Abstract
Background
Animal and human brucelloses have been reported in Rwanda, human brucellosis being linked to drinking inadequately heat‐treated milk. However, information on Brucella detection and prevalence in milk produced in Rwanda is limited.
Objectives
To determine the sero‐prevalence and risk factors of Brucella in farm bulk milk from zero and open grazing cattle production systems in Rwanda.
Methods
A total of 330 farm bulk milk samples were collected from 198 zero grazing farms and 132 open grazing farms in a cross‐sectional study in Rwanda. Sero‐prevalence of Brucella in milk was analysed using indirect enzyme‐linked immunosorbent assay. A questionnaire was administered to farmers to determine the risk factors of milk contamination with Brucella.
Results
Anti‐Brucella antibodies were prevalent in 19.7% (95% confidence interval (CI), 15.5–24.4) of the 330 collected farm bulk milk. Sero‐prevalence was significantly higher (p < 0.05) in open grazing farms (37.9% [50/132]) than in zero grazing farms (7.6% [15/198]). Practising open grazing system (odds ratio, OR = 69.5; 95% CI = 1.6–3033.6), history of abortion (OR = 19.5; 95% CI = 8.1–46.8) and placenta retention (OR = 4.2; 95% CI = 1.7–10.3) were the significant risk factors for the presence of anti‐Brucella antibodies in milk.
Conclusion
Notably, more than a third of farm bulk milk from open grazing farms in Rwanda contains Brucella antibodies. Considering the zoonotic nature of Brucella, there is a need to reinforce brucellosis control programs in the country.
Keywords: Brucella, farm, grazing, milk, Rwanda, sero‐prevalence
Short abstract
This is a cross sectional study which determined the sero‐prevalence and risk factors of Brucella prevalence in farm bulk milk in Rwanda. Brucella was found to be more prevalent in milk from open grazing farms, compared to milk from zero grazing farms. Practising open grazing system, histories of abortion and placenta retention were the significant risk factors for the presence of anti‐Brucella antibodies in farm bulk milk.
1. INTRODUCTION
Brucella species (spp) are bacteria that cause the infection known as brucellosis in different animals including livestock such as cattle, which are among natural hosts of Brucella spp (Hull & Schumaker, 2018). The most frequent clinical symptoms exhibited by brucellosis‐positive animals are reproductive disorders of abortion, stillbirths, weak calves, retained placenta and longer calving intervals in female animals such as dairy cattle (Acha & Szyfres, 2005; Boukary et al., 2013; McDermott et al., 2013). The reproductive disorders associated with brucellosis in animals result further in animal infertility and reductions to the absence of milk production (Corbel, 2006; Mangen et al., 2002; Ul‐Islam et al., 2013), translating into economic losses for the farmer.
Being zoonotic, brucellosis can be transmitted from animals to humans causing a febrile illness with intermittent undulating fevers, sweats, chills, weakness, malaise, headache, insomnia, anorexia and joint and muscle pain (Pappas et al., 2006; Food and Drug Administration, 2012). Brucellosis is transmitted to humans when the causative agent, Brucella, infect humans through contact with infected animals or infected animal's materials or through the consumption of animal products from infected animals (Corbel, 2006; Estradaa et al., 2016). The consumption of animal‐sourced foods is a common way of brucellosis transmission from infected natural host animals to humans (Corbel, 2006; Estradaa et al., 2016), and among animal‐sourced foods, unpasteurised milk and milk products are the main routes of brucellosis transmission (Dadar et al., 2019).
In Rwanda, brucellosis studies have been conducted focusing on animal health (Chatikoba et al., 2008; Manishimwe et al., 2015; Ndazigaruye et al., 2018; Ntivuguruzwa et al., 2020). Using animal sera and serological diagnostic methods, these studies reported the occurrence of animal brucellosis in Rwanda with a cattle brucellosis prevalence of 0.0% to 2.0% in peri‐urban areas of Kigali City (Manishimwe et al., 2015; Ntivuguruzwa et al., 2020), 8.3% in districts bordering the national parks (Ntivuguruzwa et al., 2020) and 9.9% to 18.9% in the district of Nyagatare (Chatikoba et al., 2008; Ndazigaruye et al., 2018). One study that investigated the general microbiological quality of milk from farms to milk collection centres in Rwanda detected anti‐Brucella antibodies in milk from two milk collection centres (Ndahetuye et al., 2020). The few studies conducted in Rwanda on human brucellosis, targeted patients attending district hospitals (Gafirita et al., 2017; Rujeni & Mbanzamihigo, 2014) and reported a prevalence of 25% among women presenting with abortion or stillbirth at Huye district hospital (Rujeni & Mbanzamihigo, 2014) and 6.1% among patients attending Nyagatare district hospital with brucellosis symptoms (Gafirita et al., 2017). In both studies, the consumption of unboiled or inadequately heat‐treated milk was reported as a risk factor for human brucellosis (Gafirita et al., 2017; Rujeni & Mbanzamihigo, 2014).
With the zoonotic nature of Brucella, with the public health complications and burdens resulting from brucellosis and with the reported associations of milk to the transmission of brucellosis from animals to humans, the aim of the current study was to investigate Brucella sero‐prevalence in milk produced across Rwanda, focusing on farm bulk milk from zero and open grazing cattle production systems and using enzyme‐linked immunosorbent assay (ELISA) methods. In addition, the study aimed to determine the risk factors associated with Brucella prevalence in farm bulk milk.
2. MATERIALS AND METHODS
2.1. Study design
A cross‐sectional study design was carried out where cow farm bulk milk samples were collected from open and zero grazing farms from five selected study districts across Rwanda. In addition, a mobile‐based electronic questionnaire was used to gather information on farms characteristics, farm management practices, cow reproduction disorders and farmers’ brucellosis awareness.
2.2. Study sites
Rwanda is a landlocked country located in central‐eastern Africa, between 1° 04′ and 2° 51′ of latitude below the equator and between 28° 45′ and 31° 15′ of longitude at the East. The country is 26,338 square km and is administratively divided into five provinces and 30 districts with a total population of 12.0 million (National Institute of Statistics of Rwanda, 2019). Rwanda is a highland country with altitudes varying between 900 and 4507 m above sea level (Ilunga et al., 2004). The climate is tropical but moderated by the high altitude (Haggag et al., 2016).
About 80.2% of all households in Rwanda are agricultural households involved in crop production, livestock production or both (National Insititute of Statistics of Rwanda, 2018). Among livestock, cattle are the most common, and up to 68.8% of rural households in Rwanda keep cattle (Ojango et al., 2012). Cattle are observed in all five provinces of the country with some regions keeping more cattle than others (National Insititute of Statistics of Rwanda, 2018). Along the important cattle keeping regions in Rwanda are also the main milk sheds. The five main milk sheds in the country are the eastern milk shed, southern milk shed, northern milk shed, north‐western milk shed and Kigali milk shed (Miklyaev et al., 2017). Cattle are raised under zero grazing and open grazing cattle production systems mainly. In the east and part of the northwest of the country with higher cattle populations and relatively more land for grazing, cattle are raised mainly under the open grazing systems in which cattle are left to graze on fenced farms. In the rest of the country with lower cattle populations and smaller holder farms, cattle are mainly raised under the zero grazing system in which cows are kept in‐doors and farmers cut and carry forage, crop residues and water to feed the cows (Feed the Future Innovation Lab, 2016; Mazimpaka, 2017). Countrywide, the zero grazing system is the most common with 80%, 17% and 3% of farms practising zero grazing, open grazing and semi‐grazing, respectively (Land O’ Lakes, 2014).
To determine the sero‐prevalence of Brucella in farm bulk milk and the risk factors of milk contamination with Brucella spp in Rwanda, five study districts (Nyanza, Gicumbi, Rwamagana, Nyagatare and Nyabihu) were selected across Rwanda (Figure 1). The five districts were selected based on their location in cattle production and milk shed areas in the country: Nyanza and Gicumbi districts are located in the southern and northern milk sheds, respectively. Rwamagana and Nyagatare districts are located in the largest eastern milk shed while Nyabihu district is located in the north‐western milk shed. The five districts were also selected to represent the two main grazing cattle production systems (zero grazing and open field grazing) practised in Rwanda: In three of the selected districts (Nyanza, Gicumbi and Rwamagana), the zero grazing system is practised, while in two of the selected districts (Nyagatare and Nyabihu), the open field grazing system is practised (Land O’ Lakes, 2014; Mazimpaka, 2017).
FIGURE 1.
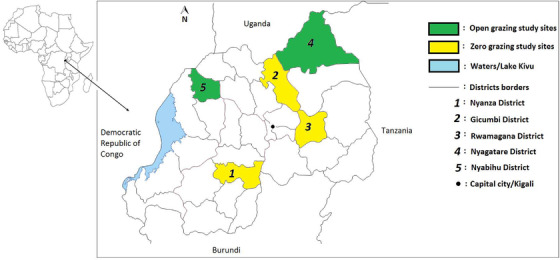
Map showing the study districts (viz., Nyanza, Gicumbi, Rwamagana, Nyagatare and Nyabihu) and the cattle production systems (open and zero grazing) practiced in the study sites
2.3. Study population
The study population consisted of rural cattle keeping households or cattle farms randomly selected from the five study districts.
2.4. Sample size
The sample size for the number of rural cattle keeping households/farms included in the study to determine the sero‐prevalence of Brucella in farm bulk milk, and the risk factors of milk contamination with Brucella spp was determined using Fischer's formula (Fisher et al., 1991):
where
-
‐
n is the sample size;
-
‐
zα is 1.96, which is the statistic corresponding to a level of confidence of 95%;
-
‐
P is 68.8%, the percentage of cattle keeping households among rural households in Rwanda (Ojango et al., 2012);
-
‐
d is the level of precision set at 5%.
Therefore, a total sample size of 330 cattle keeping households/farms was determined. An equal sample size of 330/5 = 66 of cattle keeping households/farms were then considered per study district.
