Abstract
Abstract
Background
Diabetes Mellitus (DM) is a major cause of maternal, fetal, and neonatal morbidities. Our objective was to estimate the effect of both pre-pregnancy and gestational DM on the growth parameters of newborns in the Qatari population.
Methods
In this population-based cohort study, we compared the data of neonates born to Qatari women with both pre-pregnancy and gestational diabetes mellitus in 2017 with neonates of healthy non-diabetic Qatari women.
Results
Out of a total of 17020 live births in 2017, 5195 newborns were born to Qatari women. Of these, 1260 were born to women with GDM, 152 were born to women with pre-pregnancy DM and 3783 neonates were born to healthy non-diabetic (control) women. The prevalence of GDM in the Qatari population in 2017 was 24.25%. HbA1C% before delivery was significantly higher in women with pre-pregnancy DM (mean 6.19 ± 1.15) compared to those with GDM (mean 5.28 ± 0.43) (P <0.0001). The mean birth weight in grams was 3066.01 ± 603.42 in the control group compared to 3156.73 ± 577.88 in infants born to women with GDM and 3048.78 ± 677.98 in infants born to women with pre-pregnancy DM (P <0.0001). There was no statistically significant difference regarding the mean length (P= 0.080), head circumference (P= 0.514), and rate of major congenital malformations (P= 0.211). Macrosomia (Birth weight > 4000 gm) was observed in 2.7% of the control group compared to 4.8% in infants born to women with GDM, and 4.6% in infants born to women with pre-pregnancy DM (P= 0.001). Multivariate logistic regression analysis demonstrated that higher maternal age (adjusted OR 2.21, 95% CI 1.93, 2.52, P<0.0001), obesity before pregnancy (adjusted OR 1.71, 95% CI 1.30, 2.23, P<0.0001), type of delivery C-section (adjusted OR 1.25, 95% CI 1.09, 1.44, P=0.002), and body weight to gestational age LGA (adjusted OR 2.30, 95% CI 1.64, 2.34, P<0.0001) were significantly associated with increased risk of GDM.
Conclusion
Despite the multi-disciplinary antenatal diabetic care management, there is still an increased birth weight and an increased prevalence of macrosomia among the infants of diabetic mothers. More efforts should be addressed to improve the known modifiable factors such as women's adherence to the diabetic control program. Furthermore, pre-pregnancy BMI was found to be significantly associated with gestational DM, and this is a factor that can be addressed during pre-conceptional counseling.
Keywords: Gestational Diabetes Mellitus, Women, Newborn, Infant of Diabetic Mother, Qatari
Background
Gestational Diabetes Mellitus (GDM) occurs in 2-9% of pregnant women worldwide and is defined as “any degree of glucose intolerance with onset or first recognition during pregnancy” [1]. During pregnancy, the placenta secretes certain diabetogenic hormones including growth hormone, corticotropin-releasing hormone, human placental lactogen, prolactin, and progesterone. Moreover, pregnancy is also associated with insulin resistance. If this insulin resistance is paired with insufficient pancreatic function, the risk of developing GDM increases [1, 2].
GDM is a major cause of pregnancy-related maternal morbidities [3]. Infants of women with diabetes mellitus (DM) have an increased risk for both large for gestational age (LGA) and preterm birth (PTB) compared with infants born to women without DM [2, 3]. Moreover, they have an increased risk of neonatal complications such as cardiovascular (CVS) and central nervous system (CNS) defects, hyperbilirubinemia, low iron stores, perinatal asphyxia, respiratory distress syndrome (RDS), hypoglycemia, hypocalcemia, polycythemia, transient hypertrophic cardiomyopathy [4–7], and macrosomia with its subsequent complications [8–12].
Due to the increasing prevalence of DM in Qatar, we wanted to revisit its impact on both maternal and neonatal populations by conducting this retrospective research study. Hence, we aimed to study the effects of both pre-pregnancy and gestational DM on growth parameters of neonates in the Qatari newborns and compare them with those of non-diabetic women (healthy control) in the same population.
Patients and methods
The setting for this one-year population-based cohort study was the Neonatal Intensive Care Unit (NICU) of Women’s Wellness and Research Center (WWRC) in Hamad Medical Corporation (HMC), after getting the ethical/Institutional Review Board (IRB) approval from the Medical Research Center under the number MRC-01-18-041. WWRC is a large tertiary center in Doha, Qatar, with a delivery rate of over 18,000 per year. This study was conducted following institutional policies and Good Research Practice (GRP). All methods were performed following the relevant guidelines and regulations [13–15].
Data were collected between January 1, 2017, and December 31, 2017. It comprised women’s age, pre-pregnancy body mass index (BMI), gestational age at birth, placental weight, neonatal growth parameters (weight, length, and head circumference), as well as the presence or absence of major congenital malformations (CNS, CVS or gastrointestinal anomalies).
The study data were collected by the research team members from electronic patient records and clinical documentation. All collected data were kept in an excel sheet on a password-secured computer in the principal investigator's office and the principal investigator had full controlled access to the study data as per institution and ethical policies. All data were collected using anonymized format and no patient identifications were disclosed.
The target population was Qatari women with DM, either gestational or pre-pregnancy and compared them with those born to healthy non-diabetic Qatari women. We looked at the last HbA1C% (Glycated Hemoglobin) before delivery in women with GDM and those who had pre-pregnancy DM to get an idea about glycemic control in the preceding 3 months. The data of the pre-pregnancy weight were collected from the electronic patient records. For neonatal growth parameters, we used the 2013 revised Fenton growth charts standards for comparison among the groups. As per those charts, we defined SGA as < 10th percentile for weight and LGA as > 90th percentile for weight [16].
The screening, diagnosis, and management of GDM in our hospital is usually conducted by the Diabetic Team. The Diabetic Team comprises of 5 endocrinologists, 10 obstetricians, 1 ophthalmologist, 2 diabetic educators, 2 dietitians, and 6 diabetic nurses. Medical institutions in the State of Qatar screen all pregnant ladies for diabetes at the first antenatal care visit and pregnant ladies are classified accordingly. That screening is based on the 2013-WHO Criteria. It states that gestational diabetes mellitus should be diagnosed at any time in pregnancy if one or more of the following criteria are met: fasting plasma glucose 5.1-6.9 mmol/l (92 -125 mg/dl), 1-hour plasma glucose ≥ 10.0 mmol/l (180 mg/dl) following a 75g oral glucose load, or 2-hour plasma glucose 8.5-11.0 mmol/l (153 -199 mg/dl) following a 75g oral glucose load [17]. Pre-pregnancy DM was defined by either type I or type II DM before the index pregnancy. Healthy controls are women with neither Pre-pregnancy DM nor GDM. All women with positive screening tests are referred to our diabetic team for ongoing management and monitoring of gestational diabetes [18].
