Abstract
Background and Aims
The Self-assembling Peptide Hydrogel [SAPH, PuraMatrix], a fully synthetic peptide solution designed to replace collagen, has recently been used to promote mucosal regeneration in iatrogenic ulcers following endoscopic submucosal dissection. Herein, we evaluated its utility in ulcer repair using a rat model of topical trinitrobenzene sulphonic acid [TNBS]-induced colonic injuries.
Methods
Colonic injuries were generated in 7-week-old rats by injecting an ethanol solution [35%, 0.2 mL] containing 0.15 M TNBS into the colonic lumen. At 2 and 4 days post-injury, the rats were subjected to endoscopy, and SAPH [or vehicle] was topically applied to the ulcerative lesion. Time-of-flight secondary ion mass spectrometry [TOF-SIMS] was used to detect SAPH. Colonic expression of cytokines and wound healing-related factors were assessed using real-time polymerase chain reaction or immunohistochemistry.
Results
SAPH treatment significantly reduced ulcer length [p = 0.0014] and area [p = 0.045], while decreasing colonic weight [p = 0.0375] and histological score [p = 0.0005] 7 days after injury. SAPH treatment also decreased colonic expression of interleukin [IL]-1α [p = 0.0233] and IL-6[p = 0.0343] and increased that of claudin-1 [p = 0.0486] and villin [p = 0.0183], and β-catenin staining [p = 0.0237]. TOF-SIMS revealed lesional retention of SAPH on day 7 post-injury. Furthermore, SAPH significantly promoted healing in in vivo mechanical intestinal wound models.
Conclusions
SAPH application effectively suppressed colonic injury, downregulated inflammatory cytokine expression, and upregulated wound healing-related factor expression in the rat model; thus, it may represent a promising therapeutic strategy for IBD-related colonic ulcers.
Keywords: colitis, self-assembling peptide hydrogel, ulcer
1. Introduction
Inflammatory bowel diseases [IBDs], mainly comprising ulcerative colitis1 and Crohn’s disease,2 are characterized by refractory inflammatory damage in the gastrointestinal tract caused by the predominance of injury over repair processes.3–5 IBDs are often associated with colonic ulcers that are refractory to medical treatment, and/or require surgery.
Over the past decade, advances in our understanding of IBD pathogenesis have facilitated the development of directed therapies. For example, previous studies showed that 5-aminosalicylic acid, corticosteroids and thiopurines are of limited use as IBD treatments, whereas selective blockade of inflammatory cytokines with anti-tumour necrosis factor [TNF]-α agents provides considerable clinical benefit.6,7 Nevertheless, the long-term safety and efficacy of these therapies remain unclear, and some patients become drug-resistant or intolerant.8–10
Recently, tissue engineering and regenerative materials, such as extracellular matrix scaffold materials [ECM-SM], have been developed to restore damaged tissues to a normal structural and functional state. The Self-assembling Peptide Hydrogel [SAPH, PuraMatrix; 3D Matrix Co., Ltd] is a novel, fully synthetic, 16-amino-acid polypeptide with a repeating sequence of arginine, alanine and aspartic acid, and a self-assembling peptide solution that functions like ECM-SM in its ability to replace collagen. Within the 16-amino-acid monomer sequence, the alternating positively and negatively charged amino acids [arginine and aspartic acid], along with non-polar alanine located between the charged amino acids, create two distinct structural surfaces, one hydrophilic and the other hydrophobic. The electronic interaction between positive and negative charges, as well as the hydrophobic bonds between the side chains of neutral amino acids and hydrogen bonds of peptide main chains, facilitate the proper positioning of adjacent peptide molecules. These monomer building blocks form β-sheet structures via non-covalent interactions. When exposed to physiological pH [~7] or tissue, the β-sheet structures increase in number and the gel thickens, resulting in formation of a nanofibrous hydrogel that mimics a natural extracellular matrix scaffold, thus facilitating cell and tissue retention during healing.11 Recent studies have reported that SAPHs can be used to deliver medicinal substances and promote the regeneration of various organs,.12,13 Specifically, SAPHs have proven effective in controlling bleeding after endoscopic mucosal resection for gastric cancer.14 However, their capacity to alleviate inflammation-induced ulcers in IBD has not yet been evaluated.
Trinitrobenzene sulphonic acid [TNBS]-induced colitis was originally developed by Morris et al. as an animal model of colitis, in which TNBS [acting as a hapten] is administered rectally with ethanol [which acts as a barrier breaker].15 Recent reports have since modified this model to inject an ethanol solution containing TNBS to generate local ulcers and facilitate the observation of mucosal healing.16,17
Herein, we evaluated the effect of an SAPH, applied topically, on the repair of TNBS-induced ulcers in a rat model. In addition, we evaluated the effect of SAPH on intestinal wound healing using a different model based on mechanically induced wounds, independent of colitis.18,19 Our results provide useful insights for future studies aimed at developing novel therapeutic strategies for patients with IBD.
2. Materials and Methods
The present study used an experimental protocol approved by the Animal Research Committee of Kurume University and was undertaken in strict accordance with the tenets of the Declaration of Helsinki, including extra care to avoid animal suffering.
2.1. Experimental animals
Male Sprague-Dawley rats were obtained from Charles River Laboratories Japan and housed in standard wire-mesh cages. They were provided with ad libitum access to standard chow and tap water.
2.2. Colonic injury induction
Colonic injury was induced in 7-week-old rats [weighing 300–320 g] using a method described previously. Briefly, rats were anaesthetized via isoflurane inhalation and subjected to laparotomy. A section of the proximal colon was clamped with ringed forceps [10-mm inner diameter], and an ethanol solution [35%, 0.2 mL] containing 0.15 M TNBS [Tokyo Chemical Industry Co., Ltd] was injected into the colon lumen using a 29-gauge needle.16,17 The clamp was maintained for 2 min and then released before the colon was returned to the abdominal cavity, and the incision was sutured.
