Abstract
Background
Heat stress in tropics is generally associated with significant economic losses resulting from reduced performance, morbidity, and mortality of livestock. To avoid serious consequences of heat stress, it is imperative to better understand the physiological responses and biochemical changes under the state of altered body homeostasis during different seasons of the year.
Objectives
This study aimed to evaluate the seasonal dynamics of physiological, oxidative and metabolic responses of lactating Nili‐Ravi buffaloes to the tropical climate of South China.
Methods
Physiological responses including rectal temperature (RT), body surface temperature (BST) and respiratory rate (RR) along with serum biochemical and antioxidant parameters of 20 lactating Nili‐Ravi buffaloes were evaluated during different seasons of the year.
Results
Higher temperature‐humidity Index (THI) during the summer season (>80) resulted in a significant increases in RR and BST as compared to the winter season. Higher oxidative stress was observed in the summer season as revealed by significantly higher MDA while lower serum antioxidant enzyme (TAC, GSH‐Px, SOD and CAT) contents. Moreover, serum cortisol was also significantly higher in summer and autumn. The levels of growth hormone and ACTH were also significantly (P < 0.05) lower in summer and autumn as compared to other seasons. The negative association of THI with physiological and antioxidant parameters was observed while it was positively associated with serum MDA and cortisol levels.
Conclusions
Our study revealed moderate heat stress in lactating buffaloes in the summer season which calls for attention to avoid economic losses and animal welfare issues.
Keywords: antioxidant enzyme, buffalo, heat stress, physiological response, temperature–humidity index
Seasonal dynamics of physiological and metabolic responses of lactating Nili‐Ravi buffaloes was studied under the tropical climate of south china. Higher temperature humidity Index (THI) during the summer season (>80) resulted in a significant increase in RR and BST as compared to the winter season. High oxidative stress was observed in the summer season as revealed by significantly higher MDA and Cortisol while lower serum antioxidant enzymes (T‐AOC, GSH‐Px, SOD and CAT) and growth hormone contents as compared to other seasons.
1. INTRODUCTION
Water buffalo (Bubalus bubalis) is a predominant bovine species in the Asian continent where more than 95% of the world buffalo population inhabits. Despite lower productivity of buffaloes as compared to dairy cattle, it still contributes more than 60% of total milk production in South Asia and 12.8% of global milk production (FAO, 2019). China possesses the third largest buffalo population after India and Pakistan. Buffalo is considered as an efficient converter of poor‐quality roughages into highly nutritious products (milk and meat) under harsh environmental conditions (hot and humid) of tropical areas (Marai & Haeeb, 2010). The skin of buffaloes possesses thick epidermis, which protects them against ultraviolet rays, and the secretion from abundant sebaceous glands makes their coating shiny that reflects heat rays to avoid excessive heat load (Shafie, 1985). However, less number of sweat glands and darker body colour make buffalo less heat tolerant than zebu cattle (Bombade et al., 2018; Das et al., 1999). Moreover, high milk production and exposure to hot and humid climates, especially under conditions such as lack of proper shelter, wallowing and/or swimming provisions, increase the susceptibility to heat stress in buffaloes (Das et al., 2014). Higher metabolic rate and heat generated during rumen fermentation make dairy animals more susceptible to heat stress (Liu et al., 2014) because higher milk yield is associated with higher metabolic heat production in the body (Purwanto et al. 1990).
Climate is one of the major constraints that hinder the efficiency of livestock production, especially in sub‐tropical and tropical areas. Heat stress in tropics is generally associated with animal welfare issues and significant economic losses resulting from reduced performance, morbidity and mortality of livestock (Marai & Haeeb, 2010). The issue of heat stress is likely to become worse in the recent climate change scenario as it will increase the potential intensity of hot and humid conditions in the future, leading to an increased frequency of heat stress episodes (Gauly et al., 2013). Adverse effects of heat stress on animal physiology have been extensively reviewed in buffalo and other ruminants (Marai & Haeeb, 2010; Yadav et al., 2013).
Heat stress is generally observed when the ambient temperature exceeds the thermoneutral zone of animal body and results in an imbalance between heat gains and/or produced in the body and its dissipation. This heat stress triggers many physiological changes in buffaloes, including decrease in feed intake, shifts in hormonal and metabolic secretions as well as increases in body surface temperature (BST), rectal temperature (RT), respiratory rate (RR) and oxidative stress (Bombade et al., 2018; Hooda & Singh, 2010). Thermal comfort is generally measured by temperature–humidity index (THI) and it has been applied to different livestock species for understanding the physiological stages of thermo‐neutral and heat stress zones. For dairy cattle, THI values have been grouped into three levels to depict the extent of thermal stress: 72–79 for mild stress, 79–89 for moderate stress and >89 for heavy stress (Akyuz et al., 2010). When THI value exceeds 70, management steps (such as cooling) are recommended to avoid a decrease in milk production in dairy cows (Brouček et al., 2009).
Buffaloes have better thermotolerance than dairy cattle, hence the THI value ≤74 is considered optimum for buffalo rearing (Somparn et al., 2004). Despite having efficient thermoregulatory capacity, buffaloes show heat stress symptoms when they were exposed to high air temperatures (36°C or above), high relative humidity and intense direct solar radiation (Gudev et al., 2007; Koga et al., 2004). Under the climate change and gradual global warming scenarios, research on the resilience and possible adaptation of animals to these new conditions is required, particularly in tropical and subtropical countries, in which the mean air temperature is expected to rise the most over the next decades (IPCC, 2013).
Wide variations have been observed in ambient temperature and relative humidity during different summer months in southern China. Hence, the physiological responses of buffaloes to different THI levels may vary during different seasons. Buffaloes can acclimatize efficiently with heat stress through various physiological and biochemical responses, but this compromises production and reproduction (Das et al., 2014; Marai & Haeeb, 2010). Therefore, it is important to understand variations in physiological and metabolic responses of the lactating buffaloes during different seasons, especially during harsh environmental conditions. It may help devise strategies to mitigate the adverse effects of heat stress to avoid economic losses and animal welfare issues resulting from heat stress. Therefore, this experiment was conducted to study seasonal variations in the physiological, metabolic, hormonal and oxidative responses of lactating Nili‐Ravi buffaloes under tropical climate in southern China.
2. MATERIALS AND METHODS
2.1. Geographical location and environmental conditions
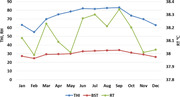
This study was conducted from April 2017 to March 2018 at the Guangxi Buffalo Research Institute, located in Nanning, the capital city of Guangxi province of China (N 22° 53′22.59″; N, E 108° 21′51.19″ E; 122 meters above sea level). This area is located in the subtropical monsoon climate zone, with sufficient sunshine, abundant rainfall, long summer and short winter. The annual rainfall of the area is 1304 mm with a range of 1108–1497 mm. Annual minimum and maximum ambient temperatures were recorded as 4.3 and 37.9°C, respectively, with an average of 25°C. Average humidity was quite high (82%), ranging from 42.1% to 96.6%. Environmental variables were recorded twice daily in the morning (at 8.00 AM) and afternoon (at 2.30 PM) and used to depict monthly meteorological variations (Figure 1). Average daily temperature and relative humidity were recorded and THI was calculated by using the following formula (Thom, 1959):
FIGURE 1.