2.5. Sampling
Selection of study farms or households was conducted in all five selected districts with 66 cattle keeping households being sampled per district. The sampling was done randomly and systematically by selecting the first household, skipping the next household and selecting the next one until the required sample size was reached. In addition to the random and systematic sampling, some inclusion and exclusion criteria were considered. To be considered, the randomly selected household/farm had to have at least one lactating cow, to have a household member available and able to provide the needed information for the questionnaire and to be geographically located within the district of interest. A household/farm was excluded if the present lactating cow or cows had just calved and the milk was still colostrum. A household/farm was also excluded if the lactating cow or cows were recently purchased or introduced and the respondent did not have past information on the cows. For each selected cattle keeping household/farm fulfilling the criteria, a questionnaire was administered to collect information on farm/household characteristics and potential brucellosis risk factors. Following the questionnaire administration, farm/household bulk raw milk was sampled and collected in duplicate sterile 15‐ml conical sampling tubes.
2.6. Data collection
2.6.1. Farmers’ interviews with a questionnaire
During the questionnaire administration at selected cattle keeping households, data were collected on the farm/household characteristics and the potential risk factors of milk contamination with Brucella spp. including farm/herd management practices, cattle reproduction and farmer's awareness about brucellosis. Questionnaire data was collected using Open Data Kit (ODK) with https://ona.io as the server.
2.6.2. Serology with indirect ELISA on farm bulk milk samples
The collected farm bulk milk samples were submitted to laboratory analysis to determine contamination with Brucella spp. The presence or absence of anti‐Brucella antibodies in collected farm bulk milk samples was determined using the SVANOVIR® Brucella‐Ab indirect ELISA (i‐ELISA) kit. According to the manufacturer, SVANOVIR® Brucella‐Ab I‐ELISA kit detects antibodies to major species of Brucella (B. abortus and B. melitensis) in cattle. According to the manufacturer, the test kit's specificity with milk samples is 99% when compared to the reference complement fixation test.
Ninety‐six well microplates coated with Brucella antigen were used according to the detailed manufacturer's kit protocol for milk samples. For each used plate, milk samples were tested in duplicate together with a positive serum control (in duplicate) and a negative serum control (in duplicate). Following instructed additions of reagents, incubation periods and washing steps, optical densities of individual wells with milk samples and controls were measured using a microplate photometer (Thermo Scientific Multiscan FC) at 450 nm according to the manufacturer's instructions. To determine whether a sample is positive or negative, optical density (OD) values were calculated into percent positivity (PP) values according to the manufacturer's instructions:
A used microplate was considered valid if (1) the duplicate OD values of the positive serum control did not differ more than 25% from the mean value of the two duplicates, (2) the OD value of the positive serum control was > 1.0 and (3) the PP of the negative serum control was < 10. A milk sample was considered negative if its calculated PP value was < 10. A milk sample was considered positive if its calculated PP value was ≥ 10.
2.7. Data analysis
Collected questionnaire data was exported from ODK to Microsoft Excel for data cleaning. i‐ELISA data on the prevalence of anti‐Brucella antibodies in collected farm bulk milk samples were also entered into Microsoft Excel. Questionnaire and i‐ELISA prevalence data were then coded and exported from Excel into SPSS for analysis. IBM SPSS Statistics version 20 was used to analyse data by descriptive statistics, univariate and multivariate logistic regressions.
Farm/households characteristics and farm management practices were analysed by descriptive statistics to obtain proportions and compute averages where needed. Comparisons of characteristics and farm management practices between zero grazing and open grazing farms/households were drawn using independent samples t test (for means comparisons) or Pearson's chi‐square (for proportions’ comparisons). Farm/households characteristics and farm management practices were also compared to the proportions of anti‐Brucella antibodies detection in farm bulk milk using Pearson's chi‐square.
To understand the associations between the different surveyed potential risk factors and farm bulk milk contamination with Brucella spp, binary logistic regression was used. The potential surveyed risk factors (farm characteristics and management practices) were set as independent/predictor variables while the presence/detection of anti‐Brucella antibodies in milk (negative or positive) was set as the dependent/outcome variable. Each individual surveyed potential risk factor was run against the dependent/outcome variable using a univariate binary logistic regression model to determine the significance of association between that individual independent variable and the presence/detection of anti‐Brucella antibodies in farm bulk milk. A risk factor was considered to be statistically significant for the presence of anti‐Brucella antibodies in farm bulk milk if the p‐value for that association was ≤ 0.05. The odds (odds ratio, OR) of the presence of anti‐Brucella antibodies in farm bulk milk in relation to each individual risk factor were also determined with a 95% confidence interval (CI).
Following the Pearson's chi‐square and univariate analyses, a multivariate logistic regression model was also used to get the effect that a combination of risk factors (significant from the univariate logistic regression model) have on farm bulk milk contamination with Brucella and to determine which risk factors best predict the presence of anti‐Brucella antibodies in milk.
3. RESULTS
3.1. Farm characteristics, management practices and reproductive disorders
A total of 330 farms/households were enrolled in this study. Most farms/households’ respondents (76.4%; 252/330) were male. The majority of respondents (56.4%; 186/330) were in the age range of 41 to 60 years with an overall mean age of 46 ± 13.0 years. The average herd size per farm/household in the open grazing farms was 17.7 ± 5.8 cows and was significantly higher (P < 0.05) than the average herd size of 2.2 ± 1.2 cows in the zero grazing farms. Seventy‐seven percentage (77.0%; 254/330) of all visited farms/households owned at least a farm‐bred cow (born and raised on the farm). In the study sites (Nyanza, Gicumbi and Rwamagana districts), practising zero grazing cattle production system, more of the owned cows were from government and non‐government donating programs with 21.2% (42/198) of zero grazing farms having at least one cow from the government “Girinka” program that has been donating cows to poor families since 2006 (Rwanda Ministry of Agriculture and Animal Resources (MINAGRI), 2009) and 10.6% (21/198) of the same zero grazing farms/households having at least one cow from other donating non‐government programs supporting the “Girinka” program. Visited farms/households in the open grazing areas had no cows from donors, whether from the government or non‐government organisations.
Natural breeding with bulls was practised in 100% (132/132) of visited open grazing farms and in significantly (p < 0.05) less zero grazing farms (33.8%; 67/198; Table 1).
TABLE 1.
Farm management practices and reproductive disorders
| Description | Response | TOTAL (N = 330) | Zero grazing (N = 198) | Open grazing (N = 132) | Comparisons: zero grazing and open grazing |
|---|---|---|---|---|---|
| Proportion (%) | Proportion (%) | Proportion (%) | p‐value | ||
| Breeding method | Bull | 60.3 | 33.8 | 100 | 0.000* |
| Artificial insemination | 39.7 | 66.2 | 0.0 | ||
| History of abortion | Yes | 20.9 | 11.1 | 35.6 | 0.000* |
| No | 79.1 | 88.9 | 64.4 | ||
| History of placenta retention | Yes | 27.9 | 26.3 | 30.3 | 0.423 |
| No | 72.1 | 73.7 | 69.7 | ||
| History of longer calving intervals (> 1 year) | Yes | 59.7 | 73.2 | 39.4 | 0.000* |
| No | 40.3 | 26.8 | 60.6 | ||
| History of still births | Yes | 22.1 | 3.0 | 50.8 | 0.000* |
| No | 77.9 | 97.0 | 49.2 | ||
| History of weak calves at birth | Yes | 2.1 | 0.5 | 4.5 | 0.013* |
| No | 97.9 | 99.5 | 95.5 | ||
| History/presence of arthritis or hygromas | Yes | 0.3 | 0.5 | 0.0 | |
| No | 99.7 | 99.5 | 100.0 | ||
| Respondent heard about brucellosis | Yes | 63.6 | 55.6 | 75.8 | 0.000* |
| No | 36.4 | 44.4 | 24.2 | ||
| Vaccination against brucellosis | Yes | 2.4 | 4.0 | 0.0 | |
| No | 70.9 | 70.2 | 72.0 | ||
| Do not know | 26.7 | 25.8 | 28.0 |
*Significant difference between compared zero grazing and open grazing proportions.
Reproductive disorders were recorded in farms across both zero and open grazing study sites but at different proportions. Histories of abortion, stillbirths and weak calves at birth were recorded significantly more (p < 0.05) in open grazing farms than in zero grazing farms (Table 1). Calving intervals of more than a year were recorded significantly more (p < 0.05) in zero grazing farms (73.2%; 145/198) than in open grazing farms (39.4%; 52/132; Table 1). The history of placenta retention was recorded in both zero grazing and open grazing farms with no significant difference (p > 0.05) between proportions (Table 1). External clinical signs of arthritis or hygromas that have been linked to cattle brucellosis (Musa et al., 1990) were observed in only one zero grazing farm in which a cow had arthritis in the leg joints.
Among respondents from all visited farms, 63.6% (210/330) indicated they had heard about brucellosis. Significantly (p < 0.05) more farmers from open grazing cattle production areas had heard about brucellosis (with 75.8% [100/132] of the respondents having heard about brucellosis), compared to farmers from zero grazing cattle production areas with 55.6% (110/198) having heard about brucellosis (Table 1).
Across all study sites, only 2.4% (8/330) of farms had their cows vaccinated against brucellosis, while 70.9% (234/330) had not vaccinated their cows and 26.7% (88/330) did not know whether their cows were vaccinated against brucellosis or not. The few farms that had vaccinated cows were all from study sites practising the zero grazing cattle production system (Table 1)
3.2. Prevalence of anti‐Brucella antibodies in farm bulk milk by different farm management practices and reproduction disorders
Anti‐Brucella antibodies were detected using i‐ELISA and were found to be prevalent in 19.7% (95% CI, 15.5–24.4) of all 330 collected farm bulk raw milk samples (Table 2). Farm bulk milk from farms practising open grazing cattle production system were contaminated at a significantly (p < 0.05) higher proportion (37.9%, 50/132), compared to farm bulk milk from farms practising the zero grazing cattle production system (7.6%; 15/198; Table 3). In terms of study districts, Nyagatare district had the highest prevalence with 51.5% (34/66) of farm bulk milk samples containing anti‐Brucella antibodies, while Nyanza district had the lowest prevalence with 4.5% (3/66) of farm bulk milk samples containing anti‐Brucella antibodies (Table 2). The proportions of anti‐Brucella antibodies prevalence in farm bulk milk from zero grazing study districts (Nyanza, 4.5%; Gicumbi, 6.1% and Rwamagana, 12.1%) were not significantly different (p > 0.05) when compared to each other (Table 4). Open grazing study districts had mostly significantly (p < 0.05) higher Brucella seropositivity proportions than zero grazing study districts (Table 4). In particular, Nyagatare district had a significantly higher proportion (p < 0.05) of Brucella seropositive farm bulk milk, compared to any other study district (Table 4).
