OBJECTIVE: Hypertensive disorders of pregnancy (HDP) affect up to 5% to 10% of pregnancies and represent a leading cause of maternal and neonatal morbidity and mortality worldwide.1 The primary theory in the pathogenesis of these disorders involved both placental and systemic inflammation. It has been hypothesized that placental hypoxia because of abnormal early placentation results in oxidative stress and the increased release of placental trophoblastic debris into the maternal circulation, stimulating the systemic inflammatory response and resulting in widespread endothelial dysfunction.2 , 3
Similarly, the infection caused by SARS-CoV-2 is characterized by a substantial inflammatory response.4 Given the commonalities in the pathogenesis of both SARS-CoV-2 infection and HDP, we sought to evaluate this relationship further. Our primary objectives were to evaluate differences in the rates of HDP and hypertensive disease severity in patients who tested negative for SARS-CoV-2 vs patients who tested positive for SARS-CoV-2.
STUDY DESIGN: We conducted a retrospective medical record review of all patients who had preadmission SARS-CoV-2 nasal polymerase chain reaction testing at Columbia University Irving Medical Center–affiliated labor and delivery (L&D) units between March 2020 and June 2020. Patients with a history of chronic hypertension and multiple gestations were excluded.
The primary exposure was SARS-CoV-2 infection status on routine admission testing to L&D. The primary outcome was overall rates of HDP, including gestational hypertension (gHTN), preeclampsia (PEC) without severe features, and PEC with severe features. A primary subanalysis evaluated the group of patients with an HDP to determine whether hypertensive disease severity differed by SARS-CoV-2 testing status. A secondary subanalysis evaluated the group of patients who tested positive for SARS-CoV-2 to determine whether symptom status differed in those with an HDP compared with those without an HPD.
Univariable analyses for continuous variables were conducted using parametric or nonparametric tests as appropriate. Categorical or binary variables were analyzed using chi-square test or the Fisher exact test as appropriate. Multivariable analyses were conducted using logistic regression models to adjust for statistically significant potential confounders.
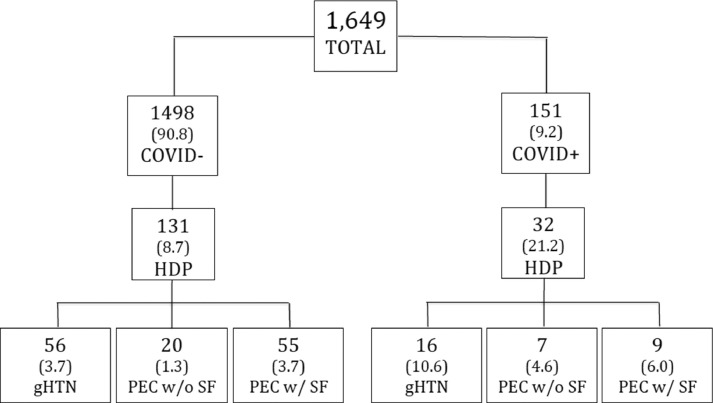
RESULTS: Our primary analysis included 1649 patients, where 1498 (90.8%) tested negative for SARS-CoV-2 and 151 (9.2%) tested positive for SARS-CoV-2 (Figure ). Patients who tested positive for SARS-CoV-2 were younger (28±6 vs 31±6 years; P<.01), less likely to be of advanced maternal age (17.2% vs 29.8%; P<.01), more likely to be Hispanic (72.8% vs 52.1%; P<.01), and more likely to have public insurance (75.7% vs 45.9; P<.01). The overall rates of HDP were higher in patients who tested positive for SARS-CoV-2 (21.2% vs 8.7%; P<.01) (Table ). Specifically, patients who tested positive for SARS-CoV-2 had higher rates of gHTN (10.6% vs 3.7%; P<.01) and PEC without severe features (4.6% vs 1.3%; P<.01). These differences remained significant on multivariable regression analysis. The rates of PEC with severe features did not differ significantly between the 2 groups (6.0% vs 3.7%; P=.17) (Table). The first subanalysis included 163 women with a diagnosis of HDP, where 32 (19.6%) tested positive for SARS-CoV-2 and 131 (80.4%) tested negative for SARS-CoV-2. Patients who tested positive for SARS-CoV-2 and patients who tested negative for SARS-CoV-2 did not show significantly different variables assessing hypertensive disease severity (Table). The final analysis included 151 patients who tested positive for SARS-CoV-2, where 32 (21.2%) were diagnosed with an HDP and 119 (78.8%) did not have an HDP. Patients who had an HDP were less likely to be asymptomatic (31.2% vs 71.4%; P<.01) and more likely to have experienced SARS-CoV-2 symptoms before presentation (37.5% vs 13.4%; P<.01).
Figure 1.
Population tree by SARS-CoV-2 infection status and rates of HDP
Table.