Statistical analysis
Quantitative and categorical data were presented as mean ± standard deviation (SD) and frequencies (percentages). For variables that were normally distributed, differences in their mean values between two independent groups (IDM and non-IDM; major and no major congenital malformation, etc.) were compared using unpaired Student's t-test or Mann Whitney U tests as appropriate. Quantitative data between three independent groups were analyzed using one-way analysis of variance (ANOVA) or the Kruskal Wallis test as appropriate. In case of significant difference observed, the pairwise difference was compared using Bonferroni post-hoc test. Associations between two or more qualitative variables were assessed using the Chi-square (χ2) test or Fisher Exact test as appropriate. Pearson’s correlation coefficient was used to assess the strength of the linear relationship between maternal HbA1C%measured before delivery and fetal and maternal characteristics. Univariate and multivariate logistic regression analysis was applied to determine and assess the mothers’ potential risk factors and neonatal outcomes associated with the development of GDM adjusted for potential predictors and confounders such as mother’s age, BMI, type of delivery, gestational age, gender, placental weight, birth weight, macrosomia, bodyweight to gestational age, major congenital anomalies. For multivariate logistic regression models, predictor variables were considered if statistical P<0.10 level in univariate analysis or if determined a priori to be clinically important. The results of logistic regression analyses were presented as odds ratios (OR) with corresponding 95% confidence intervals (CI). Thereafter, we computed a prediction model to evaluate the discriminative ability of potentially significant variables with statistical P <0.10 on the occurrence of GDM. Pictorial presentations of the key results were made using appropriate statistical graphs. All P values presented were two-tailed, and P values <0.05 were considered statistically significant. All statistical analyses were performed using statistical packages SPSS version 27.0 (Armonk, NY: IBM Corp) and Epi-info (Centers for Disease Control and Prevention, Atlanta, GA) software.
Results
In 2017, there were 17020 live births from 16765 deliveries, including 255 multiple pregnancies. Out of 17020 live births, 5195 babies were to Qatari women; all of these were singletons. Of these 5195 babies, 1260 were born to women with GDM, 152 were born to women with pre-pregnancy DM and 3783 neonates were born to non-diabetic (healthy control) women. Our data shows that the prevalence rate of GDM was 24.25% (95% CI 23.1, 25.4) in Qatari women.
The mean birth weight (grams) was 3066.01 ± 603.42 in the healthy control group compared to 3156.73 ± 577.88 in infants born to women with GDM and 3048.78 ± 677.98 in infants born to women with pre-pregnancy DM (Overall P <0.0001). There was no statistically significant difference among the 3 groups regarding the mean length (P= 0.080), and head circumference (P= 0.514). Macrosomia (Birth weight >4000 gm) was observed in 2.7% of the control group compared to 4.8% of infants born to women with GDM, and 4.6% of infants born to women with pre-pregnancy DM (P= 0.001). The rate of LGA infants was 13.8% in infants born to women with pre-pregnancy DM, compared to 5.7% in infants born to women with GDM, and 2% in infants born to healthy non-diabetic women (P <0.0001). On the other hand, the rate of SGA babies was 16.4% in infants born to healthy non-diabetic women compared to 10.1% in infants born to women with GDM and 10.5% in infants born to women with pre-pregnancy DM (Table 1).
Table 1.
Maternal and neonatal characteristics of diabetic mothers and control cases
| Variables | GDM Cases (n=1260) |
Pre-pregnancy DM (n=152) |
Control Cases (n=3783) |
P-Value |
|---|---|---|---|---|
| Mother’s Age (years) | 31.59 ± 5.87 | 34.86 ± 6.04 | 28.82 ± 5.67 | <0.0001 |
| Body Mass Index | 33.80 ± 8.31 | 36.15 ± 6.56 | 31.67 ± 5.67 | <0.0001 |
| Maternal HbA1C (%) before delivery | 5.28 ± 0.43 | 6.19 ± 1.15 | <0.0001 | |
| Type of Delivery | <0.0001 | |||
| Vaginal Delivery | 751 (59.6%) | 41 (27%) | 2415 (63.8%) | |
| Cesarean Section | 447 (35.5%) | 105 (69.1%) | 1116 (29.5%) | |
| Instrumental Delivery | 62 (4.9%) | 6 (3.9%) | 252 (6.7%) | |
| Gestational Age (weeks) | 38.12 ± 2.04 | 36.71 ± 2.34 | 38.34 ± 2.57 | <0.0001 |
| Gender | 0.068 | |||
| Male | 663 (52.6%) | 65 (42.8%) | 1969 (52%) | |
| Female | 597 (47.4%) | 87 (57.2%) | 1814 (48%) | |
| Placental Weight (gm) | 664.70 ± 120.02 | 643.95 ± 126.42 | 650.19 ± 167.31 | 0.013 |
| Birth Weight (gm) | 3156.73 ± 577.88 | 3048.78 ± 677.98 | 3066.01 ± 603.42 | <0.0001 |
| Macrosomia (Weight >4000 gm at term) | 60 (4.8%) | 7 (4.6%) | 103 (2.7%) | 0.001 |
| Length (cm) | 49.85 ± 3.40 | 49.24 ± 3.30 | 49.73 ± 3.29 | 0.080 |
| Head Circumference (cm) | 34.12 ± 1.81 | 33.93 ± 2.13 | 34.05 ± 2.56 | 0.514 |
| Body weight to Gestational Age | ||||
| SGA | 127 (10.1%) | 16 (10.5%) | 617 (16.4%) | <0.0001 |
| AGA | 1061 (84.2%) | 115 (75.7%) | 3080 (81.7%) | 0.0104 |
| LGA | 72 (5.7%) | 21 (13.8%) | 74 (2%) | <0.0001 |
| Major Congenital Anomalies | 0.211 | |||
| No | 1252 (99.4%) | 149 (98%) | 3753 (99.2%) | |
| Yes | 8 (0.6%) | 3 (2%) | 30 (0.8%) | |
The mean gestational age (weeks) of neonates born to healthy non-diabetic women was significantly higher than those born to women with pre-pregnancy DM and GDM mothers (P <0.0001). The pre-pregnancy Body Mass Index (BMI) was significantly higher (P <0.0001) in women with pre-pregnancy DM (36.15 ± 6.56) and women with GDM (33.80 ± 8.31) compared to healthy non-diabetic women (31.67 ± 5.67). The placental weight (grams) was found to be significantly higher in women who had GDM (mean 664.70 ± 120.02) than the other 2 groups (P=0.013) (Table 1).
