Abstract
A Knowledge, Attitudes, and Practices (KAP) questionnaire was designed to collect information on farmers’ knowledge of ASF and their practices surrounding that could impact the spread of the disease. The questionnaire was distributed, and data collected, from 233 backyard farmers from five selected Oblasts (Rivne, Kharkiv, Odessa, Zakarpattia and Kiev). Kruskal‐Wallis tests were conducted to identify factors that could influence knowledge, and Dunn tests were performed to determine differences between groups when the Kruskal‐Wallis tests were significant. Spearman tests were carried out to explore the association between knowledge and risky practices. Results show that comprehensive knowledge on ASF is not common in backyard farmers and that risky practices that influence the spread of ASF are regularly performed. Of the respondents, 47% felt well‐informed about how ASF can be transmitted and 31.8% felt confident about recognizing clinical signs of ASF. The independent variable “Oblast” was identified as a significant factor (p = 0.0015) associated with differences in knowledge on clinical signs. We demonstrated statistically significant differences of knowledge between backyard farmers from different Oblasts. Knowledge of preventive measures was positively correlated with risky handling practices related to edible pork products (p = 0.0053) and non‐edible pork products (p = 0.0417). In conclusion, our results show that backyard farmers have knowledge gaps on ASF and practice various risky behaviours that might favour the spread of the disease in Ukraine. There are regional differences in ASF knowledge and risky practices that should be taken into consideration in future evidence‐based ASF prevention and control programs, including public awareness activities.
Keywords: ASF, attitudes and practices, backyard farmer, knowledge, Ukraine
A Knowledge, Attitudes and Practices survey to backyard farmers in Ukraine was conducted to support control programs on African Swine Fever in the country. Results show that risky practices that influence the spread of ASF are regularly performed in backyard systems in Ukraine and that farmers would benefit from public outreach activities on ASF.

1. INTRODUCTION
African swine fever (ASF) is a viral, infectious, and notifiable swine disease with a very high case fatality rate (Galindo & Alonso, 2017; Guinat et al., 2016). The disease was detected in Ukraine in 2012 in a backyard farm. The outbreak was brought under control and no further ASF spread was reported during the following 2 years (Cwynar et al., 2019). ASF cases were confirmed in wild boar (EFSA, 2014) in 2014 and since then, additional ASF outbreaks in domestic pigs and wild boars have been reported throughout the country on a regular basis despite efforts from the authorities to control the disease (EFSA, 2014; FAO, 2013; Kovalenko et al., 2019).
ASF virus (ASFV) can be transmitted to domestic pigs and wild boar through direct contact with infected animals, contaminated fomites, and through the ingestion of contaminated meat products (Chenais et al., 2019). The virus is highly resistant to environmental conditions and can persist in contaminated fomites and meat products for several months, contributing to the disease spread and the risk of transmission through the contaminated commodities movement (Chenais et al., 2019; Mazur‐Panasiuk et al., 2019).
Currently, there is no specific treatment or available vaccine against ASF (Chenais et al., 2019; Galindo & Alonso, 2017) and, therefore, increased awareness of farmers and adequate biosecurity measures are currently the primary tools to protect pig herds from infection (Jurado et al., 2018). Formal biosecurity plans are generally focused on commercial farms or holdings that exceed a specific size defined by the national legislation of each country (Bellini, 2018). These plans are often unsuitable or impractical for very small and non‐commercial farms (Bellini, 2018; Bellini et al., 2016). Consequently, the ASFV introduction into small and non‐commercial farms is generally due to poor biosecurity standards and the movement of contaminated fomites and/or products (Chenais et al., 2019; Costard et al., 2015). These factors are relevant in backyard systems that employ risky management practices, such as swill feeding, local farmers offering butcher services in the area or engaging in pork trade, and emergency sales of animals potentially exposed to ASFV (Bellini, 2018; Chenais et al., 2019; Costard et al., 2015); circumstances that likely contribute to the ASFV establishment in this vulnerable production system (Khomenko et al., 2013) and promote the disease spread (Bellini, 2018).
The pig population in Ukraine is estimated at 8.2 million pigs, from which 56% are raised under low or non‐existent biosecurity conditions (FAO, 2013). According to Ukrainian law, backyard farms are defined as family farms whose products are used for subsistence within the household and should be registered in an official database (Ministry of Agriculture Policy and Food of Ukraine, 2003) to facilitate animal disease control. However, it is very likely that not all backyard farms are registered, a stance that is also common in other countries (EFSA 2019). Countrywide, the number of backyard farms is estimated at several hundred thousand but the proportion and density of pig farms with low biosecurity conditions predominates in the western part of the country (EFSA 2014; FAO 2013). The Ukrainian State Service on Food Safety and Consumer Protection (FSCP) estimated that there are approximately 230,000 backyard farms Ukraine's Zakarpattia Oblast (western), more than 180,000 in Rivne Oblast (northwestern) and roughly 6,000 in Kharkiv Oblast (east)(16).
The Ukrainian government's activities concerning ASF early detection (i.e., active surveillance in domestic pigs) were recognised as limited in a risk assessment conducted by the EFSA, which identified ongoing practices of swill feeding and the movement of infected pigs between backyard farms as two main factors that could result in the ASF spread in Ukraine (EFSA, 2014). The Ukrainian government establish a new control policy for the ASF prevention and control in the country (Ministry of Agriculture Policy and Food of Ukraine, 2017), which includes management measures to prevent the introduction of ASFV in farms, compulsory notification of suspected cases to the veterinary authorities, and control measures in case of an outbreak (Ministry of Agriculture Policy and Food of Ukraine, 2017).
Following the ASF detection in Ukraine in 2012, activities have been implemented countrywide to raise awareness among workers in the pig production sector including backyard farmers of ASF clinical symptoms and transmission pathways, adequate biosecurity measures and the importance of reporting cases (ASF Vet Ukraine, 2019; De Nardi et al., 2017). However, the ASF knowledge of backyard farmers in Ukraine, their attitude towards the disease and their behavioural practices relevant for ASF introduction and spread have never been assessed.
Knowledge, attitudes and practice (KAP) surveys can be used in disease control programs to identify misconceptions, misunderstandings and behaviours that might constitute barriers in the implementation of interventions (Chenais et al., 2017; Krentel et al., 2006; Matibag et al., 2007). In the context of disease control, KAP surveys are based on the assumption that knowledge leads to behaviour and practices against disease and thus, to changes that lessen the disease impact (Ahmed et al., 2020; Mascie‐Taylor et al., 2003). Therefore, a better understanding of the KAP of stakeholders towards a disease can help to identify if awareness campaigns could be necessary or if stakeholders are likely to comply with measures to reduce the disease spread. KAP surveys have been used to evaluate KAP of ASF (Chenais et al., 2017; Dione et al., 2020), to help design a program for rabies control (Matibag et al., 2007), to evaluate the impact of a communication campaign on filariasis (Krentel et al., 2006) and to assess the success of disease control and education programs for dengue (Koenraadt et al., 2006; Winch et al., 2002).
In this study, we aimed to investigate the KAP of backyard pig farmers concerning ASF and to assess if ASF knowledge is associated with demographic factors or risky practices related to the spread of the disease. Our hypothesis is that poor knowledge on ASF may be associated with the implementation of risky practices in the backyard farm sector.
