Abstract
Background
Torque teno viruses (TTVs) have been detected worldwide, from a wide range of animals. Up to date, few studies focused on the prevalence of TTVs in general and swine torque teno viruses (TTSuVs) in particular in Korean swine farms.
Objective
This study aimed to investigate the appearance of TTSuVs and TTVs in sick pigs during the 2017–2018 period.
Materials and Methods
Molecular‐based method using TTSuV1‐, TTSuV2‐ and TTV3‐specific primers was used to screen for the viruses from either sera or pooled internal organs of sick pigs. For genetic characterization, genomic sequences of TTVs were sequenced by a primer walking method. Several bioinformatic tools have been utilized to investigate the genomic organization and genetic relationship of TTVs.
Results
Two years of prevalence survey reveal that the prevalence of TTSuV2 is about twice that of TTSuV1. Furthermore, we identified TTV of genogroup 3 in swine pooled organ samples. The genome of two strains, M265_Korea_2017 and N119_Korea_2018, are 3,817 bp in size; M265_2017 has three open reading frames (ORFs); and N119_2018 strain has four ORFs. The complete genome nucleotide sequencing of the two strains shows 98.4% homology, and the phylogenetic analysis of Open reading frame (ORF)1 indicates that the strains are located close to TUPB strain subgroup C of genogroup 3.
Conclusion
Our study provided the information of TTSuVs prevalence in swine farms in Korea and highlighted the presence of TTV genogroup 3 strains in pigs.
Keywords: pig, South Korea, TTSuV, TTV3
This study was firstly identified of human like Torque Teno viruses group 3 of pig sample by the PCR method.

1. INTRODUCTION
Torque teno viruses (TTVs), a group of non‐enveloped, circular single‐strand DNA viruses with 3.6‐ to 3.9‐kb genome size, were first discovered in a Japanese patient in 1997 (Nishizawa et al., 1997). According to the International Committee on Taxonomy of viruses (ICTV), TTVs were assigned into Anelloviridae family including 14 genera (Walker et al., 2020). Of which, Alphatorquevirus, a genus mainly found in human and primates, can be divided into at least seven different genogroups with great genetic diversity (Hsiao et al., 2016; Mi et al., 2014; Ninomiya et al., 2008). Among them, genogroup 3 was the most widely spreading (AbuOdeh et al., 2015; Pinho‐Nascimento et al., 2011).
Beside in humans, TTVs were found in a wide range of other hosts such as primates, pigs, cats, and dogs. The TTVs of pig origin can be classified into two major groups: Torque teno sus virus 1 (TTSuV1, genus Iotatorquevirus) and Torque teno sus virus 2 (TTSuV2, genus Kappatorquevirus), consisting of three subtypes (1a to 1c) and seven subtypes (2a to 2 g), respectively (Li et al., 2013). TTSuV1 was considered to cause clinical symptoms in gnotobiotic pigs (Ellis et al., 2008; Krakowka et al., 2008).
It is reported that TTVs common in human infection can be found in pig's serum (Ssemadaali et al., 2016). The authors also provided evidence about the possible infection of human cells by TTSuV1, which raised the question of transmission of TTVs among humans and animals. Recently, a study based on molecular analysis supported the hypothesis of human to animal transmission event of TTVs (Sarairah et al., 2020).
Up to date, few studies focused on the prevalence of TTSuVs and other TTVs in Korean swine farms. Therefore, the aim of this study is to investigate the appearance of TTSuVs and TTV genogroup 3 in sick pigs from Korean domestic swine farms in South Korea during the 2017–2018 period.
2. MATERIALS AND METHODS
From January 2017 to December 2018, 470 clinical samples (sera, tissues in lung, kidney, liver and lymph node; samples are pooled in each group) from nine provinces of South Korea were sent to the lab for diagnosis of respiratory viral diseases. The total DNA was extracted using a viral DNA/RNA extraction kit (iNtRON Biotechnology Inc., Gyeonggi, South Korea) and was immediately used for amplification or stored at −20°C.