2.3. Protocol A: TNBS injury time course study
The extent of colonic damage [as indicated by the ulcer length, i.e. ulcer girth/colon girth] was evaluated via endoscopy or colonic resection on days 3, 7, 14, 21 and 28 after injury [TNBS/ethanol injection]. Specifically, rats undergoing endoscopy were anaesthetized via isoflurane inhalation, the colon was flushed with physiological saline, and endoscopy was performed using a mini endoscope [length, 85 mm; diameter, 5 mm] with a GASTRO PACK monitor [Karl Storz]. After euthanasia, the colon was removed, opened by longitudinal incision, and rinsed with physiological saline; the ulcer area was then analysed using the Image-Pro Plus software [Media Cybernetics]. The colonic weight [g/3-cm resected colonic section] and histological findings were evaluated and recorded. Furthermore, colonic expression levels of cytokines and wound healing-related factors were evaluated.
2.4. Protocol B: Effect of SAPH treatment on TNBS injury
On days 2 and 4 after TNBS/ethanol injection into the colonic lumen, 1 mL of SAPH or vehicle [physiological saline] was applied topically to the ulcerative lesion under endoscopy. For further comparison, we injected a group of rats with TNBS in the absence of SAPH or vehicle. Hence, the three rat groups were as follows: ‘TNBS’ group [clamped with forceps + TNBS injection without topical administration of SAPH or vehicle]; ‘TNBS + vehicle’ group [clamped with forceps + TNBS injection + topical administration of vehicle]; ‘TNBS + SAPH’ group [clamped with forceps + TNBS injection + topical administration of SAPH]. Ulcer length was evaluated via endoscopy on days 2, 4 and 7 after injury. Animals were killed on day 7 after injury, and ulcer area, colonic weight [g/3-cm resected colonic section], and histological score [sum of the extent, damage, inflammation and regeneration scores],20 as well as colonic expression levels of cytokines and wound healing-related factors were evaluated.
2.5. Real-time polymerase chain reaction [RT-PCR]
Total colonic RNA was isolated using TRIzol [Invitrogen] and reverse-transcribed into cDNA using the ReverTra Ace qPCR RT Master Mix [Toyobo].21 Tissue expression levels of interleukin-1α [Il1a], Il1b, Il6 and tumor necrosis factor-α [Tnfa], Il4, Il10, claudin-1 [Cldn1], claudin-2 [Cldn2], claudin-3 [Cldn3], occludin [Ocln], zonula occludens-1 [Zo1], vascular endothelial growth factor A [Vegfa], hepatocyte growth factor [Hgf], villin [Vil1] and leucine-rich repeat-containing G-protein-coupled receptor-5 [Lgr5] were assessed via RT-PCR using the ABI Step One Plus system [Applied Biosystems].
Specifically, Ila1, Il1b, Il6, Tnfa and β-actin [Actb] levels were evaluated using SYBR Green and the following primers: IL-1α Forward (F), 5′-AAGACCAGCCCGTGTTGCTGAAGG-3′; IL-1α Reverse (R), 5′-TCCAGAAGAAAATGAGGTCGGTC-3′; IL-1β F, 5′-TGAC CCATGTGAGCTGAAAG-3′; IL-1β R, 5′-AGGGATTTTGTCGT TGCTTG-3′; IL-6 F, 5′-TGTTCTCAGGGAGATCTTGG-3′; IL-6 R, 5′-TCCAGGTAGAAACGGAACTC-3′; TNF-α F, 5′-ATGATC CGAGATGTGGAACTGGCA-3′; TNF-α R, 5′-AATGAGAAGAGG CTGAGGCACAG’-3’; β-actin F, 5′-CTGGAGAAGAGCTATGAGC TG-3′; β-actin R, 5′-AATCTCCTTCTGCATCCTGTC-3′.
In contrast, Il4, Il10, Cldn1, Cldn2, Cldn3, Ocln, Zo1, Vegfa, Hgf, Vil1, Lgr5, and glyceraldehyde 3-phosphate dehydrogenase [Gapdh] mRNA levels were assessed using the TaqMan probe and primer sets, comprising: Il4, Rn01456866_m1; Il10, Rn01483988_g1; Cldn1, Rn00581740_m1; Cldn2, Rn02063575_s1; Cldn3, Rn00581751_s1; Ocln, Rn00580064_m1; Zo1, Rn02116071_s1; VEGFA, Rn01511601_m1; Hgf, Rn00566673_m1; Vil1, Rn01254356_g1; Lgr5, Rn01509662_m1; and Gapdh, Rn01775763_g1 [Applied Biosystems].
2.6. Immunohistochemical analysis
Immunohistochemistry was performed on paraffin sections using rabbit monoclonal CD3 primary antibody [ab16669, Abcam], and rabbit polyclonal β-catenin [ab6302] and rabbit polyclonal villin [ab97512] primary antibodies using a previously described method.21 The percentage of immunohistochemically stained positive cells for CD3, villin and β-catenin was measured in three randomly selected fields under a light microscope at 200× magnification in sections from a colon adjacent to the ulcer using ImageJ software (National Institutes of Health [NIH]) with the immunohistochemistry profiler plugin.22,23
2.7. Protocol C: Detection of SAPH on TNBS injury
SAPH was topically applied to the ulcerative lesions under endoscopy on days 2 and 4 post-TNBS injection, and rats were killed on day 7 after injury. To assess SAPH stability on colonic ulcers, 10-μm-thick sections of the frozen colonic sample on glass slides were analysed at Material Science and Technology of Japan using time-of-flight secondary ion mass spectrometry [TOF-SIMS].24,25 TOF-SIMS is an analytical technique that uses a primary ion beam to probe the surface of a solid material. The secondary ions that desorb from the sample surface are analysed, and their mass is determined with high accuracy. As a result, TOF-SIMS allows spectroscopy for the characterization of chemical composition, imaging for mapping the surface distribution of species, and depth profiling.
