Abstract
Previously, we developed a solid lipid nanoparticle (SLN) formulation of 4-(N)-docosahexaenoyl 2′, 2′-difluorodeoxycytidine (DHA-dFdC), a compound with promising antitumor activity. Herein, we studied the feasibility of administering the DHA-dFdC by the oral route using the solid lipid nanoparticles (i.e. DHA-dFdC-SLNs). In simulated gastrointestinal fluids, the DHA-dFdC-SLNs did not aggregate. The release of the DHA-dFdC from the solid lipid nanoparticles in simulated gastrointestinal fluid was slow, but was slightly faster in simulated intestinal fluid than in simulated gastric fluid. In mice orally administered with DHA-dFdC-SLNs, plasma DHA-dFdC concentration vs. time curve has a Tmax of ~1.7 h and a Cmax of 17.01 μg/mL. The absolute oral bioavailability of DHA-dFdC when given as DHA-dFdC-SLNs was ~68% (based on AUC0-24 h values), while the relative oral bioavailability DHA-dFdC (compared to DHA-dFdC in a Tween 80/ethanol-in-water solution) was 126%. Finally, in mice with pre-establish B16-F10 murine melanoma, oral DHA-dFdC-SLNs increased their survival significantly, as compared to oral administration of the DHA-dFdC solution. It is concluded that the solid lipid nanoparticle formulation increased the bioavailability of the DHA-dFdC upon oral administration, as compared to the DHA-dFdC solution.
Keywords: Oral, nanoparticles, bioavailability, tumor, pharmacokinetics, mouse survival
INTRODUCTION
Oral route is preferred for drug administration due to advantages such as painlessness, easiness for self-administration, flexibility in dosage regimen, convenience, and high patient compliance (1, 2). Furthermore, oral product manufacturing does not require sterile conditions that are necessary for products intended for parenteral administration (1). In cancer chemotherapy, there are data showing that cancer patients prefer oral administration to intravenous infusion, especially when the chemotherapy is a palliative treatment and/or given chronically (2-4). However, developing oral dosage forms of cancer chemotherapeutic agents is not without challenges, in part because the gastrointestinal (GI) tract presents various physiological, enzymatic, and chemical barriers, hindering efficient oral absorption (2, 5). In addition, factors such as low solubility and intestinal permeability of many drugs and high level of P-glycoprotein (P-gp) expressed by enterocytes also limit the oral bioavailability of many cancer chemotherapeutic agents (e.g. paclitaxel, docetaxel, doxorubicin, etc.) (2). Nanocarriers such as solid lipid nanoparticles (SLNs), liposomes, nanoemulsions, micelles, nanocrystals, and polymeric nanoparticles have shown promise in improving the oral delivery of anticancer drugs due to their ability to increase the apparent solubility of drugs, reduce degradation of drugs within the GI tract, and increase drug absorption (1, 2, 5).
Previously, we developed an SLN formulation of DHA-dFdC, DHA-dFdC-SLNs, to improve the apparent solubility and stability of DHA-dFdC, a compound synthesized by conjugating docosahexaenoic acid (DHA), an omega-3 polyunsaturated fatty acid, to 2′,2′-difluorodeoxycytidine (dFdC) on the 4-NH2 position (6). DHA-dFdC showed strong antitumor activity against pancreatic tumor cells in vitro and tumors in vivo (6). In addition, studies with NCI DTP-60 human cell lines showed that DHA-dFdC was effective against leukemia cells, non-small cell lung cancer cells, renal cancer cells, and melanoma cells (6). DHA-dFdC-SLNs were prepared by encapsulating DHA-dFdC into SLNs engineered with lecithin and glycerol monostearate (GMS)-in-water emulsions emulsified with Tween 20 and D-α-tocopherol polyethylene glycol 1000 succinate (vitamin E TPGS or TPGS) (7). DHA-dFdC-SLNs were ~100 nm in diameter, increased the apparent water solubility of DHA-dFdC by ~200-fold, and improved the chemical stability of DHA-dFdC (7). In the present work, the bioavailability of DHA-dFdC when it was administered orally as DHA-dFdC-SLNs in suspension were evaluated and compared to when it was administered orally as a DHA-dFdC in Tween 80/ethanol-in-water solution. The effect of the DHA-dFdC-SLNs after oral administration on the survival of mice with pre-established B16-F10 tumors was also evaluated and compared to that of oral DHA-dFdC solution.
MATERIALS AND METHODS
Materials and cell lines
Soy lecithin was from Alfa Aesar (Ward Hill, MA). GMS, mannitol, Tween 20, TPGS, sodium chloride (NaCl), hydrochloric acid (HCl, 37 %), monobasic potassium phosphate (KH2PO4), sodium hydroxide (NaOH), and Tween 80 were from Sigma-Aldrich (St. Louis, MO). Gemcitabine HCl was from Biotang, Inc. (Lexington, MA). DHA-dFdC was synthesized as previously described (6). Ethyl acetate, tetrahydrofuran, isopropanol, and methanol were from Thermo Fisher Scientific (Waltham, MA). Float-A-Lyzer®G2 dialysis device with a molecular weight cutoff (MWCO) of 50 kDa was from Spectrum Chemicals & Laboratory Products (New Brunswick, NJ). B16-F10 murine melanoma cells were from the American Type Culture Collection (Manassas, VA) and were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with fetal bovine serum (FBS, 10%, v/v), penicillin (100 U/mL), and streptomycin (100 μg/mL), all from Invitrogen Corporation (Carlsbad, CA).
