Abstract
Acute lung injury (ALI), or its more severe form, acute respiratory distress syndrome (ARDS), is a disease with high mortality and is a serious challenge facing the World Health Organization because there is no specific treatment. The excessive and prolonged immune response is the hallmark of this disorder, so modulating and regulating inflammation plays an important role in its prevention and treatment. Resolvin D1 (RvD1) as a specialized pro-resolving mediator has the potential to suppress the expression of inflammatory cytokines and to facilitate the production of antioxidant proteins by stimulating lipoxin A4 receptor/formyl peptide receptor 2 (ALX/FPR2). These changes limit the invasion of immune cells into the lung tissue, inhibit coagulation, and enhance cell protection against oxidative stress (OS). In particular, this biomolecule reduces the generation of reactive oxygen species (ROS) by blocking the activation of inflammatory transcription factors, especially nuclear factor-κB (NF-κB), and accelerating the synthesis of antioxidant compounds such as heme oxygenase 1 (HO-1) and superoxide dismutase (SOD). Therefore, the destruction and dysfunction of important cell components such as cytoplasmic membrane, mitochondria, Na+/k + adenosine triphosphatase (ATPase) and proteins involved in the phagocytic activity of scavenger macrophages are attenuated. Numerous studies on the effect of RvD1 over inflammation using animal models revealed that Rvs have both anti-inflammatory and pro-resolving capabilities and therefore, might have potential therapeutic value in treating ALI. Here, we review the current knowledge on the classification, biosynthesis, receptors, mechanisms of action, and role of Rvs in ALI/ARDS.
Keywords: Acute lung injury, Anti-inflammatory, SPM, ALX/FPR2, Reactive oxygen species
Abbreviations
- activator protein 1
(AP-1)
- activin receptor-like kinase 5
(ALK-5)
- acute lung injury
(ALI)
- acute respiratory distress syndrome
(ARDS)
- adenosine triphosphate
(ATP)
- allyl-isothiocyanate
(AITC)
- aspirin-triggered resolvin D1
(AT-RvD1)
- bactericidal/permeability-increasing protein
(BPI)
- B-cell lymphoma 2
(Bcl-2)
- B-cell lymphoma 2-antagonist-killer 1
(BAK)
- B-cell lymphoma 2-associated X
(BAX)
- B-cell lymphoma 2 homology 3
(BH3)
- bleomycin
(BLM)
- bone morphogenetic protein
(BMP)
- bovine serum albumin
(BSA)
- bronchoalveolar lavage fluids
(BALF)
- cAMP response elements-binding protein
(CREB)
- capsaicin
(CAPS)
- CCAAT/enhancer binding protein
(C/EBP)
- cecal ligation and puncture
(CLP)
- chemokine (C-X-C motif) ligand
(CXCL)
- c-Jun N-terminal kinase
(JNK)
- cluster of differentiation
(CD)
- collagen type I alpha 1 chain
(COL1A1)
- complement receptor
(CR)
- connective tissue growth factor
(CTGF)
- cyclooxygenase
(COX)
- cytokine-induced neutrophil chemoattractant
(CINC)
- docosahexaenoic acid
(DHA)
- docosapentaenoic acid
(DPA)
- eicosapentaenoic acid
(EPA)
- endothelial-leukocyte adhesion molecule 1
(ELAM-1)
- epithelial sodium channel
(ENaC)
- epithelial-mesenchymal transition
(EMT)
- G protein-coupled receptor 32
(GPR32)
- glutathione
(GSH)
- glycogen synthase kinase 3 beta
(GSK3β)
- haemotoxylin and eosin
(H&E)
- heme oxygenase 1
(HO-1)
- high mobility group box protein 1
(HMGB1)
- human cationic antibacterial protein of 18 kDa
(LL-37)
- human lung fibroblasts
(HLF)
- idiopathic pulmonary fibrosis
(IPF)
- immunoglobulin
(Ig)
- inducible nitric oxide synthase
(iNOS)
- inhibitor of nuclear factor kappa B kinases
(IKKs)
- intercellular adhesion molecule
(ICAM)
- interferon gamma
(IFNγ)
- interleukin
(IL)
- interleukin-1 receptor associated kinase
(IRAK)
- ischemia/reperfusion injury
(IRI)
- keratinocytes-derived chemokine
(KC)
- leukotriene
(LT)
- lipopolysaccharides
(LPS)
- lipoxin A4 receptor/formyl peptide receptor 2
(ALX/FPR2)
- lipoxin
(LX)
- lipoxygenase
(LOX)
- macrophage inflammatory protein
(MIP)
- mothers against decapentaplegic homolog
(Smad)
- malondialdehyde
(MDA)
- metallopeptidase inhibitor 1
(TIMP1)
- mitogen-activated protein kinase
(MAPK)
- monocyte chemoattractant protein 1
(MCP-1)
- myeloid differentiation factor 2
(MD-2)
- myeloid differentiation factor 88
(MyD88)
- myeloid-epithelial-reproductive tyrosine kinase
(MerTK)
- myeloperoxidase
(MPO)
- NAD(P)H: quinone oxidoreductase
(NQO-1)
- nitric oxide
(NO)
- nuclear factor erythroid 2-related factor 2
(Nrf2)
- nuclear factor-κB
(NF-κB)
- oxidative stress (OS); paraquat
(PQ)
- peroxisome proliferator-activated receptor gamma
(PPARγ or PPARG)
- phosphatidylinositol 3-kinase (PI3K)/protein kinase B
(AKT)
- polycystic ovary syndrom
(PCOS)
- polymorphonuclear leukocytes
(PMN)
- prostaglandin (PG); reactive oxygen species
(ROS)
- receptor for advanced glycation end-products
(RAGE)
- resolvin
(Rv)
- serine/threonine-protein kinase or serum and glucocorticoid-regulated kinase 1
(SGK1)
- signal transducer and activator of transcription 3
(STAT3)
- signal-regulated kinase 1
(ERK1)
- single Ig IL-1-related receptor
(SIGIRR)
- sirtuin 1
(SIRT1)
- specialized pro-resolving mediator
(SPM)
- superoxide dismutase
(SOD)
- systemic inflammatory response syndrome
(SIRS)
- thromboxane
(TX)
- toll-like receptor
(TLR)
- transforming growth factor-beta 2
(TGF-β2)
- transient receptor potential vanilloid 1 and ankyrin 1
(TRPV1, TRPA1)
- tumor necrosis factor-alpha
(TNF-α)
- tumor necrosis factor-alpha-induced protein 3
(A20)
- tumor necrosis factor receptor associated factor 6
(TRAF6)
- vascular cell adhesion molecule 1
(VCAM-1)
- vascular endothelial growth factor
(VEGF)
- ventilator-induced lung injury
(VILI)
- wet-to-dry weight ratio
(W/D ratio)
- white blood cells
(WBCs)
- zonula occludens 1 or tight junction protein 1
(ZO-1)
- α-smooth muscle actin
(α-SMA)
- ω3 polyunsaturated fatty acids
(ω3-PUFAs)
1. Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are syndromes that can lead to progressive respiratory failure through damage to the alveolar and vascular walls, extravasation of serum proteins, and eventually pulmonary edema. ALI is a disorder of acute inflammation that causes disruption of the lung endothelial and epithelial barriers and is practically defined as PaO2/FIO2 ≤ 300 mmHg with diffuse bilateral pulmonary infiltration on chest radiograph in the absence of clinical evidence of left atrial hypertension (Abedi et al., 2020a; Bernard et al., 1994; Matthay et al., 2019). The most common causes are sepsis and aspiration of gastric contents, followed by pneumonia, smoke inhalation, drowning, trauma, major surgery, burns, blood transfusions, and rarely uremia (Bersten et al., 2002).
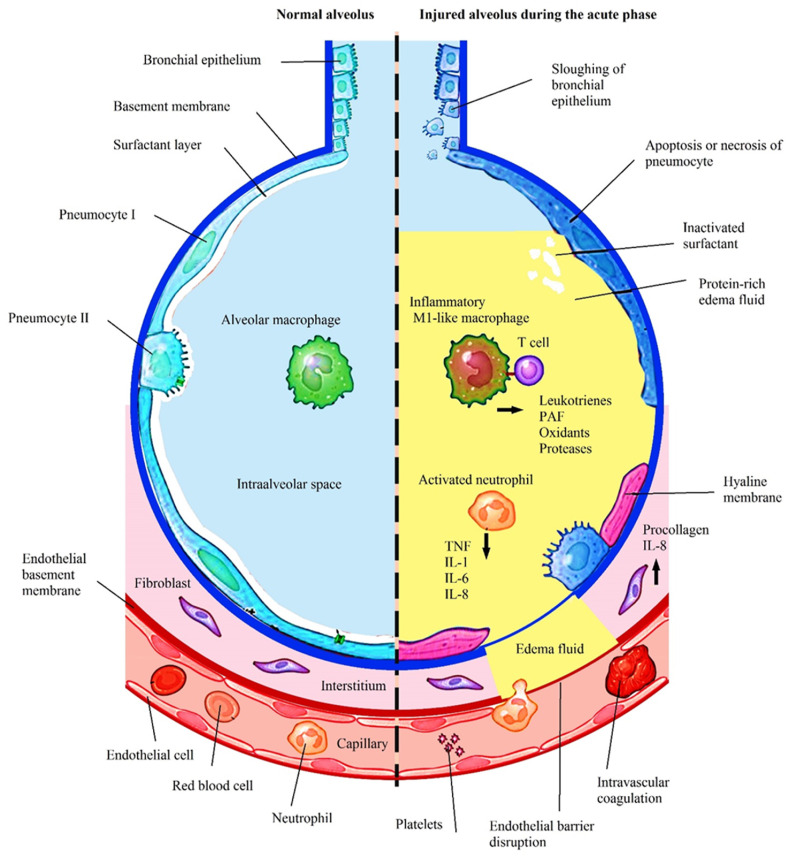
The acute phase is an inflammatory cytokine surge in lung tissue accompanied by capillary congestion, neutrophil invasion, and alveolar edema. After this, with the entry of lung tissue into the repair phase, fibrotic processes proceed with the proliferation of pneumocytes type II and increased connective tissue deposition (Kasper et al., 2015) (Fig. 1 and 2 ).
Fig. 1.