Methods for detection of TTSuV1 and TTSuV2 were following the previous studies (Li et al., 2013). We further investigated the most widely spread Torque teno virus of genogroup 3 (TTV3) to confirm cross species infection. Detection of TTV3 uses AI‐1F and AI‐1R as mentioned below (Dencs et al., 2009). The polymerase chain reaction (PCR) was performed using an i‐StarMaster mix PCR kit (iNtRON Biotechnology Inc.). For genetic characterization, we followed TTSuVs of the three strains (M117, N86 and N116), completely sequenced by a primer walking method (Li et al., 2013). The specific PCR products were purified by the gel extraction method and further processed for TA cloning and transformation (Kim et al., 2014). Putative ORFs of the obtained sequences were predicted using ORFfinder tool (https://www.ncbi.nlm.nih.gov/orffinder/) with the minimum length of 50 amino acids, and the start codon was selected as ATG and alternative initiation codons as suggested by Tanaka et al. (2001). Functional analyses of the putative proteins were detected by BLAST (Johnson et al., 2008). Sequence alignment was applied by MAFFT using default options (Katoh & Standley, 2013).
For phylogenetic study, the best nucleotide substitution model and the complete genome sequence model were selected automatically by specifying the ‘‐m TEST’ option in IQ‐TREE version 1.3.8 (Nguyen et al., 2014). In this study, the best plot model (GTR + G4) was used for phylogenetic analysis. For genotyping, we collected from the TTSuVs reference sequences (Li et al., 2013) and the TTVs reference sequences (Hsiao et al., 2016).
3. RESULTS
In this study, pigs showing signs of respiratory problems (n = 470) were collected on the detection rates of TTSuVs in 2017 and 2018; the positive rates of TTSuV1 were 17% (47/280), 15% (28/190) in 2017 and 2018, and for TTSuV2, they were 34% (95/280) and 39% (73/190) in 2017 and 2018, respectively. In total, positive rates of 16% (75/470) and 36% (168/470) were detected for TTSuV1 and TTSuV2. Co‐infection of both groups (TTSuV1 and TTSuV2) was 8% (38/470) of the total sample. Among the positive samples, three strains (M117, N86 and N116) were registered in GenBank with accession numbers MK452763–MK452765. Regarding the genetic relationship within the complete genome references in TTSuVs, two strains of M117 and N86 belonged to 1b and 1c of TTSuV1, and the other N116 strain is located in subtype 2b (Figure S1). From the collected samples (n = 470), we further investigated the most widely spread TTV3 genogroup to confirm cross species infection. Interestingly, the results detected only two field strains (M265_Korea_2017, MK452766; and N119_Korea_2018, MK452767) for which the PCR amplicon band site is 350 bps. In the sequencing blast results, the strains of M265 and N199 were shown 96% and 94% homology with TUPB (AF247137) strain. For genetic characterization, the two strains (M265_Korea_2017 and N119_Korea_2018) were completely sequenced by using a primer walking method (Biagini et al., 2000).
Focusing on the TTV 3 strains, we found that each M265 and N119 strains has a 3,817 full‐length genome and the M265 has three ORFs (ORF1, ORF2 and ORF3) while the N119 strain has four ORFs (ORF1, ORF2, ORF 2‐2 and ORF 3) (Figure 1a,b). The strains showed full‐length G+C contents of 50.79% and 50.87%. ORF1, ORF2 and ORF3 sequences encoded 760, 156 and 98 aa. ORF2‐2 was also found in N119, encoding 163 aa.
FIGURE 1.
