Abstract
Background:
The class of antibiotics known as β-lactams are a commonly used due to their effectiveness and safety. Therapeutic drug monitoring has been proposed but requires an accurate assay along with well-characterized preanalytic stability, as β-lactams are known to be relatively unstable.
Methods:
A high-throughput LC-MS/MS assay validation and stability study was performed for cefepime, meropenem, and piperacillin and tazobactam in serum. Patient samples, standards, and QCs were crashed with acetonitrile containing internal standard. Following centrifugation, an aliquot of the supernatant was diluted with clinical laboratory reagent water and analyzed by LC-MS/MS.
Results:
The assay showed linearity between 0.5 and 60 μg/mL for each analyte. The intra- and interassay reproducibility at 3 different concentrations (approximately 2, 25, and 40 μg/mL) was <5% for each analyte. Accuracy studies for each analyte were compared using linear regression and demonstrated: slope = 1.0 ± 0.1; r2 ≥ 0.980; and y intercept 95% CI that included zero. Minimal ion suppression or enhancement was observed, and no significant carryover was observed up to 500 μg/mL of each analyte. Stability studies demonstrated significant loss in serum for each analyte at ambient and refrigerated temperatures (2–8 °C) and at −20 °C over days or weeks. In contrast, when stored at −80 °C, no significant loss was observed.
Conclusions:
The LC-MS/MS assay showed acceptable performance characteristics for quantitation of β-lactams. With well-characterized stability, this assay can be used with residual specimens for pharmacokinetic modeling, which may lead to individualized dosing and improved patient care.
INTRODUCTION
Antipseudomonal β-lactams such as cefepime (FEP), meropenem (MEM), and piperacillin/tazobactam (TZP) are the backbone of treatment protocols for infected patients who are critically ill (1, 2). As hydrophilic small molecules with predominant renal elimination, they exhibit wide intra- and interindividual pharmacokinetic (PK) variability (3). Estimates suggest that the current “one size fits all” approach to β-lactam dosing in patients who are critically ill may result in at least a 30-fold intraindividual variation in concentrations observed (4), which makes the therapeutic response unpredictable. These drugs exhibit time-dependent bacterial killing. Concentrations of β-lactam that are too low increase the risk of clinical and microbiologic failure and the development of antibiotic resistance (4–7). Concentrations of β-lactam that are too high increase the risk of dose-dependent adverse effects, most notably, potentially irreversible neurotoxicity (8). Individualized β-lactam PK models developed to optimize the prediction of drug concentrations from the start of therapy and β-lactam therapeutic drug monitoring (TDM) to adjust doses in response to observed drug levels may reduce this variability. Unfortunately, the lack of a commercially available assay approved by the US Food and Drug Administration for monitoring β-lactam concentrations hinders the ability of many healthcare organizations to test locally, to develop individualized initial dosing models, and to subsequently adjust dosing if concentrations are too high or too low during a course of treatment.
Recently, the use of scavenged samples for PK modeling has been suggested (9, 10). This approach uses residual specimens collected for routine clinical care to build PK models, especially in difficult-to-sample populations such as patients who are critically ill in the intensive care units (ICUs) where β-lactams are often utilized. However, previous literature has raised significant concerns about concentration and temperature-dependent β-lactam degradation over time in plasma (11–13). One study found, for example, significant piperacillin degradation after 24 h at ambient temperature, which biased the clearance and volume of distribution estimates in PK models by 30% (11). The purpose of this study was to validate a rapid LC-MS/MS method for TDM of FEP, MEM, and TZP in serum and to further characterize the stability of each drug at different concentrations (e.g., low, medium, high), time intervals, and various storage conditions to facilitate the use of residual patient samples for future development of potential dosing models.
MATERIALS AND METHODS
The chemicals used in this study were, at the minimum, HPLC grade. FEP, MEM, and TZP were purchased from Sigma-Aldrich. Cefepime-d3, meropenem-d6, piperacillin-d5, and tazobactam sodium salt-15N3 were purchased from Toronto Research Chemicals Inc.
Standard and Quality Control Samples
Standards (0.5, 5, 20, 40, and 60 μg/mL) and 3 levels of QC samples (2.5, 15, and 45 μg/mL) were prepared in pooled blank normal human serum (Utak Laboratories Inc.), aliquoted, and stored frozen (−80 °C).
Internal Standards
Cefepime-d3, meropenem-d6, piperacillin-d5, and tazobactam sodium salt-15N3 at 1 μg/mL were prepared in 80% acetonitrile and 20% clinical laboratory reagent water (CLRW).
Patient Samples
Mayo Clinic Institutional Review Bboard approval was obtained (number 18-004992). Utilization reports were used to identify patients in the ICU who were treated with FEP, MEM, or TZP. Human residual serum samples stored at 4 °C after routine work were pulled, deidentified, and assigned a unique code by designated study staff. The samples were then immediately stored at −80 °C until further use.
Sample Preparation
Serum samples, standards, and QC samples (50 μL) were precipitated with acetonitrile (250 μL) containing isotopically labeled internal standards (cefepime-d3, meropenem-d6, piperacillin-d5, and tazobactam sodium salt-15N3). Following mixing and centrifugation (2050 RCF/3000×g; 5 min), an aliquot (50 μL) of the supernatant was diluted with 800 μL CLRW.