The sequence of events that occurred in the exudative phase of ARDS.
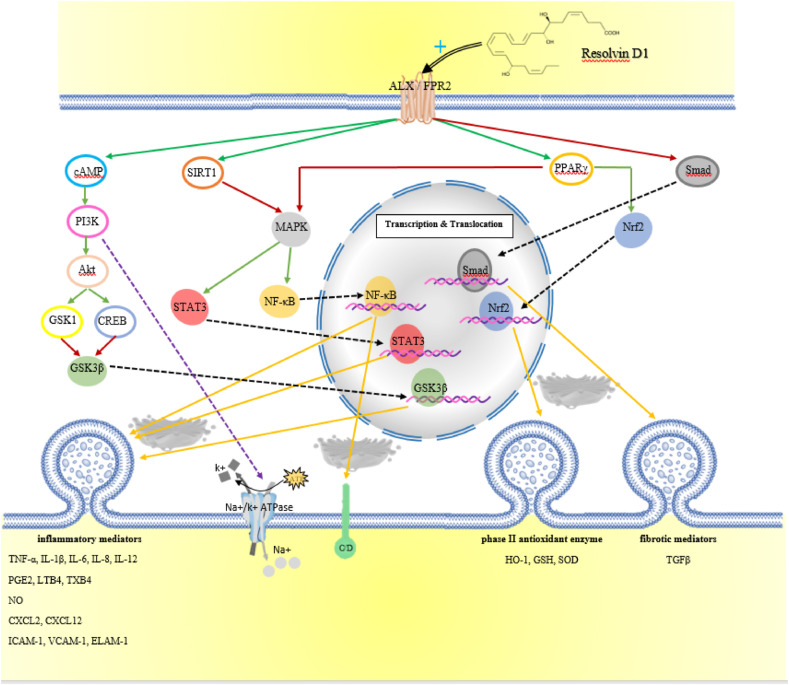
Fig. 2.
Schematic representation of the activity mechanism of RvD1 and the downstream pathways involved.
Although an understanding of the physiopathology of ALI has resulted in several treatment options, most of the medications and pharmacotherapies do not reverse completely ALI. Because inflammation is a response against infection or injury, one adjunctive therapy that has been suggested recently for ALI is to use anti-inflammatory compounds to induce cells to repair and regenerate (Nieman and Zerler, 2001).
Resolvins (Rvs) are lipid biomolecules that are part of the specialized pro-resolving mediators (SPMs) family derived from omega-3 fatty acids, primarily eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as well as docosapentaenoic acid (DPA) and clupanodonic acid. The primary substrates of Rvs are EPA and DHA and accordingly are classified into two series Rvs E and Rvs D, respectively. The synthesis of RvD as a polyhydroxyl metabolite of DHA initiates in a wide range of cells by the involvement of lipoxygenase 15 (LOX-15), which converts DHA to 17S-hydroperoxy-DHA, then this intermediate under the influence of LOX-5 generates RvD subtypes which differ in the number, position, and chirality of their hydroxyl residues as well as the position and cis-trans isomerism of their 6 double bonds. In addition, the production of certain types of these molecules in the presence of aspirin is catalyzed by acetylated cyclooxygenase-2 (COX-2), known as aspirin-triggered RvD (AT-RvD). Following the change of RvD1 to 17-oxo-RvD1 or into 8-oxo-RvD1, this biomolecule is inactivated (Demarquoy and Borgne, 2014; Kohli and Levy, 2009).
The role of SPMs is even more pronounced when it is showed that a major factor in tissue damage is chronic inflammation. The end of this process is not completely passive, and homeostasis between SPMs and inflammatory cytokines determines the onset of resolution or persistence of inflammation. In fact, one side is the secretion of cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin 1β (IL-1β), IL-6, IL-8, IL-12, prostaglandin E2 (PGE2), leukotriene B4 (LTB4), thromboxane B4 (TXB4) and nitric oxide (NO). The other side are anti-inflammatory and pro-resolving mediators, especially RvD1, which bind to ALX/FPR2 and G protein-coupled receptor 32 (GPR32) on immune cells, including T cells, macrophages, monocytes and neutrophils, and not only suppress the activation of inflammatory transcription factors but also accelerate the production of antioxidant proteins like HO-1, GSH and SOD (Norling et al., 2012). For these compounds, analgesic effects have also been proposed, which occur through surrounding lipid raft disruption and inhibiting the release of neuropeptides from the stimulated sensory nerve terminals by Transient Receptor Potential (TRP) Vanilloid 1 (TRPV1), Ankyrin 1 (TRPA1) activators capsaicin (CAPS) and allyl-isothiocyanate (AITC), respectively (Payrits et al., 2020).
RvD1 has been suggested as a novel treatment option for several inflammatory diseases. The evaluation of RvD1 in the management of a range of disorders including neurodegenerative diseases (Krashia et al., 2019; Miyazawa et al., 2020), fibrotic/electrical remodeling (Hiram et al., 2020), diabetes mellitus and its complications (Bathina and Das, 2021; Bathina et al., 2020; Bu et al., 2019; Yorek, 2018), acute kidney injury (Luan et al., 2020), colitis-associated cancers (Zhong et al., 2018), rheumatoid arthritis (Özgül Özdemir et al., 2020), Sjogren's syndrome (Yellepeddi et al., 2021), ocular allergic responses (Dartt et al., 2019), and polycystic ovary syndrom (PCOS) (Regidor et al., 2020) has shown promising results. In addition, lung diseases that have shown improvement following RvD1 administration include smoking-related asthma and COPD (Bhat et al., 2020, 2021), acute immune response and fibrotic lesions due to exposure to dust and nanomaterial (Dominguez et al., 2020; Lim et al., 2020), lung adenocarcinoma (Vannitamby et al., 2021), and airway inflammation in cystic fibrosis (Ringholz et al., 2018), as well as ARDS. Here, we review the current knowledge on the effectiveness of RvD1 along with its mechanism of action and signaling routes in ALI/ARDS.
2. Resolvin D1 in different models of ARDS
2.1. Gram-negative bacterial pneumonia and lipopolysaccharides (LPS)-induced ALI
Bacterial pneumonia, an inflammation of the lungs due to a bacterial infection, is at or near the top of the list of the most common hospital-acquired infections. Although different types of bacteria can cause pneumonia, the increasing incidence of infections caused by the Gram-negative bacteria Pseudomonas aeruginosa and Klebsiella pneumoniae, as well as a host of other Gram-negative organisms, is of growing concern (Zhang et al., 2013). These bacteria contain LPS which are highly acylated saccharolipids and are a major component of the outer membrane of Gram-negative bacteria that plays a key role in host-pathogen interactions with the innate immune system (Lu et al., 2008). The lipopolysaccharide is critical to maintaining the barrier function preventing the passive diffusion of hydrophobic solutes such as antibiotics and detergents into the bacterial cell. Lipid A is the immunologically active part of the LPS molecule that can bind to the cluster of differentiation 14 (CD14)/toll-like receptor 4 (TLR4)/myeloid differentiation factor 2 (MD-2) receptor complex on immune cells (monocytes, dendritic cells, macrophages, and B cells) thereby triggering the release of inflammatory cytokines and intensifying OS (Lu et al., 2008). Intracellular signaling molecules of this receptor complex are myeloid differentiation factor 88 (MyD88), interleukin-1 receptor associated kinase (IRAK), and tumor necrosis factor receptor associated factor 6 (TRAF6), which interact with inhibitor of nuclear factor kappa B kinases (IKKs) (IᴋB-α and IκB-β) and MAPKs to activate NF-κB and activator protein 1 (AP-1), respectively. The end product of this chain is increased production and release of TNF-α and IL-8 that exacerbate tissue damage by utilizing neutrophils (Domscheit et al., 2020).
It has been demonstrated in several mouse models of LPS-induced lung injury that treatment with RvD1 significantly reduced leukocyte infiltration and the number of neutrophils in the bronchoalveolar lavage fluid (BALF). The tissue concentration of myeloperoxidase (MPO) was reduced and the wet-to-dry weight ratio (W/D) of lung tissue decreased, suggesting pulmonary edema and vascular damage. These changes were reported to be the result of suppressing the biosynthesis and release of inflammatory cytokines such as TNF-α, IL-1β, and IL-6, the secretion of chemokine (C-X-C motif) ligand 2 (CXCL-2) and CXCL-12 (or macrophage inflammatory protein 2 (MIP-2)) chemokines, the expression of COX-2 and inducible nitric oxide synthase (iNOS), and the appearance of adhesion proteins such as intercellular adhesion molecule 1 (ICAM-1) (P-selectin), vascular cell adhesion molecule 1 (VCAM-1), and endothelial-leukocyte adhesion molecule 1 (ELAM-1) (Liao et al., 2012; Wang et al., 2011, 2014a; Yaxin et al., 2014; Zhang et al., 2019). Antagonization of RvD1 receptor with peptide WRW4 in mice with pneumococcal pneumonia indicated that RvD1 contribute to the resolution of lung injury by augmenting bacterial clearance and reducing pulmonary edema via the restoration of lung alveolar-capillary barrier permeability (Siegel et al., 2021).
RvD1 was reported to prevent the activation and migration of NF-ᴋB into the nucleus by inhibiting the degradation of IᴋB-α with an increase in peroxisome proliferator-activated receptor gamma (PPARγ) activity due to the interaction of ALX/FPR2 by RvD1. In addition, inhibition of mitogen-activated protein kinases (MAPKs) phosphorylation by RvD1 had a similar effect on NF-ᴋB (Liao et al., 2012; Wang et al., 2011). In both human airway epithelial cell culture and in a mouse model using LPS or E. coli to evoke lung injury, it was reported that stimulation of ALX/FPR2 by various specialized pro-resolving mediators (SPMs) improved pathogen clearance by elevating antimicrobial substances including bactericidal/permeability-increasing protein (BPI) and human cationic antibacterial protein of 18 kDa (LL-37) and by decreasing the secretion of CXCL-8 neutrophilic chemoattractant by downregulating NF-ᴋB activity. Among the SPMs, 15-epi-lipoxin A4 (LXA4) accelerated pacification of NF-ᴋB by increasing tumor necrosis factor-alpha-induced protein 3 (A20) and SIGIRR transcription, while 17-epi-RvD2 and 17-epi-RvD3 only affected SIGIRR expression while 17-epi-RvD1 1 showed no activity (Sham et al., 2018). Another in vitro study suggested that the inactivation of glycogen synthase kinase 3 beta (GSK3β) by RvD1 was also involved in reducing the production of several inflammatory mediators. Augmentation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling by RvD1 inducted phosphorylation of GSK3β and reduced interference of this protein with TLR4. In addition, this study suggested that the effect of NF-ᴋB in the development of RvD1 included the anti-inflammatory axes cAMP response elements-binding protein (CREB) and serine/threonine-protein kinase or serum and glucocorticoid-regulated kinase 1 (SGK1) in the suppression of NF-ᴋB activity (Gu et al., 2016).
