Abstract
There is an evidence that ginger enhance semen quality via improving different sperm parameters mainly count, viability, motility, morphology and DNA integrity. According to research results in various species, ginger seems to have strong antioxidant properties (due to the presence of active phenolic compounds) and androgenic activity. Ginger improves semen quality and increases fertility of sperm by disrupting the production of free radicals, dissolving oxidative chain reactions, reducing oxidative stress and altering the levels of gonadotropin hormones (LH, FSH) and sex hormones (such as testosterone). The antioxidant and androgenic properties of ginger give a sperm with normal morphological structure (head, middle and tail) and more integrated chromatin. The rate of DNA failure and damage to the mitochondrial genome in these cells is minimal and they have the most progressive motility, the highest viability and the best fertility. Therefore, the use of the ginger significantly improves the biological parameters of sperm (number, total motility, survival rate and normal morphology) and also increases all specialized fertility indicators of sperm. Tacking account of lacking literature and possibility of toxicity and adverse effect of ginger on vital organ, further clinical trial especially on evaluating the safety and clinical effect must be considered. Also, dose and duration of consumption by monitoring of health indicators and biochemical changes in all species such as human, animal and poultry must be applied.
Keywords: fertility, ginger, sperm
Abstract
The manuscript reviewed the published data related to the biological effect of ginger on the health status of sperm in infertility.
1. INTRODUCTION
The spermatogenesis occurs during three consecutive stages (spermatocytogenesis, spermiogenesis and spermation) in the seminiferous ducts of testis. The duration of spermatogenesis in birds is much shorter than mammals which are approximately 25 days (Farner & King, 1972). During this process, spermatogonia stem cells proliferate and differentiate into more specific cells (primary spermatocytes and then secondary spermatocytes; Razi et al., 2010). In the sixth and tenth weeks of the developmental process, the primary spermatocytes and then secondary spermatocytes appear in the epithelium of the seminiferous ducts and begin to proliferate. Testicular growth accelerates after the 15th week, with each reaching approximately 12–22 g weight in the 23rd week. At this time, almost all the seminiferous ducts contain secondary spermatocyte cells, and their process of differentiation into spermatid cells and eventually adult sperm is completed. The maximum testicular weight and the highest fertility rate in roosters are obtained at the age of 28–30 weeks (Rothwell, 1973). Testicular weight, semen volume and fertility usually decline significantly after 35 weeks of age. In roosters, the highest fertility rate (more than 95%) is obtained at the beginning of the reproductive period (30–40 weeks of age). Fertility levels drop sharply after 40–45 weeks and reach their lowest levels in 65–70 weeks. At this age, roosters are usually removed from the herd and replaced with young fertile roosters that called spiking of rooster (Farner & King, 1972; Razi et al., 2010; Rothwell, 1973).
One of the important factors that affect reproductive age, semen quality, and the rooster reproductive potential, is nutrition (Jorsaraei et al., 2008). It appears that the administration of natural or artificial antioxidants in the diet can reduce the amount of oxidative stress in sperm and improve semen quality and increase sperm fertility (El‐Shahat et al., 2009). Oxidative stress in semen leads to the lipid peroxidation of the cytoplasmic membrane of the sperm, the damage of the acrosomal membrane, the oxidation and breakdown of the DNA and eventually chromosomal abnormalities in the sperm (Sikka, 1996). On the other hand, increased oxidative stress by lowering testosterone production, degeneration of Sertoli cells, and rupture of the blood‐testicular barrier disrupts the process of spermogenesis and ultimately leads to a decrease in epidermal sperm counts and fertility (El‐Shahat et al., 2009; Jorsaraei et al., 2008; Sakr & Badawy, 2011). Therefore, in order to reduce oxidative stress, improvement in spermatogenesis and an increase in sperm fertility potential, the use of natural or artificial antioxidant compounds is necessary (Khaki et al., 2014). Herbal medicines with antioxidant properties such as ginger, remove the intermediate free radicals, end the oxidation chain reactions and ultimately lead to the improvement of specific sperm fertility indicators and increase the fertility potential of sperm in roosters (Khaki et al. 2014; Kubra & Jaganmohanrao, 2012; Shokri Mashhadi et al., 2013).
Ginger (Zingiber officinale) contains gingerol, gingerdiol and gingerdione, which may promote the functioning of the antioxidant defense system (Baliga et al., 2011). The antioxidant capacity was enhanced in chickens and laying hens when their diet was supplemented with ginger (Zhang et al., 2009; Zhao et al., 2011). These findings suggest that ginger might enhance the fertility of male poultry. Indeed, adding 15 g of ginger root powder/kg diet increased the fertility of aged Cobb male breeders because of the increased number of live sperm, total antioxidant capacity of the seminal plasma, sperm membrane integrity, forward motility and sperm penetration (Akhlaghi et al., 2014), while consuming 100 μl of ginger essential oil/kg body weight maximized the fertility of male quails (Herve et al., 2018). Ginger increased sperm production because of improved testes growth by enhancing development of the seminiferous tubules and germ cells and semen quality by suppressing the oxidative damage induced in the testes via the activation of antioxidant enzymes, such as superoxide dismutase and catalase (Herve et al., 2018; Saeid et al., 2011; Shanoon, 2011). Saeid et al. reported that Zingiber offcinale had useful effects on spermatogenesis and sperm parameters in broiler breeder males (Saeid et al., 2011). The 24 week old broiler breeders were administered aqueous extract of Zingiber offcinale in drinking water at 5% and 10% daily for 20 weeks. It increased FSH, testosterone and LH levels, but decreased MDA and TAC levels. There were increases in testes weight, ejaculated volume, sperm concentration and motility but decreases in dead sperm and abnormal sperm. These findings showed that Zingiber offcinale could enhance spermatogenesis.
In this review, the effects of ginger on the biological parameters of sperm and specific sperm fertility indicators in laboratory animal, rooster and human have been collected and analysed based on available sources.
2. MATERIALS AND METHODS
2.1. Search strategy
In this systematic review, the specialized databases Google Scholar, Science Direct, Elsevier, Springer, Scopus and PubMed, were used for the literature search from April 2000 to April 2020, with the purpose of limiting the search to the latest findings, using different combinations of the following keywords: ginger, sperm, Zingiber officinale and fertility. In Google Scholar, Direct, Elsevier and Springer, we used the following search equation strategy: (sperm, fertility AND Zingiber officinale). The search equation used in Scopus was: “ginger AND sperm” AND fertility. In PubMed, we used the following search equation strategy: (“Zingiber officinale” OR “Ginger”) AND sperm.
2.2. Selection criteria
Articles were organized by the Zingiber officinale (Ginger) effects on sperm fertility especially about poultry. One member of the team (MGA) extracted information about the characteristics of the articles. The information extracted from the articles included Zingiber officinale, in vitro and in vivo studies, concentrations, species and biological characteristics of sperm. After that, the quality evaluation and selection were performed by three authors (MGA, MKD and AAJ) who independently worked according to the main criteria of PICO (Population, Intervention, Comparison, Outcome; Table 1).
TABLE 1.