The rate of caesarian section, in healthy non-diabetic women, was significantly lower than in women with pre-pregnancy DM and women with GDM (P <0.0001). On the other hand, instrumental delivery was significantly higher in the control group compared to the other groups (P <0.0001). (Table 1).
A total of 41 infants were born with major congenital malformations in 2017, of Qatari women. Interestingly, major congenital malformations were seen in only 0.6% of the infants of women with GDM, compared to 0.8% in healthy control, and 2% of infants born to women with pre-pregnancy DM (P= 0.211) (Table 1). Those anomalies included 26 cases with Congenital Heart Disease (CHD), 9 cases of GIT malformations, 3 cases of CNS malformations, and 3 cases of multiple congenital anomalies. The presence of congenital malformation was not significantly associated with any of the neonatal or maternal characteristics (Table 2).
Table 2.
Maternal and neonatal characteristics of infants born with and without major congenital malformation
| Variables | Presence of major congenital malformation (n=41) |
No major congenital malformation (n=5154) |
P-Value |
|---|---|---|---|
| Mother’s Age (years) | 31.46 ± 6.70 | 29.66 ± 5.90 | 0.51 |
| DM Status | 0.211 | ||
| GDM | 8 (0.6%) | 1252 (99.4%) | |
| Pre-pregnancy DM | 3 (2%) | 149 (98%) | |
| Controls | 30 (0.8%) | 3753 (99.2%) | |
| Body Mass Index | 33.70 ± 6.37 | 32.31 ± 6.53 | 0.180 |
| Maternal HbA1C (%) before delivery | 5.66 ± 0.49 | 5.40 ± 0.65 | 0.202 |
| Type of Delivery | 0.446 | ||
| VD | 24 (0.7%) | 3183 (99.3%) | |
| CS | 16 (1%) | 1652 (99 %) | |
| ID | 1 (0.3%) | 319 (99.7%) | |
| Gestational Age (weeks) | 38.78 ± 1.351 | 38.23 ± 2.470 | 0.157 |
| Gender | 0.687 | ||
| Male | 20 (0.7%) | 2677 (99.3%) | |
| Female | 21 (0.8%) | 2477 (0.8%) | |
| Placental Weight (gm) | 645.98 ± 111.72 | 653.58 ± 156.46 | 0.756 |
| Birth Weight (gm) | 3142.56 ± 527.60 | 3087.08 ± 601.40 | 0.556 |
| Length (cm) | 49.61 ± 3.33 | 49.75 ± 3.32 | 0.789 |
| Head Circumference (cm) | 34.05 ± 1.82 | 34.06 ± 2.39 | 0.974 |
| Body weight to Gestational Age | 0.960 | ||
| SGA | 6 (0.8%) | 754 (99.2%) | |
| AGA | 4 (0.8%) | 4222 (99.2%) | |
| LGA | 1 (0.6%) | 166 (99.4%) |
The mean maternal glycosylated hemoglobin (HbA1C%%) before delivery was 6.19 ± 1.15 in women with pre-pregnancy DM, compared to 5.28 ± 0.43 in women with GDM (P <0.0001). Pearson correlation analysis showed maternal glycosylated hemoglobin (HbA1C%%) before delivery had a significantly high positive correlation with the mother’s age (Pearson r= 0.70, P=0.017). However, both BMI (Pearson r =0.12, P<0.001) birth weight (Pearson r =0.11, P<0.001) had a significant but weakly positive correlation with maternal HbA1C%. In contrast, the correlation between gestational age and maternal HbA1C% showed an inverse and weak correlation (Pearson r = -0.13, P<0.0001).
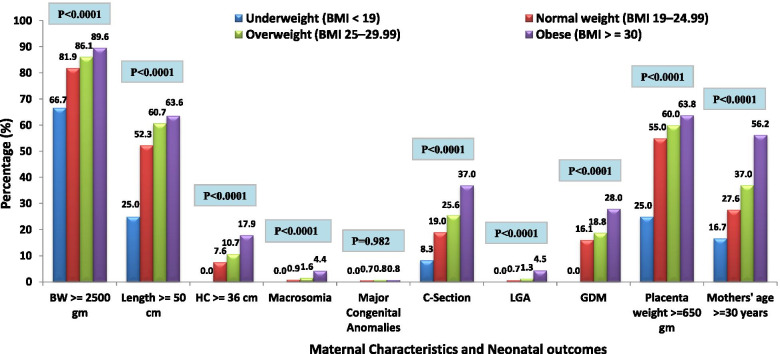
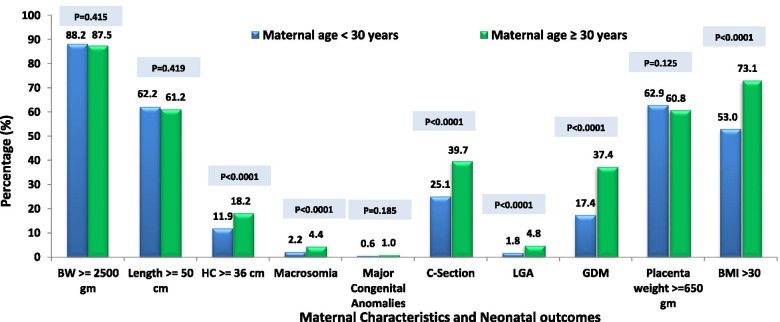
There were significant associations between maternal characteristics and pregnancy and neonatal outcomes across various BMI categories. A significant increasing trend was observed when compared to both maternal and neonatal outcomes in obese and overweight with those in normal and underweight groups (P<0.0001). However, pre-pregnancy overweight and obesity didn’t affect major congenital anomalies (P=0.982) (Fig. 1). As depicted in Fig. 2, there were significant associations observed between maternal characteristics and pregnancy and neonatal outcomes with maternal age categories. Maternal age ≥ 30 years was significantly associated with a higher rate of HC ≥ 36 cm, macrosomia, C-section, LGA, GDM, and BMI>30 (P<0.0001). Whereas birth weight, length, major congenital anomalies, and placental weight showed insignificant differences between maternal age categories (P>0.05) shown in Fig. 2.
Fig. 1.
Maternal Characteristics and Pregnancy and Neonatal outcomes across various BMI categories
Fig. 2.
