Abstract
Background
The aim of this study was to compare the clinical efficacy and postoperative recovery between uterine artery embolization (UAE) and infrarenal aortic balloon occlusion (IABO) in planned cesarean sections for placenta accreta spectrum disorders.
Material/Methods
A retrospective analysis using the clinical data of 62 patients with placenta previa combined with placenta accreta for planned cesarean between January 2014 and December 2019 was performed at the First People’s Hospital in Lianyungang. Thirty-five cases undergoing UAE during cesarean section were defined as group A, while the other 27 cases undergoing IABO were defined as group B. Intraoperative and postoperative parameters including intraoperative blood loss, blood transfusion volume, radiation duration, radiation dose, hysterectomy rate, operation duration, Intensive Care Unit hospitalization, complications, and neonatal outcomes as well as the maternal recovery during follow-up were compared between the 2 groups.
Results
Intraoperative blood loss, transfusion volume, radiation time, radiation dose, hysterectomy rate, duration of surgery, Intensive Care Unit admission, and complications were higher in group A than group B, with differences being statistically significant (P<0.05). There were no significant differences in birth weight, 1-min Apgar score, neonatal asphyxia rate, admission to Neonatal Intensive Care Unit, breastfeeding time, duration of postpartum lochia, and data related to menstruation between the 2 groups (P>0.05).
Conclusions
IABO, which was more effective than UAE in cesarean section of patients with placenta accreta spectrum, could be further applied in treatment.
Keywords: Placenta Accreta, Balloon Occlusion, Uterine Artery Embolization
Background
The term placenta accreta was first introduced in 1908 by Baisch [1], and modern pathologists define this abnormality as a spectrum disorder. A newer and more accurate term, placenta accreta spectrum (PAS) disorders, which include abnormal adherence and invasive placenta, is now used worldwide [2]. The 2018 International Federation of Gynecology and Obstetrics (FIGO) has classified 3 subtypes depending on the depth of invasion of placental tissue: placenta accreta (attachment to the superficial myometrium), placenta increta (invasion into the deeper myometrium), and placenta percreta (penetration through the uterus serosa with or without infiltration of adjacent pelvic organs) [3]. The term “accreta” was once the common term for all 3 conditions. Placenta percreta is the rarest and most severe condition, and it carries the risk of severe postpartum hemorrhage (PPH). Severe PPH may be associated with massive transfusion requirements, hysterectomy, disseminated intravascular coagulation, surgical complications, multi-organ failure, and death [4]. Major risk factors are previous cesarean section, advanced maternal age, multiparity, and a history of endouterine maneuvers. A history of previous cesarean section and placenta previa are the most common risk factors for PAS [3].
The incidence of PAS shows an obviously increasing trend in China, given continuously increasing cesarean section rates and women choosing to have a second pregnancy following implementation of a policy allowing families to have 2 children [5]. In PAS, the abnormal placenta is frequently supplied by extensive arterial collaterals, including the uterine artery; the ovarian, cervical, and vaginal artery system; and the lumbar, sacral, mesenteric, and iliolumbar arteries. Arterial anastomosis of the external iliac artery is also possible [6,7]. Therefore, cesarean section for patients with PAS is extremely complex and uncontrolled hemorrhage often occurs. Hysterectomy was once the only choice of treatment to save the lives of patients [8]. How to reduce the amount of intraoperative bleeding, stop bleeding quickly and effectively, and retain the fertility function has become a pressing problem for obstetricians.
With the rapid development of interventional medicine, interventional therapy has played an increasingly important role in the multidisciplinary treatment of PAS [9–15], including internal iliac artery embolization, uterine artery embolization, internal iliac artery balloon block, and abdominal aortic balloon block. However, the curative effects of some techniques are limited due to extensive collateral blood supply in the uterus of PAS patients. Interventional technologies in cesarean section for PAS have been applied in our hospital since 2012 and are widely used in clinical practice now. Uterine artery embolization (UAE) and infrarenal aortic balloon occlusion (IABO) are the most common auxiliary surgical methods. By limiting uterine blood perfusion, good therapeutic effects have been achieved clinically. Previous clinical studies have not yielded definite conclusions about comparisons between UAE and IABO, and our study is therefore designed to compare their intraoperative efficacy and postpartum recovery, so as to provide evidence for the treatment of patients with PAS.