PICO (Population, Intervention, Comparison, Outcome) criteria for inclusion of studies
| Parameter | Inclusion criteria |
|---|---|
| Population | Studies accomplish in vitro and in vivo |
| Intervention | Anti‐infertility with Zingiber officinale (ginger) |
| Comparison | Ginger versus control |
| Outcome | Anti‐infertility properties of Zingiber officinale (ginger) especially in poultry |
2.3. Data handling, analyses and extraction
The inclusion criteria for handling of studies outlined according PRISMA guidelines were as follows: (1) studies with Zingiber officinale (ginger) with Anti‐infertility properties in vitro and in vivo; (2) clinical trial studies; and (3) studies with significant results collected via statistical analysis. The exclusion criteria used were as follows: (1) studies written in non‐English language; (2) the use of bioactive components of aromatic plant, instead of Zingiber officinale (ginger); and (3) studies without controls. After removing duplicates, the title and abstract of each article were reviewed by one member of the team. After that, acceptability for inclusion was analysed based on reading the title and abstract by three authors. Data were extracted by three authors (MGA, MHS and MO) into forms on Microsoft Excel 2013. Article selection and data extraction differences were resolved through discussion. The main results of the selected articles were arranged according to the anti‐infertility properties of ginger.
3. RESULTS
3.1. Study identification and selection
Of the 143 full texts reviewed, ninety‐five relevant articles were identified, which was in agreement with our inclusion and exclusion criteria. The selected articles were grouped into the antifungal properties of the EOs. The complete process is showed in Figure 1, which is based on a PRISMA flow chart.
FIGURE 1.
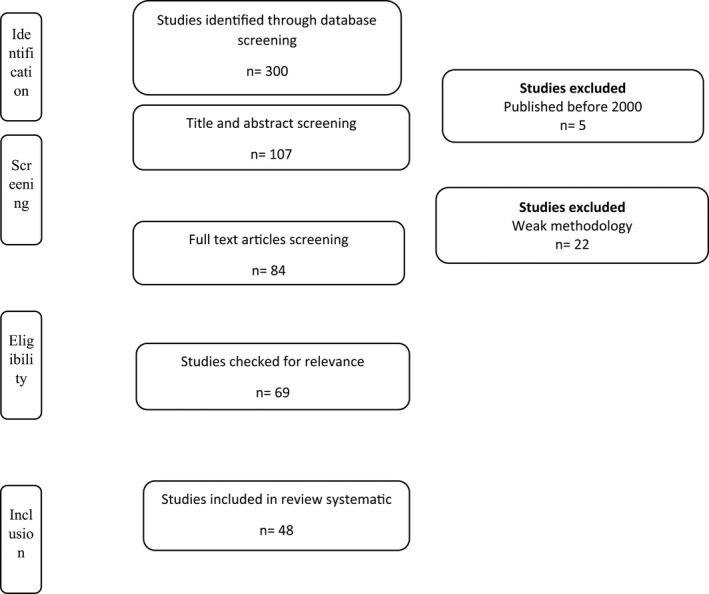
PRISMA flow chart for studies related with antifungal properties of essential oils
3.2. Pathophysiology of the effect of oxidants on fertility reduction
Iskandar et al. (2006) reported that in the Arabian roosters, the total volume of semen and pH was 300 μl and 6.95 (respectively), and the macroscopic and mass movement of sperm was good (+3) or very good (+4; Iskandar et al., 2006). In a similar study, Nataamijaya et al. (2003) stated that the total volume of semen and the pH were 260 μl and 7.02, respectively. The colour was milky white, macroscopic motion was good (+3), and microscopic mobility was 80% (Nataamijaya et al., 2003). In general, the volume of semen in roosters, ducks and quail is 300–1,000 μl, 0.1–1 ml and 50–150 ml, respectively. This depends on factors such as age, breed, body size, nutrition, environmental temperature and the concentration of vitamin A and E in the diet (Almahdi et al., 2014; Froman et al., 1995; Rouvier et al., 1984). The cause of the low volume of semen in poultry, compared with mammals, is the lack of sexual glands. The absence of glandular secretions reduces the overall volume of semen plasma and significantly increases sperm concentration in poultry.
The mean pH of semen in roosters of Kedu‐Lingen, Bangkok, and the Arab breeds is 6.92, 6.98 and 7.04, respectively. The semen colour in all of these breeds is milky white and the mass movement of sperm is reported to be +4 (Almahdi et al., 2014). Studies have shown that the mean sperm motility in the semen of Arabian roosters is 80% and in Bangkok, Kedu and Lingen are 84%. On the other hand, sperm abnormalities in Lingen, Bangkok, Kedu and Arabian roosters were reported to be 10.40%, 13.78%, 17.03% and 9.07%, respectively (Almahdi et al., 2014). Factors such as racial differences, age influence, environmental factors (especially ambient temperature), nutritional factors (especially dietary protein levels, vitamin E and calcium), disease or other structural differences in different breeds could affect the sperm motility and morphology (Hafez, 1987; Selvan, 2007). Edirisinghe et al. (1997) stated that the normal rate of sperm abnormalities in each ejaculation was 5%–30% and divided sperm abnormalities into three categories: primary, secondary and tertiary (Iskandar et al., 2006). Primary abnormalities occur in the process of spermatogenesis and in seminiferous ducts of testis. In this type of abnormality, the main parts of the sperm (head, middle and tail) are affected and lead to the formation of sperm with small, large, broadheads, or double and abnormal tails.
Secondary abnormalities occur when sperm pass through the epididymis or during ejaculation and lead to sperm with end‐cytoplasmic droplets. The third type of abnormalities occur during the ejaculation of the semen (Edirisinghe et al., 1997; Hafez, 1987).
The quality of semen, biological parameters of sperm and specialized fertility indicators are greatly reduced under the influence of interfering factors such as the production of free radicals. Free radicals have a high reactivity in the body due to their free electrons. These molecules are created by white blood cells or sperm during intracellular oxidative reactions and due to the lack of antioxidants or the release of free electrons (Zohreh et al., 2015). Studies have shown that there is a significant inverse relationship between high amounts of free radicals and sperm quality and fertility. Sperm cells are highly sensitive to free radicals due to their high levels of unsaturated fatty acids in the cytoplasmic membrane as well as low concentrations of antioxidant enzymes in their cytoplasm (Ansari et al., 2014; Asgari Jahromi et al., 2013). After exposure to high levels of the active oxygen molecule, the plasma membrane of the sperm gets damaged, and severe destruction of nuclear DNA and mitochondrial genome occurs. (Hafez, 2010). Normally, the production of large amounts of free radicals is modulated by the body's antioxidant defense system. By preventing free radical formation, the antioxidant defense system regulates damaged tissues and cells, increases the defense of damaged molecules and minimizes cell mutations (Weisiger & Fridovich, 1973).