2.8. In vivo wound healing experiments
The effect of SAPH in vivo was also evaluated using a rat intestinal wound healing model that is based on mechanical wounding and is independent of colitis.18,19 For this purpose, mucosal biopsies were taken from the distal colon of a live rat using endoscopically guided biopsy forceps, resulting in defined wounds. SAPH or vehicle was topically applied to the wound under endoscopy each day after mechanical wounding. The healing process was subsequently monitored by daily endoscopies. The diameters and the wound areas were calculated relative to the original wound size by video analysis of endoscopic recordings using the ImageJ software [NIH].
2.9. Statistical analysis
Where appropriate, results are presented as mean ± SEM. Parametric and non-parametric analyses were used where appropriate after testing for normal distribution using the Shapiro–Wilk test. A p value < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Assessment of the TNBS-induced injury time course
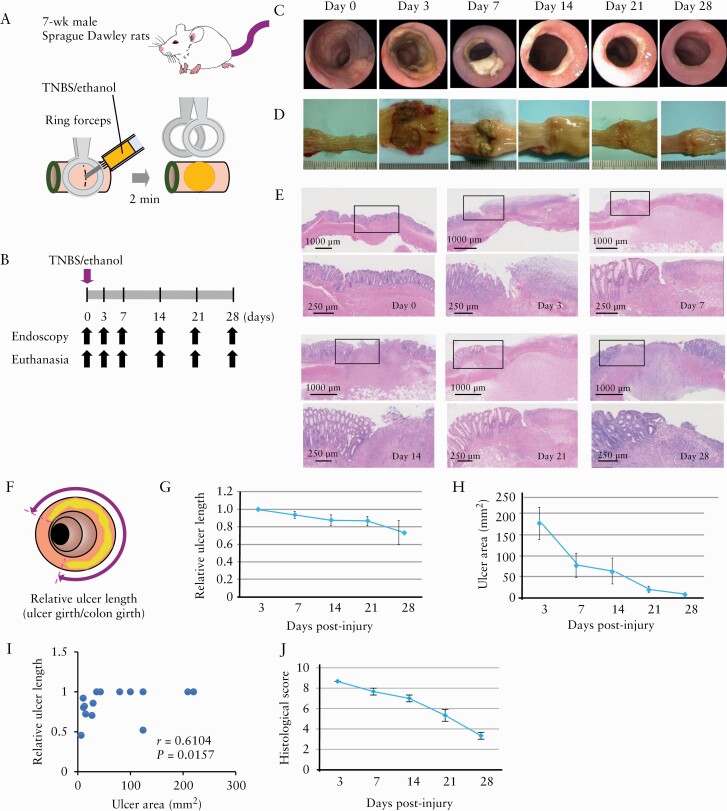
The extent of the induced colonic injuries was evaluated via endoscopy and/or colon resection on days 3, 7, 14, 21 and 28 post-induction [Figure 1A, B]. The endoscopic observation conducted [Figure 1C], which has not been previously performed as part of the modified TNBS model, revealed a well-defined local ulcer on day 3 after injury, which was gradually reduced over the experimental time course, producing scarring between 21 and 28 days post-injury.
Figure 1.
Characterization of colonic-ulcer induction in rats by trinitrobenzene sulphonic acid [TNBS] injection. [A] Schematic representation of induction of colonic ulcers in 7-week-old rats via injection of ethanol solution [35%, 0.2 mL] containing 0.15 M TNBS into the colon lumen after clamping the proximal colon using ringed forceps. [B] Time course of the experimental protocol. [C–E] Representative endoscopic [C], macroscopic [D] and histological [E] images [haematoxylin and eosin staining; the magnification of the black box in the upper panel is shown in the lower panel] taken immediately before [0], and 3, 7, 14, 21 and 28 days after TNBS injection. [F–J] Relative ulcer lengths [F, G] and areas [H]. Correlation between relative ulcer length and area [I], and histological score [J] [n = 3–15 per group]. Relative ulcer length was obtained by dividing ulcer girth by colon girth.
Macroscopic observations of resected colon sections [Figure 1D] also showed a well-defined local ulcer with surrounding oedematous mucosa that recovered gradually over the experimental time course.
Histological analysis showed that TNBS injection induced ulcers that extended toward the muscularis propria with leukocyte infiltration and inflammatory exudate observed at the ulcer base. These findings gradually improved over the experimental time course, with regenerated epithelia growing around, or covering, the ulcer base, effectively replacing granulation tissues [Figure 1E].
Quantitative measurements taken on days 3, 7, 14, 21 and 28 after colonic injury showed that colonic ulcer length and area [Figure 1F–I], as well as histological score [Figure 1J; n = 3–15 per group] gradually decreased over the experimental time course. To assess the validity of ulcer length as an index of the ulcer area, we compared the two measurements on days 3, 7, 14, 21 and 28 after TNBS/ethanol injection. As expected, we found a statistically significant correlation between ulcer length and area [r = 0.6104, p < 0.0157] [Figure 1I].
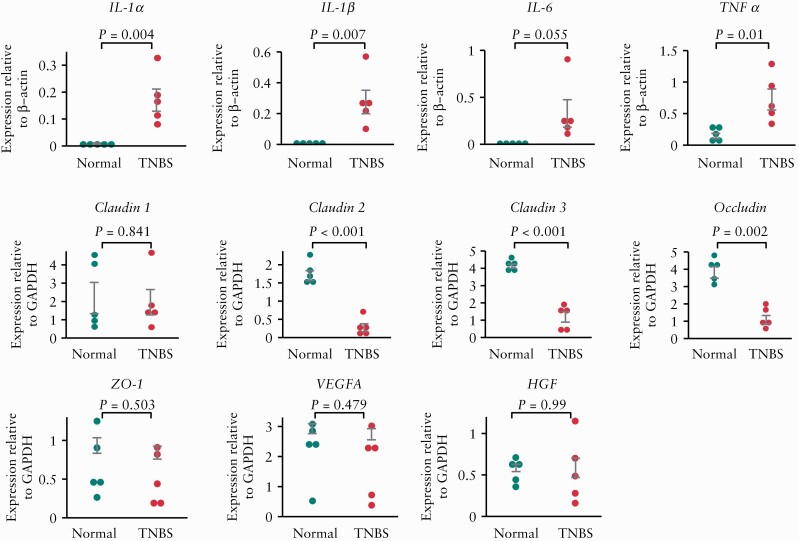
Next, we evaluated colonic mRNA expression of inflammatory cytokines [Il1a, Il1b, Il6 and Tnfa], tight-junction-related factors [Cldn1, Cldn2, Cldn3, Ocln, Zo1], and growth factors [Vegfa and Hgf; Figure 2]. We found that TNBS-induced colonic injury resulted in the upregulation of Il1a [p = 0.004], Il1b [p = 0.007] and Tnfa [p = 0.01], as well as the downregulation of Cldn2 [p < 0.001], Cldn3 [p < 0.001] and Ocln [p = 0.002].