LC-MS/MS Analysis
Next, 10 μL was analyzed by LC-MS/MS with chromatographic separation achieved using an Agilent Select Stream LC system equipped with an Agilent Eclipse Plus C18 (2.1 × 50 mm, 1.8 μm) column heated to 30 °C. Compounds were eluted off the column using a linear gradient. Mobile phase A was 10 mM ammonium formate in water with 0.1% formic acid. Mobile phase B was 0.1% formic acid in acetonitrile. The total run time was 9 min with a flow rate of 0.3 mL/min; the first 2-min mobile phase B was set at 2%, followed by 10% mobile phase B for 3 min, and then 95% mobile phase B for 1 min, after which it went back to 2% mobile phase B. Eluting compounds were detected using an Agilent 6495 Mass Spectrometer operated in positive (electrospray ionization) ion mode with dynamic multiple-reaction monitoring. The source parameters were as follows: gas temperature, 180 °C; gas flow, 19 L/min; sheath gas temperature, 350 °C; sheath gas flow, 11 L/min; and capillary voltage, 3000 V. Each analyte of interest was identified by retention time, and quadrupole 1/quadrupole 3 mass/charge (Q1/Q3 m/z) ion-pair ratios using Agilent MassHunter software. The mass transitions for all drugs and internal standards are listed in Supplemental Table 1.
Reproducibility
Blank human serum pools were spiked with certified reference standards of each compound at low, middle, and high concentrations (approximately 2, 25, and 40 μg/mL) and were measured 20 times within 1 run for intra-assay precision and over 20 days for interassay precision. The intraday and interday precision was expressed as the CV at each concentration.
Linearity
A minimum of 5 samples replicated over 5 days with concentrations spanning the linear range were analyzed, with a new calibration curve established for each run. The highest and lowest concentrations were within 10% of the upper limit of quantification (ULOQ) and lower limit of quantification (LLOQ), respectively. Acceptable samples could be another lot of calibrators run as patient samples, combined patient samples, spiked samples, or serially diluted high patient samples.
Accuracy and Recovery Studies
Accuracy was assessed based on comparison of at least 40 samples across the analytic measuring range (AMR) assayed by an outside reference laboratory (ARUP; quantitative bioassay) for MEM and piperacillin (data not shown) or by recovery studies and direct comparison to the spiked value for all compounds. Studies were performed over a minimum of 5 days, and linear regression analysis was performed.
Specificity
The top 25 prescribed drugs in the United States, common concomitant drugs, other antibiotics, antifungals, aminoglycosides, and common drugs of abuse were tested at supratherapeutic concentrations to assess their propensity to cause interferences that could result in a false-positive or -negative result (Supplemental Table 2). Additional studies at low, medium, and high concentrations of each analyte for hemolysis, icterus, and lipemia were performed using pooled serum spike with 5 levels of hemoglobin (0, 100, 250, 500, and 1000 mg/dL), intralipid (0, 50, 250, 500, and 1000 mg/dL), and bilirubin (0, 3, 15, 30, and 60 mg/dL), respectively.
Detection Limit
Twenty blank and LLOQ samples were run over a minimum of 10 days. The limit of detection was defined as the lowest concentration tested that had a peak area that was greater than or equal to the average of 20 blanks +3 SD.
Ion Suppression and Enhancement
Ten pools of random patient serum samples negative for any β-lactams were also compared with neat (CLRW) matrix. Three levels throughout the AMR were spiked with each drug. Samples 1–3 were low, approximately 10% ULOQ; samples 4–7 were mid, approximately 50% ULOQ; and samples 8–10 were high, approximately 80% ULOQ. The response of each compound in neat (CLRW) vs matrix (pools) was then compared. The response of the internal standard in neat vs matrix was also compared.
Stability
Analyte stability was determined by comparing the results of split pools freshly prepared and stored under ambient (20–25 °C), refrigerated (2–8 °C), and frozen (at or below −20 °C, and at or below −70 °C) conditions on days 1, 3, 7, 14, 21, and 35 and compared with day 0. The same pools were analyzed while undergoing at least 3 freeze/thaw cycles. Three different levels were analyzed in triplicate for each condition per day. Extracted sample stability was also determined by injecting a set of extracted samples and reinjecting the same samples at least 1 day later on the same instrument and under the same conditions. Extracted samples were stored at 2–8 °C.
Carryover
To assess carryover, blank matrix was spiked at a level that was at least 5 times the AMR for each analyte, followed by 5 serum blanks, and replicated over 5 days. Blanks following the high sample must have calculated concentrations of <50% of the LLOQ.
Anticoagulant and Tube Study
Determination of the effect of different collection tubes and anticoagulants was also conducted. Plasma(s)–sodium heparin, lithium heparin, K2 EDTA, sodium citrate, plasma separator tubes, and serum separator tubes were compared against serum. Prepared pools were spiked at 3 different concentrations, mixed well, and allowed to stand in the collection tubes for at least 30 min at room temperature. Tubes were centrifuged before analyzing. The content of each pool was analyzed in triplicate. The pools were then stored frozen (−70 °C or greater) in the collection tubes until the next day, when each pool was reanalyzed.
RESULTS AND DISCUSSION
Similar to previous publications (11, 14–19), we validated a robust but simple and faster method for the quantification of FEP, MEM, and TZP in serum using LC-MS/MS. With multiplexing on the Select Stream, individual sample analysis could be completed within 3 min compared with previous methods, which ranged from 4 to 30 min (11, 14). The validated analytic measurement range for each analyte was 0.5–60 μg/mL, covering the clinically relevant concentrations in serum for each analyte. The limit of detection was <10% of the LLOQ for each analyte. The average (n = 5) linear regression of each analyte demonstrated slope = 1.0 ± 0.1, r2 ≥ 0.990, and a y-intercept 95% CI that included zero. Intra- and interassay precision CVs were <5% across the analytic range for each analyte (Table 1). A minimum of 40 patient or blinded samples were validated against an existing reference assay or compared with a known spiked value. The results for each analyte were compared using linear regression and demonstrated slope = 1.0 ± 0.1, r2 ≥ 0.980, and a y-intercept 95% CI that includes zero (Fig. 1).
Table 1.