However, sometimes exposure of the body to certain factors such as drugs, toxins, environmental pollutants, nutritional disorders increases the production of free radicals and an imbalance between the production of radicals and the body's antioxidant defense which eventually leads to oxidative stress and tissue damage. Oxidative stress leads to lipid peroxidation in sperm membranes, inactivation of glycosylated enzymes, damage to Acrosome membrane, DNA oxidation and ultimately leads to a reduction of all biological parameters of sperm (number, motility and normal morphology; Sikka, 1996). On the other hand, studies have shown that increasing the amount of oxidative stress in the body reduces the production and release of testosterone by Leydig cells, followed by impaired spermogenesis and decreased epidermal sperm count (Cao et al., 2004). In testicular Sertoli cells, an increase in the level of free radicals causes cell degeneration, the disintegration of the cytoplasmic bridges between the cells which results in a decrease in sperm count and an increase in abnormalities (Aziz et al., 2004; El‐Shahat et al., 2009). The relation between oxidative stress and infertility is illustrated in Figure 2.
FIGURE 2.
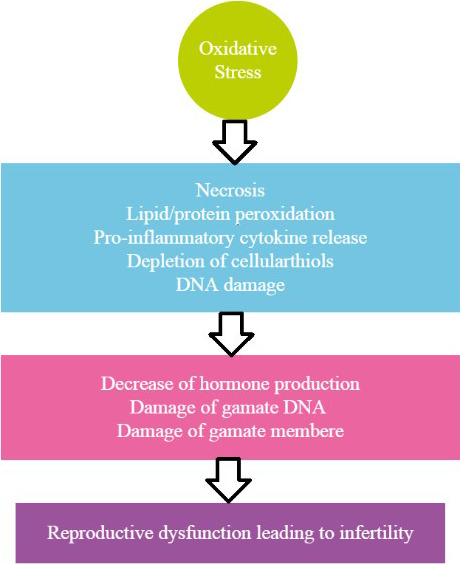
The relation between oxidative stress and infertility
3.3. The protective role of antioxidant compounds in increasing fertility
One of the body's defenses against oxidative stress is the use of antioxidant compounds. Studies have shown that treatment with natural or artificial antioxidants not only stabilizes the blood‐testicular barrier but also protects sperm DNA and increases fertility (Lombardo et al., 2011). By removing intermediate free radicals, these compounds terminate the oxidation chain reactions, and on the other hand, by oxidizing themselves, inhibit other oxidative reactions (Bjelakovic et al., 2007). Today, antioxidants such as vitamin C, vitamin E, vitamin A, zinc sulphate and selenium has been widely used in the treatment of infertility in males (Agarwal et al., 2006; Gil‐Guzman et al., 2001). It seems that the use of antioxidants of plant origin also increases sperm quality and improves the specialized parameters of sperm fertility.
3.4. Effect of ginger on sperm protection against oxidant compounds
In addition to having antioxidant properties and strong androgenic activity, ginger protects sperm‐producing cells and repairs testicular tissue against the toxic effects of some drugs and chemical compounds. Johari et al. (2010) investigated the effect of the ginger hydroalcoholic extract on body weight, testicular weight and spermatogenesis process in male rats treated with cyclophosphamide and showed that ginger eliminates the active metabolites of cyclophosphamide, repairs the DNA molecule and aides the continuation of mitotic and meiotic divisions in the testis. The researchers reported a significant increase in testicular weight, a relative increase in the number of spermatid cells and an improvement in spermatogenesis in the treated rats (Johari et al., 2010). Amin and Hamza (2006) investigated the therapeutic effects of Roselle and ginger in reducing the toxic effects of cisplatin (a drug used in chemotherapy) and found that using these herbs could significantly increase sperm motility and significantly reduce the negative effects of cisplatin on the normal sperm morphology. In this study, the concentration of ginger was 1 g/kg of body weight and added to the diet of rats for 26 days. The administration of ginger started 21 days before the first cisplatin injection. The results of this study showed that the use of Roselle and ginger significantly increased sperm motility and significantly reduced the harmful effects of cisplatin on normal sperm morphology. The use of both medicinal extracts balanced and regenerated the level of MDA (an indicator of lipid peroxidation), superoxide dismutase, glutathione and catalase in the testes of rats treated with cisplatin. In this study, the use of herbal medicines such as ginger and Roselle increased the activity of antioxidant enzymes, improve sperm motility and reduce oxidative stress in patients treated with cisplatin (Amin & Hamza, 2006).
Zahedi et al. (2012) examined the effect of ginger on the toxic effects of gentamicin on sperm fertility parameters and showed that ginger could eliminate the toxic effects of gentamicin and significantly increase serum testosterone levels in rats. Increased serum levels of testosterone led to improved spermatogenesis and a significant increase in the population of sperm cells in the epididymis (Zahedi et al., 2012). In a similar study, Mohammadi et al. (2014) examined the protective effect of ginger extract in cyclophosphamide‐treated rats and confirmed the ability of ginger to increase serum levels of testosterone and antioxidants. Evaluating the histology and pathology of testicular tissue, showed that the use of the ginger extract in 300 and 600 mg repaired damaged testicular tissue in rats treated with chemotherapy drugs. However, no change in the weight of testicular tissue and the number of free radicals was observed in the control and ginger‐treated groups (Mohammadi et al., 2014). Also, Sakr and Badawy (2011) studied the therapeutic effect of ginger on spermatogenesis and the degree of apoptosis in Albino mice. The authors investigated the effect of ginger on the level of testicular tissue damage due to the use of metiram antifungal drugs. The study first confirmed the effects of metiram on reducing the diameter of seminiferous ducts, thinning the thickness of the epithelium, reducing the number of spermatogonia and spermatocytes, apoptosis of testicular germ cells, destruction of Leydig cells and contraction of blood vessels feeding the testicular tissue. By adding ginger to the diet of treated mice, repair and regeneration of the epithelial layer of testicular tissue, as well as the proliferation of sperm‐producing germ cells in the epithelium of the seminiferous ducts was observed. In this study, the antioxidant effect of ginger extract in improving the spermatogenesis process of Albino rats was confirmed (Sakr & Badawy, 2011).
3.5. The ginger effect on spermatogenesis
In a research conducted to determine the effect of ginger [fresh rhizome powder and fresh onion water] on spermatogenesis in rats. There was significant difference between the intervention group and control group in terms of sperm count, motility percentage and vitality.
This suggested that ginger may be promising in enhancing sperm health parameters (Khaki et al., 2009, 2010). In a recent study, the toxicity of gentamicin on sperm in rats was assessed. Ginger administration caused a marked increase in the testosterone concentrations of the rats even in spite of receiving 5mg kg day‐1 gentamicin compared with the control and gentamicin treated groups. Ginger rhizome is able to overcome reproductive toxicity of gentamicin and induces spermatogenesis probably mainly through the elevation of testosterone levels (Khaki et al., 2010).
In a study, the effect of ginger extract on body weight, testes and spermatogenesis in male rats under chemotherapy with cyclophosphamide was searched. The results showed that cyclophosphamide led to a decrease in body weight, and spermatogenesis. When it was prescribed with ginger, these variables increased significantly (Johari et al., 2010). Likewise, in another research, sperm parameters significantly decreased in the streptozocin‐induced diabetic rat group. However, in the treatment group that received 100 mg/kg (oral) ginger, there was an increase in the sperm parameters in comparison to the experimental groups. Due to this preventive effect on the sperm parameters it seemed that it could be effective for treatment of diabetic rats (Nassiri et al., 2009).
