Ischemic stroke is a cerebrovascular disease with a high risk of mortality and long-lasting neurological disabilities. Medical advances have resulted in a declining trend in stroke incidence and deaths in the United States. However, increasing age and risk factors such as diabetes have contributed to an increased lifetime risk of stroke. Particularly, there is a dramatic worldwide increase in the incidence of type 2 diabetes mellitus (T2DM) and sustained increase in T2DM prevalence is projected over the next decade. T2DM is a chronic disease which increases the risk of stroke incidence, stroke recurrence and hospital readmission, and stroke related mortality and morbidity. Ischemic stroke patients with diabetes exhibit worse stroke outcome largely derived from metabolic abnormalities, extensive injury to the cerebral vasculature, white matter injury and an amplified inflammatory milieu that hinders recovery. Thus, there is a compelling need for clinical and research effort to mitigate the burden of diabetic-stroke related complications and improve stroke outcome in the diabetic population.
Ischemic stroke induced neural damage and death is rapid which limits the time frame for successful intervention with thrombolytics or other neuroprotective therapeutics. Even when T2DM stroke patients’ receive tissue plasminogen activator treatment within the stipulated time frame, they still face an increased risk of death, intracerebral hemorrhage and unfavorable 90-day outcome proportional to their admission hyperglycemia. Therefore, novel therapeutics which is effective when administered at sub-acute time points after stroke is necessary. Over the last decade, stem cells and exosomes have emerged as promising treatment options that amplify endogenous repair mechanisms in the ischemic brain when administered within a few hours or even days after stroke (Zhang et al., 2019b; Kalluri and LeBleu, 2020). Exosomes are membrane bound, small extracellular vesicles ranging ~40–160 nm in diameter that contain and transport proteins, lipids, metabolites, and nucleic acids like DNA, RNA, mRNA and microRNAs (miRs) (Zhang et al., 2019b; Kalluri and LeBleu, 2020). Exosomes play an important role in intercellular communication within the brain as well as between the brain and peripheral organs under normal physiological conditions (Zhang et al., 2019b; Kalluri and LeBleu, 2020). Interestingly, the therapeutic effects of intravenously administered mesenchymal stromal cells (MSCs) in rats subjected to stroke were found to be largely driven by exosomes and their cargo miRs (Xin et al., 2012, 2013). Exosomes administered intravenously provide therapeutic benefit at least equivalent to their cellular source, and carry low risk of vascular obstruction, immunogenicity and tumor formation (Xin et al., 2012, 2013; Doeppner et al., 2015; Zhang et al., 2019b). Therefore, exosomes are a novel and promising therapeutic agent to treat ischemic stroke. In non-diabetic rats subjected to stroke, both MSCs and MSC derived exosomes (MSC-Exo) treatment were found to improve neurological outcome by increasing neurogenesis, angiogenesis, and neurovascular remodeling in the ischemic brain (Xin et al., 2013; Doeppner et al., 2015; Zhang et al., 2019a). While the neuroprotective and neurorestorative effects of MSCs and MSC-Exo have been well studied using healthy, non-diabetic rodents, there is a paucity of pre-clinical evidence supporting the use of exosome therapy in diabetic stroke. In this article, we specifically discuss recent studies from our lab demonstrating the therapeutic efficacy and mechanisms of exosome treatment for diabetic stroke.
We employed exosomes harvested from MSCs obtained from T2DM rats (T2DM-MSC-Exo) as a treatment for ischemic stroke in T2DM rats as well as compared therapeutic efficacy with exosomes harvested from bone marrow stromal cells obtained from non-DM rats (Nor-MSC-Exo) (Venkat et al., 2020). We found that intravenous administration of T2DM-MSC-Exo at 3 days after stroke in T2DM rats significantly improves neurological function evaluated using a modified neurological severity score and adhesive removal test compared to Nor-MSC-Exo treated or vehicle treated control T2DM stroke rats, and there was no difference in behavioral outcome between rats treated with Nor-MSC-Exo and vehicle. These data are consistent with our previous study in which treatment of T1DM stroke rats with DM-MSCs improved neurological functional outcome while treatment of T1DM stroke with Nor-MSCs did not improve neurological outcome or promote neurorestorative effects (Cui et al., 2016). Further studies are required to characterize the exosomal cargo in both exosome populations i.e. derived from non-DM and DM-MSCs. Stroke with diabetic comorbidity is known to increase pathological vascular remodeling, hemorrhagic transformation, white matter damage and inflammation in the brain. It is likely that increased blood brain barrier leakage and brain hemorrhage override potential beneficial effects of Nor-MSC/Nor-MSC-Exo in DM stroke. T2DM-MSC-Exo treatment significantly increases tight junction protein expression and decreases blood brain barrier leakage, reduces hemorrhage and attenuates post-stroke weight loss compared to control T2DM stroke rats (Venkat et al., 2020). In addition to exacerbated neurovascular injury, white matter injury including axon damage/loss, demyelination, and impaired oligodendrogenesis contributes to poor post stroke recovery in diabetic stroke. Both diabetes and ischemic stroke stimulate systemic and cerebrovascular inflammatory responses which in turn increase susceptibility of brain cells to damage and death, aggravate blood brain barrier disruption, impair oligodendrogenesis and inhibit white matter repair. Therefore, modulating the neuroinflammatory status in the ischemic brain such that it is conducive for white matter remodeling is essential for long term functional recovery. T2DM-MSC-Exo treatment significantly decreases the number of M1 macrophages and activates microglia and reduces the expression of inflammatory factors such as matrix mettaloproteinase-9 and monocyte chemoattractant protein-1 in the ischemic brain compared to control T2DM-stroke rats. Increased matrix mettaloproteinase-9 has been implicated in aggravating white matter damage as well as endothelial permeability in diabetic stroke. T2DM-MSC-Exo treatment significantly reduces white matter injury and increases axon and myelin density as well as increases the number of oligodendrocytes and oligodendrocyte progenitor cells in the ischemic brain of T2DM stroke rats which in concert, likely contribute at least in part to the observed improvement in neurological function.