Intra- and interday precision of FEP at different concentrations including LLOQ.
| Intraday (n = 20) | Interday (n = 20) | |||
|---|---|---|---|---|
| Drug | Concentration | CV (%) | Concentration | CV(%) |
| FEP | 0.47 | 4.0 | ||
| 2.45 | 4.7 | 2.08 | 2.0 | |
| 24.09 | 3.0 | 21.32 | 1.9 | |
| 43.06 | 2.2 | 38.50 | 2.6 | |
| MEM | 0.45 | 2.9 | ||
| 2.33 | 4.4 | 2.29 | 2.8 | |
| 23.08 | 2.0 | 23.82 | 2.6 | |
| 37.86 | 1.9 | 43.90 | 2.4 | |
| Tazobactam | 0.42 | 2.8 | ||
| 2.25 | 4.0 | 2.06 | 1.9 | |
| 22.54 | 0.9 | 21.00 | 1.9 | |
| 37.31 | 1.3 | 38.53 | 1.6 | |
| Piperacillin | 0.43 | 3.2 | ||
| 2.31 | 4.9 | 2.05 | 3.6 | |
| 22.81 | 2.0 | 20.92 | 2.6 | |
| 39.66 | 3.3 | 39.77 | 4.5 | |
Three different spiked QC levels were also tested.
Fig. 1.
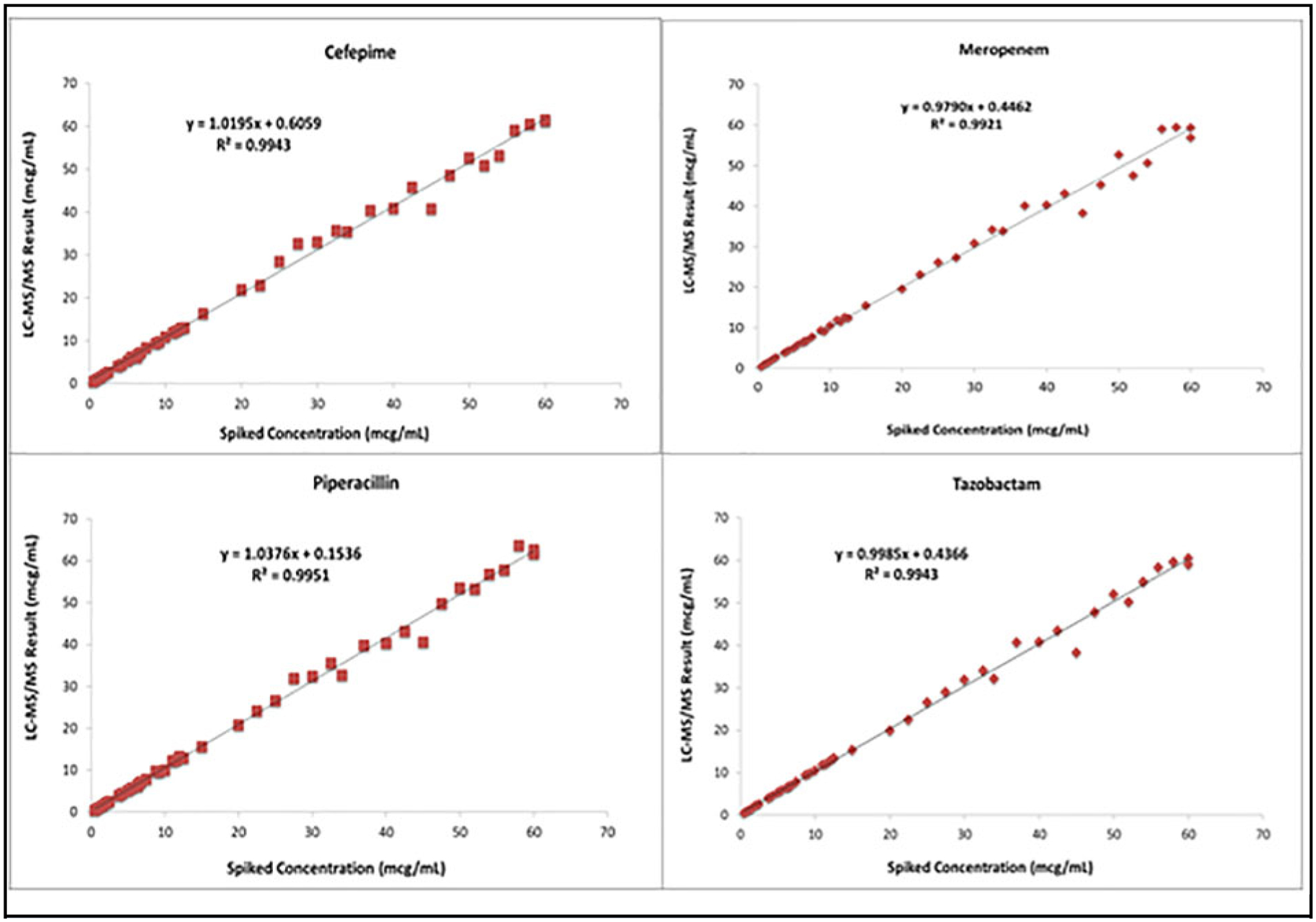
Recovery studies for (A) FEP, (B) MEM, (C) piperacillin, and (D) tazobactam.
Absence of interference from the top 25 prescribed drugs, concomitant drugs, or common drugs of abuse was observed such that no quantifiable peak was observed at the retention time of the analytes or internal standards. Values of FEP, MEM, and TZP were also unaffected in samples that were grossly lipemic, icteric, or hemolyzed with the average percentage of difference from the nonhemolyzed, nonlipemic, or nonicteric samples being ≤7% at low to high concentrations of the interferent (data not shown). Minimal ion suppression or enhancement due to the matrix effect was also observed and was consistent between the analyte and internal standard throughout the AMR. The average percentage of ion suppression for FEP, MEM, and tazobactam was 1.4%, 7.4%, and 4.5%, respectively. The average percentage of enhancement for piperacillin was 5.9%. No significant carryover was seen following a sample containing 500 μg/mL of each analyte. In addition, sample dilution effects were not observed. For all analytes, 2-, 10-, and 20-fold serum dilution demonstrated average concentrations that were ≤4% of their nominal concentration, with CVs ≤5% indicating high (toxic) concentrations of FEP, MEM, and TZP that can be accurately measured.