Antibiotics such as gentamicin, streptomycin and ofloxacin are routinely used by urologists to treat infections prior to in vitro fertilization treatment. Gentamicin and ofloxacin affect the spermatozoa by affecting their number, motility and morphology. Gentamicin induced structural changes such as sloughing of somniferous epithelium, vacuoles and gaps in the epithelium, nuclear pyknosis and atrophic changes in a few tubules. A study by Zahedi et al. (2012) to evaluate protective effect of ginger on gentamicin‐induced apoptosis in testis of rats were done. In order to study the recovery effects of ginger on testis apoptosis after treatment with gentamicin, 40 adult Wistar male rats were selected and randomly divided into four groups ofnormal salin control (group I), gentamicin control (group II), ginger control (group III) and gentamicin + ginger (group IV) each having 10 rats. The results revealed that there was a significant increase in apoptosis in the group III when compared with the other groups. However, ginger could decrease apoptosis in the group IV that received 100 mg kg−1 rat−1 of ginger. Regarding these results, it recommended that administration of ginger with gentamicin might be beneficial in men who receive gentamicin to treat infections (Zahedi et al., 2012). There are several studies related on ginger effect on sperm indices and fertility in poultry and laboratory animal that listed in Tables 2 and 3, respectively.
TABLE 2.
Studies on ginger effects on sperm parameters (Banihani, 2019)
| Affecter | Dose (mode of treatment) | Duration | Population | Effect on sperm parameters |
|---|---|---|---|---|
| Ethanol extract of ginger | 1 g kg−1 day−1 (Oral) | 26 days | Male albino rats | (+) Sperm motility (−) Extent of cisplatin‐induced sperm abnormality |
| Ethanol extract of ginger | 1 g kg−1 day−1 (Oral) | 26 days | Male albino rats | (+) Sperm viability |
| Methanolic extract | 0.1 ml of 0.1, 0.2, 0.4 | 0, 2, 4, 8 hr | Swimmed‐up human | (−) Sperm motility |
| Methanolic extracts | 100 and 200 mg kg−1 day−1 | 65 days | Spermatozoon, Male diabetic rats | (+) Sperm abnormalities (+) Sperm motility |
| Aqueous extracts of ginger | 150 and 300 mg kg−1 day−1 (Oral) | 65 days | Male diabetic rats | (+) Sperm count (+) Sperm motility |
| Ginger | 40 mg kg−1 day−1 of body weight (oral) | 60 days | Male albino rats with Aluminium‐induced toxicity | (+) Sperm viability (+) Sperm motility (+) Sperm count |
| Ginger | 3% of fed diet (Oral) | 30 days | Male diabetic rats | (+) Sperm viability |
| Dietary Ginger powder | 15, or 30 g/kg of diet oral | 14 weeks | Aged Cobb 500 breeder rooster | (−) Sperm abnormalities (+) Membrane integrity of spermatozoon (+) Sperm viability (+) Sperm motility |
| Ginger | 4% of the diet (Oral) | 14 days | Hypertensive Male Wistar rats | (+) Sperm motility Sperm viability (±) |
| Aqueous extracts of ginger + pumpkin | 300 and 600 mg/kg day−1 (Oral) | 6 weeks | Cyclophosphamide‐ induced male adult rat | Sperm motility (+) Sperm viability (+) Sperm count (+) |
| 6‐gingerol‐rich fraction from ginger | 50, 100, and 200 mg/kg (Oral) | 14 days | Male rats with carbendazim‐induced toxicity | (+) Sperm motility (+) Sperm count (−) Sperm abnormality |
| Ginger powder | 250 mg twice a day (Oral) | 3 months | Infertile men | (−) Sperm DNA fragmentation (±) Sperm count (±) Sperm motility |
| Aqueous Ginger extract | 500 mg kg−1 day−1 (Oral) | 28 days | Male rats with sodium metabisulfite‐induced toxicity | (+) Sperm motility (−) Sperm abnormality |
| Aqueous extracts of Ginger | 300 or 600 mg/day (Oral) | 6 weeks | Cyclophosphamide‐treated rats | (+) Sperm count |
TABLE 3.
Studies on ginger effects on fertility
| Host | Source | Dose | Effect | Reference |
|---|---|---|---|---|
| Wistar rats | Zingiber offcinale | 50, 100 mg/kg; 20 days | Increase of sperm healthy Parameters by increase LH, FSH, and TAC and decrease MDA | Khaki et al. (2009) |
| C57BL/6 mice | Bupleurum falcatum L., Pinellia ternata Breitenbach, Cinnamon verum J. Presl, Poria cocos Wolf, Scutellaria baicalensis Georgi, Zizyphus jujuba var. inermis, Crassostrea gigas., Panax ginseng C. A. Mey., Rinoceros spp., Rheum rhabarbarum. L., Zingiber offcinale Roscoe | 300 mg/kg; 30 days | Increase of sperm number, sperm motility by increased testosterone | Zang et al. (2016) |
| Broiler breeder | Zingiber offcinale aqueous extract | 5, 10%; 64 weeks | Increase of Spermatogenesis (by increase FSH, Testosterone, & LH), and decrease MDA & TAC | Saeid et al. (2011) |
| Male Wistar rats | Zingiber offcinale aqueous extract | 600 mg kg−1 day−1 for 8 days | Increase of both testis weight and serum testosterone levels | Kamtchouing et al. (2002) |
| Broilers breeder male (ROSS strain) | Zingiber offcinale extract | 100 g/kg feed for 140 days | Increased semen volume, sperm motility, sperm concentration, live spermatozoa, and decreased abnormal spermatozoa | Shanoon and Jassim (2012) |
| Broilers breeder male | Zingiber offcinale powder | 0, 6, 11 g/kg feed for 42 days | Increased semen volume, sperm motility, sperm concentration, live spermatozoa, and decreased abnormal spermatozoa | Shanoon (2011) |
| Native cock fowls | Zingiber offcinale powder | 0, 2.5, 5.0 g/kg feed for 84 days | Improved semen volume, sperm motility, sperm concentration, live spermatozoa, and decreased abnormal spermatozoa | Ezzat et al. (2018) |
The mechanisms by which ginger or its extracts exert such effects is mainly by increasing the level of testicular cholesterol, increasing blood flow in the testes, stimulating the synthesis of luteinising hormone, enhancing the activity of molecular defense mechanism in the testes and reducing blood glucose level. Other mechanisms include increasing testicular weight, increasing blood flow to the testes and recycling testosterone receptors. Accordingly, the positive impact of ginger on sperm parameters, and thus on semen quality, may be attributable, at least in part, to the increased levels of gonadal hormones (testosterone and luteinising hormone; Banihani, 2019).
3.6. The effect of ginger on chicken male spermatogenesis
Semen evaluation is regularly used in modern poultry enterprise to ascertain the reproductive ability of cocks to be utilized for artificial insemination (AI) program. The use of poor‐quality spermatozoa in AI program has been reported to adversely affect fertility, increases embryo mortality and makes the hen depend totally on stored spermatozoa from the sperm storage tubules (Thurston, 1995).
