Abstract
Purpose
SARS-CoV-2 RNA has been detected in ocular tissues, but their susceptibility to SARS-CoV-2 infection is unclear. Here, we tested whether SARS-CoV-2 can infect human conjunctival epithelial cells (hCECs) and induce innate immune response.
Methods
Conjunctival tissue from COVID-19 donors was used to detect SARS-CoV-2 spike and envelope proteins. Primary hCECs isolated from cadaver eyes were infected with the parental SARS-CoV-2 and its beta variant of concern (VOC). Viral genome copy number, and expression of viral entry receptors, TLRs, interferons, and innate immune response genes were determined by qPCR. Viral entry receptors were examined in hCECs and tissue sections by immunostaining. Spike protein was detected in the cell culture supernatant by dot blot.
Results
Spike and envelope proteins were found in conjunctiva from COVID-19 patients. SARS-CoV-2 infected hCECs showed high viral copy numbers at 24–72h post-infection; spike protein levels were the highest at 24hpi. Viral entry receptors ACE2, TMPRSS2, CD147, Axl, and NRP1 were detected in conjunctival tissue and hCECs. SARS-CoV-2 infection-induced receptor gene expression peaked at early time points post-infection, but gene expression of most TLRs peaked at 48 or 72hpi. SARS-CoV-2 infected hCECs showed higher expression of genes regulating antiviral response, RIG-I, interferons (α, β, & λ), ISG15 & OAS2, cytokines (IL6, IL1β, TNFα), and chemokines (CXCL10, CCL5). Compared to the parental strain, beta VOC induced increased viral copy number and innate response in hCECs.
Conclusions
Conjunctival epithelial cells are susceptible to SARS-CoV-2 infection. Beta VOC is more infectious than the parental strain and evokes a higher antiviral and inflammatory response.
Keywords: COVID-19, SARS-CoV-2, Eye, Conjunctiva, Ocular surface, Inflammation, Viral entry receptors
1. Introduction
The ongoing pandemic of Coronavirus Disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus −2 (SARS-CoV-2) is a serious global health problem [1], [2], [3]. As of 16th September 2021, 227,185,960 COVID-19 cases have been confirmed worldwide with 4,672,629 total deaths. SARS-CoV-2 belongs to the family of coronaviruses shared with SARS-CoV and MERS-CoV, causative agents of epidemics in 2002-03 and 2012, respectively [4,5]. They are enveloped, non-segmented, positive-sense RNA viruses belonging to the family Coronaviridae with a genome of 29–31 kb [5]. There have been significant efforts to contain COVID-19 with mass immunization across the globe [6]. However, due to genetic mutations in parental SARS-CoV-2, multiple variants of concern (VOC) have emerged with increased transmissibility, disease severity, and ability to evade vaccine-induced immunity. Currently, as per CDC four VOCs are prevalent in the US, B.1.1.7 (alpha), B.1.351 (beta), B.1.617.2 (delta), and P.1 (gamma) [7,8].
The main route of transmission of SARS-CoV-2 is via respiratory droplets while coughing and direct contact. However, the involvement of ocular surface tissue as a route of viral transmission was first suspected with the detection of COVID-19 in an ophthalmologist in China who was wearing an N95 mask. The human ocular surface acts as a barrier but also as a gateway for pathogen invasion. Anatomically, the nasolacrimal duct bridges the transport of ocular fluids (tears) to the respiratory tract system, thereby contributing to the pathogen spread. The respiratory RNA viruses, including SARS-CoV, MERS-CoV, and influenza virus are known to have ocular tropism as the infectious droplets can easily contaminate the mucosal surface of the cornea and conjunctiva via hand-to-eye contact or direct exposure with aerosols [[9], [10], [11]].
There have been several reports of ocular tissue harboring SARS-CoV-2 viral RNA in tears, conjunctiva, and cornea. Some clinical studies report as high as 32% of conjunctivitis cases in COVID-19 patients [[12], [13], [14], [15]]. Several published reports suggest that SARS-CoV-2 can cause red eye, conjunctivitis, and conjunctival congestion as an early sign of the disease or during hospitalization [[16], [17], [18], [19], [20]]. A recent publication also suggests that rhesus macaques can be effectively infected with SARS-CoV-2 via the ocular surface, specifically through the conjunctival route [21]. In our previous study, we have shown the presence of SARS-CoV-2 in the postmortem corneal and scleral tissues of COVID-19 affected donors by qPCR and immunofluorescence analysis [14,22].
The primary infection of host cells by SARS-CoV-2 is mediated by the attachment of viral spike (S) protein to the cell receptor, angiotensin-converting enzyme-2 (ACE2), that is primed by a type II transmembrane serine protease TMPRSS2 or cathepsin L [23,24]. Recently, other accessory receptors involved in viral attachment and infection including CD147/EMMPRIN/Basigin, Axl, and Neuropilin-1 (NRP1) have also been implicated [21,[25], [26], [27], [28], [29], [30], [31]]. The ocular surface (conjunctiva, corneal and limbal epithelium, and corneal endothelium) has been shown to express SARS-CoV-2 receptors ACE2, TMPRSS2, CD147, and NRP1. These ocular tissues have been postulated to be permissive to SARS-CoV-2 and potentially involved in its transmission [27,[32], [33], [34], [35], [36], [37]]. However, it is unclear whether the ocular surface can be a primary or secondary virus entry site. Therefore, there is an urgent need to understand ocular tropism of SARS-CoV-2. The identification of organs and cell types permissive to viral attachment, entry, and replication could help in devising preventative or therapeutic strategies against SARS-CoV-2 transmission.
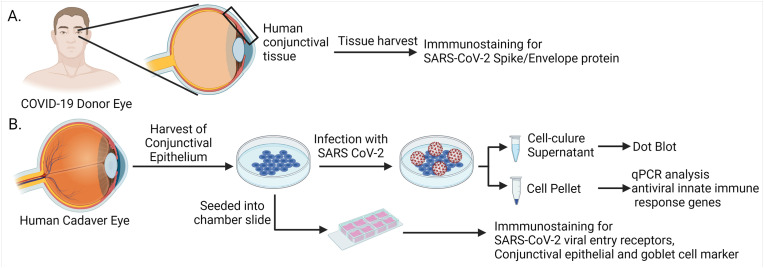
Among the ocular surface tissues, the conjunctiva has the largest surface area, and it has been shown to express viral entry receptors, ACE2 and TMPRSS2 [32]. It would seem logical to evaluate the susceptibility of the conjunctiva to SARS-CoV-2 infection. In this study we tested whether SARS-CoV-2 can infect human conjunctival epithelium and induce innate immune response. Moreover, we demonstrated differential susceptibility of parental and emerging variants of concerns (VOCs) of SARS-CoV-2. The schematic of experimental design is shown in Fig. 1 .
Fig. 1.
Schematic of the study design. (A) Conjunctival tissues recovered from healthy, and COVID-19 affected human donor eyes were used for immunostaining for viral proteins and viral entry receptors. (B) The primary human conjunctival epithelial cells (hCECs) were isolated from human cadaver eyes. The hCECs and ex vivo tissues were immunostained for the viral entry receptors and conjunctival epithelial and goblet cell markers. Cultured cells were infected with live SARS-CoV-2 and qPCR analysis was performed for innate immune response genes. Culture supernatant was used for dot blot assay. Images were prepared using BioRender.