Preparation and characterization of DHA-dFdC-SLNs
DHA-dFdC-SLNs were prepared as previously described (7). The nanoparticles, in 1 mL, comprised of 5.2 mg of DHA-dFdC, 3.5 mg of soy lecithin, 0.5 mg of GMS, 0.875 mg of TPGS, and 10 mg of Tween 20. The particle size (by intensity), polydispersity index (PDI), and zeta potential of the nanoparticles were determined by dynamic light scattering (DLS) using a Malvern Zetasizer Nano ZS (Westborough, MA). The content of DHA-dFdC within the nanoparticles was determined using HPLC after extraction with isopropanol and ethyl acetate as previously described (6). The encapsulation efficiency of DHA-dFdC in the nanoparticles was also determined by an ultracentrifugation method as previously described (6).
Stability of DHA-dFdC-SLNs in stimulated gastrointestinal fluids
Simulated gastric fluid (SGF, pH 1.2) and simulated intestine fluid (SIF, pH 6.8) without enzymes were prepared according USP XXVI and as previously described (8). DHA-dFdC-SLNs were incubated in SGF or SIF at 37°C under agitation. At various time points, samples were withdrawn and diluted in water to measure particle size, PDI, and zeta potential. As a control, DHA-dFdC-SLNs were also incubated in phosphate-buffered saline (PBS, 10 mM, pH 7.4). The experiments were repeated three times. The size and morphology of the nanoparticles before and after incubation in SGF and SIF were also examined using a transmission electron microscope (TEM) as previously described (9).
In vitro release in simulated gastrointestinal fluids
The release behavior of DHA-dFdC from the DHA-dFdC-SLNs in SGF and SIF was studied as previously described (6), except that the amount of DHA-dFdC placed in the dialysis tube was 151 μg, and the release medium, 13 mL, was SGF or SIF, with 2.5% of Tween 20. As a control, 151 μg of DHA-dFdC in 1 mL of 2.5% of Tween 20 (i.e. DHA-dFdC in Tween 20 micelles, particle size, 8.6 ± 0.1 nm, n = 3) was placed in dialysis tube to examine the release of DHA-dFdC from the Tween 20 micelles and its diffusion across the dialysis membrane to make sure that the release profiles obtained with the DHA-dFdC-SLNs represent the release of the DHA-dFdC from the DHA-dFdC-SLNs (6). Finally, to confirm that the diffusion of DHA-dFdC across the dialysis membrane was not the rate-limiting step, the diffusion of the DHA-dFdC in a 90% (v/v) ethanol in water solution across the dialysis membrane into 13 mL of 90% ethanol solution was also examined.
Pharmacokinetic (PK) studies
The Institutional Animal Care and Use Committee at The University of Texas at Austin approved the animal protocol. Female C57BL/6 mice (6-8 weeks) were from Charles River Laboratories, Wilmington, MA). Water was allowed ad libitum. Mice were fasted for 3 h and then orally gavaged with DHA-dFdC dissolved in a Tween 80/ethanol-in-water vehicle solution (i.e. Tween 80 (10%, w/v), ethanol (5.2% v/v), and mannitol (5%, w/v) in sterile water) (6, 10) or in the DHA-dFdC-SLNs suspended in a sterile mannitol solution (5%, w/v), or intravenously injected with the DHA-dFdC-SLNs suspended in a sterile mannitol solution (5%, w/v). Mice that were i.v. injected with the DHA-dFdC-SLNs were not fasted. The dose of DHA-dFdC was 2 mg per mouse. Mice (n = 3) were euthanized at various time points to collect blood. DHA-dFdC concentration in plasma samples were extracted and measured using HPLC as previously described (6). The 4-(N)-stearoyl 2′,2′-difluorodeoxycytidine (C18-dFdC) was used as an internal control during the extraction (11). Data were analyzed using the PK Solver®, assuming a two-compartmental model (12).
The effect of orally administered DHA-dFdC-SLNs on the survival of B16-F10 tumor-bearing mice
To establish tumors, B16-F10 (5 x 105 cells/mouse) were subcutaneously (s.c.) injected in the right flank of female C57BL/6 mice (18-20 g) on day 0. Mice were randomized into 4 groups (n = 7-8) on day 7 and orally gavaged with DHA-dFdC-SLNs (250 μg/mouse of DHA) dispersed in a mannitol solution (5%, w/v), DHA-dFdC (250 μg/mouse) dissolved in a Tween 80/ethanol-in-water solution with 5% (w/v) of mannitol (6, 10), or DHA-dFdC-free SLNs dispersed in a 5% mannitol solution. Mice in the control group were left untreated. Treatment was repeated every day until day 11. Mice were allowed to rest for two days, and treatment was resumed on day 13 and continued until day 20. Mice were monitored daily, and the endpoints include death, tumor size reaching 15 mm, tumor ulceration and/or bleeding, body weight loss of more than 20%, or other signs of severe distress and discomfort.
Statistical analysis
ANOVA followed by a Bonferroni post hoc test were completed to analyze data. The Mantel-Cox log-rank method was used to compare mouse survival curves. A p value of ≤ 0.05 was considered significant (2-tail). The analyses were performed with Excel or GraphPad Prism (GraphPad Software, Inc., La Jolla, CA).