Material and Methods
Data Collection and Follow-Up
The study was designed as a retrospective study and was approved by the Institutional Review Board at the First People’s Hospital of Lianyungang. Informed signed consent was obtained from each patient involved in this study.
From January 2014 to December 2019, the database of our institution was retrospectively reviewed to identify all cases of PAS. The inclusion criteria were as follows: (1) diagnosis of placenta previa combined with placenta accreta by ultrasonography and magnetic resonance imaging preoperatively and confirmed by pathological diagnosis postoperatively; (2) a history of at least 1 uterine low segment cesarean delivery; (3) underwent UAE or IABO during cesarean delivery; and (4) all patients included underwent the planned cesarean section. Exclusion criteria included (1) unstable vital signs caused by preoperative hemorrhage; (2) patients with severe medical and surgical diseases, severe dysfunction of heart, liver, and kidney, and abnormal coagulation function; (3) patients with other complications of pregnancy; and (4) patients with contrast agent allergy. Finally, 62 cases of PAS were collected. Thirty-five cases of UAE during cesarean section were defined as group A, while 27 cases of IABO were defined as group B.
Clinical data were obtained in the procedure as well as during follow-up and reviewed, including intraoperative bleeding volume, transfusion volume, operation time, radiation time, radiation dose, postoperative hospitalization stay, Intensive Care Unit (ICU) admission, hysterectomy rate, complications (eg, pain, fever, numbness, thrombosis), neonatal weight, 1-min Apgar score, Neonatal Intensive Care Unit (NICU) admission, duration of breastfeeding, duration of lochia, uterine involution, pelvic ultrasound, menstrual recovery time, first postpartum menstrual volume, the time of normal menstrual volume, menstrual cycle, duration of menstruation, and chronic pelvic pains.
Operational Procedure
A multidisciplinary team for treating PAS, including senior obstetricians, gynecologists, radiologist, vascular surgeons, anesthesiologists, neonatologists, urologists, and blood transfusion physicians, had a preoperative discussion. All operations were performed in a hybrid operating room.
In group A, the right femoral artery was punctured with 5F vascular sheaths after local anesthesia. The catheters were super-selectively inserted into bilateral uterine arteries and reserved. Cesarean section was done under general anesthesia. The uterine incision margin was clamped immediately after delivery, and gauze was placed in the lower segment of the uterus to reduce bleeding. Then uterine artery embolization was performed. Gelatin sponge particles (1000–1400 μm, Ellicon Pharmaceutical Technology Co., Ltd., China) were pushed slowly along the catheter in the bilateral uterine arteries under fluoroscopy until embolization was completed. The maximum dosage was 800 mg in this study. Attempts were then made to remove the placenta manually. Various surgical methods were used to reduce bleeding, such as local suture, gauze filling, B-Lynch suture, and so forth. According to the intraoperative conditions, a hysterectomy would be performed if necessary. After the operation, the puncture point was bandaged with pressure and the lower limbs were immobilized for 24 h.
In group B, the left brachial artery was exposed by skin incision after local anesthesia. The guide wire and catheter were descended along the left subclavian artery, descending aorta, and abdominal aorta to the upper part of the common iliac artery bifurcation and below the level of the renal artery. After removing the sheath tube, a fully compliant balloon (AB46, Medtronic, Santa Rosa, Calif.) was placed to L3–4 level (Figure 1). Normal saline was injected to inflate the balloon until the patient’s foot pulse disappeared, indicating that the abdominal aorta had been successfully blocked. The balloon was deflated, and the amount of liquid used was recorded. The cesarean section was then performed under general anesthesia. The balloon was inflated just before the uterus was incised for delivery (Figure 2). Attempts were made to remove the placenta manually. If removal was difficult or the hemostatic effect was not satisfactory, local excision of placenta and uterine tissue was performed, and the defect was subsequently repaired. If the placenta invaded or penetrated the uterine wall in a large area, hysterectomy would be performed directly. The balloon would be deflated for 1 min every 20 min until uterine surgery was completed. Clinical application generally needed 2–3 cycles in our hospital.