2. Materials and methods
2.1. Conjunctival tissue acquisition
Conjunctival tissues from two healthy and two COVID-19 affected donors were obtained through Eversight (Cleveland, OH). The histological analysis was performed with the approval of the Institutional Biosafety Committee (IBC # 20-04-2164) of Wayne State University. The presence of SARS-CoV-2 RNA and proteins in postmortem ocular tissues from Eversight was reported in our recent study [14].
2.2. Virus culture and infection
Both parental SARS-CoV-2 [USA-WA1/2020 (NR-52281)] and beta lineage [KRISP-K005325/2020 (NR-54009)] strains were obtained from BEI Resources (NIAID/NIH, USA). All experiments involving live SARS-CoV-2 virus were carried out in a CDC and USDA-approved biosafety-level 3 (BSL-3) facility at the University of California, Los Angeles (UCLA) following institutional biosafety approval. Virus stocks were propagated in Vero E6 cells (ATCC, CRL-1586), and serum in the cell culture media was reduced to 2% for all infection experiments.
The hCECs were infected with SARS-CoV-2 parental strain (USA-WA1/2020) at MOI 1 for 2 h with intermittent shaking in a serum-free tissue culture medium. The cells were rinsed thrice with 1X PBS followed by incubation in a complete growth medium with 2% fetal bovine serum (FBS) for desired time points. The comparative infection study with parental strain (USA-WA1/2020) and beta lineage (hCoV-19/South Africa/KRISP-K005325/2020) has been performed at MOI 0.1 owing to the low infectious viral titer of the beta strain. All infected samples were inactivated by lysis in TRIzol Reagent (Thermo Fisher Scientific, MA, USA) or 24 h incubation in 4% paraformaldehyde before removal from the BSL-3 facility.
2.3. Primary human conjunctival epithelial cells (hCECs)
The healthy human donor conjunctival tissue was dissected from postmortem whole globes and corneas obtained from the National Disease Research Interchange (Philadelphia, PA) (Table 1 ). Work was done under Cedars-Sinai Medical Center's approved IRB protocol Pro00019393. The tissue was cut into 1 × 2 mm pieces that were placed with the epithelial side up onto a scratched surface of the plastic plate to ensure adhesion of the tissue and incubated in DMEM with 3% FBS. After hCECs have migrated onto the dish from the explant of conjunctival tissue, the tissue explants were removed and the hCECs were further cultured in EpiLife medium containing N2, B27, HKGS (human keratinocyte growth supplement) supplements, and 10 ng/mL epidermal growth factor (EGF) (Thermo Fisher, MA, USA), with 60 μM Ca2+ [38]. Confluent (90%) hCEC cultures were passaged using TrypLE, and the cells in EpiLife medium with 10 ng/mL EGF were plated onto the dishes or 8-chamber glass slides pre-coated with a mixture of human fibronectin (BD Biosciences, NJ, USA), type IV collagen (Sigma-Aldrich, MO, USA), and laminin-521 (BioLamina, Ontario, Canada) at 0.5–1 μg/cm2 [39].
Table 1.
Characteristics of the donors for conjunctival immunostaining and epithelial cell isolation.
| Case | Age | Gender | Cause of death |
|---|---|---|---|
| N14-01 C | 86 | Female | COPD |
| N14-03 | 91 | Female | CVA |
| N15-08 C | 44 | Female | Myocardial infarction |
| N15-09 C | 15 | Male | Gunshot wound |
| N15-10 C | 5 | Male | Drowning |
| N18-37 | 53 | Male | ALS |
| N20-26 | 75 | Female | Cardiac arrest |
| N21-03 | 80 | Female | Lung cancer |
C, culture; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident (stroke); ALS, amyotrophic lateral sclerosis.
2.4. Immunohistochemistry on human conjunctival tissue
Paraformaldehyde (4%) fixed conjunctival tissues from healthy and COVID-19 affected donors were incubated in a series of sucrose gradients (10%, 20%, 30%), and embedded in OCT (Tissue-Tek®-Sakura, CA, USA). Ten-micrometer thin sections were prepared using a cryotome (HM525 NX, ThermoFisher Scientific, MA, USA) and mounted onto lysine-coated glass slides (Fisherbrand, Thermo Scientific, MA, USA). The tissue sections were permeabilized and blocked with 10% normal goat serum with 0.5% Triton X-100 for 2 h at room temperature (RT) followed by overnight incubation with primary mouse anti-SARS-CoV-2 spike [NR-616, BEI Resources, NIAID (1:100)] or envelope protein antibodies [NR-614, BEI Resources, NIAID (1:100)] at 4 °C. The next day, sections were rinsed four times with 1X PBS (10 min, each) and incubated with anti-mouse Alexa Fluor 594 (1:200) for 2 h at RT. The sections were extensively rinsed with 1X PBS, and the slides were mounted using Vectashield anti-fade mounting medium (Vector Laboratories, CA, USA) and visualized using a Keyence microscope BZ-X810 series (Keyence, CA, USA). For the viral entry receptor staining, conjunctival cryostat sections were fixed in 2% formalin for 5 min at RT and then processed as published [27].
2.5. Immunostaining of cultured hCECs
Primary hCECs cultures were fixed with 10% formalin for 5 min at RT, followed by blocking with 5% BSA (bovine serum albumin) in 1X PBS for 1 h. For the receptor staining, the cells were incubated overnight at 4 °C in primary antibody (goat anti-ACE2, 1:100, #AF933, goat anti-Axl, 1:100, #AF154, R&D Systems, MN, USA; rabbit anti-neuropilin-1 (NRP1), 1:100, #PB9300, Boster, CA, USA; mouse anti-CD147, clone HIM6, 1:100, #306202, BioLegend, CA, USA; mouse anti-TMPRSS2, clone H4, 1:100, #sc-515727, Santa Cruz Biotechnology, TX, USA), followed by incubation with Alexa Fluor 488 or 594 conjugated secondary antibody (Thermo Fisher, MA, USA) for 1 h at RT. For conjunctival goblet cell marker staining, cells were fixed in ice-cold methanol for 10 min. Primary antibodies were mouse anti-keratin 7, clone OV-TL 12/30, 1:100, #MS-1352-P; and mouse anti-MUC5AC, clone 45M1, 1:50, #MS-145-P0, Thermo Fisher, MA, USA. For conjunctival epithelial marker keratin 13 staining (mouse clone Ks13.1, 1:20, #sc-101460, Santa Cruz Biotechnology, TX, USA), cells were fixed in 10% formalin for 5 min, permeabilized with 0.5% Triton X-100 in 1X PBS for 10 min, and blocked in normal goat serum for 1 h. Cell nuclei were visualized with 4′,6′- diamidino-2-phenylindole (DAPI). Controls with the omission of primary antibodies were negative.