Hypertensive disorder rates
| Hypertensive disorder | Negative SARS-CoV-2 (n=1498) | Positive SARS-CoV-2 (n=151) | P value (univariable) | P value (multivariable)a | Odds ratio (95% CI)a | ||
|---|---|---|---|---|---|---|---|
| Overall rate of HDP | 131 (8.7) | 32 (21.2) | <.01 | <.01 | 1.98 (1.26–3.10) | ||
| Gestational hypertension | 56 (3.7) | 16 (10.6) | <.01 | .02 | 2.13 (1.15–3.92) | ||
| Preeclampsia without severe features | 20 (1.3) | 7 (4.6) | <.01 | .02 | 3.02 (1.18–7.71) | ||
| Preeclampsia with severe features | 55 (3.7) | 9 (6.0) | .17 | .65 | 1.19 (0.56–2.51) | ||
| Hypertensive disorder severity | |||||||
|---|---|---|---|---|---|---|---|
| Outcome | Negative SARS-CoV-2 (n=131) | Positive SARS-CoV-2 (n=32) | P value | ||||
| Requirement for rapid acting antihypertensive medications | 33 (25.2) | 4 (12.5) | .12 | ||||
| Preeclamptic laboratory abnormalities | 14 (10.7) | 5 (15.6) | .54 | ||||
| Platelets<100 (× 103/µL) | 3 (2.3) | 1 (3.1) | >.99 | ||||
| AST or ALT>2 times normal (U/L) | 9 (6.9) | 5 (15.6) | .15 | ||||
| Creatinine>1.1 (mg/dL) | 4 (3.0) | 0 (0) | >.99 | ||||
| Preeclamptic symptoms | .27 | ||||||
| Headache | 9 (6.9) | 0 (0) | |||||
| Vision changes | 0 (0) | 1 (3.1) | |||||
| Chest pain | 1 (0.8) | 0 (0) | |||||
| RUQ pain | 1 (0.8) | 0 (0) | |||||
| Pulmonary edema | 1 (0.8) | 0 (0) | |||||
| Magnesium intrapartum | 34 (26.0) | 6 (18.8) | .40 | ||||
| Magnesium postpartum | 55 (42.0) | 9 (28.1) | .15 | ||||
| Requirement for antihypertensive medications after birth | 48 (36.6) | 6 (18.8) | .05 | ||||
| Number of antihypertensive medications after birth | |||||||
| 1 antihypertensive | 42 (32.1) | 6 (18.8) | .14 | ||||
| >1 antihypertensive | 6 (4.6) | 0 (0) | .60 | ||||
| Readmission for preeclampsia | 9 (6.9) | 1 (3.1) | .69 | ||||
Data are presented as number (percentage), unless otherwise indicated.
ALT, alanine transaminase; AST, aspartate transaminase; HDP, hypertensive disorders of pregnancy; RUQ, right upper quadrant.
Controlled for maternal age, advance maternal age status, race, and insurance type; observations with unknown insurance status were excluded in the multivariable analysis (n=1329).
Madden. SARS-CoV-2 and hypertensive disease in pregnancy. Am J Obstet Gynecol MFM 2021.
CONCLUSION: There were several notable commonalities in the pathophysiologies of HDP and SARS-CoV-2 infection with the activation of the systemic inflammatory response and endothelial dysfunction playing prominent roles in both.2, 3, 4 Our results showed that patients who tested positive for SARS-CoV-2 had significantly higher rates of HDP, including gHTN and PEC without severe features, than patients who tested negative for SARS-CoV-2. Rates of PEC with severe features and severity of HDP did not differ in our cohort of patients with asymptomatic or mild SARS-CoV-2 infection. This was in contrast to the results from a recent review of literature that included 790,954 pregnant women, showing that the risk of PEC was significantly higher among 15,524 pregnant women with SARS-CoV-2 infection than those without SARS-CoV-2 infection (7.0% vs 4.8%; odds ratio [OR], 1.62; 95% CI, 1.45–1.82; 26 studies), as was the risk of PEC with severe features (6.9% vs 4.9%; OR, 1.76; 95% CI, 1.18–2.63; 7 studies, 11,019 women).5 Furthermore, our study showed that patients with an HDP were more likely to experience symptoms of SARS-CoV-2. In the context of significant similarities in disease mechanism, the results suggested that acute inflammation because of SARS-CoV-2 infection may mimic a hypertensive disease in pregnancy and contribute to increased rates, indicating a need for increased monitoring for hypertensive disease in patients who test positive for SARS-CoV-2.
Further research is needed to better understand how to differentiate between a true hypertensive disease of pregnancy and the effects of the acute inflammatory state of SARS-CoV-2 infection. Furthermore, the implications of this possible overlap concerning antenatal testing and surveillance, optimal timing of delivery, prevention of unindicated iatrogenic preterm delivery, and management of subsequent pregnancies need to be elucidated.
Footnotes
The authors report no conflict of interest.
C.G.B. has funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung and Blood Institute and was compensated for a talk for Medela. S.B. is partially supported by the National Institutes of Health (grant numbers RF1AG056111 and R01DK116603).
Findings were previously presented as an oral presentation at the 41st annual meeting of the Society for Maternal-Fetal Medicine, held virtually, January 25–30, 2021.
References
- 1.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 3.von Versen-Hoeynck FM, Hubel CA, Gallaher MJ, Gammill HS, Powers RW. Plasma levels of inflammatory markers neopterin, sialic acid, and C-reactive protein in pregnancy and preeclampsia. Am J Hypertens. 2009;22:687–692. doi: 10.1038/ajh.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conde-Agudelo A, Romero R. SARS-COV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.07.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]