Traditional semen quality assessment methods demand the calculation of sperm motility, viability, concentration and morphology of spermatozoa as well as semen volume and semen colour. Most of these quality assessment parameters have a direct link with spermatozoa fertilizing capacity (Donoghue & Wishart, 2000). The capability of the spermatozoa to fertilize the ovum depends on the structural and functional attributes of sperm cell membrane lipids. The abundance of polyunsaturated fatty acids (PUFAs) in the chicken sperm membrane engendered sperm to lipid peroxidation caused by reactive oxygen species (ROS) manufactured in the mitochondrial and plasma membrane (Ogbuewu et al., 2010). Naturally, the sperm antioxidant defense action against ROS attack is weak, but the seminal antioxidant defense activity tends to boost defense against ROS (Zini et al., 2009). However, excess ROS generation reduces the sperm antioxidant defense mechanism, hence resulting in infertility. Several investigators have tried to boost semen quality indicators in poultry using plant materials with antioxidant properties with mixed results (Khan et al., 2012; Nahed et al., 2014; Neuman et al., 2002; Ommati et al., 2013). Thus, the need to ascertain the potential of tropical plants with antioxidant properties such as ginger on semen production in poultry. The results of this study suggest that phytobiotic supplementation has a positive influence on semen parameters. However, a number of studies employed in the analysis to determine the effect of ginger on semen production were less than ten, and there is evidence of large heterogeneity as indicated by the inconsistency index (I2‐statistics; Higgins et al., 2003).
Therefore, the inability to ascertain the percentage of this variability explained by our chosen explanatory predictors (duration of supplementation, presentation form, strain and inclusion level) due to small nature of studies used in the analysis means that our results especially for semen volume, sperm motility and concentration should be discussed with caution. The significant decline in the number of sperm with abnormal cells could be linked to the activity of ginger bioactive elements to guard the sperm membranes against free radical attack during in vitro and in vivo storage (Zhang et al., 2009; Zhao et al., 2011).
3.7. The effect of ginger on increasing sperm fertility
Several studies have been conducted on the effect of ginger on sperm fertility in mice and rats. Khaki et al. (2009) studied the effects of ginger on spermatogenesis and semen quality in rats by using 50 and 100 mg of ginger powder per kg of diet. In this study, the use of ginger powder in both concentrations significantly increased the viability and motility of sperm. However, the use of ginger in 50 and 100 mg did not cause significant statistical changes in testicular tissue weight, total sperm count, normal sperm morphology and concentrations of LH and FSH hormones. The authors confirmed the increasing effect of ginger on serum testosterone levels as well as serum glutathione levels and the use of ginger in reducing the DNA damage and oxidative stress in sperm (Khaki et al., 2009).
In another study, Morakinyo et al. (2008) added 500 and 1,000 mg ginger hydroalcoholic extract orally to the diet of the rats for 14–28 days. Adding ginger extract significantly increased testicular and epididymal tissue weight, serum testosterone levels, total sperm count and their motility in semen. In rats treated with ginger extract, the levels of malonyldehydrate (MDA) and oxidative stress was significantly reduced. The researchers stated that the increase in fertility potential following the administration of ginger was due to the antioxidant and androgenic properties of this medicinal plant (Morakinyo et al., 2008).
Several studies investigated the effect of ginger on increasing human fertility (Hosseini et al., 2016; Saeid et al., 2011; Zahedi et al., 2012). Hosseini et al. (2016) investigated the effect of ginger extract on sperm fragmentation rate and reproductive potential in infertile men. In this study, patients used 250 mg capsules of ginger powder twice a day for 3 months. Then, the total number of sperm, sperm motility and the amount of sperm DNA fragmentation were evaluated before and after treatment. The results of this study showed that the rate of DNA damage in infertile patients receiving ginger extract was significantly lower than in the control group. However, there was no statistically significant difference in the total number and the motility of sperm between treated and control groups (Hosseini et al., 2016). In a similar study, Mares et al. (2012) measured LH, FSH and testosterone hormones in serum to investigate the effect of ginger extract on sperm parameters (total number, motility, viability and normal sperm morphology) in 19–40‐year‐old infertile men. In this study, the use of ginger for 3 months caused a significant increase in the total number of sperm (more than 16.2%), a significant improvement in sperm motility (more than 47.3%), increased viability (more than 40.7%) and normal sperm morphology. Also, the total volume of semen in patients treated with ginger extract increased by 36%. The researchers reported that the DNA damage (DNA fragmentation) significantly reduced, serum glutathione, LH, FSH, and testosterone levels increased in patients who received the ginger extract (Mares et al., 2012).
3.8. The effect of ginger on increasing sperm fertility in poultry
Akhlaghi et al. (2014) investigated the effect of ginger on the reproductive efficiency of commercial roosters and reported a significant improvement in semen quality, increased sperm fatty acid levels and increased reproductive efficiency in roosters (Akhlaghi et al., 2014). The ginger powder in 0, 15 and 30 g concentrations were added to the rooster's diet for 14 weeks, and then semen characteristics and sperm parameters were evaluated every 14 days. The researchers observed a significant increase in viability and sperm motility, decrease in structural abnormalities, and a greater integration of sperm cytoplasmic membranes in roosters receiving ginger powder. In this study, ginger increased the amount of unsaturated fatty acids and reduced the concentration of saturated fatty acids in sperm.
Also, the levels of C22: 4 (n‐6) fatty acids in sperm and the antioxidant properties of semen plasma were very high in roosters that received ginger powder. Although the use of high concentrations of ginger (30 g), significantly increased sperm penetration in the perivitelline membrane and improved fertility, there was no statistically significant difference in the rate of duration of incubation and mortality of chicks due to the use of ginger powder. The researchers recommended that ginger in the diet improves the quality of semen, especially in commercial chickens (Akhlaghi et al., 2014). In a similar study, Saeid et al. (2011) examined the effect of ginger extract on fertility potential and reproductive activity of chickens and used two concentrations of 5% and 10% ginger extract in drinking water. In this study, all sperm fertility parameters, serum hormone concentrations and semen plasma hormones were measured every 4 weeks (from week 24 to 44 weeks). Treatment with ginger significantly increased testicular tissue weight in both groups. The overall volume of semen and the number of sperm cells in the chickens that received 10% ginger extract were significantly higher than in roosters that used a lower concentration of ginger extract. Also, adding high concentrations of the ginger extract significantly reduced sperm abnormalities. The researchers found a significant increase in serum glutathione levels, increased concentrations of LH, FSH and serum testosterone, as well as decreased plasma protein levels in treated roosters. In this study, following the use of the ginger extract, the concentration of cholesterol and glucose in semen plasma also increased (Saeid et al., 2011).