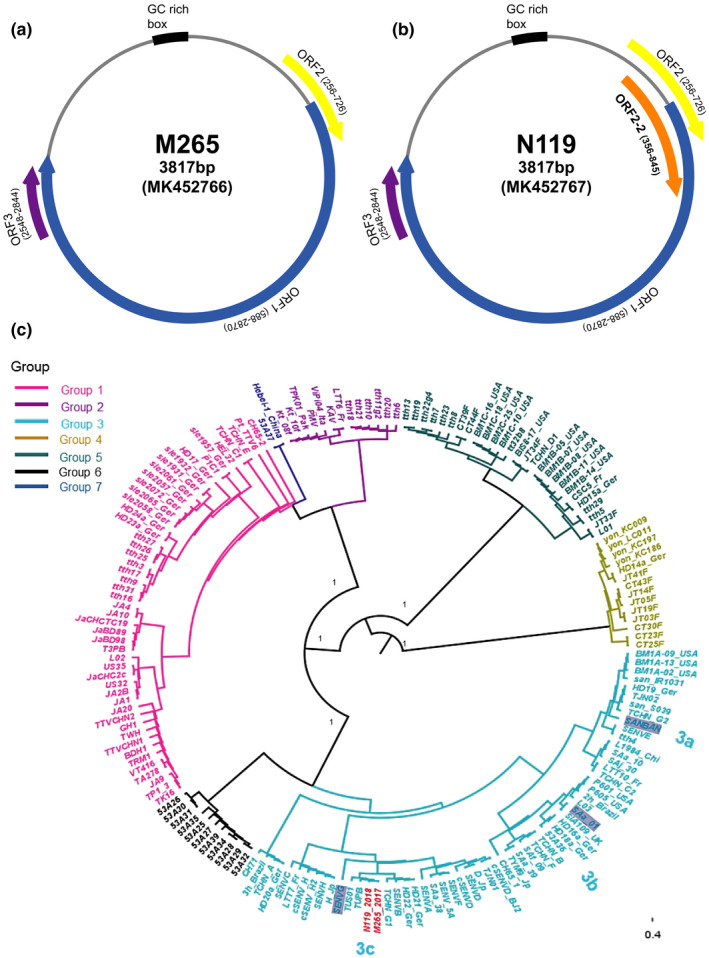
Predicted genome map of Torque teno viruses (TTVs) of M265 (a) and N119 (b) strains. Each strain including ORF frame site with arrow sign. The phylogenetic tree (c) is constructed using the maximum likelihood trees of TTVs ORF1 genomes with bootstrap 1,000, automatically best fitting model selected by IQ‐TREE. The M265 and N199 (in this study) strains were highlighted with red colour, and the posterior supported values were represented in the node bar. The SANBAN, SAa‐01 and SENVG grey colours are representative for showing subgroup 3a, 3b and 3c, respectively
The complete genome sequences of each strain have 98.4% homology. In the ORF1, the two strains showed 98.2% similarity in nucleotide and 95.4% in amino acid, and ORF 2 showed 95.9% and 92.3%, and ORF3 showed 98.9% and 98% homology, respectively. As inferred based on the ORF1 alignment with previous phylogenetic tree study within TTVs, the M265 and N119 strains belong to genogroup 3 of subgroup 3c, which are close to the TUPB strain (Figure 1c).
Further analysis indicated that the putative ORF1 of both two TTV genogroup 3 strains contained (1) the Arginine‐rich region located at N‐terminate and (2) three conserved motifs of replicate‐associated protein including motif 1 (FSL), motif 3 (YxxK) and motif 4 (GxGK: P‐loop) (Figure 2a,b). Similarly, the conserved motif (WX7HX3CXCX5H) of ORF2‐TTV was observed in M265 strain while an aa change H→Q in the first histidine of the motif was found in N119 (Figure 2c). Of the putative ORF3, a serine‐rich domain was found in the C‐terminate of both strains (Figure 2d).
FIGURE 2.
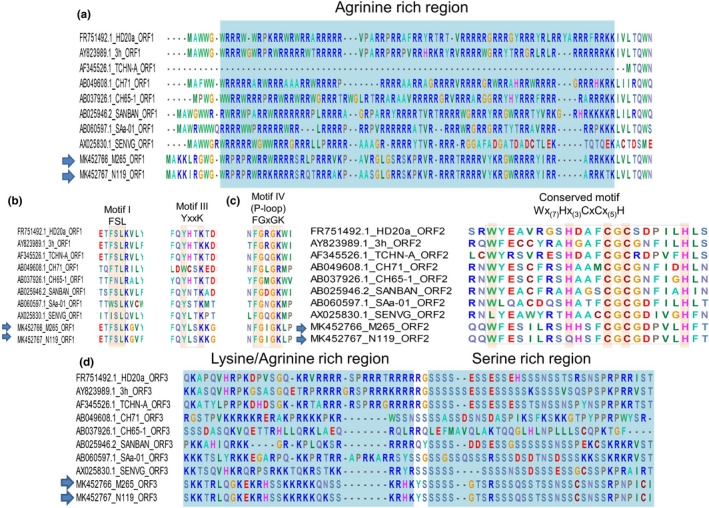
Functional domains of the putative ORF1 (a,b); ORF2 (c); and ORF3 (d). Torque teno viruses (TTVs) strains M265 and N119 (arrows) were predicted to contain several conserved regions (highlighted as light blue) and motifs (indicated as dash boxes). The well‐conserved amino acid in each motifs are highlighted; ‘x’ was any amino acid
4. DISCUSSION
It was reported that TTSuV infections existed in many countries around the world (McKeown et al., 2004). In our study, the prevalence rate of TTSuVs in Korea during 2017–2018 was 44% (the positive rates of TTSuV1 and TTSuV2 were 16% and 36%, respectively, and the co‐infection of both type of TTSuVs was approximately 8%). The prevalence of TTSuVs in Korea in this study was equivalent to the positive rate of one in Thailand (McKeown et al., 2004). However, the detection rate of TTSuV1 and/or TTSuV2 in this study was lower compared with reports in other countries (Blois et al., 2014; Li et al., 2013; Sibila et al., 2009) and even that of Korea before 2004 (McKeown et al., 2004).