Figure 1.
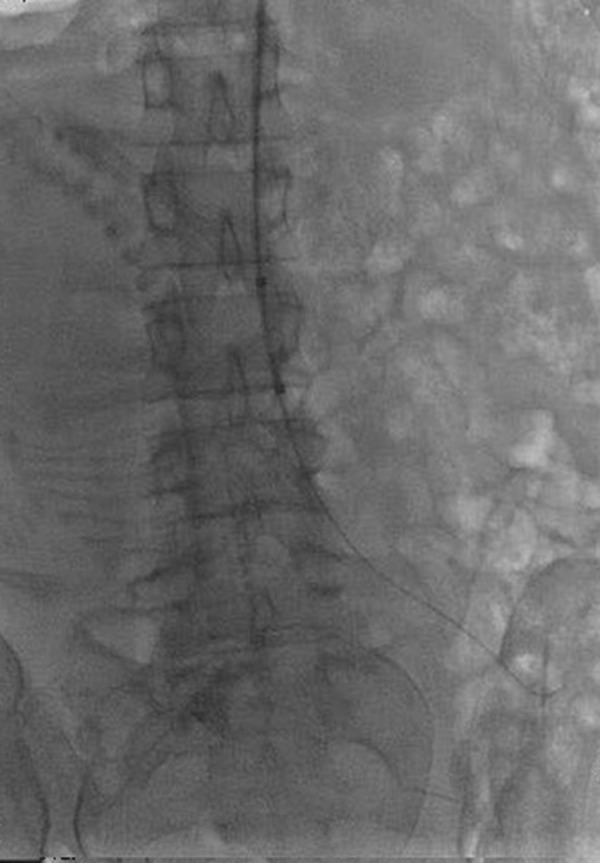
The image shows the balloon placed in a predetermined position in the abdominal aorta.
Figure 2.
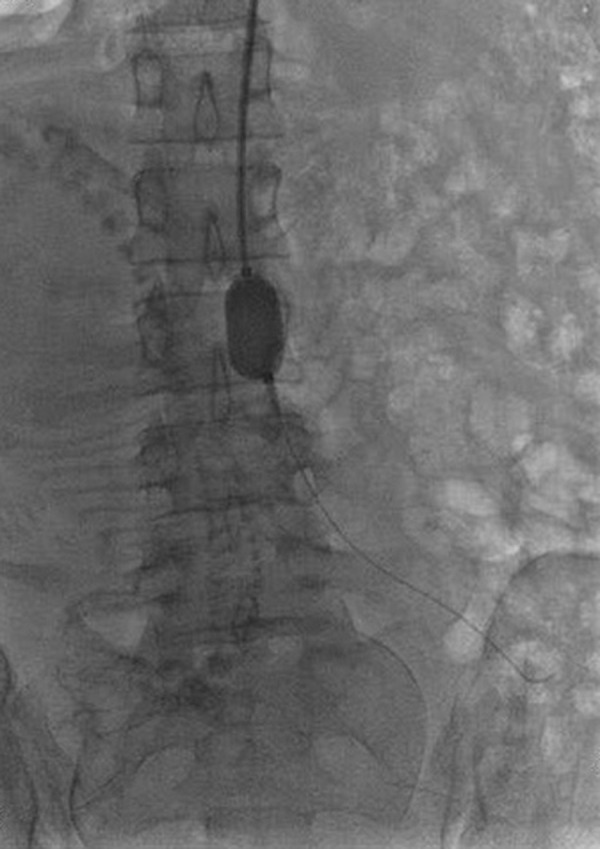
The image shows the filled balloon blocking the abdominal aorta.