2. MATERIALS AND METHODS
2.1. Study area
This study was performed in five Oblasts in Ukraine (Rivne, Kharkiv, Odessa, Zakarpattia and Kiev). An Oblast is an administrative region, of which there are a total of 24 in the country (EP, 2014). The selective Oblasts have the following geographic extension, Rivne with 20,100 km2 (Kupets, 2012), Kharkiv with 31,400 km2 (Ministry of Foreign Affairs of Ukraine, 2021), Odessa with 33,300 km2 (Ministry of Foreign Affairs of Ukraine, 2021), Zakarpattia with 12,800 km2 (Ministry of Foreign Affairs of Ukraine, 2021) and Kiev with 28,400 km2 (Ministry of Foreign Affairs of Ukraine, 2021). Selected Oblasts had reported ASF outbreaks in both domestic pigs and wild boars during the period 2014–2019 (Figure 1). and were selected because these regions reported the highest density of ASF outbreaks in domestic pigs and ASF cases in wild boars in Ukraine from 2017 to 2018 (DTRA, 2018). The total number of ASF outbreaks in 2017–2018 was 31 in Odessa, 25 in Rivne, 21 in Kakarpattia and 14 in Kyiv (DTRA, 2018).
FIGURE 1.
Map of Ukraine representing the study Oblasts where the Knowledge, Attitudes and Practice on ASF was conducted
2.2. KAP survey and data collection
A questionnaire was designed to collect data from backyard farmers regarding demographics, characteristics of the backyard pig farms, farmers’ knowledge of ASF, and their perspectives on reporting suspected ASF cases. The questionnaire comprised 31 questions in total, including five questions on demographics, 10 questions on characteristics of the backyard pig farm, four questions on knowledge of ASF, six questions on the suspicion of an ASF case, five questions on reporting a suspected ASF case, and one last question to self‐grade respondents’ honesty in answering. In total, the questionnaire contained 21 single‐select multiple choice questions and 10 multi‐select multiple choice questions. A single‐select multiple choice question was defined as a question in which only one option could be chosen from a set of possible answers, and a multiple‐select multiple choice question was defined as a question in which multiple or all applicable options could be chosen. The questionnaire was first drafted in English and then translated into Ukrainian by a professional translator. The pre‐final questionnaire was internally piloted by collaborators of the Institute of Veterinary Medicine (IVM), and the resulting feedback was integrated into the final version. The final version of the questionnaire in English is available in Appendix S1.
The questionnaires were delivered between September and December 2019 by project collaborators from two Ukrainian research institutions, IVM and the Institute of Experimental and Clinical Veterinary Medicine (IECVM). Staff from IVM delivered questionnaires in Kyiv, Rivne, and Zakarpattia Oblasts, while staff from IECVM delivered questionnaires in Kharkiv and Odessa Oblasts. Interviewers from both institutions received instructions from the team leaders involved in the project on how to administer the questionnaire, approach participants and on interview techniques.
2.3. Selection of participants
A complete list of pig backyard farmers per Oblast in Ukraine does not exist and therefore we lacked this source of information to draw our sample. A sample size of 250 respondents (50 per Oblast) was targeted. This sample size was determined without conducting statistical sample calculations but it was both considered to be a sufficient number to make inferences on the source population and a feasible number considering the available human resources for conducting interviews and time period.
Backyard farmers were approached by the interviewers either randomly at street markets and backyard farms located in convenient villages that were on the road map or based on the advice of the local veterinarians’ and pre‐existing contacts. Participants were asked if they had a pig backyard farm and only after a positive answer, consent for participation was requested. Participants were informed that participation in the study was completely voluntary that unwillingness to participate would not have negative consequences and that they could withdraw their consent at any time.
Following their consent to participate, the questionnaire was delivered. Interviewers were instructed to find a suitable place to conduct the interview undisturbed. In some cases, notebooks and bags with the logo of the institutions were given to respondents as a “thank you” for participating. The questionnaire was delivered through face‐to‐face interviews, took approximately 45–60 min and responses were noted on paper.
Before initiating each interview, a confidentiality statement was presented by the interviewers informing that personal data obtained in connection with the study would remain confidential.
2.4. Data management and data cleaning
All data were entered into a Microsoft Excel® spreadsheet. Guidelines on how to transfer the data to the spreadsheet were developed to standardize data entry into the electronic database. Upon data entry, all information was translated into English. Data cleaning was performed to check for misspellings and inconsistencies.
Answers for multiple‐select multiple choice questions were considered invalid and removed from the database if within the same question both the answer option “none of these” and other options were selected. In total, 37 responses were excluded.
2.5. Data analysis
The electronic database was analyzed in Stata® (StataCorp, 2017). First, a descriptive analysis of all questions was conducted. Then, questions related to specific topics were selected for further analysis. These specific topics were knowledge of ASF, demographics, activities and management practices related to feeding pigs and handling of edible and non‐edible pork products.
2.5.1. Knowledge of ASF
Questions 17–19 in the KAP questionnaire were used to assess knowledge of clinical signs, transmission pathways, and preventive measures of ASF. For each of these multiple‐select multiple choice questions, a set of answer options reflected correct knowledge (checked answers options in Supporting Information S1), while other options revealed incorrect knowledge or knowledge gaps. For the question concerning clinical signs of disease, nine out of 12 options were considered correct responses. The three other answer options were “vesicles around the tongue and lips”, “lameness”, and “I do not know any signs”. For the question on transmission pathways, nine out of 11 answer options were deemed correct. The two others were “through sexual contact” and “I do not know any pathways”. For the question on preventive measures, seven out of 11 options were considered adequate preventive measures. The three others were “provision of a salt block”, “vaccination,” and “I do not take any measures”. The option “other” for this last question had an empty space for respondents to provide further information. Only two respondents selected this option and added more details. These additional details were not further analysed.
2.5.2. Factors influencing knowledge of ASF
A Kruskal‐Wallis rank test was performed to assess if backyard farmers’ knowledge of ASF was correlated with selected demographic factors. For this, the number of selected answers from the three knowledge questions discussed above were identified as dependent variables, whereas Oblast, age, and educational level were selected as independent variables. A Dunn test was conducted as a post hoc test when results of the Kruskal‐Wallis tests were statistically significant (p < 0.05) to check which specific groups were significantly different using rank sums.
2.5.3. Association between knowledge of ASF and risky practices
Spearman correlation tests were performed to understand if there was a correlation between ASF knowledge, risk pathways and preventive measures, and application of risky practices that could play a role in the ASF spread. These risky practices were related to commercial activities carried out by members of the household, pig feeding practices, and handling practices of edible and non‐edible pork products after slaughtering a pig.
Regarding the activities carried out by members of the household, six out of seven options were considered risky practices. The only non‐risky option was “no member of the household carries out any of the aforementioned activities”. Concerning pig feeding practices, five out of seven options were considered risky practices. Only the options “I use commercial feed for pigs” and “Other” were considered non‐risky practices. In relation to the handling practices of edible pork products, four out of six options were considered risky practices. “Consumption within my household” and “Other” were considered non‐risky practices. For non‐edible pork products, four out of six options were identified as risky practices. The two non‐risky practices were “dispose of them at official dump” and “Other”. For all four questions, participants had the opportunity to include additional information when selection “other”. This additional information was not further analysed.
3. RESULTS
A total number of 233 backyard farmers from five Oblasts of Ukraine (Kharkiv [n = 36], Kyiv [n = 50], Odessa [n = 47], Rivne [n = 50], and Zakarpattia [n = 50]) were interviewed. The demographic characteristics of respondents are presented in Table 1. The predominant age category was 36–50 years old (41.6%, 96/231), and the most prevalent educational level among respondents was technical school (30.6%, 71/232). None of the respondents declared having primary school or post‐graduate level as the highest education level. Half of the respondents (51.3%, 118/230) declared to be the main person in taking care of the pigs and 37.4% (86/230) mentioned that another person living in the same household was the main person taking care of the pigs (Table 1). The large majority of all respondents (87.2%, 203/233) had up to five pigs. Full characteristics of backyard farms are available in Appendix S2.