The effect of different anticoagulants and separator gels (e.g., plasma separator tube and serum separator tubes) were assessed and compared with spiked drug in regular serum tubes at 3 different concentrations after 24 h. The average difference compared with the serum was <10%, with no individual difference >20% for each tube and anticoagulant tested (data not shown).
Application of the Assay
For potential opportunistic sampling for PK modeling, residual serum was validated in this method, as it is frequently available. Patients who are commonly prescribed β-lactams and are treated in the ICU have routinely drawn basic chemistry tests, which typically require a serum sample. In addition, serum is the traditional sample most often collected for TDM. Consequently, the preanalytic stability of each β-lactam was further characterized in serum under ambient (20–25 °C), refrigerated (2–8 °C), and frozen (at or below −20 °C and at or below −70 °C) conditions. β-Lactams are well known for their instability in serum samples due to nonenzymatic hydrolyzation of the lactam ring. This is consistent with our own observations, in which values for each analyte decreased significantly at ambient (20–25 °C), refrigerated (2–8 °C), and frozen (−20 °C) temperatures over a time period of 35 days but were stable when stored at −70 °C or below with up to 3 freeze/thaw cycles (Figs. 2–5). Benchtop stability at approximately 3, 15, and 45 μg/mL was also assessed and shown up to 4 h at ambient conditions, with a mean difference ≤7% for each analyte. Refrigerated stability was also shown up to 24 h for all compounds, with an average difference ≤12% and no individual difference >15%.
Fig. 2.
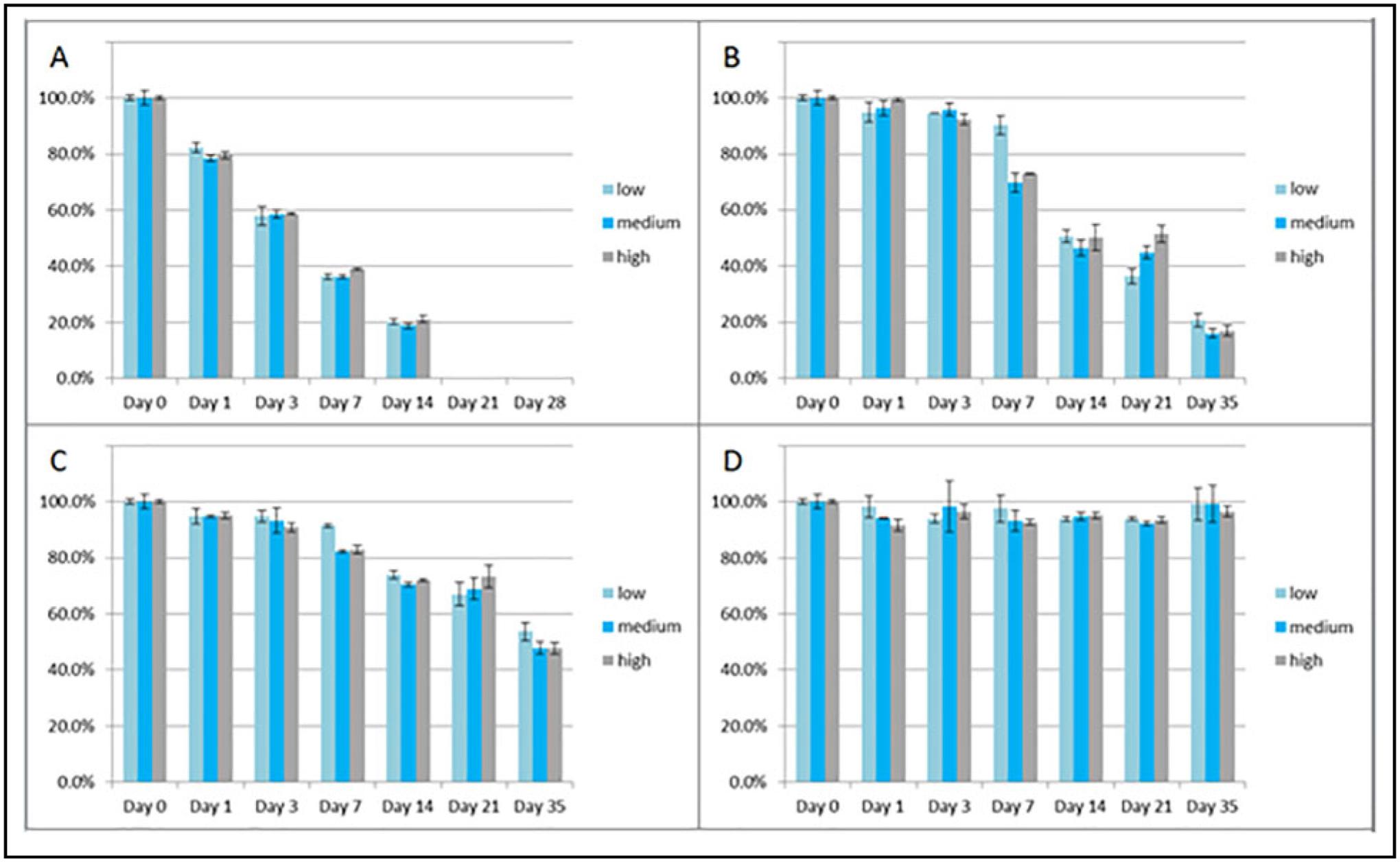
Stability of FEP in triplicate at low (approximately 3 μg/mL), medium (approximately 15 μg/mL), and high (approximately 45 μg/mL) concentrations at ambient (A: 20–25 °C), refrigerated (B: 2–8 °C), and rozen (C: at or below −20 °C; and D: at or below −70 °C) conditions up to 35 days. Note: ambient conditions were only done up to 14 days.