The principle of uterine incision selection was to avoid placental attachment to prevent massive bleeding. Preoperative ultrasound could identify the upper edge of the placenta and help guide abdominal and uterine incision. If the placenta was anterior and extended to the umbilical level, the uterus had to be incised high above the placenta or, more commonly, a transverse incision at the uterine base was made to facilitate removal of the baby. When the placenta was about to be removed or completely separated, uterotonic drugs were used based on the uterine contractions. Commonly used drugs were oxytocin, Herba Leonuri, and carboprost tromethamine. Uterotonic drugs were not used when hysterectomy was planned in patients with advanced uterine serosa perforation or bladder invasion.
Statistical Analysis
SPSS 24 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Continuous variables in a Gaussian distribution are expressed as means±standard deviations. Categorical variables are presented as numbers and proportions, and they were analyzed using chi-square or Fisher’s exact tests, as appropriate. Continuous variables between groups were compared using the 2-tailed t test. P value <0.05 was considered significant.
Results
Patient Characteristics
Sixty-two cases of placenta previa combined with placenta accreta were collected. Thirty-five cases of UAE during cesarean section were defined as group A, while the 27 cases of IABO were defined as group B. There were no significant differences between the 2 groups in age, gestational age, number of pregnancies, and number of deliveries (P>0.05), as shown in Table 1.
Table 1.
General comparison of the 2 groups.
| Parameters | Group A (n=35) | Group B (n=27) | t | P |
|---|---|---|---|---|
| Age, y | 33.11±3.73 | 33.63±3.28 | −0.568 | 0.572 |
| Gestational weeks | 35.89±1.62 | 36.39±1.62 | −1.119 | 0.235 |
| Number of pregnancies | 3.91±1.36 | 3.63±1.12 | 0.883 | 0.381 |
| Number of deliveries | 1.31±0.47 | 1.26±0.45 | 0.466 | 0.643 |
Hospitalization Data
The intraoperative bleeding volume, transfusion volume, operation time, radiation time, and radiation dose were higher in group A than in group B (Table 2), and the difference was statistically significant (P<0.05).
Table 2.
Comparison of intraoperative and postoperative conditions between the 2 groups.
| Parameters | Group A (n=35) | Group B (n=27) | t/χ2 | P |
|---|---|---|---|---|
| Operative time, min | 138.14±22.03 | 98.89±19.68 | 7.282 | <0.001 |
| Amount of bleeding, mL | 1691.43±479.81 | 940.74±381.55 | 6.662 | <0.001 |
| Transfusion volume, mL | 854.29±337.24 | 325.93±364.37 | 5.906 | <0.001 |
| Radiation time, s | 62.63±15.19 | 8.19±2.57 | 18.389 | <0.001 |
| Radiation dosage, mGy | 32.86±4.84 | 5.30±1.51 | 28.489 | <0.001 |
| Hospital stay, d | 6.51±1.74 | 5.07±0.99 | 3.841 | <0.001 |
| ICU admission, n (%) | 13 (37.14) | 3 (11.11) | 5.395 | 0.020 |
| Hysterectomy, n (%) | 8 (22.86) | 1 (3.70) | 4.506 | 0.034 |
| Postoperative complications, n (%) | 10 (28.57) | 2 (7.40) | 4.374 | 0.036 |
| Birth weight, g | 2882.54±411.07 | 2951.11±485.57 | −0.602 | 0.550 |
| Apgar score, score | 8.46±1.09 | 8.74±1.23 | −0.960 | 0.341 |
| Neonatal asphyxia, n (%) | 5 (14.29) | 3 (11.11) | 0.137 | 0.712 |
| NICU admission, n (%) | 5 (14.29) | 4 (14.81) | 0.003 | 0.953 |
ICU – Intensive Care Unit; NICU – Neonatal Intensive Care Unit.
The hospitalization time, ICU admission, and hysterectomy rate were higher in group A than in group B (P<0.05). In group A, 10 cases (28.57%) had complications, including abdominal pain in 5 cases, fever in 2 cases, buttock numbness in 2 cases, and femoral artery thrombosis in 1 case. In group B, abdominal pain occurred in 2 cases (7.40%). The difference of the incidence of complications between the 2 groups was statistically significant (P<0.05).
