Graphical abstract

Keywords: Superabsorbent, Soil conditioner, Cross-linking, Grafting, Swelling index, Slow nutrient release, Water retention
Abstract
Background
The cumulative influence of global warming, climate abrupt changes, growing population, topsoil erosion is becoming a threatening alarm for facing food challenges and upcoming global water issues. It ultimately affects the production of food in a water-stressed environment and slows down the production with more consumption of fertilizers by plants. The superabsorbent hydrogels (SAHs) have extensive applications in the agricultural field and proved very beneficial for plant growth and soil health. These polymeric materials are remarkably distinct from hygroscopic materials owing to their multidimensional network structure. It retains a lot of water in its 3D network and releases it slowly along with nutrients to plant in stressed environment.
Aim of review
A soil conditioner boosts up the topology, compactness, and mechanical properties (swelling, water retention, and slow nutrient release) of soil. The superabsorbent hydrogel plays an astonishing role in preventing the loss of nutrients during the heavy flow of rainwater from the upper surface of soil because these SAHs absorb water and get swollen to keep water for longer time. The SAHs facilitate the growth of plants with limited use of water and fertilizers. Beyond, it improves the soil health and makes it fertile in horticulture and drought areas.
Key scientific concept of review
The SAHs can be synthesized through grafting and cross-linking polymerization to introduce value-added features and extended network structure. The structure of superabsorbent hydrogel entirely based on cross-linking that prompts its use in the agricultural field as a soil conditioner. The properties of a SAHs vary due to its nature of constituents, polymerization process (grafting or cross-linking), and other parameters. The use of SAHs in agricultural field comparatively enhances the swelling rate up to 60–80%, maximum water retaining, and slowly nutrient release to plants for a longer time.
Introduction
The superabsorbent hydrogels are three-dimensional matrix (3D) constituted by linear or branched hydrophilic polymers. Hydrophilic gels usually possess polymeric chains that may resemble colloidal gels in which water acts as dispersion medium [1]. The hydrogel polymer network can swell and retain the defined amount of water within its matrix but never dissolve itself in water [2]. The water absorption tendency of hydrogels arises from the hydrophilic functional groups of the polymer, while their reluctance to dissolution in water due to cross-linking of network chains [3].
The most common of these hydrogels are grafted and cross-linked polymeric networks that may be produced by the simple reaction of one or more monomers [4]. Hydrogels mostly consisted of two- or more components system extended over a three-dimensional network of polymer chains and water fills the space created between macromolecules to exhibit swelling and water retaining properties [5]. The swelling property of hydrogels depends upon nature and density of the monomer in an extended network of the polymer chain [6].
The soil hydrogels are frequently used as soil conditioners that boost up the growth of plants by providing them nutrients and water content periodically [7]. These hydrogels facilitate the growth of plants by giving a nutrient rich environment, maximum water absorbency, and slow release of nutrients. The slow release is a tremendous quality of soil hydrogels because the valuable nutrients get released slowly from hydrogel matrix and a plant can avail these nutrients for a longer time [8].
As it is concerned with hydrogel’s historical background, the term “hydrogel” was introduced first time in the scientific literature in 1894 for the description of colloidal gels made from inorganic salts [9]. Later on, in 1960, “Wichterle and Lim” reported on hydrogel as water swollen macromolecules. Hydrogels can be classified into three generations depending upon their structural pattern (simple to complex), their properties, and different eras of research work. The first generation (1960–1980) hydrogels based on simple water swollen capability of macromolecular networks [10]. They include three categories of hydrogels, cellulose based, polymerization of water-soluble monomers like Poly-hydroxyethyl methacrylate (PHEMA), and cross-linking of water-soluble synthetic polymers like polyvinyl alcohol (PVA) and Polyethylene glycol (PEG) [1].
The second generation (1990–2000) hydrogels tend to respond to abiotic factors (pH, temperature, and concentration of monomer) of the environment [11]. The 2nd generation hydrogels include temperature-sensitive hydrogels (like poly lactide, PEG-polyester block copolymers [1], poly glycolic-co-lactic acid, poly ε-caprolactone, poly-N-isopropyl-acrylamide), degradable hydrolytic hydrogels (like N-2-hydroxypropyl methacrylamide) and pH sensitive based hydrogels (acidic or basic moieties). In this era, the hydrogels were synthesized by using different techniques like redox based radical polymerization, photo-polymerization, gamma radiation polymerization, inclusion complex formation, cross-linking through Michal addition and atom transfer radical polymerization (ATRP) [12]. In third generation, the hydrogels consisted of stereo-complexes (in 2000) and smart hydrogels (2010 onward): in situ chemically cross-linked hydrogels by enzymes, radical polymerization, double-network hydrogels, the combination of natural & synthetic polymers and composite hydrogels [13]. Shortly, for the last 50 years, the hydrogels have received attention due to their exceptional properties and 3D structural pattern of monomers either cross-linked or grafted. They exhibit a little degree of flexibility similar to the natural tissues owing to their absorption of large water content [14]. Fig. 1 is illustrating the road map for different agricultural hydrogel in last two decades.
Fig. 1.
Illustrating the road map for different agricultural hydrogels in last two decades. First arrow is Showing the hydrogels in era of 2001–2005, second arrow for 2005–2010, third arrow for 2010–2015 and fourth arrow is for 2015–2020.
The soil hydrogels are either made up of natural sources or synthetic material. The natural materials included the most common and degradable components that are polysaccharides (PS) and polypeptides (PP) whereas synthetic material (petrochemical based) consisted of acrylic acid (AA), its salts, and acrylamide (AM) [15]. The first generation hydrogels were mainly consisted of natural based hydrogels owing to their biocompatibility, feasibility, and biodegradability [16]. Later on in second generation, synthetic hydrogels were substituted with synthetic hydrogels due to their longer life and more intensifying features than natural based hydrogels [17]. During the last twenty years, synthetic hydrogels and combo of natural with synthetic hydrogels got more importance and replaced the traditional natural hydrogel [18]. The synthetic hydrogels have a comparatively high capacity of water absorption, long service life, and high gel strength. Fortunately, synthetic polymers usually have well-defined structures that can be modified to produce tailored functionality and degradability [19].
Synthesis of hydrogels through cross-linking
The hydrogels are mostly synthesized through cross-linking polymerization process and it can be done either by physical or chemical process.
Physical cross-linking
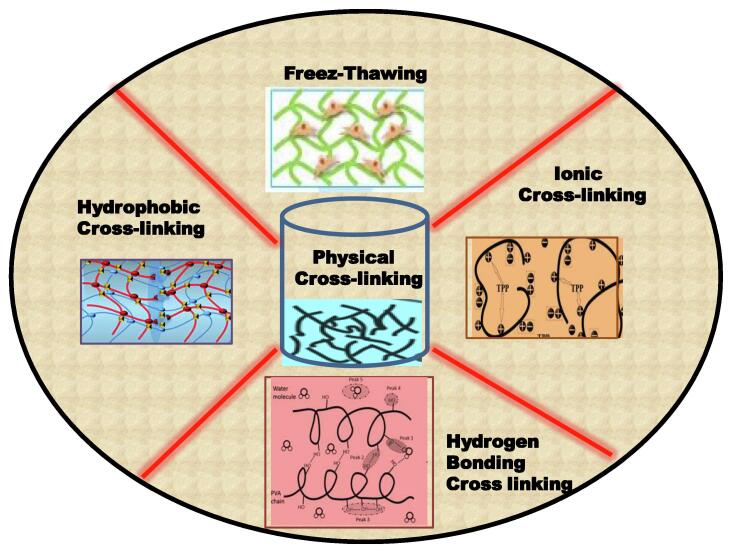
The physical cross-linking, also known as self assembled hydrogels owing to absence of any chemical bonding, generally proceeds through a very easy and convenient method to avoid the use of any cross-linking agent [20]. They are usually reversible hydrogels because of temporary junctions and reversible interactions that can be distorted by making a minor change in any condition or applying stress to the reacting conditions [21]. Physical cross-linking avoid any chemical interactions thus there is no dissolution occur. A bunch of methods can be used for physical cross-linking in the synthesis of hydrogels. Fig. 2 is showing different types of physical cross-linking processes.
Fig. 2.
Different types of physical cross-linking processed.
Freeze –thawing process: It is the best and simplest process used for the preparation of natural material based hydrogels. Thawing is exactly the contrary process of freezing and carried out in mild temperature conditions without any aid of organic solvents. The freeze-thawing process is repeated two or more times for the preparation of physical hydrogels. Mostly cellulose and cellulose derived based materials used in this process. These hydrogels also exhibit well-defined properties of swelling in soil and water [22].
Cross-Linking by Hydrophobic Interaction: The hydrogels exhibiting hydrophobic interactions must have a hydrophobic co-monomer in addition to hydrophilic monomers and are synthesized by free radical copolymerization [23]. It was experimentally observed that these hydrophobic interactions are comparatively stronger than other intermolecular forces like Vander Waal and hydrogen bonding [24]. These interactions vary according to variations in temperature, carbon atomicity, and shape of hydrophobes. There should be higher carbon atomicity to develop strong hydrophobic interactions between polymer chains. These hydrogels are very sensitive to temperature that strongly affects the hydrophobic interaction (existing between hydrophobic segments of polymer) and hydrophilic interactions (existing between hydrophilic segments of polymer). Generally alkylated cellulose based polymers exhibit hydrophobic interactions between the OH-group of an alkylated chain. Cellulose gets hydrated at low temperature while the OH-group makes interaction with each other and turns to hydrogel at high temperature [25].
Cross-Linking by Ionic Interaction: It is very easy to make ionic cross-linked materials by the addition of multivalent counter ion and follows the gelling principle of poly-electrolytic solution [26]. The polyelectrolyte can easily be cross-linked through ionic bond with multivalent counter-ions by a simple ion-exchange method. For example, sodium alginate (polymer of glucuronic and mannuric acids) solution can easily be cross-linked with opposite charges (Ca+2) of calcium chloride solution. The ionic cross-linking usually takes place at lower temperatures and broadly applicable in the medical field like drug delivery; wound healing, and tissue engineering [27].
Cross-Linking by Hydrogen Bonds: The hydrogen interactions exist between the hydroxyl groups of cellulose chain and are responsible to develop hydrogen bonding with water to make hydrogel [23]. These hydrogels are sensitive to change in concentration of polymers, temperature, solvent nature, and the degree of association of functional groups. The higher concentration of polymers will cause more hydrogen bonding thus there is more organized and stable gel prepared [28]. When polyethylene is added to the cellulose polymer then hydrogen bonding interactions developed between the oxygen of glycol and hydrophilic OH-group of cellulose polymer [29].
Chemical cross-linking
The chemical cross-linking must contains a covalent bonding in polymer chain. There are three major categories of methodologies that fall under chemical cross-linking.
Chain growth polymerization
This process follows initiation, propagation and termination steps similar to the free radical polymerization. It is necessary to follow the chain growth polymerization that a monomer must contain unsaturation (double bond) in it to makes polymer chain. When the reaction mixture turns to gel formation, at this instant, the polymerization can be classified into three categories; solution polymerization, suspension polymerization, and photo-polymerization [30].
Solution polymerization: In this polymerization the reactants, initiator, and final polymer can easily dissolve in solvent. Solution polymerization needs a very low conc. of catalyst, high conc. of monomers, and solvent. This polymerization has low cost, controllable heat of polymerization and more convenient in synthesis, so prefer to use than other homogenous polymerization [31]. For example, the solution polymerization was used for the synthesis of carboxymethyl cellulose (CMC) and hexaethyl cellulose (HEC hydrogels) in the presence of cross-linking agents and initiators. Besides, in many studies, the acrylic acid and acrylamide were grafted on CMC by using solution polymerization [32]. It yields high rate of polymerization and provides a safe environment of reactant’s dealing in aqueous media without the aid of additional heating because it takes place at room temperature [33].
Suspension Polymerization: Contrary to the solution polymerization, the suspension polymerization occurs in non-polar solvent (organic phase) media. So the hydrophilic reacting species (monomers, cross-linking agent, and initiators) are insoluble in media and get suspended in non-polar phase. The properties of hydrogels prepared from suspension polymerization entirely depend upon viscosity difference of monomers & liquid phase, agitation speed and dispersant type [34].
Photo-polymerization: Entirely opposite to aforementioned categories, photo-polymerization proceeds with the initiation of radicals in the presence of radiations like UV, visible, or infrared in situ form of cross-link networking. This polymerization also follows the radical polymerization but the photo-sensitive monomers interact with light radiation and induce radicals [35]. The initiation of radicals may occur through photo-cleavage, cationic photo-polymerization, and hydrogen abstraction. These radicals follow the same propagation and termination steps of free radical polymerization. This polymerization method got superiority in context of controlled gelation process. Photo-polymerization involves in the preparation of alginate, guar gum, and polyethylene glycol based hydrogels that depends upon the exposure and intensity of UV radiation [36].
Irradiation polymerization
The irradiation polymerization differs from photo-polymerization in aspect of high energy radiations used in former category. In some studies, the microwave radiations were also used to develop cross-linking in hydrogels [6]. In irradiation polymerization, ionizing or high energy radiation like gamma rays and electron beams are used to initiate radicals of unsaturated monomer substances. These radicals follow the chain growth mechanism for cross-linked polymer synthesis. This new forefront technology has superiority over traditional technologies is that it never requires a catalyst or modifying agent and swelling properties can be controlled by managing degree of cross-linking and hydrogel’s particle size [37].
Step-growth polymerization
The step growth polymerization proceeds slowly in steps and poly-functional monomers react together through covalent bonding. This polymerization is usually self catalyzed and covalent bond was developed with the release of water or HCl molecule. Step growth polymerization yields slowly increase in average molecular weight but high reaction rates are needed to get longer chains of polymer [38]. The distinct characteristic of step growth polymerization is that its terminal ends remains still active whereas the chain growth polymerization process terminates when all reactive sites fixed through a chemical bond [39].
Although the soil hydrogels can be classified on the basis of source material used as precursor in their synthesis methodology. Fig. 3 shows all processes of chemical cross-linking polymerization methods.
Fig. 3.
Processes of chemical cross-linking polymerization methods.
Natural precursor materials based hydrogels
Pure natural
There are three types of natural materials used as precursor in the synthesis of soil hydrogels; one is polysaccharides like cellulose, chitosan and starch (majorly used), second is by-products of agriculture like rice husk ash (RHA) and wheat straw (WS) (moderately used) and third in least priority is pectin, guar gum, sawdust and arabic guar gum [40], [41]. All the above categories of natural materials used in grafting or cross-linking mechanism for synthesis.
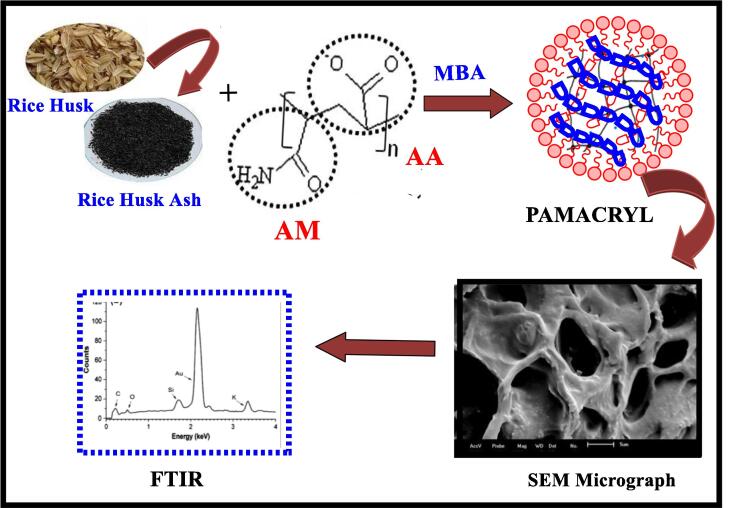
Poly (acrylamide-co-acrylate)/RHA hydrogel: The PAMACRYL; (poly acrylamide-co acrylate) hydrogel can be synthesized by cross-linking of acrylic acid (AA) and acrylamide (AM) with utilization of a cross-linker N, N-methylene bisacrylamide and a reactive catalyst tetramethylethylenediamine (TMED). The main role of K2S2O8 in hydrogel synthesis is to initiate the radicals for polymerization process. The radicals of monomers combine effectively with each other and make a valuable hydrogel. The size of particle ranged from 2 to 0.71 mm by using 9–24 mesh sieves. The rice husk ash based composite PAMACRYL/RHA was prepared as free radical polymerization in the same aforementioned method but monomers (acrylamide and potassium acrylate have been taken in 50:50% mole concentration) mixed in rice husk ash and cross-linker [42]. The rice husk ash based PAMACRYL composite obtained at different calcination temperature range (400–900 °C) to get data for their chemical properties. It has been found that the PAMACRYL hydrogel and PAMACRYL/RHA composite possess different swelling ratios; the former has least swelling ratio (645 gwater/gabsorbent) whereas the composite calcinated at 900 °C shows comparatively maximum swelling ratio than others that is 1077 gwater/gabsrobent. The swelling ratio of composite has variations according to pH change and salt concentrations of swelling media [43]. Fig. 4 illustrates the process of synthesis of PAMACRYL/RHA hydrogel.
Fig. 4.
The process of synthesis of PAMACRYL/RHA hydrogel.
