Abstract
Bovine Ephemeral fever virus (BEFV) is endemic in South Africa and has a negative economic impact on the meat and dairy industries. Bovine ephemeral fever or three‐day stiff‐sickness is controlled through annual vaccination with a live attenuated virus manufactured by Onderstepoort Biological Products (South Africa). We announce the genome sequences of two South African Bovine Ephemeral Virus strains; the live attenuated vaccine strain (14 876 nucleotides) and a field strain (14 883 nucleotides). A mutation in the alpha 3 open reading frame rendered the gene non‐functional in both genomes. Phylogenetic analysis based on the glycoprotein gene showed that the two strains clustered with the South African lineage.
Keywords: Bovine Ephemeral fever virus, glycoprotein, next generation sequencing
Bovine ephemeral fever or three‐day stiff‐sickness is controlled through annual vaccination with a live attenuated virus manufactured by Onderstepoort Biological Products (South Africa). We announce the genome sequences of two South African Bovine Ephemeral Virus strains; the live attenuated vaccine strain (14 876 nucleotides) and a field strain (14 883 nucleotides). Phylogenetic analysis based on the glycoprotein gene showed that the 2 strains clustered with the South African lineage.

1. INTRODUCTION
Bovine Ephemeral Fever (BEF) is an economically important disease that occurs in tropical and subtropical areas of Africa, the Middle East, Asia and Australia (Walker & Klement, 2015). The causative virus is classified in the genus Ephemerovirus within the Rhabdoviridae family (Pyasi et al., 2020). It is transmitted through several vector species including Culicoides and mosquitoes and it causes an acute inflammatory disease in cattle and water buffalo (Bakhshesh & Abdollahi, 2015; Cybinski et al., 1990; Pyasi et al., 2020). BEF disease negatively impacts the beef and dairy industry through reduced milk production, infertility and abortions in late‐pregnancy (Bakhshesh & Abdollahi, 2015; Gao et al., 2017). Since the first documented case in Zimbabwe in 1906, the disease has been reported in Uganda, Kenya, Sudan, South Africa and other sub‐Saharan countries (Dacheux et al., 2019). In South Africa, disease control is achieved through the annual administration of a live attenuated vaccine subcutaneously in susceptible cattle. Currently, Onderstepoort Biological Products (OBP) is the sole supplier of the registered B‐Phemeral vaccine against BEF in South Africa. Vaccine and field strains from South Africa have not been completely characterised. This is the first report of Bovine Ephemeral fever virus (BEFV) vaccine genomes of South African origin. The vaccine strain genome was comparatively analysed with a South African field strain and with published genomes from the Australian, Middle East and East Asian lineages.
2. METHODS AND MATERIALS
2.1. Virus and Cells
African Green Monkey Kidney (Vero) cells were maintained at 37°C with 5% CO2 in Glasgow Minimum Essential Media (GMEM) (Gibco) supplemented with 10% (v/v) new‐born calf serum (Cell Sera). The BEF virus strains; commercial vaccine isolated in Onderstepoort, South Africa in 1973 and a 2008 South African field strain of unknown passage history were propagated in Vero cells using GMEM media without serum. Cultures were maintained at 37°C until ≥80% cytopathic effects were observed.
2.2. Sequencing and phylogenetic analysis
Total RNA was extracted using TRI Reagent LS (Sigma) according to the manufacturer's protocol. Sequencing was carried out using the next‐generation sequencing (NGS) Illumina MiSeq Platform (Inqaba Biotechnical Industries (Pty) Ltd.), and data were assembled using the CLC Genomics Workbench 9.5.3. Genome sequences were deduced through a combination of de novo assembly and mapping to published sequence data. Gap closure was performed through cDNA synthesis using the Quantitect Reverse Transcription kit (Qiagen). Multiple sequence alignments of the glycoprotein (G) gene were conducted using BioEdit (version 7.0.5.3) (Hall, 1999). A phylogenetic tree of the G gene was constructed on MEGA 7 (Kumar et al., 2016) using Maximum Likelihood (Jukes & Cantor, 1969) with 1000 bootstraps. The tree with the highest log likelihood (−5481,8,369) is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbour‐Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree was drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 31 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. All positions containing gaps and missing data were eliminated. There were a total of 1541 positions in the final dataset. Evolutionary analyses were conducted in MEGA 7.