There was no significant difference in birth weight, 1-min Apgar score, neonatal asphyxia rate, and NICU admission rate between the 2 groups (P>0.05). More detailed information is summarized in Table 2.
Follow-Up
Six out of 62 patients were lost to follow-up, 4 in group A and 2 in group B. The remaining patients breastfed for 6–18 months, and the duration of lochia was within the normal range, with no significant difference between the 2 groups (P>0.05). Physical examination of 47 cases (excluding 9 cases of hysterectomy) at 42 days postpartum indicated that the uterus had healed well. Ultrasound examination showed diverticulum in the uterine scar in 3 cases, including 2 in group A and 1 in group B. No special treatment was done because menstruation was restored and no abnormality was observed. The menstrual cycle, menstrual period, and menstrual volume of 45 cases had no significant change compared with those before pregnancy. In group A, the menstrual volume of 2 cases decreased significantly, which was half of the original menstrual volume. The incidence of chronic pelvic pain in group A was higher than that in group B (P<0.05). More detailed information is summarized in Tables 3 and 4.
Table 3.
Comparison of breastfeeding time and chronic pelvic pain between the 2 groups.
| Parameters | Group A (n=31) | Group B (n=25) | t/χ2 | P |
|---|---|---|---|---|
| Breastfeeding time mo | 10.63±2.78 | 10.08±2.64 | 0.752 | 0.456 |
| Chronic pelvic pain, n (%) | 5 (16.13) | 0 (0) | 4.428 | 0.035 |
Table 4.
Comparison of menstruation-related conditions between the 2 groups.
| Parameters | Group A (n=23) | Group B (n=24) | t | P |
|---|---|---|---|---|
| Duration of lochia, d | 29.04±6.61 | 28.75±5.99 | 0.160 | 0.874 |
| Menstrual resurgence time, mo | 6.21±1.85 | 6.68±1.98 | −0.727 | 0.471 |
| F/N | 1.42±0.61 | 1.31±0.58 | 0.654 | 0.517 |
| RNM | 2.22±0.95 | 2.00±0.98 | 0.772 | 0.444 |
| Menstrual cycle length, d | 30.04±3.46 | 30.75±4.47 | −0.605 | 0.548 |
| Duration of menstruation, d | 5.17±0.89 | 5.29±0.99 | −0.427 | 0.672 |
F/N – first menstrual volume after operation/normal menstrual volume; RNM – menstrual cycle frequency needed to restore normal menstrual volume.
Discussion
Postpartum hemorrhage is the most common obstetric complication and the leading cause of maternal death in China. If the bleeding cannot be stopped by conservative treatment, surgery and interventional therapy or even hysterectomy are necessary [12,16]. The most important risk of PAS is intractable postpartum hemorrhage. Kassem and Alzahrani [17] found that the average amount of bleeding in patients with PAS was about 3000 mL, with bleeding in 20% of the patients exceeding 5000 mL. Some experts once thought that cesarean section combined with hysterectomy was the best way to treat placenta previa [11]. Minimizing the risk of bleeding, shock, disseminated intravascular coagulation, organ loss, and maternal mortality requires the collaboration of obstetricians, radiologist, vascular surgeons, anesthesiologists, neonatologists, and urologists. In recent decades, endovascular interventions have been used in the treatment of PAS to reduce intraoperative bleeding and hysterectomy rate. Due to collateral arterial blood supply of the abnormal placenta and extensive intrapelvic vascular anastomosis, the curative effect is limited for methods such as internal iliac artery balloon occlusion [18]. In this regard, greater benefit is expected from balloon occlusion of the common iliac artery; however, lower extremity arterial thrombosis and common iliac artery dissection and other serious complications have also been reported [19,20].
Interventional techniques for PAS patients have been used in our hospital since 2012. UAE and IABO are the most common auxiliary methods at present, and they have achieved good therapeutic effects in clinical practice [10,21]. By analyzing the cases admitted to our hospital in recent years, we found that group B was superior to group A in control of bleeding volume, reduced blood transfusion volume, shortened operation time and hospitalization days, reduced radiation time and dosage, as well as lower rates of hysterectomy, postoperative complications, and ICU admission (P<0.05). IABO provides a more proximal occlusion that blocks the infrarenal blood supply to the placenta. There was no significant difference in birth weight, 1-min Apgar score, and NICU admission between the 2 groups, indicating that the 2 surgical methods had no significant effect on neonatal prognosis.
