Abstract
Current studies have confirmed the feasibility of SARS-CoV-2 RNA detection by RT-qPCR assays in wastewater samples as an effective surveillance tool of COVID-19 prevalence in a community. Analytical performance of various RT-qPCR assays has been compared against wastewater samples based on the positive ratio. However, there is no systematic comparison work has been conducted for both analytical sensitivity and quantitative reliability against wastewater, which are essential factors for WBE. In this study, the detection performance of four RT-qPCR primer-probe sets, including CCDC-N, CDC-N1, N-Sarbeco, and E-Sarbeco, was systematically evaluated with pure synthetized plasmids, spiked wastewater mocks and raw wastewater samples. In addition to confirm RT-qPCR results, Nanopore sequencing was employed to delineate at molecular level for the analytical sensitivity and reproducibility of those primer-probe sets. CCDC-N showed high sensitivity and the broadest linearity range for wastewater samples. It was thus recommended to be the most efficient tool in the quantitative analysis of SARS-CoV-2 in wastewater. CDC-N1 had the highest sensitivity for real wastewater and thus would be suitable for the screening of wastewater for the presence of SARS-CoV-2. When applying the primer-probe sets to wastewater samples collected from different Australian catchments, increased active clinical cases were observed with the augment of SARS-CoV-2 RNA quantified by RT-qPCR in wastewater in low prevalence communities.
Keywords: SARS-CoV-2, COVID-19, RT-qPCR, Nanopore sequencing, Wastewater-based epidemiology
Graphical abstract
1. Introduction
Coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a public health emergency of international concern since its first outbreak at the end of 2019. To date, it has resulted in more than 230 million confirmed cases and more than 4.7 million deaths globally (https://covid19.who.int/table). Strict measures such as lockdowns, border closures and social distancing were employed to suppress the transmission of SARS-CoV-2. Although being effective, these measures have negative impacts on the local and global economies (Thunström et al., 2020). To manage COVID-19 effectively, the tracking and monitoring of community prevalence is critical. This is primarily done through large scale of clinical testing of individuals.
COVID-19 patients can have gastrointestinal symptoms and shed viruses into wastewater through feces. The virus shedding may last longer than the infection of respiratory tract. Thus, wastewater analysis of SARS-CoV-2, aka wastewater-based epidemiology (WBE), has been recommended as a powerful tool to reveal the COVID-19 prevalence in catchments or cities. WBE successfully detected SARS-CoV-2 in wastewater from many countries (Ahmed et al., 2020a; Ahmed et al., 2020b; Haramoto et al., 2020; Kumar et al., 2020; La Rosa et al., 2020; Medema et al., 2020; Wurtzer et al., 2020), and usually days to weeks earlier than the first clinically confirmed case occurred (Peccia et al., 2020; Randazzo et al., 2020). WBE based SARS-CoV-2 surveillance was reported as an important and informative approach to support pandemic management. RT-qPCR, as the most popular analytical method, was widely used in the detection and quantification of SARS-CoV-2 RNA in wastewater. Normally, two or more RT-qPCR assays were employed to analyze one sample to avoid false negative or positive result. Sequencing was also included to confirm the qPCR results. In addition to the hefty time, resources and cost required, the varying performance (sensitivity, specificity, repeatability, and reliability etc.) of different RT-qPCR assays lead to difficulty in the quantification of SARS-CoV-2 in wastewater and the comparability of data obtained using different methods. Therefore, it is essential to conduct a systematic evaluation for the RT-qPCR primer-probe sets to improve the application of WBE for the SARS-CoV-2 detection in wastewater (Ahmed et al., 2020b; Pérez-Cataluña et al., 2021).
At present, 13 different RT-qPCR primer-probe sets targeting various SARS-CoV-2 RNA regions, including nucleocapsid (N), envelope (E) and RNA-dependent RNA polymerase (RdRp), have been developed and applied worldwide (Li et al., 2021) (Supplementary Materials Table-S8). Several studies have been carried out to compare the sensitivity and efficiency of those primer-probe sets. Jung et al. investigated the performance of 10 primer-probe sets targeting N and RdRp/Orf1 genes for the detection of SARS-CoV-2 using the same primer-probe concentration. The results showed that the cycle number of most primer-probe sets targeting N gene (6/7) was lower (around 1–5 cycles) than that of those targeting RdRp/Orf1 gene. Moreover, N2, N3 and NIID_2019-nCOV_N primer-probe sets could achieve the detection of 15 copies/reaction with 33 RT-qPCR cycles (Jung et al., 2020). In another study, nine primer-probe sets targeting the N, E and RdRp/Orf1 genes were compared by using 10-fold dilutions of SARS-CoV-2 RNA derived from cell cultures, or by using 10-fold dilutions of SARS-CoV-2 RNA spiked into RNA extracts from negative clinical samples (Vogels et al., 2020). Under the same PCR condition, three primer-probe sets (CCDC-ORF1, HKU-ORF1, and E-Sarbeco) showed better detection performance than others at 500 copies/reaction in spiked clinical samples. Except for RdRp-SARSr and CDC-N2, all primer-probe sets were able to partially detect (Ct values <40) SARS-CoV-2 RNA at 5 or 50 viral RNA copies/reaction.