2.6. Dot-blot analysis
hCECs were infected with SARS-CoV-2 for various time points (24, 48, and 72h) and 1X PBS-treated cells were used as mock-infected controls. After incubation for various time points, the culture supernatant was collected from each well and centrifuged at 10,000×g for 10 min to remove cell debris. The supernatant was used for the dot-blot assay. The cell culture supernatants were loaded onto a 0.45 μm nitrocellulose membrane using a BIO-DOT apparatus (Bio-Rad, CA, USA) and vacuum suctioned. The membrane was fixed using 10% formaldehyde in PBS for an hour at room temperature followed by blocking in 5% skimmed milk in 1X TBS-T (TBS containing 0.05% Tween 20) for 1 h at RT. The membrane was then incubated with anti-SARS-CoV-2 spike antibody at 4 °C overnight followed by secondary antibody (anti-mouse-HRP conjugate) at room temperature for 1 h. The antibody specificity was checked using different concentrations of the SARS-CoV-2 spike protein as positive control and culture supernatant of mock infected cells as a negative control. The blots were developed using SuperSignal West Femto maximum sensitivity substrate (Thermo Scientific, MA, USA) using a molecular imaging system. The intensity of dots was analyzed using ImageJ software.
2.7. Real-time PCR
Total RNA was extracted from the cells using TRIzol reagent (Thermo Fisher, MA, USA) as per the manufacturer's instructions. The cDNA was synthesized using 1 μg of RNA using Maxima First Strand cDNA Synthesis Kit (Thermo Fisher, MA, USA) according to the manufacturer's instructions. Quantitative RT-PCR (qPCR) was performed using gene-specific primers (Table 2 ) in a StepOnePlus Real-Time PCR System (Applied Biosystems, MA, USA). Quantification of gene expression was performed using the comparative cycle threshold method and expressed as relative fold changes compared to housekeeping gene 18S rRNA (see Table 2). The viral RNA transcripts were quantified using a Taqman probe-based qPCR (Table 2) by plotting a standard curve prepared from serial 10-fold dilutions of SARS-CoV-2 RNA as described previously [40].
Table 2.
Primers used in the study.
| Name | Sequence |
|---|---|
| SARS-CoV-2 N Forward Primer | 5′- GGGGAACTTCTCCTGCTAGAAT -3′ |
| SARS-CoV-2 N Reverse Primer | 5′- CAGACATTTTGCTCTCAAGCTG -3′ |
| SARS-CoV-2 Probe | 5′-FAM-TTGCTGCTGCTTGACAGATT-TAMRA-3′ |
| 18S rRNA Forward Primer | 5′- GTAACCCGTTGAACCCCATT -3′ |
| 18S rRNA Reverse Primer | 5′- CCATCCAATCGGTAGTAGCG -3′ |
| ISG15 Forward Primer | 5′- CAGCGAACTCATCTTTGCCAG -3′ |
| ISG15 Reverse Primer | 5′- GGACACCTGGAATTCGTTGC -3′ |
| OAS2 Forward Primer | 5′- ACCATCGGAGTTGCCTCTTA -3′ |
| OAS2 Reverse Primer | 5′- GGTGAACACCATCTGTGACG -3′ |
| IFNλ1 Forward Primer | 5′- GAAGCAGTTGCGATTTAGCC -3′ |
| IFNLλ1 Reverse Primer | 5′- GAAGCTCGCTAGCTCCTGTG -3′ |
| AXL Forward Primer | 5′- CGCAGGAGAAAGAGGATGTC-3′ |
| AXL Reverse Primer | 5′- ACCTACTCTGGCTCCAGGATG-3′ |
| CD147 Forward Primer | 5′- CAGAGTGAAGGCTGTGAAGTCG-3′ |
| CD147 Reverse Primer | 5′- TGCGAGGAACTCACGAAGAAC-3′ |
| TMPRSS2 Forward Primer | 5′-CCTGTGTGCCAAGACGACTG-3 |
| TMPRSS2 Reverse Primer | 5′- TTATAGCCCATGTCCCTGCAG-3′ |
| NRP1 Forward Primer | 5ʹ-CCCAACAGCCTTGAATGCAC-3′ |
| NRP1 Reverse Primer | 5ʹ-ATTTCTAGCCGGTCGTAGCG-3ʹ |
| ACE2 Forward Primer | 5′-GGACCCAGGAAATGTTCAGA-3′ |
| ACE2 Reverse Primer | 5′-GGCTGCAGAAAGTGACATGA-3′ |
| CXCL10, IFNα2, IFNβ1, IL6, IL1β, TNFα. | Taqman based qPCR assays (IDT) |
2.8. Statistical analysis
Each experiment was repeated three times. The expression data are presented as mean ± standard error of the mean (SEM). The comparison of two groups was performed with the Student T-test, and with three or more groups, by one-way analysis of variance (ANOVA). Significance was defined as p < 0.05.
3. Results
3.1. SARS-CoV-2 proteins are detected in the conjunctival tissue of COVID-19 donors
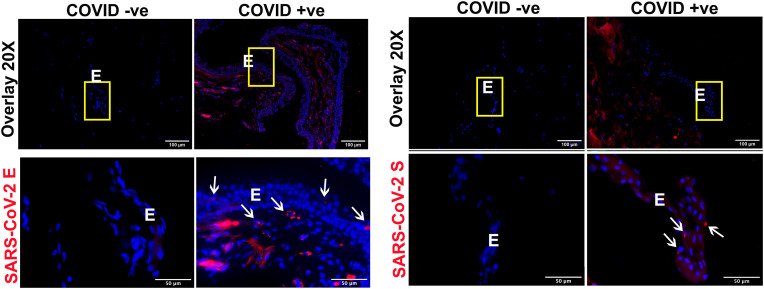
In a recent study, we reported the prevalence of SARS-CoV-2 in postmortem ocular tissues [14]. We demonstrated the presence of SARS-CoV-2 RNA in both anterior and posterior cornea, conjunctiva, and vitreous. Moreover, we detected SARS-CoV-2 spike and envelope proteins in corneal tissue of COVID-19 affected donors. Several other studies also reported SARS-CoV-2 in the conjunctiva using RT-PCR [[41], [42], [43]]. As our laboratory has a biobank of ocular tissues from COVID-19 positive and negative donor eyes, we sought to check SARS-CoV-2 proteins in conjunctival tissue. IHC analysis showed positive staining for both envelope and spike proteins of SARS-CoV-2 in the conjunctiva of COVID-19 positive donors (Fig. 2 ). Increased SARS-CoV-2 envelope protein staining was observed in both epithelial and stromal layers, whereas spike protein was mainly detected in the conjunctival epithelium. These results support the idea that SARS-CoV-2 can infect the human conjunctiva.
Fig. 2.
COVID-19 donor conjunctiva showed the presence of SARS-CoV-2 envelope and spike proteins. Conjunctival tissue from healthy and COVID-19 donors was fixed in formaldehyde and 10 μm thin sections were stained for IHC using the antibody against (A) SARS-CoV-2 envelope (E) and (B) spike (S) protein (red color). DAPI was used for nuclear staining (blue color). The image was captured at different magnifications (20X and 60X) to visualize the cellular location of the viral proteins. The region of interest has been highlighted using a yellow box and white arrows. E, conjunctival epithelium.