No data are currently available about menstrual recovery and ovarian function after interventional therapy. The blood supply of the ovary comes from the ovarian artery and the ascending branch of the uterine artery, the former from the abdominal aorta and the latter from the anterior branch of the internal iliac artery. Theoretically, interventional therapy may affect the blood supply of the ovary to some extent, possibly leading to menstrual disorders. However, in this study, we used gelatin sponge particles with effective biocompatibility as a medium-effective embolic agent that would dissolve and be absorbed 2–3 weeks after embolization, so that blood flow could be restored. Through follow-up, we found no significant difference in menstruation-related indicators between the 2 groups, indicating that the 2 treatment methods had no significant effect on puerperal ovarian function. Chronic pelvic pain was significantly higher in group A than in group B, and menstrual volume in 2 cases was significantly reduced, which may have been related to incomplete dissolution and absorption after embolization, leading to uterine ischemia and necrosis. However, balloon occlusion was temporary and had no significant effect on the ovary or uterus.
During operation, we observed that hemorrhage was significantly reduced in the surgical field of the 2 interventional methods, which subjectively confirmed the effectiveness of our radiology technology. Previous studies also pointed out that these 2 methods were effective in controlling bleeding in placenta accreta [10,14,15]. However, there are few studies comparing the 2 techniques. In our study, IABO produced better results than UAE. The possible explanation may be the collateral circulation. The abnormal placenta is frequently supplied by extensive arterial collaterals, including the uterine artery; the ovarian, cervical, and vaginal artery system; and the lumbar, sacral, mesenteric, and iliolumbar arteries. Arterial anastomosis of the external iliac artery is also possible [6,7]. After embolization of the uterine artery, collateral vessels can continue to provide blood supply to the placenta, while occlusion of infrarenal aorta can block most of the collateral vessels, including the inferior mesenteric artery and its lateral branches, the bilateral external and internal iliac arteries. Obstetricians were able to suture the bleeding point at the placental attachment site more easily in a bloodless operation field. Furthermore, because the balloon was inflated for 20 min and deflated for 1 min alternately, the bleeding point could be easily found.
The International Committee on Radiation Protection states that fetal exposure to an X-ray dose of less than 100 mGy will not cause any dysfunction of the fetus [22,23]. In our study, irradiation doses in both groups were lower than that dose. The irradiation dose of the IABO group was lower, indicating that it was safer. Andoh et al [24] suggested that the occlusion time of abdominal aorta should be within 25 min, while other researcher have indicated that the time should be within 40 min [14,25]. Considering individual differences, the occlusion time of blood flow should be minimized during the operation. In the current study, the balloon was deflated for 1 min every 20 min until uterine surgery was completed. Neither ischemic necrosis nor reperfusion injury of tissues and organs was observed with such transient flow blockage. The Medtronic AB46 balloon offers complete compliance, which can make the balloon easily conform to the shape of the blood vessel during its filling period. While completely blocking the abdominal aorta, the injury to the intima of the artery caused by the balloon was minimized. Eight patients in the UAE group and 1 patient in the IABO group underwent hysterectomy due to massive bleeding during the operation. Intravascular occlusion of the uterine artery was ineffective in some types of obstetric bleeding. The abnormal placenta was frequently supplied by extensive arterial collaterals. These failures may be associated with an extrauterine branch, such as the internal pudendal artery or some of its branches [7]. Understanding of the uterine-vaginal anastomotic system and improvement of interventional techniques will greatly help to improve the curative effect of selective devascularization.