3. RESULTS AND DISCUSSION
The genomes of two South African strains were assembled through a combination of de novo and mapping to genomes from Australia, China and Israel available on Genbank. Recovered genome sizes for the vaccine and field strains were 14 876 and 14 883 nucleotides, respectively. The genome sequences were deposited in the GenBank database under the accession numbers MW512963 and MW463337. A comparison of the length of the deduced protein sequences showed uniformity across all reading frames (Table S1) but with varied intergenic regions. The putative genome organisation of BEFV was observed in both strains (Figure S1). The α3 gene which plays a role in apoptosis during viral replication (Jiang et al., 2020) was non‐functional in both genomes due to a mutation in the start codon. This observation concurred with the recently published African BEFV genomes from Mayotte island (Dacheux et al., 2019). A pairwise alignment of all the deduced amino acid sequences showed 99.1%–100% similarity (Table S1). Variations between the two genomes were confined to 10 amino acids located on the N, M, G, Gns and L proteins (Table 1). The vaccine is derived from the 1973 wild‐type BEF isolate EF13 attenuated though serial passage in baby hamster kidney cells (Theodoridis et al., 1973). The field strain was obtained in 2008 and recently isolated strains were unavailable. Hence the degree of representation of circulating strains was unclear. Although preliminary data suggest that these amino acids may have a role in establishing the attenuation of the vaccine strain, further comparison with other whole genome sequences of South African origin is necessary. When these variant amino acids were compared with BEFV from Mayotte island, Australia, China, Israel, Turkey and Thailand, four unique vaccine amino acid residues were present in the South African vaccine strain only; Thr10 on the putative N protein, Ile435 on the G protein, Asn167 on the Gns protein and Val1 987 on the L protein. The amino acid residues were fairly conserved across the lineages in comparison to the South African genomes. The uniqueness of these amino acid substitutes may indicate that one or more of these were involved in the transformation of a wild‐type virus into an attenuated vaccine strain. Moreover the Gns, β and L amino acid sequences of the South African strains varied in length when compared to proteins from the selected regions. The Gns protein of the South African vaccine and field strains was determined at 578 amino acids (aa) whilst all other regions reported a longer length of 586aa. Also, the β amino acid sequence of isolates from Mayotte island, China, Israel and Thailand was 147aa, whereas South African isolates possessed an extra amino acid residue (Lys36) giving a length of 148aa. The corresponding protein from Australia and Turkey was determined to be 107aa.
TABLE 1.
Amino acid residue differences between the South African vaccine and field strains
| Gene | Position on Protein | Vaccine Amino Acid South Africa | Wild‐type Amino Acid South Africa | MN148800 Mayotte | NC002526 Australia | KY315724 China | MN078236 Israel | KY012742 Turkey | MH105245 Thailand |
|---|---|---|---|---|---|---|---|---|---|
| N | 10 | Thr | Ile | Ile | Ile | Ile | Ile | Ile | Ile |
| M | 198 | Asp | Glu | Asp | Asp | Asp | Gly | Asp | Asp |
| G | 108 | Glu | Asp | Asp | Glu | Glu | Glu | Glu | Glu |
| 291 | Arg | Gly | Arg | Arg | Arg | Arg | Arg | Arg | |
| 392 | Trp | Leu | Trp | Trp | Trp | Trp | Trp | Trp | |
| 435 | Ile | Thr | Thr | Asn | Thr | Thr | Thr | Thr | |
| Gns | 167 | Asn | Asp | Asp | Asp | Asp | Asp | Asp | Asp |
| L | 1 988 | Val | Ile | Glu | Ile | Ile | Ile | Ile | Ile |
| 2 055 | Val | Ile | Asn | Ile | Val | Ile | Val | Val | |
| 2 137 | Ser | Thr | Glu | Ser | Ser | Ser | Ser | Ser |
Four antigenic sites on the glycoprotein (G1, G2, G3 and G4) constitute the major neutralisation sites (Walker & Klement, 2015; Zheng & Qiu, 2012). A pairwise comparison of the two South African glycoprotein genes showed ≥98% similarity with four identified variant amino acid residues (Table 1) located outside the neutralising antigenic regions. A comparison of G proteins from various regions showed alternative residues at the G1‐3 sites (Figure 1). An assessment of the linear G1 antigenic site showed South African strains had amino acid residue Ile496 while other regions had Val496. In the G2 antigenic site, a single variation in residue was identified where South African strains contained Ala179 and other regions had Thr179 or Met179. In the discontinuous G3 antigenic site, five residue variations were present where the South African strains differed from the almost uniform residues present in other regions. A pairwise comparison of the vaccine strain with published South African glycoprotein sequences gave amino acid identities and similarities of >97%. A phylogenetic tree was constructed with glycoproteins from the three lineages (Australia, Middle East and East Asia) together with African sequences from Mayotte island (Dacheux et al., 2019) and South Africa (Omar et al., 2020) (Figure 2). The South African strains grouped into the recently described lineage (Omar et al., 2020) which was separate from strains from Mayotte island. The data are useful in vaccine product development and suggests that vaccine strains derived from one lineage may not confer protection. Efficacy against other areas will have to be proven.