Pretreatment of mice suffering LPS-induced pneumonia with RvD1 reduced the amount of TNF-α and IL-1β in the BALF, and facilitated the production of anti-inflammatory factors such as IL-10, and promoted the cellular antioxidant defense system by enhancing HO-1 expression and increasing SOD generation (Wang et al., 2014a). In addition, RvD1 had a biphasic role in the expression of COX-2. This enzyme peaked at 6 and 48 h after LPS incubation both in an human Lung Fibroblasts 1 (HLF-1) cell line and rat pulmonary fibroblasts, but a phenotypic switch resulted in different end products. Simultaneously with the first peak, the synthesis of PGE2 increased with the concentration of PGD2 being elevated at the second peak. RvD1 inhibited COX-2 expression as well as the production of PGD2 via PI3K/Akt and ERK2 intracellular pathways, while PGD2 synthesis was amplified at the second peak (Wu et al., 2013).
The penetration and collection of fluid in lung tissue are prevented in alveolar epithelial cells by resisting outflow through insulating intercellular spaces with tight junction proteins and by clearing the alveoli through ion channels and pumps (Wang et al., 2014b, 2018). The wet-to-dry weight ratio (W/D ratio) and the amount of Evans blue extravasation showed that these two functions were improved in lung tissue of mice that received RvD1 before LPS aspiration. The stimulation of ALX/FPR2 by RvD1 with a secondary messaging of cAMP/PI3K resulted in the accumulation of epithelial sodium channel (ENaC) and Na+/k + ATPase in the cells (Wang et al., 2014b). RvD1 also mitigated ROS by increasing HO-1 expression. Occludin and zonula occludens 1 (ZO-1) also were protected against degradation while the epithelial cells were protected against apoptosis, and the dysfunction of the air-blood barrier was limited (Xie et al., 2013).
Abdulnour et al. demonstrated a strong correlation between the level of aspirin-triggered resolvin D1 (AT-RvD1) activity and the immune response shifting from neutrophilic invasion to macrophagic recovery. Comparison of changes in neutrophil and macrophage counts and microbial load in the BALF of E. coli-infected mice and a sample of native lung tissue inoculated with the bacterium revealed that AT-RvD1 could improve phagocytosis of bacterial particle and efferocytosis (phagocytosis of apoptotic neutrophils) by increasing the recruitment of the infiltrating (CD11cLow CD11b+) and exudative (CD11cHi CD11b+) subsets of macrophages. Another consequence was a reduction in IL-6 and TNF-α levels without affecting IL-1α, monocyte chemoattractant protein 1 (MCP-1), and IL-10. In addition, this molecule potentiated the expression of lipocalin 2, an antimicrobial peptide derived from the host response (Abdulnour et al., 2016).
The impairment of phagocytic clearance in the development and continuation of inflammatory reactions due to bacterial lung infection showed that CpG DNA (bacterial DNA), by binding TLR9, caused this impairment and also inhibited the activity of Caspase-8/3 apoptotic enzymes in neutrophils in whole blood. Both the enzymatic cleavage of C5a receptor by neutrophil elastase and proteinase-3 and the increased C3b receptor (CD11b/CD18) expression were involved. Considering that the intraperitoneal injection of CpG DNA into the mouse model of pneumonia prevented the natural peak of 15-epi-LXA4 and 17-epi-RvD1, the administration of these lipids has the potential to reverse these effects, suggesting the possibility of an effective treatment (Sekheri et al., 2020). Co-delivery of RvD1 and ceftazidime in nanovesicles to mice infected with P. aeruginosa showed that they alleviated both inflammation and bacterial growth in the mouse lung. This study reveals a new strategy to treat infectious diseases by designing nanoparticles to target inflammatory pathways and pathogens. (Gao et al., 2020b) (Table 1 ).
Table 1.
A summary of the studies review on the beneficial role of RvD1 in managing ARDS.
| RvD1 ADMINISTRATION/DOSE/ROUTE | STUDY | MODEL | MAIN RESULTS | REF |
|---|---|---|---|---|
| 300 or 600 ng/i.v | in vivo | BALB/c mice with LPS -related ARDS | ↓leukocyte count; ↓ cytokine concentration in BAL; ↓ tissue damage severity score; ↓ IL-6 and TNF-α levels; ↑ PPARγ; ↓ IκBα and NF-ᴋB; | Liao et al. (2012) |
| 1 or 5 mg/kg/i.p | in vivo | Balb/c-mice with LPS-related ARDS | ↓ tissue damage image; ↓count of BAL white blood cells; ↓ MPO activity; ↓ inflammatory mediators such as TNF-a, COX-2, IL-6, PGE2 and NO and adhesion proteins such as ICAM-1, VCAM-1 and ELAM-1; ↓ phosphorylation of MAPKs (ERK1/2, p38, and JNK) and NF-kB | Wang et al. (2011) |
| 100 ng/mouse/i.v | in vivo | mice with LPS-related ARDS | ↑ HO-1 expression; ↑ amount of IL-10; ↓ TNF-α and IL-1β; ↑ activity level of MDA and SOD; ↓ tissue edema and W/D ratio; ↓ neutrophils in BAL flow cytometry; ↓lung damage and apoptosis in Haemotoxylin and Eosin (H&E) staining | Wang et al. (2014a) |
| 3 μg/kg/i.p | in vivo | Balb/c-mice with LPS-related ARDS | ↓ BAL neutrophil count, ↓ MPO tissue activity level; ↓ concentration of inflammatory mediators (IL-1β and TNF-α); ↓ BAL protein concentration and ↓ wet/dry weight ratio as two indicators of pulmonary vascular permeability; ↑ CXCL-12 and CXCR4 | (Yaxin et al., 2014, 2020) |
| 0.1 μg/i.p | in vivo | C57Bl/6 mice with LPS-related ARDS | ↓ CXCL2 mRNA levels; ↓ BOC-2, the ALI score, the TNF-α protein concentration; ↓neutrophil influx | Zhang et al. (2019) |
| 100 nM and 100 ng/i.v | in vitro and in vivo | C57Bl/6 mice and Calu-3 lung cells with gram-negative bacterial pneumonia and LPS-related ARDS | CXCL8 as a neutrophilic chemo attractants; ↓ NF-ᴋB; ↓ Cell counts in BALF; ↑A20 and SIGIRR; ↑ effect on ALX/FPR2 receptors; | Sham et al. (2018) |
| 10 nM | in vitro | human monocytes with gram-negative bacterial pneumonia-related ARDS | no effect on IL-10; ↓ IL-6, IL-8, IL-12, as IL-1β and TNF-α levels; ↓ GSK3β activity; stimulating TLR4; ↓ anti-inflammatory axes of CREB and SGK1; ↓ phosphorylation of NF-κBp65; no significant alteration in MAPKs signaling | Gu et al. (2016) |
| 10, 50, or 100 nM | in vitro | HFL-1 cell line with LPS-related ARDS | ↓ pro-inflammatory mediator such as PGE2, COX-1,MCP1, IL-8 and COX-2; ↓PGD2 activity; no significant change in the expression of the phosphorylated ERK1; ↓ PI3K/AKT expression | Wu et al. (2013) |
| 5 mg/kg/i.v | in vivo | rat with LPS-related ARDS | ↑ alveolar fluid clearance (AFC) in live rats; enhanced ENaC and Na-K ATPase; ↑ ALX/cAMP/PI3K; ↓ MPO activity; no significant changes occurred in cGMP and PKA and harnessing their signaling by H89 (PKA inhibitor) had no effect on this process | Wang et al. (2014b) |
| 5 μg/kg/i.p | in vivo | Balb/c-mice with LPS-related ARDS | ↓ tissue edema and wet/dry weight ratio; ↑ HO-1, occludin and ZO-1 | Xie et al. (2013) |
| 100 ng/i.v | in vivo | C57BL/6 mice with gram-negative bacterial pneumonia-related ARDS | ↓ inflammatory (CD11cLow CD11b+) and exudate (CD11cHi CD11b+) macrophages in the BAL; ↓ IL-6 and TNF-α levels; no effect on IL-1α, MCP-1 and IL-10; ↑ lipocalin 2 as an antibacterial peptide derived improved efferocytosis; ↑ phagocytosis of bacterial particles by CD11c + macrophage | Abdulnour et al. (2016) |
| 200 nM 25 ng/g/i.p | in vitro and in vivo | mice and human PMNs with gram-negative bacterial pneumonia-related ARDS | ↓ phagocytosis and the apoptotic activity of Caspase-8 and Caspase-3; ↑ CD11b expression; ↓ C5a receptor levels; ↓ neutrophil elastase and proteinase-3; ↓ Cell counts in BALF; ↓ Caspase-8 and Caspase-3 activity;; ↓ MPO activity | Sekheri et al. (2020) |
| 67 ng/i.v | in vivo | mice with LPS-related ARDS | ↓ expression of ICAM-1 on HUVECs; ↓ apoptosis; ↑ therapies of bacterial infections; ↓ cytokine levels and neutrophil lung infiltration | Gao et al. (2020a) |
| 10ng/g/i.v | in vivo | C57BL/6 mice with sepsis -related ARDS | ↓ IL-1β, IL-6 and TNF-α; ↓ MPO activity; ↓ protein content of BAL; ↓ NF-κB and STAT3; ↑ SIRT expression; ↓ phosphorylation of MAPKs | Zhuo et al. (2018) |
| 10 ng/g/i.v | in vivo | C57BL/6 mice with sepsis -related ARDS | ↓ leukocyte adhesion molecules CD18; ↓ MPO activity; ↓ ICAM-1; ↑ survival rate | Zhang et al. (2018) |
| 250–500 ng/i.v | in vivo | C57BL/6 mice with ischemia/reperfusion-related ARDS | ↓ lung PMN infiltration and MPO activity; | Shinohara et al. (2014) |