It should be emphasized that this procedure is not perfect. The severity of the disease, the level of local medical care, and the willingness of patients to retain fertility should be taken into consideration. In some cases with uterine serous perforation or bladder invasion, hysterectomy with interventional assistance may reduce bleeding even if the uterus cannot be conserved. No matter what kind of intervention measures are taken, the purpose is to reduce the intraoperative blood loss and strive for the surgeon’s suture operation time. Interventional technology is only auxiliary, and surgical expertise is needed to control massive hemorrhage. Compared with a single obstetric intervention mode, the involvement of a multidisciplinary team can reduce the incidence of complications and maternal mortality in PAS disorders to a large extent.
Conclusions
Compared with uterine artery embolization, IABO has significant advantages in the operation for placenta previa combined with placenta accreta. Current evidence is insufficient to recommend the routine use of IABO technology to control intraoperative hemorrhage in patients with PAS. To support the conclusion effectively, a prospective and random study on a large sample is needed.
Footnotes
Ethics Approval
The study was approved by the Institutional Review Board of the First People’s Hospital of Lianyungang (20141207).
Availability of Data and Material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Conflict of interest
None.
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: Departmental sources
References
- 1.Baisch K. Zur pathologischen Anatomie der Placenta accreta. Arb. a. d. pathol. Institut Tübingen, Heft. 1908:6. [in German] [Google Scholar]
- 2.Luke RK, Sharpe JW, Greene RR. Placenta accreta: The adherent or invasive placenta. Am J Obstet Gynecol. 1966;95:660–68. doi: 10.1016/s0002-9378(16)34741-x. [DOI] [PubMed] [Google Scholar]
- 3.Jauniaux E, Ayres-de-Campos D FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO consensus guidelines on placenta accreta spectrum disorders: Introduction. Int J Gynaecol Obstet. 2018;140(3):261–64. doi: 10.1002/ijgo.12406. [DOI] [PubMed] [Google Scholar]
- 4.Silver RM. Abnormal placentation: Placenta previa, vasa previa, and placenta accreta. Obstet Gynecol. 2015;126(3):654–68. doi: 10.1097/AOG.0000000000001005. [DOI] [PubMed] [Google Scholar]
- 5.Fan D, Wu S, Wang W, et al. Prevalence of placenta previa among deliveries in Mainland China: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e5107. doi: 10.1097/MD.0000000000005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chait A, Moltz A, Nelson JH. The collateral arterial circulation in the pelvis. An angiographic study. Am J Roentgenol Radium Ther Nucl Med. 1968;102(2):392–400. doi: 10.2214/ajr.102.2.392. [DOI] [PubMed] [Google Scholar]
- 7.Palacios Jaraquemada JM, García Mónaco R, Barbosa NE, et al. Lower uterine blood supply: Extrauterine anastomotic system and its application in surgical devascularization techniques. Acta Obstet Gynecol Scand. 2007;86(2):228–34. doi: 10.1080/00016340601089875. [DOI] [PubMed] [Google Scholar]
- 8.Committee on Obstetric Practice. Committee opinion no 529: Placenta accreta. Obstet Gynecol. 2012;120(1):207–11. doi: 10.1097/AOG.0b013e318262e340. [DOI] [PubMed] [Google Scholar]
- 9.Bouvier A, Sentilhes L, Thouveny F, et al. Planned caesarean in the interventional radiology cath lab to enable immediate uterine artery embolization for the conservative treatment of placenta accreta. Clin Radiol. 2012;67(11):1089–94. doi: 10.1016/j.crad.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Yang T, Liu C, Qiao C. Feasibility of infrarenal abdominal aorta balloon occlusion in pernicious placenta previa coexisting with placenta accrete. Biomed Res Int. 2018;2018:4596189. doi: 10.1155/2018/4596189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panici PB, Anceschi M, Borgia ML, Brunelli R, et al. Fetal Maternal Risk Group. Intraoperative aorta balloon occlusion: Fertility preservation in patients with placenta previa accreta/increta. J Matern Fetal Neonatal Med. 2012;25(12):2512–16. doi: 10.3109/14767058.2012.712566. [DOI] [PubMed] [Google Scholar]