The mechanisms by which T2DM-MSC-Exo treatment improves stroke outcome in T2DM rats may in part involve decreasing miR-9 expression and upregulating the adenosine triphosphate-binding cassette transporter 1 (ABCA1) and insulin-like growth factor 1 receptor 1 pathways (Venkat et al., 2020). MiR-9 is highly expressed in the developing and adult vertebrate brain and is known to exert both protective and detrimental effects under pathological conditions. While miR-9 expression in the circulation is low under normal physiological conditions, diabetes and ischemic stroke are known to increase serum and serum exosomal miR-9 expression, respectively (Ji et al., 2016). Since miR-9 is specific to the adult central nervous system, increased expression of miR-9 in the circulation may serve as an indicator of neural damage and neurotoxicity. In ischemic stroke patients, elevated miR-9 expression in the circulation is associated with increased infarct volume, inflammatory status and poor neurological outcome (Ji et al., 2016). We also found that stroke in T2DM rats significantly increases serum miR-9 expression, and treatment with T2DM-MSC-Exo significantly decreases serum miR-9 expression (Venkat et al., 2020). In the ischemic brain of T2DM stroke rats, miR-9 is expressed by neurons, endothelial cells and astrocytes (Venkat et al., 2020). ABCA1 and insulin like growth factor 1 (IGF1) are downstream targets of miR-9. ABCA1 is highly expressed in neurons, oligodendrocytes and glial cells and plays an important role in maintaining neurovascular function in the brain. Since ABCA1 mediates cholesterol transport in the central nervous system and is required for myelination, dendrite outgrowth and synaptic activity, its deficiency aggravates white matter injury in the ischemic brain and contributes to worse neurological functional deficits after stroke (Cui et al., 2015). IGF1 decreases cholesterol efflux via ABCA1 and scavenger receptor class B type I expression and is also known to mediate brain growth and development. Under ischemic conditions, IGF1 evokes neurorestorative effects such as myelination, neurogenesis and oligodendrogenesis (Sanchez-Bezanilla et al., 2020). T2DM-MSC-Exo treatment significantly increases ABCA1 and insulin-like growth factor 1 receptor 1 expression in the ischemic brain of T2DM stroke rats compared to vehicle treated T2DM stroke rats (Venkat et al., 2020). In addition, overexpression of miR-9 in T2DM-MSC-Exo (miR9+/+ T2DM-MSC-Exo) fails to improve neurological function or blood brain barrier integrity and significantly attenuates T2DM-MSC-Exo treatment induced white matter remodeling, oligodendrogensis and anti-inflammatory responses in the ischemic brain. However, miR9+/+-T2DM-MSC-Exo treatment improves myelination in T2DM-MCAo rats (Venkat et al., 2020). Therefore, it is likely that miR-9 and its targets such as ABCA1 only partially contribute to T2DM-MSC-Exo treatment induced neurorestorative effects, and it is likely that other signaling pathways are also involved. Detailed proteomics and RNA analysis may provide insights into the tapestry of therapeutic benefits mediated by T2DM-MSC-Exo in T2DM stroke and further studies are warranted.
