Abstract
This is the first report on the isolation of Lyme disease Borrelia from seabirds on the Faeroe Islands and the characteristics of its enzootic cycle. The major components of the Borrelia cycle include the puffin (Fratercula arctica) as the reservoir and Ixodes uriae as the vector. The importance of this cycle and its impact on the spread of human Lyme borreliosis have not yet been established. Borrelia spirochetes isolated from 2 of 102 sampled puffins were compared to the borreliae previously obtained from seabird ticks, I. uriae. The rrf-rrl intergenic spacer and the rrs and the ospC genes were sequenced and a series of phylogenetic trees were constructed. Sequence data and restriction fragment length polymorphism analysis grouped the strains together with Borrelia garinii. In a seroepidemiological survey performed with residents involved in puffin hunting on the Faeroe Islands, 3 of 81 serum samples were found to be positive by two commonly used clinical tests: a flagellin-based enzyme-linked immunosorbent assay (ELISA) and Western blotting. These three positive serum samples also had high optical density values in a whole-cell ELISA. The finding of seropositive Faeroe Islanders who are regularly exposed to I. uriae indicate that there may be a transfer of B. garinii by this tick species to humans.
Lyme disease, the most prevalent tick-borne zoonosis in North America and Europe, is a multisystemic disorder caused by the spirochete Borrelia burgdorferi sensu lato. In recent years, the taxonomic and phylogenetic relationships between different B. burgdorferi sensu lato species have become more extensive and complicated. Three genomic species of B. burgdorferi sensu lato with human pathogenic relevance are recognized: B. burgdorferi sensu stricto, Borrelia garinii, and Borrelia afzelii (4, 11). The main reservoir hosts for these spirochetes are small mammals and birds (17, 18, 23, 24, 39), with ticks in the Ixodes ricinus complex as the principal vectors (1). Recently, an alternative enzootic cycle involving B. garinii, Ixodes uriae ticks, and seabirds was described (31). On a mammal-free island, B. garinii was isolated from I. uriae, and B. burgdorferi sensu lato DNA was amplified from soft parts of a tick-infected razorbill (Alca torda) (31). In contrast to the terrestrial enzootic cycles of B. garinii, the circulation of Borrelia in the marine environment seems to be primarily restricted to colonial seabirds and the tick species associated with those seabirds, I. uriae (31). We have previously suggested that B. garinii is the species that infects I. uriae ticks in both the northern and the southern hemispheres (32). The occurrence of identical Borrelia flagellin (flaB) genes in the northern and southern hemispheres, as well as within the hemispheres, is compatible with the theory of an exchange of B. garinii by seabirds (9, 32).
The objectives of this study were to investigate the importance of the seabird-I. uriae-B. garinii cycle for the spread of Lyme disease Borrelia and the possibility that humans may acquire Lyme disease from I. uriae.
MATERIALS AND METHODS
Cultivation and isolation of spirochetes.
At the end of July, during the traditional puffin-hunting season at Nólsoy, Faeroe Islands, puffin (Fratercula arctica) blood was collected from the hematoma that forms immediately after cervical dislocation. Blood was inoculated into BSK-II medium (5) supplemented with 10% rabbit serum (Sigma Chemical Co., St. Louis, Mo.) and 1.3% Bacto Gelatin (Difco Laboratories, Detroit, Mich.). To avoid contamination, 100 μg of phosphomycin (Sigma) per ml and 50 μg of sulfamethoxazole (Sigma) per ml were added to the medium. The cultures were incubated at 32°C and were checked weekly by phase-contrast microscopy. Contaminated cultures were purified by filtration with 0.2-μm-pore-size, 115-ml sterile filter units (Nalgene Labware, Nalge Nunc International, Rochester, N.Y.). The origins of the Lyme disease Borrelia strains used in this study are listed in Table 1. For subsequent protein and DNA analyses, the borreliae were grown to the exponential growth phase.
TABLE 1.
Borrelia strains used in this study
| Strain | Origin (reference or source) |
|---|---|
| Far01 | I. uriae, Faeroe Islands (9) |
| Far02 | I. uriae, Faeroe Islands (9) |
| Far03 | Puffin blood, Faeroe Islands (this study) |
| Far04 | Puffin blood, Faeroe Islands (this study) |
| Fis01 | I. uriae, Iceland (9) |
| B31 | ATCC 35210. B. burgdorferi sensu stricto |
| ESP1 | I. ricinus, Spain (R. C. Johnson). B. burgdorferi sensu stricto |
| ACAI | Human isolate, Sweden (2). B. afzelii |
| Ip90 | Ixodes persulcatus, Chabarovsk (21). B. garinii |
| 20047 | I. ricinus, France (J. F. Anderson). B. garinii |
| NT29 | I. persulcatus, Japan (M. Fukunaga). B. garinii |
To investigate whether mammals living in the puffin colony were infected with Borrelia, blood samples from 28 domestic sheep (Ovis aries) were taken, and three mice (Mus musculus) were caught and tested for Borrelia. Tissue samples (kidney, liver, spleen, heart, urinary bladder, and ear) were taken from the three mice, and blood was obtained from two of the mice. The samples from the sheep were collected at the beginning of October, and the samples from the mice were collected at the end of July. All blood samples and biopsy specimens were inoculated into BSK-II medium that was supplemented with antibiotics, rabbit serum, and gelatin as described above.