Monthly trend of THI, air temperature and relative humidity during study period
where AT is the air temperature and DPT is the dew point temperature of the buffalo shed. Four seasons of a year were defined as follows: spring (March to May), summer (June to August), Autumn (September to November) and winter (December to February).
2.2. Animals, diet and lactation attributes
Twenty Nili‐Ravi buffaloes were randomly selected for this trial. All buffaloes were in their mid‐lactation (3–4 months) with an average body weight of 600 ± 50 kg. All buffaloes were multiparous (4 ± 1 lactations) and were milked twice daily in the morning and afternoon with milking machines. Buffaloes were housed in an open‐sided shed with an asbestos roof during milking only while for other times they were set free in an adjacent open area with a stocking density of 15 m2/head. Buffaloes had free access to water round the clock. Fans were installed in the shed above animal's height to facilitate airflow during three seasons except winter. Buffaloes were provided 30 min of wallowing in water before milking (once in each morning and afternoon). All buffaloes were fed a total mix ration consisting of grass (Pennisetum purpureum Schum.), cassava residue, brewers’ grains and concentrate mixture (corn, soybean meal and wheat bran). Daily‐measured quantity of total mix ration was fed to each buffalo to meet their dietary requirements as per the general routine of buffalo farm. Ingredient and chemical composition of the total mix ration are presented in Table 1. The average milk production of buffaloes was around 6.13 kg during the study period. Three buffaloes which became dry during the study period were replaced with lactating buffaloes of very similar milk yield, parity and lactation stage. Proper vaccination and deworming schedule were followed as a prophylactic measure against diseases and parasitic infestation.
Table 1.
Ingredient and chemical composition of total mix ration fed to lactating buffaloes (on air‐dry basis)
| Ingredients | Percentage |
|---|---|
| Grass (Pennisetum purpureum Schum.) | 12 |
| Brewer's grain | 21 |
| Cassava residue | 33 |
| Corn | 17.83 |
| Wheat bran | 7.51 |
| Soybean meal | 5.72 |
| Lime stone | 0.5 |
| CaHPO4 | 0.6 |
| NaHCO3 | 0.8 |
| NaCl | 0.7 |
| Vitamin–mineral premixa | 0.34 |
| Total | 100 |
| Nutrient levelsb | |
| Crude Protein | 14.6 |
| Neutral Detergent Fiber | 36.21 |
| Acid Detergent Fiber | 23.5 |
| Ash | 6.23 |
The additive premix provided the following per Kg of diets: Vit. A 550,000 IU, Vit. E 3000 IU, Vit. D3 150,000 IU, Fe (as ferrous sulphate) 4.0 g, Cu (as copper sulphate) 1.3 g, Mn (as manganese sulphate) 3.0 g, Zn (as zinc sulphate) 6.0 g, and Co (as cobalt sulphate) 80 mg.
Measured values.
2.3. Recording of physiological parameters
Online dust monitoring system (Shenzhen Greenford Environmental Technology Co., Ltd., China) was used to record air temperature (AT) and relative humidity (RH), with an interval of 30 min and at an installation height of buffalo. Weekly average BST was recorded on each Tuesday at 8:00 AM and 2:30 PM using an animal infrared thermometer from three different sites (forehead, left chest and abdomen). At the same time, RT was also recorded using a veterinary rectal thermometer (by inserting it in the rectum for 15 s), whereas RR was recorded as times per minute by observing thoracic movements using a stopwatch and a counter (for 2 min).
2.4. Determination of serum hormones and antioxidant enzymes
Blood samples were collected from jugular vein before morning feeding once during each season, namely spring (April 17th), summer (July 22nd), autumn (October 18th) and winter (January 28th). Blood samples were put in ice after collection and immediately transferred to the laboratory for separation of serum. The serum was stored at –20°C until further analysis. Serum hormones, including adrenocorticotropic hormone (ACTH), insulin, cortisol, triiodothyronine (T3), thyroxine (T4) and growth hormone (GH), were analysed using commercial ELISA Kits (CUSABIO BIOTECH CO., Wuhan, China) through ELISA assay (ELISA microplate reader) according to manufacturer's instructions. Levels of serum antioxidant enzymes – total antioxidant capacity (TAC) (Cat No. A015), malondialdehyde (MDA) (A003‐1), total superoxide dismutase (SOD) (A001‐1), glutathione peroxidase (GSH‐Px) (A005) and catalase (CAT) (A007‐1‐1) – were determined through spectrophotometer using the Nanjing Built‐in Kits (www.njjcbio.com) according to manufacturer's instructions. Average values for each season for all buffaloes are expressed as mean ± SE. Duplicate samples were analysed from each animal according to manufacturer's instructions and intra‐assay (within duplicates) and inter‐assay (plate‐to‐plate) coefficients of variation were calculated, which were <10% for these kits.
2.5. Statistical analysis
Data of physiological parameters were analysed by repeated measure analysis of variance followed by Tukey's post hoc test to compare the values among different seasons using SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA). The data of serum hormones and antioxidant enzymes were subjected to Student's t test for comparison between different seasons as described previously (Habibu et al., 2017). Pearson correlation coefficients were calculated by SAS using the CORR procedure to determine the relationship between THI and different physiological parameters.
3. RESULTS
3.1. Monthly trend of meteorological data
Monthly variations in THI, air temperature and relative humidity of buffalo shed are presented in Figure 1. Higher average THI values (above 80) were observed in the summer season (June to September). The THI started decreasing in autumn and winter. It again started to increase in the spring season. Weekly trends of THI, AT and RH in each season are presented in Figure 2.
FIGURE 2.
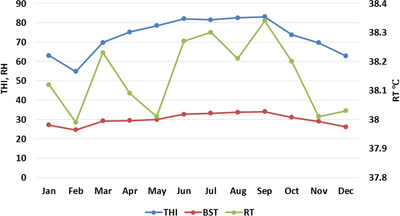
Monthly trend of THI, BST and RT in lactating Nili‐Ravi buffaloes
3.2. Physiological parameters
Average values for THI in the shed and physiological parameters of lactating buffaloes during different seasons are presented in Table 2. The THI in spring, summer and autumn was significantly higher as compared with winter (P < 0.05), with the highest THI value (82) in summer. The RR and BST were significantly higher in summer than in winter (P < 0.05). However, no significant difference (P > 0.05) was observed in the RT of buffaloes in different seasons. Monthly variations of physiological parameters showed similar trends, as observed in monthly THI (Figure 3). An increase in THI during summer months resulted in corresponding higher BST, RT and RR values in lactating buffaloes.
Table 2.