TABLE 2.
Prevalence of anti‐Brucella antibodies in collected farm bulk milk
| Cattle production system and study districts | Farm bulk milk samples (positive samples by i‐ELISA) | Anti‐Brucella antibodies sero‐prevalence (proportion, %) | Anti‐Brucella antibodies sero‐prevalence (proportion, %); 95% confidence interval (CI) |
|---|---|---|---|
| Zero grazing | |||
| Nyanza | 66 (3) | 4.5 | |
| Gicumbi | 66 (4) | 6.1 | |
| Rwamagana | 66 (8) | 12.1 | |
| Total/zero grazing | 198 (15) | 7.6 | 7.6 (4.3–12.2) |
| Open grazing | |||
| Nyagatare | 66 (34) | 51.5 | |
| Nyabihu | 66 (16) | 24.2 | |
| Total/open grazing | 132 (50) | 37.9 | 37.9 (29.6–46.7) |
| TOTAL | 330 (65) | 19.7 | 19.7 (15.5–24.4) |
TABLE 3.
Proportions of Brucella seropositive farm bulk milk samples by potential risk factors
| Risk factors | Level | Milk samples (positive samples); proportion | Milk samples (negative samples); proportion | p‐value |
|---|---|---|---|---|
| Study district | Nyanza | 66 (3); 4.5 | 66 (63); 95.5 | 0.000* |
| Gicumbi | 66 (4); 6.1 | 66 (62); 93.9 | ||
| Rwamagana | 66 (8); 12.1 | 66 (58); 87.9 | ||
| Nyagatare | 66 (34); 51.5 | 66 (32); 48.5 | ||
| Nyabihu | 66 (16); 24.2 | 66 (50); 75.8 | ||
| Cattle production system | Zero grazing | 198 (15) ; 7.6 | 198 (183) ; 92.4 | 0.000 |
| Open grazing | 132 (50) ; 37.9 | 132 (82) ; 62.1 | ||
| Herd size | 1 to 2 | 151 (11); 7.3 | 151 (140) ; 92.7 | 0.000* |
| 3 to 6 | 44 (3); 6.8 | 44 (41) ; 93.2 | ||
| > 6 | 135 (51); 37.8 | 135 (84) ; 62.2 | ||
| Breeding method | Artificial insemination | 131 (14); 10.7 | 131 (117) ; 89.3 | 0.001 |
| Bull | 199(51); 25.6 | 199(148) ; 74.4 | ||
| History of abortion | Yes | 69 (47); 68.1 | 69 (22) ; 31.9 | 0.000 |
| No | 261 (18); 6.9 | 261 (243) ; 93.1 | ||
| History of placenta retention | Yes | 92 (35); 38.0 | 92 (57) ; 62.0 | 0.000 |
| No | 238 (30); 12.6 | 238 (208) ; 87.4 | ||
| History of longer calving intervals (> 1 year) | Yes | 197 (48); 24.4 | 197 (149); 75.6 | 0.009 |
| No | 133 (17); 12.8 | 133 (116); 87.2 | ||
| History of still births | Yes | 73 (27); 37.0 | 73 (46); 63.0 | 0.000 |
| No | 257 (38); 14.8 | 257 (219); 85.2 | ||
| History of weak calves at birth | Yes | 7 (6); 85.7 | 7 (1); 14.3 | 0.000 |
| No | 323 (59); 18.3 | 323 (264); 81.7 | ||
| History of arthritis or hygroma | Yes | 1 (1); 100 | 1 (0); 0 | 0.043 |
| No | 329 (64); 19.5 | 329 (265); 80.5 | ||
| Respondent heard about brucellosis | Yes | 210 (56); 26.7 | 210 (154); 73.3 | 0.000 |
| No | 120 (9); 7.5 | 120 (111); 92.5 | ||
| Vaccination against brucellosis | Yes | 8 (2); 25.0 | 8 (6) ; 75.0 | 0.071 |
| No | 234 (53); 22.6 | 234 (181) ; 77.4 | ||
| Do not know | 88 (10); 11.4 | 88 (78) ; 88.6 |
*Comparisons of seropositivity between the different groups (levels) are shown below in Table 4.
TABLE 4.
Comparisons of Brucella seropositivity proportions in farm bulk milk from different locations and from different herd size groups
| Compared groups/levels | p‐value |
|---|---|
| Location/study districts | |
| Nyanza vs. Gicumbi | 0.698 |
| Nyanza vs. Rwamagana | 0.115 |
| Gicumbi vs. Rwamagana | 0.226 |
| Nyanza vs. Nyagatare | 0.000* |
| Gicumbi vs. Nyagatare | 0.000* |
| Rwamagana vs. Nyagatare | 0.000* |
| Nyanza vs. Nyabihu | 0.001* |
| Gicumbi vs. Nyabihu | 0.004* |
| Rwamagana vs. Nyabihu | 0.071 |
| Nyagatare vs. Nyabihu | 0.001* |
| Herd size groups | |
| 1 to 2 cows vs. 3 to 6 cows | 0.916 |
| 1 to 2 cows vs. > 6 cows | 0.000* |
| 3 to 6 cows vs. > 6 cows | 0.000* |
*Significant difference between Brucella seropositivity of the compared groups/levels.
The proportion of farm bulk milk contaminated with anti‐Brucella antibodies increased as the herd size increased (Table 3), and farms with more than six cows had a significantly higher (p < 0.05) proportion of farm bulk milk Brucella seropositivity (Table 4).
The proportion of anti‐Brucella antibodies prevalence in farm bulk milk from farms using natural breeding (25.6%; 51/199) was significantly higher (p < 0.05) than the proportion of prevalence in farm bulk milk from farms using artificial insemination (10.7%; 14/131; Table 3).
Anti‐Brucella antibodies were detected in significantly higher proportions in farms with histories of reproductive disorders (p < 0.05), compared to farms with no histories of reproductive disorders (Table 3). Anti‐Brucella antibodies were also detected in the farm bulk milk from one zero grazing farm in which a cow presented with external brucellosis clinical sign of arthritis (Table 3).
A significantly (p < 0.05) higher proportion of farm bulk milk (26.7%; 56/210) from respondent farmers who had heard about brucellosis contained anti‐Brucella antibodies, compared to the proportion of seropositive farm bulk milk (7.5%; 9/120) from respondent farmers who had not heard about brucellosis (Table 3).
Respondents from only eight farms indicated that their cattle were vaccinated against brucellosis, although no vaccination record was kept or shown. Out of these eight farms, anti‐Brucella antibodies were detected in farm bulk milk from two farms (Table 3).
3.3. Risk factors of farm bulk milk contamination with Brucell a
Potential risk factors for milk contamination with Brucella spp on which data were collected were first individually analysed using univariable binary logistic regression to determine their associations with the prevalence anti‐Brucella antibodies in farm bulk milk. Ten potential risk factors (out of 12) were found to be statistically significant factors (p < 0.05) for the presence/detection of anti‐Brucella antibodies in farm bulk milk (Table 5). They are location/study district, cattle production system, herd size, breeding method, history of abortion, history of placenta retention, history of longer calving intervals (> 1 year), history of stillbirths, history of weak calves at birth and the respondent having heard about brucellosis. In particular, history of reproductive disorders such as abortion and weak calves at birth were strong predictors of the detection of anti‐Brucella antibodies in farm bulk milk. The odds of detecting anti‐Brucella antibodies in milk from a farm/household with a history of abortion were, for example, 28.8 times more (OR = 28.8; 95% CI, 14.3–57.9) than the odds of detecting anti‐Brucella antibodies in milk from a farm/household with no history of abortion (Table 5). The practised cattle production system was also a strong predictor, and the odds of detecting anti‐Brucella antibodies in milk from a farm/household practising open grazing cattle production system were also 7.4 times more (OR = 7.4; 95% CI, 3.9–14.0) than the odds of detecting anti‐Brucella antibodies in milk from a farm/household practising zero grazing cattle production system (Table 5).
TABLE 5.
Univariable binary logistic regression analysis of associations between risk factors and the prevalence of anti‐Brucella antibodies in farm bulk raw milk
| Risk factor | Level | p‐value | Odds ratio (OR; 95% CI) |
|---|---|---|---|
| Study district | 0.000* | ||
| Nyanza | 0.004 | 0.1 (0.0–0.5) | |
| Gicumbi | 0.007 | 0.2 (0.0–0.6) | |
| Rwamagana | 0.076 | 0.4 (0.1–1.1) | |
| Nyagatare | 0.002 | 3.3 (1.6–6.9) | |
| Nyabihu | a | ||
| Cattle production system | Zero grazing | a | |
| Open grazing | 0.000* | 7.4 (3.9–14.0) | |
| Herd size | 0.000* | ||
| 1 to 2 | 0.000 | 0.1 (0.0–0.2) | |
| 3 to 6 | 0.001 | 0.1 (0.0–0.4) | |
| > 6 | a | ||
| Breeding method | Artificial insemination | a | |
| Bull | 0.001* | 2.8 (1.5–5.4) | |
| History of abortion | Yes | 0.000* | 28.8 (14.3–57.9) |
| No | a | ||
| History of placenta retention | Yes | 0.000* | 4.2 (2.4–7.5) |
| No | a | ||
| History of longer calving intervals (> 1 year) | Yes | 0.011* | 2.2 (1.2–4.0) |
| No | a | ||
| History of still births | Yes | 0.000* | 3.3 (1.8–6.0) |
| No | a | ||
| History of weak calves at birth | Yes | 0.003* | 26.8 (3.1–227.2) |
| No | a | ||
| History of arthritis or hygroma | Yes | 1.000 | 6,689,075,610 (0.0) |
| No | a | ||
| Respondent heard about brucellosis | Yes | 0.000* | 4.4 (2.1–9.4) |
| No | a | ||
| Vaccination against brucellosis | 0.079 | ||
| Yes | 0.279 | 2.6 (0.4–14.6) | |
| No | 0.026 | 2.2 (1.1–4.7) | |
| Do not know | a |
*Significant risk factor;
Reference value.