Protein profiles and Western blot analysis.
Protein analyses were performed with whole-cell Borrelia preparations as described previously (20). Ten micrograms of protein was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Proteins were stained with Coomassie brilliant blue R-250 (Sigma) or were electroblotted onto a Hybond-N nylon filter (Amersham, Little Chalfont, Buckinghamshire, England). Molecular mass standards ranged from 14.3 to 220 kDa (Rainbow markers; Amersham). The Western blot analysis was performed as described by Jonsson et al. (20). FlaB, OspA, OspB, and OspC were characterized with murine monoclonal antibodies H9724 (6), H5332 (7), and 84C (13) and rabbit polyclonal OspC-specific antiserum (9), respectively. Detection was performed with alkaline phosphatase-conjugated secondary antibodies (DAKO, Glostrup, Denmark), and the blot was developed with 5-bromo-4-chloro-3-indolyl phosphate (Sigma) as the substrate.
Serum samples.
The seroepidemiological study included 81 residents from the Faeroe Islands, where puffin hunting is a traditional annual event. All residents who are involved in the puffin hunting on these islands, and who are thereby exposed to I. uriae, were asked to participate. The ages of the participants varied from 12 to 73 years (for men [n = 62], mean ± standard deviation age, 32.4 ± 13.8 years; for women [n = 19], mean ± standard deviation age, 39.6 ± 17.7 years). Half of the blood samples were taken in the spring, before the puffin-hunting season, and the other half were taken after the season ended, in the autumn.
Previously sampled sera from 146 healthy blood donors from a tick-free area in northern Sweden served as a control group (10).
ELISA.
A whole-cell enzyme-linked immunosorbent assay (ELISA) was prepared by coating each well of a microtiter plate overnight at room temperature (RT) with 250 ng of a mixture of Far03, IP90, B31, and ACA1 whole-cell protein preparations in 50 μl of phosphate-buffered saline (PBS). Proteins, prepared as described previously (20), were quantified in a spectrophotometer by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.) according to the manufacturer’s instructions. Unbound proteins were removed by washing three times with PBS (pH 7.4) and once with distilled water before vacuum drying. Serum samples were diluted 1:200 in PBS containing 0.5% nonfat dairy milk and 0.05% Tween 20 (Merck-Schuchardt, Hohenbrunn, Germany). In each of the two wells assigned for the individual sample, 100 μl of diluted serum was placed and the plates were incubated at RT for 2 h. After extensive washing with 0.1 M NaCl containing 0.1% Tween 20, the plates were incubated for 1 h with peroxidase-conjugated rabbit anti-human immunoglobulin G (DAKO) diluted in PBS containing 0.5% nonfat dairy milk and 0.05% Tween 20. The wells were washed again, and 100 μl of citrate buffer (pH 5.0) containing chromogen (500 μg of o-phenylenediamine dihydrochloride per ml) and 0.02% hydrogen peroxide was added to the wells. After incubation in the dark for 60 min, 100 μl of 0.85 M sulfuric acid was added to stop the reaction. The optical density (OD) at 490 nm was measured with a microtiter plate photometer (Microstation; Kebo Biomed, Spånga, Sweden). Positive and negative control sera were included on each plate to standardize the OD values between runs. As a positive control, a mixture of serum from six patients with a diagnosis of Lyme disease was used. The sera were mixed to ensure a large enough volume to cover all the experiments. As a negative control, we used a serum sample from a healthy blood donor that had a low reactivity in the ELISA.
For comparison, the sera from the Faeroe Islanders were tested for antibodies against B. burgdorferi sensu lato flagellin (FlaB) by the flagellin ELISA (Lyme borreliosis ELISA; DAKO) according to the manufacturer’s instructions.
Sera that were positive by the flagellin ELISA were further confirmed to be positive by Western blotting (Lyme disease B. burgdorferi genogroup 2 Western blot IgG; MRL Diagnostics, Cypress, Calif.). Both the flagellin ELISA and the Western blot assay are clinically used for the diagnosis of Lyme disease.
Statistical analyses.