Average THI and physiological parameters of lactating buffaloes during different seasons
| Parameters | Spring | Summer | Autumn | Winter | P‐values |
|---|---|---|---|---|---|
| Temperature–humidity index | 74.48 ± 4.38a | 82.00 ± 0.53a | 75.51 ± 6.86a | 60.25 ± 4.73b | 0.003 |
| Respiratory rate (times/min) | 13.28 ± 1.72ab | 17.32 ± 1.09a | 15.17 ± 2.82ab | 10.61 ± 3.14b | 0.041 |
| Body surface temperature (°C) | 25.96 ± 1.25c | 33.17 ± 0.52a | 31.27 ± 2.50ab | 29.53 ± 0.35b | 0.002 |
| Rectal temperature (°C) | 38.04 ± 0.06 | 38.11 ± 0.11 | 38.26 ± 0.04 | 38.18 ± 0.16 | 0.135 |
The values with different superscripts in the same row differ significantly (P < 0.05).
FIGURE 3.
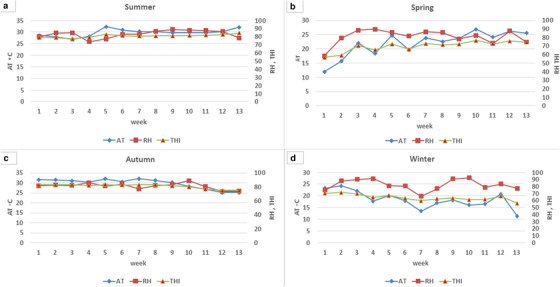
Weekly trend of THI, air temperature and relative humidity in summer (a), spring (b), autumn (c) and winter (d)
3.3. Serum antioxidant enzymes
Average concentrations of serum antioxidant enzymes during different seasons are presented in Table 3. Significantly higher (P < 0.05) TAC contents were recorded in winter than in other seasons but to be the lowest in summer and autumn. However, MDA contents were significantly higher (P < 0.05) in summer compared with other seasons. The highest MDA indicated a high oxidative stress compared with winter. Moreover, MDA contents in autumn were also significantly higher than in winter and spring (P < 0.05). There was no significant difference between MDA contents of spring and winter (P > 0.05). The highest concentrations (P < 0.05) of GSH‐Px, SOD and CAT were observed in winter compared with other seasons, whereas average concentrations of all these enzymes were the lowest in summer (P < 0.05).
Table 3.
Seasonal variations in serum antioxidant enzymes in lactating buffaloes (n = 20)
| Parameter | Spring | Summer | Autumn | Winter | P‐values |
|---|---|---|---|---|---|
| TAC (U/ml) | 3.85 ± 1.03b | 1.31 ± 0.14c | 1.78 ± 0.07c | 5.30 ± 1.16a | 0.001 |
| MDA (nmol/ml) | 4.90 ± 0.28c | 11.29 ± 0.83a | 8.51 ± 0.50b | 4.88 ± 0.23c | 0.001 |
| GSH‐Px (U/ml) | 226.63 ± 9.77b | 130.54 ± 7.95d | 169.85 ± 11.45c | 263.86 ± 15.97a | 0.001 |
| SOD (U/ml) | 36.11 ± 3.85b | 17.12 ± 2.01d | 30.61 ± 3.71c | 40.95 ± 2.32a | 0.001 |
| CAT (U/ml) | 11.53 ± 3.54b | 3.44 ± 1.04c | 4.27 ± 0.71c | 20.33 ± 1.81a | 0.001 |
Abbreviations: CAT, catalase; GSH‐Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; TAC, total antioxidant capacity. The values with different superscripts in the same row differ significantly
3.4. Serum hormone levels
Concentration of ACTH was significantly higher (P < 0.05) in winter and significantly lower in summer than in other seasons (Table 4). Cortisol contents were significantly higher (P < 0.05) in summer and autumn than in spring and winter. However, no significant difference in insulin concentrations was observed among different seasons (P > 0.05). The T3 concentration was the highest in spring and summer, whereas no difference (P > 0.05) was observed during autumn and winter. The T4 concentration was significantly (P < 0.05) higher in autumn as compared to spring and winter. The concentration of GH was the highest in winter, but the lowest in summer (P < 0.05).
Table 4.
Average serum hormone levels in buffalo during different seasons
| Hormone | Spring | Summer | Autumn | Winter | P‐values |
|---|---|---|---|---|---|
| Corticotrophin (ACTH) (pg/ml) | 59.04 ± 16.52b | 36.43 ± 6.81c | 54.84 ± 7.62b | 83.57 ± 8.87a | 0.005 |
| Cortisol (ng/ml) | 28.92 ± 3.94b | 42.86 ± 6.64a | 41.99 ± 6.42a | 25.49 ± 3.82b | 0.008 |
| Insulin (nIU/ml) | 10.36 ± 1.50 | 10.17 ± 3.92 | 12.26 ± 3.22 | 10.92 ± 1.31 | 0.784 |
| Triiodothyronine (T3) (ng/ml) | 1.82 ± 0.63a | 1.39 ± 0.56b | 1.23 ± 0.45b | 1.39 ± 0.16b | 0.000 |
| Thyroxin (T4) (ng/ml) | 57.21 ± 10.48b | 63.11 ± 15.48ab | 67.95 ± 16.53a | 40.24 ± 3.78c | 0.000 |
| Growth hormone (GH) (ng/ml) | 14.22 ± 2.61ab | 9.89 ± 2.83c | 12.28 ± 4.76bc | 16.40 ± 3.63a | 0.000 |
The values with different superscripts in the same row differ significantly (P < 0.05).
3.5. Blood metabolites
Seasonal variations in blood metabolites of buffaloes are presented in Table 5. No significant change was observed in total protein contents during different seasons (P > 0.05). However, albumin was significantly higher in winter and the lowest was in summer as compared with other seasons (P < 0.05). On the other hand, globulin contents were lower in winter than in other seasons (P < 0.05). The concentrations of ALT and AST were significantly lower in summer than in other seasons (P < 0.05). Urea nitrogen was the highest in winter, followed by spring, summer and autumn. Glucose contents were significantly higher in winter than in other seasons (P < 0.05), whereas the lowest contents were observed during spring season.
Table 5.
Levels of different blood metabolites in lactating buffaloes during different seasons
| Parameters | Spring | Summer | Autumn | Winter | P‐values |
|---|---|---|---|---|---|
| Total protein (g/L) | 80.12 ± 5.52 | 80.15 ± 6.96 | 81.77 ± 5.08 | 77.53 ± 8.00 | 0.090 |
| Albumin (g/L) | 35.96 ± 2.14bc | 33.72 ± 2.02c | 35.23 ± 2.38b | 38.02 ± 3.40a | 0.000 |
| Globulin (g/L) | 44.15 ± 5.71a | 46.42 ± 6.85a | 46.54 ± 4.97a | 39.51 ± 7.99b | 0.002 |
| Alanine aminotransferase (ALT) (U/L) | 51.54 ± 8.89b | 37.05 ± 6.37c | 59.82 ± 10.68a | 52.11 ± 9.61b | 0.000 |
| Aspartate aminotransferase (AST) (U/L) | 154.90 ± 21.66a | 129.88 ± 25.02b | 157.29 ± 28.72a | 146.23 ± 18.69a | 0.001 |
| Blood urea nitrogen (BUN) (mmol/L) | 9.56 ± 2.01b | 8.50 ± 1.61bc | 7.81 ± 1.79c | 11.95 ± 2.99a | 0.000 |
| Glucose (mmol/L) | 2.04 ± 0.32c | 3.57 ± 0.76b | 3.34 ± 0.24b | 3.95 ± 0.48a | 0.000 |
The values with different superscripts in the same row differ significantly (P < 0.05).