CSt-g-PAA/NR/PVA semi-IPN hydrogel: The cost effective and eco-friendly soil hydrogels are gathering more attention for researchers and need biodegradable material for the synthesis of superabsorbent hydrogels. This novel synthesis based on grafting polymerization of 70% neutralized acrylic acid (AA) on cassava starch followed by cross-linking of one polymeric chain with another through N,N-methylene bisacrylamide (MBA) with the aid of ammonium persulfate (APS as an initiator) in the presence of nitrogen atmosphere [44]. For interpenetration, the polyvinyl alcohol (PVA) is blended with natural rubber (NR) latex and an emulvin introduced to prohibit premature coagulation. The cassava starch cross-linked hydrogel is subsequently mixed with PVA/NR blend to develop semi-interpenetrating network with hydrogel for compact structuring organization [45].
This compact structuring enhances the flexibility and mechanical properties of hydrogel owing to the presence of hydrogen bonding between PVA and OH group of polymeric chain. The swelling rate of hydrogel is comparatively greater in de-ionized water (7 9 4) than in 0.9% wt of NaCl solution (2 4 4). The superabsorbent hydrogel exhibits maximum swelling at 1% wt cross-linking agent concentration, 7.5 g of cassava starch, 6 ml of acrylic acid, 3% wt of ammonium persulfate concentration at pH-7. The lower pH causes higher protonation and cross-linking whereas the higher pH than 7 causes in “charge shielding effect” of sodium ions so reduces the anion to anion repulsions and finally results in decreasing the swelling [46]. The water retaining capability directly depends upon temperature and the experimental results show that it has maximum water retention (63.5%) at 30 °C [45].
Wheat straw based hydrogel: A synthetic approach is used to fabricate the Wheat straw (WS) for the synthesis of hydrogel. It started to prior preparation of carboxymethyl intermediates (CM-intermediates) by using agricultural by-products like wheat straw mixed with isopropanol, sodium hydroxide, and monochloroacetate (MCA) along with anhydrous glucose unit (AGU) [47]. After getting CM- intermediates, it is cross-linked with different concentrations of citric acid monohydrate. The citric acid got priority over other cross-linking agents owing to its non-toxic effect and easily available at low cost. Cross-linking plays a crucial role in the swelling properties of the hydrogel. The reaction mixture ultimately cured at different temperature ranges and time duration to boost up gel stiffness, gel yield, and swelling properties. The particle size of hydrogel ranges from 150 to 800 μm. The fabrication of wheat straw into a soil hydrogel proved as a sustainable source and enhanced the water absorption up to 70% for sandy soil. The concentration of citric acid (4% wt), 140 °C temperature along with maximum time duration of reaction cross-linking significantly enhances the gel stiffness, strain resistance and swelling properties [48].
Sawdust cellulose based hydrogel slow release fertilizer (SC-HSRF): A novel sawdust hydrogel slow released fertilizer (HSRF) is an eco-friendly, biocompatible and bio-decomposable that has been synthesized by first converting the sawdust into its functional form of sawdust cellulose (SC) by mixing its powder in an aqueous ammonia solution in the ratio of 1:12 respectively [49]. A graft copolymerization methodology has been used for grafting of acrylic acid (AA), acrylamide (AM) in the presence of N, N-methylene bisacrylamide (MBA) as a cross-linker. The potassium persulfate (KPS) is a reaction initiator for the radical generation. The complete synthesis process of SC-HSRF hydrogel is shown in Fig. 5. The introduction of urea accomplished to make it nutrient rich fertilizer based hydrogel and plays a dual role in soil that it is a good soil conditioner as well as provides an essential nutrient rich environment for plant growth [50]. The HSRF cut into small pieces of 2 × 2 mm for storage and further utilization in soil. When its sample loaded to soil that has original water retention (WR) value is 38.7%, it found that soil exhibits 166.2% value and enhances the water retention capacity of soil 122.5% times more than its original value. Besides, the incorporation of SC exaggerated the swelling ratio (210 g/g) in de-ionized water and can absorb more water in saline solution (NaCl) in the range of 7–10 pH [49]. The presence of hydrophilic groups and SC in hydrogel provides more skeletal nodes that are responsible for water absorption.
Fig. 5.
Synthesis process of SC-HSRF hydrogel.
Tapioca starch based Urea-Encapsulated Hydrogel: The urea encapsulated hydrogel also falls in fertilizer based superabsorbent hydrogel that starts with prior synthesis of tapioca starch based hydrogel that later on encapsulated with urea fertilizer [50]. The tapioca starch usually gets from cassava root that is broadly used as precursor in the synthesis of superabsorbent soil hydrogels. The hydrogel constituted on cross-linking of tapioca starch with acrylic acid (AA) with the aid of N, N-methylene bisacrylamide (MBA as cross-linker) [51]. At the end the obtained hydrogel is mixed with urea in an aqueous NaOH solution to make urea encapsulated fertilizer hydrogel. The use of NaOH is very mandatory for neutralization of acrylic acid protons, to balance the electrical charges and helps in hydration of hydrogen upon contact with water. The superabsorbent hydrogel (SAH) shows maximum swelling rate at 1:1.05 starch to AA ratio, 0.48% cross-linking and 1.94% concentration of ammonium persulphate as an initiator [52]. Besides urea encapsulated fertilizer based hydrogel has controlled release of urea and the experiments showed that it has increased the soil porosity and water retaining capacity on implication in soil for plant growth examination [53].
Poly-g (AA-cl-EGDMA) superabsorbent hydrogel: The guar gum (GG) is a naturally occurring galactomannan polysaccharide that usually derives from guar beans. Although it has stabilizing and thickening properties so it is potentially used as a precursor for soil hydrogels as polymer backbone chain [54]. The guar gum (GG) grafted with acrylic acid (AA) and cross-linked with ethylene glycol dimethacrylic acid (EGDMA) in the presence of benzoyl peroxide (BPO; as initiator), for the synthesis of novel poly-g(AA-cl-EGDMA) hydrogel [55]. The bulky groups created steric hindrance so primary hydroxyl group (–OH) of polymer chain more vulnerable to initiator attack than secondary hydroxyl group. As a result more polar bonds have more tendency to rotate and leading to the generation of the active sites for grafting polymerization. In this procedure, cross-linking and grafting took place simultaneously [56].
The swelling ratio may alter with change in concentration of cross-linking agent and has maximum swelling at 0.5 mM and decrease signifcantly with both below and above 0.5mM concentration. Lesser quantity leads to insufficient cross-linking while greater quantity makes hydrogel structure very compact and hard due to excessive cross-linking. Similarly the initiator content also depends on swelling index, the hydrogel show maximum swelling index (625 ml/g) at 0.25 mM initiator concentration in alkaline media (pH-9). The initiator less than 0.25 mM may cause slow polymerization owing to insufficient free radicals for initiating the process. In an acidic medium (pH-4), the swelling rate decreases because of incomplete ionization of cross-linking. In soil implementation, the hydrogel has increased water retaining capacity (51.7%) and 9% porosity of soil than original at 0.3% concentration of hydrogel [55]. Owing to magnifying features, this potent hydrogel is broadly used in agriculture industry as best soil conditioner and water reservoir.
GG-cl-(poly-(AA-ipn-PANI) Hydrogel: The guar gum (GG) hydrogel is potentially synthesized by using response surface methodology to get modified surface morphology with ascending swelling ratios. This novel guar gum hydrogel comprises of cross linking polymerization of acrylic acid (AA) on guar gum by using hexamine as cross-linker and ammonium persulfate (APS) as an initiator [56]. It is subsequently cross-linked with poly-aniline, along with addition of hydrochloric acid (HCl) and sodium hydroxide (NaOH) for neutralization, to get inter-penetrating network of hydrogel [57]. The mixture’s color turns soon from brown to light green with the addition of hexamine and ammonium persulfate. The inter-penetration of polyaniline takes 16 h for completion and formed a swollen product. The SEM images explain the coarse surface of (GG-cl-poly-(AA-ipn-PANI) that proves cross-linked networking of hydrogel whereas the original guar gum has smooth surface with tiny dots. Like others, this hydrogel also depends upon initiator, monomer, cross-linking agent concentration, pH sensitive and exhibit maximum water absorbency (5307%) at 22.3 × 10−3 mol/L, 166.8 × 10−6 mol/L and 53.4 × 10−3mol/L in neutral medium respectively [58].
Porous cl-GG-g-poly (acrylate) hydrogel: The free radical grafting polymerization can be used for the synthesis of porous cl-GG-g-poly (acrylate) hydrogel by the use of redox pair initiator the persulphate- N, N, N, N tetramethylenediamine (TEMED). This method proceeds with polymerization in situ, by the addition of acrylic acid, N, N methylene bisacrylamide (MBA as crosslinker), initiator redox pair, and foaming acid (acetic acid) in distilled water step by step at 70–80 °C under nitrogen bubbling. The porosity of hydrogel depends upon bubbling of CO2 gas that evolves when foaming acid combines with NaHCO3. When the viscosity of gel increased by increasing cross-linking concentration then CO2 gas finds difficulty in releasing, hence this trapped gas results in porous structure of hydrogel that ultimately increases the swelling behaviour [59]. The backbone polymer chain to monomer ratio has considerable influences on swelling along with foaming stabilizer. The former ratio tries to keep below 0.2 while later has concentration limit up to 7.35% in acidic medium to get maximum water absorbency [40]. The illustration of synthesis schemes for guar gum based hydrogels is given in Fig. 6.
Fig. 6.
Illustration of synthesis schemes for guar gum based hydrogels. a) Synthesis of (GG-cl-poly-(AA-ipn-PANI) hydrogel. b) Synthesis of GG-poly-g (AA-cl-EGDMA) hydrogel. c) Synthesis of porous cl-GG-g-poly (acrylate) hydrogel. d) Synthesis of GG-g-P(NaA-co-St)/APT hydrogel.
Modified natural materials
Modified natural material includes chemically modification of pure natural materials and used as precursor in the synthesis of soil hydrogels [60]. This review section includes modified natural materials like carboxymethyl cellulose (CMC), sodium sulfonated corn starch (SSS), salep phosphate (SP) and cellulose acetate (CA). All these modified natural materials act as precursor base of the dispersion medium. Both categories of natural materials play important role in biodegradable hydrogel, biocompatible with soil and eco-friendly with environment [61].
GEDTA hydrogel: The GEDTA based hydrogel prepared by grafting and subsequently esterification cross-linking polymerization process in the homogenous medium using cellulose acetate (CA) with ethylenediamine tetraacetic dianhydride (EDTAD) as a crosslinker. A viscous reaction mixture obtained immediately after the addition of EDTAD and triethylamine (catalyst) [62]. The one molecule of EDTAD (possess two anhydric bonds) grafted onto a cellulose acetate through one anhydric (-CO-O-OC-) bond cleavage then simultaneously cross-linked with another CA molecule through opposite anhydric bond cleavage. The un-reacted carboxyl group of precursor (cellulose acetate) reacts with aqueous sodium bicarbonate (NaHCO3) to convert it into sodium carboxylate. The role of sodium bicarbonate is to boost up the water affinity of reactants. The product (GEDTA hydrogel) sifted through 60 mesh sieve and tested on Typical Red Oxisol soil to analyze its functional properties for growth of plant. The GEDTA hydrogel was mixed with NPK and 20% ammonium hydroxide solution to neutralize it [63]. It is necessary to neutralize to prevent protonation of carboxylate group that may decrease the water absorption capacity. The GEDTA hydrogel is efficiently proved as a large amount of water absorber, slow release behavior, and soil moisture retainer. These efficient properties of GEDTA hydrogel enforce to widely use in limited water soils (drought-prone), horticulture and agriculture [62].
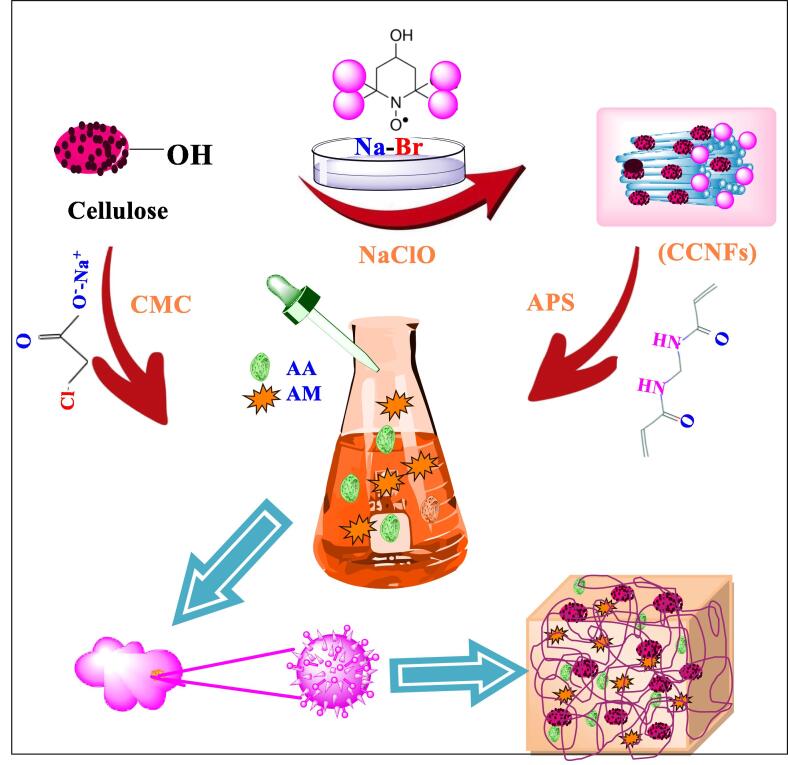
CMC-g-p(AA-co-AM)/CCNFs nanocomposite hydrogels: Nanocomposites are considered as comparatively better than micro or macro-composites in enhancing the water retaining and absorbency properties of hydrogels [64]. Carboxylated cellulose nanofibrils (CCNFs) can be synthesized by suspending microcrystalline cellulose (MCC) in distilled water, sodium bromide and tetramethylpiperidine-1-oxyl radical (TEMPO) [65], [66]. The solution subsequently oxidized by the addition of sodium hypochlorite (NaClO) solution with gentle agitation. The aqueous solution of NaOH used to maintain pH at 10.5 and then oxidized cellulose passed through ultrasonication process, by using an ultrasonic homogenizer. For the synthesis of CMC-g-p(AA-co-AM)/CCNFs, defined amount of sodium carboxymethyl cellulose (CMC), acrylamide (AM), acrylic acid (AA), methylenebisacrylamide (MBA) and carboxylated cellulose nanofibrils, (CCNFs) along with APS added with heating at 60 °C for production of free radicals. Fig. 7 is showing the schematic illustration for synthesis of CMC-g-p(AA-co-AM)/CCNFs nanocomposite hydrogels.
Fig. 7.
Schematic illustration fro synthesis of CMC-g-p (AA-co-AM)/CCNFs nanocomposite hydrogels.
The most vital and distinguishing role of CCNFs in swelling property is that it is responsible for the production of more carboxylate groups (0.94 mmol/g) at the consumption of 2.5%wt CCNFs. The presence of CCNFs (as filler) in CMC-g-p(AA-co-AM)/CCNFs hydrogel exaggerates the swelling property up to 428.4 g/g in distilled water at 10 mmol/L of NaCl solution with a smaller particle size of hydrogel. The small particle size provides interstitial volume that is more supportive for up taking extra water. Besides, the water retention increases up to 81.1% at 30 °C due to intervention of CCNFs [65].
Salep phosphate hydrogel: The introduction of salep phosphate in hydrogel, as an innovative polymer backbone substituent to cellulose, proved as a good carrier for slow release of agrochemicals with enhanced hydrogel properties [67]. The salep is usually obtained from dried tubers of natural terrestrial Orchids and it is a biopolymer constituted of multi-component polysaccharides, glucomannan (16–60%), etc. It is modified by introducing phosphate group on its backbone chain by reacting with disodium hydrogen phosphate dodecahydrate and sodium dihydrogen phosphate dihydrate in basic environment of pH-11. The salep phosphate can easily be cross-linked with acrylic acid (AA) in the presence of N,N-methylene bis-acrylamide under grafting copolymerization for cross-linking mechanisms [68]. The (salep phosphate)-g-polyacrylic acid hydrogel is biodegradable and eco-friendly for both plants and environments. The incorporation of bio-polymeric substances (salep phosphate) empowered the water absorbency with maximum water retention property achieved at moderate phosphate content [69].
Cellulose/CMC-cl-ECH hydrogel: Among the physically cross-linked hydrogels, the cellulose and its derivatives like carboxymethyl cellulose (CMC) are more prominent. A CF-11 whaatman paper (as a source of cellulose) is being transformed into the functional cellulosic group by dissolving it in a urea/NaOH solution. The functional cellulose and CMC passed through freeze-thawing process in the presence of epichlorohydrin (ECH) as a cross-linker for the synthesis of natural based hydrogels [70]. The CMC and cellulose solutions have been prepared in different ratios for studying the variations of hydrogel properties. The swelling equilibrium data shows that all solutions are proved as superabsorbent hydrogels but the composition with higher concentration of CMC (9:1) exhibits comparatively more swelling than rest of hydrogels. The hydrogel properties are greatly influenced by the nature and concentration of salt solution (CaCl2 decreases swelling ratio more than NaCl solution). Thus the hydrogel properties are controllable by managing the CMC concentration and nature of salt aqueous solution. The cellulose/CMC-cl-ECH hydrogels exhibit 1000 times more swelling ratio than that of cellulose based hydrogels. Although these hydrogels also express the slow nutrient release of bovine serum albumin (BSA) and vary with the content of CMC accordingly [71].
