2–ΔΔCt. Baseline measurements were set to 1. PCR product specificity was assessed by melting curve analysis, with each gene displaying an individual peak. The sequences of the genes used in this study are listed in Table 1.
Table 1.
Primer sequences targeting the rattus norvegicus GFAP and Iba1 genes, optimised for real-time PCR
| Gene (Ref. Seq.) | Primers (5’–3’) | Location of primers | Tm (°C) | Length (bp) |
|---|---|---|---|---|
| GFAP (NM_017009.2) | F: GCG AAG AAA ACC GCA TCA CC | 1189 | 60.01 | 150 |
| R: TCT GGT GAG CCT GTA TTG GGA | 1338 | 61.12 | ||
| Aif1 (NM_017196.3) | F: AGC AAG GAT TTG CAG GGA GG | 108 | 60.32 | 143 |
| R: TTG AAG GCC TCC AGT TTG GAC | 250 | 60.48 | ||
| 18S Ribosomal protein subunit (NM_213557.1) | F: GGC GGA AAA TAG CCT TCG CT | 113 | 61.1 | 101 |
| R: AGC CCT CTT GGT GAG GTC AA | 213 | 60.77 |
F: Forward; GFAP: glial fibrillary acidic protein; Iba1: Ionized calcium binding adaptor molecule 1; R: reverse; Tm: temperature.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis and Western blot
To obtain our protein lysates, brain tissues taken from different regions were homogenised using a sterilized conical pestle in RIPA Buffer (1:5 w/v, Sigma-Aldrich) containing a Protease Inhibitor cocktail (cOmplete™, Mini, EDTA-free Protease Inhibitor Cocktail, Sigma-Aldrich). Samples were then cleared by centrifugation at 12,000 × g for 10 minutes. Protein quantification was performed using the BCA assay (Thermo Fisher Scientific).
Denatured proteins (30 μg) were prepared by adding 4× Laemmli buffer (Bio-Rad, Gladesville, NSW, Australia) and β-mercaptoethanol (Sigma-Aldrich) and heating for 10 minutes at 70°C. Samples were run on a gradient Tris-glycine gel (4–20%, Bio-Rad), with a molecular weight ladder (Bio-Rad). Gels were transferred to a polyvinylidene difluoride (PVDF) membrane using the Trans-Blot Turbo instrument (Bio-Rad) (Giunta et al., 2010). Once transfer was completed, membranes were washed thoroughly with TBS + 0.1% Tween 20 (Sigma-Aldrich) (TBST 1×). Membranes were then blocked in 5% dry non-fat skim milk in TBST with slow agitation (50–60 r/min) for 1 hour at room temperature (RT).
Membranes were incubated with either GFAP (Abcam, Cambridge, Massachusetts, US, Cat# ab68428; dilution 1:2000) or Iba1 primary antibodies (Abcam, Cat# ab178846; dilution 1:500) in blocking buffer overnight at 4°C with slow agitation. Thereafter, membranes were washed 3× with TBST, followed by 3 × 5 minutes long washes. Finally, membranes were incubated with a secondary antibody (horse radish peroxidase-conjugated goat anti-rabbit IgG) for 1 hour at RT, diluted at 1:10,000 in blocking buffer. Membranes were finally washed to remove excess secondary antibody (Bucolo et al., 2012). Blots were revealed by chemiluminescence method (Clarity Western ECL, Bio-Rad) using the Bio-Rad ChemiDoc MP Imaging System (Bio-Rad).
Statistical analysis
This is an exploratory study. Given the intrinsic nature of these types of studies, we could not predict a priori for any obvious changes in the pattern of gene and protein expression for any of the tested markers. Therefore, we conducted a priori power calculation using the online tool ClinCalc.com (https://clincalc.com/stats/samplesize.aspx) to calculate the right sample size on the assumption that a gene or protein expression fold change of ≥ 1.5 would have been considered biologically relevant. Based on this assumption and by estimating an inter-experiment standard deviation of 0.2 (20% variation), using a power of 80% and an alpha value of 0.05, our power calculations revealed that n = 3 per group was enough to provide sufficient statistical power.
Data are reported as the mean?±?SEM. Comparisons between groups were assessed using the unpaired Student's t-test. P ≤ 0.05 was considered statistically significant. Data analyses were performed using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Surgical induction of mild spinal cord injury
There were no adverse events during our surgical procedures and all rats recovered well after surgery. Rats with SCI showed signs of hind limb movement impairment, consistent with the location and severity of the injury. Sham rats (controls) did not show any signs of locomotor impairment as reported in our previous work (Nguyen et al., 2017).