Fig. 5.
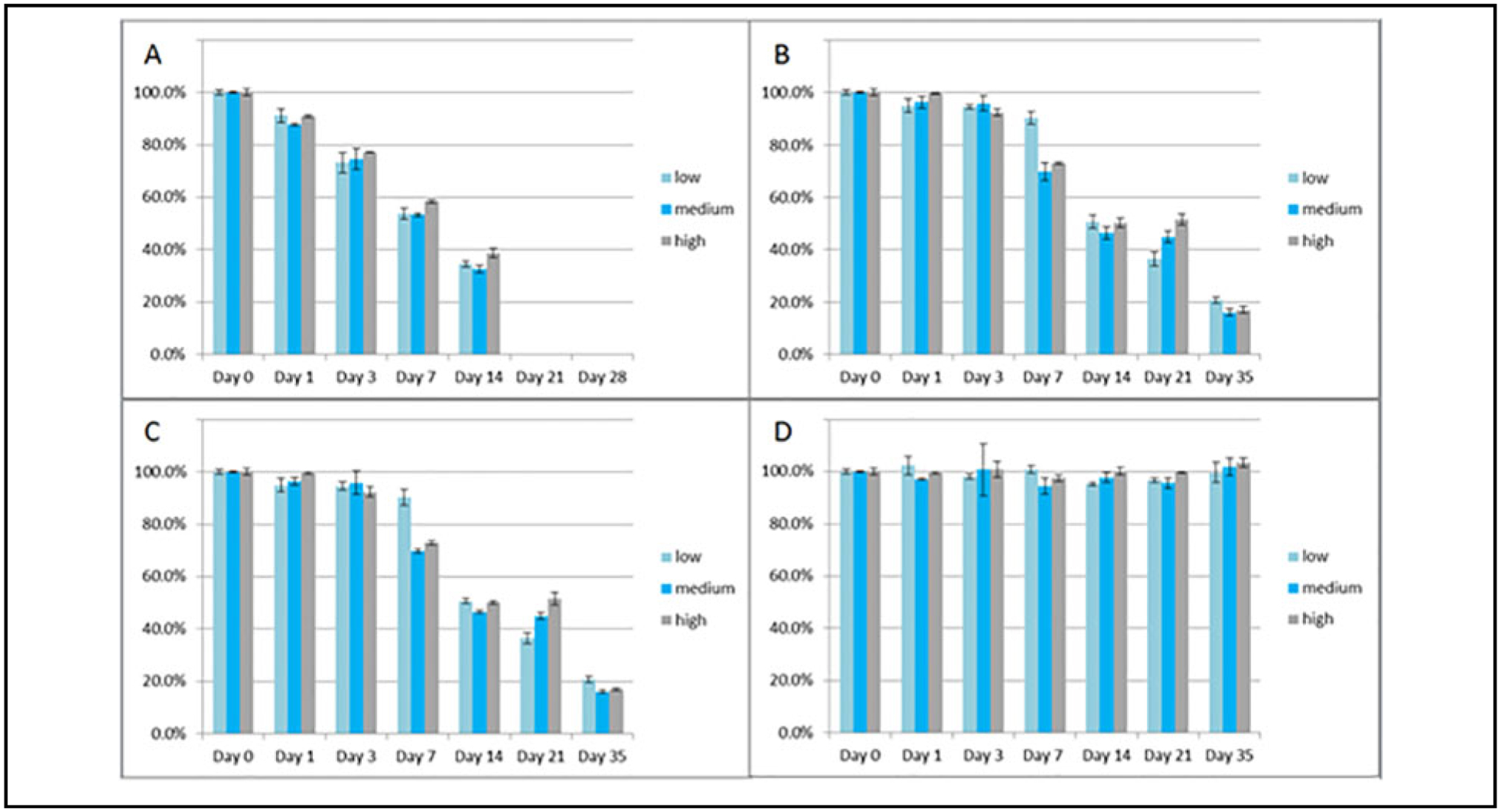
Stability of tazobactam in triplicate at low (approximately 3 μg/mL), medium (approximately 15 μg/mL), and high (approximately 45 μg/mL) concentrations at ambient (A: 20–25 °C), refrigerated (B: 2–8 °C), and frozen (C: at or below −20 °C; and D: at or below −70 °C) conditions up to 35 days. Note: ambient conditions were only done up to 14 days.
The combined validation data demonstrate a simple, rapid, and accurate LC-MS/MS assay for the determination of FEP, MEM, and TZP concentrations in serum. With tight precision characteristics (CV ≤5%) for all analytes, a clinically relevant measuring range, and good accuracy, this assay can be used to further establish the relationship between the serum concentrations and the clinical response to help clarify the role of TDM.
Clinical Significance of TDM for β-Lactams
The primary determinant of treatment success for β-lactams is the percentage of time that the drug level exceeds the organism minimum inhibitory concentration during the dosing interval, as they exhibit time-dependent bacterial killing. In critically ill patients treated with β-lactams, it has been shown that drug concentrations can vary almost 500-fold between patients (4, 20, 21), which likely contributes to noninferiority observed in studies of prolonged infusions and loading doses rather than superiority. In sepsis, in which early appropriate and adequate antibiotics determine patient survival, β-lactam levels fall below targets as early as 90 min into the 6- to 12-h dosing interval, leading to considerable time without sufficient drug at the site of action and the organism at sustained sublethal drug levels (22). Patients with low β-lactam levels risk propagation of resistance and have a 1.5-fold higher risk of a negative clinical outcome, defined by clinical failure, antibiotic escalation, antibiotic reinitiation, or death.