Synthesis of soil hydrogels based on synthetic materials
Pure synthetic material
Owing to the confined benefits of natural material based hydrogels, the researcher paid their attentions to make chemical based hydrogels that have a bunch of enhanced benefits with their prolonged life time. Synthetic materials are usually based on acrylic acid (AA), acrylamide (AM), sodium acrylate (NaA), polyvinyl alcohol (PVA), urea, aluminium zinc ferrate (AlZnFe2O4), poly-2-Acrylamide-2-Methyl-1-Propane Sulphonic Acid (PAMPS), attapulgite, sodium humate [72].
LCSH by urea and acrylic acid: The low cost superabsorbent hydrogel (LCSH) has been prepared by solution polymerization of acrylic acid (AA) with urea in the presence of reactive cross-linker N,N'-methylenebisacrylamide (MBA). This polymer was synthesized by neutralization of acrylic acid with NaOH to form sodium acrylate. The sodium acrylate reacts with urea and polymerization process started with using a chemical initiator K2S2O8 in the presence of nitrogen atmosphere [73]. The final product’s granular size is maintained up to 0.18 to 0.212 mm for use. The hydrogel’s properties are strongly affected by the weight ratio of MBA to AA (wt %), mole ratio of urea to AA and neutralization degree of AA [74].
The absorbency of LCSH depends upon the ascending mole ratio of urea to acrylic acid (AA) and its maximum value was achieved at 1.6:1 mol ratio. The absorbency of water increases with increasing urea concentration that resultantly enhances the affinity for grafting of hydrophilic groups on acrylic acid. While crossing 1.6 mol ratio of urea results in descending the absorbency of LCSH. The LCSH shows maximum absorbency 835 g/g in distilled water at 0.005% weight ratio of MBA to AA with 85% degree of neutralization of AA [33].
Poly(acrylic acid-co-acrylamide)/sodium humate/attapulgite (PAA-AM/SH/APT) superabsorbent hydrogel: For the synthesis of novel multifunctional (PAA-AM/SH/APT) hydrogel, the sodium humat (SH), attapulgite (APT), acrylic acid (AA) having 50% degree of neutralization, and acrylamide (AM) mixed in a defined ratio in the presence of N, N- methylene bisacrylamide [75]. The concentration ratio of AA to AM is very accountable because the water absorbency increases up to 1.2 M ratio and decreases sharply beyond this limit. The purging of nitrogen gas for 30 min removes dissolved oxygen in the solution. The presence of sodium humate and attapulgite plays a vital role in enhancing the polymeric network by cross-linking between the chain’s ends thus elevates the water absorbency (996 g/g in water) of hydrogel at 10%wt and 20%wt concentrations respectively. The particle size of multifunctional hydrogel ranges from 40–80 mesh [31].
Slow Release Fertilizer based Hydrogel (SRFH): The combined utilization of hydrogel and nutrient rich fertilizer is very demanding in agriculture field. The fabulous characteristics of slow release fertilizer hydrogel (SRFH) focusing on the formulations of different monomers for fertilizer based hydrogels. The neutralization of monomer is a very essential step to enhance the electrostatic repulsions among carboxylate anions of slow release fertilizer hydrogel (SRFH) [76]. The high degree of neutralization enhances the water absorbency of hydrogel owing to the attached carboxylate anions to the polymer chain that develops electrostatic repulsions to expand the network. The SRFH constituted neutralized acrylic acid (AA: 60% degree of neutralization), urea and acrylamide (AM) in the presence of N-N, methylene bisacrylamide (MBA). The use of SRFH potentially reduces the fertilizer loss rate, provides nutrients slowly. The SRFH has maximum swelling rate and water retention along with positive effect on plant growth [64].
PAMPS Hydrogel: The PAMPS hydrogel synthesis is based on the polymerization of reactive monomer, 2-Acrylamide-2-Methyl-1-Propane Sulphonic Acid (AMPS), in the presence of N, N-methylene-bis-acrylamide and ammonium persulphate (APS). The hydrogel has been examined for different parameters like cross-linking densities, water pH (acidic, basic, or neutral media), and temperature (40 °C or 60 °C) [77]. The experimental results show that PAMPS exhibit maximum water absorbency at lowest (0.03) concentration than higher at 60 °C in alkaline media [20].
Combo materials
Another surprising material for the synthesis of hydrogel as dispersion medium is combination of natural (either pure or modified) and chemical salts. This combination of materials provides astonishing qualities that elevates the demand for soil hydrogel in agriculture. The combo materials include a combination of cellulose with acrylic acid, CMC with acrylic acid and acrylamide, guar gum (GG) with acrylic acid and acrylamide, CMC with an attapulgite, agricultural by-products like rice husk ash and wheat straw with AA and AM, clinoptilolite with AA and AM, chitosan with PVA and AA [78].
CRF-hydrogels: The three different controlled release fertilizer (CRF) based hydrogels have been synthesized by mixing fertilizer solution (dihydrogen ammonium phosphate, potassium nitrate, and ammonium nitrate) with hydrogels. The first hydrogel based on the combination of chitosan solution with fertilizer solution in the presence of glutaraldehyde and labeled as CRF-CS. The second hydrogel is the combination of PVA with fertilizer solution in presence of acetic acid, H2SO4, and methanol (acting as pH controller, catalyst and cross-linking agent respectively) along with glutaraldehyde and labeled as CRF-PVA hydrogel. The third hydrogel has composition of PVA, chitosan and fertilizer solution with a suitable cross-linking agent labeled as CRF-CS/PVA [79].
All these CRF hydrogels were examined in the context of water absorbency, water retention, and potassium release factors. CRF hydrogels showed high swelling ratio ranging from 70 to 300%. The CRF-PVA hydrogel has maximum swelling that is 300% and CRF-PVA/CS hydrogel exhibit 225% while CRF-CS showed up to 70%. The water retention property of all these hydrogels vary in similar fashion, the CRF-PVA has highest water retention capacity of 25% and CRF-PVA/CS has 10% while CRF-CS has only 4% absorbencies [80]. The diffusion or release property of fertilizers depends upon swelling ratio of hydrogel. All three hydrogels showed less swelling ratio in soil than in de-ionized water. So diffusion of soluble fertilizer components is less in soil than water. Hence CRF hydrogels have a slower release of potassium in soil than water. All CRF hydrogels have less (n < 0.5) exponent of potassium release in de-ionized water showing quasi-Fickian movement; the movement in which potassium releases partially from water filled pores and swollen matrix [81].
In contrast, these hydrogels exhibit different release exponent values in soil, CRF- PVA has 0.52 and CRF-PVA/CS hydrogel exhibit greater exponent value i.e., 0.92 and both hydrogels show non-Fickian movement (anomalous movement) while the CRF-CS hydrogel has a maximum release exponent value of 1.05 and show case II transport [82]. The release of potassium from fertilizer depends upon chemical structure that affects on swelling ratio of hydrogel. The CRF-PVA hydrogel has greater initial swelling ratio and hence potassium release coefficient is also greater. The swelling ratio and potassium release follow the descending order among three CRF hydrogels [80].
CRF-PVA > CRF-PVA/CS > CRF-CS
Chitosan- PAADU (double coated) based hydrogel: A novel synthesis of double coated fertilizer based superabsorbent and water retainer hydrogel includes coatings of chitosan and polyacrylic acid diatomite on a common fertilizer (NPK). The fertilizer was taken as core material whereas chitosan is directly coated on it [83]. The diameter of NPK fertilizer granules was taken within the range of 2.36–2.85 mm. The granules with damaged or defective surfaces were separated during sieving. After adding granules into the rotary drum, the chitosan powder stuck on NPK granules in the presence of epoxy dissolved in acetone and a strong adhesive agent HYUOP at regular intervals to get the first homogeneous and compact coating. For second coating (Polyacrylic acid diatomite – containing urea; PAADU) preparation, the acrylic acid (AA) neutralized with ammonia followed the addition of urea and diatomite to the solution. Subsequently, the aqueous solution of N, N- bismethylene acrylamide and potassium persulfate added to original solution with constant, vigorous stirring and nitrogen line [84].
The chitosan coated NPK fertilizer then used to have a coating of PAADU on it. For this purpose, the chitosan coated NPK fertilizer dipped in water to get moisture on it for sticking of PAADU powder and shaken continuously. The surface product has been cross-linked with the spray of 1% v/v methanol solution of epoxy chloropropane, dried in an oven at 70 °C to get double-coated slow-release NPK compound fertilizer with superabsorbent and water-retention. This double coated superabsorbent hydrogel has swollen up to 75times of original weight after 2 h of dissolution in tap water at room temperature [85]. The slow release, biodegradable, eco-friendly, and water retention properties of superabsorbent have increased its rating to use it in agriculture and horticulture.
Sulfonated corn starch polyacrylic acid phosphate rock (SCS/PAA/PHR) hydrogel: The phosphate rock (PHR) or phosphorite is a conventional fertilizer used as the source of essential elements for growth but the plants could not avail themselves easily. The researchers utilize this rock mineral with the combination of soil hydrogels to make it available for plants with controlled release properties [86]. The solution polymerization process has been followed for the preparation of sulphonated corn starch and phosphate rock based (SCS/PAA/PHR) hydrogel [87]. For the synthesis of sulfonated corn starch (SCS), the corn starch potentially combined with sulfur trioxide pyridine solution in the presence of Chloro-sulphonic acid. The sulfonated corn starch was grafted with acrylic acid (neutralized with KOH) in the presence of N, N- methylene bisacrylamide (MBA) and potassium persulfate (KPS). The phosphate rock is penetrating the SCS/PAA network to enhance the feasibility of fertilizer along with superabsorbent hydrogel. The hydrogel dried at 100 °C and particle size maintained up to 20 mesh [87].
The hydrogel tested for its properties and results showed that it has maximum swelling capacity in distilled water (498 g/g) while decreased in 0.9 wt% saline solution (65 g/g). The high water absorbency can be achieved by sustaining 0.3% wt of cross-linking agent, 1.7 wt% of ammonium persulfate, and 60% charge density of neutralized acrylic acid in distilled water. Besides, the availability of phosphorus and potassium depends on the feeding content of PHR directly. This study has opened the gates of utilization of PHR as fertilizer with hydrogel combo to facilitate its consumption by plants and prohibits the production of hazardous ions while using the manufacturing of phosphate fertilizers. The release of soluble phosphate from SCS/PAA/PHR is enhanced by the presence of SCS. It is found that hydrogel releases 0.058% phosphorus after 15 days and 0.060% after 30 days that is comparatively 4.5 times greater than direct usage of PHR [87], [88]. Table 1 contains the details of Precursor, monomer, initiator and cross-linking agent involved in the synthesis of Superabsorbent hydrogels. The list of natural and synthetic materials is classified in Fig. 8.
Table 1.
Details of Precursor, monomer, initiator and cross-linking agent involved in the synthesis of Superabsorbent hydrogels.
| Types of hydrogel | Precursor | Monomer | Initiator | Cross-linker | Conditions temp/time/pH | Mesh size/particle size | |
|---|---|---|---|---|---|---|---|
|
Cross-linked polymerization |
Pure natural |
Rice husk ash | AA,AM, | Potassium persulfate | MBA | 25°/15hrs | 9–24/2–0.71 mm |
| Wheat straw-CM | Citric acid monohydrate | – | MBA | 25°/24hrs | 150–800 μm | ||
| Sawdust cellulose | AA,AM, Urea | Potassium persulfate | MBA | 70°/4hrs | 2 × 2 mm(small pieces) | ||
| Cassava starch | AA | Potassium persulfate | MBA | 60°/2hrs | 40 mesh | ||
| Tapoica starch | AA, Urea | Potassium persulfate | MBA | 70°/0.5hrs/7 | 40 mesh | ||
| Guar gum (GG) | AA | Benzoyl peroxide | EGDMA | 55°/4hr | 40mesh | ||
| Guar gum (GG) | AA, Aniline | Ammonium persulfate | Hexamine | 35°/16hr | 60mesh | ||
| Guar gum (GG) | AA | TEMED | MBA | 80°/2hr | 60mesh | ||
| Modified Natural | Cellulose acetate | NaHCO3Esterification process | – | EDTAD | 25°/48hrs | 60 mesh | |
| CMC, CCNFs | AA, AM | Ammonium persulfate | MBA | 60°/48hrs/10 | 20-80mesh | ||
| Salep phosphate | AA | – | MBA | 55°/1hrs | 40mesh | ||
| CMC | Urea | NaOH | ECH | 40°/6hrs | 60mesh | ||
| Pure synthetic | Urea | AA | Potassium persulfate | MBA | 0.18 to 0.212 mm | ||
| Attapulgite | AA,AM | MBA | 40–80 mesh | ||||
| Urea | AA,AM | Ammonium persulfate | MBA | ||||
| AMPS | AMPS | Ammonium persulfate | MBA | 60°/1hrs | |||
| Combo | Chitosan + DAP | PVA | Base | Ethanol | |||
| Chitosan + NPK | AA, Urea, Diatomite | Potassium persulfate | MBA, epoxy Chloropropane | 35°/6hrs, 70°/2hrs |
2.36–2.85 mm | ||
| Sulphonate-d corn starch | AA, PHR | Potassium persulfate | MBA | 60°/1hrs | 20mesh | ||
| Graft polymerization | Pure Natural | Rice husk ash, NaAlg | AA,AM | Ammonium persulfate | MBA | 40°/3hrs | 40-80mesh |
| Chitosan | HEA | Ammonium persulfate | – | 65°/1hrs | 40mesh | ||
| Guar gum | NaA | Ammonium persulfate | MBA | 45°/2hrs | 40-80mesh | ||
| Father keratin | AA | Potassium persulfate, SBS | MBA | 60°/8hrs | 60mesh | ||
| Modified Natural | Sodium starch sulfate | AA, DMDAAC | Ammonium persulfate | – | 35°/2hrs | 60mesh | |
| CMC | AA | Gamma rays | MBA | 60°/48hrs | – | ||
| Pure Synthetic | Sodium humate | AA, AM | Potassium persulfate | DEG, SMB | 45°/2hrs/4.5 | 40mesh | |
| Combo | CMC, MMT | AA, AM, AMPS | Potassium persulfate | MBA | 40°/3hrs | 10-80mesh | |
| NaAlg, clinoptilolite | Ammonium persulfate | MBA | 60°/2hrs/10 | 40mesh | |||
| CMC, Attapulgite | AA, AMPS | Ammonium persulfate | MBA | 45°/5hrs/2–6 | 200mesh | ||
| Guar gum, Attapulgite | NaA, Styrene | Ammonium persulfate | MBA | 75°/3hrs/2–7.5 | 40-80mesh | ||
Fig. 8.
Flow diagram of hydrogels explaining the material and process used for synthesis.
Synthesis of hydrogels through grafting
Contrary to the cross-linking, the graft polymer usually consisted of a consecutive sequence of one type of the monomer horizontally that makes the backbone of polymer. Whereas the second monomer consecutively joined to form short chain connected vertically at any joint of the first monomer chain, thus a graft polymer is known as copolymer [89]. The use of two different monomers imparts versatile and attractive properties to the desired polymer because of possessing different functional groups.
The graft copolymerization of a monomer onto polymer backbone chain is usually accompanied by three methods; free radical polymerization, living radical polymerization and ring opening polymerization [90]. All these methods are primarily based on three approaches; i) grafting to, ii) grafting from and iii) grafting through. A “grafting to” methodology involves the coupling mechanism of functional groups “A,” distributed randomly on backbone chain, with active ends of polymer branches, to be attached on backbone chain [91]. On the other hand, the “grafting from” methodology starts with the generation of initiating sites that will react with monomer and extend vertically in the form of graft polymer. This “grafting from” methodology can easily be used to control the percentage grafting and number of grafting branches by introducing number of initiating sites [92]. The “grafting through” or “macro-monomer” method involves the copolymerization of one macro-monomer containing acrylate functional group with low molecular weight monomer. It is comparatively convenient method to synthesize cellulose based macro-monomers. The most commonly used approach is “grafting from” because it yields high density grafted polymer by reacting chain ends of branch polymer [93].
The grafting polymerization usually proceeds with initiation of active sites for the grafting of monomer on backbone chain of polymer. In this regard, chemical initiation can be done by four different techniques like a) free radical by using ammonium persulfate (APS) or potassium persulfate (KPS) b) ionic initiation c) irradiation and d) photochemical initiation [94].
Free radical graft co-polymerization
Researchers have concentrated their attention on this most frequently used methodology for the synthesis of graft polymerization. Almost 60% of market polymers were synthesized by using free radical polymerization [95]. It is a chain reaction process mainly comprised of three major steps, initiation, propagation, and termination. The chain polymerization starts with the generation of radicals that make a covalent bond with monomer and followed by the subsequent addition of a several monomers for propagation [96]. The termination of polymerization process may ends with the combination of propagating radicals with growing polymer chain through disproportionation (abstraction of a hydrogen atom by other growing polymer) or chain transfer method [97].
All acrylic and vinyl based graft polymerization on cellulose follow the free radical mechanism by using the “grafting from” approach. This methodology includes marvelous feature like mass production of countless co-polymers, inexpensive and ease of implementation. It is mostly used for the polymerization of different vinyl based monomers (meth-acrylamide, methyl acrylates, vinyl acetate), water soluble monomers (hydroxyacrylates, acrylic acid and N-vinyl pyrrolidone) and organic monomers like styrene and butadiene [98]. In graft polymerization, potassium persulfate is the most commonly used initiator. It was reported in research that graft copolymerization of itconic acid was done on cellulose by the use of potassium persulfate, xanthate mixtures and cellulose thiocarbonate reaction systems [99].