Acute changes in GFAP mRNA and protein expression in the rat brain following SCI
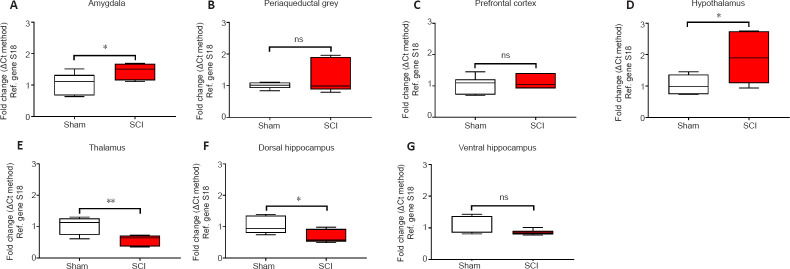
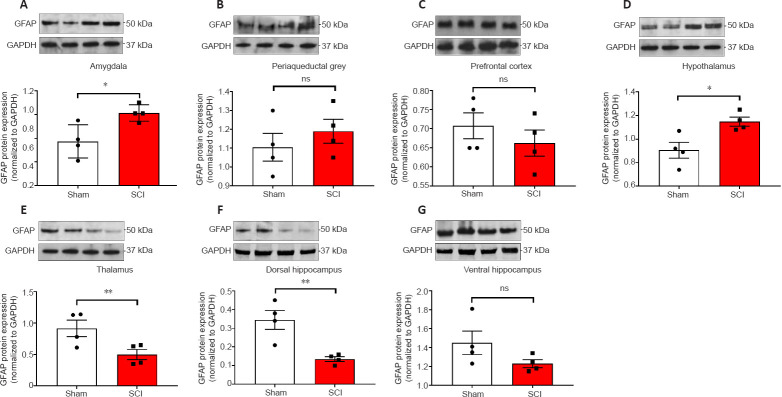
The acute effects of SCI on the expression of GFAP in the amygdala and PAG, two regions pivotal in mediating emotional coping behaviours and that play important roles in the development of the behavioural dysfunction comorbid with injury and trauma (Keay and Bandler, 2001; Phelps and LeDoux, 2005; Mor et al., 2015; Motta et al., 2017), were evaluated. Figures 3A and 4A show that GFAP mRNA and protein expression levels were significantly upregulated in the amygdala compared to Sham controls (t10 = 2.261 and t6 = 3.061, P < 0.05), whereas in the PAG, the expression of GFAP mRNA and proteins were not significantly affected 24 hours after SCI (Figures 3B and 4B; t10 = 1.144 and t6 = 0.872, P > 0.05 for GFAP). Figures 3C and 4C show that GFAP expression in the prefrontal cortex was unchanged 24 hours after SCI (t10 = 0.630 for mRNA and t6 = 0.936, P > 0.05 for GFAP), whereas hypothalamic GFAP mRNA and protein expression was significantly increased at this time (Figures 3D and 4D, t10 = 2.490 and t6 = 3.123, P < 0.05).
Figure 3.
Real-time qPCR data showing the differential mRNA expression of GFAP in the amygdala (A), periaqueductal gray (B), prefrontal cortex (C), hypothalamus (D), thalamus (E), dorsal and ventral hippocampus (F and G) in sham and spinal cord injured rats.
Real-time qPCRs were carried out using selected primer pairs that were designed and optimised to amplify small fragments (≤ 150 bp length) within the coding sequence of the gene of interest (see Table 1). Results show mean fold changes ± SEM obtained from two independent experiments run in duplicate. Fold changes for the genes of interest were calculated using the comparative ΔΔCt method after normalization to the reference (Ref.) gene S18. Baseline gene expression of the Sham groups was set to 1. Each experiment was repeated at least three times. *P< 0.05, **P< 0.01 (Student's t-test). GFAP: Glial fibrillary acidic protein; ns: not significant; qPCR: quantitative polymerase chain reaction.
Figure 4.
Western blot analyses of GFAP protein expression in the amygdala, periaqueductal gray, prefrontal cortex, hypothalamus, thalamus, ventral & dorsal hippocampus of sham-injured (Sham) and spinal cord injury rats.
(A–G) Representative GFAP immunoblots and semi-quantitative densitometric analyses are shown for the (A) amygdala, (B) periaqueductal grey, (C) prefrontal cortex, (D) hypothalamus, (E) thalamus, dorsal and ventral hippocampus (F and G). Data are the mean ± SEM of two separate experiments, each run in duplicate. *P< 0.05, **P< 0.01 (Student's t-test). GFAP: Glial fibrillary acidic protein; ns: not significant.
The thalamus receives a substantial spinal input and is the critical relay for somatosensory inputs to the cerebral cortex (Yuan et al., 2016); it also receives substantial inputs from spinal recipient brainstem regions, including projections from the ventro-lateral portion of the PAG carrying deep noxious inputs (Floyd et al., 1996), therefore we sought to determine if SCI altered GFAP expression in the thalamus. We report a surprising, and robust decline in GFAP transcript levels at this acute, 24-hour time-point (Figure 3E, t10 = 3.488, P < 0.01), which was further confirmed by protein analyses (Figure 4E, t6 = 2.684, P < 0.01).