3.2. SARS-CoV-2 infects hCECs and increases the expression of viral entry receptor genes
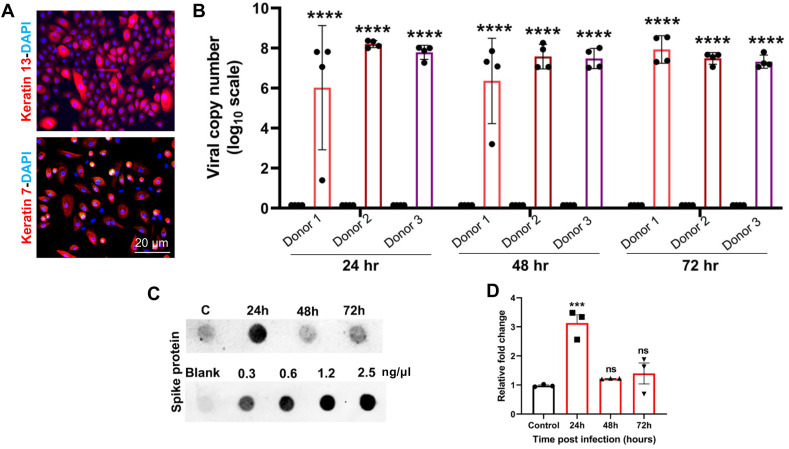
Because conjunctiva has been shown to express ACE2 and TMPRSS2, the key receptors for SARS-CoV-2 entry [32,36,37], we hypothesized that conjunctival epithelial cells are susceptible to SARS-CoV-2 infection. To test this, we isolated primary hCECs from donor eyes. Cells were characterized by the positive expression of goblet and epithelial cell markers MUC5AC (not shown here), keratin 7, and keratin 13 [44,45] (Fig. 3 A) confirming the conjunctival origin. The cells from three individual donors were infected with parental SARS-CoV-2 with MOI of 1, and the intracellular viral burden was determined by assessing the viral genome copy number by qPCR. Our data showed that hCECs were permissive to SARS-CoV-2 infection as evidenced by a significant increase in viral copy number at 24h post-infection (Fig. 3B). However, the viral copy number did not change at 48h or 72h time points, indicating stable virus infection. No significant differences were noted in the susceptibility of hCECs from three different donors. Next, we checked levels of SARS-CoV-2 spike protein in the conditioned culture medium of infected hCECs as an indicator of potential viral replication and progeny virion production. Dot-blot analysis revealed that spike protein levels in the cell culture supernatant increased at 24h post-infection. However, at 48h and 72h, the level decreased and became similar to mock-infected control cells (Fig. 3C and D). These results indicated that SARS-CoV-2 is capable of infecting hCECs, and the peak of virus production and secretion is rapidly reached by 24h post-infection.
Fig. 3.
Primary human conjunctival epithelial cells (hCECs) are permissive to SARS-CoV-2 infection. (A) Primary hCEC were isolated from donor eye globes and were characterized by the presence of conjunctival markers, keratins 7 and 13, by immunofluorescence staining. (B) hCECs from three donors in quadruplicate were infected with SARS-CoV-2 strain parental USA-WA1/2020 (MOI of 1) for indicated time points. qPCR analysis was performed to quantitate the viral RNA copy number wherein uninfected cells served as mock controls. (C) The cell culture supernatant from SARS-CoV-2 infected hCECs and the mock-infected hCECs was used for dot-blot assay to detect the presence of SARS-CoV-2 spike protein along with a gradient of spike protein as standard protein for quantification. The viral RNA copy number was calculated using a standard curve prepared from serial 10-fold diluted SARS-CoV-2 RNA by TaqMan based qPCR. (D) The intensity of dots was quantified using Image J software and relative fold change is plotted on the graph. One-way ANOVA; ns, non-significant; ***p < 0.001; ****p < 0.0001.
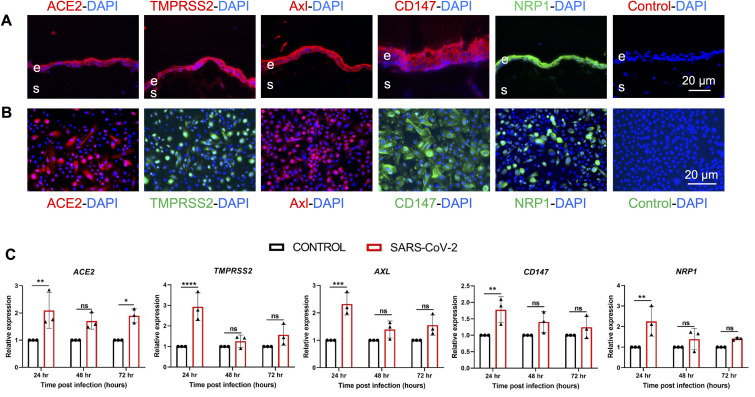
The susceptibility of hCECs to SARS-CoV-2 is likely due to the expression of viral entry receptors. Thus, we evaluated the expression of classical SARS-CoV-2 entry receptors, ACE2 and TMPRSS2, and more recently implicated receptors, Axl, CD147, and NRP1. Our data showed that the conjunctival epithelium ex vivo expressed all viral entry receptors (Fig. 4 A). Similarly, distinct expression of all these receptors was detected in most cultured hCECs (Fig. 4B). Next, we examined the modulation of these receptor genes upon SARS-CoV-2 infection using qPCR. Our data showed that the expression of all receptor genes was increased in SARS-CoV-2 infected hCECs at 24h time point. The expression levels of receptors at 48h and 72h, albeit higher than controls, did not reach a significant level (Fig. 4C).
Fig. 4.