Figure 2.
Comparison of colonic mRNA expression levels of cytokines and wound healing-related factors after trinitrobenzene sulphonic acid [TNBS] injection. Interleukin-1α [Il1a], IL-1β [Il1b], IL-6 [Il6], tumour necrosis factor [Tnfa], claudin-1 [Cldn1], claudin-2 [Cldn2], claudin-3 [Cldn3], occludin, zonula occludens-1 [Zo1], vascular endothelial growth factor A [Vegfa] and hepatocyte growth factor [Hgf] expression levels were assessed in untreated [Normal] and TNBS-treated rats 7 days after TNBS injection; n = 5 per group. The data were normalized to the expression of β-actin [Actb] or glyceraldehyde 3-phosphate dehydrogenase [Gapdh].
3.2. Efficacy of SAPH treatment against TNBS injury
Next, we evaluated the efficacy of SAPH in treating TNBS-induced ulcers among the TNBS group, TNBS + Vehicle group and TNBS + SAPH group. Based on the data from the time course studies of TNBS-induced injury mentioned above, we further evaluated the efficacy of SAPH treatment up to day 7 post-induction of injury. After 7 days, there may be complete resolution of the injury, making comparison of efficacy among the groups difficult. Hence, a 7-day observation was considered to be optimal.
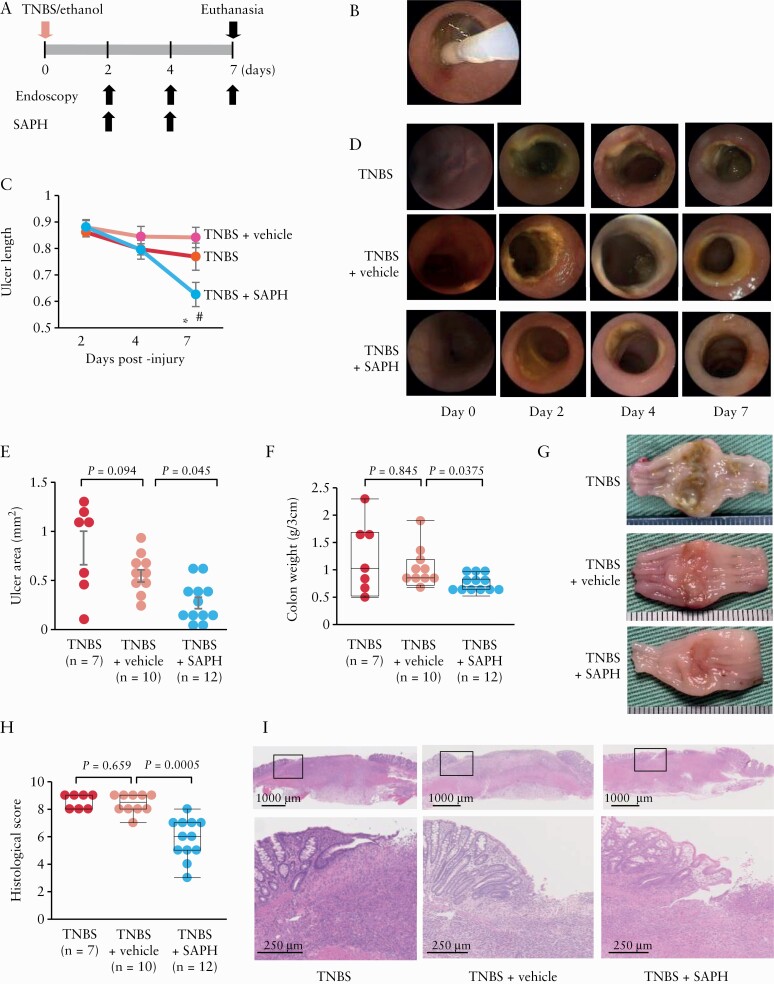
SAPH [or vehicle] was applied on days 2 and 4 after injury, and the effect of this treatment on ulcer healing was evaluated on days 4 and 7 [via endoscopy; Figure 3A, B]. Topical SAPH application was shown to significantly shorten ulcer length, in comparison to vehicle treatment [p = 0.0014] on day 7 [Figure 3C, D], suggesting that it can accelerate healing of TNBS-induced colonic injuries.
Figure 3.
Effect of the Self-assembling Peptide Hydrogel [SAPH] on trinitrobenzene sulphonic acid [TNBS]-induced colonic ulcers. [A] SAPH was applied topically [using endoscopy] to the ulcers on days 2 and 4 post-TNBS injection. The rats were killed for analysis on day 7 post-injury. [B] Image showing endoscopic topical SAPH application. [C] Serial changes in ulcer length following the topical application of SAPH or vehicle, before, and 2, 4 and 7 days after TNBS injection or no topical application of SAPH nor vehicle after TNBS injection [n = 7–14 per group]. *p = 0.0014 vs TNBS + vehicle, #p = 0.0252 vs TNBS alone. [D] Representative images showing endoscopic findings in SAPH- and vehicle-treated and non-treated TNBS rats. [E–G] SAPH administration reduced the size [area] [E] and weight and ameliorated the macroscopic features [G] of TNBS-induced colonic ulcers [3-cm colonic samples] [F]. [H, I] SAPH reduced the histological score of the TNBS-induced colonic ulcers [H]. Representative image of each group. The magnification of the black box in the upper panel is shown in the lower panel [I, haematoxylin and eosin staining].