RESULTS AND DISCUSSION
SLNs as carriers for oral drug delivery have several advantages, such as improving the stability and enhancing the bioavailability of drugs (5, 13-16). Previously, we engineered DHA-dFdC-SLNs by encapsulating DHA-dFdC into SLNs comprised of soy lecithin, GMS, TPGS, and Tween 20 (6). The mean diameter of the nanoparticles was 100 ± 8 nm, with a PDI value of 0.22 ± 0.02. Nanoparticle size significantly affects the gastrointestinal absorption, and nanoparticles smaller than 300 nm are good candidate for oral administration (2, 17). Indeed, an evaluation of the cellular uptake of polymeric nanoparticles by Caco-2 cells in culture showed that the most desirable particle size is about 100-200 nm (17). The surface properties of the nanoparticles are important for the cellular uptake of the nanoparticles; surface modification of nanoparticles with vitamin E TPGS is known to improve the cellular uptake of the nanoparticles (17). The zeta potential of the DHA-dFdC-SLNs was −41 ± 2 mV, indicating that they are stable in an aqueous suspension (17, 18). Finally, the encapsulation efficiency of the DHA-dFdC in the DHA-dFdC-SLNs was 94.5 ± 9.5% (n = 7).
Stability of DHA-dFdC-SLNs in stimulated gastrointestinal fluids
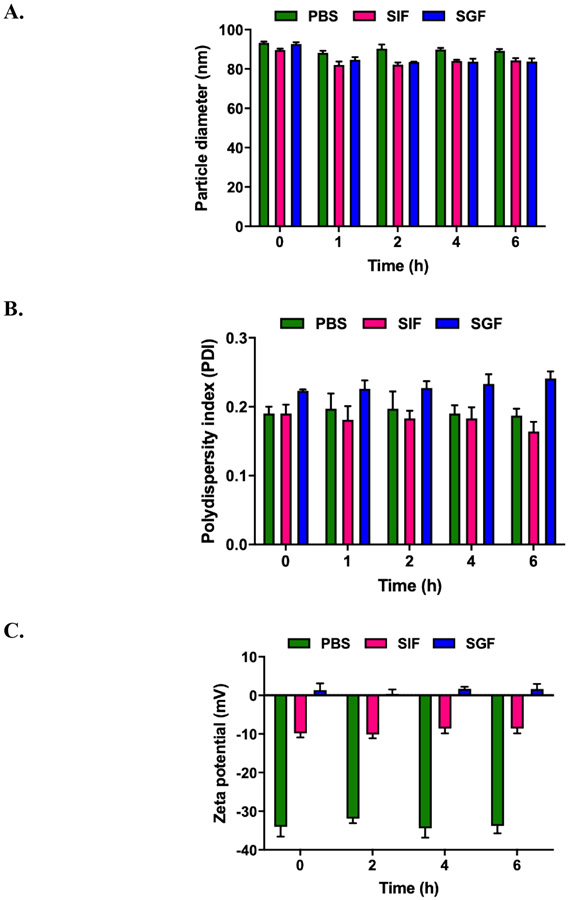
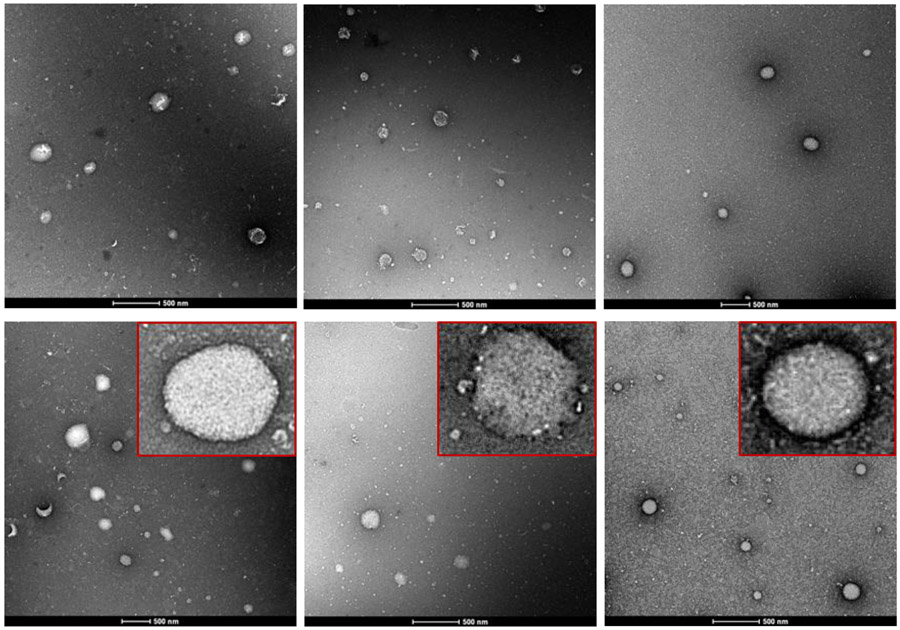
Shown in Figure 1A-C are the particle size, PDI, zeta potential values of DHA-dFdC-SLNs as a function of time when incubated in SGF or SIF. As a control, the stability of the DHA-dFdC-SLNs in PBS (10 mM, pH 7.4) was also studied. The particle size, PDI, and zeta potential values of the DHA-dFdC-SLNs, as measured by DLS, did not increase during a 6 h incubation in SGF or SIF. The particle size actually decreased slightly (i.e. ~5.4% in SIF and 6.1% in SGF, as compared to in PBS) (Fig. 1A). As expected, the medium in which the DHA-dFdC-SLNs were dispersed to significantly affected the zeta potential of the nanoparticles (Fig. 1C). Shown in Fig. 1D-I are representative TEM images of the nanoparticles before and after 6 h of incubation in SGF or SIF. Overall, the shape of the nanoparticles did not change significantly after the incubation; however, after 6 h of incubation in SIF, the surface of the DHA-dFdC-SLNs appeared rough (Fig. 1G, inset), which is not the case after the DHA-dFdC-SLNs were incubated in SGF (Fig. 1I, inset). Studies examining the degradation of SLNs in GI fluids showed that their degradation induces a decrease in particle size due to the loss of surface active agents coated on the nanoparticles, which ultimately led to an increase in the particle size, because the nanoparticles aggregate in the absence of the surface active agents (18, 19). It was reported that non-ionic surfactants such as Tween 20 and polyvinyl alcohol provide steric stabilization to particles in acidic pH (20). Tween 20 was