3.9. The synergic effect of ginger in increasing sperm fertility
Some researchers have studied the simultaneous use of ginger and other chemical or herbal compounds and examined their effect on fertility parameters and reproductive patterns. Khaki et al. (2014) investigated the antioxidant effects of ginger and cinnamon on spermatogenesis, sperm biology and oxidative stress in diabetic rats. The researchers also confirmed the effects of ginger on increasing the serum concentrations of testosterone, LH and FSH, and observed a significant increase in total sperm count, motility and sperm viability. On the other hand, the concentration of antioxidant components such as catalase, superoxide dismutase, glutathione peroxidase and total antioxidant capacity (TAC) in serum increased significantly after using ginger. The simultaneous addition of ginger and cinnamon to the diet accelerate the effects of each herb on spermatogenesis. Therefore, the simultaneous use of ginger (100 mg kg−1 day−1) and cinnamon (75 mg kg−1day−1) increases the level of androgens (LH and FSH) and serum testosterone and also improve the specialized parameters of sperm fertility in patients with diabetes (Khaki et al., 2014). Akinyemi et al. (2015) stated that oral administration of ginger and turmeric can improve reproductive activity and sperm quality in rats with high blood pressure. These researchers examined the protective effects of ginger and turmeric on biomarkers associated with the reproductive activity. Often, there is a significant decrease in serum testosterone levels and sperm motility in rats with high blood pressure. In rats suspected to hypertension, due to high oxidative stress in testicles and epididymis, the total thiol and non‐protein thiol levels, the nitrite oxide levels and glutathione S‐transferase activity were at their lowest level and the activity of Arginase and thiobarbituric acid (TBA) metabolites increased. In this study, adding ginger or turmeric to the diet of rats with high blood pressure prevented changes in the level of biomarkers associated with reproductive activity and increased serum levels of testosterone and nitric oxide, as well as significantly reduced arginase activity in the body. These changes can prevent infertility in people with high blood pressure. The researchers recommended that adding ginger and turmeric as dietary supplements to the diet of patients with infertility caused by high blood pressure can have beneficial effect on fertility (Akinyemi et al., 2016).
In conclusion, changes in lifestyle, changes in food culture, environmental pollution and excessive use of chemicals in the food industry and animal husbandry can increase the risk of oxidative stress on physiological process. Sperm can be affected by oxidants, leading to reduced viability and structural changes that prevent successful fertilization. Natural antioxidants can prevent this process. Various studies have shown that plants such as ginger have a positive effect on the process of spermatogenesis and sperm development due to their biomaterials, and can increase biological strength and increase fertility in humans and animals. The use of the ginger significantly improves the biological parameters of sperm (number, total motility, survival rate and normal morphology) and also increases all specialized fertility indicators of sperm. Therefore, the use of ginger in the diet or drinking water especially in humans or animals with low potent fertility, is recommended.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTION
Majid Gholami‐Ahangaran: Conceptualization; Investigation; Methodology; Project administration; Supervision; Writing‐original draft; Writing‐review & editing. Maryam Karimi‐Dehkordi: Resources; Software; Validation; Writing‐original draft. Arefeh Akbari Javar: Investigation; Writing‐original draft; Writing‐review & editing. Maziar Haj Salehi: Resources; Software; Validation; Writing‐review & editing. Mehrdad Ostadpoor: Search, Software; Writing‐review & editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.538.
Gholami‐Ahangaran M, Karimi‐Dehkordi M, Akbari Javar A, Haj Salehi M, Ostadpoor M.A systematic review on the effect of Ginger (Zingiber officinale) on improvement of biological and fertility indices of sperm in laboratory animals, poultry and humans.. Vet Med Sci. 2021;00:1959–1969. 10.1002/vms3.538
REFERENCES
- Agarwal, A., Gupta, S., & Sikka, S. (2006). The role of free radicals and antioxidants in reproduction. Current Opinion in Obstetrics & Gynecology, 18(3), 325–332. 10.1097/01.gco.0000193003.58158.4e [DOI] [PubMed] [Google Scholar]
- Akhlaghi, A., Ahangari, Y. J., Navidshad, B., Pirsaraei, Z. A., Zhandi, M., Deldar, H., Rezvani, M. R., Dadpasand, M., Hashemi, S. R., Poureslami, R., & Peebles, E. D. (2014). Improvements in semen quality, sperm fatty acids, and reproductive performance in aged Cobb500 breeder roosters fed diets containing dried ginger hizomes (Zingib officinale). Poultry Science, 93(5), 1236–1244. [DOI] [PubMed] [Google Scholar]
- Akinyemi, A. J., Adedara, I. A., Thome, G. R., Morsch, V. M., Rovani, M. T., Mujica, L. K. S., Duarte, T., Duarte, M., Oboh, G., & Schetinger, M. R. C. (2015). Dietary supplementation of ginger and turmeric improves reproductive function in hypertensive male rats. Toxicology Reports, 13(2), 1357–1366. 10.1016/j.toxrep.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemi, A. J., Thomé, G. R., Morsch, V. M., Bottari, N. B., Baldissarelli, J., de Oliveira, L. S., Schetinger, M. R. C. (2016). Dietary supplementation of ginger and turmeric rhizomes modulates platelets ectonucleotidase and adenosine deaminase activities in normotensive and hypertensive rats. Phytotherapy Research, 30(7), 1156–1163. [DOI] [PubMed] [Google Scholar]
- Almahdi, A. B., Ondho, Y. S., & Sutopo, A. (2014). Comparative studies of semen quality on different breed of chicken in poultry breeding center temanggung‐central java. International Journal of Engineering Science, 3(2), 94–103. [Google Scholar]
- Amin, A., & Hamza, A. A. (2006). Effect of Rosella and Ginger on cisplatin induced toxicity in rates. Asian Journal of Andrology, 8(5), 607–612. [DOI] [PubMed] [Google Scholar]
- Ansari, S., Brouki Milan, P., Mohammadnejad, D., Delazar, A., Mortazavi, M., & Mohammadi, R. A. (2014). Effects of Polygonum avicular extract on histological changes of mouse seminiferous tubules after electromagnetic field exposure. Pharmaceutical Sciences, 19(4), 139–144. [Google Scholar]
- Asgari Jahromi, M., Movahedin, M., Amanloo, M., Mowla, G., Mazaheri, Z., & Batouli, H. (2013). The effects of calligonum extract on sperm parameters and the rate of apoptosis in aged male mice testis tissue. Modares Journal of Medical Sciences: Pathobiology, 16(1), 25–38. [Google Scholar]
- Aziz, N., Saleh, R. A., Sharma, R. K., Lewis‐Jones, I., Esfandiari, N., Thomas, A. J., & Agarwal, A. (2004). Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertility and Sterility, 81(2), 349–354. 10.1016/j.fertnstert.2003.06.026 [DOI] [PubMed] [Google Scholar]
- Baliga, M. S., Haniadka, R., Pereira, M. M., D’Souza, J. J., Pallaty, P. L., Bhat, H. P., & Popuri, S. (2011). Update on the chemopreventive effects of ginger and its phytochemicals. Critical Reviews in Food Science and Nutrition, 51, 499–523. 10.1080/10408391003698669 [DOI] [PubMed] [Google Scholar]
- Banihani, S. A. (2019). Effect of ginger (Zingiber officinale) on semen quality. Andrologia, 51(6), 13296. [DOI] [PubMed] [Google Scholar]