A chief contributor to inter- and intraindividual β-lactam–level variability in the ICU is limitation of the existing approach to kidney function assessment (20, 23). Existing models use serum creatinine to estimate kidney function and to determine clearance of FEP, MEM, and TZP (24–26). Creatinine is an insensitive and nonspecific marker of kidney function in the ICU given the many nonrenal factors that alter its production and elimination (27). As the terminal byproduct of skeletal muscle catabolism, creatinine is affected by deconditioning, altered muscle mass, and dietary changes that are incompletely accounted for in creatinine clearance equations and common in the ICU. With routine creatinine-based β-lactam dosing, 30%–50% of patients in the ICU failed to achieve their target drug level (22). As a result, accessibility to a rapid TDM assay could be used to verify that appropriate therapeutic concentrations are achieved.
CONCLUSION
This study reports on the development and validation of a rapid LC-MS/MS method for TDM of FEP, TZP, and MEM in serum. With well-characterized preanalytic stability, the use of residual serum samples for PK modeling can now be performed.
If using residual serum specimens for PK modeling, samples should be processed within 4 h if kept at ambient temperature and within 24 h if stored refrigerated. After processing, long-term storage of samples should be kept frozen at −70 °C or below.
Supplementary Material
Fig. 3.
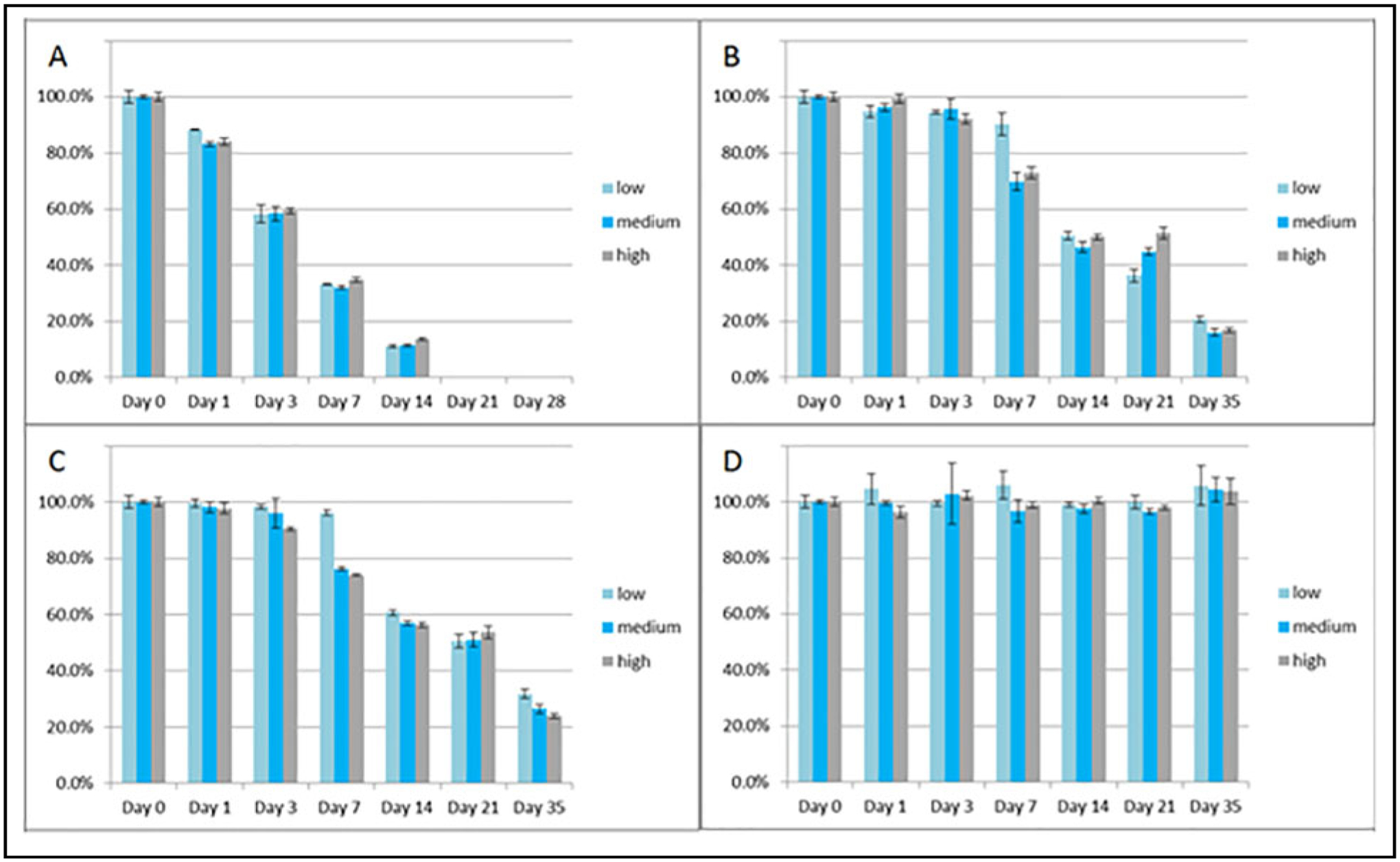
Stability of meropenem in triplicate at low (approximately 3 μg/mL), medium (approximately 15 μg/mL), and high (approximately 45 μg/mL) concentrations at ambient (A: 20–25 °C), refrigerated (B: 2–8 °C), and frozen (C: at or below −20 °C; and D: at or below −70 °C) conditions up to 35 days. Note: ambient conditions were only done up to 14 days.
Fig. 4.