Following univariable logistic regression analyses, a multivariable logistic regression model was used with all 10 significant risk factors to determine which risk factors best predicted the presence of anti‐Brucella antibodies in farm bulk milk (Table 6). Multivariable logistic regression showed that practising open grazing system, history of abortion, history of placenta retention and history of longer calving intervals (> 1 year) were the significant risk factors (p < 0.05), which better predicted the presence of anti‐Brucella antibodies in farm bulk milk (Table 6). Practising open grazing system and having a history of abortion at the farm were associated with the highest odds (OR = 69.5; 95% CI, 1.6–3033.6 and OR = 19.5; 95% CI, 8.1–46.8, respectively) for the presence of anti‐Brucella antibodies in farm bulk milk when compared to practising zero grazing system and having no history of abortion, respectively (Table 6). Using bulls (natural service) for breeding had also an effect (p = 0.053) on the presence of anti‐Brucella antibodies in farm bulk milk (Table 6). To determine the predictability of the multivariable logistic regression model, the goodness of fit of the model was tested using Hosmer–Lemeshow test and was 0.161. The overall ability of prediction of the model was 91.2%.
TABLE 6.
Multivariable binary logistic regression analysis of associations between all significant risk factors and the prevalence of anti‐Brucella antibodies in farm bulk raw milk
| Risk factor | Level | Multivariate logistic regression p‐value | OR (95% CI) |
|---|---|---|---|
| Study district | 0.922 | ||
| Nyanza | 0.530 | 0.6 (0.1–3.0) | |
| Gicumbi | 0.907 | 0.9 (0.1–4.6) | |
| Rwamagana | |||
| Nyagatare | 0.766 | 1.2 (0.3–3.9) | |
| Nyabihu | a | ||
| Cattle production system | Zero grazing | a | |
| Open grazing | 0.028* | 69.5 (1.6–3033.6) | |
| Herd size | 0.895 | ||
| 1 to 2 | 0.646 | 1.8 (0.1–24.6) | |
| 3 to 6 | 0.660 | 1.8 (0.1–24.8) | |
| > 6 | a | ||
| Breeding method | Bull | 0.053 | 0.1 (0.0–1.0) |
| Artificial insemination | a | ||
| History of abortion | Yes | 0.000* | 19.5 (8.1–46.8) |
| No | a | ||
| History of placenta retention |
Yes No |
0.002* |
4.2 (1.7–10.3) |
| History of longer calving‐ intervals (> 1 year) |
Yes No |
0.007* |
3.8 (1.4–10.2) |
| History of still births | Yes | 0.845 | 1.1 (0.4–2.9) |
| No | a | ||
| History of weak calves at‐ birth |
Yes No |
0.635 |
4.2 (0.0–1739.6) |
| Respondent heard about‐ brucellosis |
Yes No |
0.584 |
1.3 (0.4–4.0) |
*Significant risk factor;
Reference value.
4. DISCUSSION
This study was conducted to determine Brucella sero‐prevalence in farm bulk milk in Rwanda and the risk factors associated with farm bulk milk contamination with Brucella spp.
To determine Brucella prevalence in farm bulk milk, the serological method, i‐ELISA, was the preferred method due to its commercial availability, sensitivity and specificity. According to the manufacturer, the used i‐ELISA kit is highly sensitive and specific for B. abortus and B. melitensis (Boehringer Ingelheim Svanova), and B. abortus is the main Brucella species affecting cattle (Godfroid et al., 2014). High i‐ELISA sensitivity (varying from 96% to 100%) and high specificity (varying from 93.8% to 100%) have also been reported (Gall & Nielsen, 2004; Gall et al., 2001). ELISA‐based tests are also known to be the most sensitive among serological tests (Geresu & Kassa, 2015; Smirnova et al., 2013; Zhao et al., 2014). However, most ELISA methods, including i‐ELISA used in this study, detect antibodies against the Brucella smooth lipopolysaccharide and, therefore, may also detect antibodies due to vaccine strains S19 and Rev1 (Ko et al., 2012; Lim et al., 2012). This was not a setback in this study as cattle brucellosis vaccination is not yet widespread in Rwanda. In this study, very few farms (only 8/330 representing 2.4%) indicated their cattle were vaccinated against brucellosis, although no vaccination records were kept or shown.
This study revealed that anti‐Brucella antibodies are prevalent in farm bulk milk in Rwanda and especially in milk from open grazing farms. Previous studies investigating brucellosis in Rwanda have also reported the prevalence of the disease in the country. A recent study that was conducted on cattle brucellosis in Nyagatare district using cattle sera and the Rose Bengal Test reported a prevalence of 18.9% at individual cow level in the district (Ndazigaruye et al., 2018). A different study that also investigated brucellosis at an individual cow level in Kigali city reported a much lower prevalence of 2.03% using the Rose Bengal Plate test and 1.7% using competitive ELISA (Manichee et al., 2015). Our findings in this study on anti‐Brucella antibodies prevalence in farm bulk milk reflect, however, brucellosis prevalence at herd level (and not at individual cow level) and would be better compared against herd level cattle brucellosis prevalence. A herd‐level investigation on bovine brucellosis in Nyagatare district, Rwanda, reported a prevalence of 30.2% using the Rose Bengal Test on cattle sera collected from 998 cows from 205 herds in the district (Chatikoba et al., 2008). Our study findings in the Nyagatare district (Brucella sero‐prevalence in 51.5% of farm bulk milk) are, therefore, higher than the cattle herd‐level prevalence of 30.2% reported by Chatikoba et al. (2008). This indicates an increase in cattle brucellosis prevalence at the herd level in the Nyagatare district over the past 12 years. Sample types and sensitivities of the serological diagnostic methods used in both our studies were, however, different, Chatikoba et al. having used animal sera and the Rose Bengal Plate Test, which is less sensitive, compared to ELISA‐based tests (Geresu & Kassa, 2015; Smirnova et al., 2013; Zhao et al., 2014). The apparent increase in herd‐level brucellosis could also be due to the elapsed time (about 12 years) with no control measures known to have been put in place to control brucellosis. This may have then led to further transmission between herds over time, especially in the Nyagatare district where, although most farms are fenced, the majority of farms (89.7%) have no water at the farm or near the farm and use shared watering points (Mazimpaka, 2017). Sharing drinking water and interactions between cows from different herds can cause transmission of the disease between herds (Alhaji et al., 2016; Aparicio, 2013; Mekonnen et al., 2010), and farmers who used individual water wells as watering points were found to have lower proportions of seropositive animals than farmers who used communal water wells (Muma et al., 2007).
While our study is among the first study on Brucella prevalence in milk in Rwanda, data from a recent study on microbiological quality and safety of milk from farm to milk collection centres in Rwanda (Ndahetuye et al., 2020) detected anti‐Brucella antibodies in milk samples from two milk collection centres in the Eastern Province of Rwanda, although the anti‐Brucella antibodies were not detected in milk samples from farms attached and serving the milk collection centres. Other studies carried out on human brucellosis in Rwanda have also associated the prevalence in humans to raw milk consumption and implied milk contamination with Brucella in the country. A study conducted on women presenting with abortion or stillbirth of unknown origin at Huye district hospital reported a prevalence of 25% among those women. The study showed that 87.6% of the women seropositive to Brucella had the habit of consuming milk with more than half of those milk consumers consuming it raw (Rujeni & Mbanzamihigo, 2014). More recently, a study conducted on patients attending Nyagatare district hospital and presenting with key brucellosis symptoms reported a prevalence of 6.1% and found a significant association between the infection and drinking unboiled milk (Gafirita et al., 2017).
Outside Rwanda and in the East African region, studies on Brucella prevalence in cattle milk have been conducted. Using diagnosis tests including Milk Ring Test, i‐ELISA and real‐time PCR, the reported prevalence in raw milk samples from dairy farms, milk shops, street vendors, milk deliverers, boiling points, milk collection centres and dairy factories in Uganda varied between 6.5% and 49.45% (Hoffman et al., 2016; Kamwine et al., 2017; Makita et al., 2010; Rock et al., 2016). Compared to our findings, there were similar trends between Brucella prevalence in milk in Uganda and Rwanda. Our results in Nyagatare district (51.5%; 34/66) where the open grazing cattle production system is predominant are, for example, similar to the results of 49.45% prevalence obtained from raw milk collected from dairy farms and dairy factories in Southwestern Uganda where the extensive cattle production system is predominantly practised (Kamwine et al., 2017). Our results of the low prevalence of anti‐Brucella antibodies in farm bulk milk from districts practising zero grazing system (4.5%, 6.1% and 12.1% in Nyanza, Gicumbi and Rwamagana districts, respectively) are also similar to the prevalence of 11% reported in Gulu district, in Uganda, where the zero grazing cattle production was predominant (Rock et al., 2016). Similar studies in Tanzania investigating Brucella herd‐level sero‐prevalence, using farm bulk milk and using i‐ELISA to test the milk, reported a herd‐level prevalence of 44.4% in the Morogoro region, where cattle are mostly raised in semi‐extensive and extensive production systems (Asakura et al., 2018).