3.6. Correlation of THI with physiological and biochemical parameters
Pearson correlation analysis showed an effect of THI on physiological parameters of buffaloes (Table 6). Significant correlations (P < 0.01) of THI with BST and RR were detected, whereas a modest correlation of THI with RT was observed (P < 0.05). A significant negative correlation (P > 0.01) was observed between THI and ACTH (–0.76), whereas positive correlations (P < 0.05) were observed with cortisol (0.68) and T4 (0.60) concentrations (Table 6).
Table 6.
Correlation of THI with physiological and metabolic parameters of lactating buffaloes
| Parameters | THI | BST | RT | RR | ACTH | Cortisol | Insulin | T3 | T4 | GH |
|---|---|---|---|---|---|---|---|---|---|---|
| THI | 1 | |||||||||
| BST | 0.962** | 1 | ||||||||
| RT | 0.684* | 0.809** | 1 | |||||||
| RR | 0.901** | 0.929** | 0.745** | 1 | ||||||
| ACTH | –0.764** | –0.263 | 0.391 | 1 | ||||||
| Cortisol | 0.689* | 0.777** | 0.494 | 0.906** | –0.488 | 1 | ||||
| Insulin | 0.009 | 0.359 | 0.729** | 0.434 | 0.339 | 0.536 | 1 | |||
| T3 | 0.025 | –0.082 | 0.146 | 0.317 | 0.358 | 0.175 | 0.640* | 1 | ||
| T4 | 0.603* | 0.486 | 0.41 | 0.828** | –0.304 | 0.882** | 0.716** | 0.521 | 1 | |
| GH | –0.442 | –0.122 | 0.474 | –0.044 | 0.834** | –0.147 | 0.673* | 0.668* | 0.114 | 1 |
Abbreviations: ACTH, adrenocorticotropic hormone; BST, body surface temperature; GH, growth hormone; RR, respiratory rate; RT, rectal temperature; T3, triiodothyronine; T4, thyroxine; THI, temperature–humidity index.
P < 0.05;
P < 0.01.
3.7. Correlation of THI with serum antioxidant enzymes
There was a negative correlation between THI and serum antioxidant enzymes, whereas a positive correlation of THI was observed with MDA in lactating buffaloes (Table 7). The THI showed highly negative correlations (P < 0.01) with TAC, GSH‐Px, SO and CAT and positive correlation (P < 0.05) with MDA levels.
Table 7.
Correlation of THI with serum antioxidant parameters of lactating buffaloes
| Parameters | THI | TAC | MDA | GSH‐Px | SOD | CAT |
|---|---|---|---|---|---|---|
| THI | 1 | |||||
| TAC | –0.722** | 1 | ||||
| MDA | 0.696* | –0.818** | 1 | |||
| GSH‐Px | –0.777** | 0.954** | –0.913** | 1 | ||
| SOD | –0.730** | 0.869** | –0.880** | 0.952** | 1 | |
| CAT | –0.813** | 0.971** | –0.786** | 0.945** | 0.844** | 1 |
Abbreviations: CAT, catalase; GSH‐Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; TAC, total antioxidant capacity; THI, temperature–humidity index.
P < 0.05;
P < 0.01.
4. DISCUSSION
4.1. Effect of THI on physiological parameters
Significant variations were observed in the physiological responses of lactating buffaloes in different seasons of a year in the present study. Meteorological data showed higher THI values (>80) during 4 months (June to September) which corresponded well with higher RT, BST and RR in lactating buffaloes. Significantly higher BST during summer and autumn as compared with other seasons indicated the effect of higher THI on lactating buffaloes. The RR is directly associated with the thermotolerance and physiological response of an animal to extreme environmental conditions, especially under the tropical climate. Significantly higher RR was observed during summer as compared with the winter season in our study. This indicated that high temperature or THI resulted in changes in cardiovascular and respiratory functions and animals responded by increasing RR to keep body temperature down (as an adaptive strategy) through evaporative cooling. Earlier studies have reported higher RR and RT but lower PR in buffaloes exposed to high temperatures in the climatic chamber (Wankar et al., 2014). Similarly, dramatic increase in RR, RT and PR has been observed in cattle to respond to heat stress (Banerjee & Ashutosh, 2011). In our study, no difference was observed in RT in different seasons, nor did in RR (as RR was the same in three seasons). These findings are in agreement with earlier studies (Chaiyabutr et al., 1987; Chikamune, 1986) in which the effects of THI on RT, RR and pulse rate are not pronounced at an ambient temperature of 30°C, indicating the ability of swamp buffaloes to manage their body heat balance. Respiratory rate increases in buffaloes markedly when ambient temperatures exceed 30°C, owing to the stimulation of peripheral receptors (thermo‐regulatory system) which leads to the initiation of panting without allowing a rise in RT (Chikamune, 1986).
Buffaloes have been reported to exhibit more fluctuations in RT and BST than cattle when they were exposed to higher ambient temperatures owing to their dark skin and reduced sweating capacity (Koga et al., 2002). That is why buffaloes have developed an adaptive mechanism that triggers them to seek water for immersion at higher ambient temperatures (Thomas, 2004). Moreover, when buffaloes are exposed to direct sunlight with no access to a wallow, they tend to actively seek shade to avoid direct exposure to radiations. In our study, despite the higher THI (82) value observed in summer, no severe response was found in physiological parameters of buffaloes, especially in RT which is considered as an index of heat storage in the body. According to the reference values for RT (37.4–37.9°C), BST (25.6–35.5°C) and RR (18–30 times/min) in buffaloes, there was no adverse sign of heat stress in the present study (Shafie, 1985). This indicates that the management of buffaloes, particularly wallowing and sprinkling of water twice a day (in the morning and afternoon), can facilitate body heat dissipation and help buffaloes to mediate adverse effects of heat stress. This is also in line with earlier studies that reported positive effect of water sprinkling and airflow to reduce heat stress in dairy buffaloes (Kumar & Gupta, 1991; Vijayakumar et al., 2011). However, absence of a control group in the present study is a limitation to corroborate these findings as we just aimed to report seasonal dynamics of physiological and biochemical responses of lactating buffaloes. Additionally, it is also indicated that THI values are not equally applicable in buffaloes as these are basically designed for dairy cattle. A similar finding in Murrah buffalo bulls has been observed during the summer season (April to August) at an average THI value of 79.7 (ranging from 78.2 to 80.6) as no sign of thermal discomfort was present (Barros et al., 2015). But in female buffaloes, THI values >75 have shown increases in RT and RR (Armstrong, 1994) along with adverse effects of heat stress with respect to decline in fertility under subtropical climatic conditions (Dash et al., 2015; Dash et al., 2016). But our findings in the present study indicate that under appropriate management conditions, lactating buffaloes can withstand THI ∼ 80 without showing any adverse physiological responses and heat stress signs. Our companion study also supports these findings as under these environmental conditions at the same location and period of time, non‐lactating Nili‐Ravi buffaloes showed higher BST and RR in summer compared with winter (Li et al., 2020). It was attributed mainly to the effect of wallowing (swimming) that was only provided to lactating buffaloes and hence they showed no physiological response under the same climatic conditions and THI values. However, these findings suggest developing a separate index for buffalo to reveal thermal comfort under different agro‐climatic conditions and production systems.