GFAP expression in the hippocampus was also significantly reduced in the dorsal hippocampus both at the mRNA (Figure 3F, t10 = 2.500, P < 0.05) and even more robustly at the protein level (Figure 4F, t6 = 4.029, P < 0.01), but not in the ventral hippocampus of SCI rats (Figure 3G and 4G, t10 = 1.474 and t6 = 1.659, P > 0.05).
Acute changes in Iba1 mRNA and protein expression in the rat brain following SCI
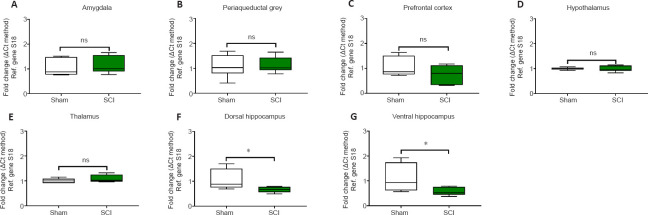
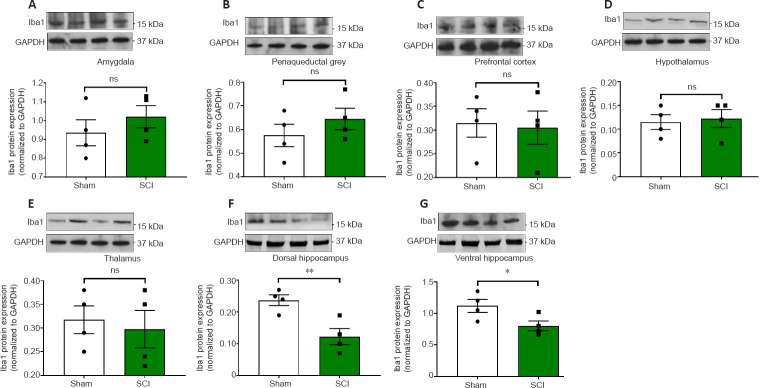
24 hours post SCI the mRNA and protein expression of the microglial marker Iba1 were unaffected in the amygdala (Figures 5A and 6A, t10 = 0.514 and t6 = 0.936, P > 0.05); the PAG (Figures 5B and 6B, t10 = 0.186 and t6 = 1.059, P > 0.05); the prefrontal cortex (Figures 5C and 6C; t10 = 1.342 and t6 = 0.216, P > 0.05); the thalamus (Figures 5E and 6E, t10 = 1.148 and t6 = 0.406, P > 0.05) and the hypothalamus (Figures 5D and 6D, t10 = 0.248 and t6 = 0.307, P > 0.05). In contrast, Iba1 mRNA and protein expression levels in the hippocampus were reduced in both the dorsal hippocampus (Figures 5F and 6F, t10 = 2.292 and t6 = 3.739, P < 0.05 and P < 0.01, respectively) and the ventral hippocampus (Figures 5G and 6G, t10 = 2.251 and t6 = 2.453, P < 0.05).
Figure 5.
Real-time qPCR data showing the differential mRNA expression of Iba1 in the amygdala (A), periaqueductal gray, (B) prefrontal cortex (C), hypothalamus (D), thalamus (E), dorsal and ventral hippocampus (F and G) of sham-injured (Sham) and spinal cord injury rats (SCI).
Target-specific amplificons were obtained using custom-designed primer pairs optimised for qPCR analyses (≤ 150?bp length). Sequences are shown in Table 1. Results shown are the mean fold changes ± SEM obtained from two independent experiments, which were each run in duplicate. Fold changes for the genes of interest were calculated using the comparative ΔΔCt method after normalization to the reference (Ref.) gene 18S. Baseline gene expression of the Sham groups was set to 1. Each experiment was repeated at least three times. *P< 0.05, vs. Sham (Student's t-test). Iba1: Ionized calcium binding adaptor molecule 1; ns: not significant; qPCR: quantitative polymerase chain reaction.
Figure 6.
Western blots analyses of Iba1 protein expression in the amygdala, periaqueductal gray, prefrontal cortex, hypothalamus, thalamus, ventral & dorsal hippocampus of sham-injured (Sham) and spinal cord injury rats.
(A–G) Representative Iba1 immunoblots and semi-quantitative densitometric analyses are shown for the (A) amygdala, (B) periaqueductal grey, (C) prefrontal cortex, (D) hypothalamus, (E) thalamus, dorsal and ventral hippocampus (F & G). Data are the mean ± SEM of two separate experiments. Results were obtained from two independent experiments, each run in duplicate. *P< 0.05, **P< 0.01 (Student's t-test). Iba1: Ionized calcium binding adaptor molecule 1; ns: not significant.