It is obvious in our study findings that the practised cattle production system (open grazing or zero grazing) had an effect on the level of Brucella sero‐prevalence in farm bulk milk, and practising open grazing cattle production system was found to be a significant risk factor associated with anti‐Brucella antibodies presence in farm bulk milk. This clear finding of significantly higher Brucella sero‐prevalence in cattle farms of Nyagatare and Nyabihu practising open grazing system, compared to cattle farms of Nyanza, Gicumbi and Rwamagana practising zero grazing system, was also reported in several studies on cattle brucellosis (Boukary et al., 2013; de Alencar Mota et al., 2016; Makita et al., 2011; Sagamiko et al., 2018; Shahid et al., 2014; Tadesse, 2016). The transmission and spread of cattle brucellosis are favoured in areas practising open grazing in which cattle freely interact within a herd and between herds. The spread is realized through shared grazing areas, shared bulls (if natural breeding is practised), shared water sources, contaminated and contaminating aborted materials, vaginal discharges and manure (Aparicio, 2013; Kaur et al., 2018; Tekle et al., 2019). With regard to our findings in this study, however, the high proportion of contaminated herds in open grazing areas may not be due to shared grazing areas as farms are predominantly fenced, but it may be explained by shared water sources and shared bulls. The high proportions of contaminated herds in both open grazing areas of Nyagatare and Nyabihu covered in this study were, however, significantly different when compared to each other. This may be explained by the water shortage and water sources sharing reported in Nyagatare (Mazimpaka, 2017) where the higher proportion of contaminated herds was found. Indeed, Nyagatare is faced with more water scarcity being located in the lower drier eastern lands of the country with an annual rainfall of 700 to 1100 mm and an annual average temperature of up to and beyond 30°C (Haggag et al., 2016; Muhire et al., 2014). Nyagatare district also experiences longer dry periods per year during which only 6% of farmers were reported to have water on farm, and the remaining majority had to trek their cattle to the nearest valley dams or rivers where the water source is shared by different herds (Mazimpaka, 2017). Nyabihu, on the other hand, has more water sources being located in the higher, more humid western lands of the country with an annual rainfall of 1300 to 1550 mm and an annual average temperature of 15 to 17°C (Haggag et al., 2016; Muhire et al., 2014). The water shortage and water sharing in Nyagatare district could, therefore, be contributing to the significantly higher prevalence proportion, compared to the prevalence proportion in Nyabihu district where open grazing is also practised.
Concerning zero grazing areas, it has been reported that the transmission and spread of cattle brucellosis are limited due to the low level of herd‐to‐herd contact and small confined herds (McDermott & Arimi, 2002). In the case of Rwanda, in particular, the low sero‐prevalence (7.6%; 15/198) reported, in this study, from zero grazing study sites could also be explained by the origin of the one to two cows per farm widely observed in the smallholder zero grazing farms. A number of the zero grazing smallholder farms (31.8%; 63/198) had cows from cow donating governmental (Girinka) and non‐governmental programs that have been distributing cattle to poor families while none (0%) of the visited open grazing larger farms had cows from the donating programs. The distributed heifers are healthy and are screened by conducting a Rose Bengal brucellosis test for each heifer prior to distribution to farmers (Rwanda Ministry of Agriculture and Animal Resources (MINAGRI), 2019). Although data on animal age was not collected in this study, the one to two cows per farm observed in zero grazing farms is relatively younger. Indeed, more farmers in zero grazing system had obtained cattle from the government and non‐governmental cattle donating programs and owned younger animals. The younger cattle in the zero grazing system have, therefore, been less exposed for brucellosis contamination, compared to older cows raised in open grazing farms. The young age of animals in zero grazing farms could also explain the low prevalence in zero grazing farms, and recent studies in Rwanda (Ndazigaruye et al., 2018; Ntivuguruzwa et al., 2020) have found older animal age to be significantly associated with brucellosis prevalence in cows. Other factors such as zero grazing and stall feeding with limited cattle movements, preference and use of artificial insemination and additional follow‐up veterinary services offered by the cattle donating programs to the benefiting farmers (Rwanda Ministry of Agriculture and Animal Resources (MINAGRI), 2019) may also contribute to preventing new brucellosis infections and explain the low sero‐prevalence in zero grazing farms. Some studies have, however, found no statistically significant associations between cattle production systems and brucellosis prevalence. No significant difference was found in the overall prevalence of brucellosis in cattle from different grazing systems in Nyagatare, Rwanda, although more cattle brucellosis seropositive cases were reported in farms practising extensive open grazing system than in the few farms that practice zero grazing in the district (Ndazigaruye et al., 2018). A higher prevalence of cattle brucellosis was reported from zero grazing cattle production systems in Nigeria, but it was argued that the higher prevalence was due to most zero grazing farms sourcing their cattle from open markets with high risks of contamination (Mai et al., 2012). A study in Ethiopia also reported a lower prevalence of cattle brucellosis in the extensive production system, and this low prevalence was attributed to reduced animal‐to‐animal contact and reduced contamination of pastures under dry conditions (Elemo & Geresu, 2018).
In terms of herd size and proportionality, our study detected anti‐Brucella antibodies more in farms with more than six cows than in smallholder farms with six or fewer cows. Independently, the herd size was also a significant risk factor associated with anti‐Brucella antibodies detection in farm bulk milk. Our findings are in line with a study conducted in Nyagatare District, Rwanda, which found that the occurrence of cattle brucellosis in herds with 40–70 cattle was 26.9% and was significantly greater than the occurrence (14.9%) in herds with 10–39 cattle (Ndazigaruye et al., 2018). Several other studies have also found herd size to have a significant effect on the herd and individual cattle brucellosis prevalence and the sero‐prevalence increased with herd size (Awah‐Ndukum et al., 2018; Boukary et al., 2013; Makita et al., 2011; Miller et al., 2016; Sagamiko et al., 2018; Sanogo et al., 2012; Tasiame et al., 2016). In larger herds, high stocking densities and associated poor hygiene contribute to within‐herd brucellosis infection (Ibrahim et al., 2010; Omer et al., 2000), and the larger the herd, the more likely there will be at least one infected cow per herd causing the pooled farm bulk milk to contain anti‐Brucella antibodies. Once a herd is infected, the infection is also likely to stay in the herd as more and more cows are exposed through common grazing lands, common water sources and contaminating aborting materials and other interactions within the herd. Although it is generally observed that the large stocking densities in larger herds result in a higher level of prevalence among larger herds, some studies found that increasing herd size did not have a significant effect on cattle brucellosis in herds (Asgedom et al., 2016; Elemo & Geresu, 2018).
In the present study, farms using natural breeding were proportionally and significantly more contaminated with Brucella than farms using artificial insemination. Natural breeding was also almost significantly associated with anti‐Brucella antibodies presence in farm bulk milk in the final multivariate analysis model. Natural breeding, the use of community bulls and the exchange of bull for mating between herds and cattle have been reported as major risk factors for cattle brucellosis (Alhaji et al., 2016; Berhe et al., 2007; Ebrahim et al., 2016). The possible contribution of bulls to Brucella infection in herds, in the present study, was also supported by the preference and use of bulls in open grazing farms in which Brucella antibodies prevalence in farm bulk milk was the highest. Indeed, all visited open grazing farms indicated they preferred and used bulls for breeding. Some studies have, however, reported that artificial insemination can, as well, contribute to brucellosis spreading. In a case–control study involving 98 newly infected farms and 93 farms that remained brucellosis‐free in Colombia, for example, natural breeding with bulls from certified brucellosis‐free farms was safer than the use of artificial insemination, whether with frozen semen (frozen semen coming from insemination centres certified as brucellosis‐free by veterinary services) or with fresh semen from un‐controlled herds (Cárdenas et al., 2019).
In this study, anti‐Brucella antibodies were detected in significantly higher proportions in farms with a history of reproductive disorders such as abortion and placenta retention, compared to farms with no history of reproductive disorders. Several other studies have also associated reproductive disorders to cattle brucellosis at animal and herd levels (Alhaji et al., 2016; Boukary et al., 2013; Hossain et al., 2014; Makita et al., 2011; Mufinda et al., 2015; Tasiame et al., 2016), and cows infected with Brucella have been reported to be three to four times more likely to abort than uninfected and unexposed cows (Boukary et al., 2013; Muma et al., 2007; Schelling et al., 2003). Reproductive disorders such as abortion are also known symptoms and the most frequent clinical signs of brucellosis in animals including cattle (Acha & Szyfres, 2001; McDermott et al., 2013; Schmutz et al., 1996). Following infection, Brucellae spread in different areas and especially in the animal's reproductive system where they cause placentitis and metritis (Poester et al., 2013), which in turn results in abortions (Ul‐Islam et al., 2013). Contrary to our findings, however, some studies did not find significant associations between reproductive disorders and brucellosis but did report higher brucellosis prevalences in cattle with a history of reproductive disorders (Al‐Majali et al., 2009; Ibrahim et al., 2010; Kebede et al., 2008; Makita et al., 2011).
The reproductive disorder of longer calving intervals (> 1 year) was a significant risk factor for anti‐Brucella antibodies presence in farm bulk milk as computed by both univariate and multivariate analyses of risk factors. However, it is interesting to note, in Table 1, that longer calving intervals were more recorded in zero grazing system farms (with significantly lower anti‐Brucella antibodies presence in farm bulk milk) than in open grazing system (with significantly higher anti‐Brucella antibodies presence in farm bulk milk). This means that the high occurrence of longer calving intervals reported across zero grazing and open grazing farms could be due to other factors such as irregularities in carrying out artificial insemination where it is practised, insufficient training or experience for identifying a cow in heat and other poor husbandry management practices.