In our previous studies, we found that T2DM mice subjected to stroke exhibit significantly reduced serum and brain miR-126 compared to non-diabetic stroke mice. Therefore, we tested the efficacy of exosomes derived from endothelial cells (EC-Exo) as well as exosomes harvested from human umbilical cord blood derived CD133+ cells (CD133+Exo), both of which are rich in miR-126 (Venkat et al., 2019, 2021) on the treatment of ischemic stroke in T2DM animals. In these studies, we employed BKS.Cg-m+/+Leprdb/J (db/db) mice, which is a widely used mouse model of T2DM. Since subcortical infarction is frequent in diabetic stroke, we subjected db/db mice to photothrombotic stroke in which occlusion of small cerebral vessels generates highly reproducible cortical infarcts. We found that intravenous administration of EC-Exo or CD133+Exo at 3 days after stroke in T2DM mice significantly improves neurological function as well as cognitive outcome at 1 month after stroke when compared to vehicle treated T2DM stroke mice. Interestingly, T2DM-stroke mice treated with EC-Exo that were deficient in miR-126, significantly attentuated EC-Exo treatment derived neurorestorative effects (Venkat et al., 2019). By fluorescently labeling exosomes, we demonstrated that intravenously injected exosomes pass the blood brain barrier, home to the ischemic brain, and are taken up by endogenous brain cells such as neurons, endothelial cells, macrophages, and microglia (Venkat et al., 2019). In the brain region immediately surrounding the infarct, significant white matter remodeling, vascular remodeling and anti-inflammatory responses were observed in exosome treated T2DM-stroke mice comapred to vehicle treated T2DM stroke mice.
We have demonstrated that even in the absence of primary cardiac disease, ischemic stroke can induce progressive cardiac deficits and adverse pathological remodeling in the heart, which is likely mediated at least in part by aggravated inflammatory responses and decreased miR-126 expression in serum and heart tissue (Chen et al., 2017). Such post stroke cardiac deficits are worse in T2DM mice compared to non-diabetic stroke mice (Venkat et al., 2021). Therefore, we tested the effect of CD133+Exo on improving post stroke cardiac function in T2DM mice. There is emerging evidence in support of a feedback loop between cardiac function and neuro-cognitive outcome following ischemic stroke or heart failure (Čelutkienė et al., 2016). Similarly, we found a strong correlation between measures of cognitive function such as escape latency in Morris water maze test and discrimination index in novel odor recognition test with measures of cardiac function such as left ventricular ejection fraction in T2DM stroke mice treated with vehicle or CD133+Exo. We found that CD133+Exo treatment significantly increases miR-126 expression and decreases its target gene expression in the heart, improves cardiac function evaluated by echocardiography, decreases cardiac fibrosis, reduces cardiomyocyte hypertrophy, increases myocardial capillary density and decreases cardiac inflammatory factor expression (Venkat et al., 2021). Overall, our studies demonstrate that exosome treatment for stroke has the potential to improve neurological outcome, cognitive function as well as cardiac function in the diabetic stroke population. Figure 1 presents a simplified schematic of the therapeutic effects of exosome treatment in diabetic stroke.
Figure 1.
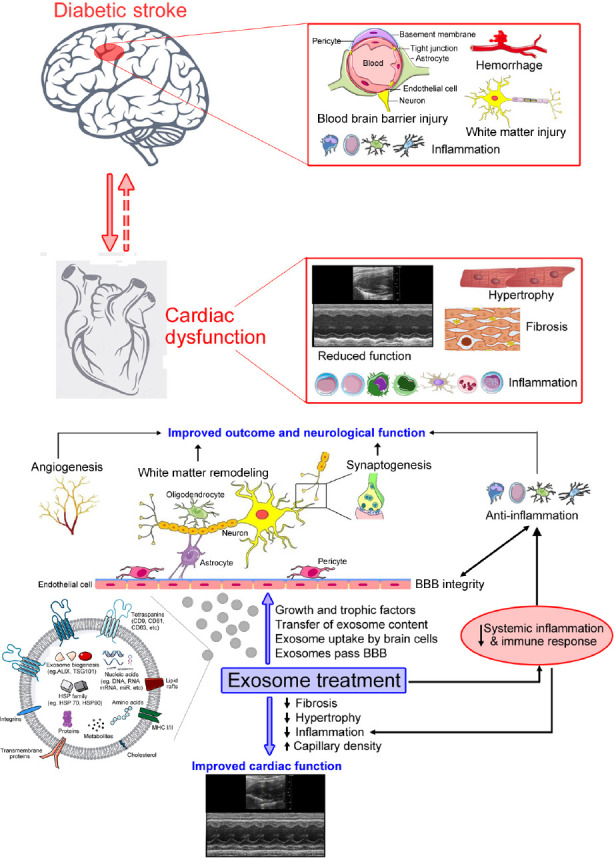
Summary of exosome derived therapeutic effects after stroke in diabetic animals.
Diabetes exacerbates stroke induced neurological injury as well as cardiac dysfunction. Intravenously administered exosomes pass the blood-brain barrier (BBB), home to the ischemic brain and are taken up by endogenous brain cells. Exosome treatment improves neurological function as well as cardiac function after stroke via transfer of exosome content such as microRNA as well as regulation of systemic inflammatory and immune responses.