4.2. Serum antioxidant indices of buffalo in different seasons
In the present study, serum GSH‐Px and SOD contents were the lowest in summer and the highest in winter. Previous studies have also shown a decrease in GSH‐Px levels under heat stress conditions, leading to H2O2 accumulation (Waiz et al., 2016). Exposure to high ambient temperature leads to the disruption of antioxidant defence system with subsequent decrease in activities of antioxidant enzymes (SOD, CAT and GSH‐Px). This eventually results in the accumulation of large amounts of reactive oxygen species (ROS) that cannot be removed in time, causing redox disorder and eventually oxidative stress (Aréchiga et al., 1998; Rahal et al., 2014). Therefore, levels of oxidative and antioxidant enzymes in ruminants are usually used as biomarkers to reveal the degree of oxidative stress (Celi, 2011). MDA is one of the most important end products of membrane lipid peroxidation, and its increased levels can aggravate cell membrane damages. Therefore, MDA contents are used as a biomarker for the degree of oxidative stress in animals.
In our study, significantly higher MDA contents in lactating buffaloes were observed in summer than in other seasons, showing a higher degree of oxidative stress as reported earlier in Murrah buffaloes (Lallawmkimi et al., 2013). Levels of antioxidant enzymes and heat shock proteins are the most important stress biomarkers that reveal animal comfort and climate adaptability (Lacetera et al., 2006). Higher levels of MDA but lower levels of antioxidant enzymes (TAC, GHS‐Px, SOD and CAT) in the summer season than in other seasons indicate a state of oxidative stress in buffaloes induced by the increased THI. But at the same time, no significant change in the physiological parameters of buffaloes suggests their adaptive ability coupled with the positive effect of management practices that facilitated evaporative cooling.
4.3. Hormonal and biochemical parameters
Climatic stress has also shown effect on the activity of thyroid gland, which is directly associated with variations in the plasma T3 and T4 levels in buffaloes. Exposure to high ambient temperature suppresses thyroid activity (Pereira et al., 2008). Thyroid activity is responsible for the regulation of metabolic heat increment and body temperature. Climatic stress leads to physiological strain resulting in metabolic burden and subsequently affects feed intake of animals. These metabolic changes are reflected through shifts in the concentrations of metabolic hormones such as T3, T4 and insulin. In the present study, T3 levels decreased significantly in summer as compared with spring, showing obvious effects of heat stress and THI on lactating buffaloes. However, surprisingly, plasma T4 levels were significantly higher in summer than winter but similar to the other two seasons (spring and autumn). Similar finding regarding the depression of T3 and T4 levels in buffaloes during summer heat stress period has been reported in earlier study (Lakhani et al., 2018). The decrease in T3 and T4 during heat stress is an adaptive strategy to reduce the basal metabolic rate to decrease net body heat production (Collier et al., 2008; Farooq et al., 2010). Contrary to our finding, no difference in plasma T3 and T4 levels in Nili‐Ravi buffaloes was observed during hot humid and hot dry seasons (Das et al., 2014). Fluctuations in T3 and T4 levels in response to heat stress are not abrupt as it takes time to change T3 and T4 levels and subsequently requires several days to reach a new steady state (Silanikove, 2000).
Heat stress stimulates the hypothalamus–pituitary–adrenal axis of central nervous system to secrete corticotrophin releasing factor that subsequently leads to secretion of ACTH from corticotrope of the anterior pituitary gland (Tilbrook et al., 2000). ACTH release further triggers the synthesis and secretion of glucocorticoids such as cortisol from the cortex of adrenal glands. A significantly lower level of ACTH was observed in lactating buffaloes in summer than in other seasons, which might be attributed to negative feedback of the hypothalamus–pituitary–adrenal axis by higher levels of cortisol observed in the summer season (Plotsky et al., 1989). It was also evident by the corresponding negative correlation of ACTH with cortisol levels observed in our study, although it was insignificant.
Plasma cortisol is a well‐established biomarker of physiological stress in dairy cattle (Alhussien & Dang, 2018; Chaiyabutr et al., 2008) and buffaloes (Dimri et al., 2010; Zhengkang et al., 1994). Significantly higher plasma cortisol was observed in summer and autumn than in winter and spring in the present study. Similar levels of plasma cortisol in summer and autumn showed the effect of thermal stress in both seasons as a higher humidity level in autumn also put stress on buffaloes. Increased plasma cortisol indicates the stimulation of the hypothalamic–pituitary–adrenal axis to secrete more cortisol, to facilitate animals to maintain homeostasis and adjust body physiology to cope with stress. Similar findings regarding higher cortisol levels during extreme summer conditions (compared with other seasons) have been reported earlier in Murrah buffaloes (Bombade et al., 2018; Lakhani et al., 2018) and Sahiwal cows (Alhussien & Dang, 2018). Moreover, it is reported that thermo‐tolerant animals show elevated cortisol levels compared to thermo‐sensitive counterparts; for instance, a higher plasma cortisol was observed in crossbred buffaloes (Nili‐Ravi × Murrah) compared with Mediterranean buffaloes (Shenhe et al., 2018).
Prolonged heat stress conditions initiate the acclimatization process to achieve physiological homeostasis in animals, which is partly achieved by decreasing GH and catecholamine (Horowitz, 2001). It seems imperative as GH is a major hormone related to energy metabolism and its activity is restricted to reduce body heat production (Bauman & Currie, 1980). Plasma GH level was also significantly reduced in lactating buffaloes in summer compared with winter and spring in our study, in agreement with earlier studies (Igono et al., 1988; Mitra et al., 1972). Furthermore, GH is also responsible for heat production by stimulating the activity of thyroid gland, in addition to calorigenesis (Yousef & Johnson, 1966). This was also evident by a modest positive correlation of GH with T3 and insulin observed in our study. Therefore, a decrease in GH is inevitable to facilitate physiological homeostasis in homeotherms such as lactating buffaloes under high ambient temperatures.
Despite the modest positive correlation of insulin with T3 and T4, we found no significant change in plasma insulin levels in lactating buffaloes during different seasons, in agreement with earlier reports (McVeigh et al., 1982). However, contrary findings such as decrease (Abdel‐Samee et al., 1989) or increase (Chaiyabutr et al., 1987) in plasma insulin in cows under heat stress have been reported earlier. Such variations are mainly attributed to different metabolic status, nutrition, physiological stage and thermo‐tolerance ability of animals.