Maternal Characteristics and Pregnancy and Neonatal outcomes across maternal age categories
The results of univariate and multivariate logistic regression analysis testing for each predictor and their possible association with GDM and neonatal outcomes are presented in Tables 3 and 4. Compared to mothers of normal weight (BMI between 19 and 24.99), noted before pregnancy or at the first visit, mothers with a BMI between 25 to <30, had an increased risk of developing GDM (unadjusted OR 1.21, 95% CI 0.91, 1.61; P=0.192). The risk of developing GDM in women who were obese before pregnancy was two folds higher than that in the group of normal weight (unadjusted OR 2.12, 95% CI 1.62, 2.76, P<0.0001). The higher maternal age (≥30 years) was significantly associated with the increased risk of developing GDM compared to the age group <30 years (unadjusted OR 2.38, 95% CI 2.09, 2.71, P<0.0001). When examined for the association between GDM (Table 3) and birth weight, higher birth weight (≥2500 gm) (unadjusted OR 1.39, 95% CI 1.13, 1.72; P=0.002) was significantly higher in the GDM group compared to control group. The presence of GDM significantly increases the risk of C-sections (unadjusted OR 1.29, 95% CI 1.12, 1.48; P<0.0001) however, the difference in instrumental delivery in the two groups were statistically insignificant (P=0.113). Women with GDM delivered babies with a higher proportion (approximately three-fold) of ‘large for gestational age’ infants than women with AGA (unadjusted OR 2.82, 95% CI 2.03, 3.94; P<0.0001). The newborns of women with GDM were at a twofold increased risk of being macrosomic (unadjusted OR 1.79, 95% CI 1.29, 2.47, P<0.0001). We did not observe that GDM significantly influenced gestational age at birth (≥37 weeks) and major congenital anomalies (P>0.05) as shown in Table 3.
Table 3.
Factors associated with GDM: Univariate Logistic regression analysis
| Variables | GDM n/N (%) |
Unadjusted Odds ratio (OR) |
95% CI for OR | P-value |
|---|---|---|---|---|
| Mother’s Age (years) | ||||
| <30 | 463/2658 (17.4%) | 1.0 (reference) | ||
| ≥30 | 797/2385 (33.4%) | 2.38 | 2.09, 2.71 | <0.0001 |
| Body Mass Index (BMI) | ||||
| Normal weight (19-24.99) | 71/437 (16.2%) | 1.0 (reference) | ||
| Overweight (25-29.99) | 278/1463 (19%) | 1.21 | 0.91, 1.61 | 0.192 |
| Obese (≥30) | 910/3125 (29.1%) | 2.12 | 1.62, 2.76 | <0.0001 |
| Type of Delivery | ||||
| Vaginal Delivery | 751/3166 (23.7%) | 1.0 (reference) | ||
| Cesarean Section | 447/1563 (28.6%) | 1.29 | 1.12, 1.48 | <0.0001 |
| Instrumental Delivery | 62/314 (19.7%) | 0.79 | 0.59, 1.06 | 0.113 |
| Gestational Age (weeks) | ||||
| <37 | 155/583 (26.6%) | 1.0 (reference) | ||
| ≥37 | 1105/4460 (24.8%) | 0.91 | 0.75, 1.11 | 0.342 |
| Gender | ||||
| Male | 663/2632 (25.2%) | 1.0(reference) | ||
| Female | 597/2411 (24.8%) | 0.98 | 0.86, 1.11 | 0.726 |
| Placental Weight (gm) | ||||
| <650 | 448/1904 (23.5%) | 1.0 (reference) | ||
| ≥650 | 806/3122 (25.8%) | 1.13 | 0.99, 1.29 | 0.069 |
| Birth Weight (gm) | ||||
| <2500 | 120/603 (19.9%) | 1.0 (reference) | ||
| ≥2500 | 1140/4438 (25.7%) | 1.39 | 1.13, 1.72 | 0.002 |
| Macrosomia | ||||
| No | 1200/4878 (24.6%) | 1.0 (reference) | ||
| Yes | 60/163 (36.8%) | 1.79 | 1.29, 2.47 | <0.0001 |
| Bodyweight to Gestational Age | ||||
| AGA | 1061/4141 (25.6%) | 1.0(reference) | ||
| SGA | 127/744 (17.1%) | 0.60 | 0.49, 0.73 | <0.0001 |
| LGA | 72/146 (49.3%) | 2.82 | 2.03, 3.94 | <0.0001 |
| Major Congenital Anomalies | ||||
| No | 1252/5005 (25%) 8/38 (21.1%) | 1.0 (reference) | ||
| Yes | 0.80 | 0.37, 1.75 | 0.575 | |
CI Confidence interval, OR Odds ratio; Outcome variable: non-GDM was considered as the reference group
LGA Large for gestational age, AGA appropriate for gestational age, SGA small for gestational age
‘n’ is the total number of GDM cases whereas ‘N’ is the total number of participants included against each specific variable/parameter
Table 4.
Factors associated with GDM: Multivariate Logistic regression analysis
| Variables | GDM n/N (%) |
Adjusted Odds ratio (OR) |
95% CI for OR | P-value |
|---|---|---|---|---|
| Mother’s Age (years) | ||||
| <30 | 463/2658 (17.4%) | 1.0 (reference) | ||
| ≥30 | 797/2385 (33.4%) | 2.21 | 1.93, 2.52 | <0.0001 |
| Body Mass Index (BMI) | ||||
| Normal weight (19-24.99) | 71/437 (16.2%) | 1.0 (reference) | ||
| Overweight (25-29.99) | 278/1463 (19%) | 1.11 | 0.83, 1.48 | 0.493 |
| Obese (≥30) | 910/3125 (29.1%) | 1.71 | 1.30, 2.23 | <0.0001 |
| Type of Delivery | ||||
| Vaginal Delivery | 751/3166 (23.7%) | 1.0 (reference) | ||
| Cesarean Section | 447/1563 (28.6%) | 1.25 | 1.09, 1.44 | 0.002 |
| Instrumental Delivery | 62/314 (19.7%) | 0.82 | 0.61, 1.20 | 0.176 |
| Bodyweight to Gestational Age | ||||
| AGA | 1061/4141 (25.6%) | 1.0(reference) | ||
| SGA | 127/744 (17.1%) | 0.70 | 0.57, 0.87 | 0.001 |
| LGA | 72/146 (49.3%) | 2.30 | 1.64, 3.24 | <0.0001 |
CI Confidence interval, OR Odds ratio, Outcome variable: non-GDM was considered as the reference group
LGA Large for gestational age, AGA appropriate for gestational age, SGA small for gestational age
‘n’ is the total number of GDM cases whereas ‘N’ is the total number of participants included against each specific variable/parameter
The multivariable logistic regression analysis indicated that higher maternal age (adjusted OR 2.21, 95% CI 1.93, 2.52, P<0.0001), obesity before pregnancy (adjusted OR 1.71, 95% CI 1.30, 2.23, P<0.0001), type of delivery C-section (adjusted OR 1.25, 95% CI 1.09, 1.44, P=0.002), and body weight to gestational age LGA (adjusted OR 2.30, 95% CI 1.64, 2.34, P<0.0001) remained significantly associated with increased risk of GDM adjusting all other potential confounder and predictors (Table 4). Therefore, we computed a prediction model to evaluate the discriminative ability of potentially significant variables with statistical P <0.10 on the occurrence of GDM. Multivariate logistic regression (stepwise variable selection approach) indicated that the final model demonstrated a modest fit (area under the curve (AUC) = 0.633, 95% CI 0.62, 0.65) and included the following variables maternal age and BMI before pregnancy as shown in Table 4.