FIGURE 1.
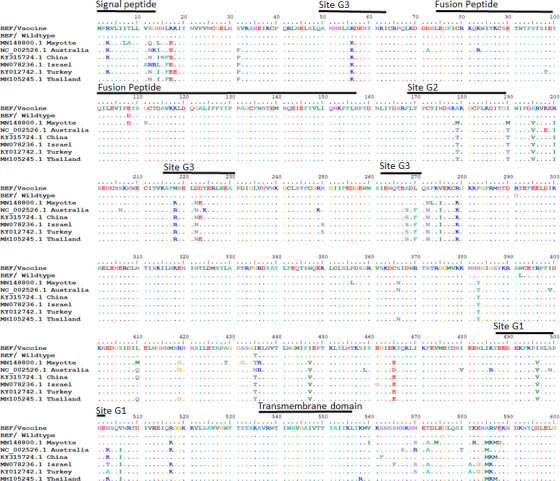
Multiple alignment of G Gene deduced amino acid sequences of isolates from South Africa, Mayotte island, Australia, China, Israel, Turkey and Thailand. The residues which differed from the South African BEFV vaccine are denoted
FIGURE 2.
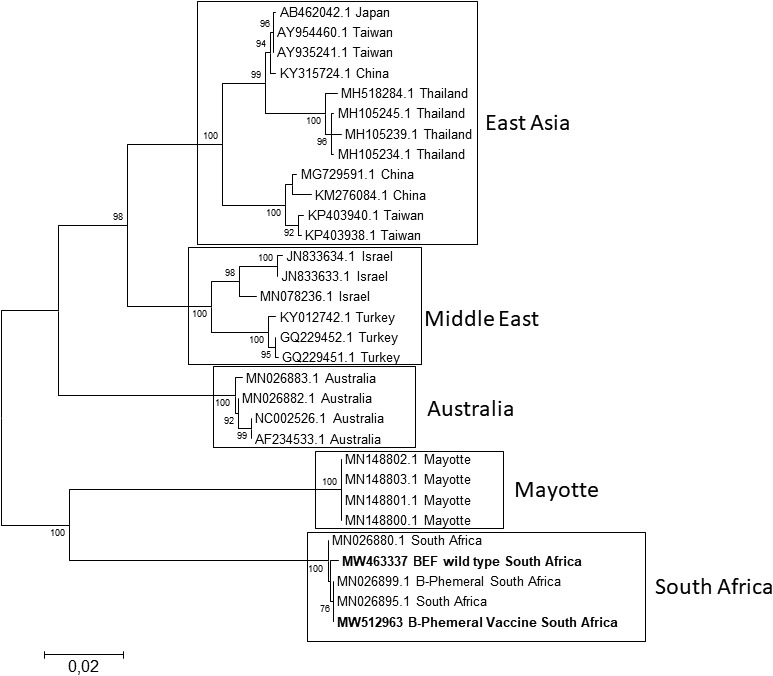
Phylogenetic profile of the BEFV G gene sequences was constructed using the Maximum Likelihood method with 1 000 bootstrap replicates shown at the node (>70). The tree divided into 5 phylogenetically distinct groups that included Australia, East Asia, the Middle East, Mayotte island and South African were evident
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
Tendai Aleck Matsa Mlingo: Formal analysis; Investigation; Methodology; Project administration; Writing‐original draft. Bethuel Mulalo Nthangeni: Conceptualization; Funding acquisition; Resources; Supervision; Writing‐review & editing. Nobalanda Betty Mokoena: Conceptualization; Formal analysis; Funding acquisition; Project administration; Supervision; Writing‐original draft; Writing‐review & editing.
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as no animal studies were conducted during data collection.
Supporting information
Fig S1
Table S1
ACKNOWLEDGEMENTS
The authors thank the members of the Research and Development unit for critical review of the manuscript.