Similarly, macroscopic observations made on day 7 post-injury revealed that ulcer area was significantly smaller [p = 0.045] and colonic weight was significantly lower [p = 0.0375] in SAPH-treated rats compared to vehicle-treated rats [Figure 3E–G].
Additionally, histological score was significantly lower in SAPH-treated rats compared to vehicle-treated rats [Figure 3H]. As shown in Figure 3I, SAPH-treated rats exhibited strongly regenerated epithelia around and firmly covering the ulcer base compared to vehicle-treated rats. Together, these findings support the conclusion that topical application of SAPH can promote ulcer healing.
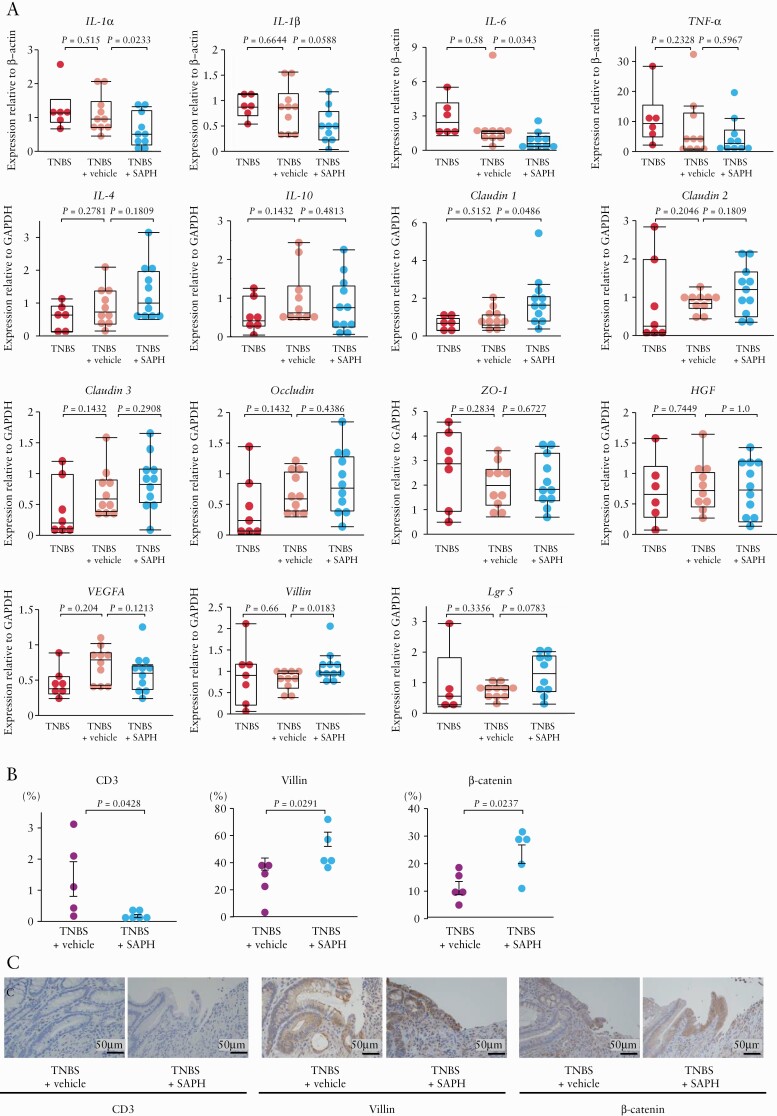
As shown in Figure 4, suppression of colonic injury by SAPH administration was associated with downregulation of inflammatory cytokines Il1a [p = 0.0233] and Il6 [p = 0.0343], which was associated with a decrease in the abundance of CD3+ cells [p = 0.0428]. In contrast, the expression of anti-inflammatory cytokines [Il4 and Il10] was not affected by SAPH administration. Moreover, SAPH administration upregulated wound healing-related factors Cldn1 [p = 0.0486] and Vil1 [p = 0.0183], while increasing the number of villin-positive cells [p = 0.0291] and β-catenin-positive cells [p = 0.0237]. SAPH also upregulated Lgr5, but this result did not reach statistical significance [p = 0.0783].
Figure 4.
Effect of the Self-assembling Peptide Hydrogel [SAPH] on the expression of cytokines and wound healing-related factors using real-time quantitative polymerase chain reaction [RT-PCR] and immunohistochemistry. [A] RT-PCR was used to assess colonic interleukin [IL]-1α, IL-1β, IL-6, tumor necrosis factor [TNF]-α, IL-4, IL-10, claudin-1 [Cldn1], claudin-2 [Cldn2], claudin-3 [Cldn3], occludin [Ocln], zonula occludens [Zo1], hepatocyte growth factor [Hgf], vascular endothelial growth factor A [Vegfa], villin [Vil1] and leucine-rich repeat-containing G-protein-coupled receptor-5 [Lgr5] expression levels; n = 5–11 per group. The data were normalized to the expression of β-actin [Actb] or glyceraldehyde 3-phosphate dehydrogenase [Gapdh]. [B] The number of CD3, villin and β-catenin-positive cells in colon cross-sections of vehicle- or SAPH-treated TNBS rats at day 7 after TNBS/ethanol injection. [C] Representative immunohistochemical localization of CD3, villin and β-catenin in colon cross-sections of vehicle- or SAPH-treated TNBS rats at day 7 after TNBS/ethanol injection [haematoxylin and eosin staining].