The OD values obtained from the whole-cell ELISA of the Faeroe Islands test group and the Swedish control group were compared by the Mann-Whitney U test. A cutoff level for the whole-cell ELISA was defined at the 95th percentile of the OD values for the control group. Sera with OD values above the cutoff level were considered positive. The proportion of seropositive samples in the two groups was compared by the chi-square test. P values of 0.05 or less were considered to indicate statistical significance. All P values were based on two-sided tests of significance. SPSS (version 7.5) for Windows (SPSS Inc., Chicago, Ill.) was used for the statistical analyses.
Restriction fragment length polymorphism (RFLP) analysis.
Total Borrelia DNA was prepared by standard methods. Briefly, the cultures were centrifuged and the pellet was washed with 50 mM Tris-HCl (pH 7.4). The cells were lysed on ice in 50 mM Tris-HCl (pH 7.4)–0.25% sucrose–50 mM EDTA–1.5 mg of lysozyme (Sigma) per ml–2% SDS. Following proteinase K (Boehringer Mannheim, Mannheim, Germany) digestion at 55°C for 30 min, the DNA was purified by repeated phenol-chloroform extractions and a final extraction with chloroform-isoamyl alcohol (24:1). RNase A (Sigma) was added to a final concentration of 10 μg/ml, the mixture was incubated at RT for 1 h, and the RNase A was removed by a phenol-chloroform and a chloroform-isoamyl alcohol extraction. DNA was precipitated with 2.5 volumes of 99.7% ethanol and 1/10 volume of 3 M sodium acetate (pH 5.2). The pellet was washed with 70% ice-cold ethanol, dried, and resuspended in a minimal volume of TE (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]). The DNA was digested with the HpaI restriction enzyme (Boehringer Mannheim), and the fragments were separated on a 0.7% agarose gel in TBE (89 mM Tris-borate, 89 mM boric acid, 2 mM EDTA) and blotted onto a Hybond N nylon filter. The membranes were prehybridized in a buffer containing 0.25 M Na2HPO4 (pH 7.2), 1% bovine serum albumin, 1 mM EDTA, and 7% SDS for 30 min and hybridized with a 32P-labeled rrf-directed probe (27), 5S94F (5′-GAGTAGGTTATTGCCAGGG-3′), for 2 h at 47°C in the same buffer. Following hybridization, the membranes were washed three times for 15 min each time at 47°C in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS, 2× SSC–0.1% SDS, and 1× SSC–0.1% SDS. After a final rinse in 0.1× SSC at RT, the membranes were exposed on a phosphor screen (Molecular Dynamics Ltd., Kemsing, England).
The variable spacer region between the rrfA and the rrlB genes was amplified by PCR with the flanking primers 5′-CTGCGAGTTCGCGGGAGA-3′ and 5′-TCCTAGGCATTCACCATA-3′ as described previously (35). The PCR products were digested with MseI (New England Biolabs, Beverly, Mass.) and the fragments were separated on a 16% acrylamide–0.8% bisacrylamide gel for 3 h at 100 V (35).
Ribosomal gene organization.
The organization of the rrl and rrf genes was examined by a multiple-primer PCR assay (27). Eight different sized fragments covering the rrlA-rrfA-rrlB region were amplified. The forward primer (5′-GGGAAGCCTTCCTCAAGA-3′) in all PCR mixtures was complementary to a region at the end of the rrl gene. The reverse primers were complementary to different parts of the rrf and rrl genes. Amplicon sizes varied from 166 to 3,090 bp.
PCR amplification, cloning, and DNA sequencing.
The amplification of approximately 600 bp from the ospC gene was performed with the primer set OspC3 (5′-AAGTGC(AG)ATATTAATGACTTTA-3′) and OspC4 (5′-TTTTTTGGACTTTCTGCCACA-3′) as described previously (28). The rrf-rrl intergenic spacer was amplified as described above. The ospC gene and the rrf-rrl intergenic spacer were sequenced with the Cy5-AutoRead sequencing kit (Pharmacia Biotech, Uppsala, Sweden) as described elsewhere (28).
For subsequent sequencing of a 1,432-bp rrs gene fragment, the DNA was amplified with the 16S-F (5′-TTGATCCTGGCTTAGAACT-3′) and 16S-R (5′-CGGGTTAGAATAATAGCTT-3′) oligonucleotides targeting each end of the gene (27). The amplicons were cloned into the pT7Blue T-vector (Novagen, Madison, Wis.) according to the manufacturer’s instructions. Competent Escherichia coli DH5α was transformed with the recombinant plasmids (3). Plasmid clones containing a fragment of the expected size were sequenced by multiple-primer-directed sequencing of the rrs gene. The dye terminator cycle sequencing ready reaction kit (Perkin-Elmer, Foster City, Calif.) and an automatic sequencer (ABI PRISM 377 DNA sequencer; Perkin-Elmer) were used.