The initiation of cellulose radical in the “grafting from” technique can also be done by chemical initiation method: the direct oxidation of cellulose through cerium+4 ions [89]. It got more significance among all redox initiation system (like Fenton’s reagent: ferrous ion with hydrogen peroxide) because of its high grafting efficiency and easy implementation [25]. In this method, the cerium ions generate cellulose radicals in the presence of nitric acid. It follows the oxidation of cellulose by cerium based salt by a single electron transfer mechanism. These cellulose radicals combine with vinyl monomers to yield graft copolymerization. This initiation mechanism involves the formation of a cellulose-Ce complex that later on follows the disproportionation process to yield cellulose radicals [100]. The purpose of this mechanism is to get high rates of polymerization at a very low temperature.
The initiation of cellulose radical can also proceed with radiation induced mechanism by using energy rich gamma radiations from electron beam or radioactive isotopes and UV light [68]. The irradiation of cellulose generates free cellulose radicals that can follow the route of graft copolymerization of acrylic and vinyl monomers. The use of gamma radiations, for generation of radical, follow two approaches, one is pre-irradiation and second is mutual irradiation [101]. The pre-irradiation got priority in application because it yields a comparatively less number of homo-polymers as in mutual irradiation. On the other hand, the use of UV-light for irradiation is more conveniently used because it has very low energy radiation that will cause less degradation of cellulose backbone chain and can have controlled grafting copolymerization [102].
Ionic & ring opening graft polymerization
The ionic graft polymerization may have proceeded in two different conditions the nature of ionic species either cation or anion [103]. The graft polymerization of isobutylene and methyl styrene initiated cationically onto a cellulose substrate. For initiation of cellulose cation, a Lewis acid or electron deficient molecule can be used. The most frequently Lewis acids used are BF3 or AlCl3, in the presence of a co-catalyst (ethanol or water) to captures the proton from cellulose, its substituent or derivative after getting adsorbed on cellulose surface [104]. The production of initiator would be the same as in free radical polymerization but the entire mechanism is different for both categories. This cellulose cation makes a complex that later on follow the graft polymerization by reacting with α-methyl styrene or isobutylene (monomers) [105].
The polymerization of methacrylonitrile, acrylonitrile, and methyl methacrylate on cellulose was done anionic graft polymerization in the presence of inert solvent like ammonia at low temperature conditions. The most commonly used initiator for anionic graft polymerization is IA metal alkoxides of cellulose. In this case, the alkoxide of cellulose attacks the vinylic carbon of acrylonitrile and shifts its negative charge to the other vinylic carbon of acrylonitrile [106]. Thus several acrylonitriles go on reacting with propagating anion and extend chain growth. The anionic polymerization will terminate by the chain transfer of proton to the hydroxyl group of other cellulose and leaves an anionic initiator. The chain transfer may occur with monomer or solvent molecules [56]. The anionically graft polymerization of cellulose possesses 15000 g/mol of its molecular weight with high degree of substitution. Although it is still a challenge for scientists and researchers because it is quite difficult to control the reactivity of cellulosic-OH group with carbanion to get avoid from side product formation [107].
Ring opening polymerization is a less common method for polymerization of cellulose through ring opening. The ring opening polymerization of paper cellulose and solid cotton was first time done with a cyclic monomer that is ε-caprolactone in the presence of tartaric acid acting as a catalyst [108]. The yield of grafting of caprolactone on cellulose paper was 490% and it has increased 11% weight of paper than original. In another study, it was reported that cellulose fibers were grafted with L-lactic acid and ε-caprolactone by using ring opening polymerization [109]. The percentage of grafting can be controlled by adjusting the ratio of concentrations of monomers to the initiators. The polymers obtained from ring opening polymerization exhibit high resistance against degradation by the enzymes [109].
Living radical polymerization
The living polymerization is also known as chain growth process that proceeds without breakage of the reaction chain. It is the opposite of irreversible termination and chain transfer processes. This polymerization facilitates the equal distribution of molecular weight, architecture, and controlled composition of polymer [110]. Although the coupling of living radical polymerization with free radical polymerization provide more options to choose a bunch of monomers under different conditions to yield outstanding featured polymers. Now a day, living radical polymerization is being applied for cellulose grafting by using the “grafting from” approach. This approach usually provides an opportunity to control the graft length, composition, architecture, and surface properties of polymer [111].
Synthesis of soil hydrogels based on natural precursors
Pure natural precursors
Sodium alginate grafted poly acrylic acid co-acrylamide rice husk ash (NaAlg-g-poly(AA-co-AAm)/RHA) hydrogel: The synthesis of superabsorbent hydrogel involved two steps: the first step includes the preparation of rice husk ash while the 2nd step follows the synthesis of NaAlg-g-poly (AA-co-AAm)/RHA superabsorbent hydrogel. First of all, the rice husk washed with distilled water and rinsed off completely to get rid of dust particles. The acid leaching process was applied for the removal of metal based impurities. The boiling process takes place in the presence of an aqueous solution of hydrochloric acid and sulphuric acid solution in two turns and followed by burning in a furnace to get white ash [112]. This rice husk ash added in a four neck flask along with monomers AA, AM, sodium alginate. The use of ammonium persulfate initiates the alginate radicals and N, N- methylenebisacrylamide (MBA) catalyzes the reaction mixture under constant stirring in the presence of a nitrogen atmosphere. The temperature of the solution elevated to terminate the graft polymerization [113]. In another study, the authors used this formulation of hydrogel to prepare a nutrient rich fertilizer containing NPK the essential elements required for the growth of plant [114].
HEA grafted chitosan based hydrogels: The hydroxy ethyl acrylate (HEA) can be easily grafted on chitosan by the addition of HEA and ammonium persulfate as an initiator into the solution of chitosan. The solution purged with argon gas in a glass ampoule. After polymerization, it gets precipitated by acetone, filtered, and separated by using exhaustive extraction of the product with ethanol. The hydrogel examined for the influence of reaction variables on grafting efficiency (%E) and grafting percentage (%G) [115]. Both parameters consistently increase as the feed of HEA monomer increased and 60 min duration for polymerization. The maximum %G and %E have been found at 2% wt of chitosan and 10 mmol of APS concentration. If the amount of initiator crosses the limit of 10 mmol then it surely breaks the ether bond (C-O-C) of chitosan backbone chain results in fragmentation of chitosan. The HEA grafted hydrogel is temperature sensitive and yields maximum %G and %E at 50–60 °C [115].
Guar gum-g-poly sodium acrylate (GG-g-PNaA)hydrogel: The novel GG-g-PNaA based hydrogel can be prepared by dispersing guar gum (GG) sodium hydroxide (NaOH), acrylic acid (AA; neutralized with an aqueous solution of sodium hydroxide) and N, N-methylene bisacrylamide (MBA) under magnetic stirring. The reaction proceeds with grafting polymerization with the initiation of radicals by the use of ammonium persulfate solution (APS).. The biodegradable hydrogel shows high water absorbency (1107 g/g) at optimum conditions and reaction proceeds at 70° [116].
Hydrolyzed feather keratin-poly acrylic acid (HFK-PAA) Hydrogel: The chicken feather is abundant by-products of Poultry farms and these feathers are chemically protein in nature that consists of keratin protein majorly. The keratin is contained a higher content of amino acid cysteine (7%) than present in canola (0.63%) and soy (1.26%). The researchers have focused to utilize these waste products of poultry farms in fruitful form for agricultural benefits. The feather keratin hydrogel is based on a protein solution of hydrolyzed feather keratin (hydrolyzed by 1 M hydrochloric acid) with subsequent addition of two initiators (potassium persulfate (KPS) and sodium bisulfate;SBS), N, N methylene bisacrylamide and neutralized acrylic acid. The stirring continues till the end of the polymerization process then hydrogel washed by ethanol (two times). The maximum water absorbency (501 g/g) in water has achieved at the optimum composition. The utilization of HFK in hydrogel makes it biodegradable and eco-friendly with significant properties required for a soil conditioner. It will be more eco-friendly with the environment if keratin hydrolysis takes place under microwave radiations or superheated steam. This hydrogel is very sensitive to a minute change in pH and saline concentration [117].
Modified natural materials
Sodium starch sulfate based amphoteric hydrogel: The amphoteric hydrogel synthesis includes the first preparation of sodium starch sulfate (SSS) from corn starch by following Schweiger’s method. The water content can be eliminated from freshly synthesized SSS by acetone. The second step includes the polymerization process for the synthesis of an amphoteric hydrogel. In this regard, the SSS solution mixed with acrylamide, diallyldimethyl ammonium chloride (DMDAAC), ammonium persulfate and N, N methylene bisacrylamide under nitrogen atmosphere. At last, un-reactive monomer and homopolymers are separated by washing the hydrogel with methanol and distilled water. The swelling properties of amphoteric hydrogel vary concerning the content of acrylamide (10 g), initiator (0.3%) and cross-linking agent (0.07%) at low temperature [118]. The low temperature facilitats the decomposition of the initiator and increases the diffusion of monomer. Besides these parameters, the amphoteric hydrogel has an important salt linkage that may also influence the swelling properties. The amphoteric hydrogel contains additional sulfate, amino, and carboxylate groups that makes intermolecular linkage thus the cross-linking agent is used in less quantity as compared to other hydrogels [119].
Synthesis of CMC-PAA radiation grafted hydrogel: Radiation based grafting for the synthesis of superabsorbent hydrogel provides marvellous benefits and reduces the consumption of energy (proceeds at room temperature), initiator, and produce contamination free hydrogel [120]. For the synthesis of radiation based grafted hydrogel, the mixture of carboxymethyl cellulose (CMC), acrylamide (AA) and MBA homogenized, bubbled with nitrogen and exposed to gamma radiations at a dose of 0.14 kGy/min with a total dose of 2 kGy. The radiation grafting increases the functionality of carboxylate groups of polymeric backbone to impart highly demanding properties. The radiated material has gone through the disposal of water soluble un-reactive monomers and subsequent purification by a soxhlet extractor for the removal of homopolymer. The gamma radiation dose is very crucial for this hydrogel because doses less than 2 kGy do not start it and beyond this dose will reduce swelling properties ultimately. For the concern of the ionic strength, the role of multivalent anion (Cl−1 > CO3−2 > PO4−3) also decrease the swelling ratio due to effective concentration of anions in surrounding medium [52].
Synthesis of soil hydrogels based on synthetic materials
Pure synthetic material
Poly (AA-co-AM)/AlZnFe2O4/Na hydrogel: The humic substances releases organic content to the soil, increases the photosynthesis & biomass absorption, and develops the soil structure. Thus, they are potentially used in the agriculture field in the form of a humic salts with low cost and high affinity for water [121]. The synthesis of graft polymer superabsorbent hydrogel nano-composite (abbreviated as PSHNC) like poly (AA-co-AM)/AlZnFe2O4/SH hydrogel synthesis based on the dissolution of acrylic acid (AA) and acrylamide (AM) in de-ionized water. The AlZnFe2O4 nanoparticles (prepared by the co-precipitation method; Shahid et al. 2012) added along with triton X-100, sodium humate (SH), diethylene glycol (DEG), metabisulfite (SMB) and potassium persulfate (KPS) into reaction mixture. The 1 M aqueous solution of sodium hydroxide (NaOH) used to maintain pH at 4.5 with elevated temperature at 75 °C. The temperature of the mixture is lowered to 45 °C with the addition of 37% formaldehyde. This superabsorbent hydrogel is specially designed and applied to the sandy soil and grass seedling growth. The PSHNC exhibit maximum water absorption (892 g/g) in distilled water at 2.5 g of AlZnFe2O4, 1.5 g of sodium humate (SH), 5 g of diethylene glycol and 0.2 g of initiator [75]. The 0.4% application of PSHNC to sandy soil has increased the 130% of water retention with prominent decrease in hydraulic conductivity of soil. Thus, it can be used broadly in agriculture for drought prone conditions, horticulture, and sandy soil to get a high yield of grass [122].
Combo materials
Sodium carboxymethyl cellulose-g-poly (AA-co-AM-co-AMPS)/MMT hydrogel: The Synthesis of sodium carboxymethyl cellulose-g-poly(AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel based on the mixing of sodium carboxymethyl cellulose (CMC) with sodium salt of montmorillonite (MMT), potassium persulfate, acting as an initiator to generate radicals, in distilled water. The temperature of reactants dropped to 40 °C, the mixture of acrylamide, acrylic acid, N, N-methylenebisacrylamide (MBA) and acrylamido-2-methyl-1-propane sulfonic acid (AMPS) mixed along with Sodium hydroxide (NaOH) to maintain the pH and heated for 2 h at 70° for completion of grafting polymerization reaction [123]. The precipitates of the product can be collected by adding methanol to water (5:1) mixture. The chemistry of cellulose-g-poly(AA-co-AM-co-AMPS)/MMT hydrogel is shown in Fig. 9.
Fig. 9.
Chemistry of cellulose-g-poly(AA-co-AM-co-AMPS)/MMT hydrogel.
The hydrogel comprises of porous structure based on cross-linking of AA, AM on CMC backbone along with MMT content. Similar to the ionic hydrogel, this is also pH-sensitive within the range of 5–9. The strongly affected factor on swelling is the ionic strength of salt solution. The monovalent ion (K+1 > Na+1) has comparatively less ionic strength but imparts maximum swelling than divalent ions (Ca+2 > Mg+2). These ions penetrate to the porous structure of hydrogel so that it compensates for the electrostatic effect of carboxylate and sulfite ions. The hydrogel exhibits high swelling and water retention properties at 10–80 mesh size to maintain the maximum interstitial volume of small particle size [97].
NaAlg-g-Poly (AA-co-AM)/Clin hydrogel nanocomposite: The clinoptilolite (natural zeolite), has microporous structure of silica and tetrahedral alumina that has highly ion exchange properties and affinity for ammonium ions, so broadly used in agriculture with a combination of alginate. This zeolite combines with alginate and dispersed in water for the synthesis of NaAlg-g-Poly (AA-co-AM)/Clin hydrogel [124]. This solution passes through ultra-sonication and then added into sodium alginate, acrylamide, acrylic acid having 70% degree of neutralization (neutralized by adding 6 mol/L freshly prepared aqueous solution of NaOH), and N, N -methylene bisacrylamide (MBA). The ammonium persulfate (APS) can generate free radicals to proceed free radical polymerization process. The process terminated after heating the mixture with continuous stirring at 1000 rpm speed under a nitrogen atmosphere. The procedure of sodium alginate graft polymerization of (AA-co-AAm)/clinoptilolite is similar to the sodium alginate graft polymerization of (AA-co-AAm) hydrogel. The introduction of 10% clinoptilolite at pH-10 to the hydrogel enhances the swelling rate and the release of nutrients to the soil required for plant growth because it also behaves as physical cross-linking agent. In the comparison of a local hydrogel, this nanocomposite hydrogel has a record of 54.23% of nutrient release in 30 days whereas the former case has 50% release within 2 days [90].
CMC-g-poly (AA-co- AMPS)/ATP Hydrogel: The attapulgite has the composition of magnesium aluminium phyllosilicate, found in clay, mostly used in medical field for treatment of diarrhea and in agriculture field for the synthesis of hydrogels [99]. For the synthesis of CMC-g-poly (AA-co- AMPS)/ATP Hydrogel, the carboxymethyl cellulose (CMC), the inorganic clay Attapulgite (ATP), PEG and Na2CO3 were dissolved in distilled water. Another mixture of ammonium persulfate (APS), N,N-methylene bisacrylamide (MBA), neutralized acrylic acid (AA) and 2-acrylamido-2- methylpropane sulfonic acid (AMPS) mixed with solution and agitated ultrasonically for 30 min. The polymerization terminates with the addition of H2O2 and NaHCO3 at 75 °C. This eco-friendly hydrogel exhibit a maximum swelling ratio of 864 g/g in distilled water at 0.25 g/g ratio of AMPS to AA, 0.2% of cross-linking concentration with 1:0.22 of CMC to ATP weight ratio in acidic medium (pH ranges from 2 to 6) [125]. The swelling may also be affected by the metallic ion activity on the coordination of carboxylate groups for the production of inter-molecular complexes. The swelling ratio varies with the nature of metallic ions in descending order (Na+1 > Mg+2 > Ca+2 > Fe+3) [99]. The water retention capacity also enhanced by the introduction of ATP at its lowest concentration (0.05) and has improved the water retention by up to 74%.
Porous GG-g-P(NaA-co-St)/APT hydrogels: The synthesis of novel fast porous GG-g-P(NaA-co-St)/APT superabsorbent hydrogel comprised of guar gum solution, acrylic acid (AA), styrene (St), attapulgite (APT) and a cross-linker MBA mixture. The ammonium persulfates (APS) generates the radical in free radical graft polymerization that terminates after 3 h while turning to a gel. The hydrogel follows the surfactant self-assembling template pore-forming technique with the use of sodium n-dodecyl sulfate (SDS) as a surfactant in gel formation [126]. The pore formation is facilitated by the use of surfactant as a template but it never becomes part of hydrogel and can be removed by washing with methanol–water (8:1) solution. The launching of SDS plays a vital role in enhancing the swelling rate, salt solution, and pH. The maximum swelling (948 g/g) achieved at the dose of 1.8 mmol/L of SDS, 5 mmol/L of Nacl solution at pH ranging 2.1 to 7.4 in distilled water. The swelling of hydrogel is strongly affected by the cation nature (Na+1 > Mg+2 > Al+3) in salt solution [56]. The synthesis process, properties and its application are summarized in Table 2.