SARS-CoV-2 modulated the expression of viral entry receptor genes. (A) Ex vivo conjunctival tissues were immunostained for SARS-CoV-2 viral entry receptors ACE2, TMPRSS2, Axl, CD147, and NRP1. For negative control, the primary antibody was omitted and DAPI was used to stain nuclei. (B) The primary hCECs were immunostained for indicated viral entry receptors, nuclei were counterstained with DAPI. (C) hCECs isolated from three human donor cadaver eyes were infected with SARS-CoV-2 parental strain USA-WA1/2020 (MOI of 1) for indicated time points. qPCR analysis was performed to check the expression of ACE2, TMPRSS2, CD147, NRP1, and AXL genes. The experiment was performed in biological triplicates and technical duplicates and statistical analysis was performed using one-way ANOVA; ns, non-significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
3.3. SARS-CoV-2 infected hCECs exhibit activation of innate immune responses
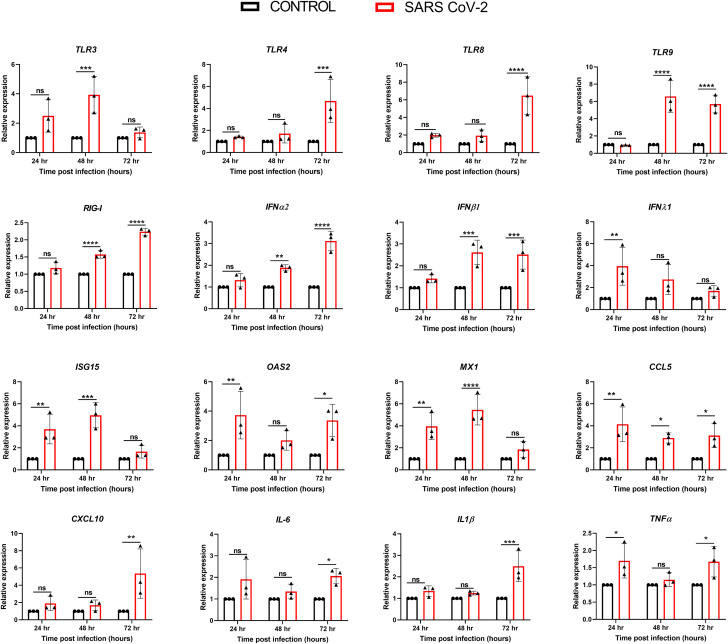
The innate immune response is triggered upon activation of the pathogen recognition receptors (PRRs) such as TLRs, RIG-I, and MDA5 leading to a signaling cascade to induce the expression of interferons (IFNs), interferon-stimulated genes (ISGs), and inflammatory mediators [46,47]. To determine hCECs response to SARS-CoV-2 infection, qPCR analysis was performed. SARS-CoV-2 was found to induce differential expression of genes regulating the host innate immune response (Fig. 5 ). Time course study revealed that the expression of TLRs in SARS-CoV-2 infected hCECs was mainly induced at 48h (TLR3, TLR9) or 72h (TLR4, TLR8, TLR9) time points, along with elevated RIG-I levels. Similarly, the transcript levels of IFNα2, and IFNβ1 increased at 48h and 72h, whereas the expression of IFNλ1 peaked at 24h post-infection followed by a gradual decline. The interferon-stimulated genes ISG15, OAS2, and MX1 increased until 48h post-infection followed by a decrease to baseline at 72h. Among the proinflammatory cytokine genes, CCL5 increased significantly (∼6-fold) at an early point (24h), whereas the others (IL1β, CXCL10, TNFα, and IL6) increased at the later time point i.e. 72h (Fig. 5). Collectively, these results indicate that hCECs possess the ability to recognize and respond to SARS-CoV-2 infection by inducing the expression of innate inflammatory and antiviral response genes.
Fig. 5.
SARS-COV-2 evoked antiviral innate and inflammatory response in hCECs. hCECs isolated from three human donor cadaver eyes were infected with SARS-CoV-2 parental strain USA-WA1/2020 (MOI of 1) for 24, 48, and 72h. qPCR analysis was performed for temporal expression of genes encoding TLRs (TLR 3, 4, 8, and 9), RIGI, IFNs (IFNα2, IFNβ1, and IFNλ1), IFN-induced antiviral response genes (ISG15, OAS2, and MX1), and inflammatory mediators (CCL5, CXCL10,IL6, IL1β, and TNFα). The experiment was performed in biological triplicates and statistical analysis was performed using one-way ANOVA; ns non-significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
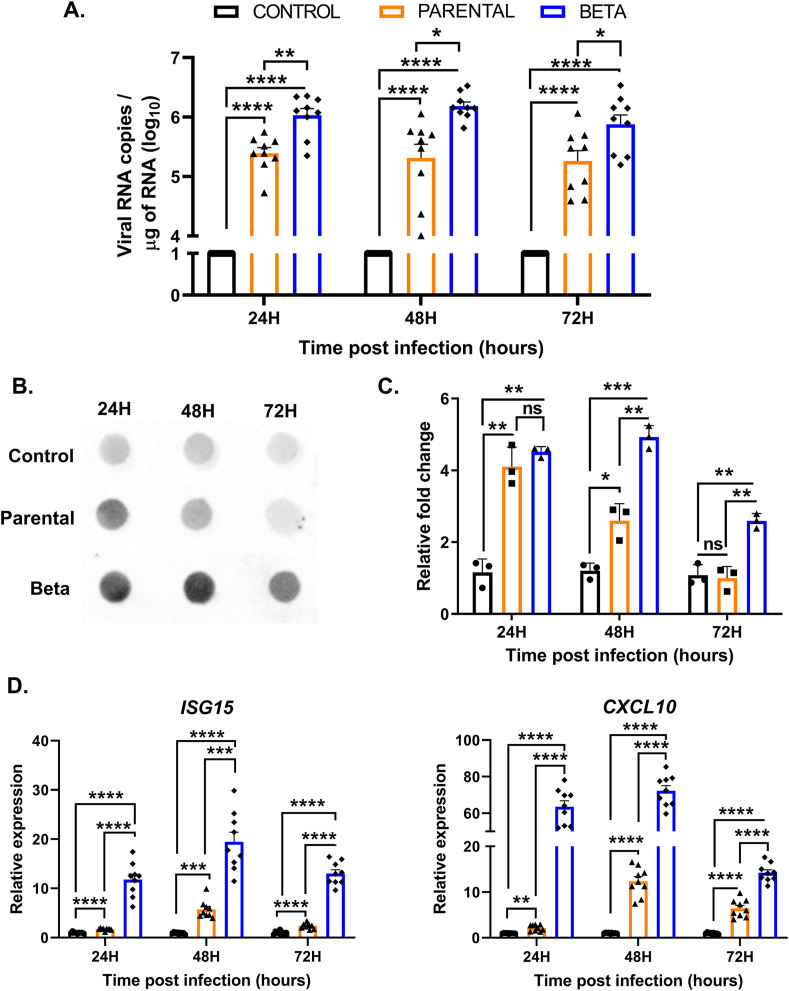
3.4. Beta variant of SARS-CoV-2 is more infectious than parental strain
The recent emergence of multiple variants of SARS-CoV-2 has become a significant concern worldwide [48]. Among the variants of concern, the beta lineage originated in South Africa has been reported to be more infectious than the parental strain. However, its ability to infect ocular cells has not been investigated. Therefore, we performed a direct comparison of parental and beta strains of SARS-CoV-2 using the same MOI i.e., 0.1. Our data showed that hCECs were more permissive to the beta VOC infection as indicated by 0.5 log10 higher viral copy number compared to the parental strain (Fig. 6 A). The difference in the infectivity was similar at all time points. The higher infectivity of hCECs with the beta lineage of SARS-CoV-2 was corroborated by finding increased SARS-CoV-2 spike protein in the conditioned culture medium compared to the parental strain at all time points, evidenced by the dot-blot assay with densitometry quantification (Fig. 6B and C). Furthermore, the beta VOC induced a higher innate antiviral immune response in hCECs with significantly higher expression of IFN-stimulated gene ISG15 along with inflammatory cytokine gene CXCL10 (Fig. 6D). Together, these results show that hCECs are more permissive to the beta variant of SARS-CoV-2 that also induces an increased inflammatory response.
Fig. 6.
Beta variant of SARS-COV-2 exhibited increased infectivity and higher induction of antiviral and inflammatory responses. hCECs from three donors were infected with SARS-CoV-2 parental strain and beta variant at a lower MOI of 0.1 for indicated time points. (A) qPCR analysis was performed to quantitate the viral RNA copy number wherein uninfected cells served as mock controls. (B) Culture media were used for dot blot detection of SARS-CoV-2 spike protein and the densitometry values were plotted with respect to the mock-infected control (C). (D) The expression of select antiviral response genes, ISG15, and inflammatory cytokine, CXCL10, was quantitated by qPCR. The experiment was performed in biological triplicates and statistical analysis was performed using one-way ANOVA; ns non-significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
4. Discussion
Although the SARS-CoV-2 RNA has been detected in the ocular surface tissues, there is an ongoing debate on the direct role of the ocular surface as a route of virus transmission. Therefore, in this study, we examined whether cultured human conjunctival cells are permissive to SARS-CoV-2 viral entry and infection. We also tested whether the SARS-CoV-2 infected hCECs elicit innate antiviral and inflammatory response.