Nucleotide sequences and the deduced amino acid sequences of OspC were aligned with sequences available in the databases with the Clustal V software (16) and with VSM software, version 2.0, written by Ruimy et al. (36). Evolutionary distances were computed with the molecular evolutionary genetics analysis (MEGA) software (22). Phylogenetic trees were constructed by the neighbor-joining method (37) and the unweighted pair group method with mathematical averages with MEGA software. Confidence intervals were assessed by bootstrap analysis (1,000 replicates).
PCR conditions.
DNA amplifications were carried out in a 50-μl solution containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 200 μM deoxynucleoside triphosphates, 1 U of Taq DNA polymerase (Boehringer Mannheim), and 10 pmol of each primer. Chromosomal DNA or boiled Borrelia cultures were used as templates. The cycling conditions were 94°C for 30 s, 55°C for 1 min, and 72°C for 1 min for a total of 35 cycles in an automated thermal incubator (Perkin-Elmer). For amplification of fragments larger than 1.5 kb, the extension time at 55°C was 2 min. The amplified DNA was visualized by agarose gel electrophoreses and subsequent ethidium bromide staining.
Nucleotide sequence accession numbers.
All sequences were deposited in the EMBL database and were assigned the following accession numbers: for rrs sequences, AJ009749 (Far01), AJ009750 (Far02), AJ009752 (Far03), AJ009753 (Far04), and AJ009751 (Fis01); for ospC sequences, AF080264 (Far02) and AF080263 (Far03); and for rrf-rrl intergenic spacer sequences, AF080262 (Far02) and AF080261 (Far03).
RESULTS
Isolation and characterization of spirochetes from I. uriae and puffins.
Spirochetes were isolated from the blood of 2 of 102 sampled puffins. Approximately two-thirds of the cultures became heavily contaminated and were discarded. The two isolates that were obtained were successfully purified by filtration before further investigation. No ticks were found on the 28 sheep or the three mice examined, and no spirochetes were found in the cultures from blood or biopsy specimens from these mammals.
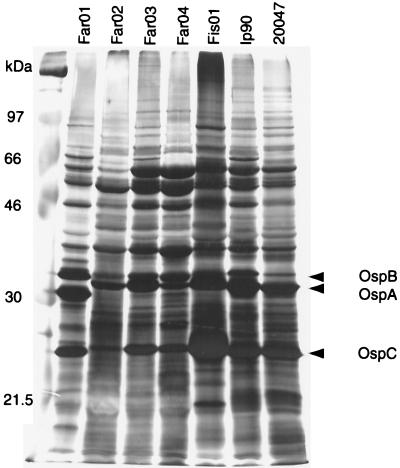
Whole-cell lysates of Borrelia were analyzed by SDS-PAGE, and the protein profiles are presented in Fig. 1. The expression of FlaB, OspA, OspB, and OspC was investigated by Western blot analysis with the monoclonal antibodies H9724, H5332, and 84C and OspC-specific antiserum, respectively (data not shown). Reactivities with monoclonal and polyclonal antibodies to the respective proteins are depicted with arrowheads in Fig. 1. All isolates expressed FlaB, OspA, OspB, and OspC.
FIG. 1.
Borrelia whole-cell protein preparations separated on SDS–12.5% polyacrylamide gels and silver stained.
Genetic characterization of ribosomal genes.
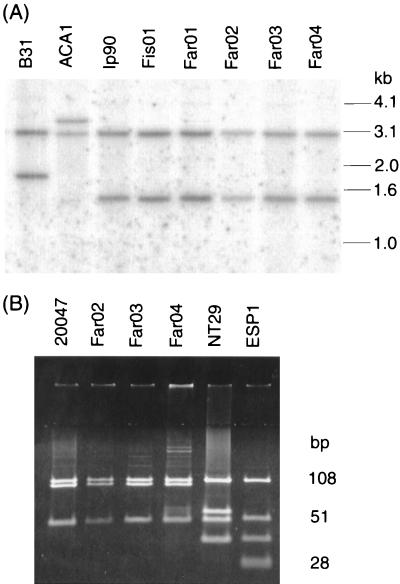
Strains Far01, Far02, Far03, Far04, and Fis01 had typical B. garinii RFLP patterns when the HpaI-digested genomic DNA was hybridized with an rrf-directed probe (25, 38) (Fig. 2A). Furthermore, separation of the MseI restriction fragments of the rrf-rrl intergenic spacer amplicons showed a pattern typical for European B. garinii isolates (35) (Fig. 2B).
FIG. 2.
RFLP analysis. (A) Hybridization of HpaI-digested total Borrelia DNA with an rrl-directed probe. (B) PCR-amplified rrf-rrl intergenic spacer fragments digested with MseI and separated by PAGE.