| ↓ levels of pro-inflammatory cytokines such as IFN-γ, IL-1β, IL-12p40, IL-6 and MCP-1, and lipid mediators affecting tissue damage and bronchoconstriction such as TXB2 and LTB4; | ||||
| ↓ adhesion molecules such as CD41a and PMN-platelet aggregation | ||||
| 100 μg/kg/i.v | in vivo | C57BL/6 mice with ischemia/reperfusion-related ARDS | ↓ serum levels of inflammatory markers such as IL-1β, IL-10 and TNF-α; except for increased (↑) IL-10 | Zhao et al. (2016a) |
| ↓ tissue concentrations of chemokines such as MCP-1, MIP-2 and CINC-1; | ||||
| ↑ activity of antioxidant proteins such as GSH-PX and SOD | ||||
| ↑ PaO2 and oxygenation index, ↓ alveolar degradation rate, improvement of pulmonary surfactant associated protein-A; ↓ ratio of total protein in BAL to the total protein in blood serum; ↓ W/D ratio; | ||||
| ↓ lactic acid content and apoptosis | ||||
| ↑ glycogen content, ATP/ADP ratio and Na+/k + ATPase activity | ||||
| 100 μg/kg/i.v | in vivo | C57BL/6 mice with ischemia/reperfusion-related ARDS | ↓ W/D ratio; ↑ PaO2 and oxygenation index; ↑ antioxidant capacity such as GSH-PX and SOD; ↓ MPO activity; ↓MDA content; ↓ apoptosis (determined by TUNEL assay); ↓ ratio of total protein in BAL to the total protein in blood serum; ↓levels of C1q, C2, C3a, C4, C5a, IgG and IgM; ↓ TLR4 and NF-κBp65 expression | Zhao et al. (2016b) |
| 500 ng/mouse/i.v | in vivo | C57BL/6 mice with ischemia/reperfusion injury -related ARDS | ↓ efferocytosis through MerTK cleavage; ↓ MPO activity; ↓ IL-6, LT, LX and PGE4; ↓ tissue damage image | Rymut et al. (2020) |
| 1000–0.1 nM | in vitro | THP1 and A549 cells with hydrogen peroxide -related ARDS | ↓ TNF-α, IL-1β, IL-6 and IL-8; ↓ phosphorylation of MAPKs; ↓ Caspase-1 activation; ↓ ICAM-1; no effect on rescue barrier function | Cox et al. (2015b) |
| 0.5–0.05 μg/i.v | in vivo | C57BL/6 mice with hyperoxia-related ARDS | ↓ level of BAL proteins and total cells; ↓ wet/dry weight ratio; ↓ TNF-α, IL-6 and MCP-1; ↓ p38MAP kinase ↓ NF-B | Cox et al. (2015a) |
| 2 ng/g/i.p | in vivo | C57BL/6 mice with hyperoxia-related ARDS | ↓ cell differentiation & organogenesis (↓ TGF-β, | Martin et al. (2014) |
| VEGF and ↑ PPAR); | ||||
| ↓ growth factor signaling (BMPR and Smad); | ||||
| ↓ extracellular matrix (Eln, | ||||
| Col1a1, LOXL2 | ||||
| MMP and TIMP1); | ||||
| ↓ Inflammation (CRP, CD46, ICAM-1, CCL5, CXCL2, TNF-α and IL-1β) | ||||
| 100 or 500 ng/i.p | in vivo | C57BL/6 mice with ventilator-related ARDS | ↑ oxygenation level (PaO2) ↓histopathological changes, ↓ pulmonary edema (W/D ratio), ↓ tissue-BAL protein; ↑stimulating PPARγ; ↑ Nrf2; ↓ NF-B; ↑ HO-1 generation; ↓expression of HMGB1; ↓MAPK-PI3K/NF-κB; ↓ IL-1β, IL-6 and TNF-α cytokines; ↓ MPO activity | Sun et al. (2019) |
| 500 ng/i.p | in vivo | C57BL/6 mice with ventilator-related ARDS | ↑ PaO2 mean levels, ↓ wet/dry weight ratio; changes in protein levels such as ↓ NF-B and ↑ PPAR- | Xia et al. (2019) |
| γ in BALF; ↓ Cell counts in BALF; ↓ TNF-α, IL-6, IL-1β and RAGE | ||||
| 1–100 nM and 0.01–0.1 μg/i.p | in vitro and in vivo | C57BL/6 mice and human lung epithelial (BEAS-2B) cells with ventilator-related ARDS | ↓ mesenchymal markers such as collagen, vimentin, α-SMA; collagen I deposition and hydroxyproline levels elevated (↑) sharply; ↓ Smad2/3 and TGFβ1; | Yang et al. (2019) |
| 10, 25, or 100 nM | in vitro | ATII cells and fibroblasts with ventilator-related ARDS | ↓ mesenchymal markers such as collagen, vimentin, α-SMA and N-cadherin; ↑ E-cadherin glycoprotein levels; ↓ Caspase-3 level; ↓ TNF-α; ↓ fibrosis and apoptosis | Zheng et al. (2018) |
| 0.5–5 μg/kg/i.v | in vivo | C57BL/6 mice with hydrochloric acid -related ARDS | ↑ ALX/FPR2 receptors expression; ↓ leukocyte recruitment; ↓ lung edema and neutrophil accumulation; ↓ lung resistance; ↑ restitution of barrier function; ↓ total/PMNs/MACs BALF cells; ↓ neutrophil – platelet interactions; ↓ LXA 4 and IL-10 levels; ↓ NF-B -p65 translocation; ↑ endogenous airway epinephrine levels | Eickmeier et al. (2013) |
| 300 ng/mouse/i.p | in vivo | C57BL/6 mice with acute pancreatitis -related ARDS | ↓ NF-B; ↓ TNF-α and IL-6; ↓ MPO activity; ↓ serum amylase and lipase; ↓ pancreatitis and lung injury score | Liu et al. (2016) |
| 500 ng/mouse/i.v | in vitro and in vivo | C57BL/6 mice and MH-S cell culture with IgG -related ARDS | ↓ level of BAL proteins and total cells; ↓ tissue damage image; ↓ levels of TNF-α, IL-6, KC and C5a; ↓ activity of NF-κB and C/EBPs | Tang et al. (2014) |
| 2 μg/mouse/i.p | in vivo | C57BL/6 mice with bleomycin-related ARDS | ↓ cell counts in BALF, inflammatory cell infiltration and IL-1b mRNA; ↓ total lung | Yatomi et al. (2015) |
| collagen levels; ↓ expression of TGF-βand CTGF mRNA; ↓ hydroxyproline contents; Improving lung tissue damage on CT scan; ↑ expression of MMP-9 and ↓ TIMP-1 mRNA | ||||
| 100 ng/i.v | in vivo | C57Bl/6 mice with paraquat related ARDS | ↓ lung W/D weight ratio; ↓ MPO activity; ↓ pro-inflammatory cytokines (IL-1β and TNF-α), total protein content, and cell count in the BALF; ↓ MDA level; ↓ platelet–neutrophil interactions; ↑ antioxidant capacity such as NQO-1 and HO-1 | Hu et al. (2019) |
2.2. Sepsis-induced ALI
Sepsis is a serious condition resulting from the presence of harmful microorganisms in the blood or other tissues and the body's response to their presence. Sepsis can result in the malfunctioning of various organs, shock, and even death if not treated properly. The lungs are one of the organs most often affected. It has been confirmed that excessive inflammation and stimulation of the production and secretion of tissue-damaging cytokines play a major role in its physiopathology (Gyawali et al., 2019).
Evaluation of the therapeutic value of RvD1 in ameliorating sepsis-induced pulmonary inflammation in cecal ligation and puncture (CLP) models has demonstrated that RvD1 tempered the inflammatory storm by enhancing sirtuin 1 (SIRT1)-mediated deacetylation of lysine residues in NF-κB and STAT3. In addition, by suppressing phosphorylation of MAPKs (ERK and p38 and no c-Jun N-terminal kinase (JNK)), RvD1 also indirectly amplified this pathway (Karbasforooshan and Karimi, 2018; Zhuo et al., 2018). Diminution of CD18 and ICAM-1 expression by RvD1 (alone or in combination with Xuebijing) also significantly reduced the level of myeloperoxidase activity and the presence of an excess of neutrophils in lung tissue (Zhang et al., 2018) (Table 1).
2.3. Ischemia/reperfusion injury (IRI)-induced ALI
Ischemia/reperfusion injury is the paradoxical exacerbation of cellular dysfunction and death, following the restoration of blood flow to previously ischaemic tissues that often occurs following cardiothoracic and vascular surgery. IRI is due to transient loss of blood flow, cessation of tissue perfusion, and subsequent recurrence. Tissue damage occurs in such a way that during ischemia, activation of the immune response causes accumulation of neutrophils and platelets, and after re-establishment of blood flow, the release of cytokines and other inflammatory mediators. An increase in OS and induction of apoptosis both play a role in IRI. IRI can cause tissue damage and dysfunction in a variety of organs. Managing this condition in the lungs is critical because it destroys the alveoli and increases the risk of ARDS (Kalogeris et al., 2016; Zarbock et al., 2014).
It has been reported that RvD1 reduced the severity of the destructive effects of IRI by blocking several pathways that disrupt lung function. Suppressing the overproduction of cytokines such as interferon gamma (IFNγ), IL-1β, IL-12p40, and IL-6, and reducing the expression of MCP-1, MIP-2, and cytokine-induced neutrophil chemoattractant 1 (CINC-1) chemokines by inhibiting the influx of white blood cells (WBCs) into lung tissue along with a disproportionate immune response plays a major role in maintaining blood-air barrier integrity. Tissue damage and bronchoconstriction were limited by decreased release of TXB2 and LTB4 lipid mediators. Decreased expression of the CD41a adhesion molecule also prevented polymorphonuclear leukocytes (PMN)-platelet aggregation and hypercoagopathy (Shinohara et al., 2014; Zhao et al., 2016a). Another mode of action was the regulation of humoral immunity and the level of complement cascade activation, which subsequently attenuated the apoptotic signal associated with TLR4/NF-κB (Zhao et al., 2016b).