TABLE 1.
Demographic characteristics of respondents to the KAP questionnaire
| Variable | Category | Number | Frequency (%) |
|---|---|---|---|
| Oblast | Kharkiv | 36 | 15.5 |
| N † = 233 | Kyiv | 50 | 21.5 |
| Odessa | 47 | 20.2 | |
| Rivne | 50 | 21.5 | |
| Zakarpattia | 50 | 21.5 | |
| Age | ≤20 years old | 9 | 3.9 |
| N † = 231 | 21‐35 years old | 31 | 13.4 |
| 36–50 years old | 96 | 41.6 | |
| 51–65 years old | 67 | 29.0 | |
| ≥ 66 years old | 28 | 12.1 | |
| Role in taking care of the pigs | Main person in taking care of the pigs | 118 | 51.3 |
| N † = 230 | Another member of the household takes care of the pigs | 86 | 37.4 |
| An external person to the household takes care of the pigs | 14 | 6.1 | |
| A member of the household and an external person of the household take care of the pigs | 12 | 5.2 | |
| Highest education level | Primary school | 0 | 0 |
| N † = 232 | Secondary school | 44 | 19.0 |
| Technical school | 71 | 30.6 | |
| Advanced technical school | 60 | 25.9 | |
| University | 57 | 24.6 | |
| Post‐graduate | 0 | 0 |
N†: Number of valid answers.
3.1. Knowledge of ASF, attitudes on ASF, and confidence in recognizing suspect ASF cases
A total of 222 respondents (95.7%) declared having heard about ASF. Fourteen of 228 (6.1%) respondents had suspected ASF in their pigs at some point in time, and these respondents were mainly from Kharkiv (6/36, 16.7%) and Kyiv (3/46, 6.5%). Almost half of the respondents (104/221, 47.0%) felt well‐informed about how ASF can be transmitted and 31.8% (70/220) felt confident about recognizing clinical signs of ASF. The two Oblasts in which respondents felt best informed were Kharkiv with a total of 69.2% (18/26) respondents and Zakarpattia with 61.2% (30/49). The Oblasts in which respondents felt the most confident in recognizing clinical signs of ASF were Zakarpattia (31/49, 63.3%) and Kharkiv (10/25, 40.0%; Table 2).
TABLE 2.
Attitudes on ASF and confidence in recognizing suspect ASF cases by participants. Values in the table represents the “Yes” option
| Questions | Kharkiv (n, %) | Kyiv (n, %) | Odessa (n, %) | Rivne (n, %) | Zakarpattia (n, %) | Total positive answers (n, %) |
|---|---|---|---|---|---|---|
| Have you ever heard about ASF? (N† = 232) | 35 (100) | 48 (96) | 43 (91.5) | 48 (96) | 48 (96) | 222 (95.7) |
| Have you ever suspected that you may have ASF in your pigs? (N† = 228) | 6 (16.7) | 3 (6.5) | 1 (2.1) | 2 (4) | 2 (4.1) | 14 (6.1) |
| Do you feel confident that you can recognize the clinical signs of ASF? (N† = 220) | 10 (40) | 19 (38) | 2 (4.3) | 8 (16) | 31 (63.3) | 70 (31.8) |
| Do you feel you are well‐informed about how ASF can be transmitted? (N† = 221) | 18 (69.2) | 26 (52) | 9 (19.6) | 21 (42) | 30 (61.2) | 104 (47) |
N†: Number of valid answers.
Almost one fourth of the respondents were able to correctly identify four clinical signs of ASF (52/221 respondents, 23.5%), three transmission pathways (52/227 respondents, 22.9%), and three preventive measures (51/227 respondents, 22.5%) (Table 3). Furthermore, 14.5% (32/221) and 10.1% (23/227) of respondents respectively declared that they do not know any clinical signs or transmission pathways, and 12.3% (28/227) mentioned that they do not take any preventive measures (Table 3).
TABLE 3.
Number of clinical signs, risk pathways, and preventive measures of ASF identified by Ukrainian backyard farmers
| Clinical signs indicative of ASF | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of clinical signs mentioned | “I do not know any signs” | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Total† |
| Number of respondents | 32 | 2 | 24 | 28 | 32 | 52 | 30 | 12 | 4 | 4 | 1 | 221 |
| Percentage (%) | 14.5 | 0.9 | 10.9 | 12.7 | 14.5 | 23.5 | 13.6 | 5.4 | 1.8 | 1.8 | 0.5 | 100 |
| Transmission pathways of ASF | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of transmission pathways mentioned | “I do not know any pathways” | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Total† |
| Number of respondents | 23 | 1 | 30 | 31 | 52 | 27 | 19 | 17 | 12 | 8 | 7 | 227 |
| Percentage (%) | 10.1 | 0.4 | 13.2 | 13.7 | 22.9 | 11.9 | 8.4 | 7.5 | 5.3 | 3.5 | 3.1 | 100 |
| Preventive measures of ASF | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of preventive measures mentioned | “I do not take any measures” | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Total† | ||
| Number of respondents | 28 | 4 | 32 | 43 | 51 | 32 | 25 | 8 | 4 | 227 | ||
| Percentage (%) | 12.3 | 1.8 | 14.1 | 18.9 | 22.5 | 14.1 | 11 | 3.5 | 1.8 | 100 | ||
: Number of valid answers.
The clinical signs indicative of ASF that were selected most frequently were “high mortality” (147/221, 66.5%), “reddening or darkening of the skin” (132/221, 59.7%), and “fever” (131/221, 59.3%). “Vesicles around the tongue and lips” and “lameness”, which had been categorized as non‐indicative ASF clinical signs, were selected by 9.1% (20/221) and 7.2% (16/221) of respondents, respectively (Table 4). The transmission pathways that were selected most frequently were “through direct contact with a diseased pig or carcass of a diseased pig” (183/227, 80.6%), “through direct contact with diseased wild boar or carcass of a diseased wild boar” (155/227, 68.3%) and “through contact with contaminated clothing, footwear and/or transport vehicles” (105/227, 46.3%). The transmission pathway that is not applicable to ASF, “through sexual contact”, was selected by 21.1% (48/227) of respondents (Table 4). In the case of preventive measures, respondents reported that their main preventive measures are “no outside access of pigs” (143/227, 63.0%), “fencing” (137/227, 60.4%) and “no introduction of new pigs in the farms from non‐commercial farms” (76/227, 33.5%). “Vaccination” and “provision of a salt block”, both preventive measures that are not relevant for ASF, were selected by 21.6% (49/227) and 2.6% (6/227) of respondents, respectively (Table 4).
TABLE 4.