It is found that the results of pure reverse-transcribed RNA transcript standards and spiked samples were different for CCDC-N and CCDC-ORF1 (Vogels et al., 2020). This indicates that the comparison of primer-probe sets should include their performance in real samples. Moreover, it was obvious that the standard curve of CDC-N1 and CDC-N3 showed varied slopes in high and low concentrations in Vogels' study (Vogels et al., 2020). These results showed that the analytical sensitivity and efficiency of different primer-probe sets vary between pure standard samples and real samples, especially when they were employed in the quantification test for low target concentration in complex matrix like wastewater. Moreover, the qPCR inhibition and quality control for SARS-CoV-2 RNA detection in wastewater was usually conducted by using a Sketa22 real-time PCR assay (Haugland et al., 2010). However, the concentration of template DNA used in this assay was 104 copies/reaction, which was around 102–103 fold higher than the concentration of SARS-CoV-2 RNA in the wastewater from low-prevalence communities. Inhibition test for low concentration gene targets by using this assay can lead to false negative result, and is not cost effective for large numbers of samples. Therefore, it is necessary to reconsider the reliability of calculating the real gc number by standard curves generated with high target concentrations.
This study aims to evaluate different SARS-CoV-2 RT-qPCR detection assays under the same optimized RT-qPCR condition for the accurate and reliable quantification in wastewater samples, thus further improving the applicability of WBE for SARS-CoV-2 surveillance. Firstly, through literature review, three N-gene targeting sets including CDC-N1, N-Sarbeco and CCDC-N were selected since they have been reported as the most sensitive ones for wastewater samples, especially CDC-N1. CCDC-N set, for the first time, was included and compared in SARS-CoV-2 detection in wastewater samples. One E-gene targeting set was also included to achieve multi-targets detection. Secondly, detailed comparison work was conducted to compare their detection sensitivity in different types of samples including pure plasmid, spiked mocks and real wastewater samples. Two kinds of standard curves were compared to evaluate the reliability of standard curve-based quantification. The importance of primer-probe selection especially for standard curve-based quantification in wastewater was highlighted. Finally, nanopore sequencing was employed to gain further insights on their analytical performance (sensitivity and reproducibility) by assessing the mutations' accumulation of RT-qPCR amplicons in this study, rather than simply confirming the RT-qPCR results in other studies. We also explored how different SARS-CoV-2 variants affect the performance of the four primer-probe sets. The expected results would provide a guidance for SARS-CoV-2 surveillance on RT-qPCR primer-probe selection by considering both the detection sensitivity and quantification reliability. All RT-qPCR tests were optimized and conducted under the same RT-qPCR condition, thus is also useful for developing multiplex RT-qPCR assay for SARS-CoV-2 detection and quantification towards wastewater sample.
2. Materials and methods
2.1. Wastewater sampling
Three sampling campaigns were carried out to collect raw wastewater samples for this study. One influent wastewater sample was collected from the membrane bioreactor of a cruise ship on 23th April 2020. In addition, three wastewater samples were collected on three consecutive days from a wastewater treatment plant (WWTP C) in August 2020. All above samples were transported to the laboratory on ice and were stored in a −80 °C freezer until analysis. In addition, influent wastewater samples were collected from 13 different wastewater treatment plants in Australia, on 3rd, 4th, 18th, 25th August and 1st, 2nd September 2020. The information for the 13 different wastewater treatment plants is provided in Table S1. Standard personal protective equipment was used during the sample collection. These samples were transported to the laboratory on ice and were stored in 4 °C fridge until extraction within 2–7 days. The above samples were primarily taken when there were reported COVID-19 cases in the community, and thus likely SARS-CoV-2 positive samples. All the wastewater samples were employed to evaluate the analytical sensitivity and efficiency of the primer-probe sets in WBE applications.
The four primer-probe sets were further evaluated using 15 wastewater RNA samples extracted at another lab in Australia. Samples were delivered with dry ice and were stored at −80 °C immediately after received. All 15 samples were extracted from 50 mL raw wastewater by using adsorption-extraction method, and they were confirmed as not PCR inhibitive.
2.2. Sample processing and RNA extraction
As the comparison of virus concentration and extraction is not the focus of this study, the most efficient methods reported in literature were adopted. Adsorption–extraction method with an electronegative membrane has been reported as the most suitable method for SARS-CoV-2 recovery from wastewater samples with the highest mean recovery of 65.7 ± 23.0% (Ahmed et al., 2020c). This method was thus adopted in this study with a few adjustments according to Ahmed et al. (2020c). No virus process control was employed but consistent virus concentration and RNA extraction methods were adopted for all wastewater samples.238 mg of MgCl2 was added to 100 mL wastewater sample to achieve a final concentration of 25 mM MgCl2. Then, 100 mL wastewater sample was firstly centrifuged at 3260g for 30 min. The supernatant was filtered with 0.45 μm pore-size, 90 mm diameter electronegative membranes (HAWP09000; Merck Millipore Ltd., Sydney, Australia). After filtration, the filter paper was transferred to a 5 mL bead tubes from the RNeasy PowerWater Kit (Qiagen, Hilden, Germany). After adding 1 mL lysis buffer composed of 990 μL of buffer PM1 and 10 μL of β-mercaptoethanol (Sigma-Aldrich, Australia), a tissue homogenizer (Minilys, Bertin Technologies, France) was used to homogenize the samples at the medium speed for 10 min. Then, all the other extraction procedure was carried out by following the instruction of the extraction kit. The pellet was extracted with RNeasy PowerMicrobiome Kit (Qiagen, Hilden, Germany) according to the kit's manual. The final extracted RNA volume of the whole 100 mL wastewater sample was 100 μL.
To investigate the effects of inactivation on the virus recovery, 100 mL of cruise ship wastewater sample was incubated at 65 °C for 30 min before the addition of MgCl2. The extraction was subsequently carried out the same as above.