Estimating the metabolic status of cells by determining glycogen content, lactate level, ATP/ADP ratio, and Na+/k + ATPase activity along with measuring the amount of glutathione-PX (GSH-PX) and SOD suggested that improving mitochondrial function by elevating the antioxidant capacity of the tissue appears to play an important role in the effectiveness of RvD1 (Zhao et al., 2016a). Following the induction of IRI in young and older mice, Rymut et al. observed that there was a correlation between gene p16INK4A as a marker for senescent cells and the amount of IL-6 as the major inflammatory cytokine. Based on this observation, Rymut and his colleagues hypothesized that a pro-inflammatory phenotypic switch was involved in the progression of damage in aging cells. The conclusion was based on the observation that the SPM:LT ratio drop was greater in older mice with the focus shifted to the possible role of SPMs. Intravenous injection of RvD1 attenuated tissue damage by a reduction in the recruitment of neutrophils. The efferocytosis power of the macrophages in each group was assessed by incubating with senescent cells generated from γ-irradiated IMR-90 cells. RvD1 inhibited ADAM17 activity by reducing ROS, thus preventing myeloid-epithelial-reproductive tyrosine kinase (MerTK) from cleavage. This accelerated the removal of damaged cells and the development of the resolution process via binding macrophages to apoptotic cells (Rymut et al., 2020) (Table 1).
2.4. Hyperoxia-induced ALI
Although hyperoxia therapy is the primary form of care for patients with impaired respiratory function, prolonged hyperoxia exposure (O2 > 65%) can exacerbate respiratory symptoms and lead to acute lung damage. Increasing oxygen concentration by producing ROS caused tissue damage, increased expression of proapoptotic proteins, and cell death. Subsequently, the release of cytokines by immune cells stimulated by inflammatory mediators completed the cycle. However, often following the reduction of the trigger factor, this cycle may degenerate into a self-limiting form and have minimal fibrosis compared to other cases of acute pulmonary injury (Kallet and Matthay, 2013).
The presence of AT-RvD1 in culture medium containing THP1 and A549 exposed to H2O2, an oxidant that plays an important role in hyperoxic ALI, diminished MAPKs (p38 and ERK) phosphorylation. As a result, the synthesis of inflammatory cytokines IL-1β 1, IL-6, and IL-8, and the expression of ICAM-1 were reduced (Cox et al., 2015b). In addition, AT-RvD1 treatment improved neutrophil infiltration, alveolar edema, and pulmonary compliance in a murine hyperoxia model. This compound also damped the stimulatory effect of NF-κB on cytokine production by inhibiting MAPK p38 activity. Nuclear factor erythroid 2-related factor 2 (Nrf2) signaling can enhanced the productions of antioxidants such as GSH. Eventually, these alterations led to a reduction in ROS levels and a calming of the B-cell lymphoma 2 homology 3 (BH3)/Caspase-3 apoptotic cascade (Cox et al., 2015a; Iranshahy et al., 2018; Molaei et al.).
A study in an animal model of hyperoxia-induced ALI suggested that the mechanism of lung damage involved cytokine over-secretion and accumulation of immune cells. This was followed by an increase in metallopeptidase inhibitor 1 (TIMP1) and a decrease in ELN, LOXL2, and collagen type I alpha 1 chain (Col1A1). The number of factors involved in cell growth and differentiation such as vascular endothelial growth factor A (VEGF-A) and transforming growth factor-beta 2 (TGF-β2) dropped. Both RvD1 and LXA4 downregulated NF-κB by triggering PPARγ and preventing the release of IL-1β, IL-8, and the expression of CXCL2. RvD1 primarily inhibited TIMP1 activity, while LXA4 also suppressed the TGF/bone morphogenetic protein (BMP)/mothers against decapentaplegic homolog (Smad) pathway (Martin et al., 2014) (Table 1).
2.5. Ventilator-induced ALI
Mechanical ventilation is an essential treatment for patients with respiratory failure (Brochard et al., 2017). Its main function is to reduce respiratory work, increase tidal volume, and improve oxygen delivery, but despite these benefits, parenchymal damage to the lungs by barotrauma and stimulation of inflammatory responses can complicate the patient's condition. VILI is an acute lung injury affecting the airways and parenchyma that is caused by or exacerbated by mechanical ventilation.
The efficacy of RvD1 in high-tidal volume ventilated rats showed that these animals had higher oxygenation levels, lower W/D ratios, and fewer histopathological changes than control rats. There was a decline in the release of IL-1β, IL-6, and TNF-α cytokines. RvD1 activated PPARγ through stimulating ALX/FPR2 and GPR32, followed by suppression of IBα phosphorylation. Then, IBα, a NF-Bp65 subunit inactivating protein, enhanced the transformation of the phenotype macrophage from proinflammatory to a pro-resolving M2-like phenotype. In addition, PPARγ reduced the production of high mobility group box protein 1 (HMGB1) by upregulating Nrf2 and increasing the concentration of HO-1. HMGB1 is derived from the passive release of necrotic cells or the active secretion of inflammatory cells resulting from stimulation of the MAPK/NF-κB pathway which in turn induces an immune response through HMGB1/receptor for advanced glycation end-products (RAGE) signaling (Sun et al., 2019; Xia et al., 2019).
RvD1 administration reversed the fibrotic processes induced by mechanical stress in two studies. Pulmonary fibrosis is characterized by a set of changes called epithelial-mesenchymal transition (EMT) which include switching from E-cadherin to vimentin and α-smooth muscle actin (α-SMA) expression, collagen I deposition, and production of surfactant protein C (a type II epithelial cell marker) instead of aquaporin 5 (a type I epithelial cell marker). TGF-β1 is an essential factor in the progression of fibrosis, which is activated by Smad2/3 molecules. RvD1 was reported to bond to ALX/FPR2 and inhibit the phosphorylation of Smad2/3 via thePI3K/AKT-dependent signaling pathway. As a result, TGFβ type I receptor (activin receptor-like kinase 5 (ALK-5)) stimulation occurred, and the subsequent effects were prevented (Yang et al., 2019; Zheng et al., 2018) (Table 1).
2.6. Aspiration pneumonia and hydrochloric acid-induced ALI
Gastric or oropharyngeal aspiration can occur as a negative event in patients with a decreased level of consciousness (e.g., general anesthesia, head trauma, alcohol or drug-induced alterations in sensorium, and cerebrovascular accidents). This condition can lead to inflammation of the walls of the alveoli (pneumonitis) or inflammation in which the air sacs fill with pus and may become solid (pneumonia) and can range from subclinical to respiratory failure. Although there is no clear demarcation to classify the pathogenesis of aspiration, aspiration of the gastric contents due to high acidity and/or the presence of food particles can cause chemical damage to lung tissue. The bacterial load is an important factor in the aspiration of the upper gastrointestinal tract (Raghavendran et al., 2011) (Table 1).
Decreased biosynthesis of inflammatory cytokines such as IL-1β, IL-6, TNF-α, and keratinocytes-derived chemokine (KC), and suppression of PMN recruitment by the use of AT-RvD1 have been reported to protect the alveolar-capillary barrier from rupture in mice following HCl -initiated ALI. Eickmeier and colleagues suggested that the inactivation of NF-κB in Kupffer and epithelial cells after ALX/FPR2 stimulation was the mechanism of action. Pretreatment with AT-RvD1 also had the potential to elevate IL-10 and LXA4, but its injection after injury did not have this property. Benefits reported following treatment with AT-RvD1 include reducing neutrophil-platelet interaction by inhibiting P-selectin and its granulocyte receptor CD24 expression and reducing airway resistance by increasing endogenous epinephrine concentration (Eickmeier et al., 2013).
2.7. Pancreatitis-induced ALI
Acute pancreatitis is an inflammatory condition of the exocrine part of the pancreas that occurs in most patients in a mild and self-limiting form. However, about one-fifth of cases are associated with the spread of inflammation to other organs (Frossard, 2008), including the lungs. These patients can develop ARDS and respiratory failure with considerable mortality. No effective treatment is currently available (Guice et al., 1988).
RvD1 attenuated the cerulein-induced histopathological changes in the pancreas including edema, vacuolization, inflammation, and necrosis in a mouse model. This treatment also lowered serum levels of amylase and lipase. As a result, the severity of the lung damage associated with pancreatitis was reduced. It was reported by the authors that RvD1 caused IKKs to inactivate NF-ᴋB in the cytoplasm, thereby suppressing the production and secretion of IL-6 and TNF-α. The neutrophil excitation increased the antioxidant concentration of MPO, and the progression of systemic inflammatory response syndrome (SIRS) was reduced (Liu et al., 2016) (Table 1).
2.8. Immunoglobulin G (IgG) immune complex-induced ALI
The IgG immune complex crosslinks to the Fcγ receptors on the alveolar macrophages to trigger an immune response that is manifested by the secretion of TNF-α and IL-6 (Guo and Ward, 2002). These cytokines stimulate transcription factors such as NF-κB and CCAAT/enhancer binding proteins (C/EBPs) to increase the expression of adhesion molecules, CXC and CC chemokines, and other inflammatory mediators. In addition, the formation of the IgG immune complex in lung tissue proceeds by employing the classical complement pathway which increases the generation of C5a as a potent chemoattractant in the tissue, and through this process, neutrophils are stimulated. Overall, the occurrence of this series of events causes tissue inflammation to flare up and acute lung damage to occur (Sun et al., 2009; Tang et al., 2014). Bovine serum albumin (BSA) was injected into mice after rabbit anti-BSA IgG was inoculated in their lungs. This was followed by RvD1 (500 ng, intravenously) to evaluate its effectiveness in alleviating the lung injury caused by the IgG immune complex. Analysis of BALF for albumin content and differential leukocyte count revealed that RvD1 decreased vascular permeability and neutrophil recurrence. The effect was the result of suppressing the activity of NF-κB and C/EBPs and reducing the levels of TNF-α, IL-6, KC, and C5a (Tang et al., 2014) (Table 1).