Summary of knowledge questions related to clinical signs, risk pathways, and preventive measures on ASF
| Which of the following clinical signs do you associate with ASF in pigs? N† = 221 | |||||
|---|---|---|---|---|---|
| Answer | Number | Percentage (%) | Answer | Number | Percentage (%) |
| Fever | 131 | 59.3 | Vomiting | 27 | 12.2 |
| Diarrhoea | 36 | 16.3 | Lethargy | 100 | 45.3 |
| Vesicles around the tongue and lips | 20 | 9.1 | Difficulty to breathing | 53 | 24.0 |
| Lameness | 16 | 7.2 | Stillborn or weak piglets | 18 | 8.1 |
| Reddening or darkening of the skin | 132 | 59.7 | Nervous signs | 31 | 14.0 |
| High mortality | 147 | 66.5 | I do not know any signs | 32 | 14.5 |
| Through which of the following pathways can pigs get infected with ASF? N† = 227 | |||||
|---|---|---|---|---|---|
| Answer | Number | Percentage (%) | Answer | Number | Percentage (%) |
| Through direct contact with a diseased pig or carcass of a diseased pig | 183 | 80.6 | Through a bite of an infected tick | 38 | 16.7 |
| Through direct contact with diseased wild boars or carcass of a diseased wild boar | 155 | 68.3 | Through use of contaminated surgical equipment | 32 | 14.1 |
| Through consumption of kitchen waste | 51 | 22.5 | Through sexual contact | 48 | 21.1 |
| Through consumption of leftovers from the slaughter process | 83 | 36.6 | Through airborne transmission | 42 | 18.5 |
| Through contact with contaminated manure | 75 | 33.0 | I do not know any pathways | 23 | 10.1 |
| Through contact with contaminated clothing, footwear and/or transport vehicles | 105 | 46.3 | |||
| Which preventive measures do you take to protect your pigs against ASF? N† = 227 | |||||
|---|---|---|---|---|---|
| Answer | Number | Percentage (%) | Answer | Number | Percentage (%) |
| No exchange of feed or bedding with other backyards | 106 | 46.7 | My pigs are not allowed to roam around freely outside of my premise | 143 | 63.0 |
| Quarantine period for new animals in a separate room | 61 | 26.9 | Using only commercial pig feed | 20 | 8.81 |
| Provision of a salt block | 6 | 2.6 | Disinfecting and cleaning the areas around the backyard | 69 | 30.4 |
| Vaccination | 49 | 21.6 | My entire premise is fenced | 137 | 60.4 |
| No introduction of pigs from non‐commercial farms | 76 | 33.5 | I do not take any measures | 28 | 12.3 |
N†: Number of valid answers.
3.2. Risky practices related to ASF spread
A total of 92.9% (210/226) of respondents mentioned performing home slaughter of their pigs, and only 7.1% (16/226) slaughter their pigs at the slaughterhouse. Among respondents that carried out home slaughter, 85.2% (179/210) slaughtered without veterinary inspection and 14.8% (31/210) with veterinary inspection. The most important economic activity that was mentioned by the respondents was carrying out slaughter and butchery services for the private sector (155/227, 68.3%) and only 9.3% of the households (21/227) did not report any potentially risky economic activity (Figure 2a). The most important pig feeding practices were a farmer's own production of pig feed (165/233, 70.8%), use of kitchen leftovers (125/233, 53.6%) and use of commercial pig feed (61/233, 26.2%) (Figure 2b). Following slaughter of one's own pigs, the most common uses of edible pork products are consumption within the household (200/231, 86.6%) and giving it to neighbours and/or relatives (72/231, 31.2%). Various trading activities of edible pork products, including selling to the local butcher, to traders and to the market in their village or a neighbouring village were mentioned by 91/231 (39.4%) of respondents (Figure 2c). The two most common handling practices of non‐edible pork products were the disposal in a composting pit for organic use (120/224, 53.6%) and the use as feed (76/224, 34.0%) (Figure 2d). The number of transmission pathways and preventive measures of ASF mentioned by backyard farmers by Oblast is available in Appendix S3.
FIGURE 2.
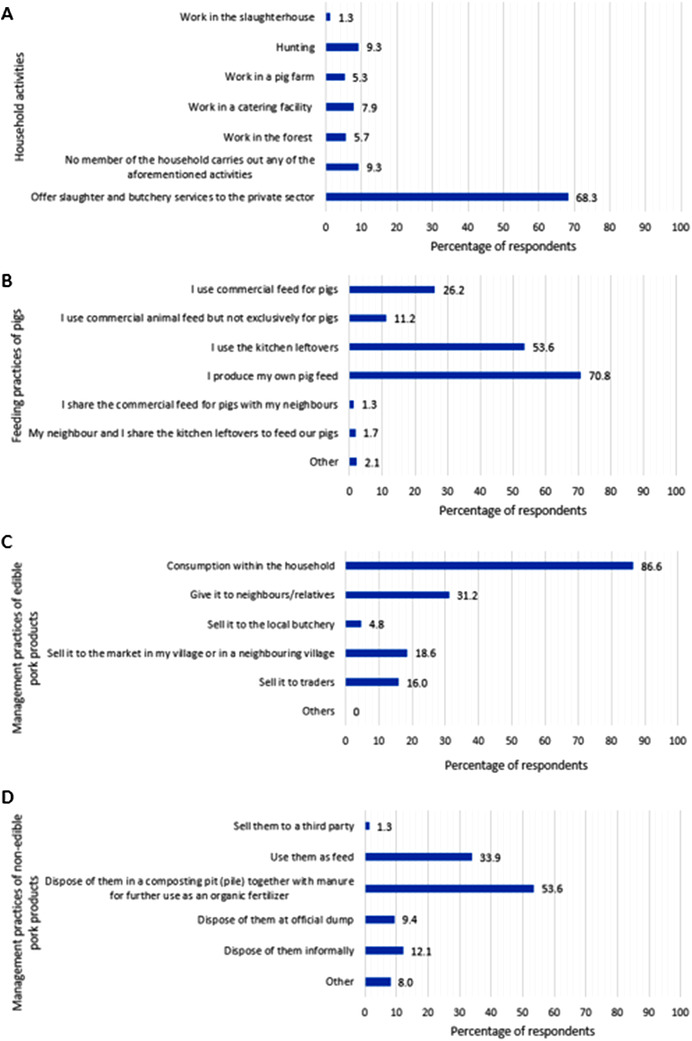
Activities carried out by any members of the household (a). Feeding habits of pigs (b). Management practices of edible food products after slaughtering the pigs (c). Management practices of non‐edible food products after slaughtering the pigs (d)
3.3. Factors influencing knowledge of ASF
Using the Kruskal‐Wallis test we tested influence of the explanatory variables education, oblast and age on the knowledge of the respondents on clinical signs, risk pathways and preventive measures. There was only a statistically significant difference in the knowledge of clinical signs between backyard farmers from different Oblasts (p = 0.0032; Table 5). The Dunn's tests showed that backyard farmers from Zakarpattia (p = 0.0026) and Rivne (p = 0.0094) had a higher knowledge of clinical signs than backyard farmers from Kharkiv. Similarly, backyard farmers from Rivne (p = 0.0034), Odessa (p = 0.0349), and Zakarpattia (p = 0.0007) had a statistically significant higher knowledge of clinical signs than backyard farmers from Kyiv. All results of the Dunn tests are presented in Table 6.
TABLE 5.
Output of the Kruskal‐Wallis tests, assessing knowledge of clinical signs, risk pathways, and preventive measures
| Response variable | Explanatory variables | Number of observations | Rank Mean | Chi‐squared | P‐ value |
|---|---|---|---|---|---|
| Clinical signs | Educational level | 220 | 20052.5 | 1.648 | 0.6485 |
| Oblasts | 221 | 4906.2 | 15.845 | 0.0032* | |
| Age | 219 | 4818.0 | 8.169 | 0.0856 | |
| Risk pathways | Educational level | 226 | 6412.6 | 5.517 | 0.1377 |
| Oblasts | 227 | 5175.6 | 6.979 | 0.1370 | |
| Age | 226 | 5130.2 | 2.868 | 0.5801 | |
| Preventive measures | Educational level | 226 | 6412.8 | 6.342 | 0.0961 |
| Oblasts | 195 | 5175.6 | 5.430 | 0.2460 | |
| Age | 225 | 5085.0 | 7.535 | 0.1102 |
P < 0.05, statistically significant.
TABLE 6.