Higher levels of MDA but lower levels of antioxidant enzymes observed in lactating buffaloes in summer indicate significant effects of heat stress. Positive correlation of MDA contents with THI but negative relationship with all antioxidant enzymes confirm its significance as a biomarker for heat stress in buffaloes. Earlier studies have reported the effect of heat stress on the fertility of buffaloes as higher MDA levels but lower antioxidant capacity were observed in acyclic buffaloes (Ahmed et al., 2010; El‐Moghazy, 2011). Moreover, it is also reported that THI above 75 makes buffaloes susceptible to reduced fertility (Dash et al., 2016). Furthermore, studies have shown that ROS and total antioxidant capacity of follicular fluids are associated with reproductive acyclicity in buffaloes because this oxidative–antioxidative imbalance leads to severe damage to follicular cells (Jan et al., 2014).
In our study, an average THI above 75 was observed in spring, summer and autumn which also correlated with the physiological and oxidative responses of lactating buffaloes. These findings might indicate the possibility of cumulative stress as same animals were used in each season. This is in agreement with earlier reports (Dash et al., 2016) that period from April to September serves as a heat stress zone for buffalo. Similar findings have also been reported in Murrah buffaloes reared under tropical climate of Eastern Amazon (Brazil), where buffaloes showed effects of heat stress in both less rainy and rainy seasons (Silva et al., 2014). Hence, it is recommended that from spring to autumn, buffaloes should be managed to alleviate the adverse effects of heat stress on the productive and reproductive performance, especially during the summer season. It is also in line with earlier studies that have suggested the use of effective stress management practices from April to September to reduce the impact of heat stress on the physiology of dairy animals (Dash et al., 2016). Our findings envisaged that lactating buffaloes can withstand THI value up to 75 (thermal comfort zone) but climatic conditions that lead to THI value >75 can cause heat stress, which requires effective cooling practices to alleviate adverse effect of heat stress. The present study indicated that wallowing (swimming in a pond) for 30 min in the morning and afternoon before milking along with suitable airflow in buffalo shed can facilitate animals to effectively withstand the adverse effects of heat stress under tropical climate. To the best of our knowledge, our study is the first report on season dynamics of physiological and metabolic responses of lactating Nili‐Ravi buffaloes to heat stress under the tropical climate of South China. It provides practical insights on the adaptive physiology of buffaloes and has several implications regarding the alleviation of heat stress in buffaloes to enhance the efficiency of production and reproduction under tropical climate.
5. CONCLUSIONS
Higher THI was observed in the summer season along with significant increases in BST, RR, serum MDA and cortisol levels in lactating buffaloes. Moreover, THI showed a negative correlation with serum total antioxidant capacity as it significantly reduced serum contents of TAC, GSH‐Px, SOD and CAT in the summer season. Plasma T3 and GH levels were also reduced in the summer season. Our study revealed that 4 months (from June to September) are critical in buffalo management as THI value exceeds 80, so appropriate management practices (such as wallowing, water sprinkling, airflow, etc.) are required to alleviate heat stress to avoid performance losses and animal welfare issues. However, further studies are required to explore the THI thresholds concerning the productive and reproductive performance of lactating buffaloes under tropical climatic conditions.
AUTHOR CONTRIBUTIONS
YC and LM conceived the idea and designed the experiment. LM performed the experiment. TZ, PL, LX and CY helped in recording of physiological parameters and laboratory analysis. FH performed data analysis and interpretation and wrote the manuscript. CY procured the funding for this experiment. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVAL
All procedures used in this experiment were approved by the Ethics Committee of the Guangxi Buffalo Research Institute, Chinese Academy of Agriculture Sciences China. All steps of animal rearing, recording of physiological parameters, and blood sample collection were conducted strictly according to the guidelines of the committee.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.570.
ACKNOWLEDGMENT
This work was supported by grants from the National Key Research and Development Program of China (2016YFD0500507 and 2018YFD0501602).
Li, M., Hassan, F.‐u., Tang, Z., Guo, Y., Liang, X., Peng, L., Xie, H. & Yang, C. (2021). Physiological, oxidative and metabolic responses of lactating water buffaloes to tropical climate of South China. Veterinary Medicine and Science, 7, 1696–1706. 10.1002/vms3.570
Mengwei Li and Faiz‐ul Hassan contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- Abdel‐Samee, A., Habeeb, A., Kamal, T., & Abdel‐Razik, M. (1989). The role of urea and mineral mixture supplementation in improving productivity of heat stressed Friesian calves in the subtropics. Proceedings of 3rd Egyptian–British conference on animal, fish and poultry production, Alexandria University, Alexandria, Vol. 2, pp. 637–641. [Google Scholar]
- Ahmed, W., El‐Khadrawy, H., El Hameed, A., & Amer, H. (2010). Applied investigations on ovarian inactivity in buffalo‐heifers. International Journal of Academic Research, 1(2), 26–32. [Google Scholar]
- Akyuz, A., Boyaci, S., & Cayli, A. (2010). Determination of critical period for dairy cows using temperature humidity index. Journal of Animal and Veterinary Advances, 9, 1824–1827. [Google Scholar]
- Alhussien, M. N., & Dang, A. K. (2018). Diurnal rhythm in the counts and types of milk somatic cells, neutrophil phagocytosis and plasma cortisol levels in Karan Fries cows during different seasons and parity. Biological Rhythm Research, 49, 187–199. [Google Scholar]
- Aréchiga, C. F., Vázquez‐Flores, S., Ortíz, O., Hernández‐Cerón, J., Porras, A., McDowell, L. R., & Hansen, P J. (1998). Effect of injection of β‐carotene or vitamin E and selenium on fertility of lactating dairy cows. Theriogenology, 50, 65–76. [DOI] [PubMed] [Google Scholar]
- Armstrong, D. V. (1994). Heat stress interactions with shade and cooling. Journal of Dairy Science, 77, 2044–2050. [DOI] [PubMed] [Google Scholar]
- Banerjee, D., & Ashutosh. (2011). Circadian changes in physiological responses and blood ionized sodium and potassium concentrations under thermal exposure in Tharparkar and Karan Fries heifers. Biological Rhythm Research, 42, 131–139. [Google Scholar]