In this study, we focused our investigation of sick pigs in the potential appearance of TTVs genogroup 3. As a result, two out of 470 samples were TTV genogroup 3 positive. Sequence comparison and phylogeny analysis indicated that the two strains share 98.4% sequence homology and belonged to the subgroup 3c. Interestingly, genome organization prediction of N119 strain revealed an additional ORF2‐2 besides the common ORFs 1‐3 found in Anelloviridae family (Biagini et al., 2012).
In this study, the TTVs genogroup 3 detected in pigs were predicted to contain the three common ORFs observed in other TTVs. Of the ORF1, the N‐terminated region was featured by the Arginine‐rich region (Figure 2a) which is similar to capsid proteins of circoviruses (Mou et al., 2019). Furthermore, three replication‐associated motifs were also observed (Figure 2b). The presented motifs in ORF1 in TTVs were previously reported elsewhere (Tanaka et al., 2001). Previous study suggested that ORF1 might encode a bifunctional structural protein: the N‐terminus played a role as capsid while the function of the C‐terminus might be a co‐response to the replication (Kakkola et al., 2008). Of the remaining ORFs, putative ORF2 of M265 contained the well‐observed motifs of Wx7Hx3CxCx5H in TTVs while a cluster of Leucine rich regions followed by Serine rich regions in C‐terminus were observed in the present strains. These features are highly conserved in other Anelloviruses (Vibin et al., 2020). As far as our knowledge, this is the first time the strains belonging to Alphaternovirus were detected and studied.
In conclusion, the present study provided information of TTSuVs prevalent in swine farms in Korea. Our results also highlight the presence of TTV genogroup 3 strains in pig.
5. COFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTION
Van Giap Nguyen: Formal analysis; Investigation. Cheong Ung Kim: Investigation. Quynh Do Hai: Formal analysis. Yong Ho Park: Conceptualization; Formal analysis. Bong‐Kyun Park: Conceptualization. Hee Chun Chung: Conceptualization; Formal analysis; Writing‐original draft.
ETHICAL STATEMENT
This article does not contain any studies with live animals performed by any of the authors.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.505.
Supporting information
Fig S1
ACKNOWLEDGEMENTS
The authors would like to thank Jung Ah Kim and Eun Ok Kim for excellent technical assistance.
Nguyen VG, Kim CU, Do HQ, Park Y‐H, Park B‐K, Chung H‐C. Torque teno virus from Korean domestic swine farms, 2017–2018. Vet Med Sci. 2021;00:1854–1859. 10.1002/vms3.505
Van Giap Nguyen, Cheong Ung Kim and Hai Quynh Do have contributed equally to this study.
Funding information
This work was supported by the Korean Science and Engineering Foundation (KOSEF) grant funded by the Korean government (no. 2020R1I1A1A01054539).
DATA AVAILABILITY STATEMENT
The data that supports the finding of this study are available in the article and its supporting information.