used as a surface active agent in the DHA-dFdC-SLNs, which might explain the stability of the DHA-dFdC-SLNs in the SGF. TPGS is a non-ionic surface active agent as well, and the presence of TPGS in the DHA-dFdC-SLNs may have also contributed to the stability of the nanoparticles in simulated GI fluids. It is worth noting that the SGF and SIF used in this study did not contain enzymes. The nanoparticles would likely be less stable if enzymes were included in the fluids, as when the SLNs are in the GI tract after oral administration.
Figure 1.
Stability of DHA-dFdC-SLNs in simulated gastrointestinal fluids. DHA-dFdC-SLNs were incubated with SGF (pH 1.2) or SIF (pH 6.8) at 37°C. Samples were collected at 0, 1, 2, 4 and 6 h to measure the particle size (A), PDI (B), and zeta potential (C). As a control, DHA-dFdC-SLNs were also incubated with PBS. Data shown are mean ± SD (n = 3). D-I. Representative TEM images of DHA-dFdC-SLNs incubated with PBS (D-E at 0 and 6 h, respectively), SIF (F-G at 0 and 6 h, respectively), and SGF (H-I at 0 and 6 h, respectively). Bar = 500 nm. Shown in insets are selected nanoparticles in a higher magnification (bar = 200 nm).
In vitro release in simulated gastrointestinal fluids
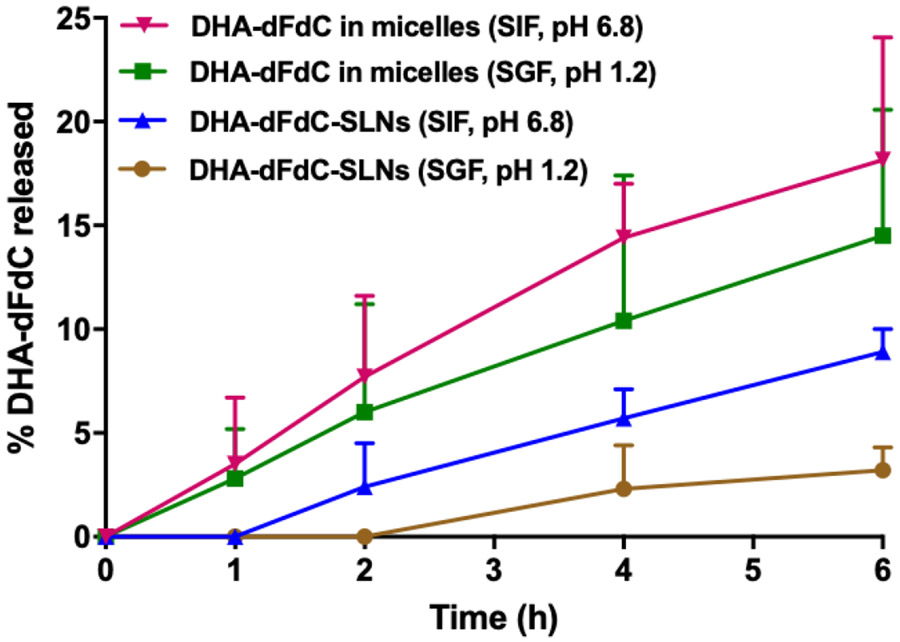
The release profiles of DHA-dFdC from the DHA-dFdC-SLNs in simulated GI fluids are shown in Fig. 2. After 6 h, the cumulative release of DHA-dFdC reached ~ 8.9% and ~ 3.2% in SIF and SIG, respectively. Similar to the stability study mentioned above, the release of DHA-dFdC from the DHA-dFdC-SLNs was monitored for 6 h only, because it was reported that the GI transition time in mice is 6-8 h (21). As shown in Fig. 1G inset, the surface of the DHA-dFdC-SLNs is not smooth after 6 h of incubation in SIF, suggesting erosion of the particles, which may explain the relatively faster release of DHA-dFdC from the DHA-dFdC-SLNs in SIF than in SGF. The release of the DHA-dFdC out of the DHA-dFdC in Tween 20 micelles was much faster than the release of the DHA-dFdC from the DHA-dFdC-SLNs (Fig. 2), indicating that the release profiles of the DHA-dFdC from the DHA-dFdC-SLNs truly represent the release, not the diffusion of the DHA-dFdC released from the SLNs across the dialysis membrane and its re-partition into Tween 20 micelles in the release medium. Finally, when the diffusion of DHA-dFdC across the dialysis membrane was measured with a DHA-dFdC in an ethanol in water solution (90, v/v), more than 20% the DHA-dFdC diffused out the dialysis device within 60 min (Fig. 2), close to 100% in 2 h (data not shown), much faster that the release of DHA-dFdC from the DHA-dFdC-SLNs or out of the DHA-dFdC in Tween 20 micelles. Taken together, it is clear that the release profiles shown in Figure 2 truly represented the in vitro release of DHA-dFdC from the DHA-dFdC-SLNs in simulated GI fluids.