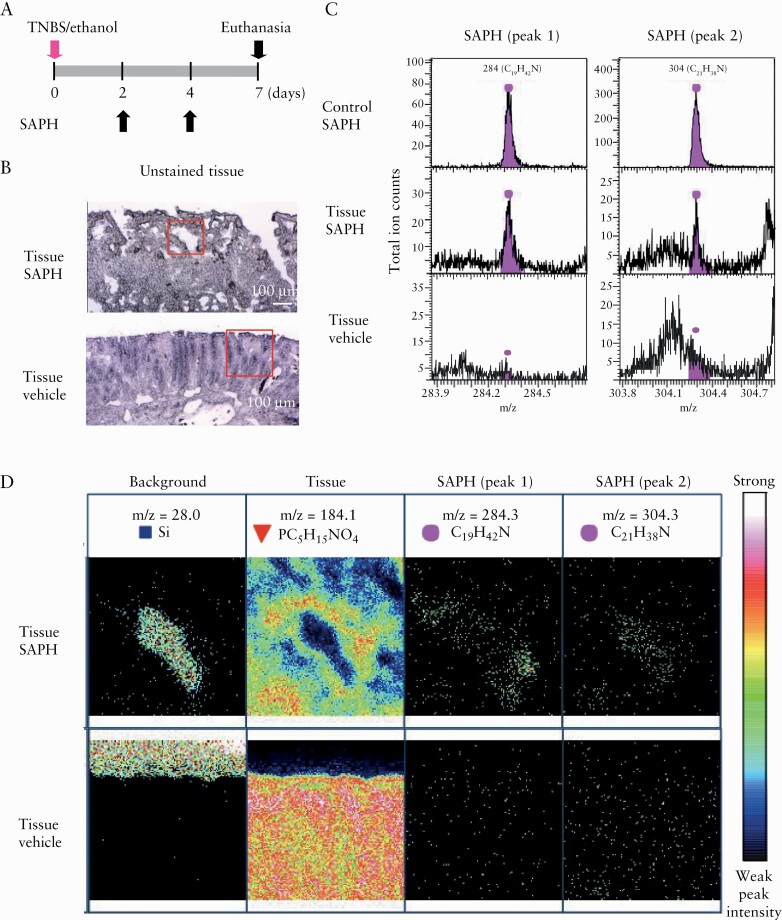
3.3. Detection of SAPH using TOF-SIMS
Endoscopic and pathological visualization of SAPH is extremely difficult. Therefore, we elected to use the TOF-SIMS technique to detect SAPH in TNBS injury. To this end, we applied SAPH or vehicle topically on days 2 and 4 after TNBS injury and performed SAPH detection on the injury surface on day 7. As expected, the fragment ion peaks of SAPH were observed, confirming that SAPH was present in the region where it was applied [Figure 5].
Figure 5.
Detection of the Self-assembling Peptide Hydrogel [SAPH] on trinitrobenzene sulphonic acid [TNBS]-induced colonic ulcers using time-of-flight secondary ion mass spectrometry [TOF-SIMS]. [A] SAPH or vehicle was topically applied to TNBS-injured sites under endoscopy at 2 and 4 days post-TNBS injection, and rats were killed on day 7 after injury. [B] Unstained tissue section of SAPH-treated TNBS injury [upper panel] and vehicle-treated TNBS injury as a negative control [lower panel]. Red square indicates measured regions. [C] Mass spectra produced by TOF-SIMS from the tissue section of SAPH-treated TNBS injury [middle panel] and vehicle-treated TNBS injury as a negative control [lower panel]. SAPH without tissue application was used as a control [upper panel]. Spectra within the tissue corresponding to SAPH showed two peaks [peaks 1 and 2]. [D] Ion images of SAPH [upper panel] or vehicle [lower panel] within the TNBS injury section. Images of the background, colonic tissue, SAPH corresponding to peak 1 and SAPH corresponding to peak 2. Representative images of one case are shown for two independent experiments.
3.4. Efficacy of SAPH treatment against in vivo wound healing models
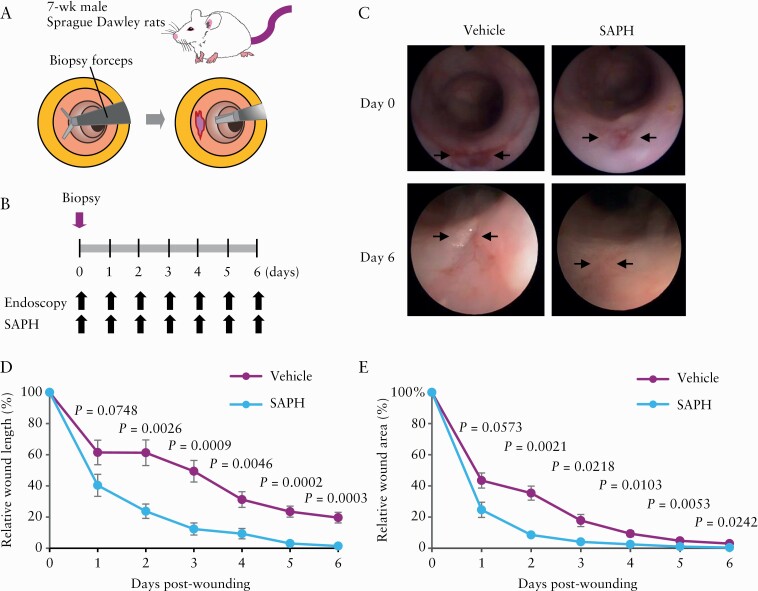
Because SAPH was found to promote wound closure in TNBS-induced ulcers, we also evaluated its effect in vivo using a rat intestinal wound healing model [Figure 6]. The healing of these wounds was monitored until day 6, and wound size was compared between vehicle-treated and SAPH-treated rats by endoscopic analysis. The reduction in wound diameters and wound areas was significantly accelerated in models treated with SAPH than with vehicle.
Figure 6.
