Abstract
Background
Acute ischemic stroke (AIS) is rare in children, and diagnosis is often delayed. Neurological involvement may occur in multisystem inflammatory syndrome in children (MIS-C), but very few cases of AIS in patients with MIS-C have been reported.
Patient Descriptions
We two patients with AIS presenting with large vessel occlusive disease in previously healthy adolescents recently exposed to SARS-CoV-2 infection.
Results
Both patients were subsequently diagnosed with and treated for MIS-C. Here, we discuss the course of their treatments and clinical responses.
Conclusion
Early recognition and diagnosis of AIS with large vessel occlusion in children with MIS-C is critical to make available all treatment options to improve clinical outcomes.
Keywords: Stroke, COVID-19, MIS-C, Acute ischemic infarction, Large vessel, SARS-Cov2
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is a global health emergency caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has affected millions of people worldwide. Initially designated as a symptomatic disease in adults, a novel symptomatic syndrome in the pediatric population has emerged.1 Multisystem inflammatory syndrome in children (MIS-C) is a condition that is temporally related to either concurrent or recent SARS-CoV-2 exposure and/or infection and can be associated with high morbidity and mortality.
While MIS-C most commonly presents with gastrointestinal, respiratory, and cardiac involvement, neurological involvement has been reported in 11% to 30% of patients.2 , 3 Because acute ischemic stroke (AIS) is uncommon in pediatric patients, there can be a delay in diagnosis leading to high morbidity and mortality. Given the time-sensitive nature of AIS treatment, it is critical that clinicians are aware of the risk of stroke in MIS-C. Here, we report two patients with MIS-C–associated large vessel occlusive (LVO) AIS in previously healthy adolescents and review the paucity of existing literature reporting similar cases.
Patient 1
This previously healthy right-handed 15-year-old girl awoke with acute aphasia and right hemiparesis. She was last known to be in her normal state of health 10 hours before symptom recognition. She had no history of head trauma. One week prior, she had tested positive for SARS-CoV-2 after several days of fevers, headache, abdominal pain, and generalized weakness.
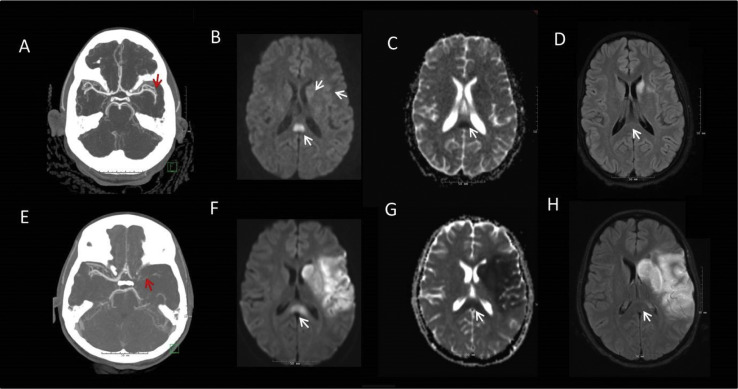
The initial National Institute of Health Stroke Scale score was 15. Computed tomography (CT), CT angiography of the head and neck, and CT perfusion demonstrated a small left insular hypodensity with a distal left middle cerebral artery M2 branch occlusion (Fig A ). She was transferred to our comprehensive stroke center for potential thrombectomy. On arrival, her symptoms had improved with complete resolution of deficits. Repeat arterial imaging demonstrated patent cerebral vasculature. She was admitted to our pediatric intensive care unit for further evaluation and management.
FIGURE.
Patient 1: Axial imaging with CTA maximum intensity projection demonstrating occlusion of the distal M2 branch of the left MCA (A), MRI diffusion weighted imaging (DWI) showing restricted diffusion in the insular ribbon, caudate head and splenium of the corpus callosum (B) with corresponding ADC changes (C) and resolution of the splenial lesion on T2 FLAIR on repeat imaging (D). Patient 2: Axial imaging with CTA maximum intensity projection demonstrating occlusion of the left ICA terminus (E), MRI DWI shows restricted diffusion in the left MCA territory and splenium of the corpus callosum (F) with corresponding ADC changes (G), and resolution of the splenial lesion on T2 FLAIR on repeat imaging (H). CTA, computerized axial tomography; DWI, diffusion weighted imaging; MCA, middle cerebral artery; ADC, apparent diffusion coefficient; FLAIR, fluid attenuation inversion recovery; ICA, internal carotid artery. The color version of this figure is available in the online edition.