- Barros, D. V., Silva, L. K., de Brito Lourenço, J., Jr, da Silva, A. O., E Silva, A. G., Franco, I. M., Oliveira, C. M., Tholon, P., Martorano, L. G., & Garcia, A. R. (2015). Evaluation of thermal comfort, physiological, hematological, and seminal features of buffalo bulls in an artificial insemination station in a tropical environment. Tropical Animal Health and Production, 47, 805–813. 10.1007/s11250-015-0792-9 [DOI] [PubMed] [Google Scholar]
- Bauman, D. E., & Currie, W. B. (1980). Partitioning of nutrients during pregnancy and lactation: A review of mechanisms involving homeostasis and homeorhesis. Journal of Dairy Science, 63, 1514–1529. [DOI] [PubMed] [Google Scholar]
- Bombade, K., Kamboj, A., Alhussien, M. N., Mohanty, A. K., & Dang, A. K. (2018). Diurnal variation of milk somatic and differential leukocyte counts of Murrah buffaloes as influenced by different milk fractions, seasons and parities. Biological Rhythm Research, 49, 151–163. [Google Scholar]
- Brouček, J., Novák, P., Vokřálová, J., Šoch, M., Kisac, P., & Uhrinčať, M. (2009). Effect of high temperature on milk production of cows from free‐stall housing with natural ventilation. Slovak Journal of Animal Science, 42, 167–173. [Google Scholar]
- Celi, P. (2011). Biomarkers of oxidative stress in ruminant medicine. Immunopharmacology and Immunotoxicology, 33, 233–240. [DOI] [PubMed] [Google Scholar]
- Chaiyabutr, N., Buranakarl, C., Muangcharoen, V., Loypetjra, P., & Pichaicharnarong, A. (1987). Effects of acute heat stress on changes in the rate of liquid flow from the rumen and turnover of body water of swamp buffalo. The Journal of Agricultural Science, 108, 549–553. [Google Scholar]
- Chaiyabutr, N., Chanpongsang, S., & Suadsong, S. (2008). Effects of evaporative cooling on the regulation of body water and milk production in crossbred Holstein cattle in a tropical environment. International Journal of Biometeorology, 52, 575–585. [DOI] [PubMed] [Google Scholar]
- Chikamune, T. (1986). Effect of environmental temperature on thermoregulatory responses and oxygen consumption in swamp buffalo and Holstein cattle. Buffalo Journal, 2, 151–160. [Google Scholar]
- Collier, R. J., Collier, J., Rhoads, R., & Baumgard, L. (2008). Invited review: Genes involved in the bovine heat stress response. Journal of Dairy Science, 91, 445–454. [DOI] [PubMed] [Google Scholar]
- Das, K. S., Singh, J., Singh, G., Upadhyay, R. C., Malik, R., & Oberoi, P. S. (2014). Heat stress alleviation in lactating buffaloes: Effect on physiological response, metabolic hormone, milk production and composition. Indian Journal of Animal Sciences, 84, 275–280. [Google Scholar]
- Dash, S., Chakravarty, A. K., Sah, V., Jamuna, V., Behera, R., Kashyap, N., & Deshmukh, B. (2015) Influence of temperature and humidity on pregnancy rate of Murrah buffaloes. Asian‐Aust. Journal of Animal Science, 28(7): 943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, S., Upadhyay, R., & Madan, M. (1999). Heat stress in Murrah buffalo calves. Livestock Production Science, 61, 71–78. [Google Scholar]
- Dash, S.Chakravarty, A. K., Singh, A., Upadhyay, A., Singh, M., & Yousuf, S. (2016). Effect of heat stress on reproductive performances of dairy cattle and buffaloes: A review. Veterinary World, 9, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri, U., Ranjan, R., Sharma, M. C., & Varshney, V. (2010). Effect of vitamin E and selenium supplementation on oxidative stress indices and cortisol level in blood in water buffaloes during pregnancy and early postpartum period. Tropical Animal Health and Production, 42, 405–410. [DOI] [PubMed] [Google Scholar]
- El‐Moghazy, F. (2011). Impact of parasitic infestation on ovarian activity in buffaloes‐heifers with emphasis on ascariasis. World Journal of Zoology, 6, 196–203. [Google Scholar]
- FAO . (2019). FAOSTAT. http://faostat.fao.org/
- Farooq, U., Samad, H., Shehzad, F., & Qayyum, A. (2010). Physiological responses of cattle to heat stress. World Applied Sciences Journal, 8, 38–43. [Google Scholar]
- Gauly, M., Bollwein, H., Breves, G., Brügemann, K., Dänicke, S., Das, G., Demeler, J., Hansen, H., Isselstein, J., König, S., Lohölter, M., Martinsohn, M., Meyer, U., Potthoff, M., Sanker, C., Schröder, B., Wrage, N., Meibaum, B., von Samson‐Himmelstjerna, G., … Wrenzycki, C. (2013). Future consequences and challenges for dairy cow production systems arising from climate change in Central Europe—A review. Animal, 7, 843–859. [DOI] [PubMed] [Google Scholar]
- Gudev, D., Popova‐Ralcheva, S., Moneva, P., Aleksiev, Y., Peeva, T., Penchev, P., & Ilieva, I., 2007. Physiological indices in buffaloes exposed to sun. Archiva Zootechnica, 10, 127–133. [Google Scholar]
- Habibu, B., Kawu, M. U., Aluwong, T., & Makun, H. J. (2017). Influence of seasonal changes on physiological variables, haematology and serum thyroid hormones profile in male Red Sokoto and Sahel goats, Journal of Applied Animal Research, 45, 508–516. 10.1080/09712119.2016.1220384 [DOI] [Google Scholar]
- Hooda, O., & Singh, G. (2010). Effect of thermal stress on feed intake, plasma enzymes and blood biochemicals in buffalo heifers. Indian Journal of Animal Nutrition, 27, 122–127. [Google Scholar]
- Horowitz, M. (2001). Heat acclimation: Phenotypic plasticity and cues to the underlying molecular mechanisms. Journal of Thermal Biology, 26, 357–363. [Google Scholar]
- Igono, M., Johnson, H., Steevens, B., Hainen, W., & Shanklin, M. (1988). Effect of season on milk temperature, milk growth hormone, prolactin, and somatic cell counts of lactating cattle. International Journal of Biometeorology, 32, 194–200. [DOI] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) . (2013). Summary for policymakers. In Stocker T. F., Qin D., Plattner G.‐K., Tignor M., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V., & Midgley P. M. (Eds.), Climate change 2013, the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (pp. 3–29). Cambridge University Press. [Google Scholar]
- Jan, M. H., Das, G., Khan, F., Singh, J., Bashir, S., Khan, S., Prasad, J., Mehrotra, S., Pathak, M. C., Singh, G., & Sarkar, M. (2014). Evaluation of follicular oxidant‐antioxidant balance and oxidative damage during reproductive acyclicity in water buffalo (Bubalus bubalis). Asian Pacific Journal of Reproduction, 3, 35–40. [Google Scholar]
- Koga, A., Kuhara, T., & Kanai, Y. (2002). Comparison of body water retention during water deprivation between swamp buffaloes and Friesian cattle. The Journal of Agricultural Science, 138, 435–440. [Google Scholar]
- Koga, A., Sugiyama, M., Del Barrio, A. N., Lapitan, R. M., Arenda, B. R., Robles, A. Y., Cruz, L. C., & Kanai, Y. (2004). Comparison of the thermoregulatory response of buffaloes and tropical cattle, using fluctuations in rectal temperature, skin temperature and haematocrit as an index. Journal of Agricultural Science, 142 (3), 351–355. [Google Scholar]