Discussion
In this study, we identified early changes in GFAP and Iba1 mRNA and protein expression levels in the female rat brain following a mild SCI. We interrogated astrocyte- and microglial-specific cell markers, as our main goal was to detect early disruptions within the glial compartment following SCI. Given the complex architectural organization of the brain and the differential involvement of specific brain regions in the development of the comorbidities associated with spinal cord injury our focus was on brain areas controlling the affective, cognitive and sensory responses to traumatic stressors, for these measurements.
There is documented evidence of sexual dimorphism in the behavioral responses to acute stress and as in several types of high order cognitive and affective functions (Rubinow and Schmidt, 2019). Apparently, these differences account for hormonal, sex chromosomes and their interaction with the environment (Rubinow and Schmidt, 2019), with studies indicating that female rats, as opposed to males, develop distinct coping strategies in response to stress (Youssef et al., 2018). Interestingly, in a recent work it has been demonstrated that stressed-susceptible brain regions such as the prefrontal cortex or the hippocampus exhibit higher activation patterns in male vs. female in rats exposed to acute immobilization stress, but not in the forced swimming test (Sood et al., 2018). These data pinpoint the sexually dimorphic response strictly depends on the specific nature of the stressor, a topic that warrants further investigations. In this exploratory study, we utilised female rats as these are conventionally used in spinal cord injury studies due to their better compliance to the surgical procedure compared with male rats (Datto et al., 2015). A further reason was ethical, as in our experience male rats subjected to SCI often develop a severe form of autotomy (self-mutilation) directed to their hind-limbs and sometimes genitalia, an adverse event that we have never observed in female rats. Interestingly, the exaggerated autotomy in male SCI rats also seems to occur after neurectomy of peripheral nerves, as shown in other studies (Wagner et al., 1995).
Our analyses identified regionally specific changes in GFAP gene and protein expression in several supraspinal structures. To contrast, changes in Iba1 expression were restricted to the dorsal and ventral hippocampus, brain regions critical in integrating memory formation, spatial navigation and emotional regulation (Schultz and Engelhardt, 2014). To our knowledge, this is the first evidence describing acute changes in supraspinal GFAP and Iba1 mRNA and protein regulation, 24 hours post-SCI.
SCI and GFAP expression in the brain
Accumulating evidence shows that the activity of astrocytes is crucial in determining the behavioral outputs of both the amygdala and hypothalamus, via a process that involves the selective regulatory activity of specific synapses by activated astrocytes (Martin-Fernandez et al., 2017; Chen et al., 2019). At the cellular level, astrocytes express receptors for both noradrenaline (β2-adrenergic receptors) and glucocorticoids (Hertz et al., 2010; Jauregui-Huerta et al., 2010), each of which play different roles in modulating the calcium influx and ATP release of individual astrocytes (Chen et al., 2019).
During the initial response to an acute stressor such as a traumatic injury, noradrenaline release precedes that of the glucocorticoids, cortisol and/or corticosterone (Pearson-Leary et al., 2015; Chen et al., 2019). This suggests that increased noradrenergic activity likely predominates in the immediate phase of the response to traumatic spinal cord injury. In view of this, and considering the different temporal activation patterns and anatomical distributions of astrocytic β2-adrenergic receptors and glucocorticoids in the brain (Gao et al., 2016), it is possible that the regionally distinct patterns of GFAP mRNA and protein regulation that we observed after SCI might be linked to differential exposure of these discrete brain regions to increasing norepinephrine (NE) and glucocorticoids after the spinal cord injury.
GFAP gene and protein levels were significantly increased in the stress-responsive amygdala and hypothalamus, each of which receives strong afferent drive from the noradrenergic locus coeruleus (Palkovits et al., 1980; Kawakami et al., 1984). The locus coeruleus is reliably activated by acute stressors and it is tempting to suggest that a strong activation of this noradrenergic region immediately following SCI, could lead to significant release of norepinephrine in the amygdala and hypothalamus, leading to increased astrocyte activity, as reflected by the induction of GFAP expression reported here.
In contrast, GFAP transcripts and proteins were reliably decreased in the lateral thalamus. The lateral thalamus, encompassing key somatosensory thalamic relays, is a critical source of somatosensory inputs both between different subcortical areas and the cortex (Herrero et al., 2002). Unlike the amygdala and hypothalamus, the thalamus is not strongly regulated by ascending noradrenergic pathways (Simpson et al., 2006). It is however particularly sensitive to the effects of deafferentation triggered by SCI, and many populations of thalamic neurons respond to SCI by immediately increasing their firing activities (Alonso-Calvino et al., 2016). It is therefore possible that the decline in GFAP mRNAs and proteins we report reflects a compensatory mechanism in which the astrocytes surrounding hyperactive thalamic neurons diminish their activity in an effort to dampen the effects of deafferentation.