2.9. Bleomycin (BLM)-induced ALI
BLM is an antineoplastic drug used to treat several types of cancer. However, the side effects of BLM, which mimic idiopathic pulmonary fibrosis (IPF), have limited its use, have limited its use. IPF is progressive inflammation in the later stages of ARDS that is characterized by alveolar epithelial injury, exaggerated expression of profibrotic cytokines, and formation of fibrotic foci (Reinert et al., 2013).
There are limited treatment options available for the management of this complication, but the use of RvD1 in mice receiving BLM showed promising results. RvD1 decreased the expression of inflammatory cytokines, such as IL-β by stimulating ALX/FPR2 resulting in fewer neutrophils migrating to the BALF. Decreased levels of TGF-β1 and connective tissue growth factor (CTGF) mediators, type 1 collagen mRNA translation, and the collagen content of lung tissue also indicated suppression of the fibrotic process by RvD1. Improved pulmonary function parameters were further indicated by a better histopathology score and adjustments in several hemodynamic variables (Yatomi et al., 2015) (Table 1).
2.10. Paraquat (PQ)-induced ALI
PQ is a non-specific, widely used herbicide that can cause edema, bleeding, and concentration of fluid in the lung tissue resulting in the collapse of the alveoli. There is no antidote for PQ-induced toxicity. In addition to releasing inflammatory mediators that play a key role in these injuries, the generation of reactive oxygen species followed by increased OS and involvement of the redox cycle are also involved in the toxicity of paraquat (Riahi et al., 2011; Xu et al., 2014).
Elevated levels of NAD(P)H:quinone oxidoreductase (NQO-1) and HO-1 and decreased malondialdehyde (MDA) occurred after RvD1 was administered to a PQ-induced ALI murine model of acute lung injury. A reduction in the severity of OS was also reported. Inhibition of IL-1β and TNF-α chemokine release and expression of P-selectin were also observed following the use of RvD1. These two mechanisms were suggested to merge by targeting Nrf2 and NF-κB, which were then able to regulate gene transcription of these antioxidant and inflammatory products and shut down the apoptotic cascades, respectively (Hu et al., 2019).
The results of the studies reviewed are summarized in Table 1.
3. Conclusion and future prospects
The physiopathology of ARDS, similar to many inflammatory diseases, involves the development of a disproportionate and prolonged immune response that is associated with increased ROS in lung tissue, destruction of vital cellular molecules, and increased dysfunctional cells. The ion channel and pumps involved in alveolar clearance and intercellular proteins forming the blood-air barrier are the primary sites of damage. Oxidation of membrane lipids, especially on the surface of the mitochondria, in addition to disrupting cellular metabolism, by stimulating the separation of B-cell lymphoma 2-associated X (BAX)/B-cell lymphoma 2-antagonist-killer 1 (BAK) proapoptotic proteins from B-cell lymphoma 2 (Bcl-2) protein leads to activation of Caspase- 9/3 and eventually cell death.
RvD1 is endogenously produced from ω3 polyunsaturated fatty acids (ω3-PUFAs) and as a lipid mediator is involved in reducing inflammation and promoting tissue repair. RvD1 has the potential to ameliorate ALI complications by stimulating ALX/FPR2 through one or all of the following mechanisms:
-
-
Inhibiting the activation of leukocytes and the release of destructive substances by suppressing the production of cytokines, chemokines, adhesion molecules, and other inflammatory enzymes and mediators
-
-
Promoting the capacity of the cellular antioxidant defense system by facilitating the production of reductive proteins
-
-
Speeding-up alveolar clearance by modifying ENaC and Na+/k + ATPase activity
-
-
Reducing PMN-platelet aggregation and improving hemodynamic status by decreasing P-selectin, CD24, and CD41a expression
-
-
Attenuating the epithelial-mesenchymal transition and pulmonary fibrosis by reducing TGF-β1 expression
Other beneficial effects that occur indirectly following the reduction of oxidative stress are:
-
-
Enhancing macrophage efferocytosis by inhibiting MerTK cleavage
-
-
Accelerating bacterial depletion by preventing C5a receptor increase
-
-
Increasing the strength of the blood-air barrier by protecting tight junction proteins against degeneration
-
-
Improving cellular metabolism by alleviating mitochondrial membrane damage
The COVID-19 pandemic is a serious public health challenge with high morbidity and mortality (Abedi et al., 2020b; Lakhani et al., 2020). The disease presents with symptoms of fever, cough, and shortness of breath and has been observed in patients with pneumonia, ARDS, and renal failure. Although the molecular mechanisms involved in the pathogenesis of COVID-19 have not been fully elucidated, it has been suggested by several authors that triggering an inflammatory storm due to T cell activity, cytokine secretion, and the humoral immune response is the mainstay of the disease progression (Pascarella et al., 2020; Wiersinga et al., 2020). RvD1 has been shown to inhibit excessive immune reactions and to strengthen the antioxidant system in ARDS. RvD1 has also been reported to prevent neutrophil infiltration, pulmonary edema due to air-blood barrier destruction, and coagulopathy, and ultimately reduced apoptosis and fibrotic complications. The inclusion of RvD1 or its precursors in the plan of patients with respiratory complications caused by COVID-19 may have a protective effect, suggesting the need for more research on the subject.
One of the gaps in studies is that many of them have been performed on animals and cell cultures. To improve the knowledge regarding the patients with ALI, it is suggested to do clinical trials by comparing the expression levels of endogenous Rvs and the proportion of them in patients with ALI whose disease course is different in terms of severity and duration.
Declaration of competing interest
Authors declare no conflict of interests.
Acknowledgment
The authors are thankful to Mashhad University of Medical Sciences for financial support.
References
- Abdulnour R.E., Sham H.P., Douda D.N., Colas R.A., Dalli J., Bai Y., Ai X., Serhan C.N., Levy B.D. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal Immunol. 2016;9:1278–1287. doi: 10.1038/mi.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedi F., Hayes A.W., Reiter R., Karimi G. Acute lung injury: the therapeutic role of Rho kinase inhibitors. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104736. [DOI] [PubMed] [Google Scholar]
- Abedi F., Rezaee R., Karimi G. Plausibility of therapeutic effects of Rho kinase inhibitors against severe acute respiratory syndrome coronavirus 2 (COVID-19) Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathina S., Das U.N. Resolvin D1 decreases severity of streptozotocin-induced type 1 diabetes mellitus by enhancing BDNF levels, reducing oxidative stress, and suppressing inflammation. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22041516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathina S., Gundala N.K.V., Rhenghachar P., Polavarapu S., Hari A.D., Sadananda M., Das U.N. Resolvin D1 ameliorates nicotinamide-streptozotocin-induced type 2 diabetes mellitus by its anti-inflammatory action and modulating PI3K/Akt/mTOR pathway in the brain. Arch. Med. Res. 2020;51:492–503. doi: 10.1016/j.arcmed.2020.05.002. [DOI] [PubMed] [Google Scholar]
- Bernard G.R., Artigas A., Brigham K.L., Carlet J., Falke K., Hudson L., Lamy M., LeGall J.R., Morris A., Spragg R. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20:225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- Bersten A.D., Edibam C., Hunt T., Moran J. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am. J. Respir. Crit. Care Med. 2002;165:443–448. doi: 10.1164/ajrccm.165.4.2101124. [DOI] [PubMed] [Google Scholar]
- Bhat T.A., Kalathil S.G., Bogner P.N., Lehmann P.V., Thatcher T.H., Sime P.J., Thanavala Y. AT-RvD1 mitigates secondhand smoke-exacerbated pulmonary inflammation and restores secondhand smoke-suppressed antibacterial immunity. J. Immunol. 2021;206:1348–1360. doi: 10.4049/jimmunol.2001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat T.A., Kalathil S.G., Miller A., Thatcher T.H., Sime P.J., Thanavala Y. Specialized proresolving mediators overcome immune suppression induced by exposure to secondhand smoke. J. Immunol. 2020;205:3205–3217. doi: 10.4049/jimmunol.2000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard L., Slutsky A., Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am. J. Respir. Crit. Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- Bu Y., Shih K.C., Kwok S.S., Chan Y.K., Lo A.C., Chan T.C.Y., Jhanji V., Tong L. Experimental modeling of cornea wound healing in diabetes: clinical applications and beyond. BMJ Open Diabet. Res. Care. 2019;7 doi: 10.1136/bmjdrc-2019-000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Jr., Phillips O., Fukumoto J., Fukumoto I., Parthasarathy P.T., Arias S., Cho Y., Lockey R.F., Kolliputi N. Enhanced resolution of hyperoxic acute lung injury as a result of aspirin triggered resolvin D1 treatment. Am. J. Respir. Cell Mol. Biol. 2015;53:422–435. doi: 10.1165/rcmb.2014-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Jr., Phillips O., Fukumoto J., Fukumoto I., Tamarapu Parthasarathy P., Mandry M., Cho Y., Lockey R., Kolliputi N. Resolvins decrease oxidative stress mediated macrophage and epithelial cell interaction through decreased cytokine secretion. PloS One. 2015;10 doi: 10.1371/journal.pone.0136755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt D.A., Hodges R.R., Serhan C.N. Immunoresolvent resolvin D1 maintains the health of the ocular surface. Adv. Exp. Med. Biol. 2019;1161:13–25. doi: 10.1007/978-3-030-21735-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarquoy J., Borgne F.L. Biosynthesis, metabolism and function of protectins and resolvins. Clin. Lipidol. 2014;9:683–693. [Google Scholar]