Application of the Self-assembling Peptide Hydrogel [SAPH] to a mechanical wound healing model. [A] Schematic representation of the creation of intestinal wounds using endoscopy-guided biopsy forceps on the distal colon of rats. [B] Time course of the experimental protocol. SAPH [n = 6] or vehicle [n = 6] was topically applied under endoscopy. Wound healing was compared between vehicle-treated and SAPH-treated rats by endoscopies every day until day 6. [C] Representative images after wound creation [arrows]. The fresh wound bed typically had a diameter of approximately 4 mm. [D] Relative wound diameters after wound creation. Data are shown as the diameters of the wound bed relative to the diameter of the fresh wound [percentage]. [E] Relative wound areas after wound creation. Data are shown as the areas of the wound bed relative to the area of the fresh wound [percentage].
4. Discussion
This study is the first to indicate the potential use of SAPH as a treatment for inflammation-induced colonic ulcers.
The TNBS-induced colitis model employed in the present study is unique in that it generates local, inflammation-induced ulcers that facilitate the detailed observation of mucosal healing.16,17 We validated the reliability of this modified TNBS colitis model by assessing ulcer progression/healing over a specified time course. The results showed that the model consistently induced ulcers of comparable size [length and area] and with histological characteristics that gradually decreased over time, reaching the scarring stage by the last assessed time point.
Using this model, we showed that topical SAPH application promotes colonic ulcer healing. Previous reports have shown that SAPHs have an exceptional capacity to promote cell proliferation, wound healing, tissue repair and drug delivery.12–14 We suggest that SAPH treatment is likely to be suitable in the treatment of colonic injuries because it functions as a synthetic extracellular matrix to replace collagen, does not incur a risk of infection because it is a fully synthetic solution, rapidly forms into a hydrogel at physiological pH, and is easily applied through a catheter from a prefilled syringe. Additionally, it has tissue engineering properties and contains regenerative substances. These characteristics are likely to facilitate the rapid translation of our findings to the clinical setting.
To elucidate the molecular mechanism by which SAPH treatment reduces TNBS-induced injuries, we assessed the colonic expression of inflammatory cytokines26 and wound healing-related factors, including tight-junction-related factors,27 growth factors28 and wound-associated epithelial cell markers.29–31 Of these, we found that Cldn1, Vil1, Lgr5 and β-catenin were upregulated. Because SAPH has been reported to promote the regeneration of damaged sites in various organs, we suggest that epithelial regeneration could be a primary effect of SAPH. The mechanism underlying the observed downregulation of Il1a and Il6 and decrease in mucosal CD3+ T cells is unclear; however, given that SAPH acts independently of the immune system, we suggest that this beneficial effect may be secondary to a reduction in the stimulation of luminal antigens caused by the observed accelerated mucosal repair.
Furthermore, an important aspect of the efficacy of topical SAPH application was its retention to ulcerative lesions for a long time. In the present study, we applied SAPH topically on days 2 and 4 after TNBS injury and, using the TOF-SIMS technique, found its presence on the injured area on day 7 after the injury. To our knowledge, this is the first study to identify SAPH using the TOF-SIMS technique, which can be applied in the future to precisely detect the length of time that SAPH is retained at the site of injury.
Colonic ulcers are caused by a range of factors other than IBD32,33; for example, they can be induced by non-steroidal anti-inflammatory drugs,34 ischaemia,35 radiation,36 infection [e.g. amoebiasis,37 tuberculosis38] or colonic resection.39 As discussed, they are occasionally refractory to medical treatment and can cause symptoms, including bleeding, anaemia and perforation, which may require surgery. Given that its demonstrated effect in ulcer healing does not appear to be mediated immunologically, SAPH is likely to be effective for treating diseases requiring controlled drug release at a distinct location and promoting the healing of post-endoscopic resection-induced stomach or iatrogenic colonic ulcers. Using an in vivo model,18,19 we demonstrated, for the first time, that topical application of SAPH can also stimulate the healing of mechanical intestinal wounds.
The primary limitations of our study include the small number of experimental animals, and the absence of a long-term follow-up; for example, we chose to kill SAPH-treated rats 7 days post-injury to assess induced histological changes. Thus, additional long-term experiments are needed to confirm our findings and test alternative SAPH formulations. Additional preclinical studies using large-animal models are needed before the clinical benefits of SAPH can be evaluated in humans.
In conclusion, topical application of SAPH described under endoscopy effectively suppressed colonic injury in the rat model employed; thus, the results of the present study support the hypothesis that SAPH is a promising potential therapeutic strategy to treat colonic ulcers in IBD. SAPH treatment is relatively straightforward and safe; nevertheless, additional comparative studies are warranted to evaluate its efficacy in this context better.
Supplementary Material
Acknowledgments
The authors thank Ms Saori Meifu for her expert technical assistance.