Mlingo TAM, Nthangeni BM, Mokoena NB. Genome sequence of Bovine Ephemeral fever virus vaccine strain of South African origin. Vet Med Sci. 2021;00:1611–1615. 10.1002/vms3.517
Funding information
The project was financially supported internally from Onderstepoort Biological Products Research budget.
DATA AVAILABILITY STATEMENT
The data that support the findings will be available in NCBI at https://www.ncbi.nlm.nih.gov/ under the following accession numbers; MW463337 and MW512963.
REFERENCES
- Bakhshesh, M., & Abdollahi, D. (2015). Bovine ephemeral fever in Iran: Diagnosis. Isolation and Molecular Characterization, 9(December), 195–203. [PMC free article] [PubMed] [Google Scholar]
- Cybinski, D. H., Walker, P. J., Byrne, K. A., & Zakrzewski, H. (1990). Mapping of antigenic sites on the bovine ephemeral fever virus glycoprotein using monoclonal antibodies. Journal of General Virology, 71, 2065–2072. 10.1099/0022-1317-71-9-2065 [DOI] [PubMed] [Google Scholar]
- Dacheux, L., Dommergues, L., Chouanibou, Y., Doméon, L., Schuler, C., Bonas, S., & Métras, R. (2019). Co‐circulation and characterization of novel African arboviruses (genus Ephemerovirus) in cattle, Mayotte island, Indian Ocean, 2017. Transboundary and Emerging Diseases, 66(6), 2601–2604. 10.1111/tbed.13323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, S., Du, J., Tian, Z., Niu, Q., Zheng, F., Huang, D., Kang, B., Luo, J., Liu, G., & Yin, H. (2017). Complete genome sequence of a bovine ephemeral fever virus JT02L strain in mainland China. Archives of Virology, 162(11), 3555–3558. 10.1007/s00705-017-3520-0 [DOI] [PubMed] [Google Scholar]
- Hall, T. A. (1999). A user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98. [Google Scholar]
- Jiang, H., Hou, P., He, H., & Wang, H. (2020). Cell apoptosis regulated by interaction between viral gene alpha 3 and host heterogeneous nuclear ribonucleoprotein K facilitates bovine ephemeral fever virus replication. Veterinary Microbiology, 240, 108510– 10.1016/j.vetmic.2019.108510 [DOI] [PubMed] [Google Scholar]
- Jukes, T. H., & Cantor, C. R. (1969). Evolution of protein molecules. In Munrow H. N. (Ed.), Mammalian Protein Metabolism (pp. 21–132). New York, NY: Academic Press. 10.1016/B978-1-4832-3211-9.50009-7 [DOI] [Google Scholar]
- Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7), 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar, R., Van Schalkwyk, A., Carulei, O., Heath, L., Douglass, N., & Williamson, A. L. (2020). South African bovine ephemeral fever virus glycoprotein sequences are phylogenetically distinct from those from the rest of the world. Archives of Virology, 165(5), 1207–1210. 10.1007/s00705-020-04568-9 [DOI] [PubMed] [Google Scholar]
- Pyasi, S., Sahu, B. P., Sahoo, P., Dubey, P. K., Sahoo, N., Byrareddy, S. N., & Nayak, D. (2020). Identification and phylogenetic characterization of bovine ephemeral fever virus (BEFV) of Middle Eastern lineage associated with 2018–2019 outbreaks in India. Transboundary and Emerging Diseases, 1–7. 10.1111/tbed.13531 [DOI] [PubMed] [Google Scholar]
- Theodoridis, A., Boshoff, S. E., & Botha, M. J. (1973). Studies on the development of a vaccine against bovine ephemeral fever. Onderstepoort Journal of Veterinary Research, 40(3), 77–82. [PubMed] [Google Scholar]
- Walker, P. J., & Klement, E. (2015). Epidemiology and control of bovine ephemeral fever. Veterinary Research, 46(124). 10.1186/s13567-015-0262-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, F., & Qiu, C. (2012). Phylogenetic relationships of the glycoprotein gene of bovine ephemeral fever virus isolated from mainland China, Taiwan, Japan, Turkey, Israel and Australia. Virology Journal, 9(268). 10.1186/1743-422X-9-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Data Availability Statement
The data that support the findings will be available in NCBI at https://www.ncbi.nlm.nih.gov/ under the following accession numbers; MW463337 and MW512963.