Table 2.
Superabsorbent hydrogel process, properties and its application.
| Hydrogel | Author | Year | Polymerization process |
Property |
Application | ||
|---|---|---|---|---|---|---|---|
| % Water retention | Swelling g/g | Slow release | |||||
| PAMACRYL/RHA | Rodrigues et al. | 2013 | Cross-linking | 67 | 1077 | – | Agriculture |
| CSt-g-PAA/NR/PVA semi-IPN | Sayant et al. | 2018 | Grafting+ Cross-linking | – | 794 | – | Agriculture |
| SC-HSRF | Panfang Lu* et al. | 2019 | Grafting+Cross-linking | 122 | 210 | – | Sandy, horticulture |
| TS-PAA-Urea encapsulated | Karak et al. | 2019 | Cross-linking | 81 | 700 | – | Agriculture |
| GG based poly-g (AA-cl-EGDMA | Thombare et al. | 2018 | Grafting+Cross-linking | 52 | 625 | – | Agriculture |
| GG-cl-poly-(AA-ipn-PANI | Kalia et al. | 2014 | Grafting+Cross-linking | – | 5307 | – | Sandy soil |
| porous cl-GG-g-poly (acrylate) | Singh et al. | 2016 | Grafting+Cross-linking | – | 523 | – | Agriculture |
| MC-g-p (AA-co-AM)/CCNFs | Shiyu Fu et al. | 2013 | Cross-linking | – | 458 | – | Agriculture |
| LCSH | Cheng et al. | 2017 | Cross-linking | – | 835 | – | |
| PAA-AM/SH/APT | Wang et al. | 2005 | Cross-linking | – | 996 | – | Horticulture |
| PAA-SRFH | Rabat et al. | 2016 | Cross-linking | – | 167 | – | Agriculture |
| PAMPS | Elazab et al. | 2017 | Cross-linking | – | 223 | – | Clay, loam, silt |
| CRF-CS/PVA | Tongsai et al. | 2010 | Cross-linking | 70 | 300 | 0.92% | Agriculture |
| SCS/PAA/PHR | Jiang et al. | 2012 | Cross-linking | – | 498 | 0.06% | Agriculture |
| NaAlg-g-poly(AA-co-AAm | Ali olad et al. | 2017 | grafting | – | 1070 | – | Agriculture |
| guar gum-g-poly sodium acrylate | Wang et al. | 2010 | grafting | 8% water retain up to 30 days | 1107 | – | Sandy soil |
| HFK-PAA | Wattie et al. | 2016 | grafting | – | 501 | – | Agriculture |
| CMC-PAA | Suwanmala et al. | 2013 | Radiation grafting | – | 190 | – | Agriculture |
| poly (AA-co-AM)/AlZnFe2O4/SH | Nada et al. | 2015 | Grafting | 130% retention | 892 | – | Sandy soil + grass seedling growth |
| NaAlg-g-Poly (AA-co-AM)/Clin | Ali Olad et al. | 2014 | grafting | – | 60% | 54.23% released in 30 days | Agriculture |
| CMC-g-poly (AA-co- AMPS)/ATP | Suliman et al. | 2016 | grafting | 74% retention | 864 | – | Agriculture |
| porous GG-g-P(NaA-co-St)/APT | Wang et al. | 2011 | grafting | – | 948 | – | Agriculture |
Properties of soil hydrogels
Soil hydrogels are substantially used in agriculture field in the perspective of soil conditioner and plant growth promoter. The plant growth promotion directly depends upon two main parameters; one is moisture content in soil which to be used by plants and the second is nutrient holding capacity of soil [127]. The soil hydrogels boost up both these parameters by making them more feasible and suitable for plant growth. A soil hydrogel has a high swelling rate, maximum water absorbency with slow nutrient release to the soil so that plants can avail nutrients for a longer time. Different soil hydrogels have a varying degree of these properties depending on the nature of monomers and polymerization process used. All soil hydrogels exhibit mainly three given properties [128].
-
•
Swelling
-
•
Water absorbency
-
•
Nutrient release
Soil hydrogel as a conditioner
Soil hydrogel as a conditioner enhances the physical, mechanical as well as fertility properties of soil. Soil conditioner is usually considered in the meanings of soil amendments and majorly a nutrient rich fertilizer or inorganic based chemicals [129]. A soil conditioner rebuilds the damaged soil, boosts up the soil features to make it feasible for proper plant growth. Soil hydrogels as a soil conditioner is an inclusive terminology that covers four major areas like water retention, soil nutrient, soil structure, and cation exchange [130]. Soil hydrogels play an important role in the first two categories.
Swelling behavior
Swelling is the consequence process of the relation between a matrix of hydrogel and a solvent [131]. Swelling is the initial process of salvation of salt or hydrogel into a solvent and extended three dimensionally [61]. It is a tool to measure the available free space between joints or cross-links of polymer hydrogel to know increased cross-link density after swelling. The swelling property of superabsorbent hydrogels overcome the worldwide issues of water consumption in agriculture, helps in improving soil water retaining property and reduction in plant’s wither rate [74].
Factors affecting swelling
The swelling behavior of a soil hydrogel directly depends upon the structure of hydrogel, polymerization process (either grafting of cross-linking), the concentration of cross-linking agent and particle size of hydrogel. All factors affect the swelling but the last factor affects the more. If the concentration of cross-linking agent rises, as a result, high densification of hydrogel and the structure of hydrogels become more congested due to the increased number of cross-links on polymer chain [131]. This densification of hydrogel prohibits the expanding of structure on water absorption. There must be flexibility in the structure of hydrogel so that it can expand and exhibit swelling behavior on absorbing a defined amount of water [154]. The swelling behavior facilitates the water absorption for sandy soils with less water holding capacity and provides water content to plant slowly for a long time. Fig. 10 is showing the factors affecting the swelling of the hydrogel.
Fig. 10.
Factors affecting the swelling of hydrogel.
Effect of hydrogel composition on swelling
The hydrogel composition is important as other factors. A study has explored the swelling dynamics of carboxymethly cellulose (CMC) hydrogels, cross-linked with acrylamide by using free radical polymerization, directly dependent on the composition and concentration of monomers [105]. In this hydrogel swelling ratio increases in the same manner as the concentration of carboxymethly cellulose (CMC) increases. The reason behind this factor is due to the affinity of hydrophilic functional groups attached to carboxymethyl cellulose to the water. As the concentration of CMC increases, the number of hydrophilic groups increases that resultantly enhances the water absorbency [64]. Beyond a defined limit of CMC consequently reduces the swelling ratio because this will result in the more densification of the backbone chain that hinders the diffusion of water and relaxation of polymeric structure [132].
The two guar gum hydrogels grafted with acrylic acid & acrylamide poly(GG-g-AA/AM) and with N-isopropyl acrylamide poly(GG-g-AA/NIPAM) have varied swelling behavior according to the factors [133]. The concentration of guar gum (GG) and cross-linking agents also affects the swelling and was experimentally measured that swelling of hydrogel decreased as the concentration of GG increases from 5%wt to 15%wt and cross-linking agent’s concentration increases from 1.5%wt to 2.5%wt [134]. It is justified by the phenomena of greater probability of increasing cross-linking that directly affects water absorption capability. If the monomer acrylic acid (AA) was replaced with N-isopropyl acrylamide (NIPAM) then it was observed that the latter category showed greater swelling than former. The temperature change effect has confliction for both these hydrogels [74].
Effect of cross-linking agent
The chitosan based glutaraldehyde cross-linked hydrogel (poly-(CS-cl-gluald)) was studied for the effect of different cross-linking concentrations on swelling behaviour [20]. The swelling kinetics has a variation with different conditions of pH, temperature and concentration of cross-linking agent. The swelling ratio decreases by the increase of glutaraldehyde because it decreases the available space for expansion of the structure. Thus diffusion of water into hydrogel decreases and affects water absorbency. In contrast to the high concentration of cross-linking agent, its low concentration makes less cross-link that also results in a decrease of swelling ratio [135]. So there must be an appropriate concentration of cross-linking agent to get maximum swelling ratios. The low cost superabsorbent hydrogel synthesized by urea and acrylic acid in the presence of 0.005% MBA cross-linking agent show maximum swelling ratio in distilled water is 835 g/g. Similarly, the swelling ratio of semi-IPN CSt-g-PAA/NR/PVA hydrogel is 794 g/g in distilled water at 0.05% concentration of MBA [136].
The rice husk ash based Poly (acrylamide-co-acrylate) hydrogel exhibit the highest swelling ratio (1077 g/g) in distilled water at 0.05% concentration of MBA cross-linking agent [137]. The variations in swelling ratios of different hydrogels by using the same cross-linking agents vary with the nature of monomers, precursor, and the ratio of monomer to MBA concentration of cross-linking agent. In the case of guar gum-polyacrylic acid–polyaniline interpenetrating network based (GG-cl-poly-(AA-ipn-PANI) hydrogel shows swelling (5307%) at a 53.4 × 10−3mol/L concentration of hexamine as a cross-linking agent. In some guar gum based hydrogels it is also found that swelling decreases with increasing cross-linking agent concentration.
Effect of pH
The cationic and anionic groups of hydrogel polymer behave differently in either acidic or basic media. When the hydrogel, containing cationic groups in polymeric chain, is immersed in acidic media (with pH < pKa), the “protonation” of cationic groups of the hydrogel network decreases thus increasing the concentration of cationic groups [78]. This enhances the electrostatic repulsion between the same or different polymer chains, and the hydrogel is thus expanded that results in an increase of swelling ratio. In basic media, the concentration of cationic groups decreases relatively and electrostatic repulsion causes the chain contraction that results in decreasing the swelling ratio [113].
The opposite behavior occurs in the case of anionic polymer chains. The anionic superabsorbent hydrogels, like cellulose-g-P (AA-co-AM-co-AMPS)/MMT, contains ionic cross-linking and exhibits ionic interaction that is directly affected by pH of the solution. The hydrogel showed maximum swelling at pH ranging from 5 to 9. At this pH, the structure of hydrogel expands as a result of increased ionic repulsions between carboxylate and sulfonate ions and show maximum swelling because hydrogen bonding was diminished.
Effect of ionic composition
The ionic nature of medium along its concentration plays a vital role in the swelling behavior of polyelectrolyte hydrogel. These hydrogels are very sensitive to a minor change in pH and ionic strength. The ionic strength along with the nature of the ions influences indirectly on the swelling of hydrogel [86]. Under the same reaction conditions, the hydrogen ions suppress the swelling capacity by developing an association with negative ions like carboxylate ions [97]. The low ionic concentration of medium results in achieving equilibrium of swelling ratio. This swelling trend is shown by the basic solutions of different pH and neutral solutions but the solvent used for a solution must be de-ionized water. In partially charged hydrogels, the situation is the same; the swelling degree tends to decrease with the increase in ionic concentration. The hydrogels having an uncharged network also follow the same pattern but the swelling ratio can be determined by interaction parameter and cross-linking density. The swelling of the polyelectrolyte hydrogels does not depends on the nature of ion and its size but is remarkably affected by ionic strength.
The studies showed that the swelling of cellulose-g-Poly (AA-co-AM-co-AMPS)/MMT based hydrogel also affected by the nature of ions. The monovalent cations having less charge with large ionic radius impart positive influence (maximum swelling) while the cations having bivalent charge with small ionic size imparts comparatively negative influence (less swelling). The cations exhibit swelling in the following order.
The swelling degree of cross linked kaolin/clay-based hydrogel composites showed higher swelling due to the presence of carboxyl and hydroxyl groups in the hydrogel network. The Donnan equilibrium theory explains that if the ionic strength is increased then the distribution of mobile ion concentration between salt solution and hydrogel decreased. Resultantly, the osmotic swelling pressure of ions inside hydrogel decreased thus it doesn’t show high swelling. Actually, it is the osmotic pressure that enforces the hydrogel to swell. If a salt contains metals then swelling degree also decreases because of metal complex formation with hydroxyl and carboxyl groups of the hydrogel. It ultimately reduces the repulsions between polymeric cross-links [7].
The weight of cross-linked ethylcellulose/poly-(acrylic acid-co-acrylamide) hydrogels increased 134 times in distilled water and 70 times in tap water with the comparison of dried weight of hydrogel. This study has experimentally proved the effect of ions on swelling behavior of hydrogel. Donnan’s equilibrium theory also favored this experiment and explains the cause of swelling due to charged ions in tap water [138]. The presence of ions in tap water decreases the water absorbency (swelling) due to the decrease of osmotic pressure difference inside and outside of the polymer. If there are multivalent ions are present then swelling will decrease more because of the ionic cross-linking on the surface of hydrogel. The water absorbency (swelling) is also affected by the screening effect of cations present in a salt solution (Na+, Mg2+ and Ca2+) to the counter ion of polymer (carboxylate and hydroxyl). In short, the cations of saline solution always compete with soil nutrients for active sites on hydrogel polymeric structure. The salt concentration must be less to achieve the goal of using superabsorbent hydrogel for water absorbency, water retention, and controlled release of nutrients in agricultural field [139].
The impact of anion’s nature, present in the medium, and its concentration has also a profound effect on swelling ratio. The anions may develop interactions with cations of medium and have a strong effect on effective concentrations in the medium. The monovalent anions (Cl−1) have more tendency of decreasing swelling ratio due to their greater effective concentrations in medium. On the contrary, the multivalent anions (Co3−2, PO4−3) make ion pair with Na+1 ion, so their effective concentration reduces and causes a decrease in swelling resultantly [120]. The nature of anions has decreasing order of their effects in swelling ratio.
Effect of temperature
The hydrogel’s behavior is dual towards temperature change. Some hydrogels show positive relation with temperature change and their swelling properties also increases with increasing temperature. Contrary to the first kind, some hydrogels show a negative relation with temperature change that is known as inverse dependence on temperature, the reason behind this is the presence of hydrophobic interactions [140]. This behavioral variation depends upon the nature of linking monomer and its LCST. The guar gum based different hydrogels were prepared with temperature ranges 15–60 °C. These hydrogels exhibit the highest swelling index value at 15 °C [141].
Water retention
Besides swelling of hydrogel, another marvelous property is water retention or water holding capacity that is different in all types of soil. It may vary due to the compactness of soil particle and their sizes [77]. The water retention ability of soil is very important for plant growth as the water is mandatory for the synthesis of glucose by plant. As we are facing the global water issue so we need to consume less water with minimum wastage of water in irrigation [142]. A soil hydrogel exhibit such amazing property that can retain limited water in its 3D structure for longer time and reduces the more consumption of water. This property is especially applicable for all those soil types that cannot retain whole irrigated water [143]. There are some factors that affect water holding capacity.
Effect of particle size of hydrogel
Particle size is a leading factor that has direct impact on two main properties that are water absorption and water retention. As the particle size of hydrogel decreases then it has greater water absorption and retention properties because the smaller size increases the surface area. The water can accommodate within the three dimensional structure of hydrogel and interstitial spaces created by swollen hydrogel particle [97]. The smaller particle size and greater interstitial spaces both increases the water absorption and water retention properties.
The superabsorbent hydrogel (cellulose-g-P (AAco- AM-co-AMPS)/MMT) has studied for water absorption and water retention with different particle sizes ranging from 10 mesh to 80 mesh. The smaller size hydrogel particle shows greater water absorption and retention properties whereas the larger size hydrogel particles exhibit less value of both properties. The interstitial spaces also increase with the decrease in particle size of superabsorbent hydrogels [144].
Effect of particle size of soil
The particle size of soil is also an important factor to hold the water content for plant growth. All types of soil have different particle sizes. The smaller particle size of soil has greater water holding capacity. In clay, the particle size is smaller so it has greater water holding capacity whereas, in loam and silt soil, the particle is comparatively large than clay so it has less water retention properties than clay [145]. Contrary to the aforementioned, the sand has largest particle size with less water retention capacity so it can’t hold water and percolates down soon. The water retention can be enhanced by using soil hydrogels for all these types of soils [146]. A hydrogel, Hyd/MMT/NPK, is applied to a soil and examined the water holding capacity (WHC). It was observed that the soil without hydrogel has 71.6% water retention on 15th and 53.4% on 30th day but when the hydrogel is applied to the soil’s water retention percentages increases as 90.4% on 15th days and 78.1% on 30th day[147]. A study has proved that water retention capacity of poly acrylic acid is less than polyacrylamide.
The obvious difference in water retention capacities calculated for the application of AA-Co-AM hydrogel on sandy, loam and clay soil. The water retention capacity determined in terms of saturated (θs), residual (θr) and available water content (AWC). The value of θs is directly related to the concentration of applied hydrogels to the soil type. It is found that the value of θs for sandy soil is 2.2 fold, for loamy soil is 1.8 fold whereas clay soil has 1.5 folds than the control group [143]. The residual water content (θr) also has same relation as the saturated water content (θs). Greater the value of applied hydrogel, the greater will be residual water content (θr). The results obtained for 8 g/kg concentration of hydrogel, the sandy soil has 5 times greater water content (θr), for loamy soil the (θr) is 3.5 times while clay soil has 2.5 times more than the control group. The reason behind the greater value of (θr) for sandy soil is that its cation holding capacity is less than loamy and clay soil and results in expansion of the polymer so it increases the water retention capacity. In the case of available water content it is calculated that for sandy soil, the AWC value is 3 folds than that of control group on the application of 8 g/kg concentration of hydrogel.