Figure 2.
In vitro release profiles of DHA-dFdC from DHA-dFdC-SLNs in simulated gastrointestinal fluids. As controls, the release of of DHA-dFdC from Tween 20 micelles and then diffusion across the dialysis membrane were monitored, as well as the diffusion of DHA-dFdC across the dialysis membrane when dissolved in a 90% ethanol in water solution. Data shown are mean ± SD (n = 3).
Oral bioavailability of DHA-dFdC
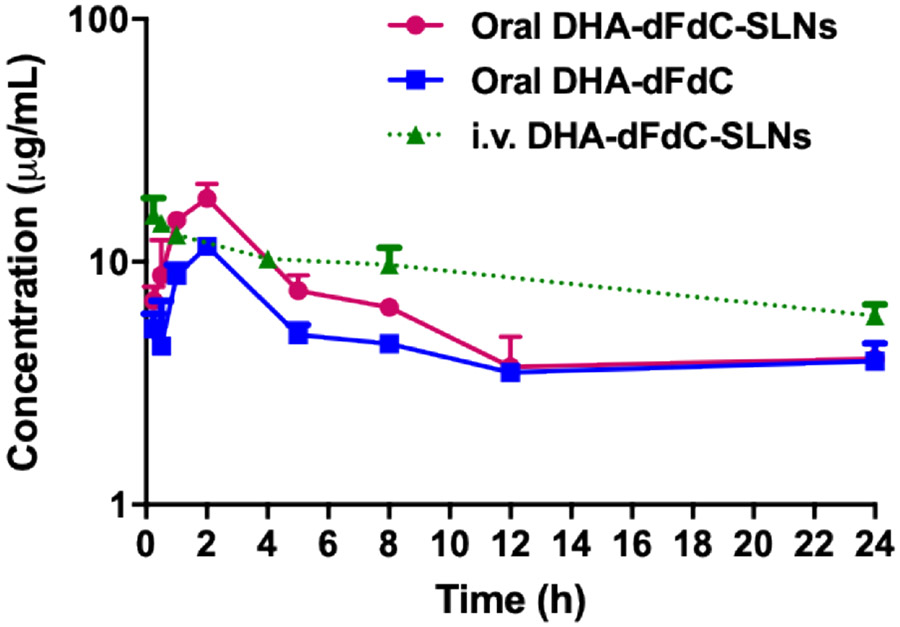
The plasma concentrations of DHA-dFdC at different time points after oral administration or intravenous injection of the DHA-dFdC-SLNs in suspension are shown in Fig. 3. Selected PK parameters of DHA-dFdC are summarized in Table 1. The plasma DHA-dFdC concentration vs. time curve after intravenous injection of the DHF-dFdC-SLNs in healthy mice was fitted in a two-compartment model, resulting an AUC0-24 h value of 210.58 μg*h/mL when calculated using PK Solver (213.0 with a 95% confident interval (CF) of 183.1-242.8, when calculated using GraphPad Prism,). On the other hand, the plasma DHA-dFdC level in mice after oral administration of the DHA-dFdC-SLNs followed an adsorption phase and then a clearance phase, with a Cmax of 17.01 μg/mL, Tmax of 1.73 h, and AUC0-24 h value of 143.44 μg*h/mL (144.3 with a 95% CI of 119.0-169.6, when calculated using GraphPad Prism). The absolute oral bioavailability of DHA-dFdC in the DHA-dFdC-solid lipid nanoparticle formulation was calculated to be 68.12% based on the AUC0-24 h values shown in Table 1.
Figure 3.
Plasma DHA-dFdC concentration-time curves after oral administration of DHA-dFdC-SLNs in suspension or DHA-dFdC in Tween 80/ethanol-in-water solution, or i.v. administration of DHA-dFdC-SLNs in suspension in healthy C57BL/6 mice. The dose of DHA-dFdC was 2 mg per mouse. Data shown are mean ± SD (n = 3). Data of the i.v. DHA-dFdC-SLNs group were adapted from reference (7) (we will request for copyright permission upon the acceptance of this manuscript).
Table 1.