Pairwise group comparisons using Dunn's test, following statistically significant results in the Kruskal‐Wallis test (Table 5)
| Kharkiv | Kyiv | Odessa | Rivne | ||
|---|---|---|---|---|---|
| Kyiv | Difference in rank sum | 0.092889 | – | – | – |
| p‐Value | 0.4630 | – | – | – | |
| Odessa | Difference in rank sum | ‐1.556525 | ‐1.813093 | – | – |
| p‐Value | 0.0598 | 0.0349* | – | – | |
| Rivne | Difference in rank sum | ‐2.350705 | ‐2.710085 | ‐0.795503 | – |
| p‐Value | 0.0094* | 0.0034* | 0.2132 | – | |
| Zakarpattia | Difference in rank sum | ‐2.790802 | ‐3.198099 | ‐1.266808 | ‐0.490549 |
| p‐Value | 0.0026* | 0.0007* | 0.1026 | 0.3119 |
p < 0.05, statistically significant.
There was a positive correlation between the knowledge of preventive measures and risky handling practices related to edible pork products (p = 0.0053) and non‐edible pork products (p = 0.0417; Table 7). This means that, with increased knowledge, more risky practices are performed.
TABLE 7.
Output of the Spearman tests, assessing the association between knowledge of ASF and risky practices
| Variables | Activities in the household | Feeding habits to the pigs | Management of edible pork products | Management of non‐edible pork products | |
|---|---|---|---|---|---|
| Number of correct answers of risk pathways | Correlation coefficient | 0.059 | 0.035 | 0.054 | 0.114 |
| p‐Value (2‐tailed) | 0.406 | 0.601 | 0.420 | 0.082 | |
| Number of correct preventive measures | Correlation coefficient | 0.040 | 0.058 | 0.185 | 0.144 |
| p‐Value (2‐tailed) | 0.572 | 0.385 | 0.005* | 0.042* |
p < 0.05, statistically significant.
4. DISCUSSION
Our study showed that comprehensive ASF knowledge is not very common among participating backyard pig farmers in the five Oblasts where this study was conducted. Furthermore, results indicated that risky practices, which are known to influence the spread and ASF persistence, are regularly performed in backyard holdings. This is a known challenge, as surveys in other countries also demonstrated that frequently, there is a lack of comprehensive disease knowledge among backyard farmers (Çakmur et al., 2015; Kumar et al., 2013). The findings of this study show that although the name of the disease is familiar, there is a clear lack of comprehensive understanding of how to identify, prevent, and control ASF. These knowledge gaps exist despite the implementation of various trainings and workshops for promoting awareness since the ASF introduction in 2012 into Ukraine (ASF Vet Ukraine, 2019; De Nardi et al., 2017).
Our findings showed that the “Oblast” category was a main factor associated with a difference in ASF knowledge among respondents. Generally, backyard farmers from Kharkiv and Kyiv had a lower general ASF knowledge. Respondents from Zakarpattia and Rivne had broader ASF knowledge. The differences in knowledge between respondents from different Oblasts could be linked to differences in the ASF epidemiological situation and the time gap from the last outbreak. The rationale behind this logic is that farmers that were more exposed to the disease could be also more familiar with it and therefore, have a higher level of knowledge. However, during the period 2012–2018, a similar number of outbreaks in backyard farms were reported in these four Oblasts (25 in Kharkiv, 22 in Zakarpattia, 31 in Rivne and 22 in Kyiv) (DTRA, 2018), which may imply that other factors such as the ones discussed below, which distant from direct exposure to the disease affect the level of ASF knowledge among the respondents of this study.
Interestingly, despite feeling well informed and having high confidence, respondents from Kharkiv showed one of the lowest ASF knowledge level as discussed above. This discrepancy between a high level of confidence among the respondents in Kharkiv and their low scores on ASF knowledge is difficult to explain and should be further investigated. In psychology a similar conflict, still under study, is described as the Dunning‐Kruger effect (Coutinho et al., 2021; Kumar et al., 2013). This effect is a cognitive bias influencing people's behaviour: people with limited knowledge on a topic are more likely to overestimate their abilities (Coutinho et al., 2021). If this holds true in the current study, this cognitive bias could prevent people to overcome deficits in knowledge hampering to some extent the efforts to control the disease (Coutinho et al., 2021). However, additional plausible factors can be found in the effectiveness of awareness strategy. It is possible that the awareness activities implemented in Ukraine were not able to achieve its intended impact, for example due to a suboptimal delivery method, or in the formulation of the key messages or in other key aspects like a proper environment for learning. We lack information on how trainings have been delivered in Ukraine. However, a study conducted by Dione et al. (2020), evaluated a participatory training on KAP of biosecurity related to ASF control in smallholder pig farmers in Uganda, showing a significantly improvement of farmers’ knowledge in biosecurity (Dione et al., 2020). This result emphasizes the need for a regular evaluation of awareness strategies.
No relevant preventive measures for ASF such as “vaccination”, was selected by 21.6% of the respondents. These results may be due to respondents might get confused with Classical Swine Fever (CSF), a disease that also affects domestic pigs and wild boars and for which there is a vaccine (OIE, 2021). CSF was eradicated in Ukraine in 1997, after mass vaccination in domestic pigs (Nevolko et al., 2015). However, sporadic cases are occasionally still reported in wild boars, posing a risk of recurrence in domestic pigs (Nychyk et al., 2018). Therefore, it is plausible that respondents might be mistaken between ASF and CSF when selecting “vaccination” as a preventive measure for ASF. However, other possible explanation is that farmers did not know that ASF vaccine does not exist when selected this answer option and therefore, have a gap knowledge.
Trading of edible pork products following the slaughter of backyard pigs were mentioned by 39.4% of respondents. These trading activities included selling to the local butcher, to traders, and to the market in their village or a neighbouring one (Figure 2c). The performance of these activities highlights the economic importance that backyard pigs represent for backyard farmers and also underscores the possibility for geographic spread of potentially contaminated pork products (EFSA, 2014; FAO, 2018). Interestingly, our results showed a positive correlation between ASF knowledge regarding preventive measures and risky practices related to edible and non‐edible pork products, indicating that even a certain awareness level on ASF preventive measures may not prevent the performance of risky practices with regard to pork products. This could be driven by the fact that enforcement actions by the authorities may be limited, and thus expected consequences for farmers are low. Results from a study conducted in Uganda showed similar results, smallholder pig farmers had a good knowledge of ASF and their control measures but this did not ensure its implementation (Chenais et al., 2017). The authors suggested that initiatives on control measures should include a financial return component to engage with farmers and compensate for the economic loss (Chenais et al., 2017). In Ukraine, although ASF control measures seem to be in compliance with the program planned, socioeconomic factors are seen as a major challenge to drive behaviour change (EC, 2018).
Backyard pig farming represents 56% of the pigs in Ukraine (FAO, 2013). Low biosecurity backyard farms have been identified to play a role in the spread and persistence of ASF in Eastern European countries (EFSA, 2010), which illustrates the significant challenge that Ukraine is facing for ASF control. Further, backyard farms could facilitate the introduction of ASFV from wild boar to domestic pigs (Guinat et al., 2016). Previous studies of the main risk factors for ASFV spread among backyard farms included movement of infected pork meat, swill feeding, underreporting, and “emergency sales” (Costard et al., 2015). Our results indicate the widespread presence of two of these risk factors in the Ukrainian pig backyard farming system, namely movement of infected pork meat and swill feeding.