A superabsorbent hydrogel nanocomposite (poly (Acrylamide-co-acrylic acid)/AlZnFe2O4) is applied to the sandy and loamy soil to check the water retention capacities. The hydrogel is applied to soil with different concentrations (0, 0.1, 0.2, 0.3 and 0.4 w/w %). It is found that water retention capacity has increased up to 60% for 0.1% and 100% for 0.4% hydrogel concentrations [148]. The water retention capacities for both sandy and loamy soils increase with the increase of hydrogel concentration with maximum moisture retention capacity observed at 0.4%. A hydrogel, having potassium polyacrylate based cross-linking, applied to sandy forest soil with different pF ranges (0, 0.5,1, 1.5, and 2). The maximum increase in water retention capacity was observed in pF 2. This shows that sandy soil enriched with superabsorbent hydrogel will retain water in it than without hydrogel and the water would percolates down rapidly. The application of superabsorbent hydrogel to soil empowered with the soil compactness along with its water retention capacity [144].
The sandy soil although has the least water retaining capacity that also decreased with the passage of time. It shows a remarkable difference in water retention capacity values for soil enriched with and without superabsorbent hydrogel. The soil has been enriched with guar gum grafted with poly-sodium acrylamide (GG-g-PNaA). The results show that sandy soil loses its absorbed water within 13 days while water retention time increased up to 30 days by using guar gum based hydrogel (GG-g-PNaA) at 2 wt% concentration applied in soil. So it is proved that superabsorbent soil hydrogel can be used as a water reservoir in agriculture [134].
Slow nutrient’s release
Nutrients are very necessary for plant growth and they are extensively used in agriculture for many decades. Beyond its benefits to plants growth and crop efficiency, it imparts some environmental hazardous effects. The major quantity of applied fertilizers, containing phosphates and nitrates, is lost during rainfall that definitely pollutes the environment and sea water [149]. To accompany the requirement of plants, the fertilizers are used in larger quantities so increasing the cost of fertilizer for a crop. Besides fertilizers, pesticides are also lost through leaching which leads to the eutrophication of surface and groundwater. Thus it pollutes the environment, unbalancing the ecosystem with increase in acute toxic and carcinogenic sources [150].
By taking this consideration, it is mandatory to resolve these social and environmental issues to save the ecosystem and maintain sustainability. There should be a substituent to this hazardous fertilizer that don’t pollute the environment and fulfill the plant’s requirements by the slow release of nutrients in soil so that plants can take them up periodically [151]. So the researchers synthesized a superabsorbent soil hydrogels that posses all these features to save the environment with slow release of nutrients to plants. Soil hydrogels increase the nutrients availability duration for plants because it has controlled release of nutrients at a required rate [152]. These are smart devices and potential barriers to degradation of fertilizer and reduced its consumption. In short, hydrogels are biodegradable, eco-friendly, cost effective and have prolonged nutrient’s availability duration with slow release of fertilizer.
Nutrients are given to plant in the form of water-soluble salts. The solubility of nutrient causes the salts to leach away from roots and plants cannot get the whole dose of nutrients. The nutrient release from fertilizers in both water and soil environments can be determined by the methods mentioned in different studies because there is no specific standard test to determine the nutrient release [73]. For the determination in water, the superabsorbent hydrogel is dissolved in distilled water without any stirring. The nutrient release from hydrogel is estimated by the use of conductometer (to check the conductance in different time intervals) or through UV –Vis Spectroscopy. On the other hand in soil, it can be estimated by placing soil, superabsorbent hydrogel and distilled water together into a leaching column. The leached water collected down periodically and nutrient release can also be determined by using the same method. A lysimeter, has 150 cm depth and 35 cm diameter with 962.5 cm2 surface area, is also used for the determination of nutrient release [153].
The nutrient release process can be understood by the interaction of GEDTA hydrogel with water step by step. First of all the GEDTA hydrogel gets water and swell up slowly in soil and followed by the exchange of ions (K+, NH4+ and H2PO4−1) between water in the soil and free water in the GEDTA. In the second step, the water of soil elutes the nutrients from GEDTA. The elution rate of nutrients depends upon the interaction of ions and GEDTA. The greater amount of NPK ions in polymeric network results in greater water absorbency and hence the GEDTA will retain more water in its structure [62].
Effect of a diffusion process on nutrient release
The hydrogel’s nutrient release is mainly dependent on many factors like that constituents, cross-linking agent and its concentration along with swelling index. The one most important factor is a solute diffusion process also plays a role in nutrient release periodically [154]. The diffusion process directly relates to the chemical interaction developed between hydrogel and solutes. The nutrient release curve for Pectin based poly-(AAm-co-AAc) hydrogels shows that the phosphate has minimum release (10%), while potassium and urea released in the 40:50 ratio. The potassium and phosphate release is enhanced by increasing acrylic acid content in hydrogel whereas the urea release is enhanced by decreasing acrylic acid content [155].
Release of nitrogen content
A synthetic polyacrylic acid urea based superabsorbent hydrogel (SASH) has slow nutrient release along with swelling and water retention properties. The SASH releases urea slowly because it retains N-content within its three dimensional matrix [62]. It found that SASH releases 0.18, 0.37, 0.58, 0.81, 1.06, 1.59, 2.67, and 3.71 wt% nitrogen content within 1, 3, 5, 7, 10, 20, 30, and 40 days respectively. This data shows that released nitrogen content is only 3.70% in 40 days so it is proved as a nutrient reservoir to plant and retain them for a longer time period. The rate of nutrient release is also profound in coated fertilizers but the mechanism is quite different from SASH granules. The coated fertilizer has less tendency of water dissolution and keeps the moisture outside the coating membrane [28]. Contrary, the SASH takes up the water and swells up slowly in distilled water. Due to its absorption, the water molecules occupy the free space of 3D network and hydrolyze urea of polymer chain. Thus the hydrolyzed urea releases slowly to the plant through dynamic water exchange [129].
The methyl cellulose polymethacrylate (MC-PAM) hydrogel is mixed with montmorillonite (MMT) to develop composites of different concentration, posses physicochemical properties accordingly and compared with urea release content. The composite 1 has 50:50 of hydrogel and MMT, composite 2 has 66:33, composite 3 has 75:25, composite 4 has 80:20 and composite 5 has only hydrogel without montmorillonite. The clay minerals in montmorillonite impart an additional role in slow release of nutrients. The urea makes strong interactions with clay nutrients so reduces the urea release. Thus the composite 1containing maximum concentration of montmorillonite (clay) has a comparatively slow release of urea [74].
Effect of pH
The effect of pH was observed for the methyl cellulose polymethacrylate (MC-PAM) hydrogel. The test was conducted at different pH ranges from 4 to 9. The nutrient release is pH-responsive along with the presence of (MMT) clay nutrients and shows remarkable results. The composite without clay releases urea soon within 24–48 h. But the incorporation of MMT to hydrogel prolongs the time period for urea release and plants can avail soil nutrients up to 72 h. The urea release curve shows that at pH 4 and 9 range, the urea has greater desorption in the absence of clay nutrients [149].
Effect of biopolymer
Agriculture is continuously facing the water stress environment so the productivity of crops should be increased with this water-stressed condition by using the principle of controlled release of nutrients. The hydrogels containing biopolymer (e.g., cellulose, alginate and chitosan, etc.), as a source or precursor material, also play a crucial role in nutrient release in soil for plant growth [52]. Among all fertilizers, urea is widely used as a big source of nitrogen to plants and it transforms the biological and physical composition of soil by releasing nutrients. The maximum portion of the applied dose of urea is wasted during leaching and side reaction that evolves nitrogen gas. The role of biopolymers is to change the kinetics of nitrogen release and reduces the nutrient loss as a result plants can get nutrient more frequently for a longer time according to their needs [105]. In short, the biopolymer helps in protecting the environment from being polluted and decomposes easily so that it can return nutrients to the environment.
Conclusion
The review summarized the most recent researches on the synthesis of superabsorbent hydrogels (SAHs) and their agricultural applications. It includes the use of natural, synthetic, and combo-based material diversity for hydrogel synthesis that develops different degrees of functional properties. The SAHs are often synthesized by grafting or cross-linking polymerization to get extended structure over three dimensions. The natural sources for hydrogel synthesis make them biodegradable and eco-friendly but the synthetic sources enhanced the functional properties in the agriculture field. This conventional trend substituted to the use of combo (natural and synthetic) source to get biodegradable with value added features.
The SAHs are extensively being used as a conditioner for soil and rapid plant growth. A soil conditioner promotes the mechanical properties of soil and facilitates the plant by providing slow nutrient release of fertilizer periodically. The use of SAHS increases the water retention property of all types of soil and has large swelling index. All these functional properties proved the best solution for the global upcoming water issue with concerning plant growth in agricultural field. The superabsorbent hydrogels facilitate the growth of plants with limited use of water and fertilizers.
Compliance with ethics requirement
This article does not contain any studies with human or animal subjects.
CRediT authorship contribution statement
Muhammad Rizwan: Data curation, Methodology, Writing - review & editing. Syeda Rubina Gilani: Conceptualization, Supervision. Arjumand Iqbal Durani: Visualization, Investigation. Sobia Naseem: Formal analysis, Software, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies

Muhammad Rizwan is a Ph.D. Chemistry candidate in the University of Engineering & Technology Lahore. He is SS in the Education department. He has five research publications in different Journals and his one book is published in Lambert Publishing house Germany. He is a keen observer, and has curiosity for research in scientific field. His research interest is synthesis of material science, nano-chemistry, and polymer. He has expertise in planning and designing of lab work for any scientific research. He is now doing research on agricultural hydrogels in the supervision of Prof. Dr. Syeda Rubina Gilani.

Prof. Dr. Syeda Rubina Gillani is Chairperson in department of chemistry, University of Engineering & Technology Lahore, Pakistan. She got her Post Doctarate degree from Queens University Kingston Ontario Canada with specialization in organo-metalic Chemistry. Her research interest is very broad covering many areas like designing of novel synthesis of functional foods, Green chemistry, phytochemistry, spectroscopic & analytical chemistry and herbs as medicines etc. She has 24 International publications in journals including Springer & others and 18 publications in National Journals. She won the Quaid-e-Azam Gold medal award in 2014.

Dr. Arjumand Iqbal Durani is an associate professor in Department of Chemistry, University of Engineering & Technology Lahore, Pakistan. She has been working for last 17 years in this institute. She is teaching organic and food chemistry. She got her doctoral degree from Institute of analytical and food chemistry, University of Vienna, Austria. She has research interest in development of new food products, packaging materials, food nanocomposites & edible nanocoatings and biosorbents. She has developed a food lab with advanced analytical instruments.
Sobia Naseem is a Ph.D. Chemistry candidate in the University of Engineering & Technology Lahore. She worked as lecturer of chemistry for 10 years in College. She has five research publications in different Journals and his one book is published in Lambert Publishing house Germany. She has research interests in inorganic, green chemistry, polymer and nanochemistry. She has expertise in operating different analytical instruments (HPLC, FTIR, etc) and Lab management. She is doing research on role of nanocomposites in maintaining the quality of food under the supervision of Dr. Arjumand Iqbal Durani.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Mondal MIH, editor. Cellulose-based superabsorbent hydrogels. Springer Science and Business Media LLC; 2019. http://link.springer.com/10.1007/978-3-319-77830-3
- 2.Maitra J., Shukla V.K. Cross-linking in hydrogels - a review. Am J Polym Sci. 2014;4(2):25–31. doi: 10.5923/j.ajps.20140402.01. [DOI] [Google Scholar]
- 3.Ahmed E.M. Hydrogel : preparation, characterization, and applications : A review. J Adv Res. 2015;6(2):105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swantomo D., Rochmadi Basuki KT, Sudiyo R. Synthesis and characterization of graft copolymer rice straw cellulose-acrylamide hydrogels using Gamma irradiation. Atom Indones. 2013;39(2):57–64. doi: 10.17146/aij.2013.232. [DOI] [Google Scholar]
- 5.Timoteo-cruz B, Torres-blancas T. OA - This study was conducted to explore the potential of superabsorbent polymers (SAPs) from used. 2017;5(4):105-117
- 6.Ranganathan N., Joseph Bensingh R., Abdul Kader M., Nayak S.K. Synthesis and properties of hydrogels prepared by various polymerization reaction systems. Published online. 2019:487–511. doi: 10.1007/978-3-319-77830-3_18. [DOI] [Google Scholar]
- 7.Rengasamy P. Soil processes affecting crop production in salt-affected soils. Funct Plant Biol. 2010;37(7):613–620. doi: 10.1071/FP09249. [DOI] [Google Scholar]
- 8.Sluckin W. Imprinting in guinea-pigs [32] Nature. 1968;220(5172):1148. doi: 10.1038/2201148a0. [DOI] [PubMed] [Google Scholar]
- 9.Ullah F., Othman M.B.H., Javed F., Ahmad Z., Akil H.M. Classification, processing and application of hydrogels: A review. Mater Sci Eng C. 2015;57:414–433. doi: 10.1016/j.msec.2015.07.053. [DOI] [PubMed] [Google Scholar]
- 10.Chirani N, Yahia LH, Gritsch L, Motta FL, Natta CG. iMedPub Journals History and Applications of Hydrogels Abstract. Published online 2015:1-23. doi:10.4172/2254-609X.100013
- 11.Ravishankar K., Dhamodharan R. Advances in chitosan-based hydrogels: Evolution from covalently crosslinked systems to ionotropically crosslinked superabsorbents. React Funct Polym. 2020;149 doi: 10.1016/j.reactfunctpolym.2020.104517. [DOI] [Google Scholar]
- 12.Wangdi K. Production of nanofertilizer-a mini review. Vol 4.; 2019. http://www.ijeast.com
- 13.Essawy H.A., Ghazy M.B.M., El-Hai F.A., Mohamed M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int J Biol Macromol. December 2015;2016(89):144–151. doi: 10.1016/j.ijbiomac.2016.04.071. [DOI] [PubMed] [Google Scholar]
- 14.Das N. Preparation methods and properties of hydrogel: A review. Int J Pharm Pharm Sci. 2013;5(3):112–117. [Google Scholar]
- 15.Zhou T., Wang Y., Huang S., Zhao Y. Synthesis composite hydrogels from inorganic-organic hybrids based on leftover rice for environment-friendly controlled-release urea fertilizers. Sci Total Environ. 2018;615:422–430. doi: 10.1016/j.scitotenv.2017.09.084. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y., Sun Y., Fu Y., Fang G., Yan X., Guo Z. Swelling behaviors of porous lignin based poly (acrylic acid) Chemosphere. 2016;163:610–619. doi: 10.1016/j.chemosphere.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Akram M, Hussain R. Nanohydrogels: History, Development, and Applications in Drug Delivery.; 2017. doi:10.1002/9783527803835.ch11
- 18.Behera S., Mahanwar P.A. Superabsorbent polymers in agriculture and other applications: a review. Polym Technol Mater. 2020;59(4):341–356. doi: 10.1080/25740881.2019.1647239. [DOI] [Google Scholar]
- 19.Stile R.A., Healy K.E. Synthesis of hydrogels. Methods Tissue Eng Published online. 2002;663–680 doi: 10.1016/b978-012436636-7/50172-5. [DOI] [Google Scholar]
- 20.Radwan M.A., Al-Sweasy O.H., Elazab H.A. Preparation of hydrogel based on acryl amide and investigation of different factors affecting rate and amount of absorbed water. Agric Sci. 2017;08(02):161–170. doi: 10.4236/as.2017.82011. [DOI] [Google Scholar]
- 21.Kumar A, Nagesh B. Foliar application of nanofertilizers in agricultural crops -A review Foliar application of nanofertilizers in agricultural crops – A review. 2019;(October).