Selected PK parameters of DHA-dFdC in mouse plasma samples after intravenous (i.v.) administration of DHA-dFdC-SLNs or oral administration of DHA-dFdC in DHA-dFdC-SLNs or in a Tween 80/ethanol-in-water solution.
| Parameter | Unit | DHA-dFdC-SLNs, i.v. |
DHA-dFdC-SLNs, p.o. |
DHA-dFdC solution, p.o. |
|---|---|---|---|---|
| Tmax | h | N/A | 1.73 | 1.75 |
| Cmax | μg/mL | N/A | 17.01 | 10.50 |
| AUC0-24 h | (μg/mL)*h | 210.58 | 143.44 | 113.55 |
| Fab | % | 68.12 | ||
| Frel | % | 126.32 |
Tmax, time at which the plasma concentration of DHA-dFdC reached the maximum; Cmax, the maximum concentration of DHA-dFdC in mouse plasma; AUC0-24 h, area under the concentration curve (t = 0-24 h); Fab%, absolute oral bioavailability in percentage; Frel %, relative oral bioavailability in percentage (as compared to DHA-dFdC solution, p.o.).
For a comparison, the plasma concentration of DHA-dFdC vs. time curve of the DHA-dFdC after it was orally administered in a Tween 80/ethanol-in-water solution was also showed in Fig. 3 as well. The Tmax was ~1.7 h, similar to that of the oral DHA-dFdC-SLNs (Table 1). However, the Cmax and AUC0-24 h values of the DHA-dFdC solution were 10.50 μg/mL and 113.55 μg*h/mL (114.3 with a 95% CI of 104.6-124.0, when calculated using GraphPad Prism), respectively. Therefore, the bioavailability of DHA-dFdC orally given as the DHA-dFdC-SLNs, relative to in solution, was 126.4%.
The exact mechanism by which the DHA-dFdC in the DHA-dFdC-SLNs was absorbed after oral administration is unknown. In general, orally administered SLNs may be absorbed as intact particles through the microfold cells in the Peyer’s patches and then transported to the lymphatic system (22). However, others suggested that SLNs suffer from digestion or degradation in the GI tract, and only a very small fraction, if any, of orally administered SLNs can reach the blood circulation intact (23). Of course, DHA-dFdC may also be released from the DHA-dFdC-SLNs in the GI tract, especially in the presence of lipases and co-lipases from pancreas, and then absorbed by passive diffusion or with the help of bile in the GI tract (24, 25).
As to the higher oral bioavailability of DHA-dFdC when given as DHA-dFdC-SLNs in suspension, relative to DHA-dFdC in solution, the DHA-dFdC in solution may be susceptible to degradation and/or precipitation when orally administered, which can lead to a decrease in its bioavailability (6). It was also thought that the exogenous lipids released from SLNs after digestion (i.e. exogenous solubilizing components) may lead to a change in the GI fluid (25), and we suspect that the change in the GI fluid may have helped to enhance the solubility of DHA-dFdC. Nonetheless, the DHA-dFdC solution contains Tween 80, which may explain the relatively high oral bioavailability of DHA-dFdC in the solution (26). Tween 80 may be digested by intestinal cells to release oleic acid, which was shown to increase the basolateral secretion of triglyceride-rich lipoproteins such as chylomicrons, increasing the lymphatic uptake of lipophilic compounds (26). In addition, it was reported that TGPS as emulsifier in a paclitaxel-polymeric nanoparticle formulation helped to increase the oral bioavailability of paclitaxel by 10-fold, as compared to oral Taxol (21). TPGS-emulsified SLNs were also shown to improve the relative oral bioavailability of docetaxel in rats (29). Therefore, the high oral bioavailability of DHA-dFdC in the DHA-dFdC-SLNs may be attributed in part to the presence of TPGS in the formulation.
Effect of DHA-dFdC-SLNs on the survival of B16-F10 tumor-bearing mice
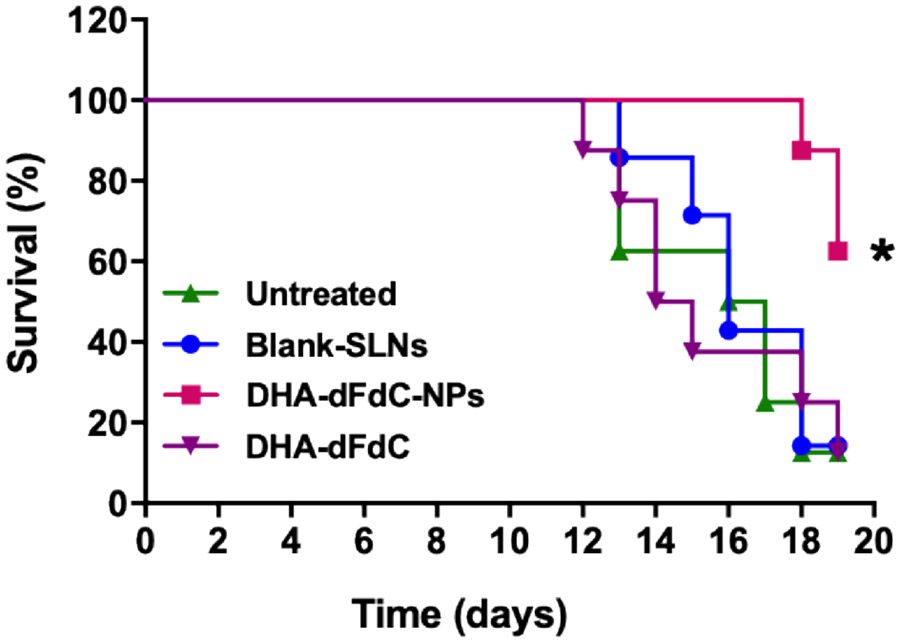