Discussion
The prevalence rate of GDM in the Qatari population in our study sample was 24.25%, which is higher than the rates observed by Bener A. et al (16.3%) [19] in 2011 but close to the rates observed by Bashir M. et al (23.5%) [20] in 2016. Studies from neighboring countries such as Oman and Bahrain reported a lower prevalence (10%) of GDM [21, 22].. Our prevalence is also higher than the rates observed in Kuwait (12.6%) [23] and in the United Arab Emirates (UAE) (13.3%) [21]. On the other hand, in Saudi Arabia, the prevalence of GDM ranged between 24% and almost 40% [24–27]. The relatively high prevalence of GDM in Qatar might be related to overweight or obesity, excessive weight gain during pregnancy, excessive central body fat deposition, positive family history of DM, and the relatively sedentary lifestyle and high socio-economic standard in Qatar compared to other populations [28].
Despite the overall statistically significant difference in the mean birth weight values among the three groups, the mean birth weight difference between infants born to women with GDM and healthy non-diabetic mothers was only 90 gm. The data from this study shows no statistically significant difference in length and head circumference in babies born to women with GDM in comparison with those who were born to healthy non-diabetic women. Other studies also did not find any difference in birth measures between the GDM-exposed and unexposed neonates [29, 30]. On the other hand, Baptiste-Roberts K. et al [31] concluded that compared to their non-diabetic counterparts, mothers with GDM gave birth to offspring that had higher weights at birth even after adjustment for other variables (β = 50 gm; 95% CI: 0.01, 0.09). Moreover, Sletner L et al [32] found that offspring of GDM mothers were smaller in mid-pregnancy but subsequently grew faster until birth, compared with offspring of non-GDM mothers.
Another interesting result in our study was the rate of macrosomia which was only 4.8% among infants born to Qatari women with GDM and 4.6% among infants born to Qatari women with pre-pregnancy DM, as compared to a previous study on the same population ( 9.3%) [20]. A literature review by Kc K. et al. [33] concluded that about 15–45% of babies born to diabetic mothers can have macrosomia, which is a 3-fold higher rate when compared to normoglycemic controls.
Macrosomia is expected in 20% of infants born to women whose postprandial glucose values average 120 mg/dL or less [34]. However, this rate can be higher (35%) when postprandial levels in women range as high as 160 mg/dL [35]. However, other studies showed different results. For instance, Vally F. et al [31] reported that macrosomia is not increased in women with diet-controlled GDM in comparison with healthy controls, and others reported low percentages of macrosomia (8-14%) among IDM mothers [36, 37].
As expected, the mean gestational age of neonates born to women with pre-pregnancy DM was significantly lower than those of GDM and healthy non-diabetic women. However, there was no difference between the incidence of preterm labor between GDM women and healthy controls. In contrast to our findings, Billionnet C. et al [38] found that the risks of preterm birth (OR 1.3 [95% CI 1.3, 1.4]) were increased in women with GDM compared with the non-diabetic population. In addition to Köck K. et al [39] also reported a significant difference in the incidence of spontaneous preterm birth (P = 0.047) between IDMs and healthy controls. The reasons for an increased risk of spontaneous preterm delivery are not clear [40, 41], but could be explained by the fast-intrauterine growth due to overexposure to the energy source [37].
Researchers believe that the hyper-insulinemic state in IDMs accompanied by the upregulation of gene expression, inflammatory mediators, and leptin in placental tissues could be the cause of excessive growth and an increase in placental weight [42]. In our study, the placental weight of neonates born to women with GDM was significantly higher than those of healthy non-diabetic women. Daskalakis et al. [43] compared the placentas of healthy pregnant women against GDM patients and had similar findings to ours. They found degenerative changes such as fibrinoid necrosis, cholangitis, and the presence of nucleated fetal erythrocytes, in addition to villous immaturity as an indicator of chronic fetal hypoxia. Macroscopically, they found the fetal/placental weight ratio was significantly decreased. On the other hand, Akarsu S. et al [44] concluded that no significant difference was found between the groups in terms of fetal/placental weight ratio.
Major congenital malformations were found in 0.6% of infants born to GDM women, compared to 0.8% in healthy controls and 2% in women with pre-pregnancy DM. The literature has reported that the overall reported risk for major malformations is approximately 5 to 6 percent with a higher prevalence rate of 10 to 12 percent when mothers require insulin therapy [45–47]. Out of the 41 cases of major congenital anomalies in our study, 29 involved the cardiovascular and central nervous systems. Becerra JE et al [45] found that two-thirds of the anomalies in infants of mothers with diabetes involve either the cardiovascular system (8.5 per 100 live births) or central nervous system (CNS) (5.3 per 100 live births) [45]. Those malformations were not significantly associated with any maternal or fetal parameters. Moreover, Prakash GT et al reported a 2.3% (3/132) rate of major congenital anomalies in infants born to GDM women [48].