Farmers were, in general, aware of cattle brucellosis (by having heard of the infection from fellow farmers mainly). In open grazing farms where anti‐Brucella were detected in a significantly higher proportion of farm bulk milk, farmers were even more aware of cattle brucellosis. Having heard about brucellosis by the respondent farmer did not, therefore, reduce the risk of farm bulk milk being contaminated with anti‐Brucella antibodies. This finding is not in line with the study by Awah‐Ndukum et al. who associated the high prevalence of cattle brucellosis to farmers being not aware or not knowing about the infection (Awah‐Ndukum et al., 2018). Our findings could, however, indicate that brucellosis is known among farmers but is neglected and not considered a serious cattle infection that should be dealt with. Indeed, the World Health Organisation has classified brucellosis as one of the top neglected zoonotic diseases (World Health Organization, 2012). Endemic zoonotic diseases, including brucellosis, are also reported to be especially neglected in low‐income countries (Halliday et al., 2015). Our findings implying the negligence of brucellosis among farmers were also supported by the rate of vaccination, which is still very low (2.4% of all visited farms) and the practice by 85.8% of farmers of not screening replacement cows for brucellosis prior to addition to existing herds as previously reported in Nyagatare district in Rwanda (Ndazigaruye et al., 2018). The majority of farmers who had heard about brucellosis in this study (86.2%) indicated they heard about the infection from fellow farmers. Hearing about brucellosis in rather informal ways from fellow farmers may also contribute to the lightness with which farmers consider brucellosis.
In this study, very few farms (eight [2.4%] of all visited farms) indicated they had vaccinated their cattle against brucellosis, although vaccination records could not be provided and concerned farmers could not recall the specific vaccine that was used. Upon contacting and consulting local veterinary officers and the Rwanda Agriculture Board, which is in charge of the brucellosis vaccination program in the country, it was established that the RB51, which is still the only vaccine used in Rwanda, was the vaccine administered at the eight farms. Following risk factors’ analysis, vaccination status was not a significant risk factor for the presence of anti‐Brucella antibodies in farm bulk milk. Also, the proportion of seropositive farms among vaccinated farms was not significantly different from the proportion of seropositive farms among non‐vaccinated farms. The overall small number of farms with vaccinated cattle (eight out of 330 farms) in this study could be the reason for the statistically non‐significant difference between the proportion of seropositivity among farms with vaccinated cattle and the proportion of seropositivity among farms with non‐vaccinated cattle. Similar results of no significant difference between the prevalence of brucellosis among vaccinated and cattle and the prevalence among non‐vaccinated cattle were reported by others (Nguna et al., 2019). Among the eight farms (out of 330 farms) that reported having vaccinated their cattle, anti‐Brucella antibodies were detected in two farms. Antibodies produced following vaccination with brucellosis vaccines such as S19 and Rev1 can be detected by i‐ELISA (Ko et al., 2012; Lim et al., 2012). However, antibodies produced from RB51 vaccine (which is the vaccine that was used) are different from antibodies induced by natural infection and do not interfere with brucellosis serological diagnostic methods (Dorneles et al., 2015) including i‐ELISA used in this study. The detection of anti‐Brucella antibodies at the two farms, which indicated (by recalling) they had vaccinated their cattle, was not expected but could suggest a natural infection rather than a positive reaction due to the vaccine's antibodies.
5. CONCLUSION
This study indicated that Brucella is prevalent in farm bulk milk in Rwanda as evidenced by the detection of anti‐Brucella antibodies in 19.7% of all farm bulk milk collected from all study sites across the country. The prevalence is especially high in farm bulk milk from open grazing farms in the Nyagatare district. Beyond the prevalence of Brucella in farm bulk milk, there is the risk of human infection as a result of consumption of raw or inadequately heat‐treated milk, especially milk from open field grazing farms and milk from cattle with a history of reproductive disorders of abortion and placenta retention. There is, therefore, an urgent need to plan for or reinforce animal brucellosis control measures in Rwanda.
ETHICAL CONSIDERATION
Data collection with the questionnaire and sampling farm bulk milk required an ethical clearance, which was applied for and obtained from competent authorities. Prior to administering the questionnaire and sampling farm bulk milk, respondents in the cattle keeping households were also explained verbally the purpose of data collection and their rights to participate or not to participate in the study. Those willing to participate in the study were further assured that their identities would remain confidential during research dissemination or publication of results.
AUTHOR CONTRIBUTIONS
Conceptualisation, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, validation, visualisation, writing‐original draft, writing‐review and editing: Juvenal Djangwani. Conceptualisation, data curation, formal analysis, methodology, project administration, resources, software, supervision, validation, visualisation, writing‐review and editing: George Abong. Conceptualisation, data curation, formal analysis, methodology, project administration, resources, software, supervision, validation, visualisation, writing‐review and editing: Lucy Gicuku Njue. Conceptualisation, data curation, formal analysis, methodology, project administration, resources, software, supervision, validation, visualisation, writing‐review and editing: Dasel Wambua Mulwa Kaindi.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.562
ACKNOWLEDGEMENT
This work was funded by the Borlaug Higher Education for Agricultural Research and Development program based at Michigan State University.
Djangwani, J., Abong’, G. O., Njue, L. G., & Kaindi, D. W. M. (2021). Sero‐prevalence and risk factors of Brucella presence in farm bulk milk from open and zero grazing cattle production systems in Rwanda. Veterinary Medicine and Science, 7, 1656–1670. 10.1002/vms3.562
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Acha Pedro N., Szyfres Boris (2005). Zoonosis y enfermedades transmisibles comunes al hombre. Revista Española de Salud Pública, 79(3), 423–423. 10.1590/s1135-57272005000300012. [DOI] [Google Scholar]
- Al‐Majali, A. M., Talafha, A. Q., Ababneh, M. M., & Ababneh, M. M. (2009). Seroprevalence and risk factors for bovine brucellosis in Jordan. Journal of Veterinary Science, 10(1), 61–65. 10.4142/jvs.2009.10.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaji, N. B., Wungak, Y. S., & Bertu, W. J. (2016). Serological survey of bovine brucellosis in Fulani nomadic cattle breeds (Bos indicus) of North‐central Nigeria: Potential risk factors and zoonotic implications. Acta Tropica, 153, 28–35. 10.1016/j.actatropica.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Aparicio, E. D. (2013). Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus . Revue Scientifique et Technique de l'OIE, 32(1), 53–60. 10.20506/rst.32.1.2187 [DOI] [PubMed] [Google Scholar]
- Asakura, S., Makingi, G., Kazwala, R., & Makita, K. (2018). Herd‐level risk factors associated with Brucella sero‐positivity in cattle, and perception and behaviours on the disease control among agro‐pastoralists in Tanzania. Acta Tropica, 187, 99–107. 10.1016/j.actatropica.2018.07.010 [DOI] [PubMed] [Google Scholar]
- Asfaw Geresu, M., & Mamo Kassa, G. (2015). A review on diagnostic methods of Brucellosis. Journal of Veterinary Science & Technology, 07(03), 1000323. 10.4172/2157-7579.1000323 [DOI] [Google Scholar]
- Asgedom, H., Damena, D., & Duguma, R. (2016). Seroprevalence of bovine brucellosis and associated risk factors in and around Alage district, Ethiopia. SpringerPlus, 5, 851. 10.1186/s40064-016-2547-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awah‐Ndukum, J., Mouiche, M. M. M., Bayang, H. N., Ngwa, V. N., Assana, E., Feussom, K. J. M., Manchang, T. K., & Zoli, P. A. (2018). Seroprevalence and associated risk factors of brucellosis among indigenous cattle in the Adamawa and north regions of Cameroon. Veterinary Medicine International, 2018, 3468596. 10.1155/2018/3468596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhe, G., Belihu, K., & Asfaw, Y. (2007). Seroepidemiological investigation of bovine brucellosis in the extensive cattle production system of Tigray region of Ethiopia. International Journal of Applied Research in Veterinary Medicine, 5(2), 65. [Google Scholar]
- Boukary, A. R., Saegerman, C., Abatih, E., Fretin, D., Bada, R. A., De Deken, R., Harouna, H. A., Yenikoye, A., & Thys, E. (2013). Seroprevalence and potential risk factors for Brucella spp. infection in traditional cattle, sheep and goats reared in urban, periurban and rural areas of Niger. PLoS ONE, 8(12), 1–12. 10.1371/journal.pone.0083175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas, L., Peña, M., Melo, O., & Casal, J. (2019). Risk factors for new bovine brucellosis infections in Colombian herds. BMC Veterinary Research, 15(1), 1–8. 10.1186/s12917-019-1825-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatikoba, P., Manzi, M., Kagarama, J., Rwemarika, J. D., & Umunezero, O. (2008). The prevalence of bovine brucellosis in milking dairy herds in nyagatare and its implications on dairy productivity and public health. The 3rd International Conference on Appropriate Technology (3rd ICAT), Kigali, Rwanda (pp. 368–376). http://www.howard.edu/library/scholarship@howard/books/2008/icat2008.pdf
- Corbel, M. J. (2006). Brucellosis in humans and animals.. World Health Organization. 10.2105/AJPH.30.3.299 [DOI] [Google Scholar]
- Dadar, M., Shahali, Y., & Whatmore, A. M. (2019). Human brucellosis caused by raw dairy products: A review on the occurrence, major risk factors and prevention. International Journal of Food Microbiology, 292, 39–47. 10.1016/j.ijfoodmicro.2018.12.009 [DOI] [PubMed] [Google Scholar]
- de Alencar Mota, A. L. A., Ferreira, F., Ferreira Neto, J. S., Dias, R. A., Amaku, M., Hildebrand Grisi‐Filho, J. H., Telles, E. O., & Picão Gonçalves, V. S. (2016). Large‐scale study of herd‐level risk factors for bovine brucellosis in Brazil. Acta Tropica, 164, 226–232. 10.1016/j.actatropica.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Dorneles, E. M. S., Sriranganathan, N., & Lage, A. P. (2015). Recent advances in Brucella abortus vaccines. Veterinary Research, 46(1), 76. 10.1186/s13567-015-0199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim, W. O. M. K., Elfadil, A. A. M., Elgadal, A. A., & Shuaib, Y. A. (2016). Seroprevalence and risk factors of anti‐brucella antibodies in cattle in Khartoum State, the Sudan. Journal of Advanced Veterinary and Animal Research, 3(2), 134–144. 10.5455/javar.2016.c141 [DOI] [Google Scholar]
- Elemo, K. K., & Geresu, M. A. (2018). Bovine brucellosis: Seroprevalence and its associated risk factors in cattle from smallholder farms in Agarfa and Berbere districts of Bale Zone, South Eastern Ethiopia. Journal of Animal and Plant Sciences, 28(2). http://www.thejaps.org.pk/docs/Accepted/2007/28‐2/41.pdf. [Google Scholar]
- Estradaa, A. I. M., R Hernández‐Castrob, A López‐Merinoa, J Singh‐Bedic, A. C.‐R. (2016). Isolation, identification, and antimicrobial susceptibility of Brucella spp. cultured from cows and goats manure in Mexico # Aislamiento, identificación y susceptibilidad antimicrobiana de Brucella spp. cultivadas de materia fecal de vacas y cabras e.235. 231–235.