- Kumar, D., & Gupta, L. R. (1991). Effect of some summer managemental practices on the growth, physiological and biochemical responses of buffalo calves. Indian Journal of Animal Production Management, 7(2):98‐101. [Google Scholar]
- Lacetera, N., Bernabucci, U., Scalia, D., Basiricò, L., Morera, P., & Nardone, A. (2006). Heat stress elicits different responses in peripheral blood mononuclear cells from Brown Swiss and Holstein cows. Journal of Dairy Science, 89, 4606–4612. [DOI] [PubMed] [Google Scholar]
- Lakhani, P., Alhussien, M. N., Lakhani, N., Jindal, R., & Nayyar, S. (2018). Seasonal variation in physiological responses, stress and metabolic‐related hormones, and oxidative status of Murrah buffaloes. Biological Rhythm Research, 49, 844–852. [Google Scholar]
- Lallawmkimi, M. C., Singh, S., Upadhyay, R., & De, S. (2013). Impact of vitamin E supplementation on heat shock protein 72 and antioxidant enzymes in different stages of Murrah buffaloes during seasonal stress. Indian Journal of Animal Sciences, 83, 909–915. [Google Scholar]
- Li, M., Hassan, F., Yanxia, G., Tang, Z., Liang, X., Xie, F., Peng, L., & Yang, C. (2020). Seasonal dynamics of physiological, oxidative and metabolic responses in non‐lactating Nili‐Ravi buffaloes under hot and humid climate. Frontiers in Veterinary Science, 7:622 10.3389/fvets.2020.00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Ye, G., Zhou, Y., Liu, Y., Zhao, L., Liu, Y., Chen, X., Huang, D., Liao, S. F., & Huang, K. (2014). Feeding glycerol‐enriched yeast culture improves performance, energy status, and heat shock protein gene expression of lactating Holstein cows under heat stress. Journal of Animal Science, 92, 2494–2502. [DOI] [PubMed] [Google Scholar]
- Marai, I., & Haeeb, A. (2010). Buffalo's biological functions as affected by heat stress—A review. Livestock Science, 127, 89–109. [Google Scholar]
- McVeigh, J., Tarrant, P., & Harrington, M. (1982). Behavioral stress and skeletal muscle glycogen metabolism in young bulls. Journal of Animal Science, 54, 790–795. [DOI] [PubMed] [Google Scholar]
- Mitra, R., Christison, G., & Johnson, H. (1972). Effect of prolonged thermal exposure on growth hormone (GH) secretion in cattle. Journal of Animal Science, 34, 776–779. [DOI] [PubMed] [Google Scholar]
- Pereira, A. M., Baccari, F., Titto, E. A., & Almeida, J. A. (2008). Effect of thermal stress on physiological parameters, feed intake and plasma thyroid hormones concentration in Alentejana, Mertolenga, Frisian and Limousine cattle breeds. International Journal of Biometeorology, 52, 199–208. [DOI] [PubMed] [Google Scholar]
- Plotsky, P. M., Cunningham Jr, E. T., & Widmaier, E. P. (1989). Catecholaminergic modulation of corticotropin‐releasing factor and adrenocorticotropin secretion. Endocrine Reviews, 10, 437–458. [DOI] [PubMed] [Google Scholar]
- Purwanto, B., Abo, Y., Sakamoto, R., Furumoto, F., & Yamamoto, S. (1990). Diurnal patterns of heat production and heart rate under thermoneutral conditions in Holstein Friesian cows differing in milk production. The Journal of Agricultural Science, 114, 139–142. [Google Scholar]
- Rahal, A., Kumar, A., Singh, V., Yadav, B., Tiwari, R., Chakraborty, S., & Dhama, K. (2014). Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Research International, 2014:761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafie, M. (1985). Physiological responses and adaptation of water buffalo. Yousef M. K (Ed.), Stress physiology in livestock (pp. 67–80). CRC Press. [Google Scholar]
- Shenhe, L., Jun, L., Zipeng, L., Tingxian, D., Rehman, Z. U., Zichao, Z., & Liguo, Y. (2018). Effect of season and breed on physiological and blood parameters in buffaloes. Journal of Dairy Research, 85, 181–184. [DOI] [PubMed] [Google Scholar]
- Silanikove, N. (2000). Effects of heat stress on the welfare of extensively managed domestic ruminants. Livestock Production Science, 67, 1–18. [Google Scholar]
- Silva, J. A., Araújo, R. D., Júnior, A. A. D., de Brito, L., Santos, N. D. F. A. D., Viana, R. B. R. B., Garcia, A. R., Rondina, D., & Grise, M. M. (2014). Hormonal changes in female buffaloes under shading in tropical climate of Eastern Amazon, Brazil. Revista Brasileira de Zootecnia, 43(1), 44–48 10.1590/S1516-35982014000100007 [DOI] [Google Scholar]
- Somparn, P., Gibb, M. J., Markvichitr, K., Chaiyabutr, N., Thummabood, S., & Vajrabukka, C., (2004). Analysis of climatic risk for cattle and buffalo production in northeast Thailand. International Journal of Biometeorology, 49 (1), 59–64. [DOI] [PubMed] [Google Scholar]
- Thom, E. C. (1959). The discomfort index. Weatherwise, 12, 57–61. [Google Scholar]
- Thomas, C. S. (2004). Milking management of dairy buffaloes (PhD thesis). Department of Animal Nutrition and Management, Swedish University of Agricultural Sciences. [Google Scholar]
- Tilbrook, A. J., Turner, A. I., & Clarke, I. J. (2000). Effects of stress on reproduction in non‐rodent mammals: The role of glucocorticoids and sex differences. Reviews of Reproduction, 5, 105–113. [DOI] [PubMed] [Google Scholar]
- Vijayakumar, P., Triveni, D., Mukesh, S., & Pandey, H. N. (2011). Effect of heat ameliorative measures on the biochemical and hormonal responses of buffalo heifers. Journal of Applied Animal Research, 39:3, 181–184, 10.1080/09712119.2011.607700 [DOI] [Google Scholar]
- Waiz, S. A., Raies‐ul‐Haq, M., Dhanda, S., Kumar, A., Goud, T. S., Chauhan, M. S., & Upadhyay, R. C. (2016). Heat stress and antioxidant enzyme activity in bubaline (Bubalus bubalis) oocytes during in vitro maturation. International Journal of Biometeorology, 60(9), 1357–1366. 10.1007/s00484-015-1129-0. [DOI] [PubMed] [Google Scholar]
- Wankar, A. K., Singh, G., & Yadav, B. (2014). Thermoregulatory and adaptive responses of adult buffaloes (Bubalus bubalis) during hyperthermia: Physiological, behavioral, and metabolic approach. Veterinary World, 7, 825–830. [Google Scholar]
- Yadav, B., Singh, G., Verma, A., Dutta, N., & Sejian, V. (2013). Impact of heat stress on rumen functions. Veterinary World, 6, 992–996. [Google Scholar]
- Yousef, M., & Johnson, H. (1966). Calorigenesis of dairy cattle as influenced by thyroxine and environmental temperature. Journal of Animal Science, 25, 150–156. [DOI] [PubMed] [Google Scholar]
- Zhengkang, H., Zhenzhong, C., Shaohua, Z., Vale, W. G., Barnabe, V. H., & Mattos, J. C. A. (1994). Rumen metabolism, blood cortisol and T3, T4 levels and other physiological parameters of swamp buffalo subjected to solar radiation. Proceedings of World Buffalo Congress, San Paulo, Brazil, Vol. 2, pp. 39–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