2.3. RT-qPCR assay
Four primer-probe sets (CDC-N1, CCDC-N, N-Sarbeco and E-Sarbeco) that targeting N and E regions of SARS-CoV-2 genome were included in the RT-qPCR assay. Information of the primer and probe sequences is shown in Supplementary Material Table-S2. For RT-qPCR assays, 2019-nCoV_N Positive Control (Catalogue No. 10006625) and 2019-nCoV_E Positive Control (Catalogue No. 10006896) were purchased from the Integrated DNA Technologies (Coralville, IA, USA) for generating the calibration curve of each set. The standard dilutions ranged from 2 to 2 × 105 copies/μL. Standard curves used 20 μL reaction system, which included 10 μL of 2 × one-step reaction mix (iTaq Universal Probes One-Step Kit, catalogue No. 1725141, Bio-Rad Laboratories, Richmond, CA), 600 nM of forward primer, 800 nM of reverse primer, 200 nM of probe, 0.5 μL of iScript reverse transcriptase and 2 μL of template plasmids. This one-step reaction mix allows 45 cycles RT-qPCR reaction to be completed within 2 h. In addition, it is compatible with the Bio-Rad PCR instrument used in this study. For each primer-probe set, 6-point serial dilution test was carried out for three times. The data from all these three results (nine Cq values for each point) were used to generate a master standard curve for calculating the gene copy number of real raw water samples.
To compare the detection limit and the reproducibility (the occurrence of false negative/inconclusive detections) of four assays in more realistic situation towards wastewater samples, four primer-probe sets were tested by spiking 2019-nCoV_N Postive Control (Catalogue No. 10006625) and 2019-nCoV_E Positive Control (Catalogue No. 10006896) into extracted RNA from SARS-CoV-2 negative wastewater samples. The 20 μL reaction system of mocked SARS-CoV-2 positive wastewater samples included 10 μL of 2 × one-step reaction mix (iTaq Universal Probes One-Step Kit, catalogue No. 1725141, Bio-Rad Laboratories, Richmond, CA), 600 nM of forward primer, 800 nM of reverse primer, 200 nM of probe, 0.5 μL of iScript reverse transcriptase, 1 μL of template plasmids(10-fold dilutions ranged from 2 to 2 × 102 copies/reaction) and 5 μL extracted RNA of SARS-CoV-2 negative raw wastewater sample.
To finally evaluate the analytical sensitivity and efficiency of each primer-probe set in WBE applications, comparison work was carried out with five positive real raw wastewater samples from one cruise ship docked in Australia and four WWTPs in Australia. The two positive samples WWTP A and WWTP B were acquired after screening a total of 52 influent wastewater samples collected in August–September 2020, by using CDC-N1 and CCDC-N assay. The 20 μL reaction system for real raw wastewater samples included 10 μL of 2 × one-step reaction mix (iTaq Universal Probes One-Step Kit, catalogue No. 1725141, Bio-Rad Laboratories, Richmond, CA), 600 nM of forward primer, 800 nM of reverse primer, 200 nM of probe, 0.5 μL of iScript reverse transcriptase, and 5 μL extracted RNA.
All RT-qPCR reactions were performed in triplicate. For each RT-qPCR run, a series of three positive and no template controls were included to eliminate the potential contamination of RT-qPCR reagents. The RT-qPCR assays were performed using a Bio-Rad CFX96 thermal cycler (Bio-Rad Laboratories). Data were collected only from the operation of the instrument in which the positive control was positive, and the non-template control was negative. Analytical efficiency (E) of RT-qPCR assays tested with corresponding plasmid standards were calculated by using the following formula (Kralik and Ricchi, 2017):
The slope of each master calibration curve was listed in Table 1 .
Table 1.
Characteristics of RT-qPCR master standard curves for four primer-probe sets.
| Primer-probe sets⁎ | Efficiency (E) (%) | Linearity (R2) | Slope | Y-intercept | |
|---|---|---|---|---|---|
| CDC-N1 | MC | 99.7 | 0.982 | −3.329 | 38.49 |
| SMC | 265.1 | 0.727 | −1.498 | 35.29 | |
| N-Sarbeco | MC | 97.9 | 0.980 | −3.374 | 40.50 |
| SMC | – | – | – | – | |
| CCDC-N | MC | 106.8 | 0.998 | −3.169 | 39.07 |
| SMC | 108.2 | 0.999 | −3.139 | 40.39 | |
| E-Sarbeco | MC | 93.2 | 0.999 | −3.496 | 39.82 |
| SMC | 153.4 | 0.995 | −2.476 | 40.67 | |
Note: *MC, Master calibration; SMC, Spiked mocks calibration.
2.4. RT-qPCR assay quality control
To determine the presence of RT-qPCR inhibition in extracted RNA of real raw wastewater samples, an experiment was conducted by targeting human RNase P gene. A known copy (2 × 103 copies/μL) of Hs_RPP30 Positive Control (Integrated DNA Technologies, Coralville, IA, USA) was used as template and the Cq value obtained acted as a reference point (no extracted RNA of wastewater sample). When the Cq value of a wastewater sample increases by more than 2 cycles compared with the reference Cq, the sample should be considered having the PCR inhibition. RNA extracted from the complex matrix of wastewater usually contains some factors that cause variation of Ct values and thus affect the RT-qPCR results. Variation within 2 cycles is acceptable according to the classical inhibition control test developed for wastewater samples (Ahmed et al., 2020c; Staley et al., 2012). RNA extraction and RT-qPCR reactions were conducted in separate laboratories to minimize the potential contamination during RNA extraction and RT-qPCR preparation.