The deleterious effects of stroke on other organs such as the heart, gut, spleen, lungs, and kidney stress the importance of treating secondary injury to peripheral organs after stroke to improve overall recovery is still being understood. Additionally, studies to understand the effects of exosome therapy on immune system and peripheral organs are needed. Likewise, it is important to investigate the mechanisms by which treatment with exosomes derived from different cell sources affect stroke outcome in diabetic animals. In addition to miRs, other components of exosomes such as circular RNA, long non-coding RNAs, and proteins may work in concert to provide therapeutic effect. Several studies have also successfully employed modified or engineered exosomes in which miR content is modulated to enhance therapeutic efficacy and improve stroke outcome (Xin et al., 2012; Zhang et al., 2019b). Therefore, future studies to characterize and optimize exosome content are warranted. In addition, safety of exosome treatment, standardization of exosome isolation methods, optimization of exosome dose, treatment route and treatment frequency are required. The optimized treatment protocol and neurovascular responses may differ between young and aged as well as between males and females, all of which require further investigation. These studies will be critical in translating exosome based therapeutics into the clinic for the treatment of stroke.
Footnotes
C-Editors: Zhao M, Zhao LJ, Qiu Y; T-Editor: Jia Y
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Čelutkienė J, Vaitkevičius A, Jakštienė S, Jatužis D. Expert opinion-cognitive decline in heart failure: More attention is needed. Card Fail Rev. 2016;2:106–109. doi: 10.15420/cfr.2016:19:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Cui C, Yang X, Xu J, Venkat P, Zacharek A, Yu P, Chopp M. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Transl Stroke Res. 2017;8:374–385. doi: 10.1007/s12975-017-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui C, Ye X, Chopp M, Venkat P, Zacharek A, Yan T, Ning R, Yu P, Cui G, Chen J. MiR-145 regulates diabetes-bone marrow stromal cell-induced neurorestorative effects in diabetes stroke rats. Stem Cells Transl Med. 2016;5:1656–1667. doi: 10.5966/sctm.2015-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui X, Chopp M, Zacharek A, Karasinska JM, Cui Y, Ning R, Zhang Y, Wang Y, Chen J. Deficiency of brain ATP-binding cassette transporter A-1 exacerbates blood-brain barrier and white matter damage after stroke. Stroke. 2015;46:827–834. doi: 10.1161/STROKEAHA.114.007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4:1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji Q, Ji Y, Peng J, Zhou X, Chen X, Zhao H, Xu T, Chen L, Xu Y. Increased brain-specific miR-9 and miR-124 in the serum exosomes of acute ischemic stroke patients. PLoS One. 2016;11:e0163645. doi: 10.1371/journal.pone.0163645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Bezanilla S, Åberg ND, Crock P, Walker FR, Nilsson M, Isgaard J, Ong LK. Growth hormone promotes motor function after experimental stroke and enhances recovery-promoting mechanisms within the peri-infarct area. Int J Mol Sci. 2020;21:606. doi: 10.3390/ijms21020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkat P, Cui C, Chopp M, Zacharek A, Wang F, Landschoot-Ward J, Shen Y, Chen J. MiR-126 mediates brain endothelial cell exosome treatment-induced neurorestorative effects after stroke in type 2 diabetes mellitus mice. Stroke. 2019;50:2865–2874. doi: 10.1161/STROKEAHA.119.025371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkat P, Zacharek A, Landschoot-Ward J, Wang F, Culmone L, Chen Z, Chopp M, Chen J. Exosomes derived from bone marrow mesenchymal stem cells harvested from type two diabetes rats promotes neurorestorative effects after stroke in type two diabetes rats. Exp Neurol. 2020;334:113456. doi: 10.1016/j.expneurol.2020.113456. [DOI] [PubMed] [Google Scholar]
- 11.Venkat P, Cui C, Chen Z, Chopp M, Zacharek A, Landschoot-Ward J, Culmone L, Yang XP, Xu J, Chen J. CD133+exosome treatment improves cardiac function after stroke in type 2 diabetic mice. Transl Stroke Res. 2021;12:112–124. doi: 10.1007/s12975-020-00807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Yu S, Tuazon JP, Lee JY, Corey S, Kvederis L, Kingsbury C, Kaneko Y, Borlongan CV. Neuroprotective effects of human bone marrow mesenchymal stem cells against cerebral ischemia are mediated in part by an anti-apoptotic mechanism. Neural Regen Res. 2019a;14:597–604. doi: 10.4103/1673-5374.247464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang ZG, Buller B, Chopp M. Exosomes — beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019b;15:193–203. doi: 10.1038/s41582-018-0126-4. [DOI] [PubMed] [Google Scholar]