The hippocampus is particularly vulnerable to both acute and chronic stressors, including those triggered by physical trauma (Jing et al., 2017). This brain structure has a highly conserved architectural organisation along its dorso-ventral axis (Schultz and Engelhardt, 2014), with the dorsal hippocampus critical for spatial navigation and memory and the ventral hippocampus regulating emotional processing and expression (Amaral and Witter, 1989). Despite the architectural similarities, several studies have pinpointed significant differences in the transcriptional and proteomic profiles of the dorsal and ventral sub-regions of the hippocampus in response to stress (Maggio and Segal, 2009; Pierard et al., 2017; Floriou-Servou et al., 2018). An important observation of this study was that SCI significantly reduced both gene and protein expression of the astrocytic marker GFAP in the dorsal, but not the ventral part of the hippocampus. Reduced GFAP expression has been previously reported in the hippocampus and prefrontal cortex in a rat model of depression (Eldomiaty et al., 2020). By contrast, mild cortical contusion has shown to increase hippocampal GFAP mRNA levels as early as after 12 hours post-injury (Hinkle et al., 1997). In line with our hypothesis that a spinal trauma can predispose an individual to the development of comorbid behavioral dysfunctions, it is perhaps not surprising that GFAP expression is reduced.
SCI and Iba1 expression in the brain
Iba1 is a microglia-specific calcium binding protein both in vitro and in vivo, whose expression reflects cellular polarization state (Ito et al., 1998). In this study, we observed that Iba1 mRNA and protein expression were selectively reduced in the dorsal and ventral regions of the hippocampus after SCI. At first consideration these findings appear counterintuitive, however, the ‘shock’ suffered by these vulnerable brain regions as a consequence of the physical trauma of spinal cord injury, might well-reflect the impacts of the shock evoked in animal models evaluating the central effects of electroconvulsive therapy (Jinno and Kosaka, 2008). Our data suggest that spinal cord trauma triggers immediate plastic changes in the hippocampus that are associated with attenuated microglia activity, in this acute post injury phase. Considering the recently identified role of microglia in synapse turnover (Wang et al., 2020), it is not unreasonable to suggest that attenuated Iba1 expression might reflect the pathological increase in neuronal plasticity that occurs after a traumatic experience such as SCI.
Conclusions
Our data provides evidence for early changes in glial activity in several brain regions involved in the development of behavioral comorbidities following SCI. Glial activity changes show clear regional specificity, and it is the activity of astrocytes that is most strongly affected during this period. We also identified attenuated Iba1 mRNA and protein expression in the hippocampus, which is consistent with rapid and adaptive neuroplasticity in this region. However, whilst the changes in the expression of the glial markers were remarkable, it should be noted that the associated comorbid changes in higher order cognitive functions and affective behaviors may require long-term modulations occurring at molecular, cellular and systemic levels. Therefore, additional investigations addressing the changes in glial activity over time are warranted. Nonetheless, these findings provide the first evidence of early supraspinal glial expression changes following spinal cord injury which could lay the foundations for the subsequent development of affective and cognitive dysfunction that is comorbid with SCI in many individuals (summarized in Table 2).
Table 2.
The topographical disruptions of GFAP and Iba1 mRNA and protein expression levels seen in response to spinal cord injury after 24 hours
| Brain Region | GFAP mRNA | GFAP protein | Iba1 mRNA | Iba1 protein |
|---|---|---|---|---|
| Amygdala | ↑ | ↑ | No change | No change |
| Periaqueductal gray | No change | No change | No change | No change |
| Prefrontal cortex | No change | No change | No change | No change |
| Hypothalamus | ↑ | ↑ | No change | No change |
| Thalamus | ↓↓ | ↓↓ | No change | No change |
| Dorsal hippocampus | ↓ | ↓↓ | ↓ | ↓↓ |
| Ventral hippocampus | No change | No change | ↓ | ↓ |
Arrows indicate the direction (upregulation or downregulation) and statistical significance (one arrow indicates P ≤ 0.05, two arrows if P ≤ 0.01, vs. sham) of the observed changes. GFAP: Glial fibrillary acidic protein; Iba1: Ionized calcium binding adaptor molecule 1.
Acknowledgments
We would like to thank Ms Mercedes Ballesteros (University of Technology Sydney, NSW, Australia) and Ms Sarah Osvath (University of Technology Sydney, NSW, Australia) for the technical support to the researchers of the Laboratory of Cellular and Molecular Neuroscience (LCMN).
Footnotes
C-Editors: Zhao M, Liu WJ, Li CH; T-Editor: Jia Y
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:The University of Technology Sydney (UTS) Start-up Research Grant 2018 funded this study (to AC).
Institutional review board statement: All procedures were carried out with the approval of the University of Technology Sydney Institutional Animal Care and Ethics Committee (UTS ACEC13-0069).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Funding:The University of Technology Sydney (UTS) Start-up Research Grant 2018 funded this study (to AC).