2.5. Nanopore sequencing
5 μL of RT-qPCR products was used as template to conduct a further amplification to ensure the DNA volume for sequencing. For nanopore sequencing, GENECLEAN® Turbo for PCR (MP Biomedicals Australia Pty Limited) was employed to conduct the first purification of each positive sample to remove unamplified primers, and probes. Then, amplicons were prepared for sequencing according to the manufacturer's protocol of Native barcoding genomic DNA (Version: NBE_9065_v109_revY_14Aug2019). Sequencing results were analysis by EPI2ME platform (Oxford Nanopore Technologies). Taxonomic classification of base-called sequences was conducted through FASTQ WIMP (human + viral) workflow, and the alignment analysis was conducted by using FASTQ Custom Alignment workflow comparing with the reference genome MT276598.1 and NC_045512.2 (GenBank accession number).
3. Results and discussion
3.1. Analytical comparisons of RT-qPCR primer-probe sets with synthesized plasmids
Several preliminary experiments were conducted with CDC-N1, N-Sarbeco and E-Sarbeco by combining the RT-qPCR conditions of CDC-N1 and N-Sarbeco at different concentrations of primer and probe, PCR cycler conditions and final reaction volumes (Data not shown). The preliminary results indicated a universal RT-qPCR condition for four primer-probe sets. Although the condition can be further optimized for better cost-effectiveness (e.g., by using lower template concentrations), each primer-probe set could generate similar or even lower Cq value than under their recommended conditions. This standard conditions for all RT-qPCR reactions are: (1) 600 nM of forward primer, 800 nM of reverse primer and 200 nM of probe; (2) The same PCR reagents, i.e., iTaq Universal Probes One-Step Kit (Bio-Rad Laboratories); and (3) Thermocycler conditions were reverse transcribed at 50 °C for 10 min and initial denaturation at 95 °C for 3 min, followed by 45 cycles of 15 s at 95 °C and 30 s at 58 °C on the Biorad CFX96 qPCR machine (Biorad). This was also applied to the CCDC-N primer-probe sets as there are no recommended RT-qPCR conditions for CCDC-N (Jung et al., 2020).
To evaluate the sensitivity and linearity, the calibration curve of each prime-probe set was established for three times by using 10-fold dilutions at six concentration points (2 × 100 - 2 × 105 plasmid copies/μL, Fig. 1 ). A master calibration curve was generated for each set by using the data acquired from all three establishments. Characteristics including the slope, Y-intercept, R2, and efficiency of each master calibration curve were listed in Table 1.
Fig. 1.
Linearity and reproducibility evaluation of each primer-probe sets by using synthetized plasmid positive control. The solid symbols indicate the median of three runs.
By measuring PCR amplification using 10-fold serial dilutions of pure synthetized plasmids of SARS-CoV-2 N and E genes (2019-nCoV_N and 2019-nCoV_E Positive Control), the efficiencies of all primer-probe sets were between 90%–110%, which matched the requirement for an efficient RT-qPCR assay (Jafferali et al., 2021). At the same concentration, CDC-N1 usually generated the lowest Cq value compared to N-Sarbeco, CCDC-N and E-Sarbeco. Moreover, although the linearity (R2) of the master calibration curve was not as good as CCDC-N and E-Sarbeco assays, CDC-N1 exhibited the best reproducibility ranged from 2 × 103 copies/μL to 2 × 105 copies/μL (Fig. 1A). However, at lower concentrations, the linearity and reproducibility of CDC-N1 was not satisfied. Also, at the concentration of 2 copies/μL, only 3/18 (16.7%) samples showed positive result (Fig. 2A; Supplementary Material Table-S3). In addition, there is an obvious variation of the slope between 2 × 102 copies/μL and 2 × 103 copies/μL, which leads to a poor linearity of the master calibration curve of CDC-N1 (R2 = 0.982).
Fig. 2.
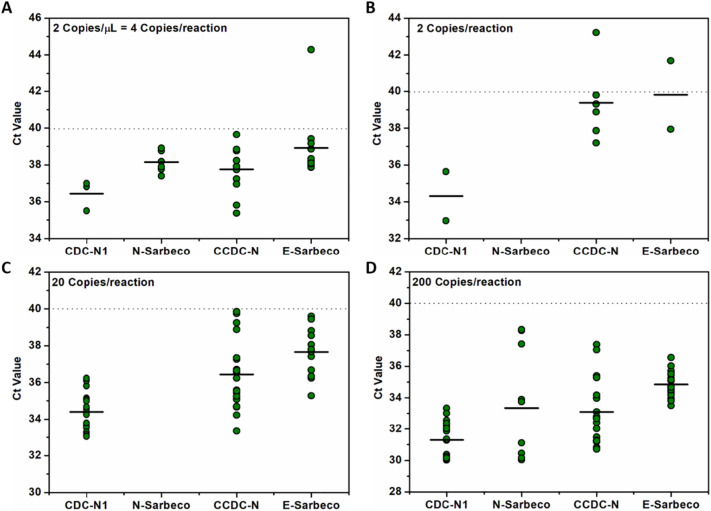
Comparison of false negative/inconclusive tests and Ct value for four primer-probe sets in plasmids-spiked mocks at low targets concentration. (A) Ct value produced with pure synthetized plasmids at 2 copies/μL (4 copies/reaction); (B) Ct value produced with plasmids-spiked mocks at 2 copies/reaction; (C) Ct value produced with plasmids-spiked mocks at 20 copies/reaction; (D) Ct value produced with plasmids-spiked mocks at 200 copies/reaction. The black line symbol is the median for each primer-probe set and the dotted line is the detection limit.