Initial laboratories demonstrated leukocytosis, renal and cardiac dysfunction, and markedly elevated inflammatory markers. Transthoracic echocardiogram (TTE) with bubble study demonstrated a left ventricle ejection fraction (LVEF) of 45% with global strain and mild mitral valve regurgitation. Cardiac magnetic resonance imaging (MRI) showed small pericardial effusion. MRI brain with diffusion weighted imaging (MRI-DWI) showed scattered infarcts in the left caudate, insula, and frontal operculum and a restricted diffusion in the splenium of the corpus callosum; the latter lesion resolved on follow-up studies (Fig B-D). Lower extremity Doppler studies were negative for deep venous thrombosis (DVT).
The patient met CDC criteria for MIS-C based on her known recent SARS-Cov2 infection, multisystem involvement (renal, cardiac, neurologic), and elevated inflammatory markers.4 She was treated with intravenous immunoglobulin (IVIG) 2 g/kg and IV methylprednisolone 2 mg/kg/day for five days, followed by a three-day oral prednisone taper and therapeutic enoxaparin 1 mg/kg twice daily for three months. A repeat TTE on day six showed improved LVEF. Upon discharge, she had subtle right hemiparesis with a Pediatric Stroke Outcome Measure (PSOM) of 1.5, which had completely resolved (PSOM 0) at 30-day follow-up.
Patient 2
This previously healthy right-handed 16-year-old girl without any history of head trauma was found obtunded and nonverbal after three days of fevers, nausea, vomiting, and diarrhea. Her family had been ill with SARS-CoV-2 about a month prior.
Upon arrival, her blood pressure was 70/40 mmHg, requiring vasopressor support. CT head showed a subtle left insular hypodensity. An initial suspicion of bacterial meningoencephalitis warranted lumbar puncture and initiation of antibiotic treatment. Five hours after initial presentation, she developed right-sided hemiparesis (National Institute of Health Stroke Scale score: 16). An urgent CT angiography head and neck showed left internal carotid artery terminus occlusion (Fig E), with unfavorable CT perfusion study. She was transferred to our institution's pediatric intensive care unit for escalation of care, where she was intubated for airway protection.
Initial laboratories showed leukocytosis, renal and cardiac dysfunction, elevated inflammatory markers, and positive SARS-CoV-2 IgG serology. TTE demonstrated an LVEF of 35% which improved after 12 hours. An apical thrombus was confirmed on cardiac MRI, and she was started on anticoagulation with heparin drip. Several subacute infarcts were noted on MRI-DWI brain in the left insula, caudate, and frontal and temporal lobes (Fig F and G), in addition to diffusion restriction with corresponding ADC changes involving the splenium of the corpus (Fig F and G); the latter had resolved on follow-up studies five days later (Fig H).
She was diagnosed with MIS-C based on the CDC criteria4 and was treated with a single dose of IVIG 2 g/kg and IV methylprednisolone 1 mg/kg/day for five days followed by a three-day oral prednisone taper. Her hospitalization was complicated by a catheter-associated right lower extremity DVT. Heparin drip was transitioned to enoxaparin 1 mg/kg twice daily and aspirin 81 mg daily. Throughout her hospital stay, she made gradual improvement with speech and mobility. She was ultimately discharged to an acute rehabilitation facility with PSOM of 3. On day 41 outpatient follow-up, she was independent in all activities of daily living with mild expressive aphasia and trace right hemiparesis with PSOM of 2.
Discussion
The COVID-19 pandemic has impacted millions of people globally. More than 4 million children have tested positive for SARS-CoV-2 thus far in the United States.5 COVID-19 in children is associated with MIS-C, a debilitating multisystem syndrome that can present in children weeks after exposure to SARS-CoV-2. We report two cases of AIS due to LVO in previously healthy adolescents with MIS-C. These two patients illustrate the diverse neurological presentations of COVID-19 in children, specifically coagulopathy associated with this infection.
The pathophysiology linking MIS-C to AIS is not fully understood, although emerging data from adult COVID-19–associated AIS suggest a combination of etiologies. These include venous stasis, hyperviscosity in critically ill patients, viral endothelial injury leading to hypercoagulability, and myocardial involvement causing reduced LVEF and subsequent cardioembolism. In adults, the connection between SARS-CoV-2 and inflammation has been proposed to involve compromise of vascular integrity activating the clotting cascade, triggering platelet reactivity, cytokine storm, and immune complexes that contribute to the formation of thromboses.1 , 6 In children, especially those with delayed MIS-C relative to initial infection, a delayed immune-mediated inflammatory response stimulated by macrophages, neutrophils, and monocytes followed by the production of antibodies from plasma and B-cells appears to be a more likely pathogenesis than direct viral invasion of tissues.