Funding
This work was supported partly by a Grants-in-Aid from the Ministry of Science and Education and Health Grant number JP19K08481 and Labour Sciences Research for research on intractable diseases from the Ministry of Health, Labour and Welfare of Japan.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
K.M. designed the research, T.A., K.M., H.Y., M.M., K.T., A.M., T.Y., S.F., K.K., S.Y. and H.T. performed the experiments, T.A., H.Y. and T.K. analyded the data, K.M., H.Y., T.K. and J.A. discussed the data, K.M. and T.T. supervised the project, and T.A., K.M. and H.Y. wrote the manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- 2.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- 3.Neurath MF, Finotto S. The many roads to inflammatory bowel diseases. Immunity 2006;25:189–91. [DOI] [PubMed] [Google Scholar]
- 4.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest 2007;117:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogler G. Resolution of inflammation in inflammatory bowel disease. Lancet Gastroenterol Hepatol 2017;2:521–30. [DOI] [PubMed] [Google Scholar]
- 6.Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol 2014;7:6–19. [DOI] [PubMed] [Google Scholar]
- 7.Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci 2017;38:127–42. [DOI] [PubMed] [Google Scholar]
- 8.Wehkamp J, Götz M, Herrlinger K, Steurer W, Stange EF. Inflammatory bowel disease. Dtsch Arztebl Int 2016;113:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreau J, Mas E. Drug resistance in inflammatory bowel diseases. Curr Opin Pharmacol 2015;25:56–61. [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Montiel MP, Casis-Herce B, Gómez-Gómez GJ, et al. Pharmacologic therapy for inflammatory bowel disease refractory to steroids. Clin Exp Gastroenterol 2015;8:257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S, Lockshin C, Cook R, Rich A. Unusually stable beta-sheet formation in an ionic self-complementary oligopeptide. Biopolymers 1994;34:663–72. [DOI] [PubMed] [Google Scholar]
- 12.Jahanbazi Jahan-Abad A, Karima S, Sahab Negah S, Noorbakhsh F, Borhani-Haghighi M, Gorji A. Therapeutic potential of conditioned medium derived from oligodendrocytes cultured in a self-assembling peptide nanoscaffold in experimental autoimmune encephalomyelitis. Brain Res 2019;1711:226–35. [DOI] [PubMed] [Google Scholar]
- 13.Akiyama N, Yamamoto-Fukuda T, Takahashi H, Koji T. In situ tissue engineering with synthetic self-assembling peptide nanofiber scaffolds, PuraMatrix, for mucosal regeneration in the rat middle-ear. Int J Nanomedicine 2013;8:2629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uraoka T, Ochiai Y, Fujimoto A, et al. A novel fully synthetic and self-assembled peptide solution for endoscopic submucosal dissection-induced ulcer in the stomach. Gastrointest Endosc 2016;83: 1259–64. [DOI] [PubMed] [Google Scholar]
- 15.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989;96:795–803. [PubMed] [Google Scholar]
- 16.Uchida M, Mogami O. Milk whey culture with Propionibacterium freudenreichii ET-3 is effective on the colitis induced by 2,4,6-trinitrobenzene sulfonic acid in rats. J Pharmacol Sci 2005;99:329–34. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi Y, Tsuji S, Tsujii M, et al. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther 2008;326:523–31. [DOI] [PubMed] [Google Scholar]
- 18.Neurath MF, Wittkopf N, Wlodarski A, et al. Assessment of tumor development and wound healing using endoscopic techniques in mice. Gastroenterology 2010;139:1837–1843.e1. [DOI] [PubMed] [Google Scholar]
- 19.Pickert G, Neufert C, Leppkes M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 2009;206:1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 1994;107:1643–52. [DOI] [PubMed] [Google Scholar]
- 21.Yamasaki H, Mitsuyama K, Yoshioka S, et al. Leukocyte apheresis using a fiber filter suppresses colonic injury through calcitonin gene-related peptide induction. Inflamm Bowel Dis 2020;26:709–19. [DOI] [PubMed] [Google Scholar]
- 22.Akimoto T, Goto O, Sasaki M, et al. Endoscopic suturing promotes healing of mucosal defects after gastric endoscopic submucosal dissection: endoscopic and histologic analyses in in vivo porcine models (with video). Gastrointest Endosc 2020;91:1172–82. [DOI] [PubMed] [Google Scholar]
- 23.Varghese F, Bukhari AB, Malhotra R, De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One 2014;9:e96801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Touboul D, Kollmer F, Niehuis E, Brunelle A, Laprévote O. Improvement of biological time-of-flight-secondary ion mass spectrometry imaging with a bismuth cluster ion source. J Am Soc Mass Spectrom 2005;16:1608–18. [DOI] [PubMed] [Google Scholar]
- 25.Desbenoit N, Schmitz-Afonso I, Baudouin C, et al. Localisation and quantification of benzalkonium chloride in eye tissue by TOF-SIMS imaging and liquid chromatography mass spectrometry. Anal Bioanal Chem 2013;405:4039–49. [DOI] [PubMed] [Google Scholar]
- 26.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014;14:329–42. [DOI] [PubMed] [Google Scholar]
- 27.Landy J, Ronde E, English N, et al. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol 2016;22:3117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Playford RJ, Ghosh S. What is the role of growth factors in IBD? Inflamm Bowel Dis 2008;14 Suppl 2:S119–20. [DOI] [PubMed] [Google Scholar]
- 29.Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A 2009;106:256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 2012;338:108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quirós M, Nusrat A. Contribution of wound-associated cells and mediators in orchestrating gastrointestinal mucosal wound repair. Annu Rev Physiol 2019;81:189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gecse KB, Vermeire S. Differential diagnosis of inflammatory bowel disease: imitations and complications. Lancet Gastroenterol Hepatol 2018;3:644–53. [DOI] [PubMed] [Google Scholar]
- 33.Nagar AB. Isolated colonic ulcers: diagnosis and management. Curr Gastroenterol Rep 2007;9:422–8. [DOI] [PubMed] [Google Scholar]
- 34.Lanas A, Sopeña F. Nonsteroidal anti-inflammatory drugs and lower gastrointestinal complications. Gastroenterol Clin North Am 2009;38:333–52. [DOI] [PubMed] [Google Scholar]
- 35.Mosińska P, Fichna J. Ischemic colitis: current diagnosis and treatment. Curr Drug Targets 2015;16:209–18. [DOI] [PubMed] [Google Scholar]
- 36.Tabaja L, Sidani SM. Management of radiation proctitis. Dig Dis Sci 2018;63:2180–8. [DOI] [PubMed] [Google Scholar]
- 37.Bercu TE, Petri WA, Behm JW. Amebic colitis: new insights into pathogenesis and treatment. Curr Gastroenterol Rep 2007;9:429–33. [DOI] [PubMed] [Google Scholar]
- 38.Kedia S, Das P, Madhusudhan KS, et al. Differentiating Crohn’s disease from intestinal tuberculosis. World J Gastroenterol 2019;25:418–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chari ST, Keate RF. Ileocolonic anastomotic ulcers: a case series and review of the literature. Am J Gastroenterol 2000;95:1239–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.