A multiple-primer PCR assay revealed that the ribosomal gene organization of the isolates was identical to that described previously for B. burgdorferi sensu lato, i.e., rrs, followed by a tandem repeat of rrl and rrf (38).
Nucleotide sequence analysis of rrs and ospC genes and rrf-rrl intergenic spacer.
The four isolates from the Faeroe Islands and the Icelandic isolate were subjected to rrs gene sequencing, and a phylogenetic tree was constructed (Fig. 3). The isolates had a mutual identity within the 1,393-bp rrs sequence. Phylogenetic analysis grouped the rrs sequence together with B. garinii strains. Four unique substitutions in the rrs sequence were found at positions 183, 185, 186, and 257 according to the numbering for E. coli rrsB. The T at position 185 and the G at position 186 are unique to B. burgdorferi sensu lato. The G at position 183 and the G at position 257 are not found in any other B. garinii rrs sequence in the nucleotide sequence databases. The ospC gene and the rrf-rrl intergenic spacer in the two strains isolated from ticks and birds (Far02 and Far03, respectively) were sequenced. Both the ospC and the rrf-rrl intergenic spacer sequences from the two strains were identical. The phylogenetic trees obtained from the rrf-rrl intergenic spacer sequences (Fig. 4) and from the ospC sequences (data not shown) were consistent with the tree obtained from the rrs sequences. Isolates Far02 and Far03 appeared on the same phylogenetic branch as the B. garinii species.
FIG. 3.
Phylogenetic tree based on a comparison of the rrs sequences and constructed by the neighbor-joining method. Bootstrap values are shown (as percentages) for each branch. The accession numbers of the sequences are noted. An arrow indicates the sequence obtained in this study.
FIG. 4.
Phylogenetic tree based on a comparison of the rrf-rrl intergenic spacer sequences constructed by the neighbor-joining method. Bootstrap values are shown (as percentages) for each branch. The accession numbers of the sequences are noted. Arrows indicate sequences obtained in this study.
Serologic investigation of the Faeroe Island human population in contact with I. uriae.
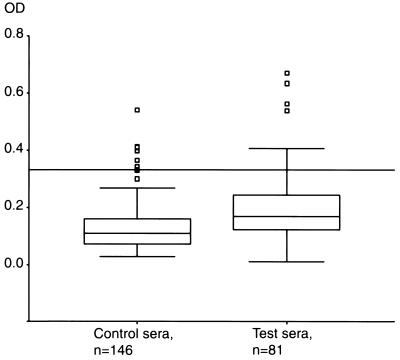
Among the 81 serum samples from I. uriae-exposed Faeroe Islanders, 3 reacted strongly in all the tests that were performed. These sera gave high OD values in the whole-cell ELISA and fulfilled the criteria for a positive diagnosis by both the flagellin ELISA and Western blotting. A total of seven serum samples were positive by the flagellin ELISA. Four of them reacted mainly with flagellin (FlaB) and with two proteins at 60 and 75 kDa in the Western blot. The OD values obtained by the whole-cell ELISA (Fig. 5) were significantly higher for the Faeroe Islands test group than for the Swedish control group (Mann-Whitney U test; P < 0.001). The 95th percentile cutoff level for the control group (OD, 0.331) resulted in 11 (13.6%) positive serum samples in the test group and six (4.1%) positive serum samples in the control group, which was a significant difference (Pearson chi-square value, 6.7; one degree of freedom; P = 0.009). Two serum samples in the test group were positive by the flagellin ELISA, Western blotting and the whole-cell ELISA. One additional serum sample was positive by the two commercial tests and by the whole-cell ELISA had an OD value (0.279) that was close to the cutoff level. We did not find gender, age, or time of sampling to be confounding factors.
FIG. 5.
Box plot of whole-cell ELISA OD values. The tops and the bottoms of the boxes define the first and the third quartiles, respectively. The horizontal lines inside the boxes indicate the median. The error bars define extreme values, and the squares define outliers. The reference line crossing the figure indicates the cutoff level for positive sera (OD, 0.331).
DISCUSSION
Previously, we have shown that seabirds may be reservoirs for Lyme disease Borrelia and may be important for the spread of this spirochete (32). To further investigate this, genotypic and phenotypic analyses were performed with Borrelia strains isolated from I. uriae and puffins from the Faeroe Islands. We believe that the Faeroe Islands are a part of the area of endemicity for the Atlantic Lyme disease Borrelia. The major participants in this marine enzootic cycle are abundantly breeding seabirds, such as the puffins, I. uriae as the vector, and the B. garinii spirochete.