In a comprehensive study of MIS-C spanning 53 pediatric health centers across the US, Feldstein et al. reported that among patients aged 13 to 20 years, the gastrointestinal, respiratory, and cardiac systems were the most commonly affected at 90%, 87%, and 80%, respectively.2 Neurological involvement has been reported in 11% to 30% of children with MIS-C.2 , 3 Commonly reported neurological symptoms include fatigue, generalized weakness, confusion, headache, and loss of sense of taste or smell.7 More severe neurologic manifestations such as seizures, encephalopathy, and cerebral edema were uncommon.7
AIS due to LVO in children is uncommon. Even in the setting of COVID-19 and MIS-C, very few cases of AIS have been reported. Beslow et al. surveyed 42 centers and found eight AIS in 971 (0.82%) pediatric patients with SARS-CoV-2, four of which were associated with LVO.8 Transient involvement of the splenium of the corpus callosum was seen in both our patients, which has been reported to be associated with various central nervous system infections including SARS-CoV-2.9 The current literature includes nine other published cases of pediatric LVO AIS in the setting of COVID-19 (Table )8 , 10, 11, 12, 13 including our two cases, the ages range from eight to 16 years, some of which were initially asymptomatic with positive polymerase chain reaction or IgG serology. Interestingly, although all patients had recent or proven concurrent SARS-CoV-2 infection, only three other reported patients met full diagnostic criteria for MIS-C.8 , 10 Both of our patients and the patient reported by Tiwari with LVO AIS and MIS-C were successfully treated with IVIG, steroids, and anticoagulation; the treatment for the two MIS-C cases reported by Beslow was not specified.8 , 10
TABLE.
COVID-19–Associated Acute Large Vessel Occlusive Stroke in Children and Adolescents
| Case | Age, Sex | Vessel Distribution | COVID | MIS-C Criteria met? | Organ Involvement | Treatment |
|---|---|---|---|---|---|---|
| CASE 1 | 15, F | L. MCA | + PCR 1 wk prior + serology |
Yes | Cardiac, renal | IVIG, methylprednisolone, prednisone, enoxaparin |
| CASE 2 | 16, F | L. ICA terminus | + serology only | Yes | Cardiac, renal, GI | IVIG, methylprednisolone, prednisone, enoxaparin, aspirin |
| Tiwari10 | 9, F | R. MCA | + PCR At presentation |
Yes | Cardiac | IVIG, methylprednisolone, dexamethasone, remdesivir, LMWH |
| Beslow (1)8 | 10, M | R. MCA + cerebellar infarcts | + PCR 1 wk prior + serology |
Yes | Not specified | Not specified |
| Beslow (2)8 | 14, M | R. MCA + R. ACA |
+ PCR 1 wk post stroke |
Yes | Respiratory, GI | Not specified |
| Beslow (3)8 | 10, M | B. PCA | + PCR 1 wk post stroke |
No | Respiratory | Not specified |
| Beslow (4)8 | 16, M | L. MCA + B. ACA |
+ PCR 1 wk prior |
No | Not specified | Not specified |
| Appavu (1)11 | 8, F | B. MCA | + PCR 3 weeks prior + serology |
No | None | Thrombectomy |
| Appavu (2)11 | 16, M | L. MCA | + PCR 4 weeks prior |
No | Cardiac, renal | Enoxaparin only |
| Gulko12 | 13, F | L. MCA | + PCR At presentation |
No | None | Not specified |
| Mirzaee13 | 12, M | L. MCA | + PCR At presentation |
No | None | “conservative” |
Abbreviations:
ACA = anterior cerebral artery
B = bilateral
ICA = internal carotid artery
IVIG = intravenous immunoglobulin
L = Left
LMWH = low molecular weight heparin
MCA = middle cerebral artery
PCA = posterior cerebral artery
PCR = polymerase chain reaction
R = right
Medical management of MIS-C is largely based on established treatments for Kawasaki disease including IVIG, aspirin, and corticosteroids.1 Although emerging data suggest improved cardiovascular function in patients with MIS-C initially treated with IVIG plus glucocorticoids compared with IVIG alone,14 other studies show no difference in recovery from MIS-C after IVIG alone, IVIG plus glucocorticoids, and glucocorticoids alone.15 Nevertheless, IVIG remains a mainstay for MIS-C treatment. Historically, IVIG has been associated with a potential increased risk of thromboembolic events including stroke, raising concerns for IVIG exacerbating a prothrombotic state owing to MIS-C, especially in patients with AIS.16 In Patient 1, IVIG was administered according to published guidelines1; Patient 2 was hematologically complex as she exhibited a larger AIS, and additional signs of hypercoagulability, including lower extremity DVT and left ventricular thrombus, thus the treating team favored discontinuing IVIG and treatment with low-molecular-weight heparin.