- 12.Rebonato A, Mosca S, Fischer M, et al. Endovascular management of massive post-partum haemorrhage in abnormal placental implantation deliveries. Eur Radiol. 2016;26(6):1620–30. doi: 10.1007/s00330-015-4001-z. [DOI] [PubMed] [Google Scholar]
- 13.Zhu B, Yang K, Cai L. Discussion on the timing of balloon occlusion of the abdominal aorta during a caesarean section in patients with pernicious placenta previa complicated with placenta accreta. Biomed Res Int. 2017;2017:8604849. doi: 10.1155/2017/8604849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Q, Li YL, Zhu MJ, et al. Prophylactic abdominalaortic balloon occlusion in patients with pernicious placenta previa during cesarean section: A systematic review and meta-analysis from randomized controlled trials. Arch Gynecol Obstet. 2019;300(5):1131–45. doi: 10.1007/s00404-019-05297-4. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Shi X, Li Y, et al. Prophylactic intraoperative uterine or internal iliac artery embolization in planned cesarean for pernicious placenta previa in the third trimester of pregnancy. Medicine (Baltimore) 2019;98(44):e17767. doi: 10.1097/MD.0000000000017767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlke JD, Mendez-Figueroa H, Maggio L, et al. Prevention and management of postpartum hemorrhage: A comparison of 4 national guidelines. Am J Obstet Gynecol. 2015;213(1):76.e1–e10. doi: 10.1016/j.ajog.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Kassem GA, Alzahrani AK. Maternal and neonatal outcomes of placenta previa and placenta accreta: Three years of experience with a two-consultant approach. Int J Womens Health. 2013;5:803–10. doi: 10.2147/IJWH.S53865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clausen C, Stensballe J, Albrechtsen CK, et al. Occlusion of the internal iliac arteries in the multidisciplinary management of placenta percreta. Acta Obstet Gynecol Scand. 2013;92(4):386–91. doi: 10.1111/j.1600-0412.2012.01451.x. [DOI] [PubMed] [Google Scholar]
- 19.Matsueda S, Hidaka N, Kondo Y, et al. External iliac artery thrombosis after common iliac artery balloon occlusion during cesarean hysterectomy for placenta accreta in cervico-isthmic pregnancy. J Obstet Gynaecol Res. 2015;41(11):1826–30. doi: 10.1111/jog.12777. [DOI] [PubMed] [Google Scholar]
- 20.Saito K, Mariya T, Fujibe Y, et al. Common iliac artery dissection as a complication of common iliac artery balloon occlusion for placenta percreta: A case report. J Obstet Gynaecol Res. 2021;47(3):1172–77. doi: 10.1111/jog.14601. [DOI] [PubMed] [Google Scholar]
- 21.Xie L, Wang Y, Man YC, Luo FY. Preliminary experience in uterine artery embolization for second trimester pregnancy induced labor with complete placenta previa, placenta implantation, and pernicious placenta previa. Clin Exp Obstet Gynecol. 2017;44(1):81–84. [PubMed] [Google Scholar]
- 22.Patel SJ, Reede DL, Katz DS, et al. Imaging the pregnant patient for nonobstetric conditions: Algorithms and radiation dose considerations. Radiographics. 2007;27(6):1705–22. doi: 10.1148/rg.276075002. [DOI] [PubMed] [Google Scholar]
- 23.Thabet A, Kalva SP, Liu B, et al. Interventional radiology in pregnancy complications: Indications, technique, and methods for minimizing radiation exposure. Radiographics. 2012;32(1):255–74. doi: 10.1148/rg.321115064. [DOI] [PubMed] [Google Scholar]
- 24.Andoh S, Mitani S, Nonaka A, et al. [Use of temporary aortic balloon occlusion of the abdominal aorta was useful during cesarean hysterectomy for placenta accrete]. Masui. 2011;60(2):217–219. [in Japanese] [PubMed] [Google Scholar]
- 25.Li K, Zou Y, Sun J, Wen H. Prophylactic balloon occlusion of internal iliac arteries, common iliac arteries and infrarenal abdominal aorta in pregnancies complicated by placenta accreta: A retrospective cohort study. Eur Radiol. 2018;28(12):4959–67. doi: 10.1007/s00330-018-5527-7. [DOI] [PubMed] [Google Scholar]