Main pigs feeding practices mentioned included their own production of pig feed (70.8%) and the use of kitchen leftovers (53.6%). The performance of these practices can facilitate the spread of the virus in healthy pigs (Bellini et al., 2016) as happened in 2012 when ASF was introduced in Ukraine due to the use of contaminated pork products in swill feeding in small farms (EFSA, 2019). Our questionnaire did not include any question about the heat treatment practice (60°C for 30 min) of swill feeding, which would inactive any ASFV present in the swill (FAO‐EMPRESS, 2021; Plowright & Parker, 1967), but leaders from FSCP and from Kharkiv Oblast mentioned that no more than 5% of backyard farmers perform heat treatment of kitchen leftovers (FSCP, 2020). It is therefore considered unlikely that adequate heat treatment of kitchen leftovers (heating sufficient to inactivate ASFV) is practiced widely in the Ukrainian backyard farms.
One way to strengthen ASF early detection in backyard holdings is through the supervision of home slaughtering by veterinary services (Bellini et al., 2016). A veterinarian is more likely to notice clinical symptoms of ASF in pigs presented for slaughter and may need to overcome fewer barriers to report suspected cases, which is the starting point for any official outbreak response measures. Our results showed that among our respondents, approximately 80% of the backyard pigs are slaughtered without veterinary supervision, jeopardizing the identification and reporting of ASF cases. According to Ukrainian law, home slaughtering must be performed under the supervision of an official veterinarian and the sale and consumption of pork products produced without veterinary inspection during slaughter are not allowed (Ministry of Agriculture Policy and Food of Ukraine, 2004). Our results suggest that compliance of backyard farmers with the compulsory veterinary inspection during home slaughter is low. This may be due to the waiting time for veterinary inspections stemming from the logistics involved in reaching remote backyard farms or due to the lack of sufficient numbers of veterinarians in rural areas. It may also be related to the lack of backyard farmers’ understanding of the importance of veterinary inspections during slaughter processes for animal health and food safety purposes or to the costs associated with veterinary inspections. Further, backyard farmers possibly fear the economic loss that will be incurred if the official veterinarian suspects ASF and the herd needs to be culled for preventive and control measures (Ministry of Agriculture Policy and Food of Ukraine, 2017).
Public awareness programs are part of the ASF contingency plan (FAO, 2009). These activities can help in recognising the ASF incursion before the disease is widely spread, also in ensuring compliance with control measures (FAO, 2009) and in promoting ownership by farmers in animal disease control (FAO, 2001). As an example, Hasanov et al. (2018) demonstrated that awareness campaigns for households were effective in raising knowledge on rabies and were a cost‐effective method to increase vaccination coverage of domestic dogs by reminding dog owners with good knowledge to have their dogs vaccinated. The increase of rabies vaccination in dogs would ultimately be beneficial for dog owners through the reduction of the burden of rabies in both the human and dog population (Hasanov et al., 2018).
Our study provided insights regarding the behaviour and ASF knowledge among backyard pig farmers in Ukraine, but it also has several limitations. A first limitation is related to the sample size and sampling strategy. The sample size was arbitrarily determined based on what was realistic to perform in the available time period rather than based on a formal sample size calculation. While this is not the preferred method, it has been used in other studies as well in situations where little was known about the target population and resources were limited (Ansari‐Lari et al., 2010; Guinat et al., 2016; Lei et al., 2019). We used convenience sampling for the selection of interviewees. The implementation of an alternative sampling approach was not feasible in the absence of a formal register of backyard farms and with the limited available resources of the project. The use of convenience sampling may have brought selection bias of participants and mobility bias as participants were selected in conveniently located villages. This would reduce the validity of extrapolation of our findings to the larger population of backyard farmers. However, we believe that through the inclusion of different geographic regions of the country in the study design, we were able to mitigate this limitation. A third limitation is that we did not gather information in the questionnaire on the gender, which would have allowed us to shed light on the role that gender plays in households regarding backyard pig farming. Fourth, we did not record formally how many people rejected to participate in the study. This would have allowed us to assess the receptivity of this study in our target population. Feedback from the interviewers however did not indicate that there was an unwillingness to participate among backyard farmers who were approached. Fifth, our questionnaire was relatively long with 31 questions, which may have affected the validity of the answers towards the end of the questionnaire (Helgeson & Ursic, 1994; Herzog & Bachman, 1981). However, our questionnaire mostly contained closed questions, which are less cognitively demanding than open‐ended questions (Connor Desai & Reimers, 2019). Also, the length of the interviews was within the average for a face‐to‐face semi‐structured interview (DiCicco‐Bloom & Crabtree, 2006). Finally, the interviews were conducted by multiple interviewers, creating a potential risk of lack of standardization (Aamodt MGEGB and EJK, 2006). This challenge was resolved through the provision of trainings and guidelines to interviewers on the importance of standardized information and data collection.
Considering the high number of backyard farmers in Ukraine, they are important actors in national efforts to prevent and control the ASF spread. To our knowledge, this is the first ASF KAP survey carried out among backyard farmers in Ukraine and therefore our study provides important insights despite its limitations. Our study provided insights into the ASF knowledge of backyard farmers and helped to identify predominant risky practices that are regularly carried out by farmers. These insights help to better understand the role of backyard farmers in the ASF epidemic in Ukraine and to inform future evidence‐based policies, including the development of new and adjusted public awareness activities. Also, these results can be used as a baseline to determine the impact of awareness activities after targeted activities.
5. CONCLUSION
Backyard pig farmers in Ukraine have important gaps in their ASF knowledge and practice various risky behaviours that might favour the spread of ASF virus. There are regional differences in ASF knowledge and risky practices that should be taken into consideration for the design and implementation of more informed ASF prevention and control programs, including public awareness activities.
AUTHOR CONTRIBUTIONS
Violeta Muñoz was associated with study conceptualization; data curation; formal analysis; methodology; visualization; and wrote the original draft. Oleksii Solodiankin was associated with investigation and reviewed and edited the manuscript. Nataliia Rudova was associated with investigation and reviewed and edited the manuscript. Anton Gerilovych was associated with investigation and reviewed and edited the manuscript. Serhiy Nychyk was associated with investigation and reviewed and edited the manuscript. Natalia Hudz was associated with investigation and reviewed and edited the manuscript. Tetiana Ukhovska was associated with investigation and reviewed and edited the manuscript. Mykola Sytiuk was associated with investigation and reviewed and edited the manuscript. David Mustra was associated with funding acquisition; project administration; and reviewed and edited the manuscript. Marco De Nardi reviewed and edited the manuscript. Isabel Lechner was associated with methodology and reviewed and edited the manuscript. Manon Schuppers was associated with study conceptualization; supervised the study; and reviewed and edited the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.578
Supporting information
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank the members involved from IVM (Larisa Muzykina, Svitlana Mandyhra, Ludmyla Ishchenko, Maksym Bezymennryi) and IECVM institutions (Yurii Dunayev, Olha Lymanska, Olena Kolchyk, Maryna Stegniy, Maxym Lohvynenko, Borys Stegniy, Olga Florova, Bogash Mikolay, Bogash Denis, Leonid Stoyanov), who participated in data collection and data transfer processes. We also would like to thank the professional translator for translating the questionnaire and facilitating communication between the institutions and to Martin Tušl for his support on the social science component. This work was funded by the U.S. Defense Threat Reduction Agency (DTRA) Biological Threat Reduction Program in Ukraine and the Cooperative Biological Research (CBR) project UP‐10 (“Regional Field‐to‐Table Risk Assessment of the spread of African swine fever virus (ASFV) across Ukraine in wild fauna and via consumer trade routes‐insight into the development of effective ASFV quarantine strategies and public policy”). The contents of this publication are the responsibility of the authors and do not necessarily reflect the views of DTRA or the United States Government. We thank members of the Black & Veatch Special Projects Corp science team for their assistance in facilitating the implementation of this study.