The tropism of a virus to its target host cell is dependent on the expression of its specific viral receptors. The presence of permissive viral entry receptors on the ocular surface cells may not only contribute to the tissue tropism of the virus but also enable the virus to use the eye as a potential site of replication and dissemination to extraocular tissues establishing a systemic infection as seen with other RNA viruses [[40], [49], [50], [51], [52]]. The well-studied receptors for SARS-CoV-2 entry are ACE2 and a co-receptor TMPRSS2. Several studies have shown the expression of these two receptors at the ocular surface, including the conjunctiva [32,53]. Our present study is the first to show that in addition to the classical receptors, hCECs also express CD147, Axl, and neuropilin-1 receptors newly implicated for SARS-CoV-2 entry [25,[27], [28], [29], [30], [31],36]. Moreover, our data showed that SARS-CoV-2 infection increases the expression of these receptor genes at an early time point post-infection (24h) followed by a return to baseline at later time points. The control of receptor expression in hCECs and its reduction presumably by the host signaling needs to be explored further to understand the SARS-CoV-2 infection kinetics in the conjunctiva.
To date, there is only one report indicating the presence of SARS-CoV-2 viral proteins in the conjunctival mucosa and a few clinical reports indicating the low presence of viral RNA in the conjunctival swabs and tears of COVID-19 patients [43]. Our results also support the hypothesis that conjunctival epithelium could be a target of SARS-CoV-2. Our experiments involving the use of live viral infection confirm that hCECs are highly permissive to SARS-CoV-2 infection evidenced by a significant viral RNA copy number at 24h post-infection. Interestingly, the viral copy number did not change at later time points, and this coincided with the decreasing levels of viral spike proteins in the culture supernatant of the infected hCECs. The constant amount of viral RNA in infected hCECs may indicate that the virus has established a low-grade infection at later time points or reached an early plateau following infection at the given MOI of 1. This observation could also be associated with the increased expression of caspases, indicating the possibility of virus infection-induced apoptosis as reported in other SARS-CoV-2 infected epithelial cells [54,55].
Microorganisms possess highly conserved motifs - pathogen-associated molecular patterns (PAMPs) that are recognized by pathogen recognition receptors (PRRs) expressed by host cells as a vital part of their innate immune system. Toll-like receptors (TLRs) are a family of PRRs capable of recognizing various PAMPs, leading to a signaling cascade and secretion of a variety of interferons, cytokines, and chemokines [56]. The human conjunctiva has been shown to express various TLRs to provide ocular surface immunity against invading pathogens [57,58]. In our prior study with Zika virus infection of corneal epithelial cells, we found induction of TLR3 leading to antiviral innate immune response [59]. Here, we observed that SARS-CoV-2 infection of hCECs induces the expression of TLR 3, 4, 8, and 9, corroborating other COVID-19 studies with different cell types [[60], [61], [62]]. The SARS-CoV-2 spike protein has been shown to interact with TLR4 and its activation increases the expression of the primary SARS-COV-2 entry receptor, ACE2 [63]. Similar interaction of Dengue virus NS1 protein has been observed for TLR4 leading to activation of immune cells, platelet aggregation, and endothelial barrier disruption [64]. Our data showing the induction TLRs mostly at later but not at early time points indicate a potential mechanism utilized by SARS-CoV-2 to evade its recognition by TLRs. Indeed, TLRs contribute to the failure of initial viral clearance but subsequent development of severe inflammation and complications in COVID-19 [65]. Therefore, the use of TLR agonists/antagonists could be used as pharmacological interventions in clinical setting against COVID-19.
The attachment and entry of SARS-CoV-2 and the activation of TLRs and antiviral receptor RIG-I triggers innate immune responses, with the expression of type I interferons. SARS-CoV-2 infection has been known to induce a limited and delayed expression of type I and type II interferon response with higher expression of pro-inflammatory cytokines and chemokines [[66], [67], [68], [69]]. Our data show that SARS-CoV-2 infection of hCECs leads to a significant increase in the expression of RIGI, IFNα2, IFNβ1, and IFNλ1. There was a simultaneous increase in the expression of interferon-stimulated genes ISG15, OAS2, and MX1 along with inflammatory response genes CCL5, CXCL10, IL1β, TNFα, and IL6. Our data showed that the expression of these innate antiviral response genes is induced at later time points, except for ISG15, OAS2, and MX1, which were elevated at 24h time point. Overall, these results indicate that hCECs are capable of mounting innate antiviral and inflammatory responses during SARS-CoV-2 infection. Temporal modulation of this response by SARS-CoV-2 and its physiological role in the conjunctiva should be examined in future mechanistic studies.
The current strategy to prevent the COVID-19 pandemic is the continued effort for mass vaccination around the globe. However, the emergence of new variant strains of SARS-COV-2 partially evading the protective effect of COVID-19 vaccines threatens the progress made so far [7]. The CDC and WHO have classified the new variants either as variants of concern (VOCs) or variants of interest (VOIs) [48]. The VOCs include lineages such as B.1.1.7 (20I/501Y.V1 variant), P.1 (20J/501Y.V3 variant), B.1.351 (20H/501Y.V2 variant), and B.1.617.2 [48,70]. These VOCs have been found highly infectious and transmissible [7]. One of the unique aspects of our study is the comparative analysis of infectivity of hCECs challenged with parental and beta (B.1.351) VOC of SARS-CoV-2. The B.1.351 strain was first detected in the Eastern Cape province of South Africa and harbors multiple mutations in the spike, NSP3, NSP5, NSP6, and NSP12 proteins [8]. Our data showed that hCECs are highly permissive to beta strain as compared to the original parental strain. The increased infectivity of beta strain could be due to higher affinity binding of its spike proteins to entry receptors (ACE2, CD147, TMPRSS2, Axl, and NRP1) widely expressed on hCECs. Alternatively, the viral genome of beta strain replicates faster than the parental strain. The underlying mechanisms of differential infectivity of beta strain need further investigation. Moreover, beta strain infected hCECs exhibited marked induction of antiviral and inflammatory response even at a low MOI. These results indicate an increased probability of potential transmission of VOCs via the ocular surface.
In summary, we demonstrate that SARS-CoV-2 can efficiently infect human conjunctival epithelial cells and elicit an innate antiviral response. Moreover, our study is the first to report that the beta variant of SARS-CoV-2 has increased tropism towards hCECs. While our knowledge of SARS-CoV-2 pathobiology continues to evolve, our current understanding of the key molecular and cellular interactions involved in SARS-CoV-2 infection at the ocular surface is limited. Thus, further studies are needed to 1) evaluate the differential susceptibility of ocular surface parts including the cornea and conjunctiva to other more prevalent VOCs such as delta variant, and 2) to determine the mechanisms underlying increased inflammatory response during VOCs infection.
Declaration of competing interest
All authors declare no conflict of interest.