- Bjelakovic, G., Nikolova, D., Gluud, L. L., Simonetti, R. G., & Gluud, C. (2007). Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta‐analysis. JAMA, 297(8), 842–857. 10.1001/jama.297.8.842 [DOI] [PubMed] [Google Scholar]
- Cao, L., Leers‐Sucheta, S., & Azhar, S. (2004). Aging alters the functional expression of enzymatic and nonenzymatic anti‐oxidant defense systems in testicular rat in Leydig cells. Journal of Steroid Biochemistry and Molecular Biology, 88(1), 61–67. [DOI] [PubMed] [Google Scholar]
- Donoghue, A. M., & Wishart, G. J. (2000). Storage of poultry semen. Animal Reproduction Science, 62, 213–232. 10.1016/S0378-4320(00)00160-3 [DOI] [PubMed] [Google Scholar]
- Edirisinghe, W. R., Murch, A., Junk, S., & Yovich, J. L. (1997). Cytogenetic abnormalities of unfertilized oocytes generated from in‐vitro fertilization and intracytoplasmic sperm injection: A double‐blind study. Human Reproduction, 12(12), 2784–2791. [DOI] [PubMed] [Google Scholar]
- El‐Shahat, A. E., Gabr, A., Meki, A. R., & Mehana, E. S. (2009). Altered testicular morphology and oxidative stress induced by cadmium in experimental rats and protective effect of simultaneous green tea extract. International Journal of Morphology, 27(3), 757–764. 10.4067/S0717-95022009000300020 [DOI] [Google Scholar]
- Ezzat, W. (2018). Effect of adding dried ginger rhizomes to diets on semen quality and fertility rate in aged local cocks under Egyptian hot summer condition. Egyptian Poultry Science Journal, 37(1), 233–249. [Google Scholar]
- Farner, D. S., & King, J. E. (1972). Avian biology (2nd ed.). Academic Press. [Google Scholar]
- Froman, D. P., Feltman, A. J., & McLean, D. J. (1995). Increased fecundity resulting from semen donor selection based upon in vitro sperm mobility. Poultry Science, 76, 73–77. [DOI] [PubMed] [Google Scholar]
- Gil‐Guzman, E., Ollero, M., Lopez, M. C., Sharma, R. K., Alvarez, J. G., Thomas, A. J., & Agarwal, A. (2001). Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Human Reproduction, 16(9), 1922–1930. 10.1093/humrep/16.9.1922 [DOI] [PubMed] [Google Scholar]
- Hafez, D. A. (2010). Effect of extracts of ginger goots and cinnamon bark on fertility of male diabetic rats. Journal of American Science, 6(10), 940–947. [Google Scholar]
- Hafez, E. S. E. (1987). Reproduction in farm animal (5th ed.). Bandung. [Google Scholar]
- Herve, T., Raphaël, K. J., Ferdinand, N., Vitrice, L., Tiwa, F., Gaye, A., Outman, M. M., Marvel, W., & Moyo, N. (2018). Growth performance, serum biochemical profile, oxidative status, and fertility traits in male Japanese quail fed on ginger (Zingiber officinale, roscoe) essential oil. Veterinary Medicine International, 1, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. BMJ, 327, 557. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini, J., Mardi Mamaghani, A., Hosseinifar, H., Sadighi Gilani, M. A., Dadkhah, F., & Sepidarkish, M. (2016). The influence of ginger (Zingiber officinale) on human sperm quality and DNA fragmentation: A double‐blind randomized clinical trial. International Journal of Reproductive BioMedicine, 14(8), 533–540. 10.29252/ijrm.14.8.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskandar, S., Mardalestari, R., Hernawati, R., Mardiah, E., & Wahyu, E. (2006). Pengaruh jenis konsentrasi krioprotektan dan metode thawing terhadap kualitas semen beku ayam Arab. Indonesian Journal of Veterinary Sciences, 11(1), 34–38. [Google Scholar]
- Johari, H., Sharifi, A., Ansari, N., Hosseini, M., & Amiri, F. (2010). Effect of hydro‐alcoholic extract of Zingiber officinale on body weight, testis weight and spermatogenesis in rats under chemotherapy with cyclophosphamide. Journal of Shahid Sadoughi ‐ Shahid Saddoghi, 17(5), 365–374. [Google Scholar]
- Jorsaraei, S. G. A., Yousefnia Pasha, Y. R., Zainalzadeh, M., Moghadamnia, A. A., Beiky, A. A., & Rayati, D. M. (2008). The effects of methanolic extracts of Ginger (Zingiber officinale) on human sperm parameters: An in vitro study. Pakistan Journal of Biological Sciences, 11(13), 1723–1727. 10.3923/pjbs.2008.1723.1727 [DOI] [PubMed] [Google Scholar]
- Kamtchouing, P., Fandio, G. M., Dimo, T., & Jatsa, H. B. (2002). Evaluation of androgenic activity of Zingiber officinale and Pentadiplandra brazzeana in male rats. Asian Journal of Andrology, 4(4), 299–302. [PubMed] [Google Scholar]
- Khaki, A., Fathiazad, F., Nouri, M., Khaki, A. A., Ozanci, C. C., Ghafari‐Novin, M., & Hamadeh, M. (2009). The effects of Ginger on spermatogenesis and sperm parameters of rat. Iranian Journal of Reproductive Medicine, 7(1), 7–12. [Google Scholar]
- Khaki, A., Khaki, A. A., Hajhosseini, L., Sadeghpour Golzar, F., & Ainehchi, N. (2014). The anti‐oxidant effects of ginger and cinnamon on spermatogenesis dys‐function of diabetes rats. African Journal of Traditional, Complementary and Alternative Medicines, 11(4), 1–8. 10.4314/ajtcam.v11i4.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaki, A., Zahedi, A., Rezazadeh, S. H., Ahmadi‐Ashtiani, H. R., & Rastegar, H. (2010). Zingiber officinal protective effects on gentamicin’s toxicity on sperm in rats. Journal of Medicinal Plants, 35, 93. [Google Scholar]
- Khan, R. U., Naz, S., Nikousefat, Z., Tufarelli, V., Javdani, M., Qureshi, M. S., & Laudadio, V. (2012). Potential applications of ginger (Zingiber officinale) in poultry diets. World's Poultry Science Journal, 68, 245–252. [Google Scholar]
- Kubra, I. R., & Jaganmohanrao, L. (2012). An overview on inventions related to ginger processing and products for food and pharmaceutical applications. Recent Patents on Food, Nutrition & Agriculture, 4(1), 31–49. 10.2174/1876142911204010031 [DOI] [PubMed] [Google Scholar]
- Lombardo, F., Sansone, A., Romanelli, F., Paoli, D., Gandini, L., & Lenzi, A. (2011). The role of antioxidant therapy in the treatment of male infertility: An overview. Asian Journal of Andrology, 13(5), 690–697. 10.1038/aja.2010.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares, A. K., Abid, W., & Najam, W. S. (2012). The effect of Ginger on semen parameters and serum FSH, LH & testosterone of infertile men. Tikrit Medical Journal, 18, 322–324. [Google Scholar]
- Mohammadi, F., Nikzad, H., Taghizadeh, M., Taherian, A., Azami‐Tameh, A., & Hosseini, S. M. (2014). Protective effect of Zingiber officinale extract on rat testis after cyclophosphamide treatment. Andrology, 46(6), 680–686. [DOI] [PubMed] [Google Scholar]
- Morakinyo, A. O., Adeniyi, O. S., & Arikawe, A. P. (2008). Effects of Zingiber officinale on reproductive functions in the male rat. African Journal of Biomedical Research, 11, 329–334. [Google Scholar]
- Nahed, S., Tamer, A., Amera, A., & Emad, G. (2014). The effects of dietary supplementation of different levels of thyme and ginger essential oils on performance, hematological, biochemical and immunological parameters of broiler chickens. Global Veterinaria, 12, 736–744. [Google Scholar]
- Nassiri, M., Khaki, A., Ahmadiashtiani, H. R., Rezazadeh, S., Rastgar, H., & Gharachurlu, S. (2009). Effects of ginger on spermatogenesis in streptozotocin‐induced diabetic rat. Iranian Herbal Drug Journal, 2, 31–34. [Google Scholar]
- Nataamijaya, A. G., Setioko, A. R., Brahmantiyo, B., & Diwyanto, K. (2003) .Performans dan karakteristik tiga galur ayam lokal (pelung, arab, dan sentul). In Prosiding Seminar Nasional Teknologi Peternakan dan Veteriner. Pusat Penelitian dan Pengembangan Peternakan, Bogor 2003 Sep.