- Feed the Future Innovation Lab . (2016). Rwanda: Animal source foods production and marketing brief. https://livestocklab.ifas.ufl.edu/media/livestocklabifasufledu/pdf‐/pdfs‐by‐country‐pre2019/Rwanda_Brief_ASFProdMkt_final.pdf
- Fisher, A., Laing, J., Stoeckel, J., & Townsend, J. (1991). Handbook for family planning operations research design (2nd ed., Vol. 1991). Population Council. [Google Scholar]
- Food and Drug Administration . (2012). Bad bug book: Handbook of foodborne pathogenic microorganisms and natural toxins (2nd ed.). U.S. Department of Health and Human Services. 10.1016/S1872-2040(10)60451-3 [DOI] [Google Scholar]
- Gafirita, J., Kiiza, G., Murekatete, A., Ndahayo, L. L., Tuyisenge, J., Mashengesho, V., Ruhirwa, R., Nyandwi, T., Asiimwe‐Kateera, B., Ndahindwa, V., & Njunwa, K. J. (2017). Seroprevalence of brucellosis among patients attending a District Hospital in Rwanda. American Journal of Tropical Medicine and Hygiene, 97(3), 831–835. 10.4269/ajtmh.16-0632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall, D., & Nielsen, K. (2004). Serological diagnosis of bovine brucellosis: A review of test performance and cost comparison. OIE Revue Scientifique et Technique, 23(3), 989–1002. 10.20506/rst.23.3.1545 [DOI] [PubMed] [Google Scholar]
- Gall, D., Nielsen, K., Forbes, L., Cook, W., Leclair, D., Balsevicius, S., Kelly, L., Smith, P., & Mallory, M. (2001). Evaluation of the fluorescence polarization assay and comparison to other serological assays for detection of brucellosis in cervids. Journal of Wildlife Diseases, 37(1), 110–118. 10.7589/0090-3558-37.1.110 [DOI] [PubMed] [Google Scholar]
- Godfroid, J., De bolle, X., Roop, R. M., O'Callaghan, D., Tsolis, R. M., Baldwin, C., Santos, R. L., McGiven, J., Olsen, S., Nymo, I. H., Larsen, A., Al Dahouk, S., & Letesson, J. J. (2014). The quest for a true One Health perspective of brucellosis. OIE Revue Scientifique et Technique, 33(2), 521–538. 10.20506/rst.33.2.2290 [DOI] [PubMed] [Google Scholar]
- Haggag, M., Kalisa, J. C., & Abdeldayem, A. W. (2016). Projections of precipitation, air temperature and potential evapotranspiration in Rwanda under changing climate conditions. African Journal Of Environmental Science and Technology, 10(1), 18–33. 10.5897/AJEST2015.1997 [DOI] [Google Scholar]
- Halliday, J. E. B., Allan, K. J., Ekwem, D., Cleaveland, S., Kazwala, R. R., & Crump, J. A. (2015). Endemic zoonoses in the tropics: A public health problem hiding in plain sight. The Veterinary Record, 176(9), 220–225. 10.1136/vr.h798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, T., Rock, K., Mugizi, D. R., Muradrasoli, S., Lindahl‐Rajala, E., Erume, J., Magnusson, U., Lundkvist, Å., & Boqvist, S. (2016). Molecular detection and characterization of Brucella species in raw informally marketed milk from Uganda. Infection Ecology & Epidemiology, 6(1), 32442. 10.3402/iee.v6.32442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain, M., Uddin, M. B., Al Hassan, A., Islam, R., & Cho, H. (2014). Potential risk factors analysis of dairy cattle management against Brucellosis. Veterinary Research International 2(4), 96–102. [Google Scholar]
- Hull, N. C., & Schumaker, B. A. (2018). Comparisons of brucellosis between human and veterinary medicine. Infection Ecology and Epidemiology, 8(1), 1500846. 10.1080/20008686.2018.1500846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim, N., Belihu, K., Lobago, F., & Bekana, M. (2010). Sero‐prevalence of bovine brucellosis and its risk factors in Jimma zone of Oromia Region, South‐western Ethiopia. Tropical Animal Health and Production, 42(1), 35–40. 10.1007/s11250-009-9382-z [DOI] [PubMed] [Google Scholar]
- Ilunga, L., Muhire, I., & Mbaragijimana, C. (2004). Pluviometric seasons and rainfall origin in Rwanda. Geo‐Eco‐Trop, 28, 61–68. [Google Scholar]
- Kamwine, M., Orikiriza, P., Taseera, K., Iramiot, J. S., Ojuka, P., Ikiriza, S., Atwebembeire, J., Otieno, D., Tweshengyereze, S., Mwanga‐Amumpaire, J., Bazira, J., & Boum, Y. (2017). Prevalence of antibodies to Brucella species in commercial raw bovine milk in Southwestern Uganda. BMC Research Notes, 10(1), 1–5. 10.1186/s13104-017-2537-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur, P., Sharma, N. S., Arora, A. K., & Deepti, (2018). Investigation of brucellosis in cattle and buffaloes by conventional and molecular assays. Indian Journal of Animal Research, 52(10), 1482–1487. 10.18805/ijar.B-3375 [DOI] [Google Scholar]
- Kebede, T., Ejeta, G., & Ameni, G. (2008). Seroprevalence of bovine brucellosis in smallholder farms in central Ethiopia (Wuchale‐Jida district). Revue de Medecine Veterinaire, 159(1), 3–9. [Google Scholar]
- Ko, K. Y., Kim, J. W., Her, M., Kang, S. Il, Jung, S. C., Cho, D. H., & Kim, J. Y. (2012). Immunogenic proteins of Brucella abortus to minimize cross reactions in brucellosis diagnosis. Veterinary Microbiology, 156(3–4), 374–380. 10.1016/j.vetmic.2011.11.011 [DOI] [PubMed] [Google Scholar]
- Land O’ Lakes, I. (2014). Baseline survey report for Rwanda Dairy Competitiveness Program (RDCP). https://silo.tips/download/baseline‐survey‐report‐rwanda‐dairy‐competitiveness‐program
- Lim, J. J., Kim, D. H., Lee, J. J., Kim, D. G., Min, W., Lee, H. J., Rhee, M. H., Chang, H. H., & Kim, S. (2012). Evaluation of recombinant 28 kDa outer membrane protein of Brucella abortus for the clinical diagnosis of bovine brucellosis in Korea. Journal of Veterinary Medical Science, 74(6), 687–691. 10.1292/jvms.11-0512 [DOI] [PubMed] [Google Scholar]
- Mai, H. M., Irons, P. C., Kabir, J., & Thompson, P. N. (2012). A large seroprevalence survey of brucellosis in cattle herds under diverse production systems in northern Nigeria. BMC Veterinary Research, 8, 144. 10.1186/1746-6148-8-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita, K., Fevre, E. M., Waiswa, C., Eisler, M. C., & Welburn, S. C. (2010). How human brucellosis incidence in urban kampala can be reduced most efficiently? A stochastic risk assessment of informally‐marketed milk. PLoS ONE, 5(12), e14188. 10.1371/journal.pone.0014188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita, K., Waiswa, C., & Mthrusfieldedacuk, M. T. (2011). Herd prevalence of bovine brucellosis and analysis of risk factors in cattle in urban and peri‐urban areas of the Kampala economic zone, Uganda. BMC Veterinary Research, 7, 60. 10.1186/1746-6148-7-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangen, M. ‐J., Otte, J., Pfeiffer, D., & Chilonda, P. (2002). Bovine brucellosis in sub‐Saharan Africa: Estimation of sero‐prevalence and impact on meat and milk off‐take potential. Food and Agriculture Organization Livestock Information and Policy Branch, AGAL, 8, 58. [Google Scholar]
- Manishimwe, R., Ntaganda, J., Habimana, R., Nishimwe, K., Byukusenge, M., Dutuze, F., Ayabagabo, J. D., And, U. L., & Rukundo, J. C. (2015). Comparison between Rose Bengal Plat Test and Competitive Enzyme Linked Immunosorbent Assay to detect bovine brucellosis in Kigali City, Rwanda. Journal of Veterinary Science & Technology, 06(01), 2–5. 10.4172/2157-7579.1000211 [DOI] [Google Scholar]
- Mazimpaka, E. (2017). Characterization of cattle production systems in Nyagatare District of Eastern Province, Rwanda. Rheology, 1(2), 107. [Google Scholar]
- McDermott, J., Grace, D., & Zinsstag, J. (2013). Economics of brucellosis impact and control in low‐income countries. OIE Revue Scientifique et Technique, 32(1), 249–261. 10.20506/rst.32.1.2197 [DOI] [PubMed] [Google Scholar]
- McDermott, J. J., & Arimi, S. M. (2002). Brucellosis in sub‐Saharan Africa: Epidemiology, control and impact. Veterinary Microbiology, 90(1–4), 111–134. 10.1016/S0378-1135(02)00249-3 [DOI] [PubMed] [Google Scholar]
- Mekonnen, H., Kalayou, Shewit, & Kyule, Moses (2010). Serological survey of bovine brucellosis in barka and arado breeds (Bos indicus) of Western Tigray, Ethiopia. Preventive Veterinary Medicine, 94(1–2), 28–35. 10.1016/j.prevetmed.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Miklyaev, M., Afra, S., & Schultz, M. (2017). Cost‐ benefit analysis of Rwanda’ s dairy value chains. https://cri‐world.com/publications/qed_dp_299.pdf