In case of CCDC-N and E-Sarbeco assay, those two primer-probe sets both showed relatively good linearity and reproducibility at low target concentrations than CDC-N1. The R2 of the master calibration curve of both sets was above 0.998. Moreover, out of the total 18 repeats, CCDC-N and E-Sarbeco sets generated 11 (61.1%) and 12 (66.7%) positive results at the lowest concentration (Fig. 2A; Supplementary Material Table-S3), respectively. Compared with other three sets, N-Sarbeco exhibited relatively lower performance in linearity (R2 = 0.980) and reproducibility, by generating only 6/18 positive result at the lowest viral RNA concentration. Although the detection limit of all primer-probe sets was 20 copies/μL (40 copies/reaction; when all replicates were detected as positive), it seems that CCDC-N (61.1%, 11/18) and E-Sarbeco (66.7%, 12/18) could be regarded as the top two sensitive sets based on results using pure synthetized plasmids since they generated the least false negative results at 2 copies/μL. In addition, the amplification efficiency of CCDC-N assay was closer to 100% than E-Sarbeco assay, and the Y-intercept was also smaller than E-Sarbeco assay. Hence, according to the comparison results of pure plasmid test, CCDC-N assay is the most sensitive and reliable quantification tool for SARS-CoV-2 RNA detection.
3.2. Detection of viral RNA at low concentrations in plasmids spiked wastewater mocks
The spiked mocks limit of detection (SMLOD) of four primer-probe sets were determined for mock samples (extracted RNA of SARS-CoV-2 negative raw wastewater) spiked with known concentrations of synthesized SARS-CoV-2 N or E gene positive plasmids control. For each primer-probe set, we performed 24 technical replicates (8 replicates × 3 separately runs) with mock samples at the concentration of 2 and 20 copies/μL, and 18 technical replicates (6 replicates × 3 separately runs) at the concentration of 200 copies/μL.
The results of testing plasmid spiked mocks demonstrated that, except for N-Sarbeco, all other primer-probe sets had a SMLOD as 200 viral gene copies per reaction. (All replicates were positive; Fig. 2D; Supplementary Material Table-S3). LoD comparison of our studies and other two studies was provided in Supplementary Material Table-S7. CDC-N1 and E-Sarbeco exhibited smaller variation in Ct values of each repeat than N-Sarbeco and CCDC-N. For CDC-N1 assay, the Cq values produced at all concentrations are lower than 40. At 20 copies per reaction, 17/24 (70.8%) of the repeats produced positive results with a Cq value lower than 37 cycles. For CCDC-N assay, 19/24 (79.2%) of the repeats generated positive results with a Cq value lower than 40 cycles at 20 copies per reaction. Even at the concentration of 2 copies per reaction, 6/24 (25%) of repeats still generated positive signal, and 5/6 (83.3%) of the positive result got a Cq value lower than 40 cycles (Fig. 2B). Under this target concentration, only 2/24 (8.3%) positive results were produced by CDC-N1 and E-Sarbeco. While for E-Sarbeco, its sensitivity was not as high as CDC-N1 and CCDC-N since only 12/24 (50%) of the repeats detected plasmid gene at 20 copies/reaction (Fig. 2C; Supplementary Material Table-S3).
To evaluate their application potential in WBE, the spiked mocks calibration of each primer-probe sets was generated, and the characteristics of the calibration were provided (Table 1). It was found that there is no obvious linearity of CDC-N1 assay at the low concentrations ranging from 2 to 200 copies per reaction. In contrast, CCDC-N and E-Sarbeco still showed relatively good linearity at such low concentrations. By comparing the characteristics of the master calibration and the spiked mocks calibration of each primer-probe sets, it is obvious that, for CCDC-N assay, the variations between the two calibrations is minimal. This may indicate that CCDC-N assay is the most reliable assay when it is used for WBE (Fig. 3).
Fig. 3.
Detection performance of four primer-probe sets in SARS-CoV-2 N and E positive control plasmids-spiked mocks. The black line symbol is the median and the dotted line is the detection limit.
In conclusion, among these four primer-probe sets, CDC-N1 and CCDC-N assay exhibited relatively good analytical sensitivity for SARS-CoV-2 RNA detection in plasmid spiked wastewater samples. However, CDC-N1 exhibited the poorest linearity at the low concentrations ranging from 2 to 200 copies per reaction. It is thus not reliable to calculate the real gene copy numbers for real raw wastewater samples using the CDC-N1 primer-probe set for low prevalence communities. Supported by consistent results with the pure plasmids assay, the spiked mock results further confirmed CCDC-N assay as the most reliable primer-probe set in the quantitative analysis of SARS-CoV-2 gene copies in wastewater samples.
3.3. Detection of SARS-CoV-2 in raw wastewater samples
Wastewater samples collected from different WWTPs and cruise ship were analyzed using the four RT-qPCR primer-probe sets. All positive results were provided in Table 2 and Table-S4. Due to the low RNA concentration in positive samples, 10-fold dilution tests were not used to detect inhibition in this study. Instead, individual inhibition control test targeting RNase P gene was conducted for each sample following previous studies (Ahmed et al., 2020c; Staley et al., 2012). And all positive samples were double checked by Nanopore sequencing to exclude false positive result. Read depths of merged reads mapping to the SARS-CoV-2 reference genome MT_276598.1 was provided in Supplementary Material Fig. S2. For five raw and three inactivated (65 o C for 30 min) cruise ship influent wastewater samples, the positive ratio of each primer-probe sets was 3/24 (12.5%), 1/24 (4.2%) and 2/24 (8.3%) of CDC-N1, N-Sarbeco and CCDC-N, respectively. The inactivation procedure showed no obvious effect on RT-qPCR results of this cruise ship wastewater sample at low RNA concentrations, since positive signal was generated from both deactivated and raw wastewater samples. Further research is needed to decide the effects of heat inactivation on the RT-qPCR detection (Table-S4). E-Sarbeco did not produce any positive results on these samples. Cq values of all positive samples ranged from 35.29 to 39.54 (Table 2). For each positive extraction, only one reaction produced positive signal among three repeats. For the three positive raw and inactivated wastewater samples, the Cq value generated by CDC-N1 were 1–2 cycles lower than other primer-probe sets, indicating the highest sensitivity. For the fourth extraction, only CCDC-N produced one positive signal with the Cq value of 35.29. Moreover, no assay produced positive signal on the fifth extraction. The supernatants of three non-inactivated samples were also tested by CDC-N1 to evaluate the presence of virus particles in the supernatant. It was found that no positive signal was produced in supernatant. The filtration with electronegative membranes thus might not have caused the loss of viral load during the sample extraction. By comparing our detection result of CDC-N1 set with Ahmed's study (Ahmed et al., 2020b), it was founded that the SARS-CoV-2 RNA concentration in cruise ship wastewater significantly decreased after being stored at −80 °C for half a year, although it still can be detected. For other positive wastewater samples, including WWTP A, B and C, only CDC-N1 assay detected SARS-CoV-2 RNA in one of the triplicate reactions with a Cq value of 34.4, 33.10 and 33.47, respectively.