Muñoz‐Gómez, V., Solodiankin, O., Rudova, N., Gerilovych, A., Nychyk, S., Hudz, N., Ukhovska, T., Sytiuk, M., Polischuk, V., Mustra, D., De Nardi, M., Lechner, I., & Schuppers, M. (2021). Supporting control programs on African swine fever in Ukraine through a knowledge, attitudes, and practices survey targeting backyard farmers. Veterinary Medicine and Science. 7, 1786–1799. 10.1002/vms3.578
REFERENCES
- Aamodt MGEGB and EJK (2006). Do structured interviews eliminate bias ? A meta‐ analytic comparison of structured and unstructured interviews. In: Annual meeting of the Society for Industrial‐Organization Psychology. Dallas Texas; p. 1–9. [Google Scholar]
- Ahmed, T., Hussain, S., Zia, U. U. R., Rinchen, S., Yasir, A., Ahmed, S., Khan, W. A., Tahir, M. F., & Ricketson, R. (2020). Knowledge, attitude and practice (KAP) survey of canine rabies in Khyber Pakhtunkhwa and Punjab Province of Pakistan. Bmc Public Health [Electronic Resource], 20(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari‐Lari, M., Soodbakhsh, S., & Lakzadeh, L. (2010). Knowledge, attitudes and practices of workers on food hygienic practices in meat processing plants in Fars, Iran. Food Control, 21(3), 260–263. [Google Scholar]
- ASF Vet Ukraine (2019). African Swine Fever Ukraine‐ Projects and seminars.
- Bellini, S. (2018). Application of biosecurity in different production systems at individual, country and regional levels [Internet]. Available from: 10.20506/TT.2934 [DOI]
- Bellini, S., Rutili, D., & Guberti, V. (2016). Preventive measures aimed at minimizing the risk of African swine fever virus spread in pig farming systems. Acta Veterinaria Scandinavica, 58(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çakmur, H., Akoğlu, L., Kahraman, E., & Atasever, M. (2015). Evaluation of farmers’ knowledge‐attitude‐practice about zoonotic diseases in Kars, Turkey. Kafkas Journal of Medical Sciences, 5(3), 87–93. [Google Scholar]
- Chenais, E., Boqvist, S., Sternberg‐Lewerin, S., Emanuelson, U., Ouma, E., Dione, M., Aliro, T., Crafoord, F., Masembe, C., & Ståhl, K. (2017). Knowledge, Attitudes and Practices Related to African Swine Fever Within Smallholder Pig Production in Northern Uganda. Transboundary and Emerging Diseases, 64(1), 101–115. [DOI] [PubMed] [Google Scholar]
- Chenais, E., Depner, K., Guberti, V., Dietze, K., Viltrop, A., & Ståhl, K. (2019). Epidemiological considerations on African swine fever in Europe 2014–2018. Porc Heal Manag, 5(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor Desai, S., & Reimers, S. (2019). Comparing the use of open and closed questions for Web‐based measures of the continued‐influence effect. Behavior Research Methods, 51(3), 1426–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costard, S., Zagmutt, F. J., Porphyre, T., & Pfeiffer, D. U. (2015). Small‐scale pig farmers’ behavior, silent release of African swine fever virus and consequences for disease spread. Scientific Reports, 5(October), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho, M. V. C., Thomas, J., Alsuwaidi, A. S. M., & Couchman, J. J. (2021). Dunning‐Kruger Effect: Intuitive Errors Predict Overconfidence on the Cognitive Reflection Test. Frontiers in Psychology, 12(April), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwynar, P., Stojkov, J., & Wlazlak, K. (2019). African swine fever status in europe. Viruses, 11(4), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nardi, M., Léger, A., Stepanyan, T., Khachatryan, B., Karibayev, T., Sytnik, I., Tyulegenov, S., Akhmetova, A., Nychyk, S., Sytiuk, M., Nevolko, O., Datsenko, R., Chaligava, T., Avaliani, L., Parkadze, O., Ninidze, L., Kartskhia, N., Napetvaridze, T., Asanishvili, Z., … Obiso, R. (2017). Implementation of a regional training program on African swine fever as part of the cooperative biological engagement program across the Caucasus region. Frontiers in Veterinary Science, 4, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCicco‐Bloom, B., & Crabtree, B. F. (2006). The qualitative research interview. Medical Education, 40(4), 314–321. [DOI] [PubMed] [Google Scholar]
- Dione, M. M., Dohoo, I., Ndiwa, N., Poole, J., Ouma, E., Amia, W. C., & Wieland, B. (2020). Impact of participatory training of smallholder pig farmers on knowledge, attitudes and practices regarding biosecurity for the control of African swine fever in Uganda. Transboundary and Emerging Diseases, 67(6), 2482–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DTRA (2018). Project proposal. Regional field‐to‐table risk assessment of the spread of African swine fever virus (ASFV) across Ukraine in wild fauna and via consumer trade routes‐ insight into the development of effective ASFV quarantine strategies and public policy.
- EC (2018). Final report of a fact‐finding mission carried out in Ukraine from 25 September 2018 to 4 October 2018 in order to evaluate the implementation of animal health controls in relation to African swine fever [Internet]. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjYr4uVhJzwAhVQr6QKHVcVBEsQFjAAegQIAxAD&url=https%3A%2F%2Fec.europa.eu%2Ffood%2Faudits‐analysis%2Fact_getPDF.cfm%3FPDF_ID%3D14337&usg=AOvVaw1gz1bfhEtWBSDMguo_LFHp
- EFSA (2010). Scientific Opinion on African Swine Fever [Internet]. Vol. 8, EFSA Journal. Parma; Available from: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2010.1556
- EFSA (2014). Scientific Opinion on African swine fever. EFSA Journal, 12(4). https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2014.3628 [Google Scholar]
- EFSA (2019). Epidemiological analyses of African swine fever in the European Union (November 2018 to October 2019). EFSA Journal, 18(1), 1–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EP (2014). Economic, Social and Territorial situation of Ukraine [Internet]. Vol. 0. Brussels; Available from: https://www.europarl.europa.eu/RegData/etudes/IDAN/2014/529089/IPOL_IDA(2014)529089_EN.pdf [Google Scholar]
- FAO (2001). Manual on the preparation of African swine fever contingency plans. Animal Health Manual No 11. Rome. [Google Scholar]
- FAO (2009). Preparation of African swine fever contingency plans. Vol. FAO Animal, Edited by M.L. Penrith, V. Guberti, K. Depner and J. Lubroth. Rome. [Google Scholar]
- FAO (2013). African swine fever in the Russian Federation: risk factors for Europe and beyond. Empres Watch, 28. [Google Scholar]
- FAO (2018). African swine fever being tackled in Ukraine. FAO Regional Office for Europe and Central Asia. [Internet]. [cited 2020 Jan 22]. Available from: http://www.fao.org/europe/news/detail‐news/en/c/1099461/ [Google Scholar]
- FAO‐EMPRESS (2021). African swine fever‐ virus survival [Internet]. [cited 2021 Apr 23]. Available from: http://www.fao.org/ag/againfo/programmes/en/empres/Gemp/cont‐plan/cp‐asf/asf1242‐virus.htm
- FSCP (2020). ASF in backyard farmers in Ukraine. Oral communication. [Google Scholar]