- 22.Varaprasad K., Raghavendra G.M., Jayaramudu T., Yallapu M.M., Sadiku R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater Sci Eng C. 2017;79:958–971. doi: 10.1016/j.msec.2017.05.096. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X.N., Wang Y.J., Sun S. A tough and stiff hydrogel with tunable water content and mechanical properties based on the synergistic effect of hydrogen bonding and hydrophobic interaction. Macromolecules. 2018;51(20):8136–8146. doi: 10.1021/acs.macromol.8b01496. [DOI] [Google Scholar]
- 24.Voorhaar L., Hoogenboom R. Supramolecular polymer networks: Hydrogels and bulk materials. Chem Soc Rev. 2016;45(14):4013–4031. doi: 10.1039/c6cs00130k. [DOI] [PubMed] [Google Scholar]
- 25.Merino C., Kuzyakov Y., Godoy K., Cornejo P., Matus F. Synergy effect of peroxidase enzymes and Fenton reactions greatly increase the anaerobic oxidation of soil organic matter. Sci Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-67953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H. Metal based nanoparticles in agricultural system: Behavior, transport, and interaction with plants. Chem Speciat Bioavailab. 2018;30(1):123–134. doi: 10.1080/09542299.2018.1520050. [DOI] [Google Scholar]
- 27.Pellá M.C.G., Lima-Tenório M.K., Tenório-Neto E.T., Guilherme M.R., Muniz E.C., Rubira A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr Polym. 2018;196(March):233–245. doi: 10.1016/j.carbpol.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 28.Moreau D., Chauvet C., Etienne F., Rannou F.P., Corté L. Hydrogel films and coatings by swelling-induced gelation. Proc Natl Acad Sci U S A. 2016;113(47):13295–13300. doi: 10.1073/pnas.1609603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olad A., Zebhi H., Salari D., Mirmohseni A., Reyhanitabar A. Synthesis, characterization, and swelling kinetic study of porous superabsorbent hydrogel nanocomposite based on sulfonated carboxymethylcellulose and silica nanoparticles. J Porous Mater. 2018;25(5):1325–1335. doi: 10.1007/s10934-017-0543-6. [DOI] [Google Scholar]
- 30.Krishna Sailaja A., Amareshwar P., Chakravarty P. Different techniques used for the preparation of nanoparticles using natural polymers and their application. Int J Pharm Pharm Sci. 2011;3(SUPPL. 2):45–50. [Google Scholar]
- 31.Zhang J.P., Li A., Wang A.Q. Study on superabsorbent composite. V. Synthesis, swelling behaviors and application of poly(acrylic acid-co-acrylamide)/sodium humate/attapulgite superabsorbent composite. Polym Adv Technol. 2005;16(11–12):813–820. doi: 10.1002/pat.657. [DOI] [Google Scholar]
- 32.Li X., Li Q., Xu X., Su Y., Yue Q., Gao B. Characterization, swelling and slow-release properties of a new controlled release fertilizer based on wheat straw cellulose hydrogel. J Taiwan Inst Chem Eng. 2016;60 doi: 10.1016/j.jtice.2015.10.027. [DOI] [Google Scholar]
- 33.Cheng D., Liu Y., Yang G., Hao G., Wang Y., Zhang A. Preparation of low cost superabsorbent hydrogel by urea and acrylic acid. Mater Lett. 2017;204:16–18. doi: 10.1016/j.matlet.2017.05.136. [DOI] [Google Scholar]
- 34.Omidian H., Hashemi S.A., Sammes P.G., Meldrum I. Modified acrylic-based superabsorbent polymers (dependence on particle size and salinity) Polymer (Guildf) 1999;40(7):1753–1761. doi: 10.1016/S0032-3861(98)00394-2. [DOI] [Google Scholar]
- 35.Liu G., Ding Z., Yuan Q., Xie H., Gu Z. Multi-layered hydrogels for biomedical applications. Front Chem. 2018;6(September):1–10. doi: 10.3389/fchem.2018.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma L., Shi Y., Siemianowski O. Hydrogel-based transparent soils for root phenotyping in vivo. Proc Natl Acad Sci U S A. 2019;166(22):11063–11068. doi: 10.1073/pnas.1820334116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y., Liu J., Huang L., Wang Z., Wang L. Design and performance of a sericin-alginate interpenetrating network hydrogel for cell and drug delivery. Sci Rep. 2015;5(July):1–13. doi: 10.1038/srep12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kakuchi R. The dawn of polymer chemistry based on multicomponent reactions. Polym J. 2019;51(10):945–953. doi: 10.1038/s41428-019-0209-0. [DOI] [Google Scholar]
- 39.Chabukswar V.V., Bhavsar S.V., Mohite K.C. Synthesis of poly(N-ethylaniline) nanoparticles synthesis and characterization of organically soluble conducting poly(N-ethylaniline) nanoparticles using acrylic acid as a soft template. J Macromol Sci Part A Pure Appl Chem. 2012;49(7):547–553. doi: 10.1080/10601325.2012.687682. [DOI] [Google Scholar]
- 40.Chandrika K.P., Singh A., Rathore A., Kumar A. Novel cross linked guar gum-g-poly(acrylate) porous superabsorbent hydrogels: Characterization and swelling behaviour in different environments. Carbohydr Polym. 2016;149:175–185. doi: 10.1016/j.carbpol.2016.04.077. [DOI] [PubMed] [Google Scholar]
- 41.Li X., Anton N., Arpagaus C., Belleteix F., Vandamme T.F. Nanoparticles by spray drying using innovative new technology: The Büchi Nano Spray Dryer B-90. J Control Release. 2010;147(2):304–310. doi: 10.1016/j.jconrel.2010.07.113. [DOI] [PubMed] [Google Scholar]
- 42.Olad A., Gharekhani H., Mirmohseni A., Bybordi A. Synthesis, characterization, and fertilizer release study of the salt and pH-sensitive NaAlg-g-poly(AA-co-AAm)/RHA superabsorbent nanocomposite. Polym Bull. 2017;74(8):3353–3377. doi: 10.1007/s00289-016-1899-5. [DOI] [Google Scholar]
- 43.Cândido J.D.S., Pereira A.G.B., Fajardo A.R. Poly(acrylamide-co-acrylate)/rice husk ash hydrogel composites. II. Temperature effect on rice husk ash obtention. Compos Part B Eng. 2013;51:246–253. doi: 10.1016/j.compositesb.2013.03.027. [DOI] [Google Scholar]
- 44.Xiao X., Yu L., Xie F. One-step method to prepare starch-based superabsorbent polymer for slow release of fertilizer. Chem Eng J. 2017;309:607–616. doi: 10.1016/j.cej.2016.10.101. [DOI] [Google Scholar]
- 45.Tanan W, Panichpakdee J, Saengsuwan S. Novel biodegradable hydrogel based on natural polymers: synthesis, characterization, swelling/reswelling and biodegradability. Vol 112. Elsevier Ltd; 2019. doi:10.1016/j.eurpolymj.2018.10.033
- 46.Nnadi F., Brave C. Environmentally friendly superabsorbent polymers for water conservation in agricultural lands. J Soil Environ Manag. 2011;2(7):206–211. [Google Scholar]
- 47.Dalias P., Neocleous D. Comparative analysis of the nitrogen effect of common agricultural practices and rotation systems in a rainfed mediterranean environment. Plants. 2017;6(4):1–17. doi: 10.3390/plants6040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heise K., Kirsten M., Schneider Y. From agricultural byproducts to value-added materials: wheat straw-based hydrogels as soil conditioners? ACS Sustain Chem Eng. 2019;7(9):8604–8612. doi: 10.1021/acssuschemeng.9b00378. [DOI] [Google Scholar]
- 49.Zhang X., Liu Y., Lu P., Zhang M. Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers. Green Process Synth. 2020;9(1):139–152. doi: 10.1515/gps-2020-0015. [DOI] [Google Scholar]
- 50.Gonçalves A.A.L., Fonseca A.C., Fabela I.G.P., Coelho J.F.J., Serra A.C. Synthesis and characterization of high performance superabsorbent hydrogels using bis[2-(Methacryloyloxy)ethyl] phosphate as crosslinker. Express Polym Lett. 2016;10(3):248–258. doi: 10.3144/expresspolymlett.2016.23. [DOI] [Google Scholar]
- 51.Al- HWA, Al-shami QMN. The effect of fertigation with nano NPK fertilizers on some parameters of growth and yield of potato (Solanum tuberosum L .). 2019;9(2):225-232.
- 52.Sarmah D., Karak N. Biodegradable superabsorbent hydrogel for water holding in soil and controlled-release fertilizer. J Appl Polym Sci. 2020;137(13):1–12. doi: 10.1002/app.48495. [DOI] [Google Scholar]
- 53.Ghobashy MM. The application of natural polymer-based hydrogels for agriculture. Elsevier Inc.; 2019. doi:10.1016/B978-0-12-816421-1.00013-6
- 54.Shariatinia Z., Barzegari A. Polysaccharide hydrogel films/membranes for transdermal delivery of therapeutics. Elsevier Ltd. 2019 doi: 10.1016/B978-0-08-102553-6.00022-2. [DOI] [Google Scholar]
- 55.Thombare N., Mishra S., Siddiqui M.Z., Jha U., Singh D., Mahajan G.R. Design and development of guar gum based novel, superabsorbent and moisture retaining hydrogels for agricultural applications. Carbohydr Polym. 2018;185:169–178. doi: 10.1016/j.carbpol.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 56.Shi X.N., Wang W.B., Wang A.Q. Effect of surfactant on porosity and swelling behaviors of guar gum-g-poly(sodium acrylate-co-styrene)/attapulgite superabsorbent hydrogels. Colloids Surf B Biointerfaces. 2011;88(1):279–286. doi: 10.1016/j.colsurfb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Matsukawa K., Masuda T., Akimoto A.M., Yoshida R. A surface-grafted thermoresponsive hydrogel in which the surface structure dominates the bulk properties. Chem Commun. 2016;52(74):11064–11067. doi: 10.1039/c6cc04307k. [DOI] [PubMed] [Google Scholar]
- 58.Kaith B.S., Sharma R., Kalia S., Bhatti M.S. Response surface methodology and optimized synthesis of guar gum-based hydrogels with enhanced swelling capacity. RSC Adv. 2014;4(76):40339–40344. doi: 10.1039/c4ra05300a. [DOI] [Google Scholar]
- 59.Wei W., Hu X., Qi X. A novel thermo-responsive hydrogel based on salecan and poly(N-isopropylacrylamide): Synthesis and characterization. Colloids Surf B Biointerfaces. 2015;125:1–11. doi: 10.1016/j.colsurfb.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 60.Kabiri K., Zohuriaan-Mehr M.J. Superabsorbent hydrogel composites. Polym Adv Technol. 2003;14(6):438–444. doi: 10.1002/pat.356. [DOI] [Google Scholar]
- 61.Yadollahi M., Gholamali I., Namazi H., Aghazadeh M. Synthesis and characterization of antibacterial carboxymethylcellulose/CuO bio-nanocomposite hydrogels. Int J Biol Macromol. 2015;73(1):109–114. doi: 10.1016/j.ijbiomac.2014.10.063. [DOI] [PubMed] [Google Scholar]
- 62.Senna A.M., Braga Do Carmo J., Santana Da Silva J.M., Botaro V.R. Synthesis, characterization and application of hydrogel derived from cellulose acetate as a substrate for slow-release NPK fertilizer and water retention in soil. J Environ Chem Eng. 2015;3(2):996–1002. doi: 10.1016/j.jece.2015.03.008. [DOI] [Google Scholar]
- 63.Alotaibi K.D., Schoenau J.J. Addition of biochar to a sandy desert soil: Effect on crop growth, water retention and selected properties. Agronomy. 2019;9(6):5–7. doi: 10.3390/agronomy9060327. [DOI] [Google Scholar]
- 64.Rabat N.E., Hashim S., Majid R.A. Effect of different monomers on water retention properties of slow release fertilizer hydrogel. Procedia Eng. 2016;148:201–207. doi: 10.1016/j.proeng.2016.06.573. [DOI] [Google Scholar]
- 65.Zhou Y., Fu S., Zhang L., Zhan H. Superabsorbent nanocomposite hydrogels made of carboxylated cellulose nanofibrils and CMC-g-p(AA-co-AM) Carbohydr Polym. 2013;97(2):429–435. doi: 10.1016/j.carbpol.2013.04.088. [DOI] [PubMed] [Google Scholar]
- 66.Erkossa T., Itanna F., Stahr K. Indexing soil quality: A new paradigm in soil science research. Aust J Soil Res. 2007;45(2):129–137. doi: 10.1071/SR06064. [DOI] [Google Scholar]
- 67.Pourjavadi A, Doulabi M, Soleyman R, Sharif S, Eghtesadi SA. Reactive & functional polymers synthesis and characterization of a novel (salep phosphate) -based hydrogel as a carrier matrix for fertilizer release. 2012;72:667-672.
- 68.Rop K., Mbui D., Njomo N., Karuku G.N., Michira I., Ajayi R.F. Biodegradable water hyacinth cellulose-graft-poly(ammonium acrylate-co-acrylic acid) polymer hydrogel for potential agricultural application. Heliyon. 2019;5(3) doi: 10.1016/j.heliyon.2019.e01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pourjavadi A., Doulabi M., Soleyman R., Sharif S., Eghtesadi S.A. Synthesis and characterization of a novel (salep phosphate)-based hydrogel as a carrier matrix for fertilizer release. React Funct Polym. 2012;72(10):667–672. doi: 10.1016/j.reactfunctpolym.2012.06.010. [DOI] [Google Scholar]
- 70.Ahmadian Y., Bakravi A., Hashemi H., Namazi H. Synthesis of polyvinyl alcohol/CuO nanocomposite hydrogel and its application as drug delivery agent. Polym Bull. 2019;76(4):1967–1983. doi: 10.1007/s00289-018-2477-9. [DOI] [Google Scholar]
- 71.Chang C., Duan B., Cai J., Zhang L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur Polym J. 2010;46(1):92–100. doi: 10.1016/j.eurpolymj.2009.04.033. [DOI] [Google Scholar]
- 72.Abdul Sisak M.A., Louis F., Matsusaki M. In vitro fabrication and application of engineered vascular hydrogels. Polym J. 2020;52(8):871–881. doi: 10.1038/s41428-020-0331-z. [DOI] [Google Scholar]
- 73.Suppan S. By Steve suppan applying nanotechnology to fertilizer the institute for agriculture and trade policy works locally and globally at the intersection of policy and practice to ensure fair and sustainable food, farm and trade systems. More at iatp.org OVERVI. 2017;(October).