Table 2.
Detection result of SARS-CoV-2 RNA in wastewater samples collected from a cruise ship and three WWTPs in Australia.
| Sampling sites (date) | Sample (extraction volume) | Cq value (Copies/100 mL) |
|||
|---|---|---|---|---|---|
| CDC-N1 | N-Sarbeco | CCDC-N | E-Sarbeco | ||
| Cruise ship (23/4/2020) |
1 (100 mL) |
38.44 (104) | 39.54 (193) | ND | ND |
| ND | ND | ND | ND | ||
| ND | ND | ND | ND | ||
| 2 (100 mL) |
ND | ND | ND | ND | |
| ND | ND | ND | ND | ||
| ND | ND | ND | ND | ||
| 3 (100 mL) |
ND | ND | ND | ND | |
| ND | ND | ND | ND | ||
| ND | ND | ND | ND | ||
| 4 (100 mL) |
ND | ND | 35.29 (1559) | ND | |
| ND | ND | ND | ND | ||
| ND | ND | ND | ND | ||
| 5 (100 mL) |
ND | ND | ND | ND | |
| ND | ND | ND | ND | ||
| ND | ND | ND | ND | ||
| WWTP A (4/8/2020) |
100 mL |
33.10 (4160) | ND | ND | ND |
| ND | ND | ND | ND | ||
| ND | ND | ND | ND | ||
| WWTP B (1/9/2020) |
100 mL | 33.47 (3221) | ND | ND | ND |
| ND | ND | ND | ND | ||
| ND | ND | ND | ND | ||
| WWTP C (11–13/8/2020) |
1 (100 mL) |
ND | ND | ND | ND |
| ND | ND | ND | ND | ||
| ND | ND | ND | ND | ||
| 2 (100 mL) |
ND | ND | ND | ND | |
| ND | ND | ND | ND | ||
| ND | ND | ND | ND | ||
| 3 (100 mL) |
ND | ND | ND | ND | |
| ND | ND | ND | ND | ||
| 34.4 (1693) | ND | ND | ND | ||
Note: ND: not detected.
Collectively speaking, based on the analytical performance evaluation of different primer-probe sets using real raw wastewater. CDC-N1 was demonstrated to be the most sensitive primer-probe set in analyzing real wastewater samples for the highest positive ratio (4 positive results out of 59 wastewater samples). For the samples reported as positive by CDC-N1 (samples of cruise ship), the positive results were also reported by CCDC-N (2 positive results ≤40 cycles) is comparable to CDC-N1 (3 positive results ≤40 cycles). In contrast, E-Sarbeco set showed the poorest analytical sensitivity without any positive result out of 39 reactions. This detection sensitivity trend of CDC-N1 and E-Sarbeco for wastewater study is consistent with other previous studies (Ahmed et al., 2020b; Medema et al., 2020; Pérez-Cataluña et al., 2021).
For the 15 extracted wastewater RNA samples, two repeats were conducted for each sample using the four primer-probe sets. The positive ratio of each primer-probe sets is 9/15 (60%), 8/15 (53.3%), 6/15 (40%) and 4/15 (26.7) of CDC-N1, CCDC-N, N-Sarbeco and E-Sarbeco, respectively (Fig. S4). CDC-N1 (60%) and CCDC-N (53.3%) showed high detection sensitivity as the top two among four primer-probe sets. E-Sarbeco generated the lowest positive ratio. These results further confirmed that, in the detection of SARS-CoV-2 RNA of wastewater samples, CDC-N1 is the most sensitive set followed by CCDC-N.
In addition, the analysis of wastewater samples from WWTP C on three consecutive days showed that the last sampling day was positive with the previous two days as negative. This indicated an increase of SARS-CoV-2 RNA in wastewater. According to the COVID-19 cases reported by clinical testing, on the next day of positive sampling day, the active cases number within the area coved by WWTP C increased from 52 to 56, which is consistent with the increase of SARS-CoV-2 RNA detected in wastewater (Supplementary Material Fig. S3).
Overall, the results of testing real wastewater samples showed that CDC-N1 exhibited superior detection sensitivity than the other sets. Using the RT-qPCR assays in this study, it was demonstrated that the increase of SARS-CoV-2 RNA in wastewater was consistent with the increase of active cases number within a low prevalence community.
3.4. Analysis of RT-qPCR products by nanopore sequencing
Various types of errors introduced during PCR, including polymerase misincorporation, structure-induced template-switching, PCR-mediated recombination and thermocycling-induced DNA damage will lead to the mutations of PCR products. These mutations will be enlarged with the increasing of cycle's number, and ultimately confound downstream genetic analysis (Potapov and Ong, 2017). According to the principle of PCR, the amplicons of last cycle will be used as the template DNA of the next cycle. We infer that the mutation can affect the combination between primer-probes and templates (PCR products of last cycle), thus altering the efficiency of amplification and detection. The average nucleotide identity (ANI) and alignment percentage (alignment accuracy >90%, AP90) of PCR products was thus determined through Nanopore sequencing for assessing the mutations' accumulation, and to gain insights at nucleotide level for the variation of analytical sensitivity and efficiency of the four primer-probe sets.