Conclusion
Pediatric AIS remains an infrequent complication of SARS-CoV-2. These two patients demonstrate an association between MIS-C and AIS due to LVO, thus expanding the literature on this rare condition. These cases illustrate the importance of early symptom recognition, timely diagnosis, and rapid intervention to improve clinical outcomes.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest: None.
Author contributions: Jaimie Chang and Zachary Bulwa provided substantial contributions to the conception and design of the work, the acquisition, analysis, and interpretation of the data. Jaimie Chang, Zachary Bulwa, and Rima Dafer were responsible for the drafting of the manuscript. All authors were responsible for revising the manuscript. All authors were responsible for patient care. All authors give final approval of the version to be published and are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of the work is appropriate.
Data sharing: Deidentified data are available upon request.
References
- 1.Nakra N.A., Blumberg D.A., Herrera-Guerra A., Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel) 2020;7:69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mavrogeni S.I., Kolovou G., Tsirimpis V., Kafetzis D., Tsolas G., Fotis L. The importance of heart and brain imaging in children and adolescents with multisystem inflammatory syndrome in children (MIS-C) Rheumatol Int. 2021;41:1037–1044. doi: 10.1007/s00296-021-04845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Health Alert Network Multisystem inflammatory syndreom in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) 2020. https://emergency.cdc.gov/han/2020/han00432.asp Available at: Accessed September 13, 2021.
- 5.American Academy of Pediatrics and the Children's Hospital Association Children and COVID-19 state data report version 6/24/21. https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/ Accessed September 13, 2021.
- 6.Spence J.D., de Freitas G.R., Pettigrew L.C., et al. Mechanisms of stroke in COVID-19. Cerebrovasc Dis. 2020;49:451–458. doi: 10.1159/000509581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaRovere K.L., Riggs B.J., Poussaint T.Y., et al. Overcoming COVID-19 Investigators Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. doi: 10.1001/jamaneurol.2021.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beslow L.A., Linds A.B., Fox C.K., et al. International Pediatric Stroke Study Group Pediatric ischemic stroke: an infrequent complication of SARS-CoV-2. Ann Neurol. 2021;89:657–665. doi: 10.1002/ana.25991. [DOI] [PubMed] [Google Scholar]
- 9.Gaur P., Dixon L., Jones B., Lyall H., Jan W. COVID-19-associated cytotoxic lesions of the corpus callosum. AJNR Am J Neuroradiol. 2020;41:1905–1907. doi: 10.3174/ajnr.A6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiwari L., Shekhar S., Bansal A., Kumar S. COVID-19 associated arterial ischaemic stroke and multisystem inflammatory syndrome in children: a case report. Lancet Child Adolesc Health. 2021;5:88–90. doi: 10.1016/S2352-4642(20)30314-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appavu B., Deng D., Dowling M.M., et al. Arteritis and large vessel occlusive strokes in children after COVID-19 infection. Pediatrics. 2021;147 doi: 10.1542/peds.2020-023440. e2020023440. [DOI] [PubMed] [Google Scholar]
- 12.Gulko E., Overby P., Ali S., Mehta H., Al-Mufti F., Gomes W. Vessel wall enhancement and focal cerebral arteriopathy in a pediatric patient with acute infarct and COVID-19 infection. AJNR Am J Neuroradiol. 2020;41:2348–2350. doi: 10.3174/ajnr.A6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirzaee S.M.M., Gonçalves F.G., Mohammadifard M., Tavakoli S.M., Vossough A. Focal cerebral arteriopathy in a pediatric patient with COVID-19. Radiology. 2020;297:E274–E275. doi: 10.1148/radiol.2020202197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Son M.B.F., Murray N., Friedman K., et al. Overcoming COVID-19 Investigators Multisystem inflammatory syndrome in children - initial therapy and outcomes. N Engl J Med. 2021;385:23–34. doi: 10.1056/NEJMoa2102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McArdle A.J., Vito O., Patel H., et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med. 2021;385:11–22. doi: 10.1056/NEJMoa2102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caress J.B., Cartwright M.S., Donofrio P.D., Peacock J.E., Jr. The clinical features of 16 cases of stroke associated with administration of IVIg. Neurology. 2003;60:1822–1824. doi: 10.1212/01.wnl.0000068335.01620.9d. [DOI] [PubMed] [Google Scholar]