- Neuman, S. L., Lin, T. L., & Hester, P. Y. (2002). The effect of dietary carnitine on semen traits of White Leghorn roosters. Poultry Science, 81, 495–503. 10.1093/ps/81.4.495 [DOI] [PubMed] [Google Scholar]
- Ogbuewu, I. P., Aladi, N. O., Etuk, I. F., Opara, M. N., Uchegbu, M. C., Okoli, I. C., & Iloeje, M. U. (2010). The relevance of oxygen free radicals and antioxidants in sperm production and function. Research Journal of Veterinary Sciences, 3, 138–164. 10.3923/rjvs.2010.138.164 [DOI] [Google Scholar]
- Ommati, M. M., Zamiri, M. J., Akhlaghi, A., Atashi, H., Jafarzadeh, M. R., Rezvani, M. R., & Saemi, F. (2013). Seminal characteristics, sperm fatty acids, and blood biochemical attributes in breeder roosters orally administered with sage (Salvia officinalis) extract. Animal Production Science, 53, 548–554. 10.1071/AN12257 [DOI] [Google Scholar]
- Razi, M., Hassanzadeh, S. H., Najafi, G. R., Feyzi, S., Amin, M., Moshtagion, M., Janbaz, H., & Amin, M. (2010). Histological and anatomical study of the White Rooster of testis, epididymis and ductus deferens. International Journal of Veterinary Science, 4, 229–236. [Google Scholar]
- Rothwell, B. (1973). The ultrastructure of Leydig cell in thetestis of thedomestic fowl. Journal of Anatomy, 116(2), 245–253. [PMC free article] [PubMed] [Google Scholar]
- Rouvier, R., Taiand, J. J. L., & Tai, C. (1984). Artificial insemination of common canes for the production of mallards in Taiwan. The current situation (Artificial insemination and genetic improvement: Critical assessment and perspective). The INRA Symposia., 29, 360–367. [Google Scholar]
- Saeid, J. M., Shanoon, A. K., & Marbut, M. M. (2011). Effects of Zingiber officinale aqueous extract on semen characteristic and some blood plasma, semen plasma parameters in the broilers breeder male. International Journal of Poultry Science, 10(8), 629–633. 10.3923/ijps.2011.629.633 [DOI] [Google Scholar]
- Sakr, S. A., & Badawy, G. M. (2011). Effect of ginger (Zingiber officinale R.) on metiram‐inhibited spermatogenesis and induced apoptosis in albino mice. Journal of Applied Pharmaceutical Science, 4, 131–136. [Google Scholar]
- Selvan, S. T. (2007). Influence of dietary protein, calcium and vitamin E on the semen quality in broiler breeder males. Tamil Nadu Veterinary and Animal Sciences, 3(2), 60–64. [Google Scholar]
- Shanoon, A. K. (2011). Effects of Zingiber officinale powder on semen characteristic and blood serum sex hormones concentration in broilers breeder male. International Journal of Poultry Science, 10, 863–866. 10.3923/ijps.2011.863.866 [DOI] [Google Scholar]
- Shanoon, A. K., & Jassim, M. S. (2012). Effects of Thymus vulgaris and Zingiber officinale aqueous on semen parameters, testes weight and histology measurements of broiler breeder male. International Journal of Poultry Science, 11(9), 594 10.3923/ijps.2012.594.598 [DOI] [Google Scholar]
- Shokri Mashhadi, N., Ghiasvand, R., Askari, G., Hariri, M., Darvishi, L., & Mofid, M. R. (2013). Anti‐oxidative and anti‐inflammatory effects of ginger in health and physical activity: Review of current evidence. International Journal of Preventive Medicine, 4(1), 36–42. [PMC free article] [PubMed] [Google Scholar]
- Sikka, S. C. (1996). Oxidative stress and role of antioxidants in normal and abnormal sperm function. Frontiers in Bioscience, 1, 78–86. 10.2741/A146 [DOI] [PubMed] [Google Scholar]
- Thurston, R. J. (1995). Storage of poultry semen above freezing for 24–48 hours. In Bakst M. R. & Cecil H. (Eds.), Proceedings of the first international symposium on artificial insemination in poultry (pp 107–122). Poultry Science Association. [Google Scholar]
- Weisiger, R. A., & Fridovich, I. (1973). Mitochondrial superoxide simutase: Site of synthesis and intramitochondrial localization. Journal of Biological Chemistry, 248(13), 4793–4796. [PubMed] [Google Scholar]
- Zahedi, A., Fathiazad, F., Khaki, A., & Ahmadinejad, B. (2012). Protective effect of ginger on gentamicin‐induced apoptosis in testis of rats. Advanced Pharmaceutical Bulletin, 2(2), 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang, Z. J., Zhang, Y. N., Gao, Y., & Zhang, B. (2016). Effects of saikokaryukotsuboreito on spermatogenesis and fertility in aging male mice. Chinese medical journal, 129(7), 846–853. 10.4103/0366-6999.178972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, G. F., Yang, Z. B., Wang, Y., Yang, W. R., Jiang, S. Z., & Gai, G. S. (2009). Effects of ginger root (Zingiber officinale) processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poultry Science, 88, 2159–2166. 10.3382/ps.2009-00165 [DOI] [PubMed] [Google Scholar]
- Zhao, X., Yang, Z. B., Yang, W. R., Wang, Y., Jiang, S. Z., & Zhang, G. G. (2011). Effects of ginger root (Zingiber officinale) on laying performance and antioxidant status of laying hens and on dietary oxidation stability. Poultry Science, 90, 1720–1727. 10.3382/ps.2010-01280 [DOI] [PubMed] [Google Scholar]
- Zini, A., San Gabriel, M., & Baazeem, A. (2009). Antioxidants and sperm DNA damage: A clinical perspective. Journal of Assisted Reproduction and Genetics, 26, 427–432. 10.1007/s10815-009-9343-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohreh, F., Nasri, S., & Kerishchi, P. (2015). The effect of quercetin on pituitary–gonadal axis, sperm parameters and testis tissue in male rats. Journal of Sabzevar University of Medical Sciences, 22(3), 18–25. [Google Scholar]