- 74.Cheng D., Liu Y., Yang G., Zhang A. Water- and fertilizer-integrated hydrogel derived from the polymerization of acrylic acid and urea as a slow-release N fertilizer and water retention in agriculture. J Agric Food Chem. 2018;66(23):5762–5769. doi: 10.1021/acs.jafc.8b00872. [DOI] [PubMed] [Google Scholar]
- 75.Nada W.M., Blumenstein O. Characterization and impact of newly synthesized superabsorbent hydrogel nanocomposite on water retention characteristics of sandy soil and grass seedling growth. Int J Soil Sci. 2015;10(4):153–165. doi: 10.3923/ijss.2015.153.165. [DOI] [Google Scholar]
- 76.Ramli R.A. Slow release fertilizer hydrogels: A review. Polym Chem. 2019;10(45):6073–6090. doi: 10.1039/c9py01036j. [DOI] [Google Scholar]
- 77.Lejcuś K., Śpitalniak M., Dabrowska J. Swelling behaviour of superabsorbent polymers for soil amendment under different loads. Polymers (Basel) 2018;10(3) doi: 10.3390/polym10030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goto K., Teramoto Y. Development of chitinous nanofiber-based flexible composite hydrogels capable of cell adhesion and detachment. Polym J. 2020;52(8):959–967. doi: 10.1038/s41428-020-0324-y. [DOI] [Google Scholar]
- 79.Chhipa H. Applications of nanotechnology in agriculture. Methods Microbiol. 2019;46(2):115–142. doi: 10.1016/bs.mim.2019.01.002. [DOI] [Google Scholar]
- 80.Jamnongkan T., Kaewpirom S. Potassium release kinetics and water retention of controlled-release fertilizers based on chitosan hydrogels. J Polym Environ. 2010;18(3):413–421. doi: 10.1007/s10924-010-0228-6. [DOI] [Google Scholar]
- 81.Novak J.M., Busscher W.J., Watts D.W. Biochars impact on soil-moisture storage in an ultisol and two aridisols. Soil Sci. 2012;177(5):310–320. doi: 10.1097/SS.0b013e31824e5593. [DOI] [Google Scholar]
- 82.Saeed A.M. Temperature effect on swelling properties of commercial polyacrylic acid hydrogel beads. Int J Adv Biol Biomed Res. 2013;1(12):1614–1627. http://www.ijabbr.com [Google Scholar]
- 83.Yang F., Wang J., Song S. Novel controlled release microspheric soil conditioner based on the temperature and pH dual-stimuli response. J Agric Food Chem. 2020;68(30):7819–7829. doi: 10.1021/acs.jafc.0c01825. [DOI] [PubMed] [Google Scholar]
- 84.Rashidzadeh A., Olad A., Reyhanitabar A. Hydrogel/clinoptilolite nanocomposite-coated fertilizer: swelling, water-retention and slow-release fertilizer properties. Polym Bull. 2015;72(10):2667–2684. doi: 10.1007/s00289-015-1428-y. [DOI] [Google Scholar]
- 85.Wu L., Liu M., Liang Rui. Preparation and properties of a double-coated slow-release NPK compound fertilizer with superabsorbent and water-retention. Bioresour Technol. 2008;99(3):547–554. doi: 10.1016/j.biortech.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 86.Guilherme M.R., Aouada F.A., Fajardo A.R. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur Polym J. 2015;72:365–385. doi: 10.1016/j.eurpolymj.2015.04.017. [DOI] [Google Scholar]
- 87.Zhong K., Lin Z.T., Zheng X.L. Starch derivative-based superabsorbent with integration of water-retaining and controlled-release fertilizers. Carbohydr Polym. 2013;92(2):1367–1376. doi: 10.1016/j.carbpol.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 88.Prasad R., Kumar M., Kumar V. Nanotechnology: An agricultural paradigm. Nanotechnol An Agric Paradig Published online. 2017;1–372 doi: 10.1007/978-981-10-4573-8. [DOI] [Google Scholar]
- 89.Sartore L., Vox G., Schettini E. Preparation and performance of novel biodegradable polymeric materials based on hydrolyzed proteins for agricultural application. J Polym Environ. 2013;21(3):718–725. doi: 10.1007/s10924-013-0574-2. [DOI] [Google Scholar]
- 90.Rashidzadeh A., Olad A., Salari D., Reyhanitabar A. On the preparation and swelling properties of hydrogel nanocomposite based on Sodium alginate-g-Poly (acrylic acid-co-acrylamide)/Clinoptilolite and its application as slow release fertilizer. J Polym Res. 2014;21(2) doi: 10.1007/s10965-013-0344-9. [DOI] [Google Scholar]
- 91.Radu I.C., Biru I.E., Damian C.M. Grafting versus crosslinking of silk Fibroin-g-PNIPAM via tyrosine-NIPAM bridges. Molecules. 2019;24(22) doi: 10.3390/molecules24224096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roy D., Semsarilar M., Guthrie J.T., Perrier S. Cellulose modification by polymer grafting: A review. Chem Soc Rev. 2009;38(7):2046–2064. doi: 10.1039/b808639g. [DOI] [PubMed] [Google Scholar]
- 93.Rashidzadeh A., Olad A. Slow-released NPK fertilizer encapsulated by NaAlg-g-poly(AA-co-AAm)/MMT superabsorbent nanocomposite. Carbohydr Polym. 2014;114:269–278. doi: 10.1016/j.carbpol.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 94.Padhi J.R. Preparation and characterization of novel gelatin and carrageenan based hydrogels for topical delivery. Published online. 2015;52 [Google Scholar]
- 95.Nawaz M.F., Bourrié G., Trolard F. Soil compaction impact and modelling. A review. Agron Sustain Dev. 2013;33(2):291–309. doi: 10.1007/s13593-011-0071-8. [DOI] [Google Scholar]
- 96.Gao Y., Zhou D., Lyu J. Complex polymer architectures through free-radical polymerization of multivinyl monomers. Nat Rev Chem. 2020;4(4):194–212. doi: 10.1038/s41570-020-0170-7. [DOI] [PubMed] [Google Scholar]
- 97.Bao Y., Ma J., Li N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr Polym. 2011;84(1):76–82. doi: 10.1016/j.carbpol.2010.10.061. [DOI] [Google Scholar]
- 98.Huang Y., Zeng M., Ren J., Wang J., Fan L., Xu Q. Preparation and swelling properties of graphene oxide/poly(acrylic acid-co-acrylamide) super-absorbent hydrogel nanocomposites. Colloids Surf A Physicochem Eng Asp. December 2017;2012(401):97–106. doi: 10.1016/j.colsurfa.2012.03.031. [DOI] [Google Scholar]
- 99.Mohammed Soliman F., Yang W., Guo H., Ibrahim Shinger M., Mahmoud Idris A., Suliman Hassan E. Preparation of carboxymethyl cellulose-g-poly (acrylic acid-2-acrylamido-2-methylpropane sulfonic acid)/attapulgite superabsorbent composite preparation of carboxymethyl cellulose-g-poly (acrylic acid-2-acrylamido-2-methylpropane sulfonic acid)/attapulgit. Compos Am J Polym Sci Technol. 2016;2(1):11–19. doi: 10.11648/j.ajpst.20160201.12. [DOI] [Google Scholar]
- 100.Chang C., Zhang L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr Polym. 2011;84(1):40–53. doi: 10.1016/j.carbpol.2010.12.023. [DOI] [Google Scholar]
- 101.Haque M.O., Mondal M.I.H. Synthesis and characterization of cellulose-based eco-friendly hydrogels. Rajshahi Univ J Sci Eng. 2016;44:45–53. doi: 10.3329/rujse.v44i0.30386. [DOI] [Google Scholar]
- 102.El-Kereti M., El-feky S., Khater M., Osman Y., El-sherbini E. ZnO nanofertilizer and He Ne laser irradiation for promoting growth and yield of sweet basil plant. Recent Pat Food Nutr Agric. 2014;5(3):169–181. doi: 10.2174/2212798405666131112142517. [DOI] [PubMed] [Google Scholar]
- 103.Olad A., Pourkhiyabi M., Gharekhani H., Doustdar F. Semi-IPN superabsorbent nanocomposite based on sodium alginate and montmorillonite: Reaction parameters and swelling characteristics. Carbohydr Polym. December 2017;2018(190):295–306. doi: 10.1016/j.carbpol.2018.02.088. [DOI] [PubMed] [Google Scholar]
- 104.Hai T.A.P., Sugimoto R. Surface functionalization of cellulose with poly(3-hexylthiophene) via novel oxidative polymerization. Carbohydr Polym. 2018;179(October):221–227. doi: 10.1016/j.carbpol.2017.09.067. [DOI] [PubMed] [Google Scholar]
- 105.Bortolin A., Aouada F.A., Mattoso L.H.C., Ribeiro C. Nanocomposite PAAm/methyl cellulose/montmorillonite hydrogel: Evidence of synergistic effects for the slow release of fertilizers. J Agric Food Chem. 2013;61(31):7431–7439. doi: 10.1021/jf401273n. [DOI] [PubMed] [Google Scholar]
- 106.Kouser R., Vashist A., Zafaryab M., Rizvi M.A., Ahmad S. Na-montmorillonite-dispersed sustainable polymer nanocomposite hydrogel films for anticancer drug delivery. ACS Omega. 2018;3(11):15809–15820. doi: 10.1021/acsomega.8b01691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pan S., Ragauskas A.J. Preparation of superabsorbent cellulosic hydrogels. Carbohydr Polym. 2012;87(2):1410–1418. doi: 10.1016/j.carbpol.2011.09.031. [DOI] [Google Scholar]
- 108.Zant E., Grijpma D.W. Synthetic biodegradable hydrogels with excellent mechanical properties and good cell adhesion characteristics obtained by the combinatorial synthesis of photo-cross-linked networks. Biomacromolecules. 2016;17(5):1582–1592. doi: 10.1021/acs.biomac.5b01721. [DOI] [PubMed] [Google Scholar]
- 109.Carlmark A., Larsson E., Malmström E. Grafting of cellulose by ring-opening polymerisation - A review. Eur Polym J. 2012;48(10):1646–1659. doi: 10.1016/j.eurpolymj.2012.06.013. [DOI] [Google Scholar]
- 110.Bozorgi H.R. Study effects of nitrogen fertilizer management under nano iron chelate foliar spraying on yield and yield components of eggplant (Solanum melongena L.) J Eng Appl Sci. 2012;7(4):233–237. [Google Scholar]
- 111.Grill L., Hecht S. Covalent on-surface polymerization. Nat Chem. 2020;12(2):115–130. doi: 10.1038/s41557-019-0392-9. [DOI] [PubMed] [Google Scholar]
- 112.Shen Y, Wang H, Li W, et al. Synthesis and characterization of double-network hydrogels based on sodium alginate and halloysite for slow release fertilizers. Vol 164. Elsevier B.V; 2020. doi:10.1016/j.ijbiomac.2020.07.154 [DOI] [PubMed]
- 113.Yadav M., Rhee K.Y. Superabsorbent nanocomposite (alginate-g-PAMPS/MMT): Synthesis, characterization and swelling behavior. Carbohydr Polym. 2012;90(1):165–173. doi: 10.1016/j.carbpol.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 114.Deshmukh Krishi Vidyapeeth P., Kadu I.P., Pharande I.A.L., Patle P., Kadu P., Pharande A. Nanotechnology: An emerging trend in soil science and plant nutrition research the review with an overarching approach. ~ 1758 ~ Int J Chem Stud. 2018;6(3):1758–1760. [Google Scholar]
- 115.Mun G.A., Nurkeeva Z.S., Dergunov S.A. Studies on graft copolymerization of 2-hydroxyethyl acrylate onto chitosan. React Funct Polym. 2008;68(1):389–395. doi: 10.1016/j.reactfunctpolym.2007.07.012. [DOI] [Google Scholar]
- 116.Meng H., Zhang X., Chen Q. Preparation of poly(aspartic acid) superabsorbent hydrogels by solvent-free processes. J Polym Eng. 2015;35(7):647–655. doi: 10.1515/polyeng-2014-0275. [DOI] [Google Scholar]
- 117.Wattie B., Dumont M.J., Lefsrud M. Synthesis and properties of feather keratin-based superabsorbent hydrogels. Waste Biomass Valorization. 2018;9(3):391–400. doi: 10.1007/s12649-016-9773-0. [DOI] [Google Scholar]
- 118.Peng G., Xu S., Peng Y., Wang J., Zheng L. A new amphoteric superabsorbent hydrogel based on sodium starch sulfate. Bioresour Technol. 2008;99(2):444–447. doi: 10.1016/j.biortech.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 119.Yacob N., Hashim K. Morphological effect on swelling behaviour of hydrogel. AIP Conf Proc. 2014;1584(February):153–159. doi: 10.1063/1.4866123. [DOI] [Google Scholar]
- 120.Hemvichian K., Chanthawong A., Suwanmala P. Synthesis and characterization of superabsorbent polymer prepared by radiation- induced graft copolymerization of acrylamide onto carboxymethyl cellulose for controlled release of agrochemicals. Radiat Phys Chem Published online. 2014 doi: 10.1016/j.radphyschem.2014.05.064. [DOI] [Google Scholar]
- 121.Wilson M.A., Tran N.H., Milev A.S., Kannangara G.S.K., Volk H., Lu G.Q.M. Nanomaterials in soils. Geoderma. 2008;146(1–2):291–302. doi: 10.1016/j.geoderma.2008.06.004. [DOI] [Google Scholar]
- 122.Cieschi M.T., Polyakov A.Y., Lebedev V.A. Eco-friendly iron-humic nanofertilizers synthesis for the prevention of iron chlorosis in soybean (Glycine max) grown in calcareous soil. Front Plant Sci. 2019;10(April):1–17. doi: 10.3389/fpls.2019.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Olad A., Gharekhani H., Mirmohseni A., Bybordi A. Superabsorbent nanocomposite based on maize bran with integration of water-retaining and slow-release NPK fertilizer. Adv Polym Technol. 2018;37(6):1682–1694. doi: 10.1002/adv.21825. [DOI] [Google Scholar]
- 124.Arno M.C., Inam M., Weems A.C. Exploiting the role of nanoparticle shape in enhancing hydrogel adhesive and mechanical properties. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-15206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ni B., Liu M., Lü S., Xie L., Wang Y. Environmentally friendly slow-release nitrogen fertilizer. J Agric Food Chem. 2011;59(18):10169–10175. doi: 10.1021/jf202131z. [DOI] [PubMed] [Google Scholar]
- 126.Kuroda N., Tounoue Y., Noguchi K. Guest-responsive supramolecular hydrogels expressing selective sol–gel transition for sulfated glycosaminoglycans. Polym J. 2020;52(8):939–946. doi: 10.1038/s41428-020-0341-x. [DOI] [Google Scholar]
- 127.Smagin A., Sadovnikova N., Smagina M. Synthetic gel structures in soils for sustainable potato farming. Sci Rep. 2019;9(1):1–15. doi: 10.1038/s41598-019-55205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Palmqvist NGM. Nanoparticles: case studies of their synthesis, properties and biological interaction.; 2017. https://pub.epsilon.slu.se/14677/7/palmqvist_m_171101.pdf
- 129.Pereira AGB, Martins AF, Paulino AT, et al. Recent advances in designing hydrogels from chitin and chitin- derivatives and their impact on environment and agriculture: a review recentes avanços em hidrogéis de chitina e derivados de chitina e seu impacto sobre meio ambiente e agricultura: uma revis. Rev Virtual Quim. 2017;9(1). http://rvq.sbq.org.br
- 130.Bohn HL, McNeal BL, 0’connor GA. Soil chemistry. Soil Chem. Published online 1979. doi:10.1201/9781315137322-6
- 131.Ranganathan N, Joseph Bensingh R, Abdul Kader M, Nayak SK. Cellulose-based hydrogels for agricultures. Published online 2018:1-21. doi:10.1007/978-3-319-76573-0_34-1
- 132.Ghormade V., Deshpande M.V., Paknikar K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol Adv. 2011;29(6):792–803. doi: 10.1016/j.biotechadv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 133.Abdel-raouf M.E. Current research in biopolymers guar gum based hydrogels for sustained water release applications in agriculture, a review. Curr Res Biopolym Abdel-Raouf. 2019;2(01):1–15. doi: 10.29011/CRBP-111.000011. [DOI] [Google Scholar]
- 134.Wang W., Wang A. Preparation, swelling and water-retention properties of crosslinked superabsorbent hydrogels based on guar gum. Adv Mater Res. 2010;96:177–182. doi: 10.4028/www.scientific.net/AMR.96.177. [DOI] [Google Scholar]
- 135.Kim S., Healy K.E. Synthesis and characterization of injectable poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable cross-links. Biomacromolecules. 2003;4(5):1214–1223. doi: 10.1021/bm0340467. [DOI] [PubMed] [Google Scholar]
- 136.Alam M.A., Takafuji M., Ihara H. Silica nanoparticle-crosslinked thermosensitive hybrid hydrogels as potential drug-release carriers. Polym J. 2014;46(5):293–300. doi: 10.1038/pj.2014.2. [DOI] [Google Scholar]
- 137.Gharekhani H., Olad A., Mirmohseni A., Bybordi A. Superabsorbent hydrogel made of NaAlg-g-poly(AA-co-AAm) and rice husk ash: Synthesis, characterization, and swelling kinetic studies. Carbohydr Polym. 2017;168:1–13. doi: 10.1016/j.carbpol.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 138.Schott H. Kinetics of swelling of polymers and their gels. J Pharm Sci. 1992;81(5):467–470. doi: 10.1002/jps.2600810516. [DOI] [PubMed] [Google Scholar]
- 139.Siddiqui M.H., Al-Whaibi M.H., Mohammad F. Nanotechnology and plant sciences: Nanoparticles and their impact on plants. Nanotechnol Plant Sci Nanoparticles Their Impact Plants Published online. 2015;1–303 doi: 10.1007/978-3-319-14502-0. [DOI] [Google Scholar]
- 140.Martwong E. Design of surface-attached hydrogel thin films with LCST/ UCST temperature-responsive properties To cite this version : HAL Id : tel-02325327 Université Pierre et Marie Curie à propriétés thermo-stimulables LCST et UCST. Published online 2019
- 141.Shariatinia Z., Jalali A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int J Biol Macromol. 2018;115:194–220. doi: 10.1016/j.ijbiomac.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 142.Akhter J., Mahmood K., Malik K.A., Mardan A., Ahmad M., Iqbal M.M. Effects of hydrogel amendment on water storage of sandy loam and loam soils and seedling growth of barley, wheat and chickpea. Plant, Soil Environ. 2004;50(10):463–469. doi: 10.17221/4059-pse. [DOI] [Google Scholar]
- 143.Montesano F.F., Parente A., Santamaria P., Sannino A., Serio F. Biodegradable superabsorbent hydrogel increaseswater retention properties of growing media and plant growth. Agric Agric Sci Procedia. 2015;4:451–458. doi: 10.1016/j.aaspro.2015.03.052. [DOI] [Google Scholar]
- 144.Shahid S.A., Qidwai A.A., Anwar F., Ullah I., Rashid U. Improvement in the water retention characteristics of sandy loam soil using a newly synthesized poly(acrylamide-co-acrylic acid)/AlZnFe 2O 4 superabsorbent hydrogel nanocomposite material. Molecules. 2012;17(8):9397–9412. doi: 10.3390/molecules17089397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Onyelowe K.C., Okafor F.O. Review of the synthesis of nano-sized ash from local waste for use as admixture or filler in engineering soil stabilization and concrete production. J Environ Nanotechnol. 2015;4(4):23–27. doi: 10.13074/jent.2015.12.154167. [DOI] [Google Scholar]
- 146.Bongiorno G. Novel soil quality indicators for the evaluation of agricultural management practices : a biological perspective. 2020;(January). doi:10.15302/ China J-FASE-2020323
- 147.Abobatta W. Impact of hydrogel polymer in agricultural sector. Adv Agric Environ Sci Open Access. 2018;1(2):59–64. doi: 10.30881/aaeoa.00011. [DOI] [Google Scholar]
- 148.Abedi-Koupai J., Sohrab F., Swarbrick G. Evaluation of hydrogel application on soil water retention characteristics. J Plant Nutr. 2008;31(2):317–331. doi: 10.1080/01904160701853928. [DOI] [Google Scholar]
- 149.Sarkar S., Datta S.C., Biswas D.R. Effect of fertilizer loaded nanoclay/superabsorbent polymer composites on nitrogen and phosphorus release in soil. Proc Natl Acad Sci India Sect B - Biol Sci. 2015;85(2):415–421. doi: 10.1007/s40011-014-0371-2. [DOI] [Google Scholar]
- 150.Jyothi T.V., Hebsur N.S. Effect of nanofertilizers on growth and yield of selected cereals - A review. Agric Rev. 2017;38(02) doi: 10.18805/ag.v38i02.7942. [DOI] [Google Scholar]
- 151.Izza N, Himmah F, Djajakirana G. Research article nutrient release performance of starch coated npk fertilizers and their effects on corn growth. 2018;15(2):104-114. doi:10.15608/stjssa.v15i2.19694
- 152.Kaur P., Purewal S.S. Biofertilizers and their role in sustainable agriculture. Published online. 2019:285–300. doi: 10.1007/978-3-030-18933-4_12. [DOI] [Google Scholar]
- 153.El-Ramady H., El-Ghamry A., Mosa A., Alshaal T. Nanofertilizers vs biofertilizers: new insights. Environ Biodivers Soil Secur. 2018;2(1):40–50. doi: 10.21608/jenvbs.2018.3880.1029. [DOI] [Google Scholar]
- 154.Ogieglo W., Wormeester H., Eichhorn K.J., Wessling M., Benes N.E. In situ ellipsometry studies on swelling of thin polymer films: A review. Prog Polym Sci. 2015;42:42–78. doi: 10.1016/j.progpolymsci.2014.09.004. [DOI] [Google Scholar]
- 155.Döring A., Birnbaum W., Kuckling D. Responsive hydrogels – structurally and dimensionally optimized smart frameworks for applications in catalysis, micro-system technology and material science. Chem Soc Rev. 2013;42(17):7391–7420. doi: 10.1039/c3cs60031a. [DOI] [PubMed] [Google Scholar]