HbA1C% is an important indicator and prognostic factor of long-term blood sugar control with the ability to reflect the cumulative blood sugar history of the preceding three months. HbA1C% level below 5.7% is considered normal [49]. In our study, the last HbA1C% results before delivery were obtained in diabetic mothers. Its mean value was 5.28 ± 0.43 in women with GDM and 6.19 ± 1.15 in women with pre-pregnancy DM (P <0.0001). A significant positive correlation with HbA1C% results was observed with maternal age, BMI, and birth weight while a significant negative correlation with HbA1C% results was observed only with gestational age. Our correlation findings are similar to those of Sweeting AN et al who stated that baseline HbA1C%>5.9% (41 mmol/mol) identifies an increased risk of large-for-gestational-age, macrosomia, cesarean section, and hypertensive disorders in standard GDM [47, 48]. Nevertheless, a 2013 systematic review and meta-analysis of randomized trials for the US Preventive Services Task Force found that reductions in pre-eclampsia, macrosomia, and shoulder dystocia were associated with appropriate management of GDM [50].
Risk for large for gestational age and congenital anomalies are higher in women with elevated HbA1c% levels during pregnancy [51]. However, the HbA1C% mostly associated with congenital anomalies is the one taken in the periconceptional period that is not known in our study. Achieving near normal levels of HbA1C% before delivery reflects the proper antenatal care and the socioeconomic standard of the population which subsequently led to proper control of GDM despite the high BMI before delivery. While this paper does not focus on identifying risk factors for diabetes in pregnancy, the high risk of overweight and obesity in Qatar is indeed a likely major contributing factor to the high prevalence of diabetes in pregnancy. Despite recent advances in its diagnosis, follow-up, and management, GDM continues to be a common complication of pregnancy and a cause of great concern because of the relatively high rates of adverse maternal, fetal and neonatal outcomes. The study outcomes emphasize the importance of collaboration between feto-maternal medicine and neonatology in weighing the fetal and maternal risks of prolonged pregnancy compared to the potential benefits of further fetal maturation among most gestational ages.
The main limitation of the study is being a retrospective cohort with no long-term follow-up outcomes. However, the number of the population tested increases the significance of its results. Getting deeper insights about the periconceptional levels of HbA1C% in the diabetic population might further lower the rate of major congenital anomalies as well as other neonatal and maternal morbidities. Understanding the pathophysiology of the disorder may allow the development of strategies and routine screening measures to prevent morbidities in those babies. Hence, studying the molecular pathogenesis of neonatal morbidities related to GDM is recommended using prospective studies with a larger sample size and long-term outcomes measurements.
Conclusion
Despite the multi-disciplinary antenatal diabetic care management, there is still an increased birth weight and an increased prevalence of macrosomia among the infants of diabetic mothers. More efforts should be addressed to improve the known modifiable factors such as women's adherence to the diabetic control program. Furthermore, pre-pregnancy BMI was found to be significantly associated with gestational DM, and this is a factor that can be addressed during pre-conceptional counseling.
Acknowledgment
Special thanks and appreciation for the entire diabetic team in WWRC who are providing high-quality family-centered antenatal care to our pregnant women. We thank our colleagues from the Medical Research Center for sharing their pearls of wisdom with us during this research. We are very grateful to our colleagues in the Health Information and Communications Technology (HICT) Department for their kind help and support in this study.
Abbreviations
- GDM
Gestational Diabetes Mellitus
- DM
Diabetes Mellitus
- IDM
Infant of Diabetic Mother
- PTB
Preterm Birth
- HbA1C
Glycosylated Hemoglobin
- LGA
Large for Gestational Age
- BMI
Body Mass Index
- SGA
Small for Gestational Age
- AGA
Appropriate for Gestational Age
- CVS
Cardiovascular System
- CNS
Central Nervous System
- WHO
World Health Organization
- VD
Vaginal Delivery
- CS
Cesarean Section
- ID
Instrumental Delivery
- GA
Gestational Age
- BW
Birth Weight
- AUC
Area Under the Curve
- OR
Odds Ratio
- CI
Confidence Interval
- WWRC
Women’s Wellness and Research Center
- IRB
Institutional Review Board
- HMC
Hamad Medical Corporation
- NICU
Neonatal Intensive Care Unit
- MRC
Medical Research Center
Authors’ contributions
The project was conceived by M.A.A.B., R.M., N.M., and M.M. The research protocol was written, and approval was obtained by M.A.A.B. from the Medical Research Center (MRC) of Hamad Medical Corporation (HMC). Data was collected by R.M., M.M., N.M., L.L., and S.D. Data analysis was conducted by P.C. and the manuscript was written by M.A.A.B., E.E., P.C., and N.O. Intellectual input and manuscript review was provided by M.A.A.B., A.G., P.C., M.S.B., S.S., M.H., and R.A. All authors have read and approved the manuscript.
Funding
This research was funded and supported by the Medical Research Center (MRC), Hamad Medical Corporation, Doha, Qatar.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This research was approved by the ethical/Institutional Review Board (IRB) in Medical Research Center (Protocol number MRC-01-18-041), Hamad Medical Corporation, Doha, Qatar. A waiver for the requirement of informed consent from the mothers whose records were analyzed was granted by the Chair of the Medical Research Center on the grounds of being a minimal risk study.
Consent for publication
Not applicable.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammad A. A. Bayoumi, Email: moh.abdelwahab@hotmail.com
Razan M. Masri, Email: rmasri@hamad.qa
Nada Y. S. Matani, Email: nmatani89@gmail.com
Mohamed A. Hendaus, Email: mrahal@sidra.org
Manal M. Masri, Email: masri83m@gmail.com
Prem Chandra, Email: pchandra@hamad.qa.
Lisa J. Langtree, Email: llangtree@hamad.qa
Sunitha D’Souza, Email: sdsouza2@hamad.qa.
Noimot O. Olayiwola, Email: nolayiwola@hamad.qa
Saad Shahbal, Email: sabdullah5@hamad.qa.
Einas E. Elmalik, Email: einas_e@yahoo.com
Mohamed S. Bakry, Email: msb03@fayoum.edu.eg
Ashraf I. Gad, Email: agad2@hamad.qa
Ravi Agarwal, Email: ravi_agarwal5@hotmail.com.