RT-qPCR products of all positive real raw wastewater samples (section 3.3) and four positive plasmid spiked mock samples (2 × 103 copies/μL) were sequenced using Oxford Nanopore Technology (ONT) GridION. A recent study found there is a notable three nucleotide substitutions (GGG → AAC) in the first three positions of CCDC-N forward primer, which induced its mismatch with around 13% of the available SARS-CoV-2 whole genomes released as of 22 March 2020 (Vogels et al., 2020). However, the sequence annealing and amplification may not be adversely affected since the replacement occurs in the first three positions of the 5 ‘end of the CCDC-N primer. Therefore, in this study, two reference genomes, GenBank accession number MT_276598.1 (GGG → AAC) and NC_045512.2 (GGG) were chosen to conduct the alignment analysis between PCR amplicons and SARS-CoV-2 reference genome.
The alignment results for reference genome MT_276598.1 were shown as the relationship between ANI and AP90 (Fig. 4 and Supplementary Material Table-S5). The similar results for reference genome NC_045512.2 were presented in Supplementary Material Fig. S1and Table-S5. It is shown that CDC-N1 had the highest ANI at 98.90% and the lowest AP90 at 74.84% (alignment accuracy >90%) in spiked mock samples. N-Sarbeco assay had the lowest ANI at 98.3%, and the highest AP90 at 84.21%. Meanwhile, CCDC-N and E-Sarbeco both showed relatively high ANI and AP90 at 98.5%, 98.4% and 84.19%, 84.00%, respectively. The sequencing results seemingly suggest that ANI and AP90 is linked to the RT-qPCR sensitivity and reproducibility. The low AP90 of CDC-N1 might be responsible for its poor reproducibility and linearity in spiked mock samples. Similarly, the relatively poor analytical performance of N-Sarbeco might be partly due to its low ANI. Also, the relatively high ANI and AP90 of CCDC-N and E-Sarbeco is consistent with their relatively good RT-qPCR performance in spiked mock samples.
Fig. 4.

Average nucleotide identity (ANI) and alignment percentage (alignment accuracy >90%, AP90) of all positive wastewater samples and four plasmid spiked mock positive control samples. Reference genome: MT_276598.1. Symbols with red edge are the ANI and AP90 of plasmid spiked mocks. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For cruise ship wastewater sample, SARS-CoV-2 RNA was detected by three sets except for E-Sarbeco. ANI and AP90 of six SARS-CoV-2 RNA positive reactions were determined (Supplementary Material Table-S5). CDC-N1 had the highest AP90 and relatively high ANI, which is consistent with its high positive result ratio (3 positive of 8 extractions). CCDC-N produced 2/8 positive, which is consistent with the highest ANI and the second high AP90 values. The lowest ANI and AP90 of N-Sarbeco was also consistent of the lowest positive ratio (1/8).
The alignment result referencing genome NC_045512.2 was similar with the result of MT_276598.1 (Supplementary Material Fig. S1). There was no significant variation between the two alignment results of each sample. The ANI and AP90 of some positive wastewater sample was slightly decreased, while this didn't cause any change on the trend between primer-probe sets (Supplementary Material Table-S5). Moreover, we found that the ANI and AP90 values of CCDC-N set in the alignment analysis with NC_045512.2 (no nucleotide substitutions) was lower (rather than higher) than MT_276598.1. This shows that the three nucleotide substitutions in the first three positions of CCDC-N forward primer might not be responsible for the alignment variation of CCDC-N with different reference genome, as suggested by Vogels et al. (2020).
All primer-probe sets (CDC-N1, CCDC-N, N-Sarbeco and E-Sarbeco) had similar ANI (0.98–0.99) but varied AP90 values (75–90%). It seems an AP90 < 80% (e.g. N-Sarbeco) indicates a poor RT-qPCR performance in real raw wastewater samples. Notably, CCDC-N usually exhibited similar ANI and AP90 among different wastewater samples which indicated its stability towards wastewater samples. The sequence alignment comparison confirmed CDC-N1 as the most sensitive primer-probe set, while the CCDC-N being the most reliable assay with a good sensitivity in real wastewater samples.
In addition, the first 1000 SARS-CoV-2 genomes from SARS-CoV-2 Data Hub of National Center for Biotechnology Information (NCBI) by setting the release date as 28 May 2021 were also aligned with the four primer-probe sets to determine their nucleotide mismatches. The mismatched nucleotides with frequencies above 0.1% are listed in Supplementary Material Table-S6. Six nucleotide mismatches were detected in the primer-probe regions with a frequency higher than 1%, i.e., mismatch in more than 10 of the 1000 SARS-CoV-2 genomes. The high-frequency mismatches were found for both CDC-N1 and CCDC-N sets. For CCDC-N set, in addition to the three notable nucleotide substitutions (GGG → AAC) (Vogels et al., 2020), six mismatches were detected. However, the three high-frequency mismatches are located near the 5’position of forward/reverse primers. Thus, these mismatches may not be detrimental to sequence annealing and amplification during the PCR. The other three mismatches were only found in less than five genomes. For CDC-N1, high-frequency mismatches were detected at the 9th position of its forward primer, and the 3rd position of the probe, which may compromise its capability in detecting SARS-CoV-2 mutant strains. For N-Sarbeco and E-Sarbeco, no obvious mismatches with significant effect were found. However, these two primer-probe sets have always been reported to be less sensitive to wastewater samples. The nucleotide mismatch of virus variants with primers and probes is not the only determining factor of the RT-qPCR analytical performance for mutant strains. Other factors such as the resistance to inhibitors in wastewater and other environmental factors can also lead to changes in their analytical performance.