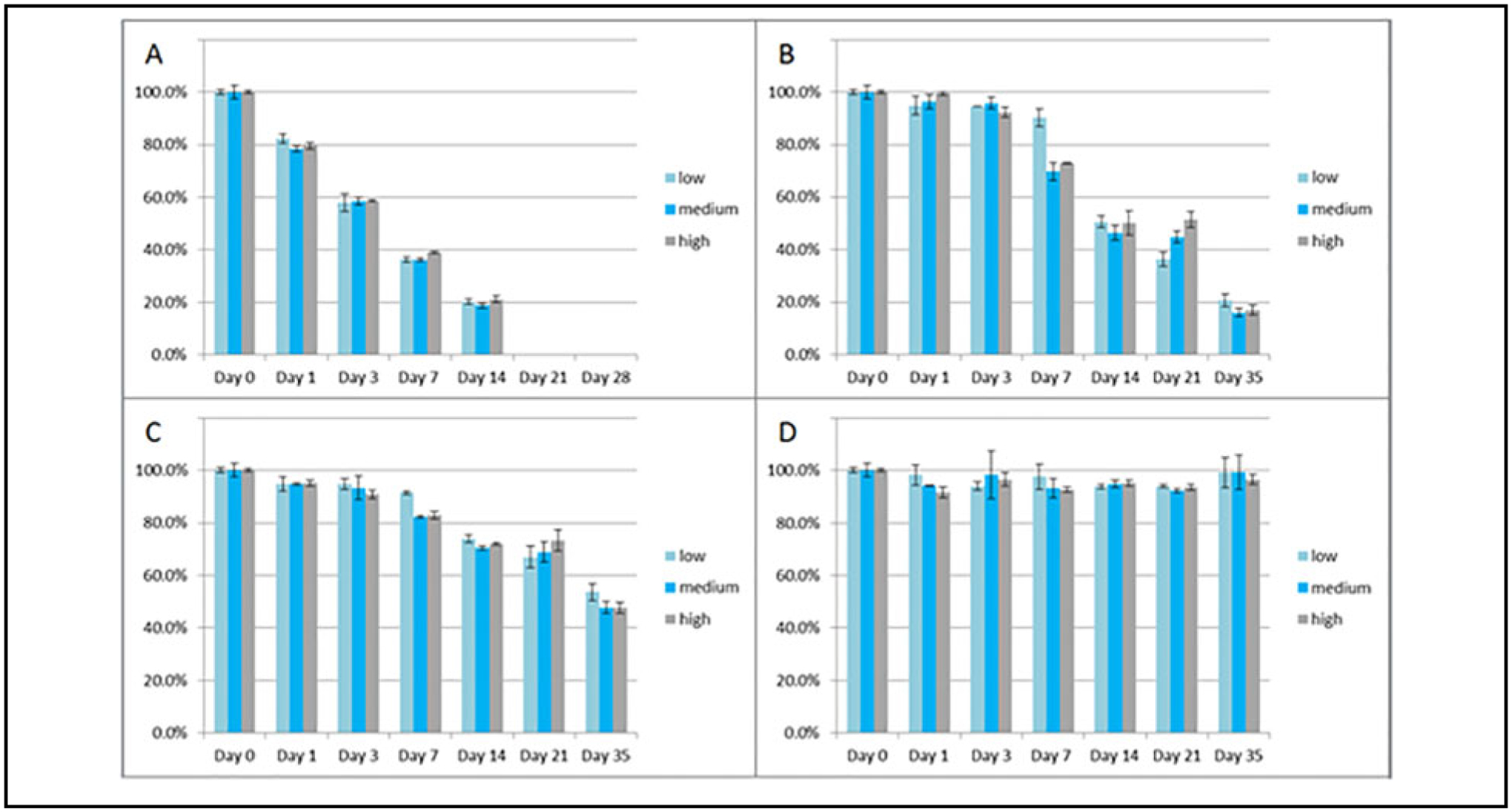
Stability of piperacillin in triplicate at low (approximately 3 μg/mL), medium (approximately 15 μg/mL), and high (approximately 45 μg/mL) concentrations at ambient (A: 20–25 °C), refrigerated (B: 2–8 °C), and frozen (C: at or below −20 °C; and D: at or below −70 °C) conditions up to 35 days. Note: ambient conditions were only done up to 14 days.
IMPACT STATEMENT.
β-Lactam antibiotics exhibit time-dependent killing, and bacterial resistance is a concern. In patients who are critically ill, in whom these medications are commonly used, altered pharmacokinetics can result in insufficient serum concentrations at standard dosages. This article describes well-characterized stability studies and a validated analytic (LC-MS/MS) method for cefepime, meropenem, piperacillin, and tazobactam in serum. Residual specimens can now be used for pharmacokinetic modeling, which may lead to individualized dosing and improved patient care.
Role of Sponsor:
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
Nonstandard Abbreviations:
- FEP
cefepime
- MEM
meropenem
- TZP
piperacillin/tazobactam
- PK
pharmacokinetics
- TDM
therapeutic drug monitoring
- ICU
intensive care unit
- CLRW
clinical laboratory reagent water
- ULOQ
upper limit of quantification
- LLOQ
lower limit of quantification
- AMR
analytic measuring range
Footnotes
Publisher's Disclaimer: Disclaimer:
Publisher's Disclaimer: The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
SUPPLEMENTAL MATERIAL
Supplemental material is available at The Journal of Applied Laboratory Medicine online.
Authors’ Disclosures or Potential Conflicts of Interest:Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:Employment or Leadership: P.J. Jannetto, AACC. Consultant or Advisory Role: P.J. Jannetto, Thermo Fisher Scientific, Roche Diagnostics. Stock Ownership: None declared. Honoraria: None declared. Research Funding: This project was supported in part by the Mayo Clinic Critical Care Independent Multidisciplinary Program and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number K23AI143882 (PI; E.F. Barreto). Expert Testimony: None declared. Patents: None declared.