References
- 1.Aguilar J, Humanes-Valera D, Alonso-Calviño E, Yague JG, Moxon KA, Oliviero A, Foffani G. Spinal cord injury immediately changes the state of the brain. J Neurosci. 2010;30:7528–7537. doi: 10.1523/JNEUROSCI.0379-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso-Calviño E, Martínez-Camero I, Fernández-López E, Humanes-Valera D, Foffani G, Aguilar J. Increased responses in the somatosensory thalamus immediately after spinal cord injury. Neurobiol Dis. 2016;87:39–49. doi: 10.1016/j.nbd.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 4.Biering-Sørensen F, Biering-Sørensen M. Sleep disturbances in the spinal cord injured: an epidemiological questionnaire investigation, including a normal population. Spinal Cord. 2001;39:505. doi: 10.1038/sj.sc.3101197. [DOI] [PubMed] [Google Scholar]
- 5.Bowley MP, Drevets WC, Ongür D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 6.Bucolo C, Leggio GM, Maltese A, Castorina A, D’Agata V, Drago F. Dopamine-3 receptor modulates intraocular pressure: implications for glaucoma. Biochem Pharmacol. 2012;83:680–686. doi: 10.1016/j.bcp.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 7.Castorina A, Vogiatzis M, Kang JWM, Keay KA. PACAP and VIP expression in the periaqueductal grey of the rat following sciatic nerve constriction injury. Neuropeptides. 2019;74:60–69. doi: 10.1016/j.npep.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Castorina A, Scuderi S, D’Amico AG, Drago F, D’Agata V. PACAP and VIP increase the expression of myelin-related proteins in rat schwannoma cells: Involvement of PAC1/VPAC2 receptor-mediated activation of PI3K/Akt signaling pathways. Exp Cell Res. 2014;322:108–121. doi: 10.1016/j.yexcr.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Castorina A, D’Amico A, Scuderi S, Leggio G, Drago F, D’Agata V. Dopamine D3 receptor deletion increases tissue plasminogen activator (tPA) activity in prefrontal cortex and hippocampus. Neuroscience. 2013;250:546–556. doi: 10.1016/j.neuroscience.2013.07.053. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Jiang Z, Fu X, Yu D, Huang H, Tasker JG. Astrocytes amplify neuronal dendritic volume transmission stimulated by norepinephrine. Cell Rep. 2019;29:4349–4361.e4. doi: 10.1016/j.celrep.2019.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu K, Lau WM, Lau HT, So KF, Chang RC. Micro-dissection of rat brain for RNA or protein extraction from specific brain region. J Vis. 2007;Exp:269. doi: 10.3791/269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull. 2001;55:585–595. doi: 10.1016/s0361-9230(01)00527-5. [DOI] [PubMed] [Google Scholar]
- 13.Coyle JT, Schwarcz R. Mind glue: implications of glial cell biology for psychiatry. Arch Gen Psychiatry. 2000;57:90–93. doi: 10.1001/archpsyc.57.1.90. [DOI] [PubMed] [Google Scholar]
- 14.Datto JP, Bastidas JC, Miller NL, Shah AK, Arheart KL, Marcillo AE, Dietrich WD, Pearse DD. Female rats demonstrate improved locomotor recovery and greater preservation of white and gray matter after traumatic spinal cord injury compared to males. J Neurotrauma. 2015;32:1146–1157. doi: 10.1089/neu.2014.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidoff GN, Roth EJ, Haughton JS, Ardner MS. Cognitive dysfunction in spinal cord injury patients: sensitivity of the Functional Independence Measure subscales vs neuropsychologic assessment. Arch Phys Med Rehabil. 1990;71:326–329. [PubMed] [Google Scholar]
- 16.Eldomiaty MA, Makarenko O, Hassan ZA, Almasry SM, Petrov P, Elnaggar AM. Contribution of glia cells specifically astrocytes in the pathology of depression: immunohistochemical study in different brain areas. Folia Morphol (Warsz) 2020;79:419–428. doi: 10.5603/FM.a2020.0007. [DOI] [PubMed] [Google Scholar]
- 17.Eng LF. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol. 1985;8(4-6):203–14. doi: 10.1016/s0165-5728(85)80063-1. [DOI] [PubMed] [Google Scholar]
- 18.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25(9-10):1439–51. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- 19.Floriou-Servou A, von Ziegler L, Stalder L, Sturman O, Privitera M, Rassi A, Cremonesi A, Thöny B, Bohacek J. Distinct proteomic, transcriptomic, and epigenetic stress responses in dorsal and ventral hippocampus. Biol Psychiatry. 2018;84:531–541. doi: 10.1016/j.biopsych.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Floyd NS, Keay KA, Bandler R. A calbindin immunoreactive “deep pain’ recipient thalamic nucleus in the rat. Neuroreport. 1996;7:622–626. doi: 10.1097/00001756-199601310-00059. [DOI] [PubMed] [Google Scholar]