The effect of the DHA-dFdC-SLNs on the survival of mice with tumor was also evaluated. Previously, we reported that DHA-dFdC-SLNs significantly inhibited the growth of B16-F10 tumor cells in culture as well as in a mouse model when given intravenously (7). Consequently, B16-F10 tumor-bearing mice were used to test the effect of DHA-dFdC-SLNs on mouse survival when given orally. DHA-dFdC-SLNs were orally gavaged at a dose of 250 μg of DHA-dFdC per mouse daily for a total of 12 days (with a two-day rest in the middle). As shown in Fig. 4, 50% of mice in the untreated group reached the endpoints on day 16. Oral DHA-dFdC-SLNs significantly improved the survival of the tumor-bearing mice, as compared to the untreated group (p < 0.05). Oral DHA-dFdC in solution did not significantly affect mouse survival as compared to mice left untreated, which is surprising because the DHA-dFdC was also orally available when given as the DHA-dFdC in Tween 80/ethanol-in-water solution (Table 1, Fig. 3). Adverse reactions associated with the DHA-dFdC solution were likely related to the lack of survival advantage of the oral DHA-dFdC solution over untreated mice, as 62.5% of mice orally gavaged with the DHA-dFdC solution, at the dosing regimen tested, showed signs of adverse effects such as a body weight decrease of more than 20% (in one mouse) or severe tumor ulceration/bleeding (in four mice) and had to be euthanized. The exact reasons underlying the adverse effects caused by the DHA-dFdC in Tween 80/ethanol-in-water solution remains unknown, but should be related to the Tween 80/ethanol-in-water solution formulation, although the amounts of Tween 80 and ethanol taken by mice from the DHA-dFdC solution were within the normal range recommended for preclinical animal study. It was reported that a 5% solution of ethanol given orally to mice for one month was well tolerated, and in rats, Tween 80 given orally at 5 ml/kg for 4 weeks was well tolerated (30, 31). It is speculated that when mice were gavaged with the DHA-dFdC in the Tween 80/ethanol-in-water solution, the GI tract of the mice was exposed to a high concentration of free DHA-dFdC immediately. In contrast, when mice were gavaged with the same amount of DHA-dFdC in the DHA-dFdC-SLNs, it took a longer time for the DHA-dFdC to be released from the DHA-dFdC-SLNs, and thus the GI tract of the mice at any time point was only directly exposed to the DHA-dFdC released from the nanoparticles.
Figure 4.
Survival curves of B16-F10 tumor-bearing mice after oral treatment with DHA-dFdC-SLNs. Tumor cells were injected (s.c.) on day 0. On day 7, mice were randomized and orally gavaged with DHA-dFdC-SLNs in suspension or DHA-dFdC in a Tween 80/ethanol-in-water solution. As controls, mice received DHA-dFdC-free SLNs (blank-SLNs) or left untreated. * p < 0.05, DHA-dFdC-SLNs vs. all other groups (Log-rank Mantel-Cox test. Data shown are mean ± SD (n = 7-8).
CONCLUSION
DHA-dFdC is a new compound with strong antitumor activity. Previously, we developed a solid lipid nanoparticle formulation of DHA-dFdC to improve its water solubility and chemical stability. In the present study, we reported that the solid lipid nanoparticle formulation also enabled the DHA-dFdC to be administered by the oral route by increasing the oral bioavailability of DHA-dFdC in a mouse model. Encapsulation of DHA-dFdC into a solid lipid nanoparticle formulation represents a viable strategy to increase its apparent water solubility, chemical stability, and oral bioavailability.
ACKNOWLEDGEMENTS
This work was supported in part by the U.S. National Institutes of Health (CA179362) and the Alfred and Dorothy Mannino Fellowship in Pharmacy at UT Austin. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. SAV was supported in part by the Becas-Chile Scholarship from Chile. RFA was supported in part by a scholarship from the King Saud University.
Footnotes
DECLARATION OF INTERESTS:
ZC was a consultant to CBM Biopharma, Inc., which licensed DHA-dFdC from The University of Texas System Board of Reagents. The terms of this arrangement have been reviewed and approved by UT Austin in accordance with its policy on objectivity in research.