The puffin is a common seabird on the Faeroe Islands, with colonies along the slopes often maintaining several thousand pairs. These dense colonies may facilitate the transmission of ectoparasites (12). The small number of isolates detected in this study is probably not a reflection of a low proportion of infected birds, but it is more likely due to sampling difficulties since this area has large numbers of infected I. uriae ticks. In a previous study, we detected B. burgdorferi sensu lato DNA in 32% of the ticks on Nólsoy, Faeroe Islands (32). This paradox, with a large number of infected vectors and a small number of positive cultures for specimens from the reservoir, may have several explanations. First, contamination of the cultures was a serious problem because inoculation was performed under field conditions. Second, the reservoir hosts, in this case, puffins, may be spirochetemic for shorter periods than mammals. In previous studies, chickens and canary finches were spirochetemic only for a short period of time following experimental infection (8, 33, 34). Third, it is easier to isolate spirochetes from the skin than from blood (40), and since we did not sample the skin, we may have underestimated the number of infected puffins.
Seabirds are natural hosts for I. uriae, although the tick is known to bite a variety of mammals including humans when given the opportunity (29). However, the rate of survival of I. uriae was shown to decrease after feeding on hosts other than seabirds (30). There are unlikely to be any other significant Borrelia reservoirs other than seabirds on the Faeroe Islands. Rodents and sheep are present only in small numbers throughout the puffin colonies (19). B. burgdorferi sensu lato was found in neither the cultures of blood and organs from three mice nor 28 cultures of blood from sheep. This could be due to the fact that culturing from blood is not a sensitive method for Borrelia detection and that only a limited number of mice were tested. However, this could indicate that mammals do not play a major role in the maintenance of B. burgdorferi sensu lato on the Faeroe Islands.
A number of different species of ticks have been identified as potential vectors of Lyme disease Borrelia. The tick species that belong to the I. ricinus complex are most important for the maintenance of the Lyme disease cycle. Migrating birds have been shown to occasionally carry I. ricinus, and there are reports of I. ricinus on the Faeroe Islands (15, 19). However, there is to our knowledge no established I. ricinus population on the Faeroe Islands. No I. ricinus was found in the puffin colony at Nólsoy over two summers, despite thorough investigations by flagging with a flannel cloth (data not shown). Further investigations at other times of the year and at different locations need to be performed. In contrast, I. uriae is prevalent on the islands, and the puffins are known to be heavily infested. This leads us to suggest that puffins are the most likely reservoir of Borrelia on the Faeroe Islands since they are so abundant and are closely associated with I. uriae ticks. The role of seabirds as reservoirs is further supported by previous findings of B. garinii in a razorbill (Alca torda) infested with I. uriae on a mammal-free island in the Baltic Sea (9, 31).
The numbers of B. burgdorferi sensu lato isolates from birds are few and are dominated by isolates from passerine birds. In this study we have successfully isolated spirochetes from puffins, and to our knowledge, this is the first report of isolates from a wild, nonpasserine species. Determination of which Borrelia genospecies can be found in seabirds is of particular interest. The isolates that we obtained were all shown to be B. garinii by RFLP analysis and DNA sequencing of parts of the rrf-rrl intergenic spacer and the ospC and the rrs genes. The RFLP analyses used in this study have previously been shown to readily discriminate between different B. burgdorferi sensu lato isolates (25, 35, 38). Construction of phylogenetic trees grouped all sequenced parts of the genome together with B. garinii.
The approximately 580-bp ospC sequence fragments from strains Far02 and Far03 differed by less than six nucleotides from Central European isolates M57 and N34 (26, 41), and this corresponds to a single amino acid substitution in the deduced sequence. This indicates a possible contact between the marine and the terrestrial Borrelia cycles by lateral transfer and recombination of the ospC gene (26). Although the marine enzootic cycle is separated from the terrestrial cycle, there are areas where interactions such as DNA transfer could theoretically occur (9).
The finding of B. burgdorferi sensu lato in the puffin population and in I. uriae on the Faeroe Islands prompted us to initiate a serological survey of the human population. People involved in the traditional puffin hunting were selected since they are regularly exposed to the Borrelia vector I. uriae. Using a number of techniques, we determined that the Faeroe Islanders had elevated titers of antibodies against B. burgdorferi sensu lato compared to those for a Swedish control group; however, none of them had symptoms of Lyme disease. To discriminate between cross-reactive and genuine Borrelia antibody responses, the use of an ELISA in concert with Western blotting increased the specificity (14). These results indicate a possible transmission of B. garinii or, at least, B. garinii antigens to humans. However, the low rate of Lyme disease on the Faeroe Islands may be explained by the residents’ protective clothing, which prevents tick bites. The ecology of Lyme disease is complicated and involves different reservoirs and tick vectors. The importance of birds, particularly seabirds, as a vehicle for Borrelia-infected ticks and of B. burgdorferi sensu lato on the Faeroe Islands is evident. Our findings indicate a transfer of B. garinii to humans by the vector I. uriae. The epidemiological and clinical importance of B. garinii on the Faeroe Islands and other regions with similar ecological components needs further investigation.