REFERENCES
- 1.Pinder N, Brenner T, Swoboda S, Weigand MA, Hoppe-Tichy T. Therapeutic drug monitoring of beta-lactam antibiotics—influence of sample stability on the analysis of piperacillin, meropenem, ceftazidime and flucloxacillin by HPLC-UV. J Pharm Biomed Anal 2017;143:86–93. [DOI] [PubMed] [Google Scholar]
- 2.Liu VX, Fielding-Singh V, Greene JD, Baker JM, Iwashyna TJ, Bhattacharya J, Escobar GJ. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med 2017;196:856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veiga RP, Paiva JA. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit Care 2018;22: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 2014;58:1072–83. [DOI] [PubMed] [Google Scholar]
- 5.Craig WA, Ebert SC. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis Suppl 1990;74:63–70. [PubMed] [Google Scholar]
- 6.McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 2008;31: 345–51. [DOI] [PubMed] [Google Scholar]
- 7.Wong G, Taccone F, Villois P, Scheetz MH, Rhodes NJ, Briscoe S, et al. Beta-lactam pharmacodynamics in gram-negative bloodstream infections in the critically ill. J Antimicrob Chemother 2020;75:429–33. [DOI] [PubMed] [Google Scholar]
- 8.Deshayes S, Coquerel A, Verdon R. Neurological adverse effects attributable to beta-lactam antibiotics: a literature review. Drug Saf 2017;40:1171–98. [DOI] [PubMed] [Google Scholar]
- 9.Laughon MM, Benjamin DK Jr, Capparelli EV, Kearns GL, Berezny K, Paul IM, et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol 2011;4:643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen-Wolkowiez M, Benjamin DK Jr., Ross A, James LP, Sullivan JE, Walsh MC, et al. Population pharmacokinetics of piperacillin using scavenged samples from preterm infants. Ther Drug Monit 2012;34:312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kipper K, Barker CIS, Standing JF, Sharland M, Johnston A. Development of a novel multipenicillin assay and assessment of the impact of analyte degradation: lessons for scavenged sampling in antimicrobial pharmacokinetic study design. Antimicrob Agents Chemother 2018;62:E01540–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshpande A, Baheti KG, Chatterjee NR. Degradation of β-lactam antibiotics. Curr Sci 2004;87:1684–95. [Google Scholar]
- 13.Bugnon D, Giannoni E, Majcherczyk P, Glauser MP, Moreillon P. Pitfalls in cefepime titration from human plasma: plasma- and temperature-related drug degradation in vitro. Antimicrob Agents Chemother 2002; 46:3654–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colin P, De BL, T’Jollyn H, Boussery K, Van BJ. Development and validation of a fast and uniform approach to quantify beta-lactam antibiotics in human plasma by solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Talanta 2013; 103:285–93. [DOI] [PubMed] [Google Scholar]
- 15.Carlier M, De Waele JJ, Verstraete AG, Stove V. Exploration of the pre-analytical stability of beta-lactam antibiotics in plasma and blood—implications for therapeutic drug monitoring and pharmacokinetic studies. Clin Chem Lab Med 2015;53:e227–30. [DOI] [PubMed] [Google Scholar]
- 16.Rigo-Bonnin R, Ribera A, Arbiol-Roca A, Cobo-Sacristan S, Padulles A, Murillo O, et al. Development and validation of a measurement procedure based on ultra-high performance liquid chromatography-tandem mass spectrometry for simultaneous measurement of beta-lactam antibiotic concentration in human plasma. Clin Chim Acta 2017;468:215–24. [DOI] [PubMed] [Google Scholar]
- 17.Verhoven SM, Groszek JJ, Fissell WH, Seegmiller A, Colby J, Patel P, et al. Therapeutic drug monitoring of piperacillin and tazobactam by RP-HPLC of residual blood specimens. Clin Chim Acta 2018;482:60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sime FB, Roberts MS, Roberts JA, Robertson TA. Simultaneous determination of seven beta-lactam antibiotics in human plasma for therapeutic drug monitoring and pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci 2014;960:134–44. [DOI] [PubMed] [Google Scholar]
- 19.Cazorla-Reyes R, Romero-Gonzalez R, Frenich AG, Rodriguez Maresca MA, Martinez VJ. Simultaneous analysis of antibiotics in biological samples by ultra high performance liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 2014;89:203–12. [DOI] [PubMed] [Google Scholar]
- 20.Ehmann L, Zoller M, Minichmayr IK, Scharf C, Maier B, Schmitt MV, et al. Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: a prospective observational study. Crit Care 2017;21:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlier M, Carrette S, Stove V, Verstraete AG, De Waele JJ. Does consistent piperacillin dosing result in consistent therapeutic concentrations in critically ill patients? A longitudinal study over an entire antibiotic course. Int J Antimicrob Agents 2014;43:470–3. [DOI] [PubMed] [Google Scholar]
- 22.Taccone FS, Laterre PF, Dugernier T, Spapen H, Delattre I, Wittebole X, et al. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care 2010;14:R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson JQ, Nolin TD. Pragmatic use of kidney function estimates for drug dosing: the tide is turning. Adv Chronic Kidney Dis 2018;25:14–20. [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Aziz MH, Sulaiman H, Mat-Nor MB, Rai V, Wong KK, Hasan MS, et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med 2016;42:1535–45. [DOI] [PubMed] [Google Scholar]
- 25.Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, et al. A multicenter randomized trial of continuous versus intermittent beta-lactam infusion in severe sepsis. Am J Respir Crit Care Med 015;192:1298–305. [DOI] [PubMed] [Google Scholar]
- 26.Pea F, Viale P, Cojutti P, Furlanut M. Dosing nomograms for attaining optimum concentrations of meropenem by continuous infusion in critically ill patients with severe gram-negative infections: a pharmacokinetics/pharmacodynamics-based approach. Antimicrob Agents Chemother 2012;56:6343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Inker LA. Assessment of glomerular filtration rate in health and disease: a state of the art review. Clin Pharmacol Ther 2017;102:405–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