- 21.Gao V, Suzuki A, Magistretti PJ, Lengacher S, Pollonini G, Steinman MQ, Alberini CM. Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc Natl Acad Sci U S A. 2016;113:8526–8531. doi: 10.1073/pnas.1605063113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giunta S, Castorina A, Adorno A, Mazzone V, Carnazza ML, D’Agata V. PACAP and VIP affect NF1 expression in rat malignant peripheral nerve sheath tumor (MPNST) cells. Neuropeptides. 2010;44:45–51. doi: 10.1016/j.npep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 23.He F, Sun YE. Glial cells more than support cells? Int J Biochem Cell Biol. 2007;39:661–665. doi: 10.1016/j.biocel.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 25.Hertz L, Lovatt D, Goldman SA, Nedergaard M. Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca(2+)]i. Neurochem Int. 2010;57:411–420. doi: 10.1016/j.neuint.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinkle DA, Baldwin SA, Scheff SW, Wise PM. GFAP and S100beta expression in the cortex and hippocampus in response to mild cortical contusion. J Neurotrauma. 1997;14:729–738. doi: 10.1089/neu.1997.14.729. [DOI] [PubMed] [Google Scholar]
- 27.Honer W, Falkai P, Chen C, Arango V, Mann J, Dwork A. Synaptic and plasticity-associated proteins in anterior frontal cortex in severe mental illness. Neuroscience. 1999;91:1247–1255. doi: 10.1016/s0306-4522(98)00679-4. [DOI] [PubMed] [Google Scholar]
- 28.Hwang IK, Lee CH, Li H, Yoo KY, Choi JH, Kim DW, Kim DW, Suh HW, Won MH. Comparison of ionized calcium-binding adapter molecule 1 immunoreactivity of the hippocampal dentate gyrus and CA1 region in adult and aged dogs. Neurochem Res. 2008;33:1309–1315. doi: 10.1007/s11064-007-9584-6. [DOI] [PubMed] [Google Scholar]
- 29.Imbe H, Kimura A, Donishi T, Kaneoke Y. Chronic restraint stress decreases glial fibrillary acidic protein and glutamate transporter in the periaqueductal gray matter. Neuroscience. 2012;223:209–218. doi: 10.1016/j.neuroscience.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke. 2001;32:1208–1215. doi: 10.1161/01.str.32.5.1208. [DOI] [PubMed] [Google Scholar]
- 31.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1 . Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 32.Jauregui-Huerta F, Ruvalcaba-Delgadillo Y, Gonzalez-Castañeda R, Garcia-Estrada J, Gonzalez-Perez O, Luquin S. Responses of glial cells to stress and glucocorticoids. Curr Immunol Rev. 2010;6:195–204. doi: 10.2174/157339510791823790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jing Y, Bai F, Chen H, Dong H. Acute spinal cord injury diminishes silent synapses in the rat hippocampus. Neuroreport. 2017;28:1139–1143. doi: 10.1097/WNR.0000000000000885. [DOI] [PubMed] [Google Scholar]
- 34.Jinno S, Kosaka T. Reduction of Iba1-expressing microglial process density in the hippocampus following electroconvulsive shock. Exp Neurol. 2008;212:440–447. doi: 10.1016/j.expneurol.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 35.Kawakami F, Fukui K, Okamura H, Morimoto N, Yanaihara N, Nakajima T, Ibata Y. Influence of ascending noradrenergic fibers on the neurotensin-like immunoreactive perikarya and evidence of direct projection of ascending neurotensin-like immunoreactive fibers in the rat central nucleus of the amygdala. Neurosci Lett. 1984;51:225–230. doi: 10.1016/0304-3940(84)90555-x. [DOI] [PubMed] [Google Scholar]
- 36.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy P, Rogers BA. Anxiety and depression after spinal cord injury: a longitudinal analysis. Arch Phys Med Rehabil. 2000;81:932–937. doi: 10.1053/apmr.2000.5580. [DOI] [PubMed] [Google Scholar]
- 38.Krishna V, Andrews H, Jin X, Yu J, Varma A, Wen X, Kindy M. A contusion model of severe spinal cord injury in rats. J Vis. 2013;Exp:50111. doi: 10.3791/50111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li D, Liu X, Liu T, Liu H, Tong L, Jia S, Wang YF. Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes. Glia. 2020;68:878–897. doi: 10.1002/glia.23734. [DOI] [PubMed] [Google Scholar]