ACKNOWLEDGMENTS
Jens-Kjeld Jensen and Jonas Bonnedahl are acknowledged for practical help during the fieldwork on the Faeroe Islands. We are indebted to Jana Jass, Pierre Martin, and Dominic McCafferty for critical reading of the manuscript. The monoclonal antibodies H9724 and H5332 and the OspC-specific antiserum were kindly provided by Alan G. Barbour. Monoclonal antibody 84C was a kind gift from Denee D. Thomas. We also thank Göran Hallmans and Sören Holmgren for kindly providing the serum samples from the Swedish blood donors and Katharina Ornstein for providing the serum samples from Lyme disease patients. Mats Sellin is acknowledged for valuable advice concerning ELISA.
This work was supported by the Swedish Medical Research Council (grant 07922), the Swedish Council for Forestry and Agricultural Research (grant 23.0161), and Fródskaparsetur Føroya. N.M.R. was supported by the Commission of the European Communities (ERBFMBI-CT96-0684).
REFERENCES
- 1.Anderson J F. Epizootiology of Borrelia in Ixodes tick vectors and reservoir hosts. Rev Infect Dis. 1989;11:1451–1459. doi: 10.1093/clinids/11.supplement_6.s1451. [DOI] [PubMed] [Google Scholar]
- 2.Åsbrink E, Hovmark A, Hederstedt B. The spirochetal etiology of acrodermatitis chronica atrophicans Herxheimer. Acta Dermatol Venereol. 1984;64:506–512. [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1992. [Google Scholar]
- 4.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J C, Assous M, Grimont P A D. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 5.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 6.Barbour A G, Hayes S F, Heiland R A, Schrumpf M E, Tessier S L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986;52:549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbour A G, Tessier S L, Todd W J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983;41:795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop K L, Khan M I, Nielsen S W. Experimental infection of northern bobwhite quail with Borrelia burgdorferi. J Wildl Dis. 1994;30:506–513. doi: 10.7589/0090-3558-30.4.506. [DOI] [PubMed] [Google Scholar]
- 9.Bunikis J, Olsen B, Fingerle V, Bonnedahl J, Wilske B, Bergström S. Molecular polymorphism of the Lyme disease agent Borrelia garinii in northern Europe is influenced by a novel enzootic Borrelia focus in the North Atlantic. J Clin Microbiol. 1996;34:364–368. doi: 10.1128/jcm.34.2.364-368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunikis J, Olsén B, Westman G, Bergström S. Variable serum immunoglobulin responses against different Borrelia burgdorferi sensu lato species in a population at risk for and patients with Lyme disease. J Clin Microbiol. 1995;33:1473–1478. doi: 10.1128/jcm.33.6.1473-1478.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canica M M, Nato F, du Merle L, Maize J C, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- 12.Clifford C M. Tick-borne viruses of seabirds. In: Kurstak E, editor. Arctic and tropical arboviruses. New York, N.Y: Academic Press, Inc.; 1979. pp. 83–100. [Google Scholar]
- 13.Comstock L E, Fikrig E, Shoberg R J, Flavell R A, Thomas D D. A monoclonal antibody to OspA inhibits association of Borrelia burgdorferi with human endothelial cells. Infect Immun. 1993;61:423–431. doi: 10.1128/iai.61.2.423-431.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 15.Hallas T E. Skovflåt fundet på Færøerne. Dansk Vet Tidsskr. 1990;73:1089–1090. [Google Scholar]
- 16.Higgins D G, Sharp P M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 17.Humair P F, Postic D, Wallich R, Gern L. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochetes. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1998;287:521–538. [PubMed] [Google Scholar]
- 18.Humair P F, Vittoz N, Siegenthaler M, Aeschlimann A, Gern L. Mammalian and avian reservoirs for Borrelia burgdorferi in a Lyme borreliosis focus in Switzerland. Rev Suisse Zool. 1990;97:783. [Google Scholar]
- 19.Jensen, J.-K. Personal communication.