- Dominguez E.C., Heires A.J., Pavlik J., Larsen T.D., Guardado S., Sisson J.H., Baack M.L., Romberger D.J., Nordgren T.M. A high docosahexaenoic acid diet alters the lung inflammatory response to acute dust exposure. Nutrients. 2020;12 doi: 10.3390/nu12082334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domscheit H., Hegeman M.A., Carvalho N., Spieth P.M. Molecular dynamics of lipopolysaccharide-induced lung injury in rodents. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.00036. 36-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickmeier O., Seki H., Haworth O., Hilberath J.N., Gao F., Uddin M., Croze R.H., Carlo T., Pfeffer M.A., Levy B.D. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 2013;6:256–266. doi: 10.1038/mi.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frossard J. Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- Gao J., Wang S., Dong X., Leanse L.G., Dai T., Wang Z. Co-delivery of resolvin D1 and antibiotics with nanovesicles to lungs resolves inflammation and clears bacteria in mice. Commun. Biol. 2020;3:1–13. doi: 10.1038/s42003-020-01410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Wang S., Dong X., Leanse L.G., Dai T., Wang Z. Co-delivery of resolvin D1 and antibiotics with nanovesicles to lungs resolves inflammation and clears bacteria in mice. Commun. Biol. 2020;3:680. doi: 10.1038/s42003-020-01410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Lamont G.J., Lamont R.J., Uriarte S.M., Wang H., Scott D.A. Resolvin D1, resolvin D2 and maresin 1 activate the GSK3β anti-inflammatory axis in TLR4-engaged human monocytes. Innate Immun. 2016;22:186–195. doi: 10.1177/1753425916628618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guice K.S., Oldham K.T., Johnson K.J., Kunkel R.G., Morganroth M.L., Ward P.A. Pancreatitis-induced acute lung injury. An ARDS model. Ann. Surg. 1988;208:71. doi: 10.1097/00000658-198807000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R.-F., Ward P.A. Mediators and regulation of neutrophil accumulation in inflammatory responses in lung: insights from the IgG immune complex model. Free Radic. Biol. Med. 2002;33:303–310. doi: 10.1016/s0891-5849(02)00823-7. [DOI] [PubMed] [Google Scholar]
- Gyawali B., Ramakrishna K., Dhamoon A.S. Sepsis: the evolution in definition, pathophysiology, and management. SAGE Open Med. 2019;7 doi: 10.1177/2050312119835043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiram R., Xiong F., Naud P., Xiao J., Sirois M., Tanguay J.F., Tardif J.C., Nattel S. The inflammation-resolution promoting molecule resolvin-D1 prevents atrial proarrhythmic remodeling in experimental right heart disease. Cardiovasc. Res. 2020;117(7):1776–1789. doi: 10.1093/cvr/cvaa186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Shen H., Wang Y., Zhang L., Zhao M. Aspirin-triggered resolvin D1 alleviates paraquat-induced acute lung injury in mice. Life Sci. 2019;218:38–46. doi: 10.1016/j.lfs.2018.12.028. [DOI] [PubMed] [Google Scholar]
- Iranshahy M., Iranshahi M., Abtahi S.R., Karimi G. The role of nuclear factor erythroid 2-related factor 2 in hepatoprotective activity of natural products: a review. Food Chem. Toxicol. : Int. J. Publ. Br. Industr. Biol. Res. Assoc. 2018;120:261–276. doi: 10.1016/j.fct.2018.07.024. [DOI] [PubMed] [Google Scholar]
- Kallet R.H., Matthay M.A. Hyperoxic acute lung injury. Respir. Care. 2013;58:123–141. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeris T., Baines C., Krenz M., Korthuis R. Ischemia/reperfusion. Comp. Physiol. 2016;7:113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbasforooshan H., Karimi G. The role of SIRT1 in diabetic retinopathy. Biomed. Pharmacother. 2018;97:190–194. doi: 10.1016/j.biopha.2017.10.075. [DOI] [PubMed] [Google Scholar]
- Kasper D., Fauci A., Hauser S., Longo D., Jameson J., Loscalzo J. vol. 19e. Mcgraw-hill; 2015. (Harrison's Principles of Internal Medicine). [Google Scholar]
- Kohli P., Levy B.D. Resolvins and protectins: mediating solutions to inflammation. Br. J. Pharmacol. 2009;158:960–971. doi: 10.1111/j.1476-5381.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashia P., Cordella A., Nobili A., La Barbera L., Federici M., Leuti A., Campanelli F., Natale G., Marino G., Calabrese V., Vedele F., Ghiglieri V., Picconi B., Di Lazzaro G., Schirinzi T., Sancesario G., Casadei N., Riess O., Bernardini S., Pisani A., Calabresi P., Viscomi M.T., Serhan C.N., Chiurchiù V., D'Amelio M., Mercuri N.B. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson's disease. Nat. Commun. 2019;10:3945. doi: 10.1038/s41467-019-11928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani H.V., Pillai S.S., Zehra M., Sharma I., Sodhi K. Systematic review of clinical insights into novel coronavirus (CoVID-19) pandemic: persisting challenges in US rural population. Int. J. Environ. Res. Publ. Health. 2020;17:4279. doi: 10.3390/ijerph17124279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z., Dong J., Wu W., Yang T., Wang T., Guo L., Chen L., Xu D., Wen F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARγ/NF-κB pathway. Respir. Res. 2012;13:110. doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C.S., Porter D.W., Orandle M.S., Green B.J., Barnes M.A., Croston T.L., Wolfarth M.G., Battelli L.A., Andrew M.E., Beezhold D.H., Siegel P.D., Ma Q. Resolution of pulmonary inflammation induced by carbon nanotubes and fullerenes in mice: role of macrophage polarization. Front. Immunol. 2020;11:1186. doi: 10.3389/fimmu.2020.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhou D., Long F.W., Chen K.L., Yang H.W., Lv Z.Y., Zhou B., Peng Z.H., Sun X.F., Li Y., Zhou Z.G. Resolvin D1 protects against inflammation in experimental acute pancreatitis and associated lung injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;310:G303–G309. doi: 10.1152/ajpgi.00355.2014. [DOI] [PubMed] [Google Scholar]
- Lu Y.-C., Yeh W.-C., Ohashi P.S. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Luan H., Wang C., Sun J., Zhao L., Li L., Zhou B., Shao S., Shen X., Xu Y. Resolvin D1 protects against ischemia/reperfusion-induced acute kidney injury by increasing Treg percentages via the ALX/FPR2 pathway. Front. Physiol. 2020;11:285. doi: 10.3389/fphys.2020.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C.R., Zaman M.M., Gilkey C., Salguero M.V., Hasturk H., Kantarci A., Van Dyke T.E., Freedman S.D. Resolvin D1 and lipoxin A4 improve alveolarization and normalize septal wall thickness in a neonatal murine model of hyperoxia-induced lung injury. PloS One. 2014;9 doi: 10.1371/journal.pone.0098773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019;5:1–22. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa K., Fukunaga H., Tatewaki Y., Takano Y., Yamamoto S., Mutoh T., Taki Y. Alzheimer's disease and specialized pro-resolving lipid mediators: do MaR1, RvD1, and NPD1 show promise for prevention and treatment? Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21165783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaei, E., Molaei, A., Abedi, F., Hayes, A.W., Karimi, G., Nephroprotective activity of natural products against chemical toxicants: the role of Nrf2/ARE signaling pathway. Food Sci. Nutr. (n/a). [DOI] [PMC free article] [PubMed]
- Nieman G., Zerler B. A role for the anti-inflammatory properties of tetracyclines in the prevention of acute lung injury. Curr. Med. Chem. 2001;8:317–325. doi: 10.2174/0929867013373570. [DOI] [PubMed] [Google Scholar]
- Norling L.V., Dalli J., Flower R.J., Serhan C.N., Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory Loci. Arterioscler. Thromb. Vasc. Biol. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özgül Özdemir R.B., Soysal Gündüz Ö., Özdemir A.T., Akgül Ö. Low levels of pro-resolving lipid mediators lipoxin-A4, resolvin-D1 and resolvin-E1 in patients with rheumatoid arthritis. Immunol. Lett. 2020;227:34–40. doi: 10.1016/j.imlet.2020.08.006. [DOI] [PubMed] [Google Scholar]
- Pascarella G., Strumia A., Piliego C., Bruno F., Del Buono R., Costa F., Scarlata S., Agrò F.E. COVID-19 diagnosis and management: a comprehensive review. J. Intern. Med. 2020;288:192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payrits M., Horváth Á., Biró-Sütő T., Erostyák J., Makkai G., Sághy É., Pohóczky K., Kecskés A., Kecskés M., Szolcsányi J., Helyes Z., Szőke É. Resolvin D1 and D2 inhibit transient receptor potential vanilloid 1 and ankyrin 1 ion channel activation on sensory neurons via lipid raft modification. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21145019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendran K., Nemzek J., Napolitano L.M., Knight P.R. Aspiration-induced lung injury. Crit. Care Med. 2011;39:818. doi: 10.1097/CCM.0b013e31820a856b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regidor P.A., Mueller A., Sailer M., Gonzalez Santos F., Rizo J.M., Egea F.M. Chronic inflammation in PCOS: the potential benefits of specialized pro-resolving lipid mediators (SPMs) in the improvement of the resolutive response. Int. J. Mol. Sci. 2020;22 doi: 10.3390/ijms22010384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert T., Baldotto C.S.d.R., Nunes F.A.P., Scheliga A.A.d.S. Bleomycin-induced lung injury. J. Canc. Res. 2013 2013. [Google Scholar]
- Riahi B., Rafatpanah H., Mahmoudi M., Memar B., Fakhr A., Tabasi N., Karimi G. Evaluation of suppressive effects of paraquat on innate immunity in Balb/c mice. J. Immunot. 2011;8:39–45. doi: 10.3109/1547691X.2010.543095. [DOI] [PubMed] [Google Scholar]
- Ringholz F.C., Higgins G., Hatton A., Sassi A., Moukachar A., Fustero-Torre C., Hollenhorst M., Sermet-Gaudelus I., Harvey B.J., McNally P., Urbach V. Resolvin D1 regulates epithelial ion transport and inflammation in cystic fibrosis airways. J. Cyst. Fibros. : Off. J. Eur. Cystic Fibrosis Soc. 2018;17:607–615. doi: 10.1016/j.jcf.2017.11.017. [DOI] [PubMed] [Google Scholar]