Acknowledgments
This study was supported by NIH grants R01EY026964 and R01EY027381 (Kumar), EY013431, and EY031377 (Ljubimov), and R01EY032149 (Arumugaswami). Our research is also supported in part by an unrestricted grant to the Kresge Eye Institute/Department of Ophthalmology, Visual, and Anatomical Sciences from Research to Prevent Blindness (RPB) Inc. The immunology resource core is supported by an NIH center grant P30EY004068. Ljubimov is the recipient of grants from the Board of Governors Regenerative Medicine Institute, and Arumugaswami received CIRM grant TRAN1COVID19-11975 and UCLA-Keck Foundation Award. The authors would like to thank Onkar Sawant, Ph.D., Director of Research at Eversight, and organ procurement organization (OPO) partners of Eversight for contributing donated ocular tissues.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical Characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahase E. Covid-19: WHO declares pandemic because of "alarming levels" of spread, severity, and inaction. BMJ. 2020;368:m1036. doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- 3.Ramaiah A., Arumugaswami V. Insights into cross-species evolution of novel human coronavirus SARS-CoV-2 and defining immune determinants for vaccine development. BioRxiv. 2021 doi: 10.1101/2020.01.29.925867. [DOI] [Google Scholar]
- 4.Medicine JHUo. CORONAVIRUS RESEARCH CENTER . John Hopkins University of Medicine: John Hopkins University of Medicine; 2021. [Google Scholar]
- 5.Abdelrahman Z., Li M., Wang X. Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza A respiratory viruses. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.552909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doroftei B., Ciobica A., Ilie O.D., Maftei R., Ilea C. Mini-Review discussing the reliability and efficiency of COVID-19 vaccines. Diagnostics. 2021:11. doi: 10.3390/diagnostics11040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C., Ginn H.M., Dejnirattisai W., Supasa P., Wang B., Tuekprakhon A., et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220–4236 e13. doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mwenda M., Saasa N., Sinyange N., Busby G., Chipimo P.J., Hendry J., et al. Detection of B.1.351 SARS-CoV-2 variant strain - Zambia, december 2020. MMWR Morb Mortal Wkly Rep. 2021;70:280–282. doi: 10.15585/mmwr.mm7008e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belser J.A., Rota P.A., Tumpey T.M. Ocular tropism of respiratory viruses. Microbiol Mol Biol Rev. 2013;77:144–156. doi: 10.1128/MMBR.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu C.W., Liu X.F., Jia Z.F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395:e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coroneo M.T. The eye as the discrete but defensible portal of coronavirus infection. Ocul Surf. 2021;19:176–182. doi: 10.1016/j.jtos.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aiello F., Gallo Afflitto G., Mancino R., Li J.O., Cesareo M., Giannini C., et al. Coronavirus disease 2019 (SARS-CoV-2) and colonization of ocular tissues and secretions: a systematic review. Eye. 2020;34:1206–1211. doi: 10.1038/s41433-020-0926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawant O.B., Singh S., Wright R.E., 3rd, Jones K.M., Titus M.S., Dennis E., et al. Prevalence of SARS-CoV-2 in human post-mortem ocular tissues. Ocul Surf. 2021;19:322–329. doi: 10.1016/j.jtos.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksen A.Z., Moller R., Makovoz B., Uhl S.A., tenOever B.R., Blenkinsop T.A. SARS-CoV-2 infects human adult donor eyes and hESC-derived ocular epithelium. Cell Stem Cell. 2021;28 doi: 10.1016/j.stem.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal K., Agarwal A., Jaiswal N., Dahiya N., Ahuja A., Mahajan S., et al. Ocular surface manifestations of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. PloS One. 2020;15 doi: 10.1371/journal.pone.0241661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim L.W., Tan G.S., Yong V., Anderson D.E., Lye D.C., Young B., et al. Acute onset of bilateral follicular conjunctivitis in two patients with confirmed SARS-CoV-2 infections. Ocul Immunol Inflamm. 2020;28:1280–1284. doi: 10.1080/09273948.2020.1821901. [DOI] [PubMed] [Google Scholar]
- 18.Ozturker Z.K. Conjunctivitis as sole symptom of COVID-19: a case report and review of literature. Eur J Ophthalmol. 2021;31:NP161–N166. doi: 10.1177/1120672120946287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X., Chen X., Chen L., Deng C., Zou X., Liu W., et al. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf. 2020;18:360–362. doi: 10.1016/j.jtos.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guemes-Villahoz N., Burgos-Blasco B., Arribi-Vilela A., Arriola-Villalobos P., Rico-Luna C.M., Cuina-Sardina R., et al. Detecting SARS-CoV-2 RNA in conjunctival secretions: is it a valuable diagnostic method of COVID-19? J Med Virol. 2021;93:383–388. doi: 10.1002/jmv.26219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong L., Collin J., Mostafa I., Queen R., Figueiredo F.C., Lako M. In the eye of the storm: SARS-CoV-2 infection and replication at the ocular surface? Stem Cells Transl Med. 2021;10:976–986. doi: 10.1002/sctm.20-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah R., Amador C., Tormanen K., Ghiam S., Saghizadeh M., Arumugaswami V., et al. Systemic diseases and the cornea. Exp Eye Res. 2021;204:108455. doi: 10.1016/j.exer.2021.108455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J., Jiang Q., Xia X., Liu K., Yu Z., Tao W., et al. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell. 2020:19. doi: 10.1111/acel.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulrich H., Pillat M.M. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep. 2020;16:434–440. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maatta M., Tervahartiala T., Kaarniranta K., Tang Y., Yan L., Tuukkanen J., et al. Immunolocalization of EMMPRIN (CD147) in the human eye and detection of soluble form of EMMPRIN in ocular fluids. Curr Eye Res. 2006;31:917–924. doi: 10.1080/02713680600932290. [DOI] [PubMed] [Google Scholar]
- 27.Leonardi A., Rosani U., Brun P. Ocular surface expression of SARS-CoV-2 receptors. Ocul Immunol Inflamm. 2020;28:735–738. doi: 10.1080/09273948.2020.1772314. [DOI] [PubMed] [Google Scholar]
- 28.Daly J.L., Simonetti B., Klein K., Chen K.E., Williamson M.K., Anton-Plagaro C., et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyrou I., Randeva H.S., Spandidos D.A., Karteris E. Not only ACE2-the quest for additional host cell mediators of SARS-CoV-2 infection: neuropilin-1 (NRP1) as a novel SARS-CoV-2 host cell entry mediator implicated in COVID-19. Signal Transduct Target Ther. 2021;6:21. doi: 10.1038/s41392-020-00460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S., Qiu Z., Hou Y., Deng X., Xu W., Zheng T., et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021;31:126–140. doi: 10.1038/s41422-020-00460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou L., Xu Z., Castiglione G.M., Soiberman U.S., Eberhart C.G., Duh E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf. 2020;18:537–544. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willcox M.D., Walsh K., Nichols J.J., Morgan P.B., Jones L.W. The ocular surface, coronaviruses and COVID-19. Clin Exp Optom. 2020;103:418–424. doi: 10.1111/cxo.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnichels S., Rohrbach J.M., Bayyoud T., Thaler S., Ziemssen F., Hurst J. [Can SARS-CoV-2 infect the eye?-An overview of the receptor status in ocular tissue] Ophthalmologe. 2020;117:618–621. doi: 10.1007/s00347-020-01160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makovoz B., Moeller R., Zebitz Eriksen A., tenOever B.R., Blenkinsop T.A. SARS-CoV-2 infection of ocular cells from human adult donor eyes and hESC-derived eye organoids. SSRN. 2020 [Google Scholar]