4. Conclusions
This study systematically compared the analytical performance of four SARS-CoV-2 RT-qPCR primer-probe sets towards various sample types to evaluate their application in wastewater-based epidemiology. Main outcomes could be concluded as follow:
CCDC-N was the most recommendable primer-probe set for its high sensitivity and reproducibility in pure plasmid assay, spiked mocks and real wastewater samples. It would provide the most reliable SARS-CoV-2 RNA quantitative results for the application in wastewater-based epidemiology. CDC-N1 showed high sensitivity of real raw wastewater but poor reproducibility and linearity at low concentrations against spiked mocks. It is more suitable to be employed for screening the presence of SARS-CoV-2 RNA in wastewater from low prevalence communities.
Increased active clinical cases were observed with the augment of SARS-CoV-2 RNA quantified by RT-qPCR in wastewater in low prevalence communities. However, the trend of both the viral detection and number of active cases over the entire period of an outbreak wave is needed to confirm that the selected primer-probe sets can detect the increase trend of active cases in the communities.
Analysis of more wastewater samples shall provide more data for the comparison of these primer-probe sets. It is expected that the analytical performance might vary in different regions of the world considering the base variations of SARS-CoV-2 RNA.
Considering the varying performance of different primer-probe sets, a multiplex RT-qPCR assay, combining the primer-probe sets that has better sensitivity and linearity, could further improve the performance of RT-qPCR analysis and quantification of SARS-CoV-2 RNA in wastewater.
CRediT authorship contribution statement
Shuxin Zhang - Study design, conducting experiment, data analysis and writing – original draft. Xuan Li - Wastewater sample processing and writing – review. Jiahua Shi - Sampling and writing – review. Muttucumaru Sivakumara - Writing – review. Stephen Lubyb - Writing – review. Jake O'Brienc - Sampling and writing – review. Guangming Jiang – Supervision and Writing – review.
Declaration of competing interest
The authors have declared no conflicts of interest.
Acknowledgements
This research was supported by the ARC Discovery project (DP190100385). Shuxin Zhang receives the support from a University of Wollongong PhD scholarship. Guangming Jiang was the recipient of an Australian Research Council DECRA Fellowship (DE170100694).
Editor: Damia Barcelo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.150572.
Appendix A. Supplementary data
Supplementary material
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Angel N., Bibby K., Bivins A., Dierens L., et al. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: a surveillance tool for assessing the presence of COVID-19 infected travellers. J. Travel Med. 2020;27 doi: 10.1093/jtm/taaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., et al. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R.A., Varma M., Sivaganesan M., Kelty C., Peed L., Shanks O.C. Evaluation of genetic markers from the 16S rRNA gene V2 region for use in quantitative detection of selected bacteroidales species and human fecal waste by qPCR. Syst. Appl. Microbiol. 2010;33:348–357. doi: 10.1016/j.syapm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Jafferali M.H., Khatami K., Atasoy M., Birgersson M., Williams C., Cetecioglu Z. Benchmarking virus concentration methods for quantification of SARS-CoV-2 in raw wastewater. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y., Park G.-S., Moon J.H., Ku K., Beak S.-H., Lee C.-S., et al. Comparative analysis of primer-probe sets for RT-qPCR of COVID-19 causative virus (SARS-CoV-2) ACS Infect. Dis. 2020;6:2513–2523. doi: 10.1021/acsinfecdis.0c00464. [DOI] [PubMed] [Google Scholar]
- Kralik P., Ricchi M. A basic guide to real time PCR in microbial diagnostics: definitions, parameters, and everything. Front. Microbiol. 2017;8:108. doi: 10.3389/fmicb.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., et al. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746 doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., et al. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang S., Shi J., Luby S.P., Jiang G. Uncertainties in estimating SARS-CoV-2 prevalence by wastewater-based epidemiology. Chem. Eng. J. 2021;415 doi: 10.1016/j.cej.2021.129039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A. 2020. SARS-CoV-2 RNA Concentrations in Primary Municipal Sewage Sludge as a Leading Indicator of COVID-19 Outbreak Dynamics. [Google Scholar]
- Pérez-Cataluña A., Cuevas-Ferrando E., Randazzo W., Falcó I., Allende A., Sánchez G. Comparing analytical methods to detect SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapov V., Ong J.L. Examining sources of error in PCR by single-molecule sequencing. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley C., Gordon K.V., Schoen M.E., Harwood V.J. Performance of two quantitative PCR methods for microbial source tracking of human sewage and implications for microbial risk assessment in recreational waters. Appl. Environ. Microbiol. 2012;78:7317–7326. doi: 10.1128/AEM.01430-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunström L., Newbold S.C., Finnoff D., Ashworth M., Shogren JFJJoB-C.A. The Benefits and Costs of Using Social Distancing to Flatten the Curve for COVID-19. Vol. 11. 2020. pp. 179–195. [Google Scholar]
- Vogels C.B.F., Brito A.F., Wyllie A.L., Fauver J.R., Ott I.M., Kalinich C.C., et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT–qPCR primer–probe sets. Nat. Microbiol. 2020;5:1299–1305. doi: 10.1038/s41564-020-0761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.-M., Maday Y., Teyssou R., Richard E. 2020. Evaluation of Lockdown Impact on SARS-CoV-2 Dynamics Through Viral Genome Quantification in Paris Wastewaters. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material