- Rymut N., Heinz J., Sadhu S., Hosseini Z., Riley C.O., Marinello M., Maloney J., MacNamara K.C., Spite M., Fredman G. Resolvin D1 promotes efferocytosis in aging by limiting senescent cell-induced MerTK cleavage. Faseb. J. : Off. Publ. Feder. Am. Soc. Exper. Biol. 2020;34:597–609. doi: 10.1096/fj.201902126R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekheri M., El Kebir D., Edner N., Filep J.G. 15-Epi-LXA(4) and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation. Proc. Natl. Acad. Sci. U. S. A. 2020;117:7971–7980. doi: 10.1073/pnas.1920193117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham H.P., Walker K.H., Abdulnour R.E., Krishnamoorthy N., Douda D.N., Norris P.C., Barkas I., Benito-Figueroa S., Colby J.K., Serhan C.N., Levy B.D. 15-epi-Lipoxin A(4), resolvin D2, and resolvin D3 induce NF-κB regulators in bacterial pneumonia. J. Immunol. 2018;200:2757–2766. doi: 10.4049/jimmunol.1602090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M., Kibi M., Riley I.R., Chiang N., Dalli J., Kraft B.D., Piantadosi C.A., Choi A.M., Serhan C.N. Cell-cell interactions and bronchoconstrictor eicosanoid reduction with inhaled carbon monoxide and resolvin D1. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307:L746–L757. doi: 10.1152/ajplung.00166.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E.R., Croze R.H., Fang X., Matthay M.A., Gotts J.E. Inhibition of the lipoxin A4 and resolvin D1 receptor impairs host response to acute lung injury caused by pneumococcal pneumonia in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2021;320:L1085–l1092. doi: 10.1152/ajplung.00046.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Guo R.-F., Gao H., Sarma J.V., Zetoune S.F., Ward P.A. Attenuation of IgG immune complex-induced acute lung injury by silencing C5aR in lung epithelial cells. Faseb. J. 2009;23:3808–3818. doi: 10.1096/fj.09-133694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Wang F., Yang Y., Wang J., Sun S., Xia H., Yao S. Resolvin D1 attenuates ventilator-induced lung injury by reducing HMGB1 release in a HO-1-dependent pathway. Int. Immunopharm. 2019;75 doi: 10.1016/j.intimp.2019.105825. [DOI] [PubMed] [Google Scholar]
- Tang H., Liu Y., Yan C., Petasis N.A., Serhan C.N., Gao H. Protective actions of aspirin-triggered (17R) resolvin D1 and its analogue, 17R-hydroxy-19-para-fluorophenoxy-resolvin D1 methyl ester, in C5a-dependent IgG immune complex-induced inflammation and lung injury. J. Immunol. 2014;193:3769–3778. doi: 10.4049/jimmunol.1400942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannitamby A., Saad M.I., Aloe C., Wang H., Kumar B., Vlahos R., Selemidis S., Irving L., Steinfort D., Jenkins B.J., Bozinovski S. Aspirin-triggered resolvin D1 reduces proliferation and the neutrophil to lymphocyte ratio in a mutant KRAS-driven lung adenocarcinoma model. Cancers. 2021;13 doi: 10.3390/cancers13133224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Gong X., Wan J.Y., Zhang L., Zhang Z., Li H.Z., Min S. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm. Pharmacol. Therapeut. 2011;24:434–441. doi: 10.1016/j.pupt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Wang L., Yuan R., Yao C., Wu Q., Christelle M., Xie W., Zhang X., Sun W., Wang H., Yao S. Effects of resolvin D1 on inflammatory responses and oxidative stress of lipopolysaccharide-induced acute lung injury in mice. Chin. Med. J. 2014;127:803–809. [PubMed] [Google Scholar]
- Wang Q., Yan S.-F., Hao Y., Jin S.-W. Specialized pro-resolving mediators regulate alveolar fluid clearance during acute respiratory distress syndrome. Chin. Med. J. 2018;131:982. doi: 10.4103/0366-6999.229890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zheng X., Cheng Y., Zhang Y.L., Wen H.X., Tao Z., Li H., Hao Y., Gao Y., Yang L.M., Smith F.G., Huang C.J., Jin S.W. Resolvin D1 stimulates alveolar fluid clearance through alveolar epithelial sodium channel, Na,K-ATPase via ALX/cAMP/PI3K pathway in lipopolysaccharide-induced acute lung injury. J. Immunol. 2014;192:3765–3777. doi: 10.4049/jimmunol.1302421. [DOI] [PubMed] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. Jama. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Wu D., Zheng S., Li W., Yang L., Liu Y., Zheng X., Yang Y., Yang L., Wang Q., Smith F.G., Jin S. Novel biphasic role of resolvin D1 on expression of cyclooxygenase-2 in lipopolysaccharide-stimulated lung fibroblasts is partly through PI3K/AKT and ERK2 pathways. Mediat. Inflamm. 2013 doi: 10.1155/2013/964012. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Wang J., Sun S., Wang F., Yang Y., Chen L., Sun Z., Yao S. Resolvin D1 alleviates ventilator-induced lung injury in mice by activating PPARγ/NF-κB signaling pathway. BioMed Res. Int. 2019 doi: 10.1155/2019/6254587. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Wang H., Wang L., Yao C., Yuan R., Wu Q. Resolvin D1 reduces deterioration of tight junction proteins by upregulating HO-1 in LPS-induced mice. Lab. Investig. J. Techn. Methods Pathol. 2013;93:991–1000. doi: 10.1038/labinvest.2013.80. [DOI] [PubMed] [Google Scholar]
- Xu L., Xu J., Wang Z. Molecular mechanisms of paraquat-induced acute lung injury: a current review. Drug Chem. Toxicol. 2014;37:130–134. doi: 10.3109/01480545.2013.834361. [DOI] [PubMed] [Google Scholar]
- Yang Y., Hu L., Xia H., Chen L., Cui S., Wang Y., Zhou T., Xiong W., Song L., Li S., Pan S., Xu J., Liu M., Xiao H., Qin L., Shang Y., Yao S. Resolvin D1 attenuates mechanical stretch-induced pulmonary fibrosis via epithelial-mesenchymal transition. Am. J. Physiol. Lung Cell Mol. Physiol. 2019;316:L1013–l1024. doi: 10.1152/ajplung.00415.2018. [DOI] [PubMed] [Google Scholar]
- Yatomi M., Hisada T., Ishizuka T., Koga Y., Ono A., Kamide Y., Seki K., Aoki-Saito H., Tsurumaki H., Sunaga N., Kaira K., Dobashi K., Yamada M., Okajima F. 17(R)-resolvin D1 ameliorates bleomycin-induced pulmonary fibrosis in mice. Physiol. Rep. 2015;3 doi: 10.14814/phy2.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaxin W., Shanglong Y., Huaqing S., Hong L., Shiying Y., Xiangdong C., Ruidong L., Xiaoying W., Lina G., Yan W. Resolvin D1 attenuates lipopolysaccharide induced acute lung injury through CXCL-12/CXCR4 pathway. J. Surg. Res. 2014;188:213–221. doi: 10.1016/j.jss.2013.11.1107. [DOI] [PubMed] [Google Scholar]
- Yaxin W., Shanglong Y., Huaqing S., Hong L., Shiying Y., Xiangdong C., Ruidong L., Xiaoying W., Lina G., Yan W. Corrigendum to resolvin D1 attenuates lipopolysaccharide induced acute lung injury through CXCL-12/CXCR4 pathway. J. Surg. Res. 2020;252:285. doi: 10.1016/j.jss.2020.03.049. [J Surg Res. 2014 may 1;188(1):213-221] [DOI] [PubMed] [Google Scholar]
- Yellepeddi V.K., Parashar K., Dean S.M., Watt K.M., Constance J.E., Baker O.J. Predicting resolvin D1 pharmacokinetics in humans with physiologically-based pharmacokinetic modeling. Clin. Transl. Sci. 2021;14:683–691. doi: 10.1111/cts.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorek M.A. The potential role of fatty acids in treating diabetic neuropathy. Curr. Diabetes Rep. 2018;18:86. doi: 10.1007/s11892-018-1046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A., Eroglu A., Erturk E., Ince C., Westphal M. Hindawi; 2014. Ischemia-reperfusion Injury and Anesthesia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Meredith T.C., Kahne D. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr. Opin. Microbiol. 2013;16:779–785. doi: 10.1016/j.mib.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.W., Wang Q., Mei H.X., Zheng S.X., Ali A.M., Wu Q.X., Ye Y., Xu H.R., Xiang S.Y., Jin S.W. RvD1 ameliorates LPS-induced acute lung injury via the suppression of neutrophil infiltration by reducing CXCL2 expression and release from resident alveolar macrophages. Int. Immunopharm. 2019;76 doi: 10.1016/j.intimp.2019.105877. [DOI] [PubMed] [Google Scholar]
- Zhang S.K., Zhuo Y.Z., Li C.X., Yang L., Gao H.W., Wang X.M. Xuebijing injection. and resolvin D1 synergize regulate leukocyte adhesion and improve survival rate in mice with sepsis-induced lung injury. Chin. J. Integr. Med. 2018;24:272–277. doi: 10.1007/s11655-017-2959-x. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Wu J., Hua Q., Lin Z., Ye L., Zhang W., Wu G., Du J., Xia J., Chu M., Hu X. Resolvin D1 mitigates energy metabolism disorder after ischemia-reperfusion of the rat lung. J. Transl. Med. 2016;14:81. doi: 10.1186/s12967-016-0835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Wu J., Lin Z., Hua Q., Zhang W., Ye L., Wu G., Du J., Xia J., Chu M., Hu X. Resolvin D1 alleviates the lung ischemia reperfusion injury via complement, immunoglobulin, TLR4, and inflammatory factors in rats. Inflammation. 2016;39:1319–1333. doi: 10.1007/s10753-016-0364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Wang Q., D'Souza V., Bartis D., Dancer R., Parekh D., Gao F., Lian Q., Jin S., Thickett D.R. ResolvinD(1) stimulates epithelial wound repair and inhibits TGF-β-induced EMT whilst reducing fibroproliferation and collagen production. Lab. Investig. J. Techn. Methods Pathol. 2018;98:130–140. doi: 10.1038/labinvest.2017.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Lee H.N., Surh Y.J. RvD1 inhibits TNFα-induced c-Myc expression in normal intestinal epithelial cells and destabilizes hyper-expressed c-Myc in colon cancer cells. Biochem. Biophys. Res. Commun. 2018;496:316–323. doi: 10.1016/j.bbrc.2017.12.171. [DOI] [PubMed] [Google Scholar]
- Zhuo Y., Zhang S., Li C., Yang L., Gao H., Wang X. Resolvin D1 promotes SIRT1 expression to counteract the activation of STAT3 and NF-κB in mice with septic-associated lung injury. Inflammation. 2018;41:1762–1771. doi: 10.1007/s10753-018-0819-2. [DOI] [PubMed] [Google Scholar]