- 36.Ma D., Chen C.B., Jhanji V., Xu C., Yuan X.L., Liang J.J., et al. Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye. 2020;34:1212–1219. doi: 10.1038/s41433-020-0939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collin J., Queen R., Zerti D., Dorgau B., Georgiou M., Djidrovski I., et al. Co-expression of SARS-CoV-2 entry genes in the superficial adult human conjunctival, limbal and corneal epithelium suggests an additional route of entry via the ocular surface. Ocul Surf. 2021;19:190–200. doi: 10.1016/j.jtos.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shatos M.A., Rios J.D., Horikawa Y., Hodges R.R., Chang E.L., Bernardino C.R., et al. Isolation and characterization of cultured human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44:2477–2486. doi: 10.1167/iovs.02-0550. [DOI] [PubMed] [Google Scholar]
- 39.Kramerov A.A., Saghizadeh M., Ljubimov A.V. Adenoviral gene therapy for diabetic keratopathy: effects on wound healing and stem cell marker expression in human organ-cultured corneas and limbal epithelial cells. JoVE. 2016 doi: 10.3791/54058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh P.K., Guest J.M., Kanwar M., Boss J., Gao N., Juzych M.S., et al. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y., Duan C., Zeng Y., Tong Y., Nie Y., Yang Y., et al. Ocular findings and proportion with conjunctival SARS-COV-2 in COVID-19 patients. Ophthalmology. 2020;127:982–983. doi: 10.1016/j.ophtha.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui K.P.Y., Cheung M.C., Perera R., Ng K.C., Bui C.H.T., Ho J.C.W., et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med. 2020;8:687–695. doi: 10.1016/S2213-2600(20)30193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krenzer K.L., Freddo T.F. Cytokeratin expression in normal human bulbar conjunctiva obtained by impression cytology. Invest Ophthalmol Vis Sci. 1997;38:142–152. [PubMed] [Google Scholar]
- 45.Nomi K., Hayashi R., Ishikawa Y., Kobayashi Y., Katayama T., Quantock A.J., et al. Generation of functional conjunctival epithelium, including goblet cells, from human iPSCs. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108715. [DOI] [PubMed] [Google Scholar]
- 46.Weber F. Antiviral innate immunity: introduction. Encyclopedia of Virology. 2021:577–583. [Google Scholar]
- 47.Pichlmair A., Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Chakraborty C., Sharma A.R., Bhattacharya M., Agoramoorthy G., Lee S.S. Evolution, mode of transmission, and mutational landscape of newly emerging SARS-CoV-2 variants. mBio. 2021;12 doi: 10.1128/mBio.01140-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varkey J.B., Shantha J.G., Crozier I., Kraft C.S., Lyon G.M., Mehta A.K., et al. Persistence of ebola virus in ocular fluid during convalescence. N Engl J Med. 2015;372:2423–2427. doi: 10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith J.R., Todd S., Ashander L.M., Charitou T., Ma Y., Yeh S., et al. Retinal pigment epithelial cells are a potential reservoir for ebola virus in the human eye. Transl Vis Sci Technol. 2017;6:12. doi: 10.1167/tvst.6.4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh S., Kumar A. Ocular manifestations of emerging flaviviruses and the blood-retinal barrier. Viruses. 2018;10 doi: 10.3390/v10100530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh P.K., Kasetti R.B., Zode G.S., Goyal A., Juzych M.S., Kumar A. Zika virus infects trabecular meshwork and causes trabeculitis and glaucomatous pathology in mouse eyes. mSphere. 2019;4 doi: 10.1128/mSphere.00173-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X., Chan J.F., Li K.K., Tso E.Y., Yip C.C., Sridhar S., et al. Detection of SARS-CoV-2 in conjunctival secretions from patients without ocular symptoms. Infection. 2021;49:257–265. doi: 10.1007/s15010-020-01524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., et al. Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–168 e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li S., Zhang Y., Guan Z., Li H., Ye M., Chen X., et al. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct Target Ther. 2020;5:235. doi: 10.1038/s41392-020-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fitzgerald K.A., Kagan J.C. Toll-like receptors and the control of immunity. Cell. 2020;180:1044–1066. doi: 10.1016/j.cell.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Redfern R.L., McDermott A.M. Toll-like receptors in ocular surface disease. Exp Eye Res. 2010;90:679–687. doi: 10.1016/j.exer.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leonardi A., Daull P., Garrigue J.S., Cavarzeran F., Docquier M., Di Stefano A., et al. Conjunctival transcriptome analysis reveals the overexpression of multiple pattern recognition receptors in vernal keratoconjunctivitis. Ocul Surf. 2021;19:241–248. doi: 10.1016/j.jtos.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Singh P.K., Singh S., Farr D., Kumar A. Interferon-stimulated gene 15 (ISG15) restricts Zika virus replication in primary human corneal epithelial cells. Ocul Surf. 2019;17:551–559. doi: 10.1016/j.jtos.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khanmohammadi S., Rezaei N. Role of Toll-like receptors in the pathogenesis of COVID-19. J Med Virol. 2021;93:2735–2739. doi: 10.1002/jmv.26826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poulas K., Farsalinos K., Zanidis C. Activation of TLR7 and innate immunity as an efficient method against COVID-19 pandemic: imiquimod as a potential therapy. Front Immunol. 2020;11:1373. doi: 10.3389/fimmu.2020.01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng M., Karki R., Williams E.P., Yang D., Fitzpatrick E., Vogel P., et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat Immunol. 2021;22:829–838. doi: 10.1038/s41590-021-00937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aboudounya M.M., Heads R.J. COVID-19 and toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Mediat Inflamm. 2021;2021 doi: 10.1155/2021/8874339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Modhiran N., Watterson D., Muller D.A., Panetta A.K., Sester D.P., Liu L., et al. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa3863. 304ra142. [DOI] [PubMed] [Google Scholar]
- 65.Onofrio L., Caraglia M., Facchini G., Margherita V., Placido S., Buonerba C. Toll-like receptors and COVID-19: a two-faced story with an exciting ending. Future Sci OA. 2020;6 doi: 10.2144/fsoa-2020-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–10345 e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Law H.K., Cheung C.Y., Ng H.Y., Sia S.F., Chan Y.O., Luk W., et al. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106:2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Menachery V.D., Eisfeld A.J., Schafer A., Josset L., Sims A.C., Proll S., et al. Pathogenic influenza viruses and coronaviruses utilize similar and contrasting approaches to control interferon-stimulated gene responses. mBio. 2014;5:e01174–14. doi: 10.1128/mBio.01174-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boehm E., Kronig I., Neher R.A., Eckerle I., Vetter P., Kaiser L., et al. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clin Microbiol Infect. 2021;27:1109–1117. doi: 10.1016/j.cmi.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]