References
- 1.Eslamian L, Akbari S, Marsoosi V, Jamal A. Effect of different maternal metabolic characteristics on fetal growth in women with gestational diabetes mellitus. Iran J Reprod Med. 2013;11(4):325–334. [PMC free article] [PubMed] [Google Scholar]
- 2.Group HSCR. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 3.Kong L, Nilsson IAK, Gissler M, Lavebratt C. Associations of Maternal Diabetes and Body Mass Index With Offspring Birth Weight and Prematurity. JAMA Pediatr. 2019. 10.1001/jamapediatrics.2018.5541. PMID: 30801637. [DOI] [PMC free article] [PubMed]
- 4.Riggins T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA. Consequences of low neonatal iron status due to maternal diabetes mellitus on explicit memory performance in childhood. Dev Neuropsychol. 2009;34(6):762–779. doi: 10.1080/87565640903265145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitanchez D, Burguet A, Simeoni U. Infants born to mothers with gestational diabetes mellitus: mild neonatal effects, a long-term threat to global health. J Pediatr. 2014;164(3):445–450. doi: 10.1016/j.jpeds.2013.10.076. [DOI] [PubMed] [Google Scholar]
- 6.Elmekkawi SF, Mansour GM, Elsafty MS, Hassanin AS, Laban M, Elsayed HM. Prediction of Fetal Hypertrophic Cardiomyopathy in Diabetic Pregnancies Compared with Postnatal Outcome. Clin Med Insights Womens Health. 2015;8:39–43. doi: 10.4137/CMWH.S32825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boghossian NS, Hansen NI, Bell EF, Brumbaugh JE, Stoll BJ, Laptook AR, Shankaran S, Wyckoff MH, Colaizy TT, Das A, Higgins RD; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Outcomes of Extremely Preterm Infants Born to Insulin-Dependent Diabetic Mothers. Pediatrics. 2016;137(6). 10.1542/peds.2015-3424. PMID: 27244849. [DOI] [PMC free article] [PubMed]
- 8.Langer O, Berkus MD, Huff RW, Samueloff A. Shoulder dystocia: should the fetus weighing greater than or equal to 4000 grams be delivered by cesarean section? Am J Obstet Gynecol. 1991;165(4 Pt 1):831–837. doi: 10.1016/0002-9378(91)90424-P. [DOI] [PubMed] [Google Scholar]
- 9.Modanlou HD, Dorchester WL, Thorosian A, Freeman RK. Macrosomia--maternal, fetal, and neonatal implications. Obstet Gynecol. 1980;55(4):420–424. [PubMed] [Google Scholar]
- 10.Boyd ME, Usher RH, McLean FH. Fetal macrosomia: prediction, risks, proposed management. Obstet Gynecol. 1983;61(6):715–722. [PubMed] [Google Scholar]
- 11.Menticoglou SM, Manning FA, Morrison I, Harman CR. Must macrosomic fetuses be delivered by a caesarean section? A review of outcome for 786 babies greater than or equal to 4,500 g. Aust N Z J Obstet Gynaecol. 1992;32(2):100–103. doi: 10.1111/j.1479-828X.1992.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Practice B-O Macrosomia: ACOG Practice Bulletin, Number 216. Obstet Gynecol. 2020;135(1):e18–e35. doi: 10.1097/AOG.0000000000003606. [DOI] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. 10.1016/j.jclinepi.2007.11.008. PMID: 18313558. [DOI] [PubMed]
- 14.Human Research Policies & Regulations. https://research.moph.gov.qa//DepartmentalDocuments/Policies,%20Regulations%20and%20Guidelines%20for%20Research%20Involving%20Human.pdf?csrt=13717471866515786407.
- 15.Good Research Practice. https://www.ukri.org/wp-content/uploads/2021/08/MRC-0208212-Good-research-practice_2014.pdf.
- 16.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. WHO Guidelines Approved by the Guidelines Review Committee. Geneva: 2013. PMID: 24199271. [PubMed]
- 18.Bashir M, Baagar K, Naem E, Elkhatib F, Alshaybani N, Konje JC, et al. Pregnancy outcomes of early detected gestational diabetes: a retrospective comparison cohort study, Qatar. BMJ Open. 2019;9(2):e023612. doi: 10.1136/bmjopen-2018-023612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bener A, Saleh NM, Al-Hamaq A. Prevalence of gestational diabetes and associated maternal and neonatal complications in a fast-developing community: global comparisons. Int J Womens Health. 2011;3:367–373. doi: 10.2147/IJWH.S26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bashir M, MEA-R, Aboulfotouh M, Eltaher F, Omar K, Babarinsa I, et al. Prevalence of newly detected diabetes in pregnancy in Qatar, using universal screening. PLoS One. 2018;13(8):e0201247. doi: 10.1371/journal.pone.0201247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajab KE, Issa AA, Hasan ZA, Rajab E, Jaradat AA. Incidence of gestational diabetes mellitus in Bahrain from 2002 to 2010. Int J Gynaecol Obstet. 2012;117(1):74–77. doi: 10.1016/j.ijgo.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Abu-Heija AT, Al-Bash M, Mathew M. Gestational and Pregestational Diabetes Mellitus in Omani Women: Comparison of obstetric and perinatal outcomes. Sultan Qaboos Univ Med J. 2015;15(4):e496–e500. doi: 10.18295/squmj.2015.15.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groof Z, Garashi G, Husain H, Owayed S, AlBader S, Mouhsen H, et al. Prevalence, Risk Factors, and Fetomaternal Outcomes of Gestational Diabetes Mellitus in Kuwait: A Cross-Sectional Study. J Diabetes Res. 2019;2019:9136250. doi: 10.1155/2019/9136250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal MM, Dhatt GS, Othman Y. Gestational diabetes mellitus prevalence: Effect of the laboratory analytical variation. Diabetes Res Clin Pract. 2015;109(3):493–499. doi: 10.1016/j.diabres.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Alfadhli EM, Osman EN, Basri TH, Mansuri NS, Youssef MH, Assaaedi SA, et al. Gestational diabetes among Saudi women: prevalence, risk factors and pregnancy outcomes. Ann Saudi Med. 2015;35(3):222–230. doi: 10.5144/0256-4947.2015.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahabi H, Fayed A, Esmaeil S, Mamdouh H, Kotb R. Prevalence and Complications of Pregestational and Gestational Diabetes in Saudi Women: Analysis from Riyadh Mother and Baby Cohort Study (RAHMA) Biomed Res Int. 2017;2017:6878263. doi: 10.1155/2017/6878263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahabi H, Fayed A, Esmaeil S, Alzeidan R, Elawad M, Tabassum R, et al. Riyadh Mother and Baby Multicenter Cohort Study: The Cohort Profile. PLoS One. 2016;11(3):e0150297. doi: 10.1371/journal.pone.0150297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catalano PM. The impact of gestational diabetes and maternal obesity on the mother and her offspring. J Dev Orig Health Dis. 2010;1(4):208–215. doi: 10.1017/S2040174410000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keshvari-Delavar M, Mozaffari-Khosravi H, Nadjarzadeh A, Farhadian Z, Khazaei S, Rezaeian Sh. Comparison of Growth Parameters, Apgar Scores, the Blood Zinc, Magnesium, Calcium and Phosphorus between Gestational Diabetic and Non-diabetic Pregnant Women. Int J Pediatr. 2016;4(5):1767–75.