REFERENCES
- AbuOdeh, R., Al‐Mawlawi, N., Al‐Qahtani, A. A., Bohol, M. F. F., Al‐Ahdal, M. N., Hasan, H. A., AbuOdeh, L., & Nasrallah, G. K. (2015). Detection and genotyping of torque teno virus (TTV) in healthy blood donors and patients infected with HBV or HCV in Qatar. Journal of Medical Virology, 87, 1184–1191. [DOI] [PubMed] [Google Scholar]
- Biagini, P., Attoui, H., Gallian, P., Touinssi, M., Cantaloube, J.‐F., de Micco, P., & de Lamballerie, X. (2000). Complete sequences of two highly divergent European isolates of TT virus. Biochemical and Biophysical Research Communications, 271, 837–841. 10.1006/bbrc.2000.2721 [DOI] [PubMed] [Google Scholar]
- Biagini, P., Bendinelli, M., Hino, S., Kakkola, L., Mankertz, A., Niel, C., Okamoto, H., Raidal, S., Teo, C. G., & Todd, D. (2012). Family ‐ Anelloviridae. In King A. M. Q., Adams M. J., Carstens E. B., & Lefkowitz E. J. (Eds.), Virus taxonomy (pp. 331–341). Elsevier. [Google Scholar]
- Blois, S., Mallus, F., Liciardi, M., Pilo, C., Camboni, T., Macera, L., Maggi, F., & Manzin, A. (2014). High prevalence of co‐infection with multiple Torque teno sus virus species in Italian pig herds. PLoS One, 9, e113720. 10.1371/journal.pone.0113720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencs, A., Hettmann, A., Szomor, K., Kis, Z., & Takács, M. (2009). Prevalence and genotyping of group 3 torque teno viruses detected in health care workers in Hungary. Virus Genes, 39, 39–45. 10.1007/s11262-009-0369-7 [DOI] [PubMed] [Google Scholar]
- Ellis, J. A., Allan, G., & Krakowka, S. (2008). Effect of coinfection with genogroup 1 porcine torque teno virus on porcine circovirus type 2–associated postweaning multisystemic wasting syndrome in gnotobiotic pigs. American Journal of Veterinary Research, 69, 1608–1614. 10.2460/ajvr.69.12.1608 [DOI] [PubMed] [Google Scholar]
- Hsiao, K.‐L., Wang, L.‐Y., Lin, C.‐L., & Liu, H.‐F. (2016). New phylogenetic groups of torque teno virus identified in eastern Taiwan indigenes. PLoS One, 11, e0149901. 10.1371/journal.pone.0149901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M., Zaretskaya, I., Raytselis, Y., Merezhuk, Y., McGinnis, S., & Madden, T. L. (2008). NCBI BLAST: A better web interface. Nucleic Acids Research, 36, W5–W9. 10.1093/nar/gkn201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkola, L., Bondén, H., Hedman, L., Kivi, N., Moisala, S., Julin, J., Ylä‐Liedenpohja, J., Miettinen, S., Kantola, K., Hedman, K., & Söderlund‐Venermo, M. (2008). Expression of all six human Torque teno virus (TTV) proteins in bacteria and in insect cells, and analysis of their IgG responses. Virology, 382, 182–189. 10.1016/j.virol.2008.09.012 [DOI] [PubMed] [Google Scholar]
- Katoh, K., & Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution, 30, 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, A. R., Chung, H. C., Kim, H. K., Kim, E. O., Nguyen, V. G., Choi, M. G., Yang, H. J., Kim, J. A., & Park, B. K. (2014). Characterization of a complete genome of a circular single‐stranded DNA virus from porcine stools in Korea. Virus Genes, 48, 81–88. 10.1007/s11262-013-1003-2 [DOI] [PubMed] [Google Scholar]
- Krakowka, S., Hartunian, C., Hamberg, A., Shoup, D., Rings, M., Zhang, Y., Allan, G., & Ellis, J. A. (2008). Evaluation of induction of porcine dermatitis and nephropathy syndrome in gnotobiotic pigs with negative results for porcine circovirus type 2. American Journal of Veterinary Research, 69, 1615–1622. [DOI] [PubMed] [Google Scholar]
- Li, K., Wang, L.‐Q., Wu, Y.‐Y., Chao, A.‐J., Lu, Q.‐W., Wei, Z.‐Y., Cui, B.‐A., & Chen, H.‐Y. (2013). Molecular detection and genomic characterization of Torque teno sus virus 1 and 2 from domestic pigs in central China. Virus Genes, 46, 479–486. [DOI] [PubMed] [Google Scholar]
- McKeown, N., Fenaux, M., Halbur, P. G., & Meng, X. J. (2004). Molecular characterization of porcine TT virus, an orphan virus, in pigs from six different countries. Veterinary Microbiology, 104, 113–117. 10.1016/j.vetmic.2004.08.013 [DOI] [PubMed] [Google Scholar]