- Galindo, I., & Alonso, C. (2017). African swine fever virus: A review. Viruses, 9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinat, C., Gogin, A., Blome, S., Keil, G., Pollin, R., Pfeiffer, D. U., & Dixon, L. (2016). Transmission routes of African swine fever virus to domestic pigs: Current knowledge and future research directions. Veterinary Record, 178(11), 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinat, C., Wall, B., Dixon, L., & Pfeiffer, D. U. (2016). English pig farmers’ knowledge and behaviour towards African swine fever suspicion and reporting. PLoS One [Internet], 11(9), 1–13. 10.1371/journal.pone.0161431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanov, E., Zeynalova, S., Geleishvili, M., Maes, E., Tongren, E., Marshall, E., Banyard, A., McElhinney, L. M., Whatmore, A. M., Fooks, A. R., & Horton, D. L. (2018). Assessing the impact of public education on a preventable zoonotic disease: Rabies. Epidemiology and Infection, 146(2), 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeson, J., & Ursic, M. L. (1994). The Role of Affective and Cognitive Decision‐Making Processes during Questionnaire Completion. Psychology & Marketing, 11, 493–510. [Google Scholar]
- Herzog, R., & Bachman, J. G. (1981). Effects of Questionnaire Length on Response Quality. The American Association for Public Opinion Research, 45(4), 549–559. [Google Scholar]
- Jurado, C., Martínez‐Avilés, M., De La Torre, A., Štukelj, M., de Carvalho Ferreira, H. C., Cerioli, M., Sánchez‐Vizcaíno, J. M., & Bellini, S. (2018). Relevant measures to prevent the spread of African Swine Fever in the European Union Domestic Pig Sector. Frontiers in Veterinary Science, 5(APR). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khomenko, S., Beltrán‐Alcrudo, D., Rozstalnyy, A., Gogin, A., Kolbasov, D., Pinto, J., Lubroth, J., & Martin, V. (2013). FAO. 2013. African swine fever in the Russian Federation: risk factors for Europe and beyond. EMPRES WATCH, Vol. 28, May 2013. Rome. Empres | [Internet]. 28(May). 10.4102/jsava.v84i1.1106{\%}0Ahttp://www.jsava.co.za/index.php/jsava/article/view/1106{\%}0Awww.fao.org/ag/empres.html [DOI] [Google Scholar]
- Koenraadt, C. J. M., Tuiten, W., Sithiprasasna, R., Kijchalao, U., Jones, J. W., & Scott, T. W. (2006). Dengue knowledge and practices and their impact on Aedes aegypti populations in Kamphaeng Phet, Thailand. American Journal of Tropical Medicine and Hygiene, 74(4), 692–700. [PubMed] [Google Scholar]
- Kovalenko, G., Ducluzeau, A. L., Ishchenko, L., Sushko, M., Sapachova, M., Rudova, N., Solodiankin, O., Gerilovych, A., Dagdag, R., Redlinger, M., Bezymennyi, M., Frant, M., Lange, C. E., Dubchak, I., Mezhenskyi, A. A., Nychyk, S., Bortz, E., & Drown, D. M. (2019). Complete Genome Sequence of a Virulent African Swine Fever Virus from a Domestic Pig in Ukraine. Microbiology Resource Announcements, 8(42), e00883‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentel, A., Fischer, P., Manoempil, P., Supali, T., Servais, G., & Rückert, P. (2006). Using knowledge, attitudes and practice (KAP) surveys on lymphatic filariasis to prepare a health promotion campaign for mass drug administration in Alor District, Indonesia. Tropical Medicine & International Health, 11(11), 1731–1740. [DOI] [PubMed] [Google Scholar]
- Kumar, S. C., Ramesh, N., Sreevatsan, S., Joseph, B., Alle, P., Belani, K. G., & Osterholm, M. T. (2013). Knowledge, attitudes and poultry‐handling practices of poultry workers in relation to avian influenza in India. Indian Journal of Occupational and Environmental Medicine, 17(1), 16–21. 10.4103/0019-5278.116368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupets, O. (2012). Characteristics and determinants of internal labor mobility in Ukraine. Background Paper for World Bank Report No 68824‐ECA “In search of Opportunities: How a more mobile workforce can propel Ukraine's Prosperity” [Internet]. Kiev. Available from: http://documents1.worldbank.org/curated/en/224161468313770215/pdf/NonAsciiFileName0.pdf
- Lei, X., Jing, S., Zeng, X., Lin, Y., Li, X., Xing, Q., Zhong, X., & Østbye, T. (2019). Knowledge, attitudes and practices towards avian influenza among live poultry market workers in Chongqing, China. Preventive Veterinary Medicine, 162(November 2018), 151–159. 10.1016/j.prevetmed.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Mascie‐Taylor, C. G. N., Karim, R., Karim, E., Akhtar, S., Ahmed, T., & Montanari, R. M. (2003). The cost‐effectiveness of health education in improving knowledge and awareness about intestinal parasites in rural Bangladesh. Economics and Human Biology, 1(3), 321–330. [DOI] [PubMed] [Google Scholar]
- Matibag, G. C., Kamigaki, T., Kumarasiri, P. V., Wijewardana, T. G., Kalupahana, A. W., Dissanayake, D. R., De Silva, D. D., Gunawardena, G. S., Obayashi, Y., Kanda, K., & Tamashiro, H. (2007). Knowledge, attitudes, and practices survey of rabies in a community in Sri Lanka. Environmental Health and Preventive Medicine, 12(2), 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur‐Panasiuk, N., Żmudzki, J., & Woźniakowski, G. (2019). African swine fever virus – persistence in different environmental conditions and the possibility of its indirect transmission. Journal of Veterinary Research, 63(3), 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Agriculture Policy and Food of Ukraine (2003). Law of Ukraine about the farm [Internet]. Available from: https://zakon.rada.gov.ua/laws/show/973‐15
- Ministry of Agriculture Policy and Food of Ukraine (2004). Order No 121/8720 Approval of Veterinary and Sanitary rules for slaughterhouses, slaughterhouses and livestock slaughter [Internet]. Available from: https://zakon.rada.gov.ua/laws/show/z0121‐04
- Ministry of Agriculture Policy and Food of Ukraine (2017). Order N111 on approval of the Instruction on the prevention and control of African swine fever [Internet]. Available from: https://zakon.rada.gov.ua/laws/show/z0432‐17
- Ministry of Foreign Affairs of Ukraine (2021). Regions of Ukraine [Internet]. [cited 2021 Apr 22]. Available from: http://old.mfa.gov.ua/en/about‐ukraine/info/regions
- Nevolko, O. M., Sytiuk, M. P., Nychyk, S. A., & Hudz, N. V. (2015). Results of serological and molecular analysis of african and classical swine fever in the population of wild boars in Ukraine. Bulletin‐ Veterinary Institute in Pulawy, 59(1), 9–17. [Google Scholar]
- Nychyk, S., Mandygra, S., Mushtuk, I., Ajshpur, O., & Sytiuk, M. (2018). Analysis of present day spread of classical swine fever in the world. Visnyk Agrarnoi Nauky's, 96(11), 113–119. [Google Scholar]
- OIE (2021). Classical Swine Fever [Internet]. [cited 2021 Apr 24]. Available from: https://www.oie.int/animal‐health‐in‐the‐world/official‐disease‐status/classical‐swine‐fever/
- Plowright, W., & Parker, J. (1967). The stability of African swine fever virus with particular reference to heat and pH inactivation. Archiv Fur Die Gesamte Virusforschung, 21(3–4), 383–402. [DOI] [PubMed] [Google Scholar]
- StataCorp (2017). Stata Statistical Software: Release 15. College Station, TX: StataCorp LP. [Google Scholar]
- Winch, P. J., Leontsini, E., Rigau‐Pérez, J. G., Ruiz‐Pérez, M., Clark, G. G., & Gubler, D. J. (2002). Community‐based dengue prevention programs in Puerto Rico: Impact on knowledge, behavior, and residential mosquito infestation. American Journal of Tropical Medicine and Hygiene, 67(4), 363–370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