- 30.Macaulay S, Munthali RJ, Dunger DB, Norris SA. The effects of gestational diabetes mellitus on fetal growth and neonatal birth measures in an African cohort. Diabet Med. 2018;35(10):1425–1433. doi: 10.1111/dme.13668. [DOI] [PubMed] [Google Scholar]
- 31.Baptiste-Roberts K, Nicholson WK, Wang NY, Brancati FL. Gestational diabetes and subsequent growth patterns of offspring: the National Collaborative Perinatal Project. Matern Child Health J. 2012;16(1):125–132. doi: 10.1007/s10995-011-0756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sletner L, Jenum AK, Yajnik CS, Morkrid K, Nakstad B, Rognerud-Jensen OH, et al. Fetal growth trajectories in pregnancies of European and South Asian mothers with and without gestational diabetes, a population-based cohort study. PLoS One. 2017;12(3):e0172946. doi: 10.1371/journal.pone.0172946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(Suppl 2):14–20. doi: 10.1159/000371628. [DOI] [PubMed] [Google Scholar]
- 34.Vally F, Presneill J, Cade T. Macrosomia Rates in Women with Diet-Controlled Gestational Diabetes: A Retrospective Study. J Pregnancy. 2017;2017:4935397. doi: 10.1155/2017/4935397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jovanovic-Peterson L, Peterson CM, Reed GF, Metzger BE, Mills JL, Knopp RH, et al. Maternal postprandial glucose levels and infant birth weight: the Diabetes in Early Pregnancy Study. The National Institute of Child Health and Human Development--Diabetes in Early Pregnancy Study. Am J Obstet Gynecol. 1991;164(1 Pt 1):103–111. doi: 10.1016/0002-9378(91)90637-7. [DOI] [PubMed] [Google Scholar]
- 36.Cordero L, Treuer SH, Landon MB, Gabbe SG. Management of infants of diabetic mothers. Arch Pediatr Adolesc Med. 1998;152(3):249–254. doi: 10.1001/archpedi.152.3.249. [DOI] [PubMed] [Google Scholar]
- 37.Domanski G, Lange AE, Ittermann T, Allenberg H, Spoo RA, Zygmunt M, et al. Evaluation of neonatal and maternal morbidity in mothers with gestational diabetes: a population-based study. BMC Pregnancy Childbirth. 2018;18(1):367. doi: 10.1186/s12884-018-2005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Billionnet C, Mitanchez D, Weill A, Nizard J, Alla F, Hartemann A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60(4):636–644. doi: 10.1007/s00125-017-4206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kock K, Kock F, Klein K, Bancher-Todesca D, Helmer H. Diabetes mellitus and the risk of preterm birth with regard to the risk of spontaneous preterm birth. J Matern Fetal Neonatal Med. 2010;23(9):1004–1008. doi: 10.3109/14767050903551392. [DOI] [PubMed] [Google Scholar]
- 40.Mimouni F, Miodovnik M, Siddiqi TA, Berk MA, Wittekind C, Tsang RC. High spontaneous premature labor rate in insulin-dependent diabetic pregnant women: an association with poor glycemic control and urogenital infection. Obstet Gynecol. 1988;72(2):175–180. [PubMed] [Google Scholar]
- 41.Reece EA, Sivan E, Francis G, Homko CJ. Pregnancy outcomes among women with and without diabetic microvascular disease (White's classes B to FR) versus non-diabetic controls. Am J Perinatol. 1998;15(9):549–555. doi: 10.1055/s-2007-994059. [DOI] [PubMed] [Google Scholar]
- 42.Arshad R, Karim N, Ara HJ. Effects of insulin on placental, fetal and maternal outcomes in gestational diabetes mellitus. Pak J Med Sci. 2014;30(2):240–244. doi: 10.12669/pjms.302.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daskalakis G, Marinopoulos S, Krielesi V, Papapanagiotou A, Papantoniou N, Mesogitis S, et al. Placental pathology in women with gestational diabetes. Acta Obstet Gynecol Scand. 2008;87(4):403–407. doi: 10.1080/00016340801908783. [DOI] [PubMed] [Google Scholar]
- 44.Akarsu S, Bagirzade M, Omeroglu S, Buke B. Placental vascularization and apoptosis in Type-1 and gestational DM. J Matern Fetal Neonatal Med. 2017;30(9):1045–1050. doi: 10.1080/14767058.2016.1199676. [DOI] [PubMed] [Google Scholar]
- 45.Becerra JE, Khoury MJ, Cordero JF, Erickson JD. Diabetes mellitus during pregnancy and the risks for specific birth defects: a population-based case-control study. Pediatrics. 1990;85(1):1–9. [PubMed] [Google Scholar]
- 46.Yang J, Cummings EA, O'Connell C, Jangaard K. Fetal and neonatal outcomes of diabetic pregnancies. Obstet Gynecol. 2006;108(3 Pt 1):644–650. doi: 10.1097/01.AOG.0000231688.08263.47. [DOI] [PubMed] [Google Scholar]
- 47.Sweeting AN, Ross GP, Hyett J, Molyneaux L, Tan K, Constantino M, et al. Baseline HbA1C%to Identify High-Risk Gestational Diabetes: Utility in Early vs Standard Gestational Diabetes. J Clin Endocrinol Metab. 2017;102(1):150–156. doi: 10.1210/jc.2016-2951. [DOI] [PubMed] [Google Scholar]
- 48.Prakash GT, Das AK, Habeebullah S, Bhat V, Shamanna SB. Maternal and Neonatal Outcome in Mothers with Gestational Diabetes Mellitus. Indian J Endocrinol Metab. 2017;21(6):854–858. doi: 10.4103/ijem.IJEM_66_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1C%Test in Diagnosis and Prognosis of Diabetic Patients. Biomark Insights. 2016;11:95–104. doi: 10.4137/BMI.S38440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med. 2013;159(2):123–129. doi: 10.7326/0003-4819-159-2-201307160-00661. [DOI] [PubMed] [Google Scholar]
- 51.Fonseca L, Saraiva M, Amado A, Paredes S, Pichel F, Pinto C, et al. Third trimester HbA1c and the association with large-for-gestational-age neonates in women with gestational diabetes. Arch Endocrinol Metab. 2021. 10.20945/2359-3997000000366. PMID: 33939909. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.