- Miller, R., Nakavuma, J. L., Ssajjakambwe, P., Vudriko, P., Musisi, N., & Kaneene, J. B. (2016). The prevalence of brucellosis in cattle, goats and humans in rural Uganda: A comparative study. Transboundary and Emerging Diseases, 63(6), e197–e210. 10.1111/tbed.12332 [DOI] [PubMed] [Google Scholar]
- Mufinda, F., Boinas, F., & Nunes, C. (2015). Prevalence and factors associated with cattle brucellosis in animal herds of the Namibe Province in Angola. Alexandria Journal of Veterinary Sciences, 47(1), 7. 10.5455/ajvs.188809 [DOI] [Google Scholar]
- Muhire, I., Ahmed, F., & Abutaleb, K. (2014). Relationships between Rwandan seasonal rainfall anomalies and ENSO events. Theoretical and Applied Climatology, 122, 271–284. 10.1007/s00704-014-1299-4 [DOI] [Google Scholar]
- Muma, J. B., Godfroid, J., Samui, K. L., & Skjerve, E. (2007). The role of Brucella infection in abortions among traditional cattle reared in proximity to wildlife on the Kafue flats of Zambia. Revue Scientifique et Technique (International Office of Epizootics), 26(3), 721–730. [PubMed] [Google Scholar]
- Musa, M. T., Jahans, K. L., & Fadalla, M. E. (1990). Clinical manifestations of brucellosis in cattle of the Southern Darfur Province, Western Sudan. Journal of Comparative Pathology, 103(1978), 3–7. [DOI] [PubMed] [Google Scholar]
- National Institute Of Statistics Of Rwanda . (2018). Agricultural household survey 2016/2017. http://www.statistics.gov.lk/agriculture/Publications/AHS/AHS2016‐17Report.pdf
- National Institute of Statistics of Rwanda . (2019). Statistical yearbook. https://www.statistics.gov.rw/publication/statistical‐yearbook‐2019
- Ndahetuye, J. B., Artursson, K., Båge, R., Ingabire, A., Karege, C., Djangwani, J., Nyman, A. K., Ongol, M. P., Tukei, M., & Persson, Y. (2020). MILK symposium review: Microbiological quality and safety of milk from farm to milk collection centers in Rwanda. Journal of Dairy Science, 103(11), 9730–9739. 10.3168/jds.2020-18302 [DOI] [PubMed] [Google Scholar]
- Ndazigaruye, G., Mushonga, B., Kandiwa, E., Samkange, A., Segwagwe, B. E., Segwagwe, B., & Province, E. (2018). Prevalence and risk factors for brucellosis seropositivity in cattle in Nyagatare District, Eastern Province, Rwanda. Journal of the South African Veterinary Association, 89, 1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguna, J., Dione, M., Apamaku, M., Majalija, S., Mugizi, D. R., Odoch, T., Kato, C. D., Tumwine, G., Kabaasa, J. D., Curtis, K., Graham, M., Ejobi, F., & Graham, T. (2019). Seroprevalence of brucellosis and risk factors associated with its seropositivity in cattle, goats and humans in Iganga district, Uganda. Pan African Medical Journal, 33, 1–10. 10.11604/pamj.2019.33.99.16960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntivuguruzwa, J. B., Kolo, F. B., Gashururu, R. S., Umurerwa, L., Byaruhanga, C., & van Heerden, H. (2020). Seroprevalence and associated risk factors of bovine brucellosis at the wildlife‐livestock‐human interface in Rwanda. Microorganisms, 8(10), 1553. 10.3390/microorganisms8101553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojango, J. M., Kariuki, K., Njehu, A. A., & Baltenweck, I. (2012). Breeding management strategies adopted for dairy production under low‐input smallholder farming systems of East Africa. https://cgspace.cgiar.org/handle/10568/34455
- Omer, M. K., Skjerve, E., Holstad, G., Woldehiwet, Z., & Macmillan, A. P. (2000). Prevalence of antibodies to Brucella spp. in cattle, sheep, goats, horses and camels in the State of Eritrea; influence of husbandry systems. Epidemiology and Infection, 125(2), 447–453. 10.1017/S0950268899004501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas, G., Papadimitriou, P., Akritidis, N., Christou, L., & Tsianos, E. (2006). The new global map of human Brucellosis. The Lancet Infectious Diseases, 6, 91–99. 10.1016/S1473-3099(06)70382-6 [DOI] [PubMed] [Google Scholar]
- Poester, F. P., Samartino, L. E., & Santos, R. I. (2013). Pathogenesis and pathobiology of brucellosis in livestock. OIE Revue Scientifique et Technique, 32(1), 105–115. 10.20506/rst.32.1.2193 [DOI] [PubMed] [Google Scholar]
- Rock, K. T., Mugizi, D. R., Ståhl, K., Magnusson, U., & Boqvist, S. (2016). The milk delivery chain and presence of Brucella spp. antibodies in bulk milk in Uganda. Tropical Animal Health and Production, 48(5), 985–994. 10.1007/s11250-016-1052-3 [DOI] [PubMed] [Google Scholar]
- Rujeni, N., & Mbanzamihigo, L. (2014). Prevalence of brucellosis among women presenting with abortion/stillbirth in Huye, Rwanda. Journal of Tropical Medicine, 2014, 3–5. 10.1155/2014/740479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rwanda Ministry of Agriculture and Animal Resources (MINAGRI) . (2009). Updating the master plan of the milk chain in Rwanda. http://www.minagri.gov.rw/fileadmin/user_upload/documents/STRAT.PLC/Milk_Master_Plan.pdf
- Rwanda Ministry of Agriculture and Animal Resources (MINAGRI) . (2019). Annual report 2018–2019.
- Sagamiko, F. D., Muma, J. B., Karimuribo, E. D., Mwanza, A. M., Sindato, C., & Hang'ombe, B. M. (2018). Sero‐prevalence of bovine brucellosis and associated risk factors in mbeya region, Southern highlands of Tanzania. Acta Tropica, 178, 169–175. 10.1016/j.actatropica.2017.11.022 [DOI] [PubMed] [Google Scholar]
- Sanogo, M., Abatih, E., Thys, E., Fretin, D., Berkvens, D., & Saegerman, C. (2012). Risk factors associated with brucellosis seropositivity among cattle in the central savannah‐forest area of Ivory Coast. Preventive Veterinary Medicine, 107(1–2), 51–56. 10.1016/j.prevetmed.2012.05.010 [DOI] [PubMed] [Google Scholar]
- Schelling, E., Diguimbaye, C., Daoud, S., Nicolet, J., Boerlin, P., Tanner, M., & Zinsstag, J. (2003). Brucellosis and Q‐fever seroprevalences of nomadic pastoralists and their livestock in Chad. Preventive Veterinary Medicine, 61(4), 279–293. 10.1016/j.prevetmed.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Schmutz, S. M., Moker, J. S., Clark, E. G., & Orr, J. P. (1996). Chromosomal aneuploidy associated with spontaneous abortions and neonatal losses in cattle. Journal of Veterinary Diagnostic Investigation, 8(1), 91–95. 10.1177/104063879600800114 [DOI] [PubMed] [Google Scholar]
- Shahid, M., Basit, A., Ullah, R., Rahim, K., & Sciences, A. (2014). Sero‐Prevalence of Brucellosis in Cattle in Southern Area of Khyber Pakhtunkhwa, Pakistan. Research Journal for Veterinary Practitioners, 2(4), 63–66. 10.14737/journal.rjvp/2014/2.4.63.66 [DOI] [Google Scholar]
- Smirnova, E. A., Vasin, A. V., Sandybaev, N. T., Klotchenko, S. A., Plotnikova, M. A., Chervyakova, O. V., Sansyzbay, A. R., & Kiselev, O. I. (2013). Current methods of human and animal brucellosis diagnostics. Advances in Infectious Diseases, 03(03), 177–184. 10.4236/aid.2013.33026 [DOI] [Google Scholar]
- Tadesse, G. (2016). Brucellosis seropositivity in animals and humans in Ethiopia: A meta‐analysis. PLoS Neglected Tropical Diseases, 10(10), e0005006. 10.1371/journal.pntd.0005006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasiame, W., Emikpe, B. O., Folitse, R. D., Fofie, C. O., Burimuah, V., Johnson, S., Awuni, J. A., Afari, E., Yebuah, N., & Wurapa, F. (2016). The prevalence of brucellosis in cattle and their handlers in North Tongu District of Volta Region, Ghana. African Journal of Infectious Diseases, 10(2), 111–117. 10.21010/ajid.v10i2.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekle, M., Legesse, M., Edao, B. M., Ameni, G., & Mamo, G. (2019). Isolation and identification of Brucella melitensis using bacteriological and molecular tools from aborted goats in the Afar region of north‐eastern Ethiopia. BMC Microbiology, 19, 108. 10.1186/s12866-019-1474-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ul‐Islam, M. R., Gupta, M. P., Gursimran, F., Sidhu, P. K., Shafi, T. A., Bhat, S. A., Hussain, S. A., & Radya, M. (2013). Sero‐epidemiology of brucellosis in organized cattle and buffaloes in Punjab (India). Advances in Animal and Veterinary Sciences, 1(3S), 5–8. [Google Scholar]
- World Health Organization . (2012). Research priorities for zoonoses and marginalized infections (Technical Report Series No, 971). World Health Organization. [PubMed]
- Zhao, X., Lin, C., Wang, J., & Oh, D. (2014). Advances in rapid detection methods for foodborne pathogens. Journal of Microbiology and Biotechnology, 24(3), 297–312. 10.4014/jmb.1310.10013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