- 40.Maggio N, Segal M. Differential modulation of long-term depression by acute stress in the rat dorsal and ventral hippocampus. J Neurosci. 2009;29:8633–8638. doi: 10.1523/JNEUROSCI.1901-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin-Fernandez M, Jamison S, Robin LM, Zhao Z, Martin ED, Aguilar J, Benneyworth MA, Marsicano G, Araque A. Synapse-specific astrocyte gating of amygdala-related behavior. Nat Neurosci. 2017;20:1540–1548. doi: 10.1038/nn.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mor D, Kang JW, Wyllie P, Thirunavukarasu V, Houlton H, Austin PJ, Keay KA. Recruitment of dorsal midbrain catecholaminergic pathways in the recovery from nerve injury evoked disabilities. Mol Pain. 2015;11:50. doi: 10.1186/s12990-015-0049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Motta SC, Carobrez AP, Canteras NS. The periaqueductal gray and primal emotional processing critical to influence complex defensive responses, fear learning and reward seeking. Neurosci Biobehav Rev. 2017;76:39–47. doi: 10.1016/j.neubiorev.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen T, Mao Y, Sutherland T, Gorrie CA. Neural progenitor cells but not astrocytes respond distally to thoracic spinal cord injury in rat models. Neural Regen Res. 2017;12:1885. doi: 10.4103/1673-5374.219051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohsawa K, Imai Y, Sasaki Y, Kohsaka S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J Neurochem. 2004;88:844–856. doi: 10.1046/j.1471-4159.2003.02213.x. [DOI] [PubMed] [Google Scholar]
- 46.Ongür D, Heckers S. A role for glia in the action of electroconvulsive therapy. Harv Rev Psychiatry. 2004;12:253–262. doi: 10.1080/10673220490886185. [DOI] [PubMed] [Google Scholar]
- 47.Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palkovits M, Zaborszky L, Feminger A, Mezey E, Fekete MI, Herman JP, Kanyicska B, Szabo D. Noradrenergic innervation of the rat hypothalamus:experimental biochemical and electron microscopic studies. Brain Res. 1980;191:161–171. doi: 10.1016/0006-8993(80)90320-0. [DOI] [PubMed] [Google Scholar]
- 49.Páv M, Kovárů H, Fiserová A, Havrdová E, Lisá V. Neurobiological aspects of depressive disorder and antidepressant treatment: role of glia. Physiol Res. 2008;57:151–164. doi: 10.33549/physiolres.930990. [DOI] [PubMed] [Google Scholar]
- 50.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. San Diego, CA: Academic Press-Elsevier; 2006. [Google Scholar]
- 51.Pearson-Leary J, Osborne DM, McNay EC. Role of glia in stress-induced enhancement and impairment of memory. Front Integr Neurosci. 2016;9:63. doi: 10.3389/fnint.2015.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 53.Pierard C, Dorey R, Henkous N, Mons N, Beracochea D. Different implications of the dorsal and ventral hippocampus on contextual memory retrieval after stress. Hippocampus. 2017;27:999–1015. doi: 10.1002/hipo.22748. [DOI] [PubMed] [Google Scholar]
- 54.Rubinow DR, Schmidt PJ. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology. 2019;44:111–128. doi: 10.1038/s41386-018-0148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 56.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 57.Schultz C, Engelhardt M. Anatomy of the hippocampal formation. Front Neurol Neurosci. 2014;34:6–17. doi: 10.1159/000360925. [DOI] [PubMed] [Google Scholar]
- 58.Simpson KL, Waterhouse BD, Lin RC. Characterization of neurochemically specific projections from the locus coeruleus with respect to somatosensory-related barrels. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:166–173. doi: 10.1002/ar.a.20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sood A, Chaudhari K, Vaidya VA. Acute stress evokes sexually dimorphic, stressor-specific patterns of neural activation across multiple limbic brain regions in adult rats. Stress. 2018;21:136–150. doi: 10.1080/10253890.2017.1422488. [DOI] [PubMed] [Google Scholar]
- 60.Wagner R, DeLeo JA, Coombs DW, Myers RR. Gender differences in autotomy following sciatic cryoneurolysis in the rat. Physiol Behav. 1995;58:37–41. doi: 10.1016/0031-9384(95)00037-j. [DOI] [PubMed] [Google Scholar]
- 61.Wall P, Egger M. Formation of new connexions in adult rat brains after partial deafferentation. Nature. 1971;232:542–545. doi: 10.1038/232542a0. [DOI] [PubMed] [Google Scholar]
- 62.Wang C, Yue H, Hu Z, Shen Y, Ma J, Li J, Wang XD, Wang L, Sun B, Shi P, Wang L, Gu Y. Microglia mediate forgetting via complement-dependent synaptic elimination. Science. 2020;367:688–694. doi: 10.1126/science.aaz2288. [DOI] [PubMed] [Google Scholar]
- 63.Youssef FF, Bachew R, Bissessar S, Crockett MJ, Faber NS. Sex differences in the effects of acute stress on behavior in the ultimatum game. Psychoneuroendocrinology. 2018;96:126–131. doi: 10.1016/j.psyneuen.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 64.Yuan R, Di X, Taylor PA, Gohel S, Tsai YH, Biswal BB. Functional topography of the thalamocortical system in human. Brain Struct Funct. 2016;221:1971–1984. doi: 10.1007/s00429-015-1018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci. 1998;18:7000–7007. doi: 10.1523/JNEUROSCI.18-17-07000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]