- Mi, Z., Yuan, X., Pei, G., Wang, W., An, X., Zhang, Z., Huang, Y., Peng, F., Li, S., Bai, C., & Tong, Y. (2014). High‐throughput sequencing exclusively identified a novel Torque teno virus genotype in serum of a patient with fatal fever. Virologica Sinica, 29, 112–118. 10.1007/s12250-014-3424-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou, C., Wang, M., Pan, S., & Chen, Z. (2019). Identification of nuclear localization signals in the ORF2 protein of porcine circovirus type 3. Viruses, 11, 1086. 10.3390/v11121086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, L.‐T., Schmidt, H. A., von Haeseler, A., & Minh, B. Q. (2014). IQ‐TREE: A fast and effective stochastic algorithm for estimating maximum‐likelihood phylogenies. Molecular Biology and Evolution, 32, 268–274. 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya, M., Takahashi, M., Nishizawa, T., Shimosegawa, T., & Okamoto, H. (2008). Development of PCR assays with nested primers specific for differential detection of three human anelloviruses and early acquisition of dual or triple infection during infancy. Journal of Clinical Microbiology, 46, 507–514. 10.1128/JCM.01703-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa, T., Okamoto, H., Konishi, K., Yoshizawa, H., Miyakawa, Y., & Mayumi, M. (1997). A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochemical and Biophysical Research Communications, 241(1), 92–97. 10.1006/bbrc.1997.7765 [DOI] [PubMed] [Google Scholar]
- Pinho‐Nascimento, C. A., Leite, J. P. G., Niel, C., & Diniz‐Mendes, L. (2011). Torque teno virus in fecal samples of patients with gastroenteritis: Prevalence, genogroups distribution, and viral load. Journal of Medical Virology, 83(6), 1107–1111. 10.1002/jmv.22024 [DOI] [PubMed] [Google Scholar]
- Sarairah, H., Bdour, S., & Gharaibeh, W. J. V. (2020). The molecular epidemiology and phylogeny of Torque Teno Virus (TTV) in Jordan. Viruses, 12, 165. 10.3390/v12020165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibila, M., Martínez‐Guinó, L., Huerta, E., Llorens, A., Mora, M., Grau‐Roma, L., Kekarainen, T., & Segales, J. (2009). Swine Torque Teno Virus (TTV) infection and excretion dynamics in conventional pig farms. Veterinary Microbiology 139, 213–218. [DOI] [PubMed] [Google Scholar]
- Ssemadaali, M. A., Effertz, K., Singh, P., Kolyvushko, O., & Ramamoorthy, S. (2016). Identification of heterologous Torque Teno viruses in humans and swine. Scientific Reports, 6(1), 1–10. 10.1038/srep26655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, Y., Primi, D., Wang, R. Y. H., Umemura, T., Yeo, A. E. T., Mizokami, M., Alter, H. J., & Shih, J.‐W.‐K. (2001). Genomic and molecular evolutionary analysis of a newly identified infectious agent (SEN Virus) and its relationship to the TT virus family. The Journal of Infectious Diseases, 183, 359–367. 10.1086/318091 [DOI] [PubMed] [Google Scholar]
- Vibin, J., Chamings, A., Klaassen, M., & Alexandersen, S. (2020). Metagenomic characterisation of additional and novel avian viruses from Australian wild ducks. Scientific Reports, 10, 22284. 10.1038/s41598-020-79413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, P. J., Siddell, S. G., Lefkowitz, E. J., Mushegian, A. R., Adriaenssens, E. M., Dempsey, D. M., Dutilh, B. E., Harrach, B., Harrison, R. L., Hendrickson, R. C., Junglen, S., Knowles, N. J., Kropinski, A. M., Krupovic, M., Kuhn, J. H., Nibert, M., Orton, R. J., Rubino, L., Sabanadzovic, S., … Davison, A. J. (2020). Changes to virus taxonomy and the Statutes ratified by the International Committee on Taxonomy of Viruses (2020). Archives of Virology, 165, 2737–2748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Data Availability Statement
The data that supports the finding of this study are available in the article and its supporting information.