- 20.Jonsson M, Noppa L, Barbour A G, Bergström S. Heterogeneity of outer membrane proteins in Borrelia burgdorferi: comparison of osp operons of three isolates of different geographic origins. Infect Immun. 1992;60:1845–1853. doi: 10.1128/iai.60.5.1845-1853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kriuchechnikov V N, Korenberg E I, Shcherbakov S V, Kovalevskii I V, Levin M L. Identification of Borrelia isolated in the USSR from Ixodes persulcatus Schulze ticks. Zh Mikrobiol Epidemiol Immunobiol. 1988;12:41–44. [PubMed] [Google Scholar]
- 22.Kumar S, Tamura K, Masatoshi N. MEGA: molecular evolutionary genetics analysis, version 1.01. University Park: The Pennsylvania State University; 1993. [Google Scholar]
- 23.Kurtenbach K, Peacey M, Rijpkema S G T, Hoodless A N, Nuttall P A, Randolph S E. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl Environ Microbiol. 1998;64:1169–1174. doi: 10.1128/aem.64.4.1169-1174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lane R S, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- 25.Liveris D, Gazumyan A, Schwartz I. Molecular typing of Borrelia burgdorferi sensu lato by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol. 1995;33:589–595. doi: 10.1128/jcm.33.3.589-595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livey I, Gibbs C P, Schuster R, Dorner F. Evidence for lateral transfer and recombination in OspC variation in Lyme disease Borrelia. Mol Microbiol. 1995;18:257–269. doi: 10.1111/j.1365-2958.1995.mmi_18020257.x. [DOI] [PubMed] [Google Scholar]
- 27.Marconi R T, Liveris D, Schwartz I. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J Clin Microbiol. 1995;33:2427–2434. doi: 10.1128/jcm.33.9.2427-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marti Ras N, Postic D, Foretz M, Baranton G. Borrelia burgdorferi sensu stricto, a bacterial species “made in the U.S.A.”? Int J Syst Bacteriol. 1997;47:1112–1117. doi: 10.1099/00207713-47-4-1112. [DOI] [PubMed] [Google Scholar]
- 29.Mehl R, Traavik T. The tick Ixodes uriae (Acari: Ixodidae) in seabird colonies in Norway. Fauna Norv Ser B. 1983;30:94–107. [Google Scholar]
- 30.Nuttall G H F. Observations on the biology of Ixodidae. Parasitology. 1913;6:68–118. [Google Scholar]
- 31.Olsén B, Jaenson T G T, Noppa L, Bunikis J, Bergström S. A Lyme borreliosis cycle in seabirds and Ixodes uriae ticks. Nature. 1993;362:340–342. doi: 10.1038/362340a0. [DOI] [PubMed] [Google Scholar]
- 32.Olsen B, Duffy D C, Jaenson T G T, Gylfe Å, Bonnedahl J, Bergström S. Transhemispheric exchange of Lyme disease spirochetes by seabirds. J Clin Microbiol. 1995;33:3270–3274. doi: 10.1128/jcm.33.12.3270-3274.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen B, Gylfe Å, Bergström S. Canary finches (Serinus canaria) as an avian infection model for Lyme borreliosis. Microb Pathog. 1996;20:319–324. doi: 10.1006/mpat.1996.0030. [DOI] [PubMed] [Google Scholar]
- 34.Piesman J, Dolan M C, Schriefer M E, Burkot T R. Ability of experimentally infected chickens to infect ticks with the Lyme disease spirochete, Borrelia burgdorferi. Am J Trop Med Hyg. 1996;54:294–298. doi: 10.4269/ajtmh.1996.54.294. [DOI] [PubMed] [Google Scholar]
- 35.Postic D, Assous M V, Grimont P A D, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 36.Ruimy R, Breittmayer V, Elbaze P, Lafay B, Boussemart O, Gauthier M, Christen R. Phylogenetic analysis and assessment of the genera Vibrio, Photobacterium, Aeromonas, and Plesiomonas deduced from small-subunit rRNA sequences. Int J Syst Bacteriol. 1994;44:416–426. doi: 10.1099/00207713-44-3-416. [DOI] [PubMed] [Google Scholar]
- 37.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz J J, Gazumyan A, Schwartz I. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 1992;174:3757–3765. doi: 10.1128/jb.174.11.3757-3765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tälleklint L, Jaenson T G T. Transmission of Borrelia burgdorferi s.l. from mammal reservoirs to the primary vector of Lyme borreliosis, Ixodes ricinus (Acari: Ixodidae), in Sweden. J Med Entomol. 1994;31:880–886. doi: 10.1093/jmedent/31.6.880. [DOI] [PubMed] [Google Scholar]
- 40.Wilske B, Preac-Mursic V. Microbiological diagnosis of Lyme borreliosis. In: Weber K, Burgdorfer W, editors. Aspects of Lyme borreliosis. Berlin, Germany: Springer-Verlag; 1993. pp. 270–272. [Google Scholar]
- 41.Wilske B, Jauris-Heipke S, Lobentanzer R, Pradel I, Preac-Mursic V, Rossler D, Soutschek E, Johnson R C. Phenotypic analysis of outer surface protein C (OspC) of Borrelia burgdorferi sensu lato by monoclonal antibodies: relationship to genospecies and OspA serotype. J Clin Microbiol. 1995;33:103–109. doi: 10.1128/jcm.33.1.103-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]