REFERENCES:
- 1.Date AA, Hanes J, and Ensign LM. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. Journal of Controlled Release. 240:504–526 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thanki K, Gangwal RP, Sangamwar AT, and Jain S. Oral delivery of anticancer drugs: challenges and opportunities. Journal of controlled release. 170:15–40 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Liu G, Franssen E, Fitch MI, and Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. Journal of Clinical Oncology. 15:110–115 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Eek D, Krohe M, Mazar I, Horsfield A, Pompilus F, Friebe R, and Shields AL. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient preference and adherence. 10:1609 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin C-H, Chen C-H, Lin Z-C, and Fang J-Y. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. Journal of food and drug analysis. 25:219–234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naguib YW, Lansakara-P D, Lashinger LM, Rodriguez BL, Valdes S, Niu M, Aldayel AM, Peng L, Hursting SD, and Cui Z. Synthesis, Characterization, and In Vitro and In Vivo Evaluations of 4-(N)-Docosahexaenoyl 2′ , 2′ -Difluorodeoxycytidine with Potent and Broad-Spectrum Antitumor Activity. Neoplasia. 18:33–48 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valdes S, Alzhrani R, Rodriguez A, Lansakara-P DSP, Thakkar SG, and Cui Z. A solid lipid nanoparticle formulation of 4-(N)-docosahexaenoyl 2′ , 2′ -difluorodeoxycytidine with increased solubility, stability, and antitumor activity, Int. J. Pharm, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wangand Y C. Zheng. Oral 4-(N)-stearoyl gemcitabine nanoparticles inhibit tumor growth in mouse models. Oncotarget. 8:89876 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu S, Lansakara-P DS, Li X, and Cui Z. Lysosomal delivery of a lipophilic gemcitabine prodrug using novel acid-sensitive micelles improved its antitumor activity. Bioconjugate chemistry. 23:966–980 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valdes S, Naguib YW, Finch RA, Baze WB, Jolly CA, and Cui Z. Preclinical Evaluation of the Short-Term Toxicity of 4-(N)-Docosahexaenoyl 2´, 2´-Difluorodeoxycytidine (DHA-dFdC). Pharmaceutical Research. 34:1224–1232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloat BR, Sandoval MA, Li D, Chung W-G, Lansakara-p DS, Proteau PJ, Kiguchi K, DiGiovanni J, and Cui Z. In vitro and in vivo anti-tumor activities of a gemcitabine derivative carried by nanoparticles. International journal of pharmaceutics. 409:278–288 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Huo M, Zhou J, and Xie S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Computer methods and programs in biomedicine. 99:306–314 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Üner M, Wissing S, Yener G, and Müller R. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for application of ascorbyl palmitate. Die Pharmazie-An International Journal of Pharmaceutical Sciences. 60:577–582 (2005). [PubMed] [Google Scholar]
- 14.Lim S-J, Lee M-K, and Kim C-K. Altered chemical and biological activities of all-trans retinoic acid incorporated in solid lipid nanoparticle powders. Journal of controlled release. 100:53–61 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Yuan Q, Han J, Cong W, Ge Y, Ma D, Dai Z, Li Y, and Bi X. Docetaxel-loaded solid lipid nanoparticles suppress breast cancer cells growth with reduced myelosuppression toxicity. International journal of nanomedicine. 9:4829 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MuÈller RH, MaÈder K, and Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. European journal of pharmaceutics and biopharmaceutics. 50:161–177 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Winand KY Feng S-S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 26:2713–2722 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Aditya N, Shim M, Lee I, Lee Y, Im M-H, and Ko S. Curcumin and genistein coloaded nanostructured lipid carriers: in vitro digestion and antiprostate cancer activity. Journal of agricultural and food chemistry. 61:1878–1883 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Müller RH, Rühl D, and Runge SA. Biodegradation of solid lipid nanoparticles as a function of lipase incubation time. International journal of pharmaceutics. 144:115–121 (1996). [Google Scholar]
- 20.Van Aken GA, Bomhof E, Zoet FD, Verbeek M, and Oosterveld A. Differences in in vitro gastric behaviour between homogenized milk and emulsions stabilised by Tween 80, whey protein, or whey protein and caseinate. Food Hydrocolloids. 25:781–788 (2011). [Google Scholar]
- 21.Zhaoand L Feng SS. Enhanced oral bioavailability of paclitaxel formulated in vitamin E‐TPGS emulsified nanoparticles of biodegradable polymers: In vitro and in vivo studies. Journal of pharmaceutical sciences. 99:3552–3560 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Li H, Zhao X, Ma Y, Zhai G, Li L, and Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. Journal of Controlled Release. 133:238–244 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Hu X, Fan W, Yu Z, Lu Y, Qi J, Zhang J, Dong X, Zhao W, and Wu W. Evidence does not support absorption of intact solid lipid nanoparticles via oral delivery. Nanoscale. 8:7024–7035 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Thomson A, Keelan M, Garg M, and Clandinin M. Intestinal aspects of lipid absorption: in review. Canadian journal of physiology and pharmacology. 67:179–191 (1989). [DOI] [PubMed] [Google Scholar]
- 25.Porter CJ, Trevaskis NL, and Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nature Reviews Drug Discovery. 6:231 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Seeballuck F, Lawless E, Ashford MB, and O’Driscoll CM. Stimulation of Triglyceride-Rich Lipoprotein Secretion by Polysorbate 80: In Vitro and in Vivo Correlation Using Caco-2 Cells and a Cannulated Rat Intestinal Lymphatic Model. Pharmaceutical Research. 21:2320–2326 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Nerurkar MM, Burton PS, and Borchardt RT. The Use of Surfactants to Enhance the Permeability of Peptides Through Caco-2 Cells by Inhibition of an Apically Polarized Efflux System. Pharmaceutical Research. 13:528–534 (1996). [DOI] [PubMed] [Google Scholar]
- 28.Guo Y, Luo J, Tan S, Otieno BO, and Zhang Z. The applications of Vitamin E TPGS in drug delivery. European Journal of Pharmaceutical Sciences. 49:175–186 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Cho H-J, Park JW, Yoon I-S, and Kim D-D. Surface-modified solid lipid nanoparticles for oral delivery of docetaxel: enhanced intestinal absorption and lymphatic uptake. International journal of nanomedicine. 9:495 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gad SC, Cassidy CD, Aubert N, Spainhour B, and Robbe H. Nonclinical vehicle use in studies by multiple routes in multiple species. International journal of toxicology. 25:499–521 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Shimizu S. Routes of administration. The laboratory mouse:527–541 (